Abstract
Background
Population‐based data on heart failure (HF)‐related death in patients with atrial fibrillation (AF) are lacking. We assessed HF‐related death in people with AF in the United States over the past 21 years and examined differences by age, sex, race, ethnicity, urbanization, and census region.
Methods and Results
Data were extracted from the Centers for Disease Control and Prevention Wide‐Ranging Online Data for Epidemiologic Research to determine trends in age‐adjusted mortality rates per 100 000 people, due to HF‐related death among subjects with AF aged ≥15 years. To calculate nationwide annual trends, we assessed the average annual percent change (AAPC) and annual percent change with relative 95% CIs using joinpoint regression. Between 1999 and 2020, 916 685 HF‐related deaths (396 205 men and 520 480 women) occurred among US adults having a concomitant AF. The overall age‐adjusted mortality rates increased (AAPC: +4.1% [95% CI, 3.8–4.4]; P<0.001), especially after 2011 (annual percent change, +6.8% [95% CI, 6.2–7.4]; P<0.001) in men (AAPC, +4.8% [95% CI, 4.4–5.1]; P<0.001), in White subjects (AAPC: +4.2% [95% CI, 3.9 to 4.6]; P<0.001) and in subjects aged <65 years (AAPC: +7.5% [95% CI, 6.7–8.4]; P<0.001). The higher percentage of deaths were registered in the South (32.8%). During the first year of the COVID‐19 pandemic, a significant excess in HF‐related deaths among patients with AF aged >65 years was observed.
Conclusions
A worrying increase in the HF‐related mortality rate among patients with AF has been observed in the United States over the past 2 decades.
Keywords: atrial fibrillation, heart failure, death, trends
Subject Categories: Atrial Fibrillation
Nonstandard Abbreviations and Acronyms
- AAMR
age‐adjusted mortality rate
- AAPC
average annual percent change
- APC
annual percent change
- CDC
Centers for Disease Control and Prevention's
- WONDER
Wide‐Ranging Online Data for Epidemiologic Research
Clinical Perspective.
What Is New?
The heart failure–related mortality rate in patients with atrial fibrillation has increased in the United States, especially in men aged <65 years, with differences across different census regions, over the past 2 decades.
This worrying increase was mainly driven by the increased of heart failure–related death in patients with atrial fibrillation having as underlying causes of death hypertensive heart disease and chronic obstructive pulmonary disease.
What Are the Clinical Implications?
Preventive and treatment strategies for atrial fibrillation in patients with heart failure have not penetrated equally with ethnic and regional disparities.
The observed increasing mortality trend warrants an intensification of public health efforts aimed to promptly identify and treat these patients, increasing the promotion of targeted health policy measures.
Atrial fibrillation (AF) is the most common sustained arrhythmia worldwide. 1 According to recent epidemiological projections, the prevalence of AF will exceed 12 million people in the United States by 2030, 2 , 3 generating important repercussion on the associated cardiovascular morbidity and death. 4 , 5 Similarly, heart failure (HF) remains a major public health issue, 6 affecting more than 5 million Americans 7 and increasing both the cardiovascular‐related death and morbidity rates, especially among older subjects. 8 AF is a common comorbidity in patients with HF with an estimated prevalence ranging from 25% to 39% in subjects with preserved ejection fraction. 9 , 10 Conversely, in patients with a reduced ejection fraction the prevalence of AF increases with worsening New York Heart Association class, ranging from 4.2% for class I to 49.8% for class IV. 9 , 10
From a pathophysiological perspective, AF and HF share mutual bidirectional interactions and risk factors that lead to a vicious circle, with AF worsening HF and vice versa. 10 , 11 , 12 Recent analyses, performed in the United States, have showed that both cardiovascular deaths related to AF 13 and HF 14 are increasing at an alarming rate. However, the contemporary burden and trends regarding HF‐related death in patients with AF have not yet been investigated. Epidemiological data assessing the demographic and regional distribution of HF‐related deaths in AF remains crucial to identify patients at highest risk who may benefit from targeted interventions. 15 Therefore, the aim of the present study is to assess current trends in HF‐related death among patients with AF over the past 2 decades and determine differences by sex, age, race, ethnicity, urbanization, and census region, using the data from US Centers for Disease Control and Prevention's (CDC) Wide‐Ranging Online Data for Epidemiologic Research (WONDER) data set. 16
Methods
Study Population
We performed a retrospective cohort study aimed to assess the HF‐related mortality rate in patients with concomitant AF in the United States over the past 2 decades. Data were retrieved through the publicly available CDC's WONDER 16 data set, which provides information from death certificates of all US residents according to the International Classification of Diseases, Tenth Revision (ICD‐10). Moreover, this data set provides mortality estimates by age, sex, race, ethnicity, urbanization category, and census region. In the analysis, we included deaths in adults (aged >15 years) occurring between 1999 and 2020. Specifically, decedents were ascertained when HF (ICD‐10 codes I11.0, I13.0, I13.2, and I50.x) 13 were listed as the primary cause of death and AF (ICD‐10 code I48) 14 was listed as a contributing cause of death. Although ICD‐10 codes were implemented in billing and care of patients at the end of 2015, the World Health Organization authorized the publication of ICD‐10 in 1999. This was implemented for mortality coding and classification for cause of death on death certificates in the United States beginning in 1999 and spanning the entire study period. 17 Additionally, to further characterize the trends in of HF‐related death in subjects with AF we also analyzed the trends of the first 5 underlying causes of deaths over the entire study period.
For sex‐, race‐, and ethnicity‐specific estimates, we used annual national population totals for sex, age group, race, and Latinx/Hispanic ethnicity obtained by the US Census Bureau. 18 The stratification between urban and rural counties was performed in accordance with the 2013 National Center for Health Statistics Urban–Rural Classification Scheme. Ethnicity was defined as Latinx/Hispanic and non‐Latinx/Hispanic, while race was categorized as White, Black, American Indian or Alaska native, and Asian or Pacific Islander. Furthermore, mortality trends were also analyzed by the US census regions (Northeast, Midwest, South, and West). The study did not require institutional review board approval since the analysis was based on anonymized and publicly available data. All data are publicly available consulting the CDC WONDER data set and can be accessed at https://wonder.cdc.gov/mcd.html.
Data Extraction
Data extraction and validation were performed separately by 2 independent investigators (M.Z. and M.B.). Specifically, the population size, number of deaths for all causes as well as data on HF‐related death in patients with AF, stratified by age class, sex, race, ethnicity, urbanization, and census region were abstracted for the entire study period.
Statistical Analysis
HF age‐adjusted mortality rates (AAMRs) in subjects with AF, per 100 000 people, with the relative 95% CI, were calculated by standardizing the related deaths using the annual national population totals from the US Census Bureau and the 2000 US standard population. 18 Proportionate death was defined as the number of HF‐related deaths in patients with AF per 1000 deaths due to all causes. To calculate nationwide annual trends in HF‐related death in patients with AF, we assessed the annual percent change (APC) as well as average annual percent change (AAPC) and relative 95% CIs. Since the abstracted data contain 22 time points, we identified a maximum of 4 potential inflection points across the study period, as currently suggested by the current guidelines. 19 Statistical analyses were performed using joinpoint regression (Joinpoint, version 4.6.0.0; National Cancer Institute, Bethesda, MD). Specifically, joinpoint regression software determines the inflection points for each population of interest, and accordingly across the study period, time intervals of interest vary. A parallelism test was used to examine whether groups have a similar or different trends. 20 A significant P value on this interaction test indicated that the 2 trends, in terms of AAPC, were statistically significantly different from each other. The trends of the first 5 of the underlying causes of death were assessed using linear models in the SPSS package version 20.0 (SPSS, Chicago, IL). Statistical significance was prespecified at P≤0.05 for findings in the entire population.
Results
Between 1999 and 2020, 916 685 HF‐related deaths (396 205 men and 520 480 women) occurred among US adults aged ≥15 years having a concomitant AF. In particular, the absolute number of deaths increased from 22 075 subjects in 1999 (8190 men and 13 885 women) to 85 311 subjects (41 827 men and 43 484 women) in 2020 (Figure 1A). HF‐related death in patients with AF increased with age, with a seemingly exponential distribution, especially in subjects aged >45 years (Figure 1B). Accordingly, the AAMR for HF‐related death in patients with AF increased from 8.15 (95% CI, 8.05–8.26) per 100 000 in 1999 to 20.48 (95% CI, 20.34–20.62) per 100 000 in 2020 (AAPC, +4.1% [95% CI, 3.8–4.4]; P<0.001; Table 1 and Figure 2). Notably, the AAMR slightly increased from 1999 to 2011 (APC, +2.1% [95% CI, 1.8–2.5]; P<0.001) and then sharply increased from 2011 to 2020 (APC, +6.8% [95% CI, 6.2–7.4]; P<0.001).
Figure 1. Proportional death and HF‐related death in patients with AF according to age groups.
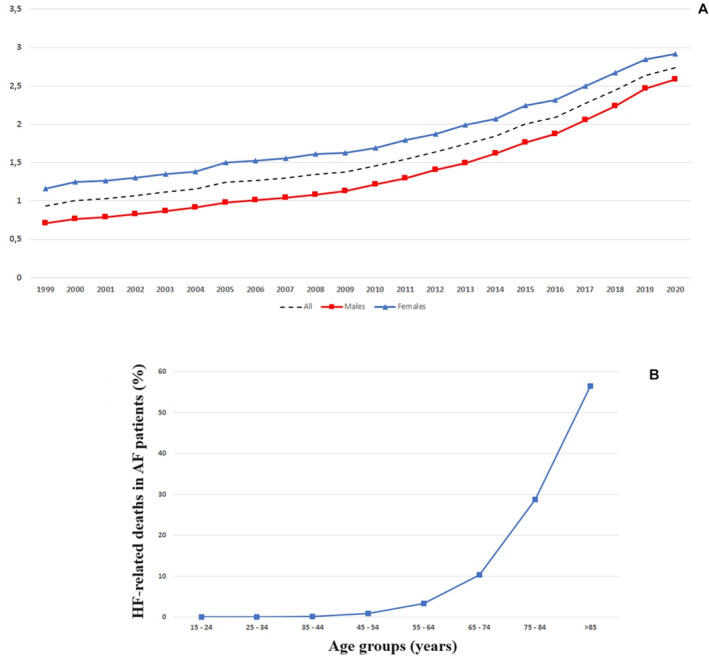
A, Proportional death over time. This is defined as the ratio between the number of HF‐related deaths in subjects with AF per 1000 deaths due to all causes. B, Percentages of HF‐related death in US subjects with AF by age groups, 1999 to 2020. AF indicates atrial fibrillation; and HF, heart failure.
Table 1.
Age‐Adjusted Mortality Rate Trend in US Subjects With Heart Failure and Atrial Fibrillation, 1999 to 2020, Stratified by Sex and Race or Ethnicity
| AAMR 1999 (95% CI) | AAMR 2020 (95% CI) | AAPC (95% CI), P value | Number of joinpoints | Period 1 [y]; APC (95% CI); P value | APC period 2 [y]; APC (95% CI); P value | P (for parallelism) | |
|---|---|---|---|---|---|---|---|
| Overall | 8.15 (8.05–8.26) | 20.48 (20.34–20.62) | +4.1 (3.8–4.4); <0.001 | 1 | [1999–2011]; +2.1 (1.8–2.5), <0.001 | [2011–2020]; +6.8; (6.2–7.4), <0.001 | … |
| Men | 8.63 (8.44–8.82) | 24.53 (24.30–24.77) | +4.8; (4.4–5.1); <0.001 | 1 | [1999–2011]; +2.6 (2.2–2.9); <0.001 | [2011–2020]; +7.8 (7.1–8.4); <0.001 | 0.002 |
| Women | 7.75 (7.62–7.88) | 17.37 (17.21–17.54) | +3.6 (3.3–4.0); <0.001 | 1 | [1999–2012]; +2.0 (1.7–2.4); <0.001 | [2012–2020]; +6.3 (5.5–7.1); <0.001 | |
| Race | |||||||
| White | 8.49 (8.37–8.60) | 21.76 (21.61–21.92) | +4.2 (3.9–4.6); <0.001 | 1 | [1999–2011]; +2.2 (1.9–2.6); <0.001 | [2011–2020]; +7.0 (6.4–7.6); <0.001 | White vs Black individuals, 0.48 |
| Black | 5.41 (5.10–5.72) | 14.62 (14.24–15.01) | +4.2 (3.6–4.9); <0.001 | 1 | [1999–2011]; +1.8 (1.0–2.5);<0.001 | [2011–2020]; +7.6 (6.4–8.9);<0.001 | White vs Asian/Pacific Islander individuals, P=0.003 |
| Asian or Pacific Islander | 4.05 (3.47–4.63) | 8.18 (7.79–8.57) | +3.5 (2.4–4.6); <0.001 | 1 | [1999–2015]; +2.6 (1.8–3.4); <0.001 | [2015–2020]; +6.5 (1.9–11.2); <0.001 | White vs Native Alaska/American Indian individuals, 0.33 |
| Native Alaskan/American Indian | 5.22 (3.84–6.02) | 12.56 (11.31–13.80) | +4.5 (3.4–5.7); <0.001 | 1 | [1999–2010]; +2.7 (1.0–4.3); <0.001 | [2010–2020]; +6.7 (4.7–8.7);<0.001 |
Black vs Asian/Pacific Islander individuals, 0.009 Black vs Native Alaskan/American Indian individuals, 0.10 Asian/Pacific Islander vs Native Alaskan/American individuals, <0.001 |
| Ethnicity | |||||||
| Latinx/Hispanic | 4.18 (3.78–4.57) | 11.23 (10.88–11.58) | +4.5 (3.9–5.0); <0.001 | 1 | [1999–2011]; +2.3 (1.6–3.0); <0.001 | [2011–2020]; +7.4 (6.3–8.6); <0.001 | … |
AAMR indicates age‐adjusted mortality rate, expressed as deaths per 100 000 population; AAPC, average annual percent change; and APC, annual percent change.
Figure 2. Trends in age‐adjusted mortality rates related to heart failure in subjects with atrial fibrillation, in the United States, 1999 to 2020.
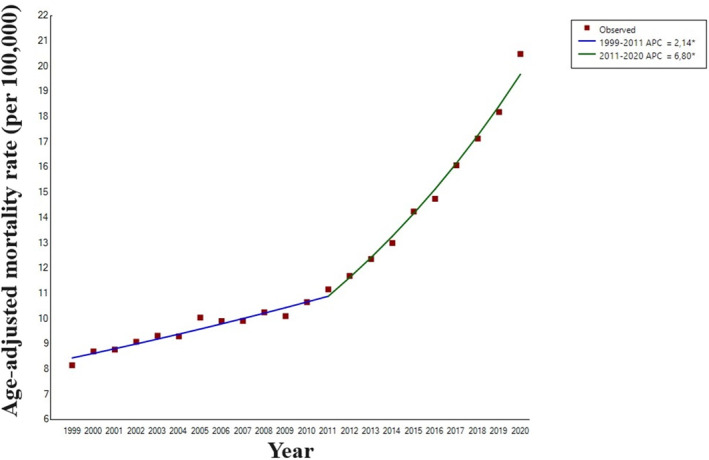
APC indicates annual percent change.
Sex
US men with HF and concomitant AF (AAPC, +4.8% [95% CI, 4.4–5.1]), compared with women (AAPC, +3.6% [95% CI, 3.3–4.0]), experienced a greater AAMR increase over the entire study period (P for parallelism=0.002). Specifically, in men, a slight increase from 1999 to 2011 (APC, +2.6% [95% CI, 2.2–2.9]) was followed by a more pronounced slope from 2011 to 2020 (APC, +7.8% [95% CI, 7.1–8.4]). Similarly, in women, the AAMR increased from 1999 to 2012 (APC, +2.0% [95% CI, 1.7–2.4]) and then further increased between 2012 and 2020 (APC, +6.3% [95% CI, 5.5–7.1]; Table 1 and Figure 3).
Figure 3. Trends in age‐adjusted mortality rates related to heart failure in subjects with atrial fibrillation, stratified by sex, in the United States, 1999 to 2020.
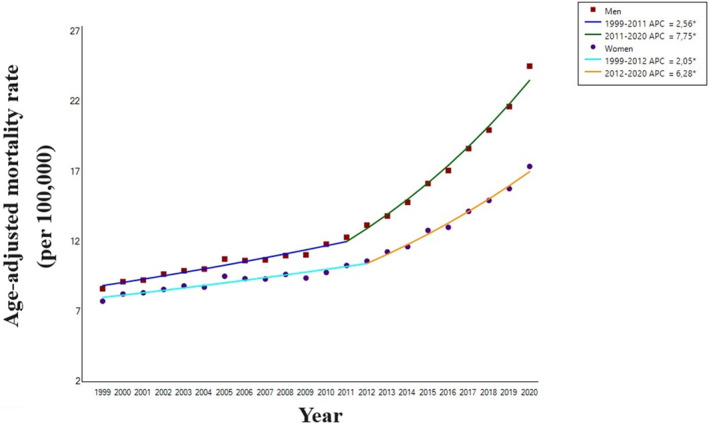
APC indicates annual percent change.
Race and Ethnicity
In White individuals, the AAMR for HF‐related death in patients with AF rose from 8.49 (95% CI, 8.37–8.60) per 100 000 in 1999 to 21.76 per 100 000 (95% CI, 21.61–21.92) in 2020 (AAPC, +4.2% [95% CI, 3.9–4.6]). The AAMR slightly increased from 1999 to 2011 (APC, +2.2 [95% CI, 1.9–2.6]; P<0.001) and then further increased from 2011 to 2020 (APC, +7.0% [95% CI, 6.4–7.6]). In non‐Latinx/Hispanic Black individuals, the AAMR rose from 5.41 (95% CI, 5.10–5.72) per 100 000 in 1999 to 14.62 (95% CI, 14.24–15.01) per 100 000 in 2020 (AAPC, +4.2% [95% CI, 3.6–4.9]). A slightly increased from 1999 to 2011 (APC, +1.8% [95% CI, 1.0–2.5]; P<0.001) was followed by a more pronounced slope from 2011 to 2020 (APC, +7.6% [95% CI, +7.6% 6.4–8.9]). In Asian/Pacific Islanders, the AAMR rose from 4.05 (95% CI, 3.47–4.63) per 100 000 in 1999 to 8.18 (95% CI, 7.79–8.57) per 100 000 in 2020 (AAPC, +3.5% [95% CI, 2.4–4.6]). The relative AAMR increased between 1999 to 2015 (APC, +2.6% [95% CI, 1.8–3.4]) and then further increased from 2015 to 2020 (APC, +6.5% [95% CI, 1.9–11.2]). Similarly, in American Indians/Alaska natives, the AAMR rose from 5.22 (95% CI, 3.84–6.02) per 100 000 in 1999 to 12.56 (95% CI, 11.31–13.80) per 100 000 in 2020 (AAPC, +4.5% [95% CI, 3.4–5.7]). Also, in this demographic group, a slight increase from 1999 to 2010 (APC, +2.7% [95% CI, 1.0–4.3]) was followed by a more pronounced slope from 2010 to 2020 (APC, +6.7% [95% CI, +4.7–8.7]). Regarding the ethnicity, only data for Latinx/Hispanic individuals were available. In this group, the AAMR rose from 4.18 (95% CI, 3.78–4.57) per 100 000 in 1999 to 11.23 (95% CI, 10.88–11.58) per 100 000 in 2020 (AAPC, +4.5% [95% CI, 3.9–5.0]). Specifically, the relative AAMR increased from 1999 to 2011 (APC, +2.3% [95% CI, 1.6–3.0]) and then further increased from 2011 to 2020 (APC, +7.4% [95% CI, 6.3–8.6]; Table 1).
Age
Decedents aged <65 years accounted for 42 247 (27 595 men and 15 652 women; 4.5%) HF‐related deaths in patients with AF. Although the AAMR was markedly lower than in patients aged ≥65 years (876 438 decedents: 368 610 men and 507 828 women), the AAPC was twice as high in the younger group (AAPC: +7.5% [95% CI, 6.7–8.4]) compared with the older group (AAPC, +4.0% [95% CI, 3.7–4.3]); P for parallelism <0.001; Table 2).
Table 2.
Age‐Adjusted Mortality Rate Trend in US Subjects With Heart Failure and Atrial Fibrillation, 1999 to 2020, Stratified by Age
| AAMR 1999 (95% CI) | AAMR 2020 (95% CI) | AAPC; (95% CI), P | Number of joinpoints | Period 1 [y]; APC (95% CI); P value | APC period 2 [y]; APC (95% CI); P value | P (for parallelism) | |
|---|---|---|---|---|---|---|---|
| <65 y (all) | 0.23 (0.21–0.25) | 1.32 (1.28–1.36) | +7.5 (6.7–8.4); <0.001 | 1 | [1999–2010]; +3.9 (2.8–5.1); <0.001 | [2010–2020]; +11.6 (10.2–13.0); <0.001 | … |
| Men | 0.33 (0.11–0.15) | 1.89 (1.83–1.96) | +8.1 (7.4–8.7); <0.001 | 1 | [1999–2010]; +4.7 (3.8–5.6), P<0.001 | [2010–2020]; +11.9 (10.7–13.1), P<0.001 | 0.46 |
| Women | 0.13 (0.11–0.15) | 0.75 (0.71–0.79) | +7.5 (6.3–8.6);<0.001 | 1 | [1999–2009]; +3.7 (1.9–5.6); <0.001 | [2009–2020]; +11.0 (9.3–12.7); <0.001 | |
| ≥65 y (all) | 62.93 (62.09–63.77) | 152.90 (151.84–153.96) | +4.0 (3.7–4.3); <0.001 | 1 | [1999–2011]; +2.1 (1.7–2.4); <0.001 | [2011–2020]; +6.6 (6.0–7.2); <0.001 | … |
| Men | 66.03 (64.54–67.53) | 181.05 (179.23–182.88) | +4.6 (4.2–4.9); <0.001 | 1 |
[1999–2011]; +2.5 (2.1–2.9); <0.001 |
[2011–2020]; +7.4 (6.8–8.1);<0.001 | <0.001 |
| Women | 60.46 (59.45–61.48) | 132.26 (130.98–133.54) | +3.5 (3.1–3.8); <0.001 | 1 | [1999–2011]; +1.7 (1.3–2.2); <0.001 | [2011–2020]; +5.8 (5.1–6.5); <0.001 |
AAMR indicates age‐adjusted mortality rate, expressed as deaths per 100 000 population; AAPC, average annual percent change; and APC, annual percent change.
Geographical Patterns
Over the study period, HF‐related death in patients with AF similarly increased in both urban (AAPC, +4.0% [95% CI, 3.7–4.4]) and rural (AAPC: +4.6% [95% CI, 4.2–4.6]) areas, respectively (P for parallelism=0.12) (Table 3). The higher AAMRs were clustered in the West (14.1 per 100 000 [95% CI, 13.1–13.2]) and in the Midwest (13.2 per 100 000 [95% CI, 13.1–13.2]), despite the fact that a higher percentage of HF‐related deaths in AF was registered in the South (32.8%; n=300 846). Full data regarding the HF‐related death in patients with AF are presented in Tables S1 and S2.
Table 3.
Age‐Adjusted Mortality Rate Trend in US Subjects With Heart Failure and Atrial Fibrillation, 1999 to 2020, Stratified by Urbanization Level
| AAMR 1999 (95% CI) | AAMR 2020 (95% CI) | AAPC (95% CI); P value | Number of joinpoints | Period 1 [y]; APC (95% CI), P value | APC period 2 [y]; APC (95% CI), P value | P value | |
|---|---|---|---|---|---|---|---|
| County‐level urbanization | |||||||
| Urban | 7.95 (7.83–8.06) | 19.58 (19.43–19.73) | +4.0 (3.7–4.4); <0.001 | 1 | [1999–2011]; +2.2 (1.8–2.6); <0.001 | [2011–2020]; +6 (6.0–7.2); <0.001 | 0.12 |
| Rural | 9.04 (8.78–9.29) | 25.00 (24.63–25.38) | +4.6 (4.2–4.9); <0.001 | 1 | [1999–2012]; +2.5 (2.1–2.9), <0.001 | [2012–2020]; +8.1 (7.2–9.0); <0.001 | |
AAMR indicates age‐adjusted mortality rate, expressed as deaths per 100 000 population; AAPC, average annual percent change; and APC, annual percent change.
Underlying Causes of Death
The first 5 underlying causes of death in patients with HF with AF were ischemic heart disease, hypertensive heart disease, cardiomyopathies, stroke, and chronic obstructive pulmonary disease, respectively. Specifically, from 1999 to 2020, the prevalence of HF decedents with AF having as underlying causes of death hypertensive heart disease (from 5.0% in 1999 to 8.2% in 2020; P for trend <0.001) or chronic obstructive pulmonary disease (from 4.2% to 4.8%; P for trend <0.001) increased. Conversely, subjects dying due to HF with AF presenting ischemic heart disease (from 38.3% in 1999 to 22.7% in 2020; P for trend <0.001) or stroke (from 3.1% in 1999 to 1.5% in 2020; P for trend <0.001) decreased. Finally, the trend of HF‐related deaths in patients with AF having cardiomyopathies as underlying causes of death remained stable over the entire study period (from 2.0 in 1999 to 2.1 in 2020; P = 0.86; Figure 4A through 4D).
Figure 4. Changes in the first underlying causes of deaths among patients with HF having concomitant AF.
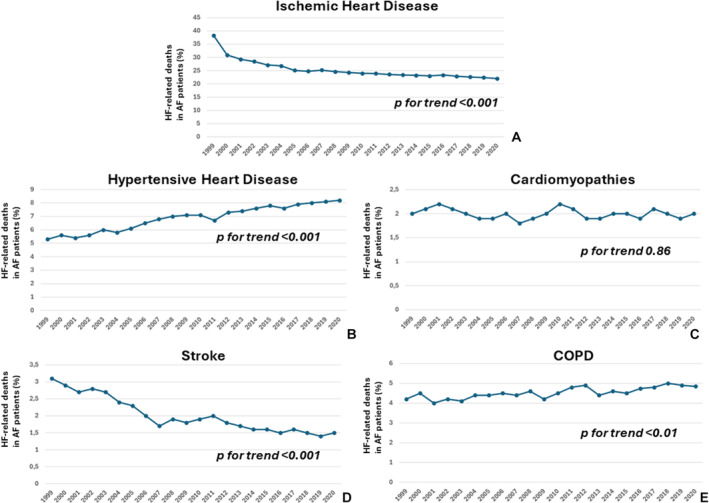
A, Ischemic heart disease; B, hypertensive heart disease; C, cardiomyopathy; D, stroke; and E, COPD. AF indicates atrial fibrillation; COPD, chronic obstructive pulmonary disease; and HF, heart failure.
Impact of COVID‐19 Pandemic
During 2020, 85 311 deaths due to HF in patients with AF were registered in the United States. Of these, 57 373 and 27 938 were recorded in subjects with and without SARS‐CoV‐2 infection (Table S3). In that year, the overall HF‐related AAMR in subjects with AF was 14.0 (95% CI, 13.9–14.1) per 100 000 and 20.6 (95% CI, 20.5–20.8) per 100 000 population in individuals with and without COVID‐19 infection, respectively. The HF‐related AAMR in COVID‐19 patients with AF was higher in men than in women (17.6; 95% CI, 17.4–17.8 per 100 000 versus 10.8; 95% CI, 10.7–11.0 per 100 000), as well as in subjects aged >65 years (97.8 [95% CI, 96.9–98.6) per 100 000 compared with those aged <65 years (1.9% [95% CI, 1.8–2.0). HF‐related deaths in subjects with COVID‐19 were observed in 88.2% of patients aged >65 years.
Discussion
In this 21‐year retrospective analysis of mortality data from the CDC WONDER data set, the overall AAMR for HF‐related deaths in patients with AF increased by ≈286.4%, especially in men. Furthermore, the higher AAPC increase was observed in patients aged <65 years. Moreover, the COVID‐19 pandemic, during its first year, determined a significant excess in HF‐related deaths among patients with AF aged >65 years. As evidenced by the complementary analysis exploring the 5 most common underlying causes of death, the observed HF‐related mortality trends in patients with AF may be explained by the increasing prevalence in subjects with hypertensive heart disease and chronic obstructive pulmonary disease. Traditionally, these conditions have been associated with a higher mortality risk either in patients with HF and patients with AF. 21 , 22 , 23 , 24
Present findings are in accordance with previous investigations, always on the basis of the CDC WONDER data set, that separately assessed the HF‐ 13 and AF‐ 14 related mortality rates and their trends in the United States over the past 2 decades. Although our findings cannot confer causation, several previous analyses have shown a pathophysiological association between AF and HF and vice versa, 25 , 26 , 27 , 28 , 29 which increases the mortality risk across all HF subtypes. 30 A variety of explanations may support the observed trends. First, the prevalence of several cardiovascular risk factors and comorbidities for both HF and AF, such as obesity, arterial hypertension, and diabetes, especially among young adults, has risen, increasing the relative risk of death. 31 , 32 , 33 , 34 At the same time, the continuous improvement in the diagnosis of both HF and AF 35 , 36 , 37 may have facilitated the recognition of some previously “unexplained” deaths. Additionally, the aging of the population may have substantially contributed to the observed trends since a larger proportion of HF‐related deaths in subjects with AF over time have been composed of older adults. 38
However, the cardiovascular‐related mortality rates may have been influenced by the presence of some competing risk factor events that could have precluded the occurrence of the cardiovascular outcome. 39 Indeed, in patients with HF, different concomitant conditions, such as diabetes, stroke, hemoglobin levels, and kidney function, may shift toward a higher rate of non–cardiovascular disease–related deaths, preventing the occurrence of cardiovascular events. 40
Although the continuous improvement in the diagnostic and therapeutic approaches to HF and AF achieved over the past 2 decades, the rise in HF‐related mortality rate among patients with AF aged <65 years is particularly concerning because it may be interpreted as unexpected. Doubtless, the continuous increase in the prevalence of cardiovascular comorbidities in US young adults, including diabetes, 41 has significantly contributed to the increasing HF‐related mortality trend in AF subjects. Furthermore, growing evidence has shown that psychosocial and lifestyle factors are important modulators of AF occurrence at a younger age. 1
Our results also highlight important racial and ethnic disparities in patients. Although previous studies have demonstrated a higher prevalence of cardiovascular disease, comorbidities, and risk factors among Black, Asian, American Indian, Alaskan Native, and Hispanic individuals, their relative AAMR for HF‐related death in subjects with AF was lower compared with White individuals. In this regard, disparities in diagnosis, awareness of the disease, access to advanced health care systems, or socioeconomic status may have influenced the observed trends. Additionally, we observed some geographic variations in HF‐related death in individuals with AF, with the Midwestern regions having the highest burden compared with other census regions. These results are consistent with a previous analysis examining the HF‐related mortality rates in the United States between 1999 and 2019 reporting a higher mortality rate in the same census regions. 14
The major inflection point observed between 2011 and 2012 in HF‐related mortality rates among patients with AF is probably due, at least in part, to the increased awareness on AF, and therefore to a greater diagnostic accuracy, as evidenced by the release of dedicated US guidelines in those years. 37 Moreover, also the publication of European guidelines on the management of patients with HF in 2012 have probably influenced the diagnostic management of these subjects in the United States. 42
As evidenced by our subanalysis, the first year of the COVID‐19 pandemic significantly contributed to the observed trends. 43 Indeed, during 2020, an excess HF‐related mortality rate in patients with AF was observed, especially in men aged >65 years. This phenomenon seems to be multifactorial. Indeed, new‐onset AF was observed in about 6% of hospitalized patients with COVID‐19, and almost half of them died during their index hospitalization. 44 Moreover, clinical evidence demonstrated that COVID‐19, by a complex interaction between inflammation, prothrombotic state, and sympathetic–vagal imbalances increases the risk of AF. 45 , 46 Additionally, cardiac consultations, electrophysiology procedures, 47 and hospitalization for HF declined 48 during the pandemic, potentially influencing the outcome of these patients. 49 , 50 Furthermore, during the first year of the pandemic, US census regions were disproportionately affected by the COVID‐19 pandemic, leading to a different mortality excess in the country. 51 Specifically, the higher COVID‐19 related mortality rates were registered in the South due to a higher infection rate, limited access to health care resources, differences in the prevalence of underlying conditions associated with higher mortality rates, such as arterial hypertension, diabetes, and obesity as well as to different implementation of COVID‐19 preventive strategies. 52
The rising trend of HF‐related death in patients with AF noted in the present analysis is concerning. This underscores the need for more robust cardiovascular surveillance in patients with HF through the implementation of opportunistic/systematic AF screening programs aimed to identify early the subjects at risk and to institute appropriate treatments. 53 Furthermore, the present data suggest that further research is warranted to understand which of the contributing factors is the primary driver of the trends and, if related to an increase in the disease burden, to reverse the observed increasing trend. 54 At the same time, the promotion of primordial, primary, and secondary prevention strategies remains fundamental to provide rapid and individualized expert‐based care. Additionally, the present findings should be used as an impetus to redouble efforts to consider AF ablation as an early therapeutic option. 55 , 56 Indeed, the degree of atrial derangement, due to the underlying atrial fibrosis, remains a major determinant for AF outcomes 57 and for the maintenance of sinus rhythm in the long‐term period. 58 In this regard, although AF ablation has been associated with a lower risk of HF hospitalization and all‐cause death by several randomized controlled trials and small observational studies, 59 the beneficial effect of such electrophysiological procedure appears to be more modest analyzing large data set. 60 This phenomenon is likely to be multifactorial. Indeed, results derived from randomized controlled trials derived from a highly selected population, whereas such strict eligibility criteria are not used in daily clinical practice. Furthermore, the prevalence of comorbidities is frequently different comparing validating studies and population derived from a large database influencing the selection of patients for AF ablation; indeed, in daily practice, several patients are often considered poor candidates for the interventional procedure. 61 Finally, the timing of the procedure, which is currently very heterogeneous in clinical practice, may significantly impact patients' outcomes. 12
Limitations
Our study has several limitations. As with any investigation relying on large nationwide administrative databases, we cannot exclude potential miscoding that may have impacted the accuracy of our results. However, death certificates remain a valuable health information source used worldwide for epidemiology, research, and public health policy. A previous analysis based on the Framingham Heart Study showed that death certificates were least accurate for individuals aged >85 years and that no change in coding accuracy was observed over time. 62 Furthermore, it has been reported that the amount of diagnostic information available to the certifying physician was associated with the reporting of chronic heart disease such as HF and AF. 63 Additionally, previous analyses, based on US subjects, although not representative for the entire country, showed an acceptable positive predictive value for both HF and AF of about 93% 64 and 88%, 65 respectively, using ICD‐10 codes for patients' identification. However, because up to one third of AF patients were asymptomatic, our findings may have conservatively underestimated the HF‐related mortality rate over the study period. Moreover, no data regarding the patient's baseline cardiovascular condition, risk factors, and left ventricular ejection fraction at presentation were provided by the CDC WONDER data set, limiting our final conclusions. In the same manner, we cannot assess how many patients received any sort of treatment for HF and/or AF, making it impossible to explore procedure usage, including advanced AF therapies. We cannot exclude that education regarding the evaluation and management of acute HF and AF, as well as increased awareness on the diseases, may have ameliorated the diagnosis on death certificates.
Conclusions
In conclusion, the HF‐related mortality rate in patients with AF has increased in the United States, especially in men with differences across different census regions. The observed mortality trend was coincident with the increase of HF‐related death in patients with AF having as underlying causes of death hypertensive heart disease and chronic obstructive pulmonary disease. This unexpected trend warrants an intensification of public health efforts aimed to promptly identify and treat AF in patients with HF, increasing promotion of targeted health policy measures.
Source of Funding
None.
Disclosures
Dr Turakhia received research grants from Bristol Myers Squibb, the American Heart Association, Bayer, Gilead Sciences, and the Food and Drug Administration; consulting fees from Medtronic, Pfizer, Johnson & Johnson, and Alivecor; And equity from iRhythm, Connect America, Forward, Evidently, and PocketRN outside the submitted work. Dr Turakhia is an employee of iRhythm Technologies Inc. Prof Boriani received small speaker fees from Bayer, Boehringer Ingelheim, Boston, Daiichi Sankyo, Microport, Janssen, and Sanofi outside the submitted work. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S3
This manuscript was sent to Yen‐Hung Lin, MD, PhD, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.033897
For Sources of Funding and Disclosures, see page 10.
References
- 1. Kornej J, Börschel CS, Benjamin EJ, Schnabel RB. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ Res. 2020;127:4–20. doi: 10.1161/CIRCRESAHA.120.316340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alonso A, Alam AB, Kamel H, Subbian V, Qian J, Boerwinkle E, Cicek M, Clark CR, Cohn EG, Gebo KA, et al. Epidemiology of atrial fibrillation in the all of us research program. PLoS One. 2022;17:e0265498. doi: 10.1371/journal.pone.0265498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dai H, Zhang Q, Much AA, Maor E, Segev A, Beinart R, Adawi S, Lu Y, Bragazzi NL, Wu J. Global, regional, and national prevalence, incidence, mortality, and risk factors for atrial fibrillation, 1990–2017: results from the global burden of disease study 2017. Eur Heart J Qual Care Clin Outcomes. 2021;7:574–582. doi: 10.1093/ehjqcco/qcaa061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta‐analysis. BMJ. 2016;354:i4482. doi: 10.1136/bmj.i4482 [DOI] [PubMed] [Google Scholar]
- 5. Lippi G, Sanchis‐Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: an increasing epidemic and public health challenge. Int J Stroke. 2021;16:217–221. doi: 10.1177/1747493019897870 [DOI] [PubMed] [Google Scholar]
- 6. Roger VL. Epidemiology of heart failure: a contemporary perspective. Circ Res. 2021;128:1421–1434. doi: 10.1161/CIRCRESAHA.121.318172 [DOI] [PubMed] [Google Scholar]
- 7. Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart disease and stroke Statistics‐2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 8. Dunlay SM, Roger VL. Understanding the epidemic of heart failure: past, present, and future. Curr Heart Fail Rep. 2014;11:404–415. doi: 10.1007/s11897-014-0220-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Karnik AA, Gopal DM, Ko D, Benjamin EJ, Helm RH. Epidemiology of atrial fibrillation and heart failure: a growing and important problem. Cardiol Clin. 2019;37:119–129. doi: 10.1016/j.ccl.2019.01.001 [DOI] [PubMed] [Google Scholar]
- 10. Trulock KM, Narayan SM, Piccini JP. Rhythm control in heart failure patients with atrial fibrillation: contemporary challenges including the role of ablation. J Am Coll Cardiol. 2014;64:710–721. doi: 10.1016/j.jacc.2014.06.1169 [DOI] [PubMed] [Google Scholar]
- 11. Boriani G, Mei DA, Imberti JF. Atrial fibrillation ablation in patients recently hospitalized for worsening heart failure: need for individualized decision‐making. JACC Clin Electrophysiol. 2023;9:1960–1963. doi: 10.1016/j.jacep.2023.06.012 [DOI] [PubMed] [Google Scholar]
- 12. Pallisgaard J, Greve AM, Lock‐Hansen M, Thune JJ, Fosboel EL, Devereux RB, Okin PM, Gislason GH, Torp‐Pedersen C, Bang CN. Atrial fibrillation onset before heart failure or vice versa: what is worst? A nationwide register study. Europace. 2023;25:283–290. doi: 10.1093/europace/euac186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tanaka Y, Shah NS, Passman R, Greenland P, Lloyd‐Jones DM, Khan SS. Trends in cardiovascular mortality related to atrial fibrillation in the United States, 2011 to 2018. J Am Heart Assoc. 2021;10:e020163. doi: 10.1161/JAHA.120.020163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Siddiqi TJ, Khan Minhas AM, Greene SJ, Van Spall HGC, Khan SS, Pandey A, Mentz RJ, Fonarow GC, Butler J, Khan MS. Trends in heart failure‐related mortality among older adults in the United States from 1999–2019. JACC Heart Fail. 2022;10:851–859. doi: 10.1016/j.jchf.2022.06.012 [DOI] [PubMed] [Google Scholar]
- 15. Boersma L, Andrade JG, Betts T, Duytschaever M, Pürerfellner H, Santoro F, Tzeis S, Verma A. Progress in atrial fibrillation ablation during 25 years of Europace journal. Europace. 2023;25:euad244. doi: 10.1093/europace/euad244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Accessed December 20, 2023. https://wonder.cdc.gov/mcd.html
- 17. Glynn P, Lloyd‐Jones DM, Feinstein MJ, Carnethon M, Khan SS. Disparities in cardiovascular mortality related to heart failure in the United States. J Am Coll Cardiol. 2019;73:2354–2355. doi: 10.1016/j.jacc.2019.02.042 [DOI] [PubMed] [Google Scholar]
- 18. US Census Bureau Population Division . Monthly population estimates by age, sex, race, and hispanic origin for the United States: April 1, 2010 to July 1, 2019 (with short‐term projections to December 2020). 2020. Accessed December 20, 2023. https://www.census.gov/data/datasets/time‐series/demo/popest/intercensal‐2000‐2010‐national.html
- 19. National Cancer Institute . Number of Joipoints. Accessed March 29, 2023. Accessed December 20, 2023. https://surveillance.cancer.gov/help/joinpoint/setting‐parameters/method‐and‐parameters‐tab/number‐of‐joinpoints
- 20. Kim HJ, Fay MP, Yu B, Barrett MJ, Feuer EJ. Comparability of segmented line regression models. Biometrics. 2004;60:1005–1014. doi: 10.1111/j.0006-341X.2004.00256.x [DOI] [PubMed] [Google Scholar]
- 21. Verdecchia P, Angeli F, Reboldi G. Hypertension and atrial fibrillation: doubts and certainties from basic and clinical studies. Circ Res. 2018;122:352–368. doi: 10.1161/CIRCRESAHA.117.311402 [DOI] [PubMed] [Google Scholar]
- 22. Aune D, Mahamat‐Saleh Y, Kobeissi E, Feng T, Heath AK, Janszky I. Blood pressure, hypertension and the risk of atrial fibrillation: a systematic review and meta‐analysis of cohort studies. Eur J Epidemiol. 2023;38:145–178. doi: 10.1007/s10654-022-00914-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dzeshka MS, Shantsila A, Shantsila E, Lip GYH. Atrial fibrillation and hypertension. Hypertension. 2017;70:854–861. doi: 10.1161/HYPERTENSIONAHA.117.08934 [DOI] [PubMed] [Google Scholar]
- 24. Proietti M, Laroche C, Drozd M, Vijgen J, Cozma DC, Drozdz J, Maggioni AP, Boriani G, Lip GY; EORP‐AF Investigators . Impact of chronic obstructive pulmonary disease on prognosis in atrial fibrillation: a report from the EURObservational research programme pilot survey on atrial fibrillation (EORP‐AF) general registry. Am Heart J. 2016;181:83–91. doi: 10.1016/j.ahj.2016.08.011 [DOI] [PubMed] [Google Scholar]
- 25. Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D'Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham heart study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E [DOI] [PubMed] [Google Scholar]
- 26. Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, Ellinor PT, Cheng S, Vasan RS, Lee DS, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133:484–492. doi: 10.1161/CIRCULATIONAHA.115.018614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boriani G, Imberti JF, Vitolo M. The burden of atrial fibrillation in patients with preserved or mildly reduced heart failure: a call to action for detecting atrial fibrillation and improving outcome. Eur J Heart Fail. 2023;25:74–76. doi: 10.1002/ejhf.2755 [DOI] [PubMed] [Google Scholar]
- 28. Saksena S, Slee A, Natale A, Lakkireddy DR, Shah D, Di Biase L, Lewalter T, Nagarakanti R, Santangeli P. Atrial fibrillation can adversely impact heart failure with preserved ejection fraction by its association with heart failure progression and mortality: a post‐hoc propensity score‐matched analysis of the TOPCAT Americas trial. Europace. 2023;25:euad095. doi: 10.1093/europace/euad095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Ferrari GM, Tavazzi L. The role of arrhythmias in the progression of heart failure. Eur J Heart Fail. 1999;1:35–40. doi: 10.1016/S1388-9842(99)00004-5 [DOI] [PubMed] [Google Scholar]
- 30. Mundisugih J, Franke KB, Tully PJ, Munawar DA, Kumar S, Mahajan R. Prevalence and prognostic implication of atrial fibrillation in heart failure subtypes: systematic review and meta‐analysis. Heart Lung Circ. 2023;32:666–677. doi: 10.1016/j.hlc.2023.02.009 [DOI] [PubMed] [Google Scholar]
- 31. Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, Long MW, Gortmaker SL; Projected U.S . State‐level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381:2440–2450. doi: 10.1056/NEJMsa1909301 [DOI] [PubMed] [Google Scholar]
- 32. Fang M. Trends in the prevalence of diabetes among U.S. adults: 1999–2016. Am J Prev Med. 2018;55:497–505. doi: 10.1016/j.amepre.2018.05.018 [DOI] [PubMed] [Google Scholar]
- 33. Chobufo MD, Gayam V, Soluny J, Rahman EU, Enoru S, Foryoung JB, Agbor VN, Dufresne A, Nfor T. Prevalence and control rates of hypertension in the USA: 2017‐2018. Int J Cardiol Hypertens. 2020;6:100044. doi: 10.1016/j.ijchy.2020.100044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Andersson C, Vasan RS. Epidemiology of cardiovascular disease in young individuals. Nat Rev Cardiol. 2018;15:230–240. doi: 10.1038/nrcardio.2017.154 [DOI] [PubMed] [Google Scholar]
- 35. Bunting KV, Gill SK, Sitch A, Mehta S, O'Connor K, Lip GY, Kirchhof P, Strauss VY, Rahimi K, Camm AJ, et al. Improving the diagnosis of heart failure in patients with atrial fibrillation. Heart. 2021;107:902–908. doi: 10.1136/heartjnl-2020-318557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leclercq C, Witt H, Hindricks G, Katra RP, Albert D, Belliger A, Cowie MR, Deneke T, Friedman P, Haschemi M, et al. Wearables, telemedicine, and artificial intelligence in arrhythmias and heart failure: proceedings of the European Society of Cardiology Cardiovascular Round Table. Europace. 2022;24:1372–1383. doi: 10.1093/europace/euac052 [DOI] [PubMed] [Google Scholar]
- 37. Svennberg E, Caiani EG, Bruining N, Desteghe L, Han JK, Narayan SM, Rademakers FE, Sanders P, Duncker D. The digital journey: 25 years of digital development in electrophysiology from an Europace perspective. Europace. 2023;25:euad176. doi: 10.1093/europace/euad176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang N, Yu Y, Sun Y, Zhang H, Wang Y, Chen C, Tan X, Wang B, Lu Y. Acquired risk factors and incident atrial fibrillation according to age and genetic predisposition. Eur Heart J. 2023;44:4982–4993. doi: 10.1093/eurheartj/ehad615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hageman SHJ, Dorresteijn JAN, Pennells L, van Smeden M, Bots ML, Di Angelantonio E, Visseren FLJ. The relevance of competing risk adjustment in cardiovascular risk prediction models for clinical practice. Eur J Prev Cardiol. 2023;30:1741–1747. doi: 10.1093/eurjpc/zwad202 [DOI] [PubMed] [Google Scholar]
- 40. Takada T, Sakata Y, Nochioka K, Miura M, Abe R, Kasahara S, Sato M, Aoyanagi H, Fujihashi T, Yamanaka S, et al. Risk of de‐novo heart failure and competing risk in asymptomatic patients with structural heart diseases. Int J Cardiol. 2020;307:87–93. doi: 10.1016/j.ijcard.2020.02.015 [DOI] [PubMed] [Google Scholar]
- 41. Gooding HC, Gidding SS, Moran AE, Redmond N, Allen NB, Bacha F, Burns TL, Catov JM, Grandner MA, Harris KM, et al. Challenges and opportunities for the prevention and treatment of cardiovascular disease among young adults: report from a National Heart, Lung, and Blood Institute working group. J Am Heart Assoc. 2020;9:e016115. doi: 10.1161/JAHA.120.016115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wann LS, Curtis AB, January CT, Ellenbogen KA, Lowe JE, Estes NA III, Page RL, Ezekowitz MD, Slotwiner DJ, Jackman WM, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (updating the 2006 guideline): a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2011;123(1):104–123. doi: 10.1161/CIR.0b013e3181fa3cf4 [DOI] [PubMed] [Google Scholar]
- 43. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the heart failure association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104 [DOI] [PubMed] [Google Scholar]
- 44. Rosenblatt AG, Ayers CR, Rao A, Howell SJ, Hendren NS, Zadikany RH, Ebinger JE, Daniels JD, Link MS, de Lemos JA, et al. New‐onset atrial fibrillation in patients hospitalized with COVID‐19: results from the American Heart Association COVID‐19 cardiovascular registry. Circ Arrhythm Electrophysiol. 2022;15:e010666. doi: 10.1161/CIRCEP.121.010666 [DOI] [PubMed] [Google Scholar]
- 45. Donniacuo M, De Angelis A, Rafaniello C, Cianflone E, Paolisso P, Torella D, Sibilio G, Paolisso G, Castaldo G, Urbanek K, et al. COVID‐19 and atrial fibrillation: intercepting lines. Front Cardiovasc Med. 2023;10:1093053. doi: 10.3389/fcvm.2023.1093053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wollborn J, Karamnov S, Fields KG, Yeh T, Muehlschlegel JD. COVID‐19 increases the risk for the onset of atrial fibrillation in hospitalized patients. Sci Rep. 2022;12:12014. doi: 10.1038/s41598-022-16113-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Boriani G, Guerra F, De Ponti R, D'Onofrio A, Accogli M, Bertini M, Bisignani G, Forleo GB, Landolina M, Lavalle C, et al. Five waves of COVID‐19 pandemic in Italy: results of a national survey evaluating the impact on activities related to arrhythmias, pacing, and electrophysiology promoted by AIAC (Italian Association of Arrhythmology and Cardiac Pacing). Intern Emerg Med. 2023;18:137–149. doi: 10.1007/s11739-022-03140-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shoaib A, Van Spall HGC, Wu J, Cleland JGF, McDonagh TA, Rashid M, Mohamed MO, Ahmed FZ, Deanfield J, de Belder M, et al. Substantial decline in hospital admissions for heart failure accompanied by increased community mortality during COVID‐19 pandemic. Eur Heart J Qual Care Clin Outcomes. 2021;7:378–387. doi: 10.1093/ehjqcco/qcab040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mishra T, Patel DA, Awadelkarim A, Sharma A, Patel N, Yadav N, Almas T, Sattar Y, Alraies MC. A National Perspective on the impact of the COVID‐19 pandemic on heart failure hospitalizations in the United States. Curr Probl Cardiol. 2023;48:101749. doi: 10.1016/j.cpcardiol.2023.101749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ostrowska M, Kasprzak M, Stolarek W, Grzelakowska K, Kryś J, Kubica A, Adamski P, Podhajski P, Navarese EP, Anielska‐Michalak E, et al. Longer hospitalizations and higher in‐hospital mortality for acute heart failure during the COVID‐19 pandemic in larger vs. smaller cardiology departments: subanalysis of the COV‐HF‐SIRIO 6 multicenter study. Rev Cardiovasc Med. 2022;23:292. doi: 10.31083/j.rcm2309292 [DOI] [Google Scholar]
- 51. Gold JAW, Rossen LM, Ahmad FB, Sutton P, Li Z, Salvatore PP, Coyle JP, DeCuir J, Baack BN, Durant TM, et al. Race, ethnicity, and age trends in persons who died from COVID‐19—United States, May–August 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1517–1521. doi: 10.15585/mmwr.mm6942e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Webb Hooper M, Nápoles AM, Pérez‐Stable EJ. COVID‐19 and racial/ethnic disparities. JAMA. 2020;323:2466–2467. doi: 10.1001/jama.2020.8598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Boriani G, Proietti M. Screening for atrial fibrillation: need for an integrated, structured approach. Eur J Intern Med. 2019;67:33–35. doi: 10.1016/j.ejim.2019.07.017 [DOI] [PubMed] [Google Scholar]
- 54. Anter E, Jessup M, Callans DJ. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation. 2009;119:2516–2525. doi: 10.1161/CIRCULATIONAHA.108.821306 [DOI] [PubMed] [Google Scholar]
- 55. Sakamoto K, Tohyama T, Ide T, Mukai Y, Enzan N, Nagata T, Ikeda M, Takase S, Nagayama T, Fujino T, et al. Efficacy of early catheter ablation for atrial fibrillation after admission for heart failure. JACC Clin Electrophysiol. 2023;9:1948–1959. doi: 10.1016/j.jacep.2023.05.038 [DOI] [PubMed] [Google Scholar]
- 56. Chew DS, Jones KA, Loring Z, Black‐Maier E, Noseworthy PA, Exner DV, Packer DL, Grant J, Mark DB, Piccini JP. Diagnosis‐to‐ablation time predicts recurrent atrial fibrillation and rehospitalization following catheter ablation. Heart Rhythm O2. 2021;3:23–31. doi: 10.1016/j.hroo.2021.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Boriani G, Vitolo M, Imberti JF. Atrial cardiomyopathy: a derangement in atrial volumes, geometry, function, and pathology with important clinical implications. J Cardiovasc Med (Hagerstown). 2022;23:359–362. doi: 10.2459/JCM.0000000000001316 [DOI] [PubMed] [Google Scholar]
- 58. Proietti M, Vitolo M, Harrison SL, Lane DA, Fauchier L, Marin F, Nabauer M, Potpara TS, Dan GA, Boriani G, et al. Real‐world applicability and impact of early rhythm control for European patients with atrial fibrillation: a report from the ESC‐EHRA EORP‐AF Long‐Term General Registry. Clin Res Cardiol. 2022;111:70–84. doi: 10.1007/s00392-021-01914-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Packer M, Kowey PR. Building castles in the sky: catheter ablation in patients with atrial fibrillation and chronic heart failure. Circulation. 2018;138:751–753. doi: 10.1161/CIRCULATIONAHA.118.034583 [DOI] [PubMed] [Google Scholar]
- 60. Noseworthy PA, Van Houten HK, Gersh BJ, Packer DL, Friedman PA, Shah ND, Dunlay SM, Siontis KC, Piccini JP, Yao X. Generalizability of the CASTLE‐AF trial: catheter ablation for patients with atrial fibrillation and heart failure in routine practice. Heart Rhythm. 2020;17:1057–1065. doi: 10.1016/j.hrthm.2020.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cappato R, Ali H. Surveys and registries on catheter ablation of atrial fibrillation: fifteen years of history. Circ Arrhythm Electrophysiol. 2021;14:e008073. doi: 10.1161/CIRCEP.120.008073 [DOI] [PubMed] [Google Scholar]
- 62. Lloyd‐Jones DM, Martin DO, Larson MG, Levy D. Accuracy of death certificates for coding coronary heart disease as the cause of death. Ann Intern Med. 1998;129:1020–1026. doi: 10.7326/0003-4819-129-12-199812150-00005 [DOI] [PubMed] [Google Scholar]
- 63. Roth GA, Dwyer‐Lindgren L, Bertozzi‐Villa A, Stubbs RW, Morozoff C, Naghavi M, Mokdad AH, Murray CJL. Trends and patterns of geographic variation in cardiovascular mortality among US counties, 1980–2014. JAMA. 2017;317:1976–1992. doi: 10.1001/jama.2017.4150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Frolova N, Bakal JA, McAlister FA, Rowe BH, Quan H, Kaul P, Ezekowitz JA. Assessing the use of international classification of diseases‐10th revision codes from the emergency department for the identification of acute heart failure. JACC Heart Fail. 2015;3:386–391. doi: 10.1016/j.jchf.2014.11.010 [DOI] [PubMed] [Google Scholar]
- 65. Yao RJR, Andrade JG, Deyell MW, Jackson H, McAlister FA, Hawkins NM. Sensitivity, specificity, positive and negative predictive values of identifying atrial fibrillation using administrative data: a systematic review and meta‐analysis. Clin Epidemiol. 2019;23(11):753–767. doi: 10.2147/CLEP.S206267 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3


