According to the National Weather Service, a thunderstorm lifecycle typically progresses through 3 stages: (1) a developing stage, marked by updrafts of moist air with no precipitation but perhaps lightning; (2) a mature stage, whereby precipitation, lightning, and wind gusts manifest and are balanced by ongoing updrafts; and (3) a dissipating stage, consisting of a shift from updrafts to downdrafts and declining precipitation. This framework serves as a fitting analogy to the present state of arrhythmia burden in adults with congenital heart disease (CHD). Although surgical and interventional advancements in CHD management have resulted in remarkable increases in patient survival over the past half century, we are now faced with an aging population of patients with combinations of chronic valvular heart disease, ventricular dysfunction, cyanosis, chamber dilation, pressure and volume overload, and surgical scar, the perfect electrical milieu for arrhythmogenesis. The developing stage of this storm has passed; we have witnessed the warning signs when adults with CHD first began presenting with sustained atrial and ventricular arrhythmias 20 years ago, resulting in seminal works describing the unique mechanisms of the arrhythmias at play. 1 , 2
In this issue of the Journal of the American Heart Association (JAHA), Pravda et al demonstrate that we are firmly in the midst of this storm's mature stage. 3 In this study, the authors use 2 national health care insurance registries that account for >75% of health care use in Israel. The data included >11 000 patients with adult congenital heart disease (ACHD) (median age ≈47 years) who received care between 2007 and 2011, although >4000 of these patients were excluded for lack of sufficient details about their disease. The population included all types of ACHD, with >70% of them comprising simple complexity (atrial septal defects, ventricular septal defects, and aortic valve disease). It was not known whether the patients were on antiarrhythmics, had prior surgical repair, or had other interventions. The patients and their health care use were tracked using the International Classification of Diseases, Ninth Revision (ICD‐9) codes to determine their specific ACHD, arrhythmias, and other diagnoses at the time of inpatient and outpatient visits. The arrhythmias were bundled into 3 categories: atrial arrhythmias (including ICD‐9 codes for atrial fibrillation, atrial tachycardia, atrial premature beats); ventricular arrhythmias (ventricular tachycardia, ventricular fibrillation, cardiac arrest, any ICD‐9 code related to presence of a defibrillator); and conduction disturbances (sinoatrial node dysfunction, second‐ and third‐degree atrioventricular block, bundle‐branch block, any ICD‐9 code related to presence of a pacemaker). The study tracked incident arrhythmias and correlations to subsequent health care use as well as mortality.
The main findings were the following: (1) At baseline, 8.7% of the patients with ACHD had atrial or ventricular arrhythmia, 1.5% had conduction disturbance, and 0.5% had both. (2) Over a median follow‐up of 5.2 years, 9.2% of patients developed incident tachyarrhythmia, 0.9% developed conduction disturbance, and 0.3% developed both. (3) The patients with incident arrhythmias had significantly more frequent outpatient visits (median 45 visits versus 33 visits over 5 years). (4) Atrial tachyarrhythmia (relative risk (RR), 1.27 [95% CI, 1.17–1.38]) and atrioventricular block (RR, 1.33 [95% CI, 1.04–1.71]) correlated with increased hospital admissions, although arrhythmias did not correlate with increased intensive care unit length of stay or hospital length of stay. (5) Atrial tachyarrhythmia (RR, 1.65 [95% CI, 1.44–1.89]) and ventricular tachyarrhythmia (RR, 2.06 [95% CI, 1.44–2.94]) correlated with mortality. (6) Atrial tachyarrhythmia did not correlate with incident stroke (RR, 1.04 [95% CI, 0.86–1.27]). Atrial tachyarrhythmias were 10 times more prevalent than ventricular tachyarrhythmias or conduction disease. The strength of the study was the use of the insurance system registries that provided comprehensive data on health care use over a long period of time. The main limitations were that clinical diagnoses were made solely based upon ICD‐9 codes, and a substantial portion of the potential target population was excluded for insufficient data. There were a multitude of ICD‐9 codes that were unspecified with regard to arrhythmia types (premature beats not otherwise specified, cardiac dysrhythmia not otherwise specified, paroxysmal tachycardia not otherwise specified) as well as ACHD types, and the lack of detail on these codes was the primary reason for exclusion of so many patients.
On the surface, these data are unsurprising, because numerous studies have reported similar statistics. A 20‐year‐old person with CHD carries a similar 20‐year risk of atrial arrhythmia development (7%) as a 55‐year‐old person in the general population. 4 , 5 Adult patients with repaired tetralogy of Fallot, the most common cyanotic CHD lesion, are faced with a 20% risk of atrial arrhythmias and 15% risk of ventricular arrhythmias by the fourth decade of life. 6 Sinus node dysfunction requiring permanent pacemaker implantation occurs in 13% to 25% of patients following atrial switch operations (for D‐looped transposition of the great arteries) and Fontan palliation (for single ventricle lesions). 7 , 8 Atrial and ventricular arrhythmias consistently are each independently associated with mortality among patients with CHD, with hazard ratios of 1.5 to 2.0. 4 , 9 , 10 , 11
Where this study adds novelty are its findings that (1) arrhythmias correlate with a significantly higher frequency of outpatient visits (on average a visit every 5 to 6 weeks), and (2) both atrial and ventricular tachyarrhythmias correlate with significantly higher mortality. Although the latter point has been demonstrated elsewhere, replicating this association in multiple populations is critical and demonstrates its potentially grave significance. With regard to mortality, the main question that comes to mind is whether the arrhythmias are the cause of death or a marker of more advanced heart failure. The answer is unknown, but it is likely a combination of both. In adults with noncongenital heart disease, this question also remains unanswered, with data showing that heart failure increases risk of arrhythmia, and arrhythmia increases risk of heart failure. 12 Diagnosis of arrhythmia and heart failure frequently happens concomitantly and are inextricably linked in the ACHD population. 13
With these data in hand, the holy grail questions become (1) how best to treat these arrhythmias and (2) whether arrhythmia treatment modifies mortality as has been shown in adults without congenital heart disease. 14 , 15 , 16 Outcome data following radiofrequency catheter ablation for atrial fibrillation and flutter in patients with ACHD exist, yet are limited to retrospective, observational study designs and by overrepresentation of simple CHD lesions. Unsurprisingly, recurrence rates following such ablation procedures are higher than those observed in adults without CHD. Antiarrhythmic drugs are frequently used to control tachyarrhythmias in patients with ACHD but have mediocre efficacy and high adverse event rates. 17 , 18 Vascular access limitations and lead‐related endocarditis and thromboembolism negatively impact transvenous pacing outcomes in the ACHD population. 19 Patient selection for primary prevention implantable cardioverter‐defibrillator placement remains challenging, and patients with ACHD are known to experience a substantial burden of implantable cardioverter‐defibrillator–related complications. 20
Some rays of optimism do exist, however. Ablation procedures are presently performed more safely than ever, with major procedural adverse event rates of only ~1%, due to advances in contact force sensing technology, intracardiac echocardiography, and transseptal puncture technology. Many electrophysiologists are shifting to fluoroless ablation techniques, eliminating radiation exposure, which is important to the ACHD population that has often already undergone numerous fluoroscopic‐based cardiac catheterizations. 21 Nonthermal pulsed field ablation received Food and Drug Administration approval just this year and will likely make ablation procedures faster (reducing time under anesthesia and its associated postoperative risks) and safer (minimizing the risks of pulmonary vein stenosis, phrenic nerve injury, and atrioesophageal fistula formation observed with thermal ablation). 22 Conduction system pacing is now routinely performed in patients with ventricular pacing indications and preserves cardiac function and synchrony better than traditional biventricular cardiac resynchronization therapy (with less transvenous hardware and not reliant on suitable coronary venous tributaries). 23 Leadless pacing technology now includes atrial and ventricular implant options and obviates the infectious and thromboembolic risks associated with transvenous leads. 24 Finally, subcutaneous implantable cardioverter‐defibrillators have been shown to be safe and effective in CHD populations, eliminating transvenous lead‐related complications while maintaining acceptable inappropriate shock rates. 25
We find ourselves at a critical juncture in the evolution of ACHD arrhythmia care. The proverbial storm is here (Figure), and it will only get worse as evidenced by the data presented by the authors. Our patients are experiencing more complications, occupying more health care resources, and having higher mortality, much of which is related to development of arrhythmias. The question we must ask ourselves is how can we alter this storm's course and be confident we are entering the dissipating phase? Can we apply the exciting new technological developments in interventional electrophysiology to our patients with ACHD? Will aggressive comorbidity management and earlier ablation referral alter arrhythmia course (as has been shown in adults with atrial fibrillation)? Does quality evidence supporting certain interventional strategies in adults without CHD translate to the ACHD population? These questions will only be answered, and our patients will only benefit, via (1) close collaboration among cardiologists and surgeons with expertise in ACHD, electrophysiology, advanced cardiac therapies, and structural interventions; and (2) organized research efforts across ACHD centers that will design appropriate studies and iteratively analyze outcomes.
Figure 10.1002/(ISSN)2047‐9980. Stages of the storm of arrhythmias in adults with CHD.
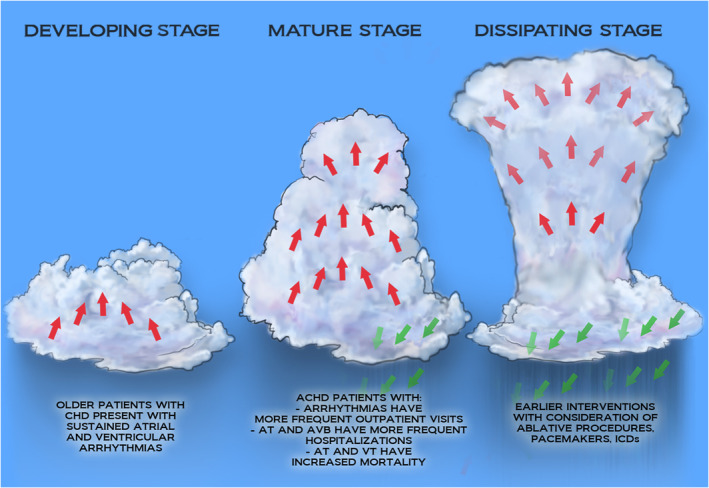
ACHD indicates adult congenital heart disease; AT, atrial tachycardia; AVB, atrioventricular block; CHD, congenital heart disease; ICD, implantable cardioverter‐defibrillator; and VT, ventricular tachycardia.
Disclosures
None.
See Article by Pravda et al.
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
This article was sent to John L. Jefferies, MD, MPH, Guest Editor, for editorial decision and final disposition.
For Disclosures, see page 4.
References
- 1. Triedman JK, Bergau DM, Saul JP, Epstein MR, Walsh EP. Efficacy of radiofrequency ablation for control of intraatrial reentrant tachycardia in patients with congenital heart disease. J Am Coll Cardiol. 1997;30:1032–1038. doi: 10.1016/S0735-1097(97)00252-0 [DOI] [PubMed] [Google Scholar]
- 2. Zeppenfeld K, Schalij MJ, Bartelings MM, Tedrow UB, Koplan BA, Soejima K, Stevenson WG. Catheter ablation of ventricular tachycardia after repair of congenital heart disease. Circulation. 2007;116:2241–2252. doi: 10.1161/CIRCULATIONAHA.107.723551 [DOI] [PubMed] [Google Scholar]
- 3. Pravda NS, Kalter‐Leibovici O, Nir A, Lorber A, Dadashev A, Hirsch R, Benderly M, Israeli Congenital Heart Disease Research Group . Arrhythmia burden amongst adult patients with congenital heart disease. J Am Heart Assoc. 2024;13:e031760. doi: 10.1161/JAHA.123.031760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bouchardy J, Therrien J, Pilote L, Ionescu‐Ittu R, Martucci G, Bottega N, Marelli AJ. Atrial arrhythmias in adults with congenital heart disease. Circulation. 2009;120:1679–1686. doi: 10.1161/CIRCULATIONAHA.109.866319 [DOI] [PubMed] [Google Scholar]
- 5. Lloyd‐Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, D'Agostino RB, Massaro JM, Beiser A, Wolf PA, et al. Lifetime risk for development of atrial fibrillation. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42 [DOI] [PubMed] [Google Scholar]
- 6. Khairy P, Aboulhosn J, Gurvitz MZ, Opotowsky AR, Mongeon FP, Kay J, Valente AM, Earing MG, Lui G, Gersony DR, et al. Arrhythmia burden in adults with surgically repaired tetralogy of Fallot. Circulation. 2010;122:868–875. doi: 10.1161/CIRCULATIONAHA.109.928481 [DOI] [PubMed] [Google Scholar]
- 7. Broberg CS, Van DA, Minnier J, Aboulhosn J, Kauling RM, Ginde S, Krieger EV, Rodriguez F 3rd, Gupta T, Shah S, et al. Long‐term outcomes after atrial switch operation for transposition of the great arteries. J Am Coll Cardiol. 2022;80:951–963. doi: 10.1016/j.jacc.2022.06.020 [DOI] [PubMed] [Google Scholar]
- 8. Dahlqvist JA, Sunnegårdh J, Hanséus K, Strömvall Larsson E, Nygren A, Dalén M, Berggren H, Ramgren JJ, Urban Wiklund U, Rydberg A. Pacemaker treatment after Fontan surgery—a Swedish national study. Congenit Heart Dis. 2019;14:582–589. doi: 10.1111/chd.12766 [DOI] [PubMed] [Google Scholar]
- 9. Egbe AC, Najam M, Banala K, Vojjini R, Bonnichsen C, Ammash NM, Faizee F, Khalil F, Deshmukh AJ, Connolly HM. Impact of atrial arrhythmia on survival in adults with tetralogy of fallot. Am Heart J. 2019;218:1–7. doi: 10.1016/j.ahj.2019.08.013 [DOI] [PubMed] [Google Scholar]
- 10. Egbe AC, Connolly HM, Khan AR, Niaz T, Said SS, Dearani JA, Warnes CA, Deshmukh AJ, Kapa S, McLeod CJ. Outcomes in adult Fontan patients with atrial tachyarrhythmias. Am Heart J. 2017;186:12–20. doi: 10.1016/j.ahj.2016.12.015 [DOI] [PubMed] [Google Scholar]
- 11. Yang H, Kuijpers JM, De GJR, Konings TC, van Dijk A, Tj Sieswerda G, Post MC, Mulder BJM, Bouma BJ. Impact of atrial arrhythmias on outcome in adults with congenital heart disease. Int J Cardiol. 2017;248:152–154. doi: 10.1016/j.ijcard.2017.06.073 [DOI] [PubMed] [Google Scholar]
- 12. Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, Ellinor PT, Cheng S, Vasan RS, Lee DS, et al. Atrial fibrillation begets heart failure and vice versa. Circulation. 2016;133:484–492. doi: 10.1161/CIRCULATIONAHA.115.018614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moore JP, Marelli A, Burchill LJ, Chubb H, Roche SL, Cedars AM, Khairy P, Zaidi AN, Janousek J, Crossland DS, et al. Management of heart failure with arrhythmia in adults with congenital heart disease JACC state‐of‐the‐art review. J Am Coll Cardiol. 2022;80:2224–2238. doi: 10.1016/j.jacc.2022.09.038 [DOI] [PubMed] [Google Scholar]
- 14. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–427. doi: 10.1056/NEJMoa1707855 [DOI] [PubMed] [Google Scholar]
- 15. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, Fetsch T, van Gelder IC, Haase D, Haegeli LM, et al. Early rhythm‐control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383:1305–1316. doi: 10.1056/NEJMoa2019422 [DOI] [PubMed] [Google Scholar]
- 16. Sapp JL, Wells GA, Parkash R, Stevenson WG, Blier L, Sarrazin JF, Thibault B, Rivard L, Gula L, Leong‐Sit P, et al. Ventricular tachycardia ablation versus escalation of antiarrhythmic drugs. New Engl J Medicine. 2016;375:111–121. doi: 10.1056/NEJMoa1513614 [DOI] [PubMed] [Google Scholar]
- 17. Iwasawa S, Uyeda T, Saito M, Ishii T, Inage A, Hamamichi Y, Yazaki S, Yoshikawa T. Efficacy and safety of low‐dose amiodarone therapy for tachyarrhythmia in congenital heart disease. Pediatr Cardiol. 2018;39:1016–1022. doi: 10.1007/s00246-018-1853-4 [DOI] [PubMed] [Google Scholar]
- 18. Moore BM, Cordina RL, McGuire MA, Celermajer DS. Adverse effects of amiodarone therapy in adults with congenital heart disease. Congenit Heart Dis. 2018;13:944–951. doi: 10.1111/chd.12657 [DOI] [PubMed] [Google Scholar]
- 19. Assaad IE, Pastor T, O'Leary E, Gauvreau K, Rathod RH, Gurvitz M, Wu F, Fynn‐Thompson F, DeWitt ES, Mah DY. Atrial pacing in Fontan patients: the effect of Transvenous Lead on clot burden. Heart Rhythm. 2021;18:1860–1867. doi: 10.1016/j.hrthm.2021.06.1191 [DOI] [PubMed] [Google Scholar]
- 20. Khairy P, Harris L, Landzberg MJ, Fernandes SM, Barlow A, Mercier LA, Viswanathan S, Chetaille P, Gordon E, Dore A, et al. Sudden death and defibrillators in transposition of the great arteries with intra‐atrial baffles. Circ Arrhythm Electrophysiol. 2008;1:250–257. doi: 10.1161/CIRCEP.108.776120 [DOI] [PubMed] [Google Scholar]
- 21. Razminia M, Willoughby MC, Demo H, Keshmiri H, Wang T, D'Silva OJ, Zheutlin TA, Jibawi H, Okhumale P, Kehoe RF. Fluoroless catheter ablation of cardiac arrhythmias: a 5‐year experience. Pacing Clin Electrophysiol. 2017;40:425–433. doi: 10.1111/pace.13038 [DOI] [PubMed] [Google Scholar]
- 22. Reddy VY, Gerstenfeld EP, Natale A, Whang W, Cuoco FA, Patel C, Mountantonakis SE, Gibson DN, Harding JD, Ellis CR, et al. Pulsed field or conventional thermal ablation for paroxysmal atrial fibrillation. N Engl J Med. 2023;389:1660–1671. doi: 10.1056/NEJMoa2307291 [DOI] [PubMed] [Google Scholar]
- 23. Felix IF, Collini M, Fonseca R, Guida C, Armaganijan L, Healey JS, Carvalho G. Conduction system pacing versus biventricular pacing in heart failure with reduced ejection fraction: a systematic review and meta‐analysis of randomized controlled trials. Heart Rhythm. 2024;S1547‐5271(24)00207‐8. in press. doi: 10.1016/j.hrthm.2024.02.035 [DOI] [PubMed] [Google Scholar]
- 24. Knops RE, Reddy VY, Ip JE, Doshi R, Exner DV, Defaye P, Canby R, Bongiorni MG, Shoda M, Hindricks G, et al. A dual‐chamber leadless pacemaker. N Engl J Med. 2023;388:2360–2370. doi: 10.1056/NEJMoa2300080 [DOI] [PubMed] [Google Scholar]
- 25. Waldmann V, Marquié C, Bessière F, Perrot D, Anselme F, Badenco N, Barra S, Bertaux G, Blangy H, Bordachar P, et al. Subcutaneous implantable cardioverter‐defibrillators in patients with congenital heart disease. J Am Coll Cardiol. 2023;82:590–599. doi: 10.1016/j.jacc.2023.05.057 [DOI] [PubMed] [Google Scholar]


