Abstract
Background
Cognitive decline may progress for decades before dementia onset. Better cardiovascular health (CVH) has been related to less cognitive decline, but it is unclear whether this begins early, for all racial subgroups, and all domains of cognitive function. The purpose of this study was to determine the impact of CVH on decline in the 2 domains of cognition that decline first in White and Black women at midlife.
Methods and Results
Subjects were 363 Black and 402 White women, similar in baseline age (mean±SD, 46.6±3.0 years) and education (15.7±2.0 years), from the Chicago site of the Study of Women's Health Across the Nation. Cognition, measured as processing speed and working memory, was assessed annually or biennially over a maximum of 20 years (mean±SD, 9.8±6.7 years). CVH was measured as Life's Essential 8 (blood pressure, body mass index, glucose, non‐high‐density lipoprotein cholesterol, smoking, physical activity, diet, sleep). Hierarchical linear mixed models identified predictors of cognitive decline with progressive levels of adjustment. There was a decline in processing speed that was explained by race, age, and the 3‐way interaction of race, CVH, and time (F 1,4308=8.8, P=0.003). CVH was unrelated to decline in White women but in Black women poorer CVH was associated with greater decline. Working memory did not decline in the total cohort, by race, or by CVH.
Conclusions
In midlife Black women, CVH promotion may be a target for preventing the beginnings of cognitive decline, thereby enhancing independent living with aging.
Keywords: cardiovascular health, cognitive decline, processing speed, racial differences
Subject Categories: Epidemiology, Lifestyle, Race and Ethnicity, Women, Cardiovascular Disease
Nonstandard Abbreviations and Acronyms
- CVH
cardiovascular health
- SWAN
Study of Women's Health Across the Nation
Research Perspective.
What Is New?
In midlife Black women, optimization of cardiometabolic risk factors may slow early decline in the cognitive domain of processing speed, which is essential to the maintenance of independence in aging.
What Question Should Be Addressed Next?
Confirmation in a clinical trial could determine whether optimization of cardiometabolic health in midlife Black women could slow cognitive aging, maximize independence, and reduce racial inequality in dementia risk.
Knowledge about midlife women's decline in cognitive function is limited for several reasons. With some exceptions, 1 , 2 , 3 , 4 , 5 , 6 longitudinal studies of cognitive function have focused on those 65 years of age and older, in part because dementia is rare before this age. However, dementia is often preceded by a decade or more of accelerated cognitive decline, 7 , 8 suggesting that subtle cognitive changes, indicative of later life dementia risk, may be occurring as early as the fourth decade of life. This was shown in primarily White people 9 but has not been studied in a more diverse population. Good cardiovascular health (CVH) has been associated with lower rates of cognitive decline in White 10 , 11 and Hispanic 12 individuals, suggesting that promotion of CVH may delay or prevent later life cognitive impairment. The ARIC (Atherosclerosis Risk in Communities) study evaluated the impact of CVH on 20‐year cognitive decline in a large cohort of Black and White participants 6 and found an association for both subgroups but one that was weaker for Black than White participants. Although the Black participants had lower education and lower CVH than the White participants, this heterogeneity was not explored as an explanation for the weaker effect between CVH and cognitive decline in Black participants, nor were gender‐specific associations reported.
A recent review in adults aged 65 and older 13 found that the prevalence of individual chronic health conditions (eg, hypertension, obesity, diabetes) and lifestyle factors (eg, smoking, low physical activity) differs between the races but does not fully explain differences in cognitive decline. However, these modifiable risk factors have typically been studied in isolation and not in combination. An exception is the ARIC study that conceptualized CVH as Life's Simple 7. 6 This index has recently been updated as Life's Essential 8, featuring a new scoring algorithm and the inclusion of sleep, in addition to blood pressure, body mass index, glucose, cholesterol, smoking, physical activity, and diet. 14 Using National Health and Nutrition Examination Survey data, Life's Essential 8 was found to be more sensitive to disparities in cardiovascular health across sociodemographic groups than Life's Simple 7. 15
By the 1990s, it was becoming evident that the first aspects of cognition to decline with age were processing speed and working memory. 16 , 17 Processing speed is speed of recognition, assessed as the ability to compare similarities and differences quickly and accurately among sets of letters, numbers, objects, pictures, or patterns. In women who were 5 years older than those in the present study, heart age, an index of cardiovascular health based on the Framingham equation, predicted decline in processing speed, but racial differences were not studied. 18 Working memory, an aspect of executive function, is assessed as the ability of a small amount of information to be held in mind and used in the execution of cognitive tasks. Because these 2 aspects are most sensitive to early aging, they were chosen for cognitive testing when the Chicago SWAN (Study of Women's Health Across the Nation) cohort was initiated in 1997 in women between the ages of 42 and 52 years.
CVH is vital for a healthy heart, a healthy brain, and healthy aging. Long‐term benefits on the brain have been less discernible among Black individuals relative to their White counterparts. The purpose of this report is to examine Black‐White differences among midlife women in the early decline of 2 aspects of cognition and the relationship between CVH and decline. Hypotheses of interest, based upon the literature, 6 , 9 were:
There will be a significant decline in processing speed and working memory that begins in early midlife and continues over the course of a maximum of 20 years in both Black and White women.
Better CVH will reduce the rate of decline in processing speed and working memory, and this association will be stronger in White than in Black women.
METHODS
SWAN provides access to public use data sets that include data from SWAN screening, the baseline visit, and follow‐up visits (https://agingresearchbiobank.nia.nih.gov/). To preserve participant confidentiality, some, but not all, of the data used for this article are contained in the public use data sets. A link to the public use data sets is also located on the SWAN website: http://www.swanstudy.org/swan‐research/data‐access/. Investigators who require assistance accessing the public use data set may contact the SWAN Coordinating Center at the following email address: swanaccess@edc.pitt.edu.
Study Population
Participants were women enrolled in SWAN at the Chicago site. SWAN is a 7‐site multiracial and multiethnic longitudinal study of women transitioning through menopause featuring ongoing annual interviews. Women were eligible for SWAN in 1996 to 1997 if they were between the ages of 42 and 52 years; not pregnant, lactating, or breastfeeding; had an intact uterus and at least 1 ovary; and had menstruated and were not using hormone therapy within 3 months of enrollment. The Chicago SWAN site features 857 participants recruited using a complete community census from 3 contiguous neighborhoods with a 72% participation rate and comparable socioeconomic status between the Black and White women. 1 Cognitive testing started in 1997 with annual or biennial follow‐up visits over the next 20 years. The approximate comparability on socioeconomic status between the Black and White women at the Chicago SWAN site provided design control for the common confound of race and socioeconomic status. Details of the SWAN recruitment and study protocol 19 and those of the Chicago SWAN cohort have been reported. 1 Cognitive assessment was conducted at the Chicago SWAN site between January 1997 and January 2017. The study was approved by the Rush University Medical Center Institutional Review Board, and all women provided written informed consent at every visit.
Procedures
All SWAN participants completed a standard protocol at every visit. At each follow‐up visit, time since baseline was calculated as the difference between exam date at the follow‐up visit and exam date at baseline. Women were eligible for this analysis if they had not reported a stroke before cognitive assessments started. Observations were censored at the time of a stroke after the first cognitive test. The analytic sample for this report was White and Black women who had at least 1 observation on both cognitive outcomes. Women were excluded if they never participated in the cognitive assessments (n=40) or did not report years of education (N=52). The analytic sample consisted of 765 women who provided 5079 processing speed and 4933 working memory assessments. The comparability between the total, included, and excluded cohorts is presented as Table S1.
Measures
Main Cognitive Outcomes
Processing speed was assessed using the interviewer‐administered Symbol Digit Modalities Test, in which participants identify as many of the 110 symbol‐digit matches as possible in 90 seconds. 20 Possible scores range from 0 to 110, with higher scores indicating better cognition. Working memory was assessed with the Digit Span Backward test, in which participants repeat backward as many increasingly long strings (3 to 8) of digits as possible without error, scored according to the Wechsler‐Memory Scale—Revised manual. 21 Possible scores range from 0 to 12, with higher scores indicating better cognition.
Predictors
Women self‐identified as Black or White at baseline and reported the number of years of education completed. Age at baseline was calculated from self‐reported date of birth and baseline exam date. CVH was assessed as Life's Essential 8 which includes blood pressure, non‐high‐density lipoprotein cholesterol, glucose level, body mass index, physical activity, smoking, diet, and sleep. 14 All of these variables were assessed at the SWAN baseline. Figure S1 presents the race‐specific distribution of Life's Essential 8.
Body mass index was calculated as weight in kilograms divided by height in meters squared. Standardized protocols were used to measure height and weight. Height was measured without shoes using a stadiometer. Weight was measured without shoes and in light indoor clothing using scales that were calibrated to a standard monthly. Resting blood pressure was measured with a mercury sphygmomanometer, using a standard protocol following at least a 5‐minute rest with participants seated. Blood pressure was measured in the right arm, using an appropriately sized cuff. Two sequential blood pressure readings were obtained, 2 minutes apart, and averaged. Blood samples for glucose and lipids were collected after a 12‐hour fast. Total cholesterol and high‐density lipoprotein cholesterol were analyzed on EDTA‐treated plasma using standard methods. 22 , 23 Use of antihypertensive as well as cholesterol‐lowering medication was self‐reported annually in SWAN and confirmed via medication review. Smoking status was assessed using standard questions from the American Thoracic Association, 24 with questions on ever smoking, amount smoked, and quit date. Physical activity was assessed with the sports and exercise questions of the Kaiser Physical Activity Survey. 25 , 26 Each type of sport/exercise activity was classified as moderate or vigorous. Diet was assessed by the Block Food Frequency Questionnaire 27 , 28 and scored according to Mellen. 29 Length of sleep was self‐reported in answer to the question “During the past month, how many hours of actual sleep did you get at night? (This may be different than the number of hours you spend in bed.)”
After converting glucose to hemoglobin A1c according to the American Diabetes Association, 30 we used the Life's Essential 8 scoring algorithm 14 to calculate the CVH component scores. CVH was calculated as the average of the component scores. Possible scores for CVH as well as each component score range from 0 to 100 with higher scores indicating better CVH.
Statistical Analysis
To make the cognitive outcomes comparable over time, observations were converted to Z scores by subtracting the baseline mean and dividing by the baseline SD. CVH as well as its components scores were also converted to Z scores. Hypotheses were examined using linear mixed models. Random effects were incorporated for individual starting level (ie, intercept) and individual rate of change (ie, time). Race, age at baseline, education, and CVH were modeled as fixed effects. Education and age at baseline were centered at their baseline mean. The main effect for time is interpreted as the slope of change where a negative coefficient reflects cognitive decline. The 2‐way interaction of a variable with time assesses the impact of that variable on decline in cognition.
Because practice effects can create a spurious improvement in studies of cognitive decline, we first determined whether there was a practice effect in either of the cognitive tests, which would be reflected in a positive estimate for time. Eliminating the first 2 observations minimizes practice effects. 31 Because practice effects were evident for working memory but not for processing speed, analyses for working memory deleted the first 2 assessments while analyses for processing speed began at the cognitive baseline.
Hierarchical modeling was progressive. Model 1 included main effects for time, race, age, education, and CVH as predictors of cognitive function at baseline. Model 2 added the 2‐way interactions of race, age, education, and CVH with time to determine the impact of these predictors on decline in cognitive function and, for completeness, the 2‐way interaction of race and CVH. Model 3 added the 3‐way interaction of race, CVH, and time to determine differential impact of race on the association between CVH and cognitive decline. The significant 3‐way interaction was confirmed in race‐specific models. In response to a reviewer request, further modeling was conducted replacing the total CVH score with each CVH component as predictor of decline in processing speed.
RESULTS
Baseline Characteristics
Table 1 shows the characteristics of the cohort at baseline. There were 363 Black and 402 White women, who were middle aged (mean±SD, 46.6±3.0 years) with no significant age difference by race. White women had slightly though significantly more education than Black women (years of education 15.9±2.1 versus 15.5±2.1, P=0.007, respectively). The mean±SD cognitive scores were 58.2±11.1 for processing speed and 6.7±2.1 for working memory with significantly higher (better) scores for White versus Black women, mean score differential 4.4 (95% CI, 2.8–6.0) for processing speed and 0.8 (95% CI, 0.5–1.1) for working memory (P<0.001 for both). CVH was lower for Black women than for White women on the total CVH score and all component scores except diet, tobacco use, and non‐high‐density lipoprotein. Participants were followed up for a maximum of 20 years (mean±SD, 9.8±6.7 years) with no difference in follow‐up time by race. The mean±SD time between assessments was 1.8±1.0 years. Table S1 compares the entire Chicago SWAN cohort to those included in this analytic sample and shows that those included were representative of the total cohort in sociodemographic factors, cognitive scores, and CVH, with the only exception being lower (worse) glucose scores in the excluded group. The distribution of the CVH scores by race (Figure S1) shows that scores were lower for Black than White women but that the variation was similar.
Table 1.
Characteristics at Cognitive Baseline Overall and by Race
| Overall | Black race | White race | P value | |
|---|---|---|---|---|
| N (%) | 765 | 363 (47.5) | 402 (52.5) | |
| Age at baseline, y, mean±SD | 46.6±3.0 | 46.8±3.0 | 46.4±2.9 | 0.057 |
| Education, y, mean±SD | 15.7±2.1 | 15.5±2.1 | 15.9±2.1 | 0.007 |
| Follow‐up time, y, mean±SD | 9.8±6.7 | 9.7±7.0 | 9.9±6.6 | 0.727 |
| Processing speed score, mean±SD | 58.2±11.1 | 55.9±12.2 | 60.3±9.7 | <0.001 |
| Working memory score, mean± SD | 6.7±2.1 | 6.3±2.0 | 7.1±2.0 | <0.001 |
| Cardiovascular health score*, mean±SD | 63.3±16.1 | 58.2±15.1 | 67.8±15.6 | <0.001 |
| Cardiovascular health score*, median (interquartile range) | 63.8 (52.9–75.6) | 58.8 (46.9–68.8) | 68.8 (57.5–80.0) | <0.001 |
| Diet score, mean±SD | 46.5±28.5 | 44.8±28.3 | 48.0±28.7 | 0.124 |
| Physical activity score, mean±SD | 45.2±43.4 | 35.3±40.8 | 54.2±43.8 | <0.001 |
| Tobacco score, mean±SD | 58.5±37.6 | 60.2±38.5 | 57.0±36.7 | 0.245 |
| Sleep score, mean±SD | 79.4±24.7 | 72.2±26.6 | 85.2±21.5 | <0.001 |
| Body mass index score, mean±SD | 59.2±34.3 | 51.8±32.7 | 65.9±34.3 | <0.001 |
| Non‐high‐density lipoprotein score, mean±SD | 70.4±29.9 | 68.6±30.0 | 72.1±29.8 | 0.110 |
| Glucose score, mean±SD | 88.6±22.4 | 85.5±24.9 | 91.4±19.6 | <0.001 |
| Blood pressure score, mean±SD | 59.3±33.4 | 49.6±33.8 | 68.1±30.5 | <0.001 |
| Financial strain, N (%) | 276 (36.1) | 149 (41.0) | 127 (31.6) | 0.007 |
Cardiovascular health assessed as Life's Essential 8 with the total score and each component score ranging from 0 to 100, with higher scores indicating better cardiovascular health.
Processing Speed
Hierarchical models are presented in Table 2. Model 1 presents main effects of variables assessed at baseline. There was a significant negative main effect of time, indicating that in the total cohort there was a small decline in processing speed by 0.010 units on the Z score scale per year. There were significant main effects for age (older women scored lower than younger women), race (Black women scored lower than White women), and education (women with more years of education scored higher than women with fewer years of education). CVH was unrelated to processing speed at baseline.
Table 2.
Hierarchical Models for Processing Speed (Z Score) Starting at Baseline
| Effect | Model 1, estimate (95% CI) | Model 2, estimate (95% CI) | Model 3, estimate (95% CI) |
|---|---|---|---|
| Intercept | 0.309 (0.233 to 0.385)‡ | 0.284 (0.204 to 0.364)‡ | 0.274 (0.193 to 0.354)‡ |
| Time, y | −0.010 (−0.015 to −0.005)‡ | −0.003 (−0.010 to 0.004) | 0 (−0.007 to 0.007) |
| Race* | −0.469 (−0.579 to −0.359)‡ | −0.419 (−0.534 to −0.304)‡ | −0.415 (−0.530 to −0.300)‡ |
| Age, y | −0.025 (−0.043 to −0.007)‡ | −0.017 (−0.035 to 0.002) | −0.017 (−0.035 to 0.002) |
| Education, y | 0.094 (0.068 to 0.121)‡ | 0.096 (0.069 to 0.124)‡ | 0.095 (0.068 to 0.123)‡ |
| CVH† | 0.030 (−0.029 to 0.088) | 0.023 (−0.058 to 0.105) | 0.047 (−0.036 to 0.130) |
| Race*×time | −0.015 (−0.025 to −0.005)‡ | −0.017 (−0.027 to −0.007)‡ | |
| Age×time | −0.003 (−0.004 to −0.001)‡ | −0.002 (−0.004 to −0.001)‡ | |
| Education×time | −0.001 (−0.003 to 0.002) | 0 (−0.003 to 0.002) | |
| CVH†×time | 0.004 (−0.002 to 0.009) | −0.004 (−0.011 to 0.004) | |
| Race*×CVH | −0.012 (−0.127 to 0.102) | −0.063 (−0.182 to 0.056) | |
| Race*×CVH†×time | 0.016 (0.005 to 0.026)‡ |
Time, baseline age, education in years; baseline age and education centered. Model 1: main effects. Model 2: Model 1 plus 2‐way interactions. Model 3: Model 2 plus 3‐way interaction. CVH indicates cardiovascular health.
Race: Referent is White.
CVH=Z score of Life's Essential 8.
Significant at P<0.05.
Model 2 adjusts for main effects at baseline and examines decline in processing speed using 2‐way interactions between each of the variables and time. It shows that independent of the significant race differential at baseline, there was a significant interaction of race by time (Black women declined faster than White women, independent of baseline differences). It also shows a significant interaction of age by time (women who started testing at an older age declined faster than younger women). CVH and education were unrelated to decline in processing speed.
Model 3 adjusts for all variables in Model 2 but adds the 3‐way interaction of race, CVH, and time. This significant 3‐way interaction shows that independent of racial differences at baseline and racial differences in decline, there was a significant difference by race in the association between CVH and decline in processing speed. This 3‐way interaction was confirmed in models stratified by race (Table S2) and is portrayed in the Figure. In contrast to the continuous CVH scores used in Table 2, the Figure divides women into 4 groups defined by race and CVH, dichotomized above or below the median, and shows predicted values with 95% confidence bands. White women with higher CVH scored better on processing speed throughout the follow‐up than White women with lower CVH scores, but neither group appeared to decline over time. Black women had lower scores than their White counterparts at baseline, but those with higher CVH did not show much decline in processing speed in contrast to those with lower CVH who showed a significant decrease of ≈10% from their initial score over the 2 decades of the study.
Figure . Processing speed by time, race, and cardiovascular health (Life's Essential 8).
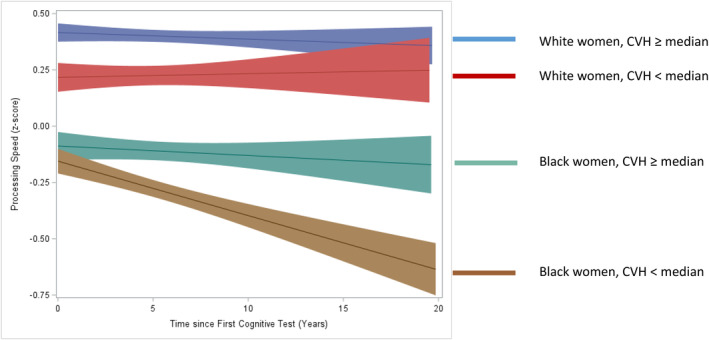
Median refers to the overall median of cardiovascular health (63.8). CVH indicates cardiovascular health.
Practice effects were not detected for processing speed, given that the time variable was negative; however, a sensitivity analysis deleting the first 2 observations produced the same results showing that the impact of CVH on cognitive decline was restricted to Black women. CVH component‐specific analyses are presented in Table S3. They show that blood pressure and smoking were the components that were primarily responsible for the significant 3‐way interaction. Additional models were run with further adjustments for medical conditions, number of medications, financial strain, menopausal status, but these further adjustments had no impact on the significance of the 3‐way interaction.
Working Memory
Practice effects in working memory were evident when all observations starting at baseline were included, suggested by a positive time effect (ie, participants appeared to be improving in working memory over time) (data not shown). Thus, working memory analyses adjusted for practice effects by starting at the third cognitive assessment. Table 3 presents hierarchical models for working memory. Model 1 shows that the control for practice effects worked based upon a negative, nonsignificant coefficient for Time interpreted as a small decline in working memory over time in the total cohort. Race and education were significant showing that Black women and those with lower education had lower scores on working memory at baseline. These main effects were unchanged when 2‐way interactions with time were added (Model 2) or the 3‐way interaction of race, CVH and time was added., none of which were significant. There was no impact of CVH on baseline working memory, decline in working memory, or differential decline in working memory by race.
Table 3.
Hierarchical Models for Working Memory (Z Score) Starting at Third Cognitive Assessment
| Effect | Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) |
|---|---|---|---|
| Intercept | 0.692 (0.581 to 0.803)‡ | 0.699 (0.581 to 0.817)‡ | 0.702 (0.584 to 0.820)‡ |
| Time, y | −0.006 (−0.013 to 0) | −0.006 (−0.016 to 0.003) | −0.008 (−0.018 to 0.003) |
| Race* | −0.693 (−0.854 to −0.532)‡ | −0.684 (−0.851 to −0.517)‡ | −0.686 (−0.853 to −0.519)‡ |
| Age, y | −0.076 (−0.159 to 0.006) | −0.054 (−0.140 to 0.031) | −0.054 (−0.139 to 0.031) |
| Education, y | 0.115 (0.077 to 0.153)‡ | 0.113 (0.074 to 0.152)‡ | 0.113 (0.074 to 0.153)‡ |
| CVH† | 0.039 (−0.047 to 0.125) | 0.004 (−0.117 to 0.125) | −0.003 (−0.125 to 0.120) |
| Race*×time | −0.004 (−0.019 to 0.010) | −0.004 (−0.018 to 0.011) | |
| Age×time | −0.008 (−0.016 to 0) | −0.008 (−0.016 to 0) | |
| Education×time | 0.001 (−0.003 to 0.004) | 0.001 (−0.003 to 0.004) | |
| CVH†×time | 0.005 (−0.002 to 0.013) | 0.008 (−0.003 to 0.018) | |
| Race*×CVH† | 0.039 (−0.128 to 0.206) | 0.052 (−0.120 to 0.224) | |
| Race*×CVH†×time | −0.005 (−0.019 to 0.010) |
Time, age at 3rd cognitive assessment, education in years; age and education centered. Model 1: main effects. Model 2: Model 1 plus 2‐way interactions. Model 3: Model 2 plus 3‐way interaction. CVH indicates cardiovascular health.
Race: Referent is White.
CVH= Z score of Life's Essential 8.
Significant at P<0.05.
DISCUSSION
An innovation of this report is its focus on specific domains of cognitive function. Because most studies evaluate cognitive decline with a global cognitive index, it precludes an ability to determine whether different cognitive domains have different patterns of decline. The domains chosen were processing speed and working memory because they are among the first to decline with aging 16 , 17 and thus an appropriate focus for a study aimed at detecting early cognitive decline in young women who began follow‐up at an average age of 46.6 years.
The first hypothesis tested was that there would be a significant decline in processing speed and working memory over the course of a maximum of 20 years of follow‐up in both Black and White women. This was based upon data from the Whitehall study that showed cognitive decline in early midlife in primarily White people 9 and is consistent with other reports from the national SWAN cohort study. 18 , 31 However, all of these prior reports were based upon participants who were an average of 7 years older than the participants in this current report. In these younger women, there was no evidence of decline in working memory. There was a decline in processing speed, but it differed by age and race. Older women and Black women declined faster than younger women or White women. Prior studies of racial differences in cognitive decline have produced inconsistent results, resulting in calls for further research. 32 , 33 The current findings contribute to this research by showing that in young women with an average starting age of 46.6 years, cognitive decline on the 2 measures expected to decline first was inconsistent across these measures of cognition. Decline was not observed in working memory. Decline in processing speed was evident overall but better explained by differential decline within race and age subgroups.
The second hypothesis tested was that better CVH would slow the rate of decline in processing speed and working memory for the total cohort and that this association would be stronger in White women than in Black women. This hypothesis was derived from the ARIC study that found an association between CVH and a global measure of cognitive decline in both White and Black participants, but one that was weaker in the Black participants. 6 In our study, CVH was unrelated to early decline in processing speed or working memory. The discrepancy between the results of these 2 studies is likely due to the differences in the cohorts studied. Participants in ARIC, relative to SWAN, were older (54 versus 47 years, respectively), and less well educated (≤12 years of education: 63% versus 13%, respectively), factors that have been related to cognition. 34
The ARIC finding of a weaker association between CVH and cognitive decline in Black versus White participants triggered our interest in exploring potential racial differences. The results provide a key finding of this report. Although Black women declined faster in processing speed than White women, there was heterogeneity within the Black subgroup. Those with lower CVH declined faster than those with higher CVH.
To study the biologic plausibility of this finding, we conducted supplementary components analyses of the global CVH score and found that the main drivers of this heterogeneity within the Black subgroup were blood pressure and smoking status (Table S3). The subgroup of Black women with low CVH (CVH below the median) had a significantly higher rate of hypertension than the Black women with high CVH (CVH above the median; 77.8% versus 41.6%, respectively) . Black Americans have greater exposure to the adverse effects of elevated blood pressure with earlier onset of about 5 years before their White counterparts and lower hypertension control. 35 Moreover, Black Americans have greater exposure to the adverse effects of nicotine, higher exposure to second‐hand smoke, 36 and greater use of menthol cigarettes 37 that mask the harshness of tobacco smoke and make it harder to quit. 36 , 37 , 38 , 39 Serum cotinine, a marker of nicotine, is higher in Black than White individuals possibly because of their lower cessation rates 38 , 39 and has been associated with worse cognitive functioning, including processing speed. 40
Strengths of the current study include the large population‐based cohort, a domain‐specific approach to cognitive decline, and use of a state‐of‐the‐art summary measure of CVH. The population‐based cohort featured large numbers of both Black and White middle‐aged women from a well‐characterized sample obtained by community‐based random sampling of a geographically defined population with low in‐ and outmigration, and a high 72% participation rate. Participants came from 3 contiguous neighborhoods on the south side of Chicago that differed in socioeconomic status across neighborhoods but equated socioeconomic status across racial subgroups within each neighborhood. This provided design control for the confound between race and socioeconomic status, resulted in more comparability between Black and White participants in education, age, and CVH than is commonly observed in studies of racial differences, and made long‐term longitudinal evaluations feasible. The domain‐specific approach to cognitive function was sensitive to early decline in young participants and made it possible to detect modifiable predictors of early decline. The cognitive domain of processing speed is a leading indicator of early cognitive aging and maintenance of independence. 17 , 41 , 42 It measures the ability to identify visual and verbal information quickly and accurately and is important for such activities of daily living as driving 42 and walking speed. 43 In contrast, we did not detect any decline in working memory, consistent with a prior report in slightly older women. 18 Use of a state‐of‐the‐art combined measure of CVH provides a novel contribution to a literature on cognitive aging that is characterized primarily by studies of individual medical conditions (ie, hypertension and diabetes) 44 and lifestyle factors (ie, physical activity and smoking). 4 , 44 , 45 , 46 , 47
Limitations are that the assessment of CVH included several self‐reported measures (ie, smoking, physical activity, diet, and sleep), a problem that is characteristic of many large epidemiological studies such as SWAN. Thus, differential accuracy of reporting across race cannot be ruled out. In addition, it was not possible to examine a range of potential explanations for the heterogeneity observed within the Black women on the association between CVH and decline in processing speed. In particular, structural racism leads to lower quality of health care and CVH 33 and could be a more fundamental explanation for the decline in processing speed than that provided by CVH. But unfortunately, structural racism was not part of the SWAN assessment battery.
Conclusions
In summary, decline in processing speed, a leading indicator of age‐related cognitive decline, 41 appears to begin early in the fourth decade of life primarily in those Black women with poorer CVH. These data provide a new rationale for optimizing CVH, particularly blood pressure control and smoking cessation, in Black women at midlife. By so doing, it could foster maintenance of independence with aging and reduction of health disparities in cognitive disorders.
Sources of Funding
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health, US Department of Health and Human Services, through the National Institute on Aging, the National Institute of Nursing Research, and the National Institutes of Health Office of Research on Women's Health (grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495, and U19AG063720). The Chicago site of the SWAN study was also supported by the Charles J. and Margaret Roberts Trust. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Disclosures
None.
Supporting information
Tables S1–S3
Figure S1
Acknowledgments
We thank Cheryl Gilhooly, PhD, MPH, RD for providing code for the diet score. Dr Janssen had full access to all the data in the study and takes responsibility for its integrity and the data analysis. We thank the study staff at each site and all the women who participated in SWAN. Clinical Centers: University of Michigan, Ann Arbor—Carrie Karvonen‐Gutierrez, PI 2021 to present, Siobán Harlow, principal investigator (PI) 2011 to 2021, MaryFran Sowers, PI 1994 to 2011; Massachusetts General Hospital, Boston, MA—Sherri‐Ann Burnett‐Bowie, PI 2020 to Present; Joel Finkelstein, PI 1999 to 2020; Robert Neer, PI 1994 to 1999; Rush University, Rush University Medical Center, Chicago, IL—Imke Janssen, PI 2020 to Present; Howard Kravitz, PI 2009 to 2020; Lynda Powell, PI 1994 to 2009; University of California, Davis/Kaiser—Elaine Waetjen and Monique Hedderson, PIs 2020 to Present; Ellen Gold, PI 1994 to 2020; University of California, Los Angeles—Arun Karlamangla, PI 2020 to Present; Gail Greendale, PI 1994 to 2020; Albert Einstein College of Medicine, Bronx, NY—Carol Derby, PI 2011 to present, Rachel Wildman, PI 2010 to 2011; Nanette Santoro, PI 2004 to 2010; University of Medicine and Dentistry—New Jersey Medical School, Newark—Gerson Weiss, PI 1994 to 2004; and the University of Pittsburgh, Pittsburgh, PA—Rebecca Thurston, PI 2020 to Present; Karen Matthews, PI 1994 to 2020. NIH Program Office: National Institute on Aging, Bethesda, MD—Rosaly Correa‐de‐Araujo 2020 to present; Chhanda Dutta 2016 to present; Winifred Rossi 2012 to 2016; Sherry Sherman 1994 to 2012; Marcia Ory 1994 to 2001; National Institute of Nursing Research, Bethesda, MD—Program Officers. Central Laboratory: University of Michigan, Ann Arbor—Daniel McConnell (Central Ligand Assay Satellite Services). Coordinating Center: University of Pittsburgh, Pittsburgh, PA—Maria Mori Brooks, PI 2012 to present; Kim Sutton‐Tyrrell, PI 2001 to 2012; New England Research Institutes, Watertown, MA—Sonja McKinlay, PI 1995 to 2001. Steering Committee: Susan Johnson, Current Chair; Chris Gallagher, Former Chair.
This article was sent to Jose R. Romero, MD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.031619
For Sources of Funding and Disclosures, see page 8.
References
- 1. Meyer PM, Powell LH, Wilson RS, Everson‐Rose SA, Kravitz HM, Luborsky JL, Madden T, Pandey D, Evans DA. A population‐based longitudinal study of cognitive functioning in the menopausal transition. Neurology. 2003;61:801–806. doi: 10.1212/01.WNL.0000079051.91602.E2 [DOI] [PubMed] [Google Scholar]
- 2. Epperson CN, Sammel MD, Freeman EW. Menopause effects on verbal memory: findings from a longitudinal community cohort. J Clin Endocrinol Metabol. 2013;98:3829–3838. doi: 10.1210/jc.2013-1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gottesman RF, Schneider AL, Albert M, Alonso A, Bandeen‐Roche K, Coker L, Coresh J, Knopman D, Power MC, Rawlings A. Midlife hypertension and 20‐year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol. 2014;71:1218–1227. doi: 10.1001/jamaneurol.2014.1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lo AH, Woodman RJ, Pachana NA, Byrne GJ, Sachdev PS. Associations between lifestyle and cognitive function over time in women aged 40–79 years. J Alzheimers Dis. 2014;39:371–383. doi: 10.3233/JAD-130971 [DOI] [PubMed] [Google Scholar]
- 5. Yaffe K, Bahorik AL, Hoang TD, Forrester S, Jacobs DR, Lewis CE, Lloyd‐Jones DM, Sidney S, Reis JP. Cardiovascular risk factors and accelerated cognitive decline in midlife: the CARDIA study. Neurology. 2020;95:e839–e846. doi: 10.1212/WNL.0000000000010078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. González HM, Tarraf W, Harrison K, Windham BG, Tingle J, Alonso A, Griswold M, Heiss G, Knopman D, Mosley TH. Midlife cardiovascular health and 20‐year cognitive decline: Atherosclerosis Risk in Communities Study results. Alzheimers Dement. 2018;14:579–589. doi: 10.1016/j.jalz.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Amieva H, Le Goff M, Millet X, Orgogozo JM, Pérès K, Barberger‐Gateau P, Jacqmin‐Gadda H, Dartigues JF. Prodromal Alzheimer's disease: successive emergence of the clinical symptoms. Ann Neurol. 2008;64:492–498. doi: 10.1002/ana.21509 [DOI] [PubMed] [Google Scholar]
- 8. Wilson RS, Leurgans SE, Boyle PA, Bennett DA. Cognitive decline in prodromal Alzheimer disease and mild cognitive impairment. Arch Neurol. 2011;68:351–356. doi: 10.1001/archneurol.2011.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singh‐Manoux A, Kivimaki M, Glymour MM, Elbaz A, Berr C, Ebmeier KP, Ferrie JE, Dugravot A. Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. BMJ. 2012;344:d7622. doi: 10.1136/bmj.d7622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Samieri C, Perier M, Gaye B, Proust‐Lima C, Helmer C, Dartigues J, Berr C, Tzourio C, Empana J. Association of cardiovascular health level in older age with cognitive decline and incident dementia. JAMA. 2018;320:657–664. doi: 10.1001/jama.2018.11499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Speh A, Wang R, Winblad B, Kramberger MG, Bäckman L, Qiu C, Laukka EJ. The relationship between cardiovascular health and rate of cognitive decline in young‐old and old‐old adults: a population‐based study. J Alzheimers Dis. 2021;84:1523–1537. doi: 10.3233/JAD-210280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gardener H, Wright CB, Dong C, Cheung K, DeRosa J, Nannery M, Stern Y, Elkind MS, Sacco RL. Ideal cardiovascular health and cognitive aging in the Northern Manhattan Study. J Am Heart Assoc. 2016;5:e002731. doi: 10.1161/JAHA.115.002731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Peterson RL, Fain MJ, A. Butler E, Ehiri JE, Carvajal SC. The role of social and behavioral risk factors in explaining racial disparities in age‐related cognitive impairment: a structured narrative review. Aging Neuropsychol Cognit. 2020;27:173–196. doi: 10.1080/13825585.2019.1598539 [DOI] [PubMed] [Google Scholar]
- 14. Lloyd‐Jones DM, Allen NB, Anderson CA, Black T, Brewer LC, Foraker RE, Grandner MA, Lavretsky H, Perak AM, Sharma G. Life's Essential 8: updating and enhancing the American Heart Association's construct of cardiovascular health: a presidential advisory from the American Heart Association. Circulation. 2022;146:e18–e43. doi: 10.1161/CIR.0000000000001078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lloyd‐Jones DM, Ning H, Labarthe D, Brewer L, Sharma G, Rosamond W, Foraker RE, Black T, Grandner MA, Allen NB. Status of cardiovascular health in US adults and children using the American Heart Association's new “Life's Essential 8” metrics: prevalence estimates from the National Health and Nutrition Examination Survey (NHANES), 2013 through 2018. Circulation. 2022;146:822–835. doi: 10.1161/CIRCULATIONAHA.122.060911 [DOI] [PubMed] [Google Scholar]
- 16. Farmer ME, White LR, Kittner SJ, Kaplan E, Moes E, Mcnamara P, Wolz MM, Wolf PA, Feinleib M. Neuropsychological test performance in Framingham: a descriptive study. Psychol Rep. 1987;60:1023–1040. doi: 10.1177/0033294187060003-201.1 [DOI] [PubMed] [Google Scholar]
- 17. Salthouse TA. Trajectories of normal cognitive aging. Psychol Aging. 2019;34:17–24. doi: 10.1037/pag0000288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Derby CA, Hutchins F, Greendale GA, Matthews KA, Sternfeld B, Everson‐Rose SA, Kazlauskaite R, Whitmer RA, Brooks MM. Cardiovascular risk and midlife cognitive decline in the Study of Women's Health Across the Nation. Alzheimers Dement. 2021;17:1342–1352. doi: 10.1002/alz.12300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sowers MF, Crawford S, Sternfeld B. SWAN: a multicenter, multiethnic, community‐based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, eds. Menopause: Biology and Pathobiology. Academic; 2000:175–188. [Google Scholar]
- 20. Smith A. Symbol Digit Modality Test Manual. Western Psychological Services; 1982. [Google Scholar]
- 21. Wechsler D. WMS‐R: Wechsler Memory Scale‐Revised: Manual. Psychological Corporation; 1987. [Google Scholar]
- 22. Warnick GR, Albers JJ. A comprehensive evaluation of the heparin‐manganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res. 1978;19:65–76. doi: 10.1016/S0022-2275(20)41577-9 [DOI] [PubMed] [Google Scholar]
- 23. Steiner PM, Freidel J, Bremner W, Stein JH. Standardization of micromethods for plasma cholesterol, triglyceride and HDL‐cholesterol with the lipid clinics' methodology. J Clin Chem Clin Biochem. 1981;19:850. [Google Scholar]
- 24. Ferris BG. Epidemiology standardization project (American Thoracic Society). Recommended respiratory disease questionnaires for use with adult and children in epidemiological research. Am Rev Respir Dis. 1978;118:7–53. [PubMed] [Google Scholar]
- 25. Ainsworth BE, Sternfeld B, Richardson MT, Jackson K. Evaluation of the Kaiser Physical Activity Survey in women. Med Sci Sports Exerc. 2000;32:1327–1338. doi: 10.1097/00005768-200007000-00022 [DOI] [PubMed] [Google Scholar]
- 26. Sternfeld B, Ainsworth BE, Quesenberry CP. Physical activity patterns in a diverse population of women. Prev Med. 1999;28:313–323. doi: 10.1006/pmed.1998.0470 [DOI] [PubMed] [Google Scholar]
- 27. Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data‐based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–469. doi: 10.1093/oxfordjournals.aje.a114416 [DOI] [PubMed] [Google Scholar]
- 28. Block G, Thompson FE, Hartman AM, Larkin F, Guire KE. Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1‐year period. J Am Diet Assoc. 1992;92:686–693. doi: 10.1016/S0002-8223(21)00707-0 [DOI] [PubMed] [Google Scholar]
- 29. Mellen PB, Gao SK, Vitolins MZ, Goff DC. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988–1994 and 1999–2004. Arch Intern Med. 2008;168:308–314. doi: 10.1001/archinternmed.2007.119 [DOI] [PubMed] [Google Scholar]
- 30. Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ; A1c‐Derived Average Glucose (ADAG) Study Group . Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31:1473–1478. doi: 10.2337/dc08-0545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Karlamangla AS, Lachman ME, Han W, Huang M, Greendale GA. Evidence for cognitive aging in midlife women: Study of Women's Health Across the Nation. PLoS One. 2017;12:e0169008. doi: 10.1371/journal.pone.0169008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Avila JF, Vonk JMJ, Verney SP, Witkiewitz K, Rentería MA, Schupf N, Mayeux R, Manly JJ. Sex/gender differences in cognitive trajectories vary as a function of race/ethnicity. Alzheimers Dement. 2019;15:1516–1523. doi: 10.1016/j.jalz.2019.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Johnson EE, Alexander C, Lee GJ, Angers K, Ndiaye D, Suhr J. Examination of race and gender differences in predictors of neuropsychological decline and development of Alzheimer's disease. Clin Neuropsychol. 2022;36:327–352. doi: 10.1080/13854046.2021.1940299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lövdén M, Fratiglioni L, Glymour MM, Lindenberger U, Tucker‐Drob EM. Education and cognitive functioning across the life span. Psychol Sci Public Interest. 2020;21:6–41. doi: 10.1177/1529100620920576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aggarwal R, Chiu N, Wadhera RK, Moran AE, Raber I, Shen C, Yeh RW, Kazi DS. Racial/ethnic disparities in hypertension prevalence, awareness, treatment, and control in the United States, 2013 to 2018. Hypertension. 2021;78:1719–1726. doi: 10.1161/HYPERTENSIONAHA.121.17570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Publication of Surgeon General's report on smoking and health. J Am Med Assoc. 1998;279:1776. doi: 10.1001/jama.279.22.1776-JWR0610-3-1 [DOI] [Google Scholar]
- 37. Villanti AC, Collins LK, Niaura RS, Gagosian SY, Abrams DB. Menthol cigarettes and the public health standard: a systematic review. BMC Public Health. 2017;17:1–13. doi: 10.1186/s12889-017-4987-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Caraballo RS, Holiday DB, Stellman SD, Mowery PD, Giovino GA, Muscat JE, Eriksen MP, Bernert JT, Richter PA, Kozlowski LT. Comparison of serum cotinine concentration within and across smokers of menthol and nonmenthol cigarette brands among non‐Hispanic black and non‐Hispanic white US adult smokers, 2001–2006. Cancer Epidemiol Biomarkers Prev. 2011;20:1329–1340. doi: 10.1158/1055-9965.EPI-10-1330 [DOI] [PubMed] [Google Scholar]
- 39. Holford TR, Levy DT, Meza R. Comparison of smoking history patterns among African American and white cohorts in the United States born 1890 to 1990. Nicotine Tob Res. 2016;18:S16–S29. doi: 10.1093/ntr/ntv274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fu Z, Qi G, Qu Z, Lin X, Xu L, Shen B, Dong F, Ge S. Higher blood cotinine level is associated with worse cognitive functioning in non‐smoking older adults. Front Neurosci. 2022;16:1080066. doi: 10.3389/fnins.2022.1080066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Finkel D, Reynolds CA, McArdle JJ, Pedersen NL. Age changes in processing speed as a leading indicator of cognitive aging. Psychol Aging. 2007;22:558–568. doi: 10.1037/0882-7974.22.3.558 [DOI] [PubMed] [Google Scholar]
- 42. Wadley VG, Bull TP, Zhang Y, Barba C, Bryan RN, Crowe M, Desiderio L, Deutsch G, Erus G, Geldmacher DS. Cognitive processing speed is strongly related to driving skills, financial abilities, and other instrumental activities of daily living in persons with mild cognitive impairment and mild dementia. J Gerontol A Biol Sci Med Sci. 2021;76:1829–1838. doi: 10.1093/gerona/glaa312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Welmer A, Rizzuto D, Qiu C, Caracciolo B, Laukka EJ. Walking speed, processing speed, and dementia: a population‐based longitudinal study. J Gerontol A Biol Sci Med Sci. 2014;69:1503–1510. doi: 10.1093/gerona/glu047 [DOI] [PubMed] [Google Scholar]
- 44. Reis JP, Loria CM, Launer LJ, Sidney S, Liu K, Jacobs DR, Zhu N, Lloyd‐Jones DM, He K, Yaffe K. Cardiovascular health through young adulthood and cognitive functioning in midlife. Ann Neurol. 2013;73:170–179. doi: 10.1002/ana.23836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Blondell SJ, Hammersley‐Mather R, Veerman JL. Does physical activity prevent cognitive decline and dementia? A systematic review and meta‐analysis of longitudinal studies. BMC Public Health. 2014;14:510. doi: 10.1186/1471-2458-14-510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yaffe K, Fiocco AJ, Lindquist K, Vittinghoff E, Simonsick EM, Newman AB, Satterfield S, Rosano C, Rubin SM, Ayonayon HN. Predictors of maintaining cognitive function in older adults: the Health ABC study. Neurology. 2009;72:2029–2035. doi: 10.1212/WNL.0b013e3181a92c36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vásquez E, Botoseneanu A, Bennett JM, Shaw BA. Racial/ethnic differences in trajectories of cognitive function in older adults: role of education, smoking, and physical activity. J Aging Health. 2016;28:1382–1402. doi: 10.1177/0898264315620589 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figure S1


