Abstract
Background
Although cardiovascular mortality continued declining from 2000 to 2019, the rate of this decrease decelerated. We aimed to assess the trends and disparities in risk factor control and treatment among US adults with atherosclerotic cardiovascular disease to find potential causes of the deceleration.
Methods and Results
A total of 55 ,021 participants, aged ≥20 years, from the 1999 to 2018 National Health and Nutrition Examination Survey were included, of which 5717 were with atherosclerotic cardiovascular disease. Risk factor control was defined as hemoglobin A1c <7%, blood pressure <140/90 mm Hg, and non–high‐density lipoprotein cholesterol <100 mg/dL. The prevalence of atherosclerotic cardiovascular disease oscillated between 7.3% and 8.9% from 1999 to 2018. A significant increasing trend was observed in the prevalence of diabetes, obesity, heavy alcohol consumption, and self‐reported hypertension within the population with atherosclerotic cardiovascular disease (P trend≤0.001). Non–high‐density lipoprotein cholesterol <100 mg/dL increased from 7.1% in 1999 to 2002 to 15.7% in 2003 to 2006, before plateauing. Blood pressure control (<140/90 mm Hg) increased until 2011 to 2014, but declined to 70.1% in 2015 to 2018 (P trend<0.001, P joinpoint=0.14). Similarly, the proportion of participants achieving hemoglobin A1c control began to decrease after 2006 (P joinpoint=0.05, P trend=0.001). The percentage of participants achieving all 3 targets increased significantly from 4.5% to 18.6% across 1999 to 2018 (P trend=0.02), but the increasing trend decelerated after 2005 to 2006 (P joinpoint<0.001). Striking disparities in risk factor control and medication use persisted between sexes, and between different racial and ethnic populations.
Conclusions
Worsened control of glycemia, blood pressure, obesity, and alcohol consumption, leveled lipid control, and persistent socioeconomic disparities may be contributing factors to the observed deceleration in decreasing cardiovascular mortality trends.
Keywords: cardiovascular disease prevalence, deceleration in mortality decline, health care disparity, risk factor control
Subject Categories: Cardiovascular Disease, Epidemiology, Race and Ethnicity, Risk Factors
Nonstandard Abbreviations and Acronyms
- NHANES
National Health and Nutrition Examination Survey
- non‐HDL‐C
non–high‐density lipoprotein cholesterol
Clinical Perspective.
What Is New?
In the population with atherosclerotic cardiovascular disease, we observed a stagnation in the initial improvements in blood pressure and non–high‐density lipoprotein cholesterol control, a decline in glycemic control, a deceleration in the increase of participants achieving all 3 targets (non–high‐density lipoprotein cholesterol, blood pressure, and glycemic control) after 2005 to 2006 and an increase in the prevalence of diabetes, obesity, and self‐reported hypertension from 1999 to 2018, which could be contributing factors to the decelerated mortality decrease.
Female and the non‐Hispanic Black populations with atherosclerotic cardiovascular disease had worse risk factor control and lower treatment rate.
What Are the Clinical Implications?
Despite some advancements in risk factor control and treatment among US adults with atherosclerotic cardiovascular disease, significant challenges remain, including the need to address the persistent inequities and disparities in risk factor control and treatment and rising prevalence of obesity and diabetes.
Atherosclerotic cardiovascular disease (ASCVD) remains the leading cause of morbidity and mortality worldwide and in the United States. 1 , 2 , 3 In 2017, cardiovascular disease caused 868 662 deaths in the United States, nearly 60% of which were attributable to coronary heart disease and stroke, the most prevalent forms of ASCVD. 4 Although the age‐adjusted mortality from cardiovascular disease in the United States has seen a continued decline from 2000 to 2019, the rate of this decrease decelerated from 3.7% per year during 2000 to 2011 to a mere 0.7% during 2011 to 2019. 1 Even more concerning, the age‐adjusted death rates increased by 4.1% for heart disease and 4.9% for stroke from 2019 to 2020. 2 This alarming trend calls for immediate action to identify and address the underlying causes.
The reasons for the deceleration in the decline of cardiovascular mortality or the recent surge in heart disease and stroke mortality remain unclear. Previous studies have linked this unsatisfactory mortality rate reduction to uncontrolled ASCVD risk factors, such as a rising prevalence of obesity and diabetes 5 , 6 and decreased blood pressure (BP) and glycemic control among both the general and diabetic populations. 5 , 7 , 8 However, most of these studies have not thoroughly evaluated the treatment and risk factor control among US adults with ASCVD, a key contributor to ASCVD mortality. In addition, disparities in cardiovascular mortality persist across sex, racial and ethnic, and socioeconomic groups. 4 Understanding these disparities in treatment and risk factor control within populations with ASCVD may assist in identifying potential causes and solutions for these concerning mortality trends.
Therefore, we aimed to evaluate the prevalence of ASCVD in the United States, assess the trends in risk factor control and treatment within the population with ASCVD, and identify subgroups within this population who exhibit poor treatment adherence and risk factor control.
METHODS
The National Health and Nutrition Examination Survey (NHANES) is a nationally representative and continuous cross‐sectional study designed to assess the health and nutritional status of noninstitutional US residents. 9 All data and materials have been made publicly available at the National Center for Health Statistics website and can be accessed at https://www.cdc.gov/nchs/nhanes/index.htm. All data were collected through in‐home interviews and mobile examination center visits. 9 In the present study, we analyzed 20‐year data (1999–2018) from NHANES. For the ASCVD prevalence analysis, all participants aged ≥20 years (n=55 021) were included. For ASCVD risk factor control and treatment analysis, after excluding 7 pregnant participants, we included 5717 adults with ASCVD. The National Center for Health Statistics Institutional Review Board approved the NHANES study protocols, and all participants provided written informed consent.
Definitions of ASCVD
We adopted a narrow definition of ASCVD limited by data availability, including only coronary heart disease and stroke, following the 2013 American College of Cardiology/American Heart Association Guidelines. 10 Participants who had been told by physicians or other health professionals to have coronary heart disease, angina, heart attack, or stroke were defined as having diagnosed ASCVD (Table S1), which was referred to as ASCVD below.
Definitions and Control of Risk Factors
Cardiovascular risk factors, 11 , 12 , 13 , 14 , 15 including alcohol consumption, weight, cholesterol level, BP, and hemoglobin A1c (HbA1c), were collected at the mobile examination center. Alcohol consumption was categorized as nonalcohol consumption, low‐moderate consumption (≤14 drinks/week for men and ≤7 drinks/week for women), and heavy alcohol consumption (>14 drinks/week for men and >7 drinks/week for women). 16 Body mass index (BMI; kg/m2) was calculated as weight in kilograms divided by height in meters squared and was categorized into 4 groups: underweight (BMI <18.5 kg/m2), normal weight (BMI 18.5 to <25 kg/m2), overweight (BMI 25 to <30 kg/m2), or obese (BMI ≥30 kg/m2). 5 Smoking status was collected during in‐home interview and categorized as current smoker or not.
Serum lipids were measured using an enzymatic method. We calculated non–high‐density lipoprotein cholesterol (non–HDL‐C) by subtracting HDL‐C from total cholesterol. Lipid control was defined as non–HDL‐C <100 mg/dL or low‐density lipoprotein cholesterol (LDL‐C) <70 mg/dL for established ASCVD following earlier guidelines. 12 , 17 , 18 We also analyzed percentage of participants with achievement of non–HDL‐C of <130 mg/dL and LDL‐C <100 mg/dL. 12 , 18 Because of the small number of participants with LDL‐C, we mainly focused on the non–HDL‐C for lipid control to make more accurate assessments. BP control was defined as BP <140/90 mm Hg. 7 , 19 Self‐reported hypertension was determined by a positive answer to the question “Have you ever been told by a doctor or other health professional that you had hypertension, also called high blood pressure?” Hypertension was defined as self‐reported hypertension, systolic BP ≥140 mm Hg, or diastolic BP ≥90 mm Hg. 7 , 19 Fasting plasma glucose was also measured using the enzymatic method and was calibrated according to the equations provided by the National Center for Health Statistics as performed in previous studies. 20 Although different equipment or methods were used over time, calibration of HbA1c is not necessary as recommended by NHANES. 21 We set the target for HbA1c control as <7% or secondarily as <8%. 15 Self‐reported diabetes was determined by a positive answer to the question “Have you ever been told by a doctor or health professional that you have diabetes or sugar diabetes.” Diabetes was defined as self‐reported diabetes, fasting plasma glucose ≥126 mg/dL, HbA1c ≥6.5%, or taking any antidiabetic agents. 5 , 8 , 22 Participants who simultaneously achieved the goal of HbA1c <7%, BP <140/90 mm Hg, and non–HDL‐C <100 mg/dL were defined as having all risk factors controlled. The detailed diagnosis of diseases is presented in Table S1.
We performed a sensitivity analysis defining hypertension as BP ≥130/80 mm Hg or self‐reported hypertension following the 2017 American College of Cardiology/American Heart Association guideline 13 (abbreviated as American Heart Association hypertension). In addition, in this sensitivity analysis, BP control was defined as BP <130/80 mm Hg.
Medication Use
All participants were asked if they had taken any prescription medications in the past 30 days, and all medications were converted to standard generic drug names and the Multum MediSource Lexicon classification system was used to categorize all medications according to their therapeutic effect. 8 , 23 The categories of BP‐lowering, lipid‐lowering, and glucose‐lowering agents are presented in Table S2.
Socioeconomic and Demographic Characteristics
Age (20–44, 45–64, or ≥65 years), sex (male or female adults), race and ethnicity (non‐Hispanic White population, non‐Hispanic Black population, Hispanic population [including Mexican American and other Hispanic population], or other race [including Asian American, American Indian or Alaskan Native, Native Hawaiian or Pacific Islander, multiple races or ethnicities, or unknown]), educational level (less than high school, high school, or more than high school), poverty/income ratio (<1.3, 1.3–3, or ≥3), 5 , 8 and health insurance (any or none) were all acquired from questionnaires.
Statistical Analysis
We examined the ASCVD prevalence among US adults over time and estimated the prevalence of risk factor control and treatment. To mitigate the impact of small sample sizes and enhance the accuracy of estimates, original 2‐year cycles were combined into 4‐year intervals in the analysis of risk factor control and treatment in ASCVD. 5 , 8 The overall trend of ASCVD prevalence and risk factor control/medication use rate from 1999 to 2018 were analyzed using logistics regression modeling the survey cycle as a continuous variable. To exclude potential confounding effects of demographic characteristics on the trend of risk factor control and medication use, we conducted a sensitivity analysis by additionally adjusting for age, sex, and race and ethnicity in the logistic regression. Joinpoint regression allowing 1 joinpoint was used to identify whether a change (joinpoint) in the trend occurred, with P joinpoint>0.05 indicating no joinpoint and thus a linearity association. To account for multiple comparisons in joinpoint regression, 2‐tailed tests with Bonferroni‐corrected significance levels were used. 5 , 24 In analysis of potential disparities in medication use and risk factor control among different population subgroups with ASCVD, logistic regression adjusting for age, sex, racial and ethnic group, educational level, family income, insurance, and BMI was used to estimate the odds ratio (OR) with 95% CI for different population subgroups. For all analyses, a 2‐tailed P<0.05 was considered statistically significant. To ensure nationally representative estimates, we performed all analyses using appropriate NHANES sampling weights. All analyses were conducted using R software, version 4.1.2 (R Core Team, Vienna, Austria), SPSS Statistics, version 27 (IBM Corp, Armonk, NY), and Joinpoint Regression Program, version 4.5.0.1 (Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute).
RESULTS
Of the 55 021 adults, 48.0% were men, 67.9% were non‐Hispanic White population, 11.3% were non‐Hispanic Black population, 13.8% were Hispanic population, and the average age was 47.03±0.17 years.
From 1999 to 2018, the secular trend of the ASCVD prevalence among US adults was stable and ranged from 7.3% to 8.9% (P trend=0.60, P joinpoint=0.27) (Figure 1A, Table S3). Characteristics of US adults with ASCVD are presented in Table S4.
Figure 1. Prevalence of atherosclerotic cardiovascular diseases among US adults and risk factor prevalence among participants with atherosclerotic cardiovascular diseases.
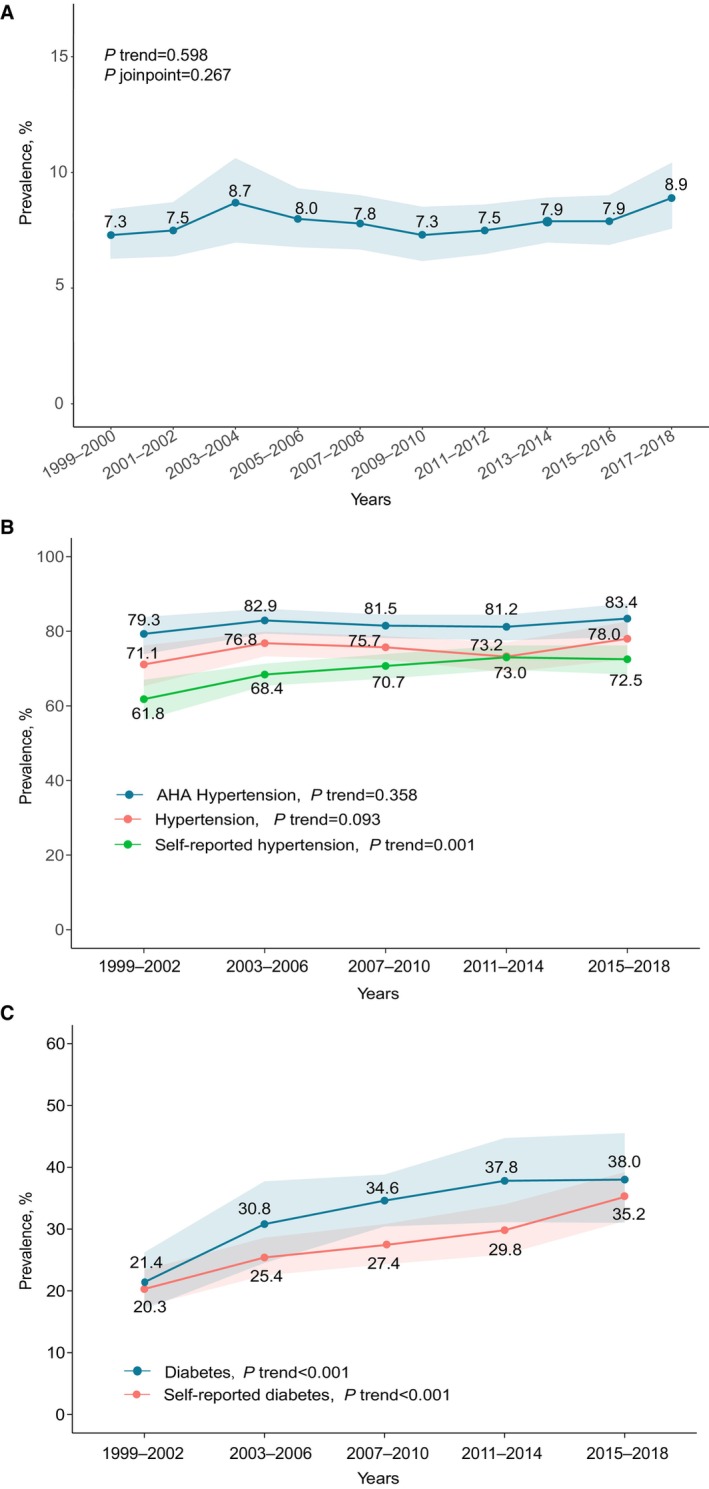
A, Atherosclerotic cardiovascular disease prevalence. B, Prevalence of hypertension among US adults with atherosclerotic cardiovascular diseases. C, Prevalence of diabetes among US adults with atherosclerotic cardiovascular diseases 1999 to 2018. Shaded areas indicate 95% CIs. Hypertension was defined as self‐reported hypertension, blood pressure ≥140/90 mm Hg, or use of any antihypertensive agents. American Heart Association (AHA) hypertension was defined as self‐reported hypertension, blood pressure ≥130/80 mm Hg, or use of any antihypertensive agents. Diabetes was defined as self‐reported diabetes, a fasting plasma glucose level of ≥126 mg/dL, hemoglobin A1c level of ≥6.5%, or taking any antidiabetic agents. P trend was analyzed using logistics regression modeling the survey cycle as a continuous variable. P joinpoint was analyzed using joinpoint regression, and statistical significance of the joinpoint was tested using the Monte Carlo permutation method.
Among US adults with ASCVD, the prevalence of both diabetes (from 21.4% to 38.0%) and self‐reported diabetes (from 20.3% to 35.2%) increased significantly from 1999 to 2002 to 2015 to 2018 (P trend<0.001) (Figure 1C). The prevalence of self‐reported hypertension also increased from 61.8% in 1999 to 2002 to 72.5% in 2015 to 2018 (P trend=0.001); however, no significant increase of the prevalence of hypertension (P trend=0.36) or the strict American Heart Association hypertension (P trend=0.09) was observed (Figure 1B).
Trends in ASCVD Risk Factor Control Among Participants With ASCVD
As shown in Table 1, the percentage of participants in whom non–HDL‐C control <100 mg/dL increased from 7.1% (95% CI, 5.4%–9.2%) in 1999 to 2002 to 15.7% (95% CI, 12.5%–19.5%) in 2003 to 2006, and then leveled off (P trend=0.01, P joinpoint<0.001; Table 1, Table S5, Figure S1A). Similar trends were observed for non–HDL‐C control <130 mg/dL, LDL‐C control <100 mg/dL, and LDL‐C control <70 mg/dL, although test for joinpoint of LDL‐C control <70 mg/dL was not statistically significant (Table 1). Decreasing trend of average non–HDL‐C levels among participants with ASCVD also slowed down after 2006 (Figure S2A).
Table 1.
Risk Factor Control Among US Adults With ASCVD, 1999 to 2002 to 2015 to 2018, Weighted
| Risk factor control | Adults with ASCVD, % (95% CI)* | P joinpoint † | P trend ‡ | ||||
|---|---|---|---|---|---|---|---|
| 1999–2002 (n=1096) | 2003–2006 (n=1129) | 2007–2010 (n=1246) | 2011–2014 (n=1049) | 2015–2018 (n=1197) | |||
| LDL‐C <70 mg/dL | 6.9 (4.4–10.8) | 14.2 (10.7–18.7) | 17.8 (14.5–21.5) | 21.0 (17.7–24.6) | 24.6 (19.0–31.2) | 0.42 | <0.001 |
| LDL‐C <100 mg/dL | 30.7 (23.7–38.7) | 46.2 (39.2–53.4) | 54.8 (50.0–59.4) | 59.2 (51.4–66.4) | 58.7 (53.3–63.9) | 0.01 | <0.001 |
| Non–HDL‐C <100 mg/dL | 7.1 (5.4–9.2) | 15.7 (12.5–19.5) | 22.5 (19.8–25.3) | 27.3 (23.1–31.8) | 30.9 (25.9–36.3) | 0.01 | <0.001 |
| Non–HDL‐C <130 mg/dL | 30.1 (24.9–35.9) | 42.7 (38.3–47.2) | 55 (51.8–58.2) | 55.2 (51.9–58.4) | 58.3 (54.0–62.5) | 0.02 | <0.001 |
| BP <130/80 mm Hg | 41.4 (36.6–46.3) | 42.7 (38.0–47.5) | 50.1 (46.4–53.9) | 52.6 (48.5–56.8) | 49.5 (44.9–54.2) | 0.04 | <0.001 |
| BP <140/90 mm Hg | 62.6 (58.2–66.8) | 64.1 (59.5–68.4) | 71.9 (69.0–74.7) | 74.1 (70.4–77.4) | 70.1 (65.8–74.1) | 0.14 | <0.001 |
| HbA1c <7% | 87.4 (84.9–89.5) | 89.6 (86.8–91.9) | 86.1 (82.9–88.8) | 84.2 (80.3–87.5) | 81.7 (77.6–85.2) | 0.05 | 0.001 |
| HbA1c <7% in those with self‐reported diabetes | 46.5 (38.3–54.9) | 63.3 (55.1–70.8) | 55.1 (48.1–62.0) | 49.7 (39.7–59.6) | 51.5 (42.5–60.4) | 0.07 | 0.009 |
| HbA1c <8% | 92.6 (90.4–94.3) | 95.4 (94.0–96.5) | 93.5 (91.3–95.1) | 91.2 (87.5–93.8) | 93.3 (90.8–95.1) | 0.05 | 0.51 |
| HbA1c <8% in those with self‐reported diabetes | 68.2 (59.6–75.7) | 83.4 (78.6–87.3) | 78.1 (71.9–83.3) | 70.8 (60.3–79.4) | 81.7 (74.8–87.0) | 0.09 | 0.32 |
| Obesity | 39.9 (35.2–44.7) | 40.4 (36.9–43.9) | 44.8 (41.9–47.7) | 44.9 (40.4–49.5) | 50.2 (45.7–54.8) | 0.70 | <0.001 |
| Current smoker | 20.1 (16.3–24.5) | 21.9 (19.1–24.9) | 19.8 (16.2–23.8) | 23.1 (19.7–26.8) | 22.9 (18.7–27.8) | 0.91 | 0.55 |
| Heavy alcohol consumption | 15.7 (11.8–20.4) | 17.8 (14.8–21.1) | 16.4 (14.1–19.1) | 19.4 (15.7–23.7) | 28.4 (22.8–34.9) | 0.06 | <0.001 |
| All controlled§ | 4.5 (3.0–6.7) | 9.4 (7.3–12.0) | 14.8 (12.5–17.5) | 18.4 (15.1–22.2) | 18.6 (14.8–23.2) | 0.02 | <0.001 |
ASCVD indicates atherosclerotic cardiovascular disease; BP, blood pressure; HbA1c, hemoglobin A1c; LDL‐C, low‐density lipoprotein cholesterol; and Non–HDL‐C, non–high‐density lipoprotein cholesterol (calculate as total cholesterol minus HDL‐C).
National Health and Nutrition Examination Survey weights were adjusted to generate nationally representative percentages.
Joinpoint regression was used to identify whether 1 joinpoint occurred, and statistical significance of the joinpoint was tested using the Monte Carlo permutation method.
The overall trend of risk factor control from 1999 to 2018 was analyzed using logistics regression modeling the survey cycle as a continuous variable.
All controlled was defined as HbA1c <7%, BP <140/90 mm Hg, and non–HDL <100 mg/dL.
The percentage of participants with ASCVD who achieved BP control <140/90 mm Hg increased from 62.6% (95% CI, 58.2%–66.8%) in 1999 to 2002 to 74.1% (95% CI, 70.4%–77.4%) in 2011 to 2014, and then slightly decreased to 70.1% (95% CI, 65.8%–74.1%) in 2015 to 2018 (P trend<0.001, P joinpoint=0.14; Table 1, Table S5, Figure S1B). Similar trends were observed for BP control <130/80 mm Hg (Table 1, Table S5, Figure S1B). Consistently, the average systolic and diastolic BP decreased from 1999 to 2014 and then began to increase among adults with ASCVD (Figure S2B, S2C).
The proportion of HbA1c control <7% among participants with ASCVD increased from 87.4% (95% CI, 84.9%–89.5%) in 1999 to 2002 to 89.6% (95% CI, 86.8%–91.9%) in 2003 to 2006 and then decreased to 81.7% (95% CI, 77.6%–85.2%) in 2015 to 2018 (P joinpoint=0.05, P trend=0.001; Table 1, Table S5, Figure S1C). Identical trends were observed for HbA1c control <8%, HbA1c control <7%, and HbA1c control <8% in participants complicated with self‐reported diabetes (Table 1, Table S5, Figure S1C). Accordingly, an abrupt increase of average HbA1c level among all participants with ASCVD was observed in 2007 to 2008 (Figure S2D).
The proportion of adults with ASCVD who simultaneously achieved HbA1c <7%, BP <140/90 mm Hg, and non–HDL‐C <100 mg/dL increased significantly from 4.5% (95% CI, 3.0%–6.7%) to 18.6% (95% CI, 14.8%–23.2%) from 1999 to 2018 (P trend<0.001) (Table 1), but the increasing trend slowed down after 2005 to 2006 (Figure 2, Figure S1D; P joinpoint=0.02), and even began to decrease after 2013 (Figure S1D). Notably, as shown in Figure 2, the percentage of female adults who achieved all control for 3 risk factors was much lower than that in male adults across 1999 to 2018, with only 13.6% female adults versus 22.6% male adults having all 3 risk factors controlled in 2015 to 2018 (Figure 2).
Figure 2. Trend of all risk factors control (hemoglobin A1c <7%, blood pressure <140/90 mm Hg, and non–high‐density lipoprotein cholesterol <100 mg/dL) among US adults with atherosclerotic cardiovascular disease.
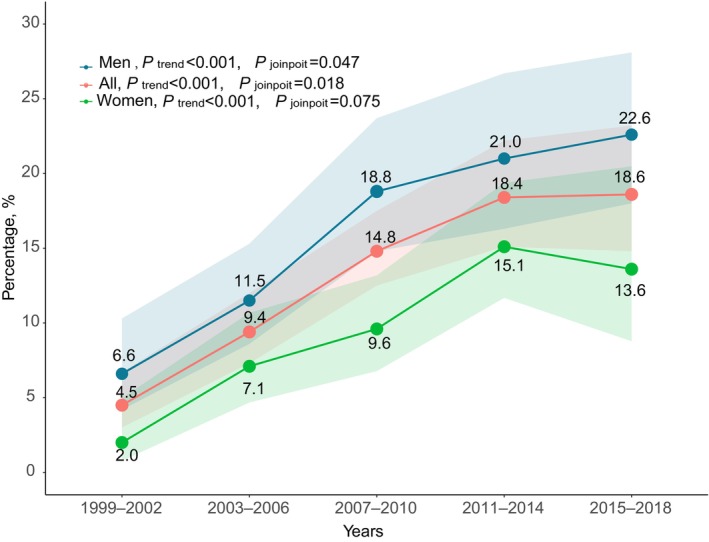
Shaded areas indicate 95% CIs. Diagnosed atherosclerotic cardiovascular disease was defined as self‐reported coronary heart disease, angina, heart attack, or stroke. P trend was analyzed using logistics regression modeling the survey cycle as a continuous variable. P joinpoint was analyzed using joinpoint regression, and statistical significance of the joinpoint was tested using the Monte Carlo permutation method.
In US adults with ASCVD, prevalence of obesity (P trend=0.03, P joinpoint=0.70) and heavy alcohol consumption (P trend<0.001, P joinpoint=0.06) increased linearly from 39.9% (95% CI, 35.2%–44.7%) and 15.7% (11.8%–20.4%) to 50.2% (95% CI, 45.7%–54.8%) and 28.4% (95% CI, 22.8%–34.9%), respectively, from 1999 to 2018, but prevalence of current smokers remained stable across 1999 to 2018 (P trend=0.19, P joinpoint=0.55) (Table 1, Figure S3).
In sensitivity analysis adjusting for age, sex, and race and ethnicity on trend of risk factor control, similar to the unadjusted model, all changes were statistically significant (P trend<0.05) except for HbA1c <8%, HbA1c <8% in those with self‐reported diabetes, and current smokers (P trend>0.05).
Trends in ASCVD Treatment
Trends in the use of any lipid‐lowering agents were nonlinear (P joinpoint=0.005), increasing from 41.8% (95% CI, 37.6%–46.2%) in 1999 to 2002 to 66.7% (95% CI, 62.4%–70.8%) in 2011 to 2014, and then leveled off (Table 2). A similar trend was observed with statin use (P joinpoint<0.001; Table 2, Table S6). Although the percentage of participants with self‐reported hypertension increased significantly over the past 20 years, the use of any antihypertensive agents among all adults with ASCVD or among those also with self‐reported hypertension did not increase (P trend>0.05, P joinpoint>0.05; Table 2, Table S6). The use of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, β‐blockers, and diuretics all increased significantly before 2006; subsequently, the use of angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers and β‐blockers plateaued, whereas diuretic use decreased (Table 2, Table S6). The use of calcium channel blockers decreased linearly over the past 20 years (P trend=0.006, P joinpoint=0.66). Along with the increasing prevalence of diabetes, the use of any antidiabetic agents among those with ASCVD also increased (P<0.001). Notably, antidiabetic agent use among participants with self‐reported diabetes increased from 74.5% (95% CI, 66.5%–81.2%) in 1999 to 2002 to 86.0% (95% CI, 81.4%–89.5%) in 2007 to 2010, but then decreased to 80.7% (95% CI, 73.9%–86.1%) in 2015 to 2018 (P joinpoint=0.004, P trend<0.001). Clopidogrel use increased from 4.2% in 1999 to 2002 to 14.9% in 2003 to 2006 and then leveled off (P joinpoint<0.001).
Table 2.
Medication Use in Adult NHANES Participants With ASCVD, 1999 to 2002 to 2015 to 2018, Weighted
| Treatment | Adults with ASCVD, % (95% CI)* | P joinpoint † | P trend ‡ | ||||
|---|---|---|---|---|---|---|---|
| 1999–2002 (n=1096) | 2003–2006 (n=1129) | 2007–2010 (n=1246) | 2011–2014 (n=1049) | 2015–2018 (n=1197) | |||
| ≥1 Lipid‐lowering agents | 41.8 (37.6–46.2) | 49.7 (45.6–53.9) | 58.6 (55.1–62.1) | 66.7 (62.4–70.8) | 63.3 (58.3–68.1) | 0.005 | <0.001 |
| Statin | 38.8 (34.6–43.2) | 45.5 (41.3– 49.8) | 54.5 (51.2–57.7) | 64.2 (60.1–68.1) | 61.1 (56.0–65.9) | 0.01 | <0.001 |
| Ezetimibe | 5.0 (2.9–8.4) | 9.4 (7.3–11.9) | 4.3 (2.8–6.5) | 2.5 (1.6–4.1) | |||
| ≥1 Antihypertensive agents | 74.2 (69.9–78.2) | 76 (71.1–80.2) | 77.3 (74.5–79.9) | 78.4 (74.6–81.7) | 78.8 (75.7–81.7) | 0.38 | 0.06 |
| ACEI/ARB | 34.2 (30.1–38.5) | 45 (41.4–48.7) | 50.4 (47.5–53.3) | 51.6 (47.3–55.8) | 50.5 (46.4–54.5) | 0.003 | <0.001 |
| β‐Blocker | 33.3 (29.2–37.7) | 45.2 (40.8–49.7) | 49.8 (46.5–53) | 46.9 (43.1–50.7) | 51.8 (47.6–55.9) | 0.05 | <0 0.001 |
| CCB | 28.1 (24.3–32.2) | 25 (21.9–28.4) | 23 (20.4–25.7) | 23.4 (20.4–26.7) | 20.4 (16.8–24.6) | 0.66 | 0.006 |
| Diuretics | 32.1 (27.9–36.5) | 37.2 (33.1–41.5) | 36.8 (33.3–40.5) | 30.5 (26.5–34.9) | 28.7 (24.7–33.2) | 0.002 | 0.02 |
| ≥1 Antihypertensive agents in those with self‐reported hypertension | 87.0 (83.5–89.8) | 88.0 (84.0–91.2) | 88.4 (84.7–91.3) | 89.3 (85.9–92.0) | 89.8 (86.4–92.5) | 0.57 | 0.23 |
| ≥1 Antidiabetic agents | 16.0 (13.4–19.1) | 20.1 (16.8–23.7) | 24.8 (22–27.9) | 27.1 (23.2–31.3) | 29.5 (26.7–32.5) | 0.23 | <0.001 |
| Insulin | 4.4 (2.9–6.8) | 5.7 (4–8) | 7.3 (5.6–9.4) | 10.2 (8.2–12.7) | 9.6 (7.9–11.6) | 0.20 | <0.001 |
| Metformin | 5.6 (4.3–7.2) | 10.1 (8–12.6) | 13.4 (11.3–15.8) | 15.4 (12.6–18.6) | 18.9 (16.1–22.1) | 0.13 | <0 0.001 |
| Sulfonylureas | 8.8 (7.3–10.6) | 10.1 (8.1–12.5) | 12.6 (10.8–14.7) | 10.4 (8–13.5) | 9.6 (7.9–11.5) | 0.27 | 0.68 |
| Dipeptidyl peptidase 4 inhibitor | 1.6 (0.8–3) | 3.6 (2.4–5.3) | 4.3 (2.9–6.2) | ||||
| Thiazolidinedione | 1.9 (1.1–3.2) | 6.5 (5.1–8.3) | 5.1 (3.5–7.2) | 1.8 (0.9–3.6) | 1.5 (0.6–3.3) | 0.005 | 0.002 |
| SGLT2 inhibitor or GLP‐1 | 0.2 (0–1.2) | 0.03 (0.004–0.2) | 1.1 (0.5–2.1) | 3.1 (1.9–5.1) | |||
| ≥1 Antidiabetic agents in those with self‐reported diabetes | 74.5 (66.5–81.2) | 75.0 (66.4–82.0) | 86.0 (81.4–89.5) | 85.3 (79.5–89.7) | 80.7 (73.9–86.1) | 0.004 | <0.001 |
| Aspirin among those aged ≥40 y§ | 63.5 (59.4–67.5) | 65.5 (60.5–70.3) | |||||
| Clopidogrel | 4.2 (3.3–5.4) | 14.9 (11.9–18.5) | 17 (14.2–20.4) | 16.9 (13.9–20.4) | 17.5 (14.3–21.2) | <0.001 | <0.001 |
| Prasugrel | 0.2 (0.0–1.5) | 1.4 (0.5–3.6) | 0.4 (0.1–1.4) | ||||
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASCVD, atherosclerotic cardiovascular disease; CCB, calcium channel blocker; GLP‐1, glucagon‐like peptide‐1; NHANES, National Health and Nutrition Examination Survey; and SGLT2, sodium‐dependent glucose transporter 2.
NHANES weights were adjusted to generate nationally representative percentages.
Joinpoint regression was used to identify whether 1 joinpoint occurred, and statistical significance of the joinpoint was tested using the Monte Carlo permutation method.
The overall trend of risk factor control from 1999 to 2018 was analyzed using logistics regression modeling the survey cycle as a continuous variable.
The aspirin data were presented from 2011 to 2012, because low‐dose aspirin is usually an over‐the‐counter medication in the United States and prescription aspirin use collected in the prescription medication data files before the 2011 to 2012 cycle was mainly to assess aspirin therapy in arthritis and musculoskeletal use or to assess general analgesic use prevalence.
Disparities in Risk Control and Medication Use Among Participants With ASCVD
Disparities in risk factor control and treatment were observed between different populations. As shown in Table 3 and Table S7, men were more likely to achieve the target of all risk factor control (ie, HbA1c <7%, BP <140/90 mm Hg, and non–HDL‐C <100 mg/dL) (OR, 1.68 [95% CI, 1.30–2.16]) or use statins (OR, 1.74 [95% CI, 1.47–2.70]) than women. Compared with the non‐Hispanic White population, the non‐Hispanic Black population was less likely to achieve the BP targets (<140/90 mm Hg; OR, 0.51 [95% CI, 0.41–0.62]), and less likely to use statins (OR, 0.75 [95% CI, 0.61–0.93]), but more likely to have non–HDL‐C controlled <100 mg/dL (OR, 1.56 [95% CI, 1.25–1.95]). The Hispanic population (OR, 0.53 [95% CI, 0.40–0.70]) and the non‐Hispanic Black population (OR, 0.60 [95% CI, 0.47–0.75]) were less likely to achieve glycemic control of HbA1c <7% than the non‐Hispanic White population. Participants with insurance and higher income were more likely to use statins. Unexpectedly, middle‐aged adults with ASCVD were less likely to have all risk factors controlled compared with younger adults (Table 3).
Table 3.
Adjusted ORs (95% CIs) for Medication Use and Risk Factor Control Among US Adults With ASCVD, Weighted
| Socioeconomic subgroups | Use of statin (n=2897)* | Use of antihypertensive agents among those with self‐reported hypertension (n=3642)* | Use of antidiabetic in those with self‐reported diabetes (n=1435)* | All controlled (BP <140/90 mm Hg, non–HDL‐C <100 mg/dL, HbA1c <7%) (n=627)* | Non–HDL‐C <100 mg/dL (n=1076)* | BP <140/90 mm Hg (n=3269)* | HbA1c <7% (n=4189)* |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Female adults | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Male adults | 1.74 (1.47–2.70) | 1.01 (0.73–1.40) | 1.47 (0.94–2.28) | 1.68 (1.30–2.16) | 1.47 (1.19–1.8) | 1.56 (1.33–1.82) | 0.65 (0.52–0.83) |
| Race and ethnicity† | |||||||
| Non‐Hispanic White population | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Hispanic population | 0.84 (0.66–1.05) | 0.59 (0.41–0.86) | 0.97 (0.60–1.56) | 0.88 (0.62–1.24) | 1.11 (0.85–1.46) | 0.89 (0.69–1.15) | 0.53 (0.40–0.70) |
| Non‐Hispanic Black population | 0.75 (0.61–0.93) | 0.87 (0.63–1.21) | 1.05 (0.68–1.62) | 1.03 (0.76–1.38) | 1.56 (1.25–1.94) | 0.51 (0.41–0.62) | 0.60 (0.47–0.75) |
| Other population | 1.59 (1.15–2.22) | 1.65 (0.85–3.20) | 0.84 (0.38–1.88) | 0.94 (0.57–1.54) | 1.24 (0.85–1.82) | 1.09 (0.79–1.52) | 0.59 (0.38–0.93) |
| Education | |||||||
| Less than high school | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| High school | 1.33 (1.90–1.62) | 1.37 (0.90–2.07) | 1.01 (0.61–1.69) | 1.25 (0.92–1.71) | 1.43 (1.14–1.80) | 0.94 (0.76–1.18) | 1.28 (0.96–1.72) |
| More than high school | 1.50 (0.86–1.29) | 0.80 (0.56–1.14) | 0.61 (0.37–1.00) | 1.68 (1.31–2.16) | 1.55 (1.26–1.92) | 1.03 (0.85–1.25) | 2.01 (1.54–2.65) |
| Insurance | |||||||
| No | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| Yes | 2.34 (1.61–3.39) | 3.13 (2.15–4.56) | 1.00 (0.51–1.97) | 1.42 (0.81–2.51) | 1.42 (0.87– 2.30) | 1.27 (0.92–1.74) | 1.01 (0.66–1.56) |
| Age groups, y | |||||||
| 20–44 | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 45–64 | 4.35 (3.30–6.23) | 3.39 (2.12–5.43) | 3.73 (1.75–7.96) | 0.59 (0.37–0.93) | 0.79 (0.52–1.22) | 0.50 (0.34–0.73) | 0.42 (0.23–0.74) |
| ≥65 | 6.21 (4.27–9.40) | 7.14 (4.50–11.36) | 4.20 (1.99–8.87) | 1.00 (0.65–1.55) | 1.7 (1.15–2.53) | 0.25 (0.17–0.38) | 0.39 (0.22–0.69) |
| Poverty/income ratio‡ | |||||||
| <1.3 | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 1.3 to <3 | 1.24 (1.40–1.48) | 1.28 (0.91–1.79) | 0.84 (0.55–1.28) | 1.12 (0.86–1.46) | 1.13 (0.91–1.39) | 1.11 (0.93–1.33) | 0.89 (0.70–1.14) |
| ≥3 | 1.83 (1.52–2.19) | 1.52 (0.98–2.36) | 1.25 (0.73–2.14) | 1.35 (1.2–1.79) | 1.33 (1.4–1.7) | 1.35 (1.10–1.66) | 0.94 (0.68–1.30) |
| BMI categories, kg/m2 | |||||||
| <18.5 | 0.59 (0.22–1.62) | 1.11 (0.32–3.78) | 1.28 (0.24–6.92) | 2.15 (0.77–6.40) | 1.95 (0.81–4.67) | 0.99 (0.50–1.97) | 1.73 (0.51–5.84) |
| 18.5 to <25 | Reference | Reference | Reference | Reference | Reference | Reference | Reference |
| 25 to <30 | 1.34 (1.50–1.72) | 1.08 (0.70–1.67) | 0.94 (0.55–1.62) | 0.88 (0.64–1.21) | 0.71 (0.54–0.93) | 1.11 (0.88–1.40) | 0.73 (0.50–1.07) |
| ≥30 | 1.48 (1.17–1.88) | 1.76 (1.11–2.77) | 1.48 (0.85–2.58) | 0.65 (0.45–0.95) | 0.65 (0.49–0.86) | 1.14 (0.92–1.40) | 0.22 (0.15–0.33) |
ASCVD indicates atherosclerotic cardiovascular disease; BMI, body mass index; BP, blood pressure; HbA1c, hemoglobin A1c; non–HDL‐C, non–high‐density lipoprotein cholesterol (calculated as total cholesterol minus HDL‐C); and OR, odds ratio.
ORs with 95% CIs were adjusted for age, sex, racial and ethnic group, educational level, family income, insurance, and weight status.
Race and ethnicity was determined by self‐report in fixed categories. Other race included Asian American, American Indian or Alaskan Native, Native Hawaiian or Pacific Islander, multiple races or ethnicities, or unknown.
Ratio of family income/federal poverty threshold in a particular year. A higher poverty/income ratio corresponds to a higher level of family income.
DISCUSSION
In this nationally representative analysis, we did not observe significant increases in the prevalence of ASCVD among US adults from 1999 to 2018. Nevertheless, risk factor control or medication use among those with ASCVD plateaued or even worsened mostly after 2005 to 2006, around 2007 to 2010. Only 18.6% of US adults with ASCVD simultaneously achieved non–HDL‐C <100 mg/dL, hemoglobin A1c <7%, and BP <140/90 mm Hg in 2015 to 2018. Striking disparities in risk factor control and medication use persisted between male and female adults, and between non‐Hispanic Black and non‐Hispanic White populations.
Lipid control is especially critical for ASCVD in preventing recurrent cardiovascular events. A log‐linear association between LDL‐C and cardiovascular events has been observed, with a 10% reduction of all‐cause mortality associated with each 1.0‐mmol/L or 38.7‐mg/dL reduction in LDL‐C. 25 However, in the present analysis, we found that after an initial sharp increase in lipid control, the percentage of participants with controlled lipid profiles leveled off after 2006. In 2015 to 2018, only 30.9% and 58.3% adults with ASCVD achieved non–HDL‐C control of <100 and <130 mg/dL, respectively, and only 63.3% were using lipid‐lowering agents. Controversy over the risk‐benefit balance of intensive cholesterol control might contribute to the plateau. In 2007, Alsheikh‐Ali et al reported in a meta‐analysis of 23 trials that cancer incidence was associated with lower achieved LDL‐C levels, 26 which provoked great dispute over safety of intensive cholesterol control. Although a larger meta‐analysis conducted later by the Cholesterol Treatment Trialists' Collaborators showed no such association, safety concerns persist, which may conceivably be related to the slight decline in lipid‐lowering medication or statin use after 2011 to 2014. 27 , 28 In addition, clinicians or participants might also be concerned by the low absolute benefit over intensive cholesterol control. 29
Consistent with previous findings on BP control among general US adults, 7 we found that although the awareness (self‐reported hypertension) of hypertension among US adults with ASCVD improved over the years, the BP control rate stalled around 2013 to 2014 and slightly decreased thereafter, which may result from the guidelines published by the eighth Joint National Committee in 2013 advising higher BP goals for some adults. 30 In the present analysis, we found that the prevalence of diabetes among US adults with ASCVD almost doubled from 1999 to 2002 to 2015 to 2018, in line with a previous study among general adults. 31 We also found that glycemic control worsened after 2006 among participants with both ASCVD and diabetes, which may be attributed to 3 major trials demonstrating that no cardiovascular benefit but increased risk of hypoglycemia was observed with intensive glycemic control (HbA1c <6.0% or <6.5%) in 2008 and 2009. 32 , 33 , 34 Concurrently, antidiabetic medication use among participants with diabetes and ASCVD declined since 2009 to 2010.
Striking disparities in risk factor control and medication use among different population groups were observed in the present analysis, especially between male and female adults and between non‐Hispanic Black and White populations. In the present analysis, we found female adults with ASCVD were much less likely to receive statin therapy and less likely to have all risk factors controlled, which is in accordance with previous findings. 35 , 36 This could potentially be attributed to sex biases in health care, where female adults' symptoms are often underrecognized or misdiagnosed. Therefore, more attention and strategies are needed to resolve the sex disparities in ASCVD management, which may potentially further reduce overall mortality rate from cardiovascular disease. Similarly, the non‐Hispanic Black population showed lower likelihood of achieving blood pressure and glycemic control targets compared with the non‐Hispanic White population. This suggests that racial and ethnic disparities in health care persist, possibly attributable to factors such as lower health care access, linguistic barriers, and cultural differences in health‐seeking behaviors. Our findings that participants with insurance and higher income were more likely to use statins also underscore the role of socioeconomic status in modulating cardiovascular risk status. These findings may partially explain the higher age‐adjusted cardiovascular mortality for Black adults compared with their White counterparts. 37 Thus, strategies are urgently needed to address the racial and ethnic disparities in risk factor control and treatment. Finally, the unexpected observation that middle‐aged adults with ASCVD were less likely to have all risk factors controlled compared with younger adults is concerning and warrants further investigation as younger adults with ASCVD or diabetes usually had greater risk of all‐cause and cardiovascular mortality, 38 , 39 It may suggest gaps in health care delivery or differing health behaviors among different age groups. Further research is needed to better understand these disparities and develop targeted interventions.
The alarmingly low risk factor control rate, the increasing prevalence of obesity, heavy alcohol consumption, and the observed disparities in ASCVD management between different socioeconomic subgroups, especially male and female adults and non‐Hispanic Black and White populations, may inform policy makers and health care professionals to take action to bridge these gaps to further reduce the cardiovascular disease mortality.
Study Limitations
Several limitations should be noted for the present study. First, we did not use the combined data set NHANES 2017 to March 2020 cycle to get the 2017 to 2020 data on ASCVD management, as the survey units and survey weights of NHANES 2017 to March 2020 were only designed for the whole population; trend analysis for population subgroups was not recommended for this combined data set. 40 Second, when analyzing racial and ethnic disparities, we used the non‐Hispanic White population as the reference group. This approach could introduce biases as it inherently assumes that risk factor control in the non‐Hispanic White population is the “norm” or “standard,” which might not necessarily be the case. Also, this approach might have overlooked the diversity and heterogeneity within the non‐Hispanic White group itself. Third, the results of our findings could be confounded by unmeasured factors, as although NHANES is a rich source of data, it does not capture all potential confounding variables. Fourth, some of our subgroup analyses (eg, those with BMI of ≤18.5 kg/m2) might be underpowered, and thus should be interpreted with caution. Another potential limitation is the validity of the collected data. Although NHANES is a well‐established and validated survey, the possibility of measurement errors or biases cannot be excluded. For instance, when collecting data on sex information, surveyors were instructed to ask about gender “if not obvious,” 41 instead of asking the participants about their sex at birth. This approach could misclassify participants and introduce bias to the observed sex difference. Additionally, although NHANES is a national representative survey of the United States, its findings may not be generalizable to other countries with different health systems and population characteristics. Finally, we were unable to assess the prevalence of aspirin, or any antiplatelet medication use over the past 20 years, because low‐dose aspirin is usually an over‐the‐counter medication in the United States.
CONCLUSIONS
Worsened glycemic, BP, obesity, and alcohol consumption control, the plateau of lipid control, and socioeconomic disparities in risk factor control and treatment might contribute to the deceleration in the cardiovascular mortality decline. The socioeconomic disparities underscore the need for tailored interventions to improve cardiovascular health across these demographic groups.
Sources of Funding
This study was supported by a grant from the National Natural Science Foundation of China (82004301) and the Scientific and Technological Innovation Project of China Academy of Chinese Medical Sciences (CI2021B004). Dr Somers was supported in part by a grant from the National Institutes of Health (HL160619).
Disclosures
Dr Somers has served as a consultant for Zoll, Lilly, Apnimed, Know Labs, and Jazz Pharmaceuticals; and serves on the Sleep Number Research Advisory Board. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S7
Figures S1–S3
This manuscript was sent to Monik C. Jiménez, SM, ScD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.032527
For Sources of Funding and Disclosures, see page 10.
References
- 1. Sawyer A, Flagg LA. State declines in heart disease mortality in the United States, 2000–2019. NCHS Data Brief . 2021:1–8. [PubMed]
- 2. Murphy SL, Kochanek KD, Xu J, Arias E. Mortality in the United States, 2020. NCHS Data Brief . 2021:1–8. [PubMed]
- 3. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart disease and stroke statistics‐2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 5. Wang L, Li X, Wang Z, Bancks MP, Carnethon MR, Greenland P, Feng YQ, Wang H, Zhong VW. Trends in prevalence of diabetes and control of risk factors in diabetes among US adults, 1999–2018. JAMA. 2021;326:704–716. doi: 10.1001/jama.2021.9883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ogden CL, Fryar CD, Martin CB, Freedman DS, Carroll MD, Gu Q, Hales CM. Trends in obesity prevalence by race and Hispanic origin‐1999–2000 to 2017–2018. JAMA. 2020;324:1208–1210. doi: 10.1001/jama.2020.14590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Muntner P, Hardy ST, Fine LJ, Jaeger BC, Wozniak G, Levitan EB, Colantonio LD. Trends in blood pressure control among US adults with hypertension, 1999–2000 to 2017–2018. JAMA. 2020;324:1190–1200. doi: 10.1001/jama.2020.14545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fang M, Wang D, Coresh J, Selvin E. Trends in diabetes treatment and control in US adults, 1999–2018. New Engl J Med. 2021;384:2219–2228. doi: 10.1056/NEJMsa2032271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johnson CL, Paulose‐Ram R, Ogden CL, Carroll MD, Kruszon‐Moran D, Dohrmann SM, Curtin LR. National Health and nutrition examination survey: analytic guidelines, 1999–2010. Vital Health Stat 2. 2013;1–24. [PubMed] [Google Scholar]
- 10. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a [DOI] [PubMed] [Google Scholar]
- 11. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455 [DOI] [PubMed] [Google Scholar]
- 12. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella‐Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APHA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e1143. doi: 10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 14. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd‐Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e563–e595. doi: 10.1161/CIR.0000000000000677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. American Diabetes Association . 6. Glycemic targets: standards of medical care in diabetes‐2018. Diabetes Care. 2018;41:S55–S64. doi: 10.2337/dc18-S006 [DOI] [PubMed] [Google Scholar]
- 16. Raza SA, Sokale IO, Thrift AP. Burden of high‐risk phenotype of heavy alcohol consumption among obese US population: results from National Health and nutrition examination survey, 1999–2020. Lancet Reg Health Am. 2023;23:100525. doi: 10.1016/j.lana.2023.100525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jellinger PS, Handelsman Y, Rosenblit PD, Bloomgarden ZT, Fonseca VA, Garber AJ, Grunberger G, Guerin CK, Bell DSH, Mechanick JI, et al. American Association of Clinical Endocrinologists and American College of endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23:1–87. doi: 10.4158/EP171764.APPGL [DOI] [PubMed] [Google Scholar]
- 18. Jacobson TA, Ito MK, Maki KC, Orringer CE, Bays HE, Jones PH, McKenney JM, Grundy SM, Gill EA, Wild RA, et al. National Lipid Association recommendations for patient‐centered management of dyslipidemia: part 1—executive summary. J Clin Lipidol. 2014;8:473–488. doi: 10.1016/j.jacl.2014.07.007 [DOI] [PubMed] [Google Scholar]
- 19. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, et al. The seventh report of the joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560 [DOI] [PubMed] [Google Scholar]
- 20. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314:1021–1029. doi: 10.1001/jama.2015.10029 [DOI] [PubMed] [Google Scholar]
- 21. Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National Health and nutrition examination survey: plan and operations, 1999–2010. Vital Health Stat 1. 2013;1–37. [PubMed] [Google Scholar]
- 22. He J, Zhu Z, Bundy JD, Dorans KS, Chen J, Hamm LL. Trends in cardiovascular risk factors in US adults by race and ethnicity and socioeconomic status, 1999–2018. JAMA. 2021;326:1286–1298. doi: 10.1001/jama.2021.15187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Centers for Disease Control and Prevention . National Health andnd nutrition examination survey 1999–2000 data documentation, codebook, and frequencies: prescription medications—drug information (rxq_drug). National Center for health Statistics 2021b. Accessed October 10, 2022. https://wwwn.cdc.gov/nchs/nhanes/1999‐2000/rxq_drug.Htm.
- 24. Bhattacharya K, Bentley JP, Ramachandran S, Chang Y, Banahan BF III, Shah R, Bhakta N, Yang Y. Phase‐specific and lifetime costs of multiple myeloma among older adults in the US. JAMA Netw Open. 2021;4:e2116357. doi: 10.1001/jamanetworkopen.2021.16357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cholesterol treatment Trialists' (CTT) collaboration . Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alsheikh‐Ali AA, Maddukuri PV, Han H, Karas RH. Effect of the magnitude of lipid lowering on risk of elevated liver enzymes, rhabdomyolysis, and cancer: insights from large randomized statin trials. J Am Coll Cardiol. 2007;50:409–418. doi: 10.1016/j.jacc.2007.02.073 [DOI] [PubMed] [Google Scholar]
- 27. Stein EA, Raal FJ. Targeting LDL: is lower better and is it safe? Best Pract Res Clin Endocrinol Metab. 2014;28:309–324. doi: 10.1016/j.beem.2013.10.010 [DOI] [PubMed] [Google Scholar]
- 28. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, et al. Ezetimibe added to statin therapy after acute coronary syndromes. New Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489 [DOI] [PubMed] [Google Scholar]
- 29. Byrne P, Demasi M, Jones M, Smith SM, O'Brien KK, DuBroff R. Evaluating the association between low‐density lipoprotein cholesterol reduction and relative and absolute effects of statin treatment: a systematic review and meta‐analysis. JAMA Intern Med. 2022;182:474–481. doi: 10.1001/jamainternmed.2022.0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. James PA, Oparil S, Carter BL, Cushman WC, Dennison‐Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth joint National Committee (JNC 8). JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427 [DOI] [PubMed] [Google Scholar]
- 31. Antonio‐Villa NE, Fernandez‐Chirino L, Vargas‐Vazquez A, Fermin‐Martinez CA, Aguilar‐Salinas CA, Bello‐Chavolla OY. Prevalence trends of diabetes subgroups in the United States: a data‐driven analysis spanning three decades from NHANES (1988–2018). J Clin Endocrinol Metab. 2022;107:735–742. doi: 10.1210/clinem/dgab762 [DOI] [PubMed] [Google Scholar]
- 32. Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. New Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431 [DOI] [PubMed] [Google Scholar]
- 34. The ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. New Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987 [DOI] [PubMed] [Google Scholar]
- 35. Setny M, Jankowski P, Krzykwa A, Kamiński KA, Gąsior Z, Haberka M, Czarnecka D, Pająk A, Kozieł P, Szóstak‐Janiak K, et al. Management of dyslipidemia in women and men with coronary heart disease: results from POLASPIRE study. J Clin Med. 2021;10:2594. doi: 10.3390/jcm10122594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peters SAE, Muntner P, Woodward M. Sex differences in the prevalence of, and trends in, cardiovascular risk factors, treatment, and control in the United States, 2001 to 2016. Circulation. 2019;139:1025–1035. doi: 10.1161/CIRCULATIONAHA.118.035550 [DOI] [PubMed] [Google Scholar]
- 37. Kyalwazi AN, Loccoh EC, Brewer LC, Ofili EO, Xu J, Song Y, Joynt Maddox KE, Yeh RW, Wadhera RK. Disparities in cardiovascular mortality between black and white adults in the United States, 1999 to 2019. Circulation. 2022;146:211–228. doi: 10.1161/CIRCULATIONAHA.122.060199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sattar N, Rawshani A, Franzen S, Rawshani A, Svensson AM, Rosengren A, McGuire DK, Eliasson B, Gudbjornsdottir S. Age at diagnosis of type 2 diabetes mellitus and associations with cardiovascular and mortality risks. Circulation. 2019;139:2228–2237. doi: 10.1161/CIRCULATIONAHA.118.037885 [DOI] [PubMed] [Google Scholar]
- 39. Rubin JB, Borden WB. Coronary heart disease in young adults. Curr Atheroscler Rep. 2012;14:140–149. doi: 10.1007/s11883-012-0226-3 [DOI] [PubMed] [Google Scholar]
- 40. Akinbami LJ, Chen TC, Davy O, Ogden CL, Fink S, Clark J, Riddles MK, Mohadjer LK. National Health and nutrition examination survey, 2017‐march 2020 prepandemic file: sample design, estimation, and analytic guidelines. Vital Health Stat 1. 2022;1–36. [PubMed] [Google Scholar]
- 41. Schier HE, Gunther C, Landry MJ, Ohlhorst SD, Linsenmeyer W. Sex and gender data collection in nutrition research: considerations through an inclusion, diversity, equity, and access lens. J Acad Nutr Diet. 2023;123:247–252. doi: 10.1016/j.jand.2022.09.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S7
Figures S1–S3


