ABSTRACT
Although intravenous thrombolysis with alteplase remains the primary treatment for acute ischemic stroke, tenecteplase has shown potential advantages over alteplase. Animal studies have demonstrated the favorable pharmacokinetics and pharmacodynamics of tenecteplase. Moreover, it is easier to administer. Clinical trials have demonstrated that tenecteplase is not inferior to alteplase and may even be superior in cases of acute ischemic stroke with large vessel occlusion. Current evidence supports the time and cost benefits of tenecteplase, suggesting that it could potentially replace alteplase as the main option for thrombolytic therapy, especially in patients with large vessel occlusion.
Keywords: alteplase, ischemic stroke, tenecteplase, thrombolysis
Subject Categories: Ischemic Stroke
Nonstandard Abbreviations and Acronyms
- AcT
Intravenous Tenecteplase Compared With Alteplase for Acute Ischaemic Stroke in Canada
- AIS
acute ischemic stroke
- ATTEST
Alteplase‐Tenecteplase Trial Evaluation for Stroke Thrombolysis
- EXTEND‐IA TNK
Tenecteplase Versus Alteplase Before Thrombectomy for Ischemic Stroke
- ICA
internal carotid artery
- LVO
large vessel occlusion
- MCA
middle cerebral artery
- mRS
modified Rankin Scale
- MSU
mobile stroke unit
- NIHSS
National Institutes of Health Stroke Scale
- NOR‐TEST
Norwegian Tenecteplase Stroke Trial
- RD
risk difference
- sICH
symptomatic intracranial hemorrhage
- TRACE
Tenecteplase Versus Alteplase in Acute Ischemic Cerebrovascular Events
Stroke is the second‐leading cause of death globally and ischemic stroke constituted more than 60% of all incident strokes in 2019. 1 Intravenous alteplase thrombolysis was approved by the US Food and Drug Administration in 1996, and has become the first‐line treatment for ischemic stroke due to its high‐quality evidence for nearly 30 years, 2 but there are concerns including hemorrhagic complications, restricted fibrinolytic efficacy, and achieving arterial recanalization in less than 40% of patients. 3 Tenecteplase (TNK–tissue‐type plasminogen activator) has a longer half‐life, with more fibrin specificity and possibly a lower rate of intracranial hemorrhage than alteplase, which may make it a better thrombolytic agent. 4 , 5 , 6 When compared with alteplase, tenecteplase is easier to administer, 7 which reduces the potential for medication errors, dose interruption, and time delays. This improved ease of administration also facilitates interhospital transfer.
Tenecteplase has been suggested to improve recanalization and reperfusion without increasing the risk of hemorrhage and may lyse large vessel clots more effectively. 8 , 9 Based on these findings, some national guideline committees have endorsed tenecteplase in lieu of alteplase for intravenous thrombolysis in patients with intracranial large vessel occlusions (LVOS) eligible for thrombectomy, while grading these recommendations as being of weak strength and low quality of evidence. 2 , 10
Studies about tenecteplase are emerging and, globally, more than 20 trials have been completed or are ongoing. The phase 3 trials AcT (Intravenous Tenecteplase Compared With Alteplase for Acute Ischaemic Stroke in Canada) 11 , TRACE‐2 (Tenecteplase Versus Alteplase in Acute Ischemic Cerebrovascular Events‐2), 12 and ATTEST‐2 (Alteplase‐Tenecteplase Trial Evaluation for Stroke Thrombolysis‐2) 13 recently provided robust results that 0.25 mg/kg tenecteplase was not inferior to alteplase with a similar safety profile within 4.5 hours of symptom onset. The latest 2023 European Stroke Organisation guideline 14 strongly recommended that 0.25 mg/kg tenecteplase can be used as an alternative to 0.9 mg/kg alteplase for patients with acute ischemic stroke (AIS) or stroke due to LVO within 4.5 hours of onset. The 2023 edition of the National Clinical Guideline for Stroke for the United Kingdom and Ireland also recommended that thrombolysis with alteplase or tenecteplase should be considered for patients with AIS within 4.5 hours of known onset. 15 In the real‐world setting, many centers have switched from alteplase to the off‐label use of tenecteplase based on the clinical trial data and guideline recommendations.
This review provides a comprehensive summary of the recent advancements in the use of tenecteplase for AIS.
Pharmacokinetics and Pharmacodynamics of Tenecteplase
Cross‐linked fibrin proteins, as one of the main components of a thrombus, can be dissolved through the process of fibrinolysis. 16 Vascular endothelial cells secrete tPA (tissue plasminogen activator), which has the ability to convert plasminogen to plasmin. Subsequently, plasmin enzymatically cleaves the fibrin skeleton, leading to the dissolution of the thrombus and the recanalization of the occluded vessel. 17 , 18
Tenecteplase is a genetically engineered mutant tissue plasminogen activator that possesses 3 sites of amino acid replacements as compared with alteplase 19 These structural alterations endow tenecteplase with advantages in pharmacodynamics and pharmacokinetics (Figure 1). 4 , 5 , 6 , 20 Tenecteplase exhibits a more prolonged half‐life and slower plasma clearance, facilitating its administration as a single bolus instead of a bolus accompanied by continuous infusion, which makes it better for interhospital transfers. 7 , 21 , 22 Furthermore, tenecteplase has a 14‐fold increase in fibrin specificity and an 80‐fold higher resistance to PAI‐1 (plasminogen activator inhibitor‐1) compared with alteplase. 19 Theoretically, tenecteplase should have a greater rate of recanalization and a lower risk of hemorrhagic events when compared with alteplase. 23 When considering these pharmacokinetic and pharmacodynamic characteristics, tenecteplase is a reasonable and promising alternative to alteplase.
Figure 1. Pharmacodynamics and pharmacokinetics of alteplase vs tenecteplase.
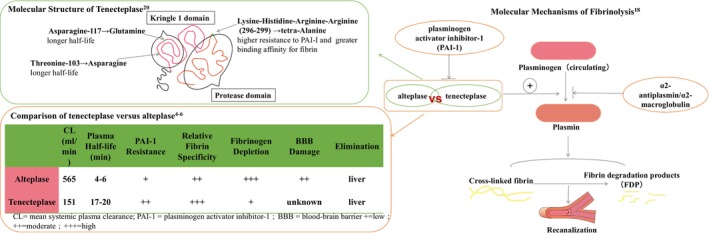
Evidence for Advantages of Tenecteplase in Animal Studies
The advantages of tenecteplase in pharmacodynamics and pharmacokinetics were proven in animal experiments. Tenecteplase has the following characteristics compared with alteplase. (1) Lower clearance: the clearance of alteplase (16.1 mL/min per kg) was significantly faster in rabbits compared with tenecteplase (1.9 mL/min per kg). 19 (2) Enhanced selectivity of fibrin: from plasma samples of rabbits with the use of either tenecteplase (0.6 mg/kg) or alteplase (6.3 mg/kg), investigators reported that tenecteplase had only slight effect on reducing fibrinogen, plasminogen, and α2‐antiplasmin levels whereas alteplase caused a significant degree of reduction of these materials. Stewart et al 24 also indicated that tenecteplase has higher fibrin selectivity than alteplase. (3) Less platelet aggregation and more potent lysis of platelet‐rich clot: different from alteplase, tenecteplase does not enhance collagen‐ or arachidonic acid–facilitated platelet aggregation at the site of thrombolysis, which may cause the reocclusion of the recanalized vessel. 25 , 26 In addition, tenecteplase has been found to reduce the inhibition of PAI‐1 by 80 times when compared with alteplase. As a result, the dose of tenecteplase needed to achieve 50% dissolution of platelet‐rich clots in vivo was 13.5 times lower than that of alteplase. 19 , 27 (4) Greater thrombolytic efficiency: Keyt et al 19 indicated that to achieve 50% lysis, tenecteplase (0.18 mg/kg) spent 3‐fold less time compared with an equivalent dose of 0.18 mg/kg alteplase (35 minutes compared with 120 minutes, respectively). In a rabbit model of carotid artery thrombosis, investigators found that tenecteplase was significantly better than alteplase with faster reperfusion, more durable, and more complete recanalization. 25 (5) Fewer bleeding‐related events: in animal studies, Thomas et al 28 found that compared with excipients, tenecteplase (0.6 mg/kg) caused significantly fewer intracranial hemorrhages and the bleeding time did not substantially elevate in the first hour, which was different from alteplase (6.3 mg/kg). In addition, Benedict et al 25 reported that in comparison with alteplase, the amount of blood loss from a deep surgical incision site was 35% less with tenecteplase.
Evidence for Advantages of Tenecteplase in Clinical Studies
Tenecteplase Thrombolysis in Clinical Trials
Efficacy and Safety
In myocardial ischemia, an intravenous bolus of 0.5 mg/kg tenecteplase resulted in similar mortality rates but less systemic bleeding than those rates in patients treated with front‐loaded alteplase 23 and led to Food and Drug Administration approval of tenecteplase. Subsequently, a series of tenecteplase trials in AIS started (Figure 2). 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 We summarized the baseline characteristics and outcomes of these clinical trials in alteplase and tenecteplase, which are shown in Tables S1–S4 and S2.
Figure 2. History of alteplase and tenecteplase trials in acute ischemic stroke.
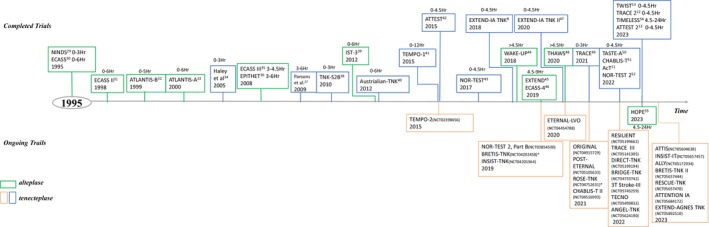
AcT indicates Intravenous Tenecteplase Compared With Alteplase for Acute Ischaemic Stroke in Canada; ALLY, Adjunctive Intra‐Arterial Tenecteplase Following Mechanical Thrombectomy Pilot Trial; ANGEL‐TNK, Intra‐Arterial Recombinant Human TNK Tissue‐type Plasminogen Activator (rhTNK‐tPA) Thrombolysis for Acute Large Vascular Occlusion After Successful Mechanical Thrombectomy Recanalization; ATLANTIS, Recombinant Tissue‐Type Plasminogen Activator (Alteplase) for Ischemic Stroke 3–5 hours After Symptom Onset; ATTENTION IA, Intra‐Arterial TNK Following Endovascular Thrombectomy in Patients With Large Vessel Occlusion of Posterior Circulation; ATTEST, Alteplase Tenecteplase Trial Evaluation for Stroke Thrombolysis; ATTIS, Alteplase Versus Tenecteplase for Thrombolysis After Ischaemic Stroke; BRETIS‐TNK, Intra‐Arterial Tenecteplase During First Thrombectomy Attempt for Acute Stroke; BRIDGE‐TNK, Endovascular Treatment With Versus Without Intravenous rhTNK‐tPA in Stroke; CHABLIS‐T, Chinese Acute Tissue‐Based Imaging Selection for Lysis In Stroke‐Tenecteplase; DIRECT‐TNK, Randomization to Endovascular Treatment Alone or Preceded by Systemic Thrombolysis With Tenecteplase in Ischemic Stroke; ECASS, European Cooperative Acute Stroke Study; EPITHET, Effects of Alteplase Beyond 3 Hours After Stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial; ETERNAL‐LVO, Extending the Time Window for Tenecteplase by Effective Reperfusion in Patients With Large Vessel Occlusion; EXTEND, Extending the Time for Thrombolysis in Emergency Neurological Deficits; EXTEND‐AGNES, Post‐Thrombectomy Intra‐Arterial Tenecteplase for Acute Management of Non‐Retrievable Thrombus and No‐Reflow in Emergent Stroke; EXTEND‐IA TNK:Tenecteplase Versus Alteplase Before Thrombectomy for Ischemic Stroke; HOPE, Treatment With Intravenous Alteplase in Ischemic Stroke Patients With Onset Time Between 4.5 and 24 Hours; INSIST‐IT, Improving Neurological Outcome for Acute Basilar Artery Occlusion With Sufficient Recanalization After Thrombectomy by Intraarterial Tenecteplase; INSIST‐TNK, Improving Neuroprotective Strategy for Ischemic Stroke With Poor Recanalization After Thrombectomy by Intra‐Arterial TNK; IST‐3, The Benefits and Harms of Intravenous Thrombolysis With Recombinant Tissue Plasminogen Activator Within 6 Hours of Acute Ischaemic Stroke (The Third International Stroke Trial; NINDS, the National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study; NOR‐TEST, Norwegian Tenecteplase Stroke Trial; ORIGINAL, A Study in Chinese Patients to Compare How Tenecteplase and Alteplase Given After a Stroke Improve Recovering of Physical Activity; POST‐ETERNA, Extending the Time Window for Tenecteplase by Recanalization of Basilar Artery Occlusion in Posterior Circulation Stroke; RESCUE‐TNK, Rescue Thrombolysis for Medium Vessel Occlusion; RESILIENT (EXTEND‐IV), Randomization to Extend Stroke Intravenous Thrombolysis in Evolving Non‐Large Vessel Occlusion With TNK; ROSE‐TNK, MRI‐Guided Thrombolysis for Stroke Beyond Time Window by TNK; TASTE‐A, Comparison of Tenecteplase With Alteplase for the Early Treatment of Ischaemic Stroke in the Melbourne Mobile Stroke Unit‐A; TECNO, Safety and Efficacy of Intra‐Arterial Tenecteplase for Noncomplete Reperfusion of Intracranial Occlusions; TEMPO, TNK‐tPA Evaluation for Minor Ischemic Stroke With Proven Occlusion; THAWS, Thrombolysis With Alteplase at 0.6 mg/kg for Stroke With Unknown Time of Onset; TIMELESS, Tenecteplase in Stroke Patients Between 4.5 and 24 hours; TNK‐S2B, Phase IIB/III Trial of Tenecteplase in Acute Ischemic Stroke Results of a Prematurely Terminated Randomized Clinical Trial; TRACE, Tenecteplase Versus Alteplase in Acute Ischemic Cerebrovascular Events; TWIST, Tenecteplase in Wake‐Up Ischemic Stroke Trial; and WAKE‐UP, MRI‐Guided Thrombolysis for Stroke With Unknown Time of Onset.
* The BRETIS‐TNK (NCT04202458) and ROSE‐TNK (NCT04752631) trials were completed but not published.
Initial Investigation in the Optimal Dose of Tenecteplase
In 2005, a pilot dose‐escalation safety study 34 was conducted on 88 patients with AIS within 3 hours of symptom onset. The study evaluated the safety of tenecteplase doses ranging from 0.1 to 0.4 mg/kg, with the primary end point being symptomatic intracranial hemorrhage (sICH). However, the study did not determine the optimal dose for achieving favorable functional outcomes at 3 months. A phase IIB/III randomized, multicenter, double‐blind trial 38 was conducted on 112 patients to determine the optimal dose of tenecteplase among 0.1, 0.25, and 0.4 mg/kg. The study used an adaptive, sequential dose selection procedure. However, the trial was prematurely terminated as the 0.4 mg/kg dose displayed a high sICH rate. Furthermore, it was not possible to distinguish between the 0.1 mg/kg and 0.25 mg/kg doses as neither showed clear efficacy. It should be noted that these findings represent early results that informed subsequent studies. In 2012, the Australian‐TNK (Low‐Dose Tenecteplase Versus Standard‐Dose Alteplase for Acute Ischemic Stroke) trial, 40 another phase IIB trial based on a perfusion imaging mismatch and a proximal vessel occlusion within 6 hours after onset, demonstrated that tenecteplase 0.25 mg/kg was superior to tenecteplase 0.1 mg/kg and alteplase 0.9 mg/kg for recanalization and 24‐hour clinical improvement, with no additional risk of hemorrhage. A longer‐term clinical benefit (0–2 score of the modified Rankin Scale [mRS] at 90 days) was also seen in the 0.25 mg/kg tier (72% versus 40%, P=0.02). Although the sample size was very small, it was the first trial with no truncation to test the optimal dose for tenecteplase. Multimodal computed tomography (CT) imaging selection criteria in the trial resulted in exclusion of 79% (477/604) of patients who were otherwise eligible for intravenous alteplase. Instead, The ATTEST (Alteplase‐Tenecteplase Trial Evaluation for Stroke Thrombolysis) phase 2 trial, 42 using imaging criteria as exploratory outcome measures rather than selection criteria, found a similar percentage of penumbral salvage on CT perfusion imaging when comparing 0.25 mg/kg tenecteplase and standard 0.9 mg/kg alteplase (68% versus 68%) administered within 4.5 hours of stroke onset. This was the first study comparing 1 dose of tenecteplase against alteplase with patients selected by criteria currently used in clinical practice to assess eligibility for thrombolysis in a 4.5‐hour window. The application of advanced imaging for outcome assessment rather than patient selection avoided restrictions of generalizability and some delay in treatment initiation. However, the use of advanced imaging did reveal some baseline imbalances, such as differences in the proportion of patients with large artery occlusion and baseline ischemic core volume. Although these imbalances were not statistically significant, they could potentially affect the interpretation of the neutral results. As a result, larger sample size phase 3 trials are warranted. The pooled analysis of these 3 trials (Haley et al, Parsons et al, and ATTEST) found that a dose of 0.25 mg/kg tenecteplase had the greatest odds of achieving early neurological improvement (odds ratio [OR], 3.3 [95% CI, 1.5–7.2], P=0.093) and excellent functional outcome (mRS score 0–1) at 3 months (OR, 1.9 [95% CI, 0.8–4.4], P=0.28), with reduced odds of ICH (OR, 0.6 [95% CI, 0.2–1.8], P=0.43) compared with alteplase. 56
Tenecteplase Thrombolysis Followed by Endovascular Therapy
The EXTEND‐IA TNK study (Tenecteplase Versus Alteplase Before Thrombectomy for Ischemic Stroke) 8 compared the efficacy of tenecteplase (administered at a dose of 0.25 mg/kg) to alteplase in thrombectomy candidates with occlusion of the internal carotid artery, basilar artery, or middle cerebral artery within 4.5 hours of symptom onset. The results of the study showed that tenecteplase significantly increased the absolute rate of successful recanalization of large vessels by 12% compared with alteplase. The subsequent EXTEND‐IA TNK part 2 trial 47 is the first substantial head‐to‐head comparison of the 2 candidate doses of tenecteplase for ischemic stroke and showed a similar percentage (19.3%) in >50% reperfusion of the previously occluded vascular territory comparing tenecteplase doses of 0.4 and 0.25 mg/kg. There were no significant differences in functional outcomes, all‐cause deaths, and sICH between the 2 groups. These findings suggested that the higher dose of 0.4 mg/kg of tenecteplase did not provide any advantage over the lower dose of 0.25 mg/kg in patients with major vessel occlusion who were scheduled for endovascular thrombectomy. In the 2019 American Heart Association/American Stroke Association acute stroke guidelines, it was suggested that choosing tenecteplase (administered as a single intravenous bolus of 0.25 mg/kg, with a maximum dose of 25 mg) for bridging therapy patients may be a reasonable option over alteplase (class of recommendation IIb, level of evidence B‐R [moderate level of evidence from randomized clinical trials]). 10
Further Investigation of the Optimal Dose of Tenecteplase in Phase 3 Trials
These results from the preliminary trials, though with small samples, encouraged tenecteplase use and lent support for phase 3 randomized trials comparing tenecteplase with alteplase, preferably incorporating penumbral/angiographic imaging selection. The NOR‐TEST (Norwegian Tenecteplase Stroke Trial), 43 the first phase 3 and the only superiority clinical trial of tenecteplase at a high dose (0.4 mg/kg) versus alteplase, enrolled 1100 patients who fulfilled standard thrombolysis eligibility criteria not selected by advanced neuroradiological modalities in 13 stroke units in Norway. Although tenecteplase was not superior to alteplase for excellent outcome (mRS score 0–1), the study added important evidence of safety. The 0.4 mg/kg tenecteplase dose was almost discarded by previous researchers because of safety concerns. However, the similar frequency of sICH (2%–3%) in the NOR‐TEST trial showed that tenecteplase 0.4 mg/kg has a similar safety profile to alteplase 0.9 mg/kg within 4.5 hours of symptom onset or of awakening with symptoms. A meta‐analysis 57 included 5 randomized clinical trials (the TNK‐S2B [Phase IIB/III Trial of Tenecteplase in Acute Ischemic Stroke Results of a Prematurely Terminated Randomized Clinical Trial], Australian‐TNK, ATTEST, NOR‐TEST, and EXTEND‐IA TNK trials) and 1585 patients (828 tenecteplase, 757 alteplase) identified a 4% risk difference for the disability‐free 3‐month outcome (mRS score 0–1) (95% CI, −1% to 8%). The lower 95% CI bound fell well within the noninferiority margin of 1.3%. The additional efficacy end points such as functional independence (mRS score 0–2) (risk difference [RD], 2% [95% CI, −3% to 6%]) also met the prespecified noninferiority margin of 5%. The rates of sICH and mortality were low and comparable (sICH: 3% versus 3%, RD 0% [95% CI, –1% to 2%]; 90‐day death: 7.6% versus 8.1%, RD 0% [95% CI, −3% to 2%]). The evidence was, however, strongly dependent on NOR‐TEST, which had a probable selection bias in favor of minor stroke and 17% had stroke mimics, which diluted the efficacy of tenecteplase over alteplase. The latest published NOR‐TEST 2 52 trial, a phase 3 and noninferiority trial, enrolled patients at 11 hospitals with stroke units in Norway with National Institutes of Health Stroke Scale (NIHSS) score >5 and investigated the efficacy and safety of tenecteplase 0.4 mg/kg versus alteplase within 4.5 hours after symptom onset or patients with wakeup stroke with a diffusion weighted imaging‐fluid‐attenuated inversion recovery mismatch. The 0.4 mg/kg dose of tenecteplase yielded worse safety and functional outcomes in moderate and severe ischemic stroke compared with alteplase. The study failed to show that 0.4 mg/kg tenecteplase was noninferior to alteplase in moderate and severe ischemic stroke, and a part B trial (NCT03854500) is ongoing with a lower dose of tenecteplase (0.25 mg/kg).
The Efficacy and Safety of 0.25 mg/kg Tenecteplase in Phase 3 Trials
A series of phase 2 trials proved that 0.25 mg/kg tenecteplase achieved better reperfusion and early neurological improvement with no increased risk of sICH and mortality, especially in patients with LVO and intention to proceed to endovascular treatment. Additionally, secondary clinical outcomes analysis showed a tendency for better functional outcomes at 3 months. The phase 3, multicenter, and noninferiority trial AcT 11 reported that 0.25 mg/kg tenecteplase dose was noninferior to standard of care with alteplase in mRS score of 0–1 at 90 to 120 days (36.9% versus 34.8%, unadjusted RD, 2.1% [95% CI, –2.6 to 6.9]), meeting the prespecified noninferiority threshold of −5% for patients with AIS presenting within 4.5 hours of symptom onset and eligible for thrombolysis per Canadian guidelines. The rates of sICH at 24 hours and all‐cause death within 3 months were similar between the 2 groups. This was the first phase 3 randomized controlled trial to show that intravenous thrombolysis with 0.25 mg/kg tenecteplase was comparable to alteplase in terms of efficacy and safety in patients with AIS presenting within 4.5 hours of stroke symptom onset. The large sample size, pragmatic eligibility criteria, and consistency of results across multiple secondary outcomes and subgroups attest to the generalizability of the trial's results. However, these trials were all aimed to establish the safety and efficacy profile of tenecteplase in White patients. In East Asian patients, the rhTNK‐tPA (recombinant human tenecteplase tissue‐type plasminogen activator) used in China had the same terminal amino acid sequence and a different production process for the tenecteplase made by Boehringer (Metalyse) and Genentech (TNKase). 49 The TRACE (Tenecteplase Reperfusion therapy in Acute ischemic Cerebrovascular Events) trial 49 enrolled 236 Chinese patients with AIS within 3 hours of symptom onset, and suggested that rhTNK‐tPA was well tolerated with similar rates of improvements in neurological deficits in Chinese patients at 0.1, 0.25, and 0.32 mg/kg doses. However, the sICH rate was numerically lower and the mRS score 0–1 rate was numerically higher with 0.25 mg/kg. The subsequent phase 3 trial TRACE‐2 12 informed by TRACE‐1 enrolled 1430 patients with AIS who were eligible for standard intravenous thrombolysis within 4.5 hours but ineligible for endovascular thrombectomy from 53 centers in China and established the noninferiority of 0.25 mg/kg tenecteplase compared with 0.9 mg/kg alteplase with a noninferiority margin of 0.937 for the risk ratio (RR; mRS score 0–1: 62% in tenecteplase group versus 58% in alteplase group; RR, 1.07 [95% CI, 0.98–1.16]). The risk of sICH within 36 hours and mortality within 90 days was similar between the 2 groups. Our latest published meta‐analysis 58 of phase 3 clinical trials including NOR‐TEST, AcT, and TRACE‐2 revealed that tenecteplase was noninferior to alteplase for achieving excellent functional outcome (mRS score 0–1) at 90 days (53% versus 50.5%, RD, 0.03 [95% CI, –0.00 to 0.06], meeting the prespecified noninferiority threshold of −4%) without increasing safety concerns. The meta‐analysis including 4068 patients of different races strongly recommends tenecteplase as an alternative to alteplase. Another phase 3 trial ATTEST‐2 13 has just been presented at the World Stroke Congress 2023. In the 0.25 mg/kg tenecteplase group, 885 patients with AIS who were clinically eligible for thrombolysis within 4.5 hours from last known well, achieved noninferior functional outcomes at 90 days compared with 891 patients in the alteplase group (90‐day mRS score distribution: adjusted conditional OR, 1.07 [95% CI, 0.90–1.27]; noninferiority test P<0.0001). Although superiority tests were not significant, all mRS outcomes at 90 days favored tenecteplase and there were no significant safety differences in this trial. The findings from the phase 3 trial in the United Kingdom added the evidence in favor of tenecteplase as a standard of care for thrombolysis.
Given the ease of use of tenecteplase versus alteplase, results from the AcT, TRACE‐2, and ATTEST‐2 trials, when combined with other evidence to date, provide a compelling rationale and solid evidence to switch the global standard for thrombolysis to tenecteplase at a dose of 0.25 mg/kg in patients with AIS who present within 4.5 hours of symptom onset, indicating action and modification of the current intravenous thrombolysis guidelines and protocols. 59
Recommendations of Tenecteplase in Societal/Organizational Guidelines
Based on the limited evidence, tenecteplase was considered in specific populations based upon the inclusion criteria of previous clinical trials 8 , 43 according to the 2019 American Heart Association/American Stroke Association acute stroke guidelines. 10 It may be reasonable to choose the 0.25 mg/kg tenecteplase over alteplase in patients without contraindications for thrombolysis who are also eligible for mechanical thrombectomy (class of recommendation IIb, level of evidence B‐R [moderate level of evidence from randomized clinical trials]). The AcT and TRACE‐2 trials have provided strong evidence of tenecteplase's noninferioirty. The pooled analysis including AcT in the latest 2023 European Stroke Organisation Guideline 14 showed an OR of 1.17 (95% CI, 0.98–1.39) for a 90‐day mRS score 0–1 in the 0.25 mg/kg tenecteplase group with a similar safety profile when compared with alteplase and strongly recommended that 0.25 mg/kg tenecteplase can be used as a safe and effective alternative to alteplase 0.9 mg/kg f or patients with AIS and LVO within 4.5 hours of onset based on the moderate quality of evidence. The updated 2023 National Clinical Guideline for Stroke for the United Kingdom and Ireland also recommended that thrombolysis with alteplase or tenecteplase should be considered for patients with AIS, regardless of age or stroke severity, within 4.5 hours of known onset. 15
Use of Tenecteplase in Specific Populations
A pooled analysis of the ATTEST and Australian‐TNK trials suggested that the benefits of tenecteplase were possibly more prominent in the subgroup of patients with a defined target mismatch (absolute mismatch volume >15 mL, mismatch ratio >1.8, baseline ischemic core <70 mL, and a volume of severely hypoperfused tissue <100 mL), regarding early clinical improvement (median NIHSS score change: 6 versus 1; P<0.001) and late independent recovery (mRS 0–1: OR, 2.33 [95% CI, 1.13–5.94]; P=0.032). 60
Another pooled analysis of these 2 trials showed that patients with baseline complete vessel occlusion had a significantly better rate of compete vessel recanalization at 24 hours (71% versus 43%, P<0.001), approaching rates seen in the recent endovascular trials, 9 , 61 , 62 larger reduction in 24‐hour NIHSS score from the baseline (9 versus 1, P=0.001) early clinical improvement, and higher rate of mRS score of 0 to 1 at 90 days (49% versus 25%, OR, 4.82 [95% CI, 1.02–7.84]; P=0.05), with much lower risk of hemorrhage (3% versus 7%, P=0.002) and no sICH in tenecteplase group. The finding suggested that tenecteplase may offer greater recanalization efficacy compared with alteplase, possibly more so in patients with complete vessel occlusions on baseline CT angiography. 63 A recent meta‐analysis conducted by Katsanos et al 64 included ATTEST, the Australian‐TNK, and EXTEND‐IA TNK randomized controlled clinical trials with a total of 433 patients with confirmed LVO. They found that patients with AIS and LVO receiving intravenous thrombolysis with tenecteplase had a 3‐fold higher rate of achieving successful recanalization (OR, 3.05 [95% CI, 1.73–5.40]) and a 2‐fold higher rate of having a favorable clinical outcomes of mRS score 0–2 at 3 months (OR, 2.06 [95% CI, 1.15–3.69]) compared with patients receiving intravenous alteplase. The 2 treatments had a similar safety profile. The meta‐analysis was the first to date that provided clear evidence of superiority for tenecteplase compared with alteplase for the treatment of AIS due to LVO.
The TEMPO‐1 (TNK–Tissue‐Type Plasminogen Activator Evaluation for Minor Ischemic Stroke With Proven Occlusion) study, 41 a dose escalation, safety, and feasibility trial, included 50 patients with an NIHSS score ≤5, intracranial arterial occlusion on CT angiography, and absence of well‐evolved infarction within 12 hours of symptom onset. No drug‐related serious adverse events as a primary outcome were observed in this trial. The single patient in the 0.25 mg/kg tier with a symptomatic parenchymal ICH had a small temporal lobe hemorrhage (20 mL), which was transient, and she had an independent outcome at 90 days (mRS score 2). Compared with 0.1 mg/kg tenecteplase, the dose of 0.25 mg/kg was confirmed to achieve higher rates of complete recanalization (52% versus 39%) and excellent functional outcome (76% versus 56%). However, the study was a proof‐of‐concept safety study in a small sample that did not compare tenecteplase to a matched control group of patients who used standard antiplatelet treatment or alteplase. The ongoing TEMPO‐2 (NCT02398656) compares tenecteplase 0.25 mg/kg with an antiplatelet agent(s) in patients with minor stroke with LVO within 12 hours of symptom onset, targeting a sample size of 1274.
Intravenous alteplase is the only approved thrombolytic agent for ischemic stroke, including patients ≥80 years old. 65 The subgroup analysis from NOR‐TEST including 273 patients ≥80 years with a medium baseline NIHSS score of 7 compared the efficacy and safety of 0.40 mg/kg tenecteplase (n=130) and 0.90 mg/kg alteplase (n=143) given within 4.5 hours from stroke onset. It identified no significant differences in the 2 treatment groups regarding the rates of excellent functional outcome (mRS score 0–1) after 3 months (43.2% in tenecteplase group versus 39.9% in alteplase group, OR, 1.14 [95% CI, 0.70–1.85], P=0.59) and frequency of sICH during the first 48 hours (8.5% in the tenecteplase group versus 7.0% in the alteplase group, OR, 1.23 [95% CI, 0.50–3.00], P=0.65). 66 In the subgroup analysis of the EXTEND‐IA TNK trials including 137 patients>80 years and with LVO in the bridging endovascular thrombectomy setting, a lower dose of 0.25 mg/kg tenecteplase was significantly associated with improved 90‐day mRS score compared with 0.40 mg/kg tenecteplase and alteplase after adjusting for baseline NIHSS score, age, and time from symptom onset to arterial puncture. There was no sICH associated with tenecteplase thrombolysis. 67
Tenecteplase use in extended time windows (4.5–24 hours, wakeup stroke, or unknown onset time) has not been fully investigated. The subgroup analysis of NOR‐TEST 68 included 40 patients with wakeup stroke and found that there was no difference in the number of patients achieving a good clinical outcome (mRS score 0–1) in either treatment group (68.8% versus 65.2%, P=0.82). However, patients treated with tenecteplase showed better early neurological improvement (87.5% versus 54.2%, P=0.027). No sICH or mortality was detected after thrombolysis. The findings were moderate for the small sample size but encouraged further investigation in this population. The latest reported TWIST (Tenecteplase in Wake‐Up Ischemic Stroke Trial), 53 a phase 3 trial conducted at 77 hospitals in 10 countries, aimed to determine whether tenecteplase given within 4.5 hours of awakening improves functional outcome in patients with ischemic wake‐up stroke selected using noncontrast CT instead advanced imaging selection. In this trial, 578 eligible patients without intracranial hemorrhage and large infarct core on noncontrast CT were randomly assigned to either 0.25 mg tenecteplase or control (no thrombolysis). The results did not support treatment with tenecteplase in patients selected with noncontrast CT with an assumption of a clinically relevant treatment effect with an OR of 1.50 (90‐day mRS distribution: adjusted OR [aOR], 1.18 [95% CI, 0.88–1.58], P=0.27). However, the trial failed to reach the inclusion target of 600 patients and thus was underpowered. The placebo‐controlled TIMELESS (Tenecteplase in Stroke Patients Between 4.5 and 24 hours) trial has just been completed. 54 The neuroimaging inclusion criteria were internal carotid artery, M1, or M2 occlusion stroke by magnetic resonance angiography/CT angiography with target mismatch profile on CT perfusion or magnetic resonance perfusion (ischemic core volume < 70 mL, mismatch ratio is ≥1.8 and mismatch volume is ≥15 mL). Note that 77.2% in the tenecteplase arm and 77.4% in the placebo arm were patients who had undergone endovascular thrombectomy. In the primary efficacy end point analysis, there was no significant difference in the odds of a lower mRS score at 90 days (common OR, 1.13 [95% CI, 0.82–1.57], adjusted P=0.45). However, complete recanalization at 24 hours was increased in the tenecteplase group as compared with placebo (76.7% versus 63.9%, OR, 1.89 [95% CI, 1.21–2.95]). With imaging selection, several other trials exploring the efficacy and safety of tenecteplase in the extended time windows are ongoing to provide more evidence.
The results of pooled analyses, subgroup studies, and proof‐of‐concept trials in specific populations provide suggestions for patient selection for tenecteplase thrombolysis. Tenecteplase use was clearly noninferior overall and superior to alteplase in patients with LVO and target mismatch but was uncertain in patients with minor stroke, oldest patients, and extended time windows.
Time and Economic Benefits
The ease of administering tenecteplase, which involves a bolus‐administered medication that does not require infusion monitoring during intrahospital or interhospital transfer, may aid in decreasing dosing errors, as well as enhancing patient workflow and potentially improving outcomes. The completed phase 2 TASTE‐A (Comparison of Tenecteplase With Alteplase for the Early Treatment of Ischemic Stroke in the Melbourne Mobile Stroke Unit) trial 50 in 5 tertiary hospitals in Melbourne aimed to test the hypothesis that tenecteplase administered in a mobile stroke unit (MSU) would result in superior reperfusion at hospital arrival, when compared with alteplase. In this study, 104 adult patients with ischemic stroke who were eligible for thrombolytic treatment were randomly allocated in the MSU to receive, within 4.5 hours of symptom onset, either standard‐of‐care alteplase (n=49) or 0.25 mg/kg tenecteplase (n=55), before being transported to the hospital for ongoing care. Treatment with tenecteplase on the MSU in Melbourne resulted in a superior rate of volume on the posttreatment perfusion lesion on arrival at the hospital assessed by CT‐perfusion imaging compared with alteplase (median, 12 mL [interquartile range, 3–28]) versus (35 mL 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 ; adjusted incidence rate ratio, 0.55 [95% CI, 0.37–0.81]; P=0.0030), and no safety concerns were noted. Notably, compared with patients allocated to alteplase, those allocated to tenecteplase had a shorter median time between MSU CT imaging and thrombolysis initiation and time from MSU arrival to thrombolytic treatment. This trial provides evidence to support the use of tenecteplase and MSUs in an optimal model of stroke care. This is the first trial to provide evidence of tenecteplase use in the prehospital setting. However, the phase 2 trial was underpowered to show a difference in 3‐month outcomes between tenecteplase and alteplase. Phase 3 randomized clinical trials comparing ultra‐early tenecteplase use with alteplase are ongoing to investigate the long‐term benefit (ACTRN12613000243718, NCT03889249).
From an economic standpoint, the cost savings from switching to tenecteplase can be significant. In the United States, the substitution of intravenous alteplase with tenecteplase saves approximately $3000 per treatment. 69 Outside the United States, switching to tenecteplase was estimated to represent a 50% cost savings for the medication. 55 In the TRACE‐2 trial in China, the total cost of rh tenecteplase‐alteplase therapy was also lower compared with alteplase (11 255.45 versus 12 094.25 yuan). 12 The EXTEND‐IA TNK investigators suggest that in Australia, tenecteplase was associated with less additional lifetime cost (96 357 versus 106 304 Australian dollars) compared with alteplase and greater benefits in the long term. 70
Whether the transition in acute stroke thrombolysis from alteplase to tenecteplase will be cost effective or reduce key system metrics associated with improved outcomes (eg, door‐to‐needle time, door‐in‐door‐out time, and transport times) at a population level remains to be seen.
Tenecteplase in the Real‐World Practice
Tenecteplase use was investigated in the real world, through mainly retrospective and small sample size observational studies (Table S3). In 2021, to compare the safety and effectiveness of intravenous tenecteplase (0.25 mg/kg) with intravenous alteplase (0.9 mg/kg) for patients with AIS and LVO in everyday clinical settings, Psychogios et al 71 from Greece conducted a prospective study enrolling 58 patients (tenecteplase=19, alteplase=39) for acute reperfusion therapies (intravenous thrombolysis with or without mechanical thrombectomy). A nonsignificant higher rate of both averted thrombectomies (31.5% versus 17.9%, P=0.243) and sICH (15.8% versus 5.1%, P=0.318) in the tenecteplase group was observed, and the 90‐day mRS score of 0–2 did not differ between the 2 groups. A single‐center cross‐sectional retrospective observational study in 2022, including 184 patients (tenecteplase=45, alteplase=139) with AIS due to LVO confirmed by CT angiography/magnetic resonance angiography/transcranial Doppler, also showed that tenecteplase (0.25 mg/kg) could be superior with a preendovascular thrombectomy recanalization rate of 22.2% and higher odds of excellent functional outcome (mRS score 0–2) at 90 days than alteplase (0.9 mg/kg). The rates of sICH between the 2 groups had no significant difference. 72 Estella et al 73 conducted a multicenter, retrospective, observational cohort study to compare intravenous tenecteplase (0.25 mg/kg) versus alteplase (0.9 mg/kg) in the bridging therapy for patients diagnosed with AIS due to LVO. One hundred patients (tenecteplase= 0, alteplase=80) who underwent fibrinolysis at the primary stroke center and then transferred to a comprehensive stroke center for thrombectomy were included. The effectiveness measured by successful recanalization (modified Thrombolysis in Cerebral Infarction score 2b‐3) rate and excellent functional outcome (mRS score 0–1) at 90 days, as well as the safety assessed by mortality and intracranial hemorrhage, showed no significant differences between tenecteplase and alteplase.
In the CERTAIN (The Comparative Effectiveness of Routine Tenecteplase Versus Alteplase in Acute Ischemic Stroke Collaboration), which involved 9238 patients with ischemic stroke treated with either alteplase or tenecteplase, the group receiving 0.25 mg/kg of tenecteplase demonstrated a significantly lower rate of symptomatic intracranial hemorrhage compared with the alteplase group (1.8% versus 3.6%, aOR, 0.42 [95% CI, 0.30–0.58]; P<0.01). 74
In addition, a multicenter prospective registry of 588 patients with stroke (tenecteplase=234, alteplase=354) found that tenecteplase had higher proportion of patients achieving target door‐to‐needle time within 45 minutes (41% versus 29%, aOR, 1.85 [95% CI, 1.27–2.71], P=0.001) and target door‐in‐door‐out time within 90 minutes (37% versus 14%, aOR, 3.62 [95% CI, 1.30–10.74], P=0.02), and unfavorable outcomes (sICH, in‐hospital mortality, or discharge to hospice) was numerically lower in the tenecteplase group (7.3% versus 11.9%, aOR, 0.77 [95% CI, 0.42–1.37]). Furthermore, the total hospital cost was lower in tenecteplase group ($13 382 versus $15 841, P<0.001). 75 Another prospective study from New Zealand confirmed that patients treated with tenecteplase had a shorter door‐to‐needle time (53 minutes versus 61 minutes, P=0.0002) and no significant difference in sICH rates or death by day 7. 76
The real‐world findings suggest that tenecteplase is relatively effective and safe, with time‐saving benefits in acute stroke settings. Many centers in the United States, Canada, and Australia have switched from using alteplase to off‐label use of tenecteplase, particularly during the COVID‐19 pandemic. 77 However, certain logistics must be considered when making the switch. First, tenecteplase should be used as an alternative to alteplase only if it is recommended by guidelines or approved by local hospitals. Second, based on current evidence, 0.25 mg/kg tenecteplase is the recommended dosage. However, the drug package may vary between tenecteplase (Guangzhou Recomgen Biotech Co., Ltd.), Metalyse (Boehringer Ingelheim), and TNKase (Roche/Genentech) in different countries and regions. Therefore, special attention should be given to the preparation of intravenous tenecteplase.
Potential Future Advances
The introduction of tenecteplase has had a significant impact on stroke thrombolysis. When administered intravenously, tenecteplase has been found to improve reperfusion and provide similar clinical effectiveness to alteplase in patients with AIS due to LVO. Importantly, the use of tenecteplase has not been associated with increased risk of intracranial hemorrhage. One of the major advantages of tenecteplase is its ease of administration, which could potentially lead to shorter treatment delays compared with alteplase. Recent trials have expanded the time window for tenecteplase use to 12 hours, using advanced imaging techniques for patient selection. Ongoing trials are further exploring the extension of the time window. However, there are still some unanswered questions related to tenecteplase (Table). Therefore, further studies are needed to investigate the use of tenecteplase in an extended treatment window, in patients with minor strokes, in combination with endovascular therapy, and in the prehospital ambulance setting (Table S4).
Table .
Directions for Further Investigation of Tenecteplase
| Directions for further investigation | Ongoing trials |
|---|---|
| Tenecteplase use in extended time windows (4.5–24 hours, wakeup stroke or unknown onset time) |
CHABLIS‐T II (NCT04516993), RESILIENT (or EXTEND‐IV, NCT05199662), ETERNAL‐LVO (NCT04454788), POST‐ETERNAL (NCT05105633), TRACE III (NCT05141305) |
| Tenecteplase in the bridge EVT vs direct EVT |
DIRECT‐TNK (NCT05199194), BRIDGE‐TNK (NCT04733742) |
| Tenecteplase use for minor ischemic stroke (with proven occlusion) | TEMPO‐2 (NCT02398656) |
| Tenecteplase use in posterior circulation stroke | POST‐ETERNAL (NCT05105633) |
| Adjunction of tenecteplase with other drugs in the early treatment | ATTIS (NCT05604638) |
| Adjunctive Intra‐arterial tenecteplase |
INSIST‐IT (NCT05657457), INSIST‐TNK (NCT04201964), ALLY (NCT05172934), TECNO (NCT05499832), BRETIS‐TNK II (NCT05657444), RESCUE‐TNK (NCT05657470), ATTENTION IA (NCT05684172), ANGEL‐TNK (NCT05624190), EXTEND‐AGNES TNK (NCT05892510) |
| Tenecteplase use in mobile stroke units |
ALLY indicates Adjunctive Intra‐Arterial Tenecteplase Following Mechanical Thrombectomy Pilot Trial; ANGEL‐TNK, Intra‐Arterial Recombinant Human TNK Tissue‐Type Plasminogen Activator (rhTNK‐tPA) Thrombolysis for Acute Large Vascular Occlusion After Successful Mechanical Thrombectomy Recanalization; ATTENTION IA, Intra‐Arterial TNK Following Endovascular Thrombectomy in Patients With Large Vessel Occlusion of Posterior Circulation; ATTIS, Alteplase Versus Tenecteplase for Thrombolysis After Ischaemic Stroke; BRETIS‐TNK Intra‐Arterial Tenecteplase During First Thrombectomy Attempt for Acute Stroke; BRIDGE‐TNK, Endovascular Treatment With Versus Without Intravenous rhTNK‐tPA in Stroke; CHABLIS‐T, Chinese Acute Tissue‐Based Imaging Selection for Lysis In Stroke‐Tenecteplase; DIRECT‐TNK, Randomization to Endovascular Treatment Alone or Preceded by Systemic Thrombolysis With Tenecteplase in Ischemic Stroke; ETERNAL‐LVO, Extending the Time Window for Tenecteplase by Effective Reperfusion in Patients With Large Vessel Occlusion; EVT, endovascular thrombectomy; EXTEND‐AGNES, Post‐Thrombectomy Intra‐Arterial Tenecteplase for Acute Management of Non‐Retrievable Thrombus and No‐Reflow in Emergent Stroke; INSIST‐IT, Improving Neurological Outcome for Acute Basilar Artery Occlusion With Sufficient Recanalization After Thrombectomy by Intraarterial Tenecteplase; INSIST‐TNK, Improving Neuroprotective Strategy for Ischemic Stroke With Poor Recanalization After Thrombectomy by Intra‐Arterial TNK; ORIGINAL, A Study in Chinese Patients to Compare How Tenecteplase and Alteplase Given After a Stroke Improve Recovering of Physical Activity; POST‐ETERNA, Extending the Time Window for Tenecteplase by Recanalization of Basilar Artery Occlusion in Posterior Circulation Stroke; RESCUE‐TNK, Rescue Thrombolysis for Medium Vessel Occlusion; RESILIENT (EXTEND‐IV), Randomization to Extend Stroke Intravenous Thrombolysis in Evolving Non‐Large Vessel Occlusion With Tenecteplase; TECNO, Safety and Efficacy of Intra‐Arterial Tenecteplase for Noncomplete Reperfusion of Intracranial Occlusions; TEMPO‐2, A Randomized Controlled Trial of TNK‐tPA Versus Standard of Care for Minor Ischemic Stroke With Proven Occlusion; and TRACEIII, Tenecteplase Reperfusion Therapy in Acute Ischemic Cerebrovascular Events‐III.
Sources of Funding
This work was supported by grants from the National Natural Science Foundation (82171272), Beijing Municipal Science & Technology Commission (Z211100003521019), and Beijing Hospitals Authority (PX2022019).
Disclosures
None.
Supporting information
Tables S1–S4
References 78–82
This article was sent to Kori S. Zachrison, MD, MSc Associate Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.123.031692
For Sources of Funding and Disclosures, see page 10.
REFERENCES
- 1. GBD 2019 Stroke Collaborators . Global, regional, and national burden of stroke and its risk factors, 1990‐2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20:795–820. doi: 10.1016/S1474-4422(21)00252-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berge E, Whiteley W, Audebert H, De Marchis GM, Fonseca AC, Padiglioni C, de la Ossa NP, Strbian D, Tsivgoulis G, Turc G. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. 2021;6:I–LXII. doi: 10.1177/2396987321989865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. American Academy of Emergency Medicine Work Group on Thrombolytic Therapy in Stroke . Position Statement of the American Academy of Emergency Medicine on the Use of Intravenous Thrombolytic Therapy in the Treatment of Stroke. Available at: www.aaem.org/. Accessed January 21, 2002. Updated March 11, 2002. https://www.aaem.org/statements/aaem‐position‐statement‐on‐tpa‐the‐use‐of‐intravenous‐thrombolytic‐therapy‐in‐the‐treatment‐of‐stroke/
- 4. Logallo N, Kvistad CE, Nacu A, Thomassen L. Novel thrombolytics for acute ischemic stroke: challenges and opportunities. CNS Drugs. 2016;30:101–108. doi: 10.1007/s40263-015-0307-2 [DOI] [PubMed] [Google Scholar]
- 5. Baruah DB, Dash RN, Chaudhari MR, Kadam SS. Plasminogen activators: a comparison. Vascul Pharmacol. 2006;44:1–9. doi: 10.1016/j.vph.2005.09.003 [DOI] [PubMed] [Google Scholar]
- 6. Li G, Wang C, Wang S, Xiong Y, Zhao X. Tenecteplase in ischemic stroke: challenge and opportunity. Neuropsychiatr Dis Treat. 2022;18:1013–1026. doi: 10.2147/NDT.S360967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van De Werf F, Adgey J, Ardissino D, Armstrong PW, Aylward P, Barbash G, Betriu A, Binbrek AS, Califf R, Diaz R, et al. Single‐bolus tenecteplase compared with front‐loaded alteplase in acute myocardial infarction: the ASSENT‐2 double‐blind randomised trial. Lancet. 1999;354:716–722. doi: 10.1016/s0140-6736(99)07403-6 [DOI] [PubMed] [Google Scholar]
- 8. Campbell BCV, Mitchell PJ, Churilov L, Yassi N, Kleinig TJ, Dowling RJ, Yan B, Bush SJ, Dewey HM, Thijs V, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018;378:1573–1582. doi: 10.1056/NEJMoa1716405 [DOI] [PubMed] [Google Scholar]
- 9. Campbell BCV, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, et al. Endovascular therapy for ischemic stroke with perfusion‐imaging selection. N Engl J Med. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 10. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 11. Menon BK, Buck BH, Singh N, Deschaintre Y, Almekhlafi MA, Coutts SB, Thirunavukkarasu S, Khosravani H, Appireddy R, Moreau F, et al; AcT Trial Investigators . Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open‐label, registry‐linked, randomised, controlled, non‐inferiority trial. Lancet. 2022;400:161–169. doi: 10.1016/S0140-6736(22)01054-6 [DOI] [PubMed] [Google Scholar]
- 12. Wang Y, Li S, Pan Y, Li H, Parsons MW, Campbell BCV, Schwamm LH, Fisher M, Che F, Dai H, et al; TRACE‐2 Investigators . Tenecteplase versus alteplase in acute ischaemic cerebrovascular events (TRACE‐2): a phase 3, multicentre, open‐label, randomised controlled, non‐inferiority trial. Lancet. 2023;401:645–654. doi: 10.1016/S0140-6736(22)02600-9 [DOI] [PubMed] [Google Scholar]
- 13. Muir GFK, Ford I, Wardlaw JM, Mcconnachie A, Greenlaw N, Mair G, Sprigg N, Price C, Macleod MJ. Tenectplase versus alteplase for acute stroke within 4.5H of onset: the second alteplase‐tenecteplase trial evaluation for stroke thrombolysis (ATTEST‐2). Paper presented at: 15th World Stroke Congress, 10–12 October 2023, Toronto, Canada. Accessed October 12, 2023. https://worldstrokecongress.org/clinical‐trials/
- 14. Alamowitch S, Turc G, Palaiodimou L, Bivard A, Cameron A, De Marchis GM, Fromm A, Kõrv J, Roaldsen MB, Katsanos AH, et al. European Stroke Organisation (ESO) expedited recommendation on tenecteplase for acute ischaemic stroke. Eur Stroke J. 2023;8:8–54. doi: 10.1177/23969873221150022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. London: Intercollegiate Stroke Working Party . National Clinical Guideline for Stroke for the UK and Ireland. 2023. Accessed May 04, 2023. www.strokeguideline.org
- 16. Pechet L. Fibrinolysis. N Engl J Med. 1965;273:966–973. doi: 10.1056/NEJM196510282731805 [DOI] [PubMed] [Google Scholar]
- 17. Medcalf RL. What drives "fibrinolysis"? Hamostaseologie. 2015;35:303–310. doi: 10.5482/HAMO-14-10-0050 [DOI] [PubMed] [Google Scholar]
- 18. Cesarman‐Maus G, Hajjar KA. Molecular mechanisms of fibrinolysis. Br J Haematol. 2005;129:307–321. doi: 10.1111/j.1365-2141.2005.05444.x [DOI] [PubMed] [Google Scholar]
- 19. Keyt BA, Paoni NF, Refino CJ, Berleau L, Nguyen H, Chow A, Lai J, Peña L, Pater C, Ogez J. A faster‐acting and more potent form of tissue plasminogen activator. Proc Natl Acad Sci U S A. 1994;91:3670–3674. doi: 10.1073/pnas.91.9.3670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bivard A, Lin L, Parsonsb MW. Review of stroke thrombolytics. J Stroke. 2013;15:90–98. doi: 10.5853/jos.2013.15.2.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Modi NB, Eppler S, Breed J, Cannon CP, Braunwald E, Love TW. Pharmacokinetics of a slower clearing tissue plasminogen activator variant, TNK‐tPA, in patients with acute myocardial infarction. Thromb Haemost. 1998;79:134–139. doi: 10.1055/s-0037-1614232 [DOI] [PubMed] [Google Scholar]
- 22. Tsikouris JP, Tsikouris AP. A review of available fibrin‐specific thrombolytic agents used in acute myocardial infarction. Pharmacotherapy. 2001;21:207–217. doi: 10.1592/phco.21.2.207.34103 [DOI] [PubMed] [Google Scholar]
- 23. Warach SJ, Dula AN, Milling TJ. Tenecteplase thrombolysis for acute ischemic stroke. Stroke. 2020;51:3440–3451. doi: 10.1161/STROKEAHA.120.029749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stewart RJ, Fredenburgh JC, Leslie BA, Keyt BA, Rischke JA, Weitz JI. Identification of the mechanism responsible for the increased fibrin specificity of TNK‐tissue plasminogen activator relative to tissue plasminogen activator. J Biol Chem. 2000;275:10112–10120. doi: 10.1074/jbc.275.14.10112 [DOI] [PubMed] [Google Scholar]
- 25. Benedict CR, Refino CJ, Keyt BA, Pakala R, Paoni NF, Thomas GR, Bennett WF. New variant of human tissue plasminogen activator (TPA) with enhanced efficacy and lower incidence of bleeding compared with recombinant human TPA. Circulation. 1995;92:3032–3040. doi: 10.1161/01.cir.92.10.3032 [DOI] [PubMed] [Google Scholar]
- 26. Fitzgerald DJ, Wright F, FitzGerald GA. Increased thromboxane biosynthesis during coronary thrombolysis. Evidence that platelet activation and thromboxane A2 modulate the response to tissue‐type plasminogen activator in vivo. Circ Res. 1989;65:83–94. doi: 10.1161/01.res.65.1.83 [DOI] [PubMed] [Google Scholar]
- 27. Madison EL, Goldsmith EJ, Gerard RD, Gething MJ, Sambrook JF. Serpin‐resistant mutants of human tissue‐type plasminogen activator. Nature. 1989;339:721–724. doi: 10.1038/339721a0 [DOI] [PubMed] [Google Scholar]
- 28. Thomas GR, Thibodeaux H, Errett CJ, Badillo JM, Keyt BA, Refino CJ, Zivin JA, Bennett WF. A long‐half‐life and fibrin‐specific form of tissue plasminogen activator in rabbit models of embolic stroke and peripheral bleeding. Stroke. 1994;25:2072–2078. doi: 10.1161/01.str.25.10.2072 [DOI] [PubMed] [Google Scholar]
- 29. National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group . Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401 [DOI] [PubMed] [Google Scholar]
- 30. Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, Boysen G, Bluhmki E, Höxter G, Mahagne MH. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA. 1995;274:1017–1025. doi: 10.1001/jama.1995.03530130023023 [DOI] [PubMed] [Google Scholar]
- 31. Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, Larrue V, Bluhmki E, Davis S, Donnan G, et al. Randomised double‐blind placebo‐controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European‐Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245–1251. doi: 10.1016/s0140-6736(98)08020-9 [DOI] [PubMed] [Google Scholar]
- 32. Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. Recombinant tissue‐type plasminogen activator (alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS study: a randomized controlled trial. Alteplase thrombolysis for acute noninterventional therapy in ischemic stroke. JAMA. 1999;282:2019–2026. doi: 10.1001/jama.282.21.2019 [DOI] [PubMed] [Google Scholar]
- 33. Clark WM, Albers GW, Madden KP, Hamilton S. The rtPA (alteplase) 0‐ to 6‐hour acute stroke trial, part a (A0276g): results of a double‐blind, placebo‐controlled, multicenter study. Thromblytic therapy in acute ischemic stroke study investigators. Stroke. 2000;31:811–816. doi: 10.1161/01.str.31.4.811 [DOI] [PubMed] [Google Scholar]
- 34. Haley EC, Lyden PD, Johnston KC, Hemmen TM. A pilot dose‐escalation safety study of tenecteplase in acute ischemic stroke. Stroke. 2005;36:607–612. doi: 10.1161/01.STR.0000154872.73240.e9 [DOI] [PubMed] [Google Scholar]
- 35. Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- 36. Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, Barber PA, Bladin C, De Silva DA, Byrnes G, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo‐controlled randomised trial. Lancet Neurol. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9 [DOI] [PubMed] [Google Scholar]
- 37. Parsons MW, Miteff F, Bateman GA, Spratt N, Loiselle A, Attia J, Levi CR. Acute ischemic stroke: imaging‐guided tenecteplase treatment in an extended time window. Neurology. 2009;72:915–921. doi: 10.1212/01.wnl.0000344168.05315.9d [DOI] [PubMed] [Google Scholar]
- 38. Haley EC, Thompson JLP, Grotta JC, Lyden PD, Hemmen TG, Brown DL, Fanale C, Libman R, Kwiatkowski TG, Llinas RH, et al. Phase IIB/III trial of tenecteplase in acute ischemic stroke: results of a prematurely terminated randomized clinical trial. Stroke. 2010;41:707–711. doi: 10.1161/STROKEAHA.109.572040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. IST‐3 Collaborative Group ; Sandercock P, Wardlaw JM, Lindley RI, Dennis M, Cohen G, Murray G, Innes K, Venables G, Czlonkowska A, Kobayashi A, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST‐3]): a randomised controlled trial. Lancet. 2012;379:2352–2363. doi: 10.1016/S0140-6736(12)60768-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Parsons M, Spratt N, Bivard A, Campbell B, Chung K, Miteff F, O'Brien B, Bladin C, McElduff P, Allen C, et al. A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Engl J Med. 2012;366:1099–1107. doi: 10.1056/NEJMoa1109842 [DOI] [PubMed] [Google Scholar]
- 41. Coutts SB, Dubuc V, Mandzia J, Kenney C, Demchuk AM, Smith EE, Subramaniam S, Goyal M, Patil S, Menon BK, et al. Tenecteplase‐tissue‐type plasminogen activator evaluation for minor ischemic stroke with proven occlusion. Stroke. 2015;46:769–774. doi: 10.1161/STROKEAHA.114.008504 [DOI] [PubMed] [Google Scholar]
- 42. Huang X, Cheripelli BK, Lloyd SM, Kalladka D, Moreton FC, Siddiqui A, Ford I, Muir KW. Alteplase versus tenecteplase for thrombolysis after ischaemic stroke (ATTEST): a phase 2, randomised, open‐label, blinded endpoint study. Lancet Neurol. 2015;14:368–376. doi: 10.1016/S1474-4422(15)70017-7 [DOI] [PubMed] [Google Scholar]
- 43. Logallo N, Novotny V, Assmus J, Kvistad CE, Alteheld L, Rønning OM, Thommessen B, Amthor K‐F, Ihle‐Hansen H, Kurz M, et al. Tenecteplase versus alteplase for management of acute ischaemic stroke (NOR‐TEST): a phase 3, randomised, open‐label, blinded endpoint trial. Lancet Neurol. 2017;16:781–788. doi: 10.1016/S1474-4422(17)30253-3 [DOI] [PubMed] [Google Scholar]
- 44. Thomalla G, Simonsen CZ, Boutitie F, Andersen G, Berthezene Y, Cheng B, Cheripelli B, Cho T‐H, Fazekas F, Fiehler J, et al. MRI‐guided thrombolysis for stroke with unknown time of onset. N Engl J Med. 2018;379:611–622. doi: 10.1056/NEJMoa1804355 [DOI] [PubMed] [Google Scholar]
- 45. Ma H, Campbell BCV, Parsons MW, Churilov L, Levi CR, Hsu C, Kleinig TJ, Wijeratne T, Curtze S, Dewey HM, et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. N Engl J Med. 2019;380:1795–1803. doi: 10.1056/NEJMoa1813046 [DOI] [PubMed] [Google Scholar]
- 46. Ringleb P, Bendszus M, Bluhmki E, Donnan G, Eschenfelder C, Fatar M, Kessler C, Molina C, Leys D, Muddegowda G, et al. Extending the time window for intravenous thrombolysis in acute ischemic stroke using magnetic resonance imaging‐based patient selection. Int J Stroke. 2019;14:483–490. doi: 10.1177/1747493019840938 [DOI] [PubMed] [Google Scholar]
- 47. Campbell BCV, Mitchell PJ, Churilov L, Yassi N, Kleinig TJ, Dowling RJ, Yan B, Bush SJ, Thijs V, Scroop R, et al. Effect of intravenous tenecteplase dose on cerebral reperfusion before thrombectomy in patients with large vessel occlusion ischemic stroke: the EXTEND‐IA TNK part 2 randomized clinical trial. JAMA. 2020;323:1257–1265. doi: 10.1001/jama.2020.1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Koga M, Yamamoto H, Inoue M, Asakura K, Aoki J, Hamasaki T, Kanzawa T, Kondo R, Ohtaki M, Itabashi R, et al. Thrombolysis with alteplase at 0.6 mg/kg for stroke with unknown time of onset: a randomized controlled trial. Stroke. 2020;51:1530–1538. doi: 10.1161/STROKEAHA.119.028127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li S, Pan Y, Wang Z, Liang Z, Chen H, Wang D, Sui Y, Zhao X, Wang Y, Du W, et al. Safety and efficacy of tenecteplase versus alteplase in patients with acute ischaemic stroke (TRACE): a multicentre, randomised, open label, blinded‐endpoint (PROBE) controlled phase II study. Stroke Vasc Neurol. 2022;7:47–53. doi: 10.1136/svn-2021-000978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bivard A, Zhao H, Churilov L, Campbell BCV, Coote S, Yassi N, Yan B, Valente M, Sharobeam A, Balabanski AH, et al. Comparison of tenecteplase with alteplase for the early treatment of ischaemic stroke in the Melbourne Mobile stroke unit (TASTE‐A): a phase 2, randomised, open‐label trial. Lancet Neurol. 2022;21:520–527. doi: 10.1016/S1474-4422(22)00171-5 [DOI] [PubMed] [Google Scholar]
- 51. Cheng X, Hong L, Churilov L, Lin L, Ling Y, Zhang J, Yang J, Geng Y, Wu D, Liu X, et al; CHABLIS‐T Collaborators . Tenecteplase thrombolysis for stroke up to 24 hours after onset with perfusion imaging selection: the umbrella phase IIa CHABLIS‐T randomised clinical trial. Stroke Vasc Neurol. 2024:svn‐2023‐002820. doi: 10.1136/svn-2023-002820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kvistad CE, Næss H, Helleberg BH, Idicula T, Hagberg G, Nordby LM, Jenssen KN, Tobro H, Rörholt DM, Kaur K, et al. Tenecteplase versus alteplase for the management of acute ischaemic stroke in Norway (NOR‐TEST 2, part a): a phase 3, randomised, open‐label, blinded endpoint, non‐inferiority trial. Lancet Neurol. 2022;21:511–519. doi: 10.1016/S1474-4422(22)00124-7 [DOI] [PubMed] [Google Scholar]
- 53. Roaldsen MB, Eltoft A, Wilsgaard T, Christensen H, Engelter ST, Indredavik B, Jatužis D, Karelis G, Kõrv J, Lundström E, et al. Safety and efficacy of tenecteplase in patients with wake‐up stroke assessed by non‐contrast CT (TWIST): a multicentre, open‐label, randomised controlled trial. Lancet Neurol. 2023;22:117–126. doi: 10.1016/S1474-4422(22)00484-7 [DOI] [PubMed] [Google Scholar]
- 54. Albers GW, Jumaa M, Purdon B, Zaidi SF, Streib C, Shuaib A, Sangha N, Kim M, Froehler MT, Schwartz NE, et al; TIMELESS Investigators . Tenecteplase for stroke at 4.5 to 24 hours with perfusion‐imaging selection. N Engl J Med. 2024;390:701–711. doi: 10.1056/NEJMoa2310392 [DOI] [PubMed] [Google Scholar]
- 55. Luo Z, Zhou Y, He Y, Yan S, Chen Z, Zhang X, Chen Y, Tong L‐S, Zhong W, Hu H, et al. Treatment with intravenous alteplase in ischaemic stroke patients with onset time between 4.5 and 24 hours (HOPE): protocol for a randomised, controlled, multicentre study [published online August 1, 2023]. Stroke Vasc Neurol. doi: 10.1136/svn-2022-002154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Huang X, MacIsaac R, Thompson JL, Levin B, Buchsbaum R, Haley EC, Levi C, Campbell B, Bladin C, Parsons M, et al. Tenecteplase versus alteplase in stroke thrombolysis: an individual patient data meta‐analysis of randomized controlled trials. Int J Stroke. 2016;11:534–543. doi: 10.1177/1747493016641112 [DOI] [PubMed] [Google Scholar]
- 57. Burgos AM, Saver JL. Evidence that Tenecteplase is noninferior to alteplase for acute ischemic stroke: meta‐analysis of 5 randomized trials. Stroke. 2019;50:2156–2162. doi: 10.1161/STROKEAHA.119.025080 [DOI] [PubMed] [Google Scholar]
- 58. Xiong Y, Wang L, Li G, Yang K‐X, Hao M, Li S, Pan Y, Wang Y. Tenecteplase versus alteplase for acute ischaemic stroke: a meta‐analysis of phase III randomised trials. Stroke Vasc Neurol. 2023;28:svn‐2023‐002396. doi: 10.1136/svn-2023-002396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sandset EC, Tsivgoulis G. Tenecteplase for acute ischaemic stroke. Lancet. 2022;400:138–139. doi: 10.1016/S0140-6736(22)01107-2 [DOI] [PubMed] [Google Scholar]
- 60. Bivard A, Huang X, McElduff P, Levi CR, Campbell BCV, Cheripelli BK, Kalladka D, Moreton FC, Ford I, Bladin CF, et al. Impact of computed tomography perfusion imaging on the response to tenecteplase in ischemic stroke: analysis of 2 randomized controlled trials. Circulation. 2017;135:440–448. doi: 10.1161/CIRCULATIONAHA.116.022582 [DOI] [PubMed] [Google Scholar]
- 61. Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira RG, Clark W, Budzik R, Zaidat OO. Solitaire flow restoration device versus the merci retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel‐group, non‐inferiority trial. Lancet. 2012;380:1241–1249. doi: 10.1016/S0140-6736(12)61384-1 [DOI] [PubMed] [Google Scholar]
- 62. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 63. Bivard A, Huang X, Levi CR, Spratt N, Campbell BCV, Cheripelli BK, Kalladka D, Moreton FC, Ford I, Bladin CF, et al. Tenecteplase in ischemic stroke offers improved recanalization: analysis of 2 trials. Neurology. 2017;89:62–67. doi: 10.1212/WNL.0000000000004062 [DOI] [PubMed] [Google Scholar]
- 64. Katsanos AH, Safouris A, Sarraj A, Magoufis G, Leker RR, Khatri P, Cordonnier C, Leys D, Shoamanesh A, Ahmed N, et al. Intravenous thrombolysis with tenecteplase in patients with large vessel occlusions: systematic review and meta‐analysis. Stroke. 2021;52:308–312. doi: 10.1161/STROKEAHA.120.030220 [DOI] [PubMed] [Google Scholar]
- 65. Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, Brott T, Cohen G, Davis S, Donnan G, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta‐analysis of individual patient data from randomised trials. Lancet. 2014;384:1929–1935. doi: 10.1016/S0140-6736(14)60584-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Thommessen B, Næss H, Logallo N, Kvistad CE, Waje‐Andreassen U, Ihle‐Hansen H, Ihle‐Hansen H, Thomassen L, Morten RO. Tenecteplase versus alteplase after acute ischemic stroke at high age. Int J Stroke. 2021;16:295–299. doi: 10.1177/1747493020938306 [DOI] [PubMed] [Google Scholar]
- 67. Yogendrakumar V, Churilov L, Mitchell PJ, Kleinig TJ, Yassi N, Thijs V, Wu TY, Shah DG, Ng FC, Dewey HM, et al. Safety and efficacy of tenecteplase in older patients with large vessel occlusion: a pooled analysis of the EXTEND‐IA TNK trials. Neurology. 2022;98:e1292–e1301. doi: 10.1212/WNL.0000000000013302 [DOI] [PubMed] [Google Scholar]
- 68. Ahmed HK, Logallo N, Thomassen L, Novotny V, Mathisen SM, Kurz MW. Clinical outcomes and safety profile of tenecteplase in wake‐up stroke. Acta Neurol Scand. 2020;142:475–479. doi: 10.1111/ane.13296 [DOI] [PubMed] [Google Scholar]
- 69. Gao L, Moodie M, Mitchell PJ, Churilov L, Kleinig TJ, Yassi N, Yan B, Parsons MW, Donnan GA, Davis SM, et al. Cost‐effectiveness of tenecteplase before thrombectomy for ischemic stroke. Stroke. 2020;51:3681–3689. doi: 10.1161/STROKEAHA.120.029666 [DOI] [PubMed] [Google Scholar]
- 70. Johansen MC, Campbell BCV. ANA investigates: tenecteplase. Ann Neurol. 2021;90:1–3. doi: 10.1002/ana.26093 [DOI] [PubMed] [Google Scholar]
- 71. Psychogios K, Palaiodimou L, Katsanos AH, Magoufis G, Safouris A, Kargiotis O, Spiliopoulos S, Papageorgiou E, Theodorou A, Voumvourakis K, et al. Real‐world comparative safety and efficacy of tenecteplase versus alteplase in acute ischemic stroke patients with large vessel occlusion. Ther Adv Neurol Disord. 2021;14:1756286420986727. doi: 10.1177/1756286420986727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Teivane A, Jurjāns K, Vētra J, Grigorjeva J, Kupcs K, Masiliūnas R, Miglāne E. Tenecteplase or alteplase better in patients with acute ischemic stroke due to large vessel occlusion: a single center observational study. Medicina (Kaunas). 2022;58:1169. doi: 10.3390/medicina58091169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Estella Á, Pérez Ruiz M, Serrano JJ. Effectiveness and safety of tecneplase vs. alteplase in the acute treatment of ischemic stroke. J Pers Med. 2022;12:1525. doi: 10.3390/jpm12091525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Warach SJ, Ranta A, Kim J, Song SS, Wallace A, Beharry J, Gibson D, Cadilhac DA, Bladin CF, Kleinig TJ, et al. Symptomatic intracranial hemorrhage with tenecteplase vs alteplase in patients with acute ischemic stroke: the comparative effectiveness of routine tenecteplase vs alteplase in acute ischemic stroke (CERTAIN) collaboration. JAMA Neurol. 2023;80:732–738. doi: 10.1001/jamaneurol.2023.1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhong CS, Beharry J, Salazar D, Smith K, Withington S, Campbell BCV, Wilson D, Le Heron C, Mason D, Duncan R, et al. Routine use of tenecteplase for thrombolysis in acute ischemic stroke. Stroke. 2021;52:1087–1090. doi: 10.1161/STROKEAHA.120.030859 [DOI] [PubMed] [Google Scholar]
- 76. Mahawish K, Gommans J, Kleinig T, Lallu B, Tyson A, Ranta A. Switching to tenecteplase for stroke thrombolysis: real‐world experience and outcomes in a regional stroke network. Stroke. 2021;52:e590–e593. doi: 10.1161/STROKEAHA.121.035931 [DOI] [PubMed] [Google Scholar]
- 77. Warach SJ, Saver JL. Stroke thrombolysis with tenecteplase to reduce emergency department spread of coronavirus disease 2019 and shortages of alteplase. JAMA Neurol. 2020;77:1203–1204. doi: 10.1001/jamaneurol.2020.2396 [DOI] [PubMed] [Google Scholar]
- 78. Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, Hennerici MG, Kaste M, Kuelkens S, Larrue V, et al. Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke‐monitoring study (SITS‐MOST): an observational study. Lancet. 2007;369:275–282. doi: 10.1016/S0140-6736(07)60149-4 [DOI] [PubMed] [Google Scholar]
- 79. Wahlgren N, Ahmed N, Dávalos A, Hacke W, Millán M, Muir K, Roine RO, Toni D, Lees KR. Thrombolysis with alteplase 3‐4.5 h after acute ischaemic stroke (SITS‐ISTR): an observational study. Lancet. 2008;372:1303–1309. doi: 10.1016/S0140-6736(08)61339-2 [DOI] [PubMed] [Google Scholar]
- 80. Belkouch A, Jidane S, Chouaib N, Elbouti A, Nebhani T, Sirbou R, Bakkali H, Belyamani L. Thrombolysis for acute ischemic stroke by tenecteplase in the emergency department of a Moroccan hospital. Pan Afr Med J. 2015;21:37. doi: 10.11604/pamj.2015.21.37.6491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. George M, Baby N, Paul R, Zabeer M, Thomas C. Comparison of thrombolytic agents in treatment of patients with acute ischemic stroke; findings from a single centre follow up study in real‐life settings. J Clin Neurosci. 2021;91:299–305. doi: 10.1016/j.jocn.2021.07.004 [DOI] [PubMed] [Google Scholar]
- 82. Warach SJ, Dula AN, Milling TJ, Miller S, Allen L, Zuck ND, Miller C, Jesser CA, Misra LR, Miley JT, et al. Prospective observational cohort study of tenecteplase versus alteplase in routine clinical practice. Stroke. 2022;53:3583–3593. doi: 10.1161/STROKEAHA.122.038950 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4
References 78–82


