Abstract
Background
A significant percentage of patients with congenital heart disease surviving into adulthood will develop arrhythmias. These arrhythmias are associated with an increased risk of adverse events and death. We aimed to assess arrhythmia prevalence, risk factors, and associated health care usage in a large national cohort of patients with adult congenital heart disease.
Methods and Results
Adults with a documented diagnosis of congenital heart disease, insured by Clalit and Maccabi health services between January 2007 and December 2011, were included. We assessed the associations between arrhythmia and subsequent hospitalization rates and death with mixed negative binomial and Cox proportional hazard models, respectively. Among 11 653 patients with adult congenital heart disease (median age, 47 years [interquartile range, 31–62]), 8.7% had a tachyarrhythmia at baseline, 1.5% had a conduction disturbance, and 0.5% had both. Among those without a baseline arrhythmia, 9.2% developed tachyarrhythmias, 0.9% developed a conduction disturbance, and 0.3% developed both during the study period. Compared with no arrhythmia (reference group), arrhythmia in the previous 6 months was associated with a higher multivariable adjusted hospitalization rate, 1.33‐fold higher than the rate of the reference group (95% CI, 1.00–1.76) for ventricular arrhythmia, 1.27‐fold higher (95% CI, 1.17–1.38) for atrial arrhythmias, and 1.33‐fold higher (95% CI, 1.04–1.71) for atrioventricular block. Atrial tachyarrhythmias were associated with an adjusted mortality hazard ratio (HR) of 1.65 (95% CI, 1.44–2.94), and ventricular tachyarrhythmias with a >2‐fold increase in mortality risk (HR, 2.06 [95% CI, 1.44–2.94]).
Conclusions
Arrhythmias are significant comorbidities in the adult congenital heart disease population and have a significant impact on health care usage and survival.
Keywords: adult congenital heart disease, arrhythmias, health care usage
Subject Categories: Arrhythmias, Congenital Heart Disease
Nonstandard Abbreviations and Acronyms
- ACHD
adult congenital heart disease
- CD
conduction disturbance
Clinical Perspective.
What Is New?
Based on a large cohort, almost 20% of community‐dwelling patients with adult congenital heart disease (ACHD) present with arrhythmia at baseline or develop arrhythmia over the next 5 years.
Arrhythmias have a significant impact on health care usage and survival among patients with ACHD, and in patients with ACHD, ventricular and atrial tachyarrhythmias and atrioventricular block are associated with 30% higher hospitalization rates.
Tachyarrhythmia is associated with a substantially increased risk of death, beyond age, comorbidities, and defect complexity.
What Are the Clinical Implications?
The high risk of arrhythmias among patients with ACHD should be accounted for in risk stratification aimed at improving prognosis.
With the increasing number and age of patients with ACHD, clinicians and health care systems must prepare for the rising prevalence of arrhythmias in this population and their consequential effect on health care service usage.
A significant proportion of patients with congenital heart disease reaching adulthood will develop arrhythmias. 1 , 2 Patients with adult congenital heart disease (ACHD) are vulnerable to arrhythmias due to the underlying heart defect and its sequelae including persistent pressure and volume changes, as well as due to postintervention scarring. The arrhythmias affecting patients with ACHD, ranging from bradyarrhythmias to tachyarrhythmias, are often poorly tolerated. Moreover, the rate of sudden cardiac death in patients with ACHD is up to 100 times more frequent that sudden cardiac death in patients with acquired heart disease. 3 With medical and surgical advances and the ensuing longer life expectancy of this patient population, the incidence and prevalence of arrhythmias in the adult years is expected to increase. 4 , 5 A large body of data support the significant burden of arrhythmias in patients with ACHD with a prevalence of up to 25%. 6 These arrhythmias are associated with increased risk of adverse events and death. 7
The aims of our study were multifold. First, we aimed to assess the extent of the arrhythmia burden, prevalence and range of both bradyarrhythmias and tachyarrhythmias, in a large national cohort of patients with ACHD. Second, we aimed to assess the risk factors for specific arrhythmias among these patients and their clinical outcomes. Third, we aimed to assess the health care usage of these patients, in terms of both interventional procedures performed and hospitalizations. This information is important in understanding the expected course of these conditions and can guide patient‐specific care.
Methods
The study population has been previously described. 8 In brief, this study was conducted in Israel in a framework of national health insurance. It was based on deidentified data retrieved from electronic records of 2 health care providers (Maccabi and Clalit health services) covering 77% of the population at the time of the study.
The data that support the findings of this study are available upon reasonable request. Requests from qualified researchers trained in human subject confidentiality protocols may be sent to Michal Benderly.
Adult patients with a diagnosis of congenital heart disease insured between January 2007 and December 2011 were included. Patients were aged ≥18 years and had at least 1 documented congenital heart lesion or a specific congenital heart malformation repair procedure.
Patients who switched providers during the data collection period (N=628) were excluded to avoid overlap of deidentified records from different providers. We also excluded 1809 patients for whom congenital heart defect diagnosis could not be ascertained on the basis of the available data, and 3547 patients for whom disease complexity could not be determined on the basis of diagnosis codes.
Arrhythmia and related procedures were determined on the basis of the International Classification of Diseases, Ninth Revision (ICD‐9; Data S1). It is important to note that tachyarrhythmias comprised atrial or ventricular tachyarrhythmias, whereas bradyarrhythmias were regarded as a conduction abnormality.
Four patients with recorded ablation and no other record of tachyarrhythmia were considered to have tachyarrhythmia. Similarly, 23 additional patients identified as having conduction disturbances (CDs) solely on the basis of recorded diagnosis of a cardiac pacemaker.
Congenital heart disease complexity was categorized according to the 32nd Bethesda Conference report as simple, moderate, or severe by a hierarchal algorithm according to the most severe congenital heart defect. 9
Comorbidities were summarized with the Charlson comorbidity score. 10 Outcomes assessed were health care usage, death, stroke, and a composite of death and stroke during the follow‐up period.
The health care services examined included primary care and cardiology outpatient visits, emergency department visits, hospital admissions, and intensive care unit admissions. Maternity ward hospitalizations were excluded. Only face‐to‐face patient–doctor encounters were counted. Several visits to the same specialty clinic (general practice or cardiology) on the same day were counted once. Data limitation prevented distinction between specialized ACHD clinics and other outpatient cardiology visits.
This study was approved by the institutional review boards of the Sheba Medical Center and the participating health care providers. Informed consent was not required.
Statistical Analysis
Age‐adjusted rates of arrhythmia by congenital disease complexity and defect type was calculated by a direct method, with the entire cohort as the reference group. We used quintile regression to compute age‐adjusted median number of health services usage.
Arrhythmia variables considered in multivariable analysis included atrial tachyarrhythmia, ventricular tachyarrhythmia, other tachyarrhythmia, atrioventricular block, bundle branch block, and premature beat.
The association between arrhythmia status in the previous 6‐month period and subsequent hospitalization rates in repeated 6‐month follow‐up periods were assessed with a multivariable negative binomial mixed model. The results are therefore counts per 6 months. Random intercept for each patient, allowed for correlation between periods within patient. The model also included a variable representing the period to account for the effect of time.
The cumulative mortality rate was assessed using Kaplan–Meier curves. The probability of difference between the age‐adjusted curves was assessed by a Cox proportional hazard model adjusting for age, with group as a time‐dependent covariate.
The associations with stroke, death, and the combined end point of stroke or death were examined with multivariable Cox proportional hazard models. Arrhythmia variables were entered in the model as time‐dependent variables (coded as 0 until first recorded diagnosis and 1 thereafter). The validity of the proportional hazard assumption was ascertained by including a time‐dependent explanatory variable for each variable in the model to test the assumption of no time‐dependent effect. In case of a violation of the assumption, an interaction term between each violating variable and time was added to the model (defect severity in all models and age in models predicting the combined end point). Patients with a baseline history of stroke (N=586) were excluded from the models predicting stroke or the combined stroke/death end point. Data were analyzed with SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
The study cohort comprised 11 653 patients with ACHD with a median age of 46.9 years (interquartile range [IQR], 31–62 years). The majority of the cohort (83.3%) had a single heart defect, and most of the patients (74.1%) had only a simple defect. The most common heart defects were atrial septal defects (30.4%), aortic valve disease (26.0%), and ventricular septal defects (14.2%). Hypertension was the most prominent baseline cardiovascular comorbidity, present in 17.9% of the cohort.
A baseline diagnosis of tachyarrhythmia was recorded for 1017 patients (8.7%). Among those with tachyarrhythmias, 611 (60.1%) had an atrial tachyarrhythmia, and 58 (5.7%) had a ventricular tachyarrhythmia. There were 30 patients (2.9%) with both an atrial and ventricular tachyarrhythmia diagnosis at baseline. Of 169 (1.5%) patients who had a CD at baseline, 69 patients (40.8%) had atrioventricular node block. The other tachyarrhythmia and CD diagnoses are detailed in Data S1. Both a tachyarrhythmia and CD at baseline were found in 60 patients (0.5%).
Patient Characteristics by Arrhythmia Type
As depicted in Table 1, patients with no arrhythmia at baseline were younger (median age, 45 years [IQR, 30–61]) compared with patients with arrhythmia (median age, 50 years [IQR, 34–64], 61 years [IQR, 47–73], 64 years [IQR 49–77] for patients with CD, tachyarrhythmia, or both, respectively).
Table 1.
Baseline Characteristics of 11 653 Patients With Adult Congenital Heart Disease by Arrhythmia at Baseline
| Baseline arrhythmia | |||||
|---|---|---|---|---|---|
| None | Tachyarrhythmia | Conduction disturbances | Both | P value | |
| N | N=10 527 | N=957 | N=109 | N=60 | |
| Age, y, median (IQR) | 45 (30–61) | 61 (47–73) | 50 (34–64) | 64 (49–77) | <0.0001 |
| Number of heart defects, N (%) | |||||
| 1 | 8845 (84.0) | 749 (78.3) | 71 (65.1) | 46 (76.7) | <0.0001 |
| 2 | 1389 (13.2) | 150 (15.7) | 27 (24.8) | 11 (18.3) | |
| 3+ | 293 (2.8) | 58 (6.1) | 11 (10.1) | 3 (5.0) | |
| Other congenital anomaly, N (%) | |||||
| 0 | 9893 (94.0) | 848 (88.6) | 100 (91.7) | 52 (86.7) | <0.0001 |
| 1 | 595 (5.7) | 97 (10.1) | 8 (7.3) | 7 (11.7) | |
| 2+ | 39 (0.4) | 12 (1.3) | 1 (0.9) | 1 (1.7) | |
| Defect complexity, N (%) | |||||
| Simple | 7856 (74.6) | 680 (71.1) | 62 (56.9) | 39 (65.0) | <0.0001 |
| Intermediate | 2193 (20.8) | 215 (22.5) | 38 (34.9) | 11 (18.3) | |
| Complex | 478 (4.5) | 62 (6.5) | 9 (8.3) | 10 (16.7) | |
| Heart defect type, N (%) | |||||
| Patent ductus arteriosus | 513 (4.9) | 27 (2.8) | 4 (3.7) | 3 (5.0) | 0.036 |
| Aortic valve stenosis/insufficiency | 2799 (26.6) | 193 (20.2) | 29 (26.6) | 11 (18.3) | 0.0001 |
| Transposition of great arteries | 67 (0.6) | 6 (0.6) | 4 (3.7) | 0 (0.0) | 0.0014 |
| Atrial septal defect | 3186 (30.3) | 306 (32.0) | 34 (31.2) | 13 (21.7) | 0.3 |
| Mitral valve anomaly | 855 (8.1) | 213 (22.3) | 11 (10.1) | 11 (18.3) | <0.0001 |
| Pulmonary valve anomaly | 377 (3.6) | 25 (2.6) | 9 (8.3) | 4 (6.7) | 0.01 |
| Ebstein anomaly of tricuspid valve | 133 (1.3) | 11 (1.1) | 1 (0.9) | 1 (1.7) | 0.96 |
| Tetralogy of Fallot | 352 (3.3) | 20 (2.1) | 8 (7.3) | 5 (8.3) | 0.002 |
| Ventricular septal defect | 1551 (14.7) | 81 (8.5) | 14 (12.8) | 8 (13.3) | <0.0001 |
| Endocardial cushion | 608 (5.8) | 137 (14.3) | 21 (19.3) | 9 (15.0) | <0.0001 |
| Anomalies of the aorta | 849 (8.1) | 78 (8.2) | 10 (9.2) | 0 (0.0) | 0.14 |
| Other anomaly | 1102 (10.5) | 90 (9.4) | 12 (11.0) | 5 (8.3) | 0.7 |
| Single/common ventricle | 147 (1.4) | 44 (4.6) | 3 (2.8) | 7 (11.7) | <0.0001 |
| Comorbidities, N (%)* | |||||
| Malignancy | 198 (2.0) | 70 (4.8) | 6 (5.1) | 5 (5.6) | <0.0001 |
| Diabetes | 442 (4.4) | 159 (11.2) | 14 (11.8) | 7 (7.1) | <0.0001 |
| Hypertension | 1487 (14.6) | 516 (38.7) | 38 (32.1) | 41 (51.7) | <0.0001 |
| Dyslipidemia | 1408 (13.8) | 419 (31.8) | 36 (30.4) | 32 (50.0) | <0.0001 |
| Ischemic heart disease | 504 (5.0) | 265 (18.8) | 15 (12.8) | 23 (28.9) | <0.0001 |
| Heart failure | 180 (1.8) | 150 (11.2) | 7 (6.0) | 15 (30.5) | <0.0001 |
| Other heart disease† | 432 (4.2) | 225 (18.2) | 97 (89.8) | 52 (92.8) | <0.0001 |
| Stroke/TIA | 432 (4.2) | 167 (12.8) | 5 (4.3) | 11 (10.2) | <0.0001 |
| Charlson comorbidity score, median (IQR) | 0 (0–1) | 3 (1–5) | 2 (0–3) | 4 (1–7) | <0.0001 |
IQR indicates interquartile range; and TIA, transient ischemic attack.
Age‐adjusted percentage.
Excluding arrhythmia.
Although the majority of the cohort had a single heart defect, patients with a CD were more likely to have >1 heart defect compared with the other subgroups (Table 1).
A higher proportion of patients with both CD and arrhythmias had a complex defect (16.7%) compared with those with only CD (8.3%), only tachyarrhythmias (6.5%) or no arrhythmia (4.5%). The most prevalent defect in all groups was atrial septal defect followed by aortic valve disease in second place (third place amongst patients with tachyarrhythmia; Table 1).
The baseline combination of tachyarrhythmia and CD was associated with a higher burden of comorbidities (baseline median Charlson comorbidity score, 4 [IQR, 1–7]) compared with only a CD (median, 2 [IQR, 0–3]), only tachyarrhythmia (median, 3 [IQR, 1–5]), or no arrhythmias (median, 0 [IQR, 0–1]).
The combination of CD and tachyarrhythmia was also associated with the highest prevalence of individual comorbidities and cardiovascular risk factors (Table 1), with the exception of diabetes (11.2%, 11.8%, 7.1%, and 4.4% among those with tachyarrhythmia, CD, both, and no arrhythmia, respectively) and stroke or transient ischemic attack, for which the highest frequency was found amongst patients with tachyarrhythmia (Table 1).
Among those with tachyarrhythmias, those with atrial tachyarrhythmias were on average older than those with ventricular arrhythmias. The prevalence of atrial tachyarrhythmias was relatively higher among those with atrial septal defect and single/common ventricles. Ventricular arrhythmia was more common among those with tetralogy of Fallot and ventricular septal defects compared with other cardiac defects (Table S1).
Progression of Arrhythmias in ACHD
Of 10 527 patients with no history of arrhythmia at baseline, 974 (9.2%) developed tachyarrhythmias during the study period (median follow‐up, 5.2 years), 97 (0.9%) developed CD, and 33 (0.3%) developed both tachyarrhythmia and CD. Those who developed tachyarrhythmias during follow up were significantly older and had more baseline comorbidities compared with those who did not. The number of heart defects and defect complexity did not differ between the groups. However, atrial septal defects were more prevalent among those who subsequently developed tachyarrhythmias (Table S2). Among 957 who had only tachyarrhythmia at baseline, 23 (2.4%) developed an additional CD during follow‐up, and of the 109 with only a CD at baseline, 11 (10.0%) developed tachyarrhythmia.
Health Care Usage
Almost all patients in all subgroups visited a primary care physician at least once, with an age‐adjusted median of 45 (IQR, 36–55) visits per 5 years in those with both tachyarrhythmia and CD, median of 43 (IQR, 41–46) visits among those with tachyarrhythmias, median of 33 (IQR, 26–39) visits among those with CD, and median of 33 (IQR, 33–34) visits among those without an arrhythmia (Table 2). The proportion of patients under cardiology follow‐up during the study period ranged between 76.2% for patients without any arrhythmia and 89.2% for patients with both CD and tachyarrhythmia, with an age‐adjusted median number of visits per 5 years ranging between 3 in patients without arrhythmia and 9 among patients with both CD and tachyarrhythmia (Table 2).
Table 2.
Health Service Usage
| Baseline arrhythmia | |||||
|---|---|---|---|---|---|
| None | Tachyarrhythmia | Conduction disturbances | Both | P value | |
| Used the service at least once,* N (%†) | |||||
| Primary care | 10 388 (98.7) | 949 (99.3) | 108 (99.1) | 58 (98.5) | 0.2 |
| Outpatient cardiology | 7995 (76.2) | 866 (88.6) | 98 (88.1) | 56 (89.2) | <0.0001 |
| Emergency department only visits | 5846 (56.6) | 640 (58.1) | 64 (55.6) | 46 (64.2) | 0.5 |
| Hospital admissions | 5433 (52.7) | 601 (54.0) | 58 (50.4) | 45 (63.4) | 0.3 |
| Intensive care unit | 856 (15.1) | 62 (11.6) | 6 (11.3) | 3 (3.5) | <0.0001 |
| Frequency/5 y,‡ median (IQR) | |||||
| Primary care visits | 33 (33–34) | 43 (41–46) | 33 (26–39) | 45 (36–55) | |
| Outpatient cardiology visits | 3 (2.7–2.8) | 6 (5.6–7.0) | 4 (3.4–5.1) | 9 (6.2–12.0) | |
| Emergency department only visits | 1.0 (0.7–1.3) | 0.0 (0.0–0.6) | 1.0 (0.6–1.3) | 0.0 (0.0–0.8) | |
| Hospital admissions | 0.9 (0.9–1.0) | 1.0 (0.8–1.1) | 0.8 (0.5–1.1) | 2.2 (1.1–3.3) | |
| Days in hospital | 7 (6.7–7.2) | 8 (6.0–9.2) | 6 (1.8–9.6) | 13 (5.1–20.1) | |
IQR indicates interquartile range.
Used the service at least once in the data collection period (2007–2011).
Age‐adjusted percentage.
Age‐adjusted.
Over half of the patients were hospitalized during the follow‐up period, with more admissions and longer hospital stays among those with both arrhythmias, compared with the other subgroups (Table 2).
Following adjustment for age, defect complexity, baseline chronic comorbidities, and time, atrial or ventricular tachyarrhythmia or atrioventricular block recorded in the previous 6 months or earlier was associated with a higher hospitalization rate compared with no arrhythmia (Figure 1).
Figure 1. Relative number of hospital admissions and days in hospital among 11 653 patients with ACHD by arrhythmia type.
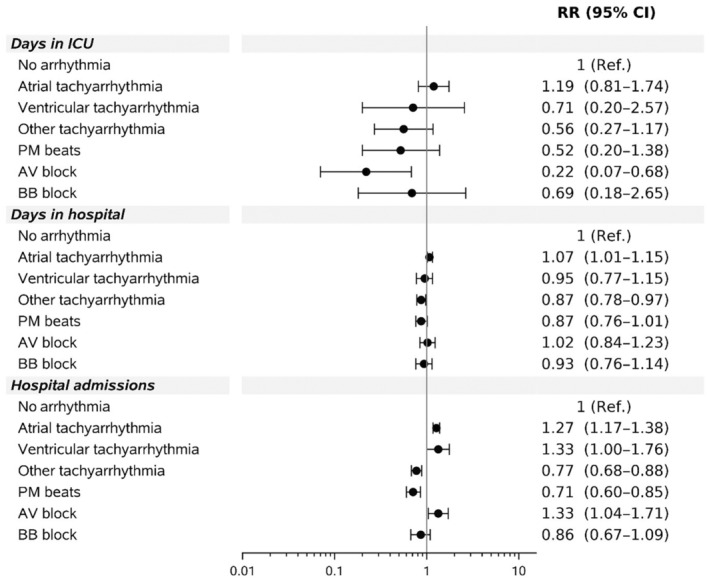
Reference=no arrhythmia. AV indicates atrioventricular; BB, bundle branch; ICU, intensive care unit; PM, premature; and RR, relative rate.
The number of hospitalizations significantly increased to 1.33‐fold higher (95% CI, 1.00–1.76) in those with ventricular arrhythmia, 1.27‐fold higher (95% CI, 1.17–1.38) in those with atrial arrhythmias, and to 1.33‐fold higher (95 CI, 1.04–1.71) in those with atrioventricular block compared with the reference group with no arrhythmia. Those with a diagnosis of premature beats or other arrhythmias, on the other hand, had significantly fewer hospitalizations, when other arrhythmias were included in the model.
Arrhythmia type was not significantly associated with the adjusted number of days spent in the hospital (Figure 1). The same is true for days in the intensive care unit with the exception of atrioventricular block, which was associated with fewer days in the intensive care unit than no arrhythmia (relative rate, 0.22 [95% CI, 0.07–0.68]).
Other characteristics associated with a higher hospitalization rate were older age (1.48‐fold increase [95% CI, 1.31–1.67] among patients aged 25–44 years; 3.12‐fold increase [95% CI, 2.77–3.52] among those aged 45–64 years; and 8.46‐fold increase [95% CI, 7.48–9.57] among those aged >64 years than in patients aged 18–25 years; P<00.1), male sex (1.09‐fold increase [95% CI, 1.02–1.15] compared with female sex; P=0.01), a more severe congenital heart lesion (intermediate lesion, 1.10‐fold increase [95% CI, 1.02–1.18]; complex lesion, 1.87‐fold increase [95% CI, 1.63–2.14]; P<0.001, respectively, compared with simple lesions), and a higher baseline comorbidity score (1.08‐fold increase with every addition of 1 point to the Charlson comorbidity score [95% CI, 1.05–1.10]; P<0.001).
On a separate model with individual baseline comorbidities, patients with heart failure had increased hospitalization rate (relative rate, 1.37 [95% CI, 1.22–1.53]), while those with hypertension had significantly fewer hospitalizations (relative rate, 0.90 [95% CI, 0.84–0.97]) compared with those without other chronic comorbidities.
Among those with any arrhythmias, 43.2% were treated with a β blocker at baseline (65.2% over the follow‐up period), 55.9% were on an antithrombotic drug (72.8% over the follow‐up), and 20.8% were on an antiarrhythmic drug (33.6% during the follow‐up).
Clinical Outcomes
During the follow‐up period, 1133 (9.7%) patients died. The age‐adjusted death rate was 17.2 per 1000 person‐years among patients with no baseline arrhythmia, 18.5 per 1000 person‐years for patients with a baseline CD, and 25.0 per 1000 person‐years for patients with tachyarrhythmias. Those with both conditions had a heightened risk of 29.6 per 1000 person‐years age‐adjusted risk of death. The crude mortality rate was significantly higher among those with both arrhythmias than among those with tachyarrhythmias and CD, and those with no arrhythmias at baseline had the lowest mortality rates. (Figure 2). However, the mortality rate did not differ among the subgroups when adjusted for age. (Figure S1) Following multivariable adjustment for age, sex, defect severity, and baseline comorbidity (Charlson index), atrial tachyarrhythmias were associated with a hazard ratio (HR) of 1.6 (95% CI, 1.4–1.9; P<0.001) for death, and ventricular tachyarrhythmias were associated with a >2‐fold increased risk of death (HR, 2.06 [95% CI, 1.44–2.94]; P<0.001).
Figure 2. Crude mortality rate per arrhythmia subgroup.
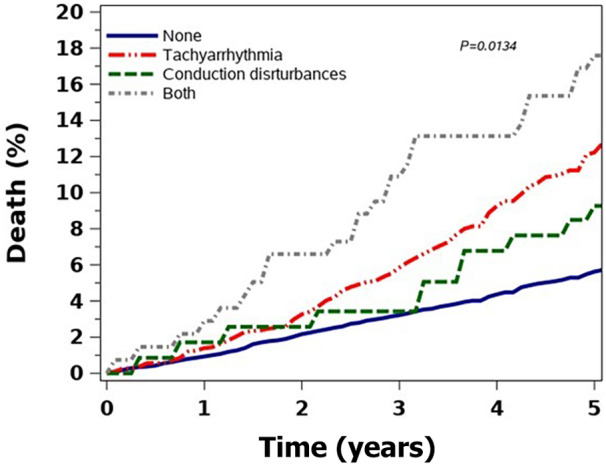
When individual baseline comorbidities were included in the model, hypertension was associated with a lower mortality hazard (HR, 0.31 [95% CI, 0.22–0.43]; P<0.001), and heart failure with a significant mortality hazard of almost 3‐fold (HR, 2.94 [95% CI, 2.00–4.32]; P<0.001).
The combined outcome of death or stroke occurred in 1805 (16.3%) of 11 067 patients with no history of stroke at baseline. Following multivariate analysis, there was no significant increased risk associated with the subtypes of arrhythmias in terms of stroke. There was an increased risk of the combined outcome of stroke/death among those with an atrial or ventricular arrhythmia (HR, 1.33 [95% CI, 1.17–1.51]; HR, 1.57 [95% CI, 1.09–2.26], respectively) driven by an increased risk of death as shown in Figure 3. Age, male sex, and severity of congenital lesion were all associated with an increased risk of death.
Figure 3. Multivariable adjusted outcome HR by arrhythmia type.
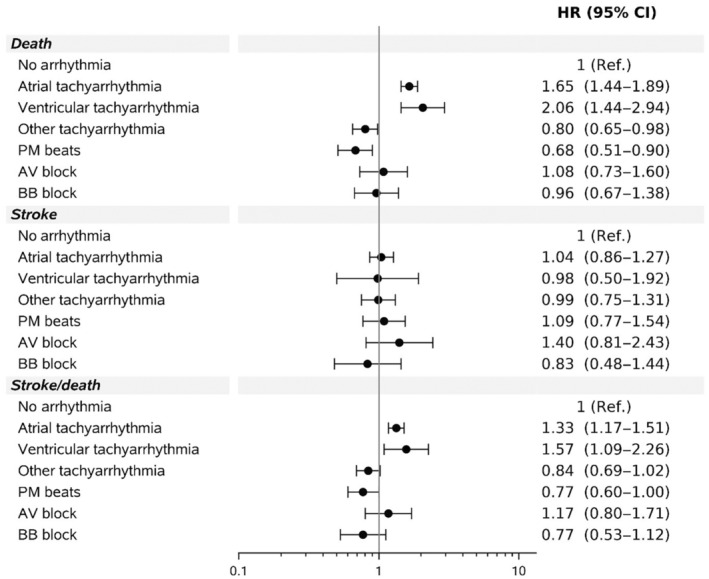
Arrhythmia variables considered in multivariable analysis included atrial tachyarrhythmia, ventricular tachyarrhythmia, other tachyarrhythmia, atrioventricular block, bundle branch block, and premature beat. AV indicates atrioventricular; BB, bundle branch; HR, hazard ratio; and PM, premature.
Discussion
Our main findings are that arrhythmias are a significant comorbidity in the ACHD population and have a significant impact on health care usage and health outcomes. The prevalence of arrhythmias is associated with both the severity of the congenital defect itself and other traditional cardiovascular risk factors that are more common in adulthood.
With the improvement of medical and surgical techniques, the number of patients with congenital heart disease surviving into adulthood is expected to increase as well as the complications and clinical sequelae associated with ACHD. A significant proportion of patients with ACHD surviving into adulthood will have a diagnosis of an arrhythmia or develop an arrhythmia during adulthood. 1 , 2
The prevalence of arrhythmia at baseline and the incidence rate during follow‐up are in line with other data reporting the prevalence of arrhythmias in patients with ACHD up to 25%. 6 A large cohort study from Taiwan on 122 518 patients with ACHD assessed the epidemiological profile of these patients, including the prevalence of arrhythmias. Concurrent with our findings, the prevalence of tachyarrhythmias in this cohort was 7.22% and the prevalence of bradyarrhythmias was low and reported as 0.99%. 11 They found that patients with all types of ACHD had a >10% risk of experiencing a tachyarrhythmia by age 50 years.
Comorbidities are prevalent among patients with ACHD. 12 In our study, we found that those with both arrhythmias had a higher comorbidity burden than those with tachyarrhythmias, bradyarrhythmias, and no arrhythmias, respectively. Chronic diseases are known to be risk factors for progression of heart failure and tachyarrhythmias in adult patients without ACHD, and are increasingly recognized as a potent risk factor in those with ACHD. 13 With the increasing survival of these patients into later adulthood, the risk of these comorbidities is expected to increase. 14
Stroke and Death
Arrhythmias increase the risk for adverse outcomes. 15 We found that atrial and ventricular arrhythmias were independently associated with a significantly increased risk of stroke and death. This is concurrent with other studies reporting an association between arrhythmias and increased risk of adverse events and death. 7 , 15
Arrhythmias themselves may reflect the hemodynamic status of the patients and be an early indicator of worsening heart failure. 15 Arrhythmias can additionally cause rapid hemodynamic decline in patients with ACHD. This is particularly noted in patients with complex and altered anatomy with less hemodynamic reserve for arrhythmias and are more dependent on atrial kick in sinus rhythm. For this reason, there is a class I recommendation to maintain sinus rhythm in all patients with ACHD. 5
In the current study, the congenital lesion's severity, age, sex, and comorbidities were all independent risk factors for death. The seemingly protective effect of hypertension against death could suggest a stronger systemic ventricle due to the chronically increased afterload that may improve resilience to arrhythmias.
Health Care Usage
To our knowledge, this is the first report on health care usage in association with arrhythmias in the patient population with ACHD. This population requires lifelong follow‐up and has an increased risk of later complications. Data from Germany has shown that as this population ages, the health care usage increases. 14 , 16 The main complications among patients with ACHD are heart failure and arrhythmias, both associated with higher hospitalization rates. 15 The cost for ACHD hospitalization has increased by 258% in a decade in a US nationwide inpatient sample. 17 We found that those with an arrhythmia had a higher usage of the health care system than those without. Interestingly, we found that patients with a diagnosis of atrioventricular block in the prior 6 months had significantly fewer days in intensive care compared with other subgroups. It could be that these patients had pacemakers inserted and thus would be protected from the hemodynamic adverse effects of bradycardias. Furthermore, in patients with dual‐chamber pacemakers, these pacemakers are programmed with a maximum tracking rate that avoids the ventricle tracking a tachyarrhythmia and prevents pacing fast atrial arrhythmias. This could prevent major hemodynamic changes and could possibly explain this finding.
It is important to note that our study was conducted in the setting of a national health insurance granting every resident, since 1995, universal coverage for primary to tertiary care with a broad benefits package, including physician consultations, hospitalizations, imaging, laboratory services, medications, and advanced technologies, with low copayment or no additional charge. The outpatient cardiology care in the majority of the patients could have been a factor that prevented hospitalizations by implementing preemptive measures and ensuring adequate follow‐up in this unique population. In contrast, in health care systems based on fee for service, patients may have lower access to regular cardiology follow‐up due to financial constraints. Thus, our hospitalization rate may be lower than in other health care systems where universal access is not available.
The population of ACHD is heterogeneous, comprising many different lesions and different arrhythmia risks. One of the strengths of our study is that the ACHD lesions and associated arrhythmia risk were assessed as well as outcomes related to the cohort as a whole. Furthermore, our study included a large cohort of patients; data were derived from administrative databases, and thus the information on health care usage and adverse outcomes are accurate. ICD‐9 codes were used to identify and classify patients with ACHD and the diagnosis of arrhythmia. This was based on administrative data and was limited in nature, mainly due to the need for comprehensive and valid input of ICD‐9 codes. Another limitation was that data were available following the date of the development of the electronic database, and thus prior data on surgical procedures and childhood clinical status were lacking in many patients. Our data are reporting on the ACHD population and have inherent survival bias by including only those that reached adulthood. Our study was observational in nature and has inherited biases (including indication bias and residual confounding) and therefore was not suited to assess causal relationships such as that of medication efficacy or ablation therapy. In conclusion, arrhythmias are common comorbidities in the ACHD population and have a significant impact on health care usage and survival. This should be taken into consideration in this population in which lifelong follow‐up is needed.
Sources of Funding
This study was supported by an unrestricted grant from the Israel National Institute for Health Policy Research, Ramat Gan, Israel.
Disclosures
None.
Supporting information
Data S1
Tables S1–S2
Figure S1
This manuscript was sent to John L. Jefferies, MD, MPH, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.031760
For Sources of Funding and Disclosures, see page 9.
See Editorial by O’Leary et al.
References
- 1. Lee VWY, Yan BP, Fong TMC, Fung AKP, Cheng FWT. Long‐term health‐related burden of adult congenital heart diseases in Hong Kong. J Med Econ. 2019;22:814–817. doi: 10.1080/13696998.2019.1613239 [DOI] [PubMed] [Google Scholar]
- 2. Mandalenakis Z, Rosengren A, Lappas G, Eriksson P, Gilljam T, Hansson P‐O, Skoglund K, Fedchenko M, Dellborg M. Atrial fibrillation burden in young patients with congenital heart disease. Circulation. 2018;137:928–937. doi: 10.1161/CIRCULATIONAHA.117.029590 [DOI] [PubMed] [Google Scholar]
- 3. Moore JP, Khairy P. Adults with congenital heart disease and arrhythmia management. Cardiol Clin. 2020;38:417–434. doi: 10.1016/j.ccl.2020.04.006 [DOI] [PubMed] [Google Scholar]
- 4. Baumgartner H, De Backer J, Babu‐Narayan SV, Budts W, Chessa M, Diller G‐P, Lung B, Kluin J, Lang IM, Meijboom F, et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. 2021;42:563–645. doi: 10.1093/eurheartj/ehaa554 [DOI] [PubMed] [Google Scholar]
- 5. Hernández‐Madrid A, Paul T, Abrams D, Aziz PF, Blom NA, Chen J, Chessa M, Combes N, Dagres N, Diller G, et al. Arrhythmias in congenital heart disease: a position paper of the European Heart Rhythm Association (EHRA), Association for European Paediatric and Congenital Cardiology (AEPC), and the European Society of Cardiology (ESC) Working Group on Grown‐Up Congenital Heart Disease, endorsed by HRS, PACES, APHRS, and SOLAECE. Europace. 2018;20:1719–1753. doi: 10.1093/europace/eux380 [DOI] [PubMed] [Google Scholar]
- 6. Loomba RS, Buelow MW, Aggarwal S, Arora RR, Kovach J, Ginde S. Arrhythmias in adults with congenital heart disease: what are risk factors for specific arrhythmias? Pacing Clin Electrophysiol. 2017;40:353–361. doi: 10.1111/pace.12983 [DOI] [PubMed] [Google Scholar]
- 7. Bouchardy J, Therrien J, Pilote L, Ionescu‐Ittu R, Martucci G, Bottega N, Marelli AJ. Atrial arrhythmias in adults with congenital heart disease. Circulation. 2009;120:1679–1686. doi: 10.1161/CIRCULATIONAHA.109.866319 [DOI] [PubMed] [Google Scholar]
- 8. Benderly M, Buber J, Kalter‐Leibovici O, Blieden L, Dadashev A, Lorber A, Nir A, Yalonetsky S, Chodick G, Weitzman D, et al. Health service utilization patterns among adults with congenital heart disease: a population‐based study. J Am Heart Assoc. 2021;10:e018037. doi: 10.1161/JAHA.120.018037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Warnes CA, Liberthson R, Danielson GK, Dore A, Harris L, Hoffman JI, Somerville J, Williams RG, Webb GD. Task force 1: the changing profile of congenital heart disease in adult life. J Am Coll Cardiol. 2001;37:1170–1175. doi: 10.1016/S0735-1097(01)01272-4 [DOI] [PubMed] [Google Scholar]
- 10. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 11. Wu M‐H, Lu C‐W, Chen H‐C, Kao F‐Y, Huang S‐K. Adult congenital heart disease in a nationwide population 2000‐2014: epidemiological trends, arrhythmia, and standardized mortality ratio. J Am Heart Assoc. 2018;7:e007907. doi: 10.1161/JAHA.117.007907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maurer SJ, Bauer UMM, Baumgartner H, Uebing A, Walther C, Tutarel O. Acquired comorbidities in adults with congenital heart disease: an analysis of the German National Register for Congenital Heart Defects. J Clin Med. 2021;10:314. doi: 10.3390/jcm10020314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 14. Tutarel O, Kempny A, Alonso‐Gonzalez R, Jabbour R, Li W, Uebing A, Dimopoulos K, Swan L, Gatzoulis MA, Diller G‐P. Congenital heart disease beyond the age of 60: emergence of a new population with high resource utilization, high morbidity, and high mortality. Eur Heart J. 2014;35:725–732. doi: 10.1093/eurheartj/eht257 [DOI] [PubMed] [Google Scholar]
- 15. Moore JP, Marelli A, Burchill LJ, Chubb H, Roche SL, Cedars AM, Khairy P, Zaidi AN, Janousek J, Crossland DS, et al. Management of heart failure with arrhythmia in adults with congenital heart disease. J Am Coll Cardiol. 2022;80:2224–2238. doi: 10.1016/j.jacc.2022.09.038 [DOI] [PubMed] [Google Scholar]
- 16. Baumgartner H. Geriatric congenital heart disease: a new challenge in the care of adults with congenital heart disease? Eur Heart J. 2014;35:683–685. doi: 10.1093/eurheartj/eht358 [DOI] [PubMed] [Google Scholar]
- 17. Burchill LJ, Gao L, Kovacs AH, Opotowsky AR, Maxwell BG, Minnier J, Khan AM, Broberg CS. Hospitalization trends and health resource use for adult congenital heart disease‐related heart failure. J Am Heart Assoc. 2018;7:e008775. doi: 10.1161/JAHA.118.008775 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S2
Figure S1


