Increased blood pressure variability (BPV) is a risk factor for Alzheimer disease and cerebral small‐vessel disease (CSVD), 1 independent of blood pressure. Studies suggest BPV‐associated risk for Alzheimer disease may be more prominent in apolipoprotein E4 (APOE4) carriers, 2 but no studies to date have examined BPV‐associated risk for CSVD in APOE4 carriers. It also remains uncertain whether BPV elevation is a cause or a consequence of CSVD 3 or to what degree central autonomic network (CAN) dysfunction may contribute to BPV‐associated risk for CSVD.
Seventy independently living older adults (65.7% women) aged 55 to 89 years (70±7.31) with no history of stroke or Alzheimer disease underwent 5 minutes of resting beat‐to‐beat BPV monitoring, genetic testing for APOE genotype, and brain structural/functional magnetic resonance imaging to determine CSVD burden and CAN resting‐state functional connectivity. CSVD presence was defined by visual rating of the presence and extent of white matter lesions (WMLs), microbleeds, lacunes, and perivascular spaces. The CAN was based on a meta‐analysis of 43 studies 4 (Figure [A]). Causal mediation and simple moderation analysis evaluated BPV and CAN connectivity effects on CSVD in APOE4 carriers (n=37) and noncarriers (n=33) using a cross‐sectional study design. This study was approved by the University of Southern California and University of California, Irvine Institutional Review Boards, and all participants gave informed consent. The data that support the findings of this study are available upon reasonable request from the corresponding author.
Figure 10.1002/(ISSN)2047‐9980. The central autonomic network moderates the relationship between blood pressure variability and cerebral small‐vessel disease.
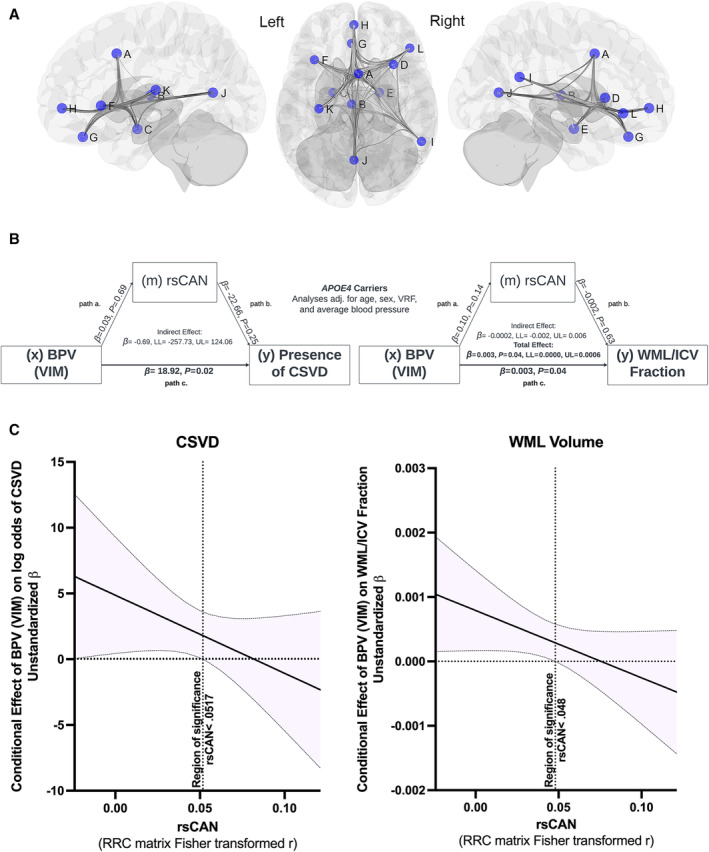
A, Central autonomic network (CAN) regions of interest (ROI), 4 defined by 5‐mm spheres within the A: midcingulate cortex; B: thalamus; C: left amygdala/hypothalamus; D: right anterior insula; E: right amygdala/hippocampal formation; F: left anterior insula; G: ventromedial prefrontal cortex/subgenual anterior cingulate cortex; H: pregenual anterior cingulate cortex; I: right angular gyrus/supramarginal gyrus; J: ventral posterior cingulate cortex/precuneus/lingual gyrus; K: left posterior insula; and L: right frontoinsular cortex. B, Simple mediation analyses showing the relationship between blood pressure variability (BPV) as variability independent of the mean (VIM) and log odds of cerebral small vessel disease (CSVD) presence and white matter lesion volume fraction of total intracranial volume (WML/ICV fraction) mediated by resting state central autonomic network functional connectivity (rsCAN) in APOE4 carriers. Significant relationships indicated by bold font (P<0.05). β=unstandardized regression coefficient. Path a×b=indirect effect, path c=direct effect, and direct effect+indirect effect=total effect. C, The conditional effect of BPV on odds of CSVD presence and WML/ICV fraction as a function of rsCAN in APOE4 carriers. Region of significance indicated by a vertical dashed line on each plot. The rsCAN metric is the Fisher transformation of the ROI‐to‐ROI bivariate correlation coefficient matrix (RRC) of the 12 CAN regions (average of 66 ROI‐pair correlations).
No significant differences in age, sex, vascular risk factors, blood pressure, CSVD features, or CAN connectivity were observed between APOE4 carrier status group. In subgroup linear and logistic regression analysis, higher BPV, quantified as variability independent of the mean, 2 was associated with increased log odds of CSVD presence (B=18.92, P=0.02), and was also associated with increased WML volume fraction (B=0.003, P=0.04) in APOE4 carriers, independent of age, sex, CAN connectivity, average blood pressure, and vascular risk factor burden. A similar relationship was not observed in noncarriers. Any potential mediation effect of CAN connectivity on the relationship between BPV and CSVD was not statistically significant in this analysis (Figure [B]). Instead, CAN connectivity was a statistically significant moderator of the relationship between BPV and CSVD in APOE4 carriers, whereby BPV effects on CSVD presence (B=36.43, P=0.02) and WML volume fraction (B=0.0006, P=0.01) were greater in those with lower CAN connectivity (−1 SD). The conditional effect of BPV on odds of CSVD presence and WML volume illustrates how BPV is more strongly correlated with CSVD as CAN connectivity diminishes (Figure [C]). Mediation and moderation analyses were performed in R (R Foundation for Statistical Computing, Vienna, Austria) using Hayes PROCESS macro model 1 (simple moderation) and model 4 (simple mediation) with x (independent variable)=BPV, y (dependent variable)=WML/IC fraction/Presence of CSVD, and w (moderator)/m (mediator)=CAN connectivity.
Older APOE4 carriers with elevated beat‐to‐beat BPV exhibited increased CSVD presence and severity, independent of age, average blood pressure, vascular risk factor burden, and CAN function. To our knowledge, this is the first study to report a relationship between BPV and CSVD specifically in APOE4 carriers. These findings are consistent with prior studies indicating stronger links between BPV and neuropathologic change in APOE4 carriers versus noncarriers. 2 These findings could also suggest that BPV and central autonomic dysfunction may have synergistic effects on CSVD in older APOE4 carriers, which may be related to their higher risk of cerebral amyloid angiopathy and blood–brain barrier disruption.
These results add to mounting evidence for the role of BPV as a risk factor for cerebrovascular disease, raising the question of what drives BPV elevation in older adults. There are multiple regulatory mechanisms underlying age‐related changes in BPV, including autonomic functions controlled by the CAN. CSVD could damage white matter connections that link central autonomic nodes, potentially disrupting blood pressure regulation. 3 , 4 However, causal mediation analysis showed that changes in the CAN could not account for the relationship between BPV and CSVD presence or extent, suggesting that increased BPV is more likely a cause rather than a consequence of CSVD. 1 Nevertheless, the relationship between higher BPV and greater CSVD burden was driven by APOE4 carriers with lower CAN connectivity. These findings could suggest that BPV and central autonomic dysfunction may have synergistic effects on CSVD in older APOE4 carriers. The mechanism is unclear, but autonomic dysfunction may hamper the ability of the cerebrovasculature to adapt to increased BPV. 5 Further study of autonomic and cerebrovascular autoregulatory functions related to CAN connectivity and BPV may yield further insights into potential treatments.
A strength of the present study is the use of a CAN definition derived from a voxel‐level meta‐analysis. 4 Additional strengths include use of multimodal imaging to characterize CSVD presence and severity. Examination of APOE4 gene effects and mediation/moderation analyses are further strengths. Future studies should evaluate the potential mediating and moderating effects of subsystems with the broader CAN. Study limitations include the cross‐sectional design, which limits causal inference, and the relatively small sample size.
Sources of Funding
This research was supported by National Institutes of Health grants (Dr Nation: R01AG064228, R01AG060049, R01AG082073, P01AG052350), (Dr Han: K24AG081325) and the Canadian Institutes of Health Research (A. Kapoor: DFD‐170763).
Disclosures
None.
This manuscript was sent to Jose R. Romero, MD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Preprint posted on MedRxiv December 14, 2023. doi: https://doi.org/10.1101/2023.12.13.23299556.
For Sources of Funding and Disclosures, see page 3.
References
- 1. Tully PJ, Yano Y, Launer LJ, Kario K, Nagai M, Mooijaart SP, Claassen JAHR, Lattanzi S, Vincent AD, Tzourio C, et al. Association between blood pressure variability and cerebral small‐vessel disease: a systematic review and meta‐analysis. J Am Heart Assoc. 2020;9:e013841. doi: 10.1161/JAHA.119.013841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sible IJ, Nation DA; on behalf of Alzheimer's Disease Neuroimaging I . Visit‐to‐visit blood pressure variability and CSF Alzheimer disease biomarkers in cognitively unimpaired and mildly impaired older adults. Neurology. 2022;98:e2446–e2453. doi: 10.1212/WNL.0000000000200302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ma Y, Song A, Viswanathan A, Blacker D, Vernooij MW, Hofman A, Papatheodorou S. Blood pressure variability and cerebral small vessel disease. Stroke. 2020;51:82–89. doi: 10.1161/STROKEAHA.119.026739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beissner F, Meissner K, Bär KJ, Napadow V. The autonomic brain: an activation likelihood estimation meta‐analysis for central processing of autonomic function. J Neurosci. 2013;33:10503–10511. doi: 10.1523/jneurosci.1103-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Claassen J, Thijssen DHJ, Panerai RB, Faraci FM. Regulation of cerebral blood flow in humans: physiology and clinical implications of autoregulation. Physiol Rev. 2021;101:1487–1559. doi: 10.1152/physrev.00022.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]


