Abstract
Background:
People with opioid use disorder (OUD) frequently present at the emergency department (ED), a potentially critical point for intervention and treatment linkage. Peer recovery support specialist (PRSS) interventions have expanded in US-based EDs, although evidence supporting such interventions has not been firmly established.
Methods:
Researchers conducted a pragmatic trial of POINT (Project Planned Outreach, Intervention, Naloxone, and Treatment), an ED-initiated intervention for harm reduction and recovery coaching/treatment in two Indiana EDs. Cluster randomization allocated patients to the POINT intervention (n=157) vs. a control condition (n=86). Participants completed a structured interview, and all outcomes were assessed using administrative data from an extensive state health exchange and state systems. Target patients (n=243) presented to the ED for a possible opioid-related reason. The primary outcome was overdose-related ED re-presentation. Key secondary outcomes included OUD medication treatment linkage, duration of medication in days, all-cause ED re-presentation, all-cause inpatient re-presentation, and Medicaid enrollment. All outcomes were assessed at 3-, 6-, and 12-months post-enrollment. Ad hoc analyses were performed to assess treatment motivation and readiness.
Results:
POINT and standard care participants did not differ significantly on any outcomes measured. Participants who presented to the ED for overdose had significantly lower scores (3.5 vs. 4.2, p<.01) regarding readiness to begin treatment compared to those presenting for other opioid-related issues.
Conclusions:
This is the first randomized trial investigating overdose outcomes for an ED PRSS intervention. Though underpowered, results suggest no benefit of PRSS services over standard care. Given the scope of PRSS, future work in this area should assess more recovery- and harm reduction-oriented outcomes, as well as the potential benefits of integrating PRSS within multimodal ED-based interventions for OUD.
Keywords: opioid use disorder, overdose, peer support, emergency department, opioid poisoning, medications for opioid use disorder
INTRODUCTION
Opioid overdose is a public health priority as fatalities remain high across the world.1,2 People with opioid use disorder (OUD) frequently use emergency department (ED) services, making an ED presentation a potentially critical point for intervention.3,4 Indeed, ED-based interventions targeting individuals who use opioids have gained traction recently.5 Two popular strategies utilized by ED-based OUD interventions are a) buprenorphine induction and b) engagement by a certified peer recovery support specialist (PRSS). While a substantial evidence base supports the former,6–8 evidence for the latter is mixed.9,10 This paper adds to the developing knowledge base for ED-based PRSS interventions by describing results from a pragmatic trial of Project Planned Outreach, Intervention, Naloxone, and Treatment (POINT), a PRSS-delivered ED intervention for OUD.
PRSS have lived experience in substance use recovery and often provide behavioral intervention, harm reduction education and supplies to reduce risks of future drug use, and recovery support.11–13 People with OUD and other substance use disorders may feel more trust and comfort interacting with a professional who has lived experience.14,15 PRSSs are trained to “meet patients where they’re at”, providing support services even when patients are not interested in treatment.13 As another advantage, supporting patients through active treatment and service linkage is within PRSSs’ practice scope, and they are able to dedicate more effort to these time-intensive activities than clinical ED personnel.12,16,17 Additionally, EDs with barriers to immediate buprenorphine induction—such as opposing provider attitudes or lack of available community-based options for treatment linkage—may find it easier to implement PRSS services.18,19 Thus, PRSSs can provide alternative harm reduction and recovery paths for patients who decline treatment or who face greater access barriers.20,21 Prior research has demonstrated ED-based PRSSs’ ability to engage patients and distribute the overdose-reversing drug naloxone.22,23 However, their capability to improve treatment linkage, including linkage to evidence-based medications for OUD (MOUD; e.g., methadone, buprenorphine, and injectable, long-acting naltrexone) or to improve clinical outcomes such as overdose and ED re-presentations have not been fully demonstrated.
POINT is a PRSS-delivered intervention developed in Indianapolis, Indiana ED to serve patients presenting following an opioid overdose. POINT informed the expansion of PRSS services in EDs across the state.24,25 The current study’s objective was to evaluate POINT’s ability to improve outcomes in two new EDs not previously exposed to the intervention. The primary hypothesis, based on state-defined goals was that patients receiving POINT (vs. a control condition) would have lower rates of overdose-related ED re-presentations. The secondary hypotheses predicted patients receiving POINT would have greater MOUD treatment access, linkage and engagement, and reduced all-cause ED re-presentations and inpatient admissions. Notably, overdose re-presentation has not been assessed as an outcome in prior trials of ED-based PRSS interventions.
METHODS
This study employed a pragmatic trial design due to an ethical imperative and research funding requirement to deliver immediate and generalizable solutions to the growing opioid crisis through collaborations between researchers and state entities.26,27 The study was pre-registered (NCT03336268) and all human subjects procedures were approved by Chestnut Health Systems’ Institutional Review Board (#1706859955).
The POINT intervention
POINT services are delivered by state-certified PRSSs28 who receive orientation and on-site training to work in the ED environment. PRSSs are alerted to the presence of a patient with concerns indicating possible OUD based on their admitting diagnosis (e.g., overdose, altered mental status, cardiac arrest [if under 35 years of age], drug withdrawal, abscess, endocarditis) through the electronic health record’s (EHR) ED tracking board. After receiving the required approval from nursing staff indicating the patients’ medical stability, the PRSS meets the patient at their bedside, conducts a brief assessment of the patient’s high-risk drug use behaviors, and provides a) harm reduction coaching (discussion of overdose risks, naloxone education, and instructions how to access naloxone and syringe services), b) naloxone, c) linkage to recovery supports, and d) treatment referral (e.g., MOUD, inpatient, outpatient), as well as assistance eliminating any associated barriers such as the ability to pay/insurance. While this process aims to motivate the patient to accept and engage in evidence-based MOUD services, the PRSS remains open to whatever path the patient chooses, consistent with the PRSS scope of practice.13 After initial contact, the PRSS arranges transportation assistance, if needed, for treatment intake appointments and provides continued phone-based support, attempting contact every 2-3 days. They will continue contact attempts until the patient is linked to MOUD, requests service discontinuation, or has two weeks of unsuccessful follow-up attempts.
Participants
Study participants (n=243) were recruited from two Indiana EDs in a university health system. The first, a Level 1 Trauma Center, began data collection February 12, 2018, and ended March 16, 2020. The second, a Level 3 Trauma Center, began April 29, 2019, and ended May 28, 2021. Participation eligibility requirements were a) presenting to the ED for an opioid overdose or related opioid health issue (e.g., opioid withdrawal or complications related to injection drug use), b) meeting at least one criterion for DSM-5 OUD diagnosis to ensure patients intentionally use opioids vs. other possible opioid-adulterated drug, c) approval for ED discharge by hospital staff, d) being at least 18 years of age, and e) having medical stability and capability to provide consent.
Measures
The primary outcome was post-enrollment overdose-related ED re-presentation. Secondary outcomes included: a) MOUD linkage, b) MOUD treatment engagement duration, c) all-cause ED re-presentation, d) all-cause inpatient hospital admission, and e) Medicaid enrollment (for patients uninsured at baseline); and d) mortality. All outcome measures were assessed using administrative data only for three years prior to participant enrollment and up to 12 months following. Administrative data were accessed from: a) state-wide hospital admissions data from the Indiana Network for Patient Care (a health information exchange that includes all hospitals within the study’s geographic areas,29 b) methadone treatment data from Indiana’s single state authority, c) controlled drug dispensing data from Indiana’s prescription drug monitoring system (buprenorphine dispensation), d) retail pharmacy dispensing data from SureScripts,30 e) Medicare and Medicaid claims data, and f) Indiana vital records data.
Procedures
To minimize the burden on ED staff, a shift-based cluster randomization approach was used.31,32 Each workday was divided into three shifts: 8:00 am–3:59 pm, 4:00 pm–11:59 pm, and 12:00 pm–7:59 am. Simple randomization selected which shift received POINT services or the control condition (blinding was impracticable with these procedures). At the onset of the study, the participating hospital sites had not enacted standard care procedures for patients who misuse opioids, and following advice from the study’s Data Safety and Monitoring Board, research assistants provided both naloxone and a list of community treatment options to control patients without providing harm reduction coaching.
When someone presented to the ED for a possible opioid-related reason, research staff were alerted through the ED tracking board that they monitored using the EHR. Staff would arrive at the ED and, before approaching the patient, confirm with nursing staff a) the reason for the ED presentation and b) that the patient had been medically cleared for discharge. Patients were approached by a research assistant during control shifts and a PRSS during POINT shifts. Research assistants and PRSSs met patients at their bedside, screened them for eligibility, and completed the consent process. When medical discharge clearance had yet to occur, research staff and PRSSs communicated with the nursing staff to monitor patient progress and entered the room after clearance was received. Research staff and PRSSs could not engage patients who left the ED before a formal discharge was processed or were formally discharged before POINT staff received clearance from medical staff.
After providing consent, all patients participated in a 30–60 minute structured baseline interview to provide: a) demographics, b) social support, c) drug use history, d) drug use treatment history, e) physical and mental health history, f) adverse childhood experiences, g) interest in recovery services, and h) drug use risk-reduction behaviors. Readiness for treatment was assessed using two items from the Circumstances, Motivation, Readiness, and Suitability Scales (CMRS)33 and two original items, all rated on a five-point Likert scale (“strongly disagree” to “strongly agree”). The four items were: 1) It is more important to me than anything else that I stop using drugs (CMRS); 2) I am confident that I can start treatment if I want to (original); 3) If I went to treatment, it would work for me (original); and 4) I’m willing to enter treatment as soon as possible (CMRS). Participants received a $30 incentive.
The enrollment process included participant consent to obtain administrative data from the previously discussed hospital and state-level systems. Data-sharing agreements were established between researchers and all relevant entities. Original baseline data with identifiers were sent to an independent data broker for deterministic linkage with medical record data using patient name, date of birth, sex, race, and partial social security number. The broker then sent an encrypted file to a state data management agency using the same identifiers to link all other administrative data. Finally, the state agency de-identified the data before granting research team access.
Modifications from the original study protocol
The pragmatic nature of this study required researchers to modify the original protocol due to challenges beyond their control. The original target recruitment was 712 participants to detect a minimum 6% reduction in subsequent overdose at 80% power. This assumed a 12% rate of subsequent overdose for the control arm at the 5% significance level, a conservative estimate based on observed overdose rates obtained from Indianapolis’s emergency medical services. However, the final sample size was a third of this number. Enrollment was impacted by a) prevention of study expansion to a third recruitment site that implemented a different program, b) patients leaving or being discharged prior to contact by research personnel, and c) patient refusals. An alternate enrollment plan was implemented just as the COVID-19 pandemic began. However, data collection was stopped early at the study’s largest-volume site due to procedures limiting non-essential personnels’ ED access.
Finally, the researchers originally planned to assess justice and child welfare-related outcomes. However, the number of study participants identified within relevant databases was inadequate for the planned statistical analyses and these data are reported on ClinicalTrials.gov.
Analytic plan
Descriptive statistics were calculated for all participants using means and standard deviations or medians and interquartile range for continuous variables, and proportions for categorical variables. Differences in demographics, baseline behaviors (12 months before index ED presentation), and primary and secondary outcomes of interest were compared between study arms. Specifically, Chi-square tests compared binary measures, t-tests compared continuous variables, and length of MOUD treatment was compared using the Wilcoxon rank-sum test.
Regardless of consent to participate, all patients discharged during POINT-designated shifts were eligible to receive PRSS services, so researchers conducted a secondary intention-to-treat analysis using a dataset of all POINT-eligible patients treated in the two EDs during the study window (IRB exemption #2006108993). These patients were identified in the medical record using ICD-10 codes for opioid-related disorders. This secondary analysis compared ED patients identified by an algorithm (N=628) discharged during a POINT (n=328) versus a control shift (n=300). Due to data availability and time restrictions, outcomes of focus for this analysis were limited to a) overdose-related ED presentation, b) MOUD linkage, c) all-cause ED re-presentation, and d) MOUD treatment engagement duration. The analysis adjusted for gender, as initial arm comparisons demonstrated significant differences between men and women. Finally, a post hoc t-test assessing motivation and readiness items was performed to compare participants by presenting condition (overdose vs. other reason). The decision to conduct this test was based on prior research suggesting baseline motivation might influence client outcomes for intervention similar to POINT.9 All analyses were performed using SAS 9.4.
RESULTS
The consort diagram displaying the research participants’ flow through the primary study procedures is shown in Figure 1. Descriptive sample characteristics for the 243 participants in the primary analysis are displayed by arm in Table 1. Participants in the two conditions did not significantly differ on any demographic variables examined, except for presenting issue (p=.04), with considerably more overdose presentations in the intervention arm.
Figure 1.
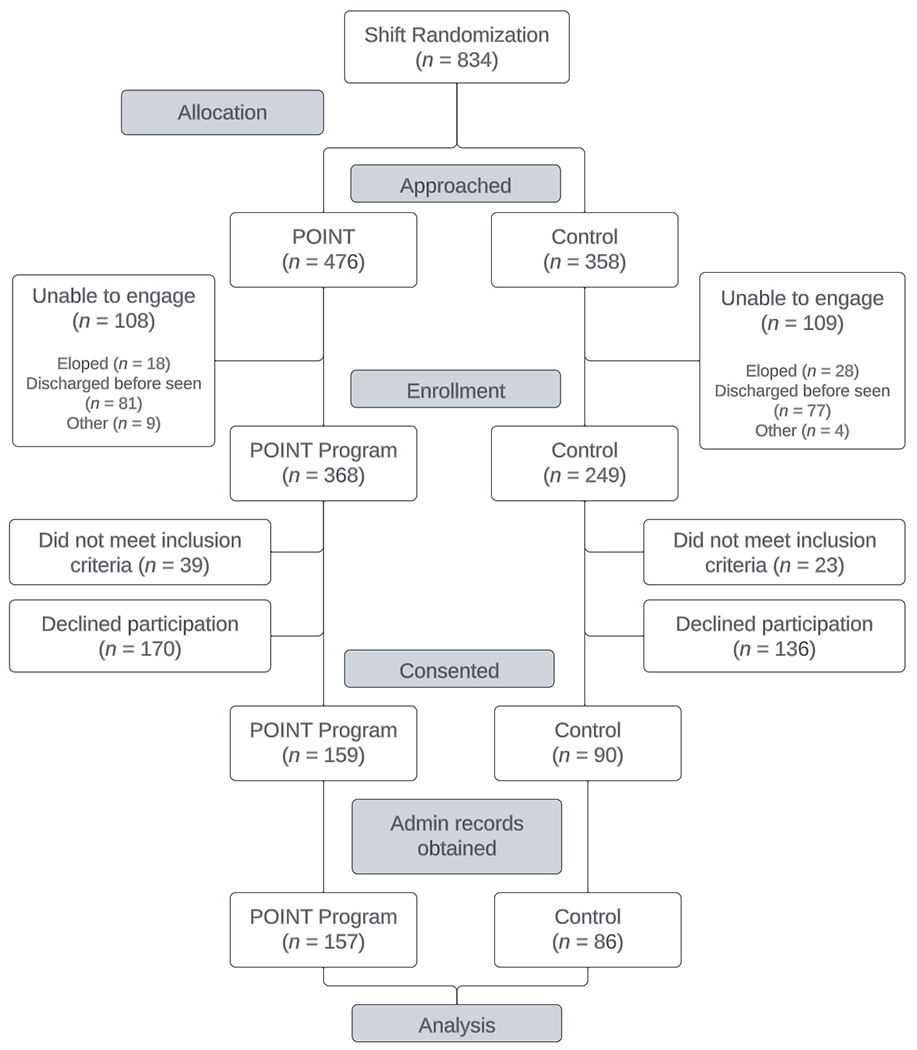
POINT trial CONSORT diagram.
Note: The allocation phase of this study preceded the enrollment phase due to the use of a cluster-randomized design.
Table 1.
Baseline sample characteristics (N = 243)
| Intervention (n = 157) |
Control (n = 86) |
|||
|---|---|---|---|---|
| n(%) or M(SD) | n(%) or M(SD) | χ2 or t | p | |
| Age | 35.0 (10.1) | 37.2 (11.7) | −1.50 | 0.13 |
| Gender | 1.48 | 0.83 | ||
| Female | 68 (43.3) | 32 (37.2) | ||
| Male | 88 (56.1) | 54 (62.8) | ||
| Other | 1 (0.6) | 0 (0.0) | ||
| Race | 0.59 | 0.48 | ||
| African American | 17 (10.8) | 11 (12.8) | ||
| White | 139 (88.5) | 75 (87.2) | ||
| Did not answer | 1 (0.6) | 0 (0.0) | ||
| Insurance | 7.32 | 0.24 | ||
| Health Indiana Plan | 91 (58.3) | 45 (52.3) | ||
| Medicaid | 17 (10.9) | 8 (9.3) | ||
| Medicare | 7 (4.5) | 6 (7.0) | ||
| None | 30 (19.2) | 20 (23.3) | ||
| Unknown | 1 (0.6) | 0 (0.0) | ||
| Other | 1 (0.6) | 4 (4.7) | ||
| Private (exchange, employer based) | 10 (6.4) | 3 (3.5) | ||
| Presenting issue | 6.58 | 0.04 | ||
| Overdose | 76 (48.4) | 56 (65.1) | ||
| Intoxication or withdrawal | 46 (29.3) | 19 (22.1) | ||
| Other | 35 (22.3) | 11 (12.8) | ||
| Preferred rout of administration | 2.20 | 0.14 | ||
| Injection | 85 (54.1) | 38 (44.2) | ||
| Other | 72 (45.9) | 48 (55.8) | ||
| Have you ever been enrolled in treatment where you received… | ||||
| Methadone | 42 (46.8) | 22 (25.6) | 0.03 | 0.84 |
| Buprenorphine (Suboxone®) | 60 (38.2) | 31 (36.0) | 0.11 | 0.73 |
| Extended-release injectable naltrexone (Vivitrol®) | 25 (15.9) | 10 (11.6) | 0.83 | 0.36 |
| Age at first opioid use | 18.4 (6.7) | 20.1 (9.4) | −1.48 | 0.18 |
| Treatment motivation and readiness | ||||
| Confident can start treatment if want | 4.2 (0.96) | 4.3 (0.92) | −1.12 | 0.27 |
| Important to stop using drugs | 4.3 (1.03) | 4.4 (1.09) | −0.42 | 0.68 |
| Treatment would work | 3.8 (0.99) | 3.9 (0.98) | −1.09 | 0.28 |
| Willing to enter treatment soon | 3.9 (1.21) | 3.9 (1.27) | 0.11 | 0.91 |
Note: Descriptive statistics and non-parametric comparison of gender, race, and insurance status by group.
Table 2 displays results from administrative data describing participants’ characteristics during the 12 months before the index ED presentation. Participants did not differ significantly on any of these characteristics.
Table 2:
Participant characteristics during 12 months before index emergency department presentation (N = 243)
| Intervention (n = 157) |
Control (n = 86) |
|||
|---|---|---|---|---|
| N (%) | N (%) | Test statistics | p-value | |
|
| ||||
| Overdose-related ED presentation | 18 (11.5%) | 9 (10.5%) | 0.06 | 0.81 |
| All-cause ED presentation | 99 (63.1%) | 52 (60.5%) | 0.16 | 0.69 |
| All-cause inpatient hospital admission | 44 (28.0%) | 23 (26.7%) | 0.05 | 0.83 |
| MOUD linkage | 48 (30.6%) | 17 (19.8%) | 3.31 | 0.07 |
| Median (IQR) | Median (IQR)) | |||
| Duration of MOUD engagement (days)* | 41.5 (17.0,184.5) | 44.0 (26.0, 139.0) | −0.18 | 0.69 |
Note: Test statistics and p-values are based on Pearson chi-square test for binary variables and Wilcoxon rank-sum test for continuous variables.
For participants with MOUD engagement only.
Table 3 displays outcomes by group at one month, three months, and twelve months post-ED. No significant differences were observed between groups for any outcomes examined. Analysis of the 634 identified patients included in the secondary analysis yielded similar results to the primary analysis with no significantly different outcomes between arms (see online Appendix for results table).
Table 3.
Participant 1-, 3-, and 12-month outcomes (N = 243)
| 1 month after index emergency department encounter | |||||
|---|---|---|---|---|---|
|
| |||||
| Intervention (n = 157) | Control (n = 86) | Test statistic | p-value | Odds Ratio (OR)(95% CI) | |
|
| |||||
| n (%) | n (%) | ||||
|
| |||||
| Overdose related ED re-presentation | 3 (1.9%) | 3 (3.5%) | 0.57 | 0.45 | 0.54 (0.11, 2.73) |
| MOUD linkage | 36 (22.9%) | 14 (16.3%) | 1.50 | 0.22 | 1.53 (0.77, 3.03) |
| All-cause ED re-presentation | 34 (21.7%) | 18 (20.9%) | 0.02 | 0.90 | 1.04 (0.55, 1.99) |
| All-cause inpatient admission | 48 (30.6%) | 17 (19.8%) | 3.31 | 0.07 | 1.79 (0.95, 3.36) |
| Medicaid enrollment (among baseline uninsured) | --- | --- | --- | --- | 0.54 (0.11, 2.73) |
| Overdose mortality | 0 | 0 | --- | --- | --- |
| All-cause mortality | 0 | 0 | --- | --- | --- |
| Median (IQR) | Median (IQR) | ||||
| Duration of MOUD engagement (days)* | 17.7 (6.7, 24.9) | 15.9 (8.0, 30.0) | −0.51 | 0.76 | 0.74 (0.25, 2.18) |
|
| |||||
| 3 months after index emergency department encounter | |||||
|
| |||||
| Intervention (n = 157) | Control (n = 86) | Test statistic | p-value | Odds Ratio (OR)(95% CI) | |
|
| |||||
| n (%) | n (%) | ||||
|
| |||||
| Overdose related ED re-presentation | 11 (7.0%) | 6 (7.0%) | 0.00 | 0.99 | 1.01 (0.32, 1.65) |
| MOUD linkage | 49 (31.2%) | 26 (30.2%) | 0.02 | 0.87 | 1.05 (0.59, 0.85) |
| All-cause ED re-presentation | 63 (40.1%) | 30 (34.9%) | 0.64 | 0.42 | 1.25 (0.72, 2.16) |
| All-cause inpatient admission | 52 (33.1%) | 20 (23.3%) | 2.59 | 0.11 | 1.63 (0.90, 2.98) |
| Medicaid enrollment (among baseline uninsured) | 10 (33.3%) | 7 (35.0%) | 0.01 | 0.90 | 0.85 (0.47, 1.56) |
| Overdose mortality | 0 | 0 | --- | --- | --- |
| All-cause mortality | 3 (1.9%) | 1 (1.2%) | 0.38 | 1.00 | 0.60 (0.06, 5.90) |
| Median (IQR) | Median (IQR) | ||||
| Duration of MOUD engagement (days)* | 39.0 (21.0, 64.3) | 30.3 (8.0, 63.3) | −1.14 | 0.25 | 1.69 (0.73, 3.88) |
|
| |||||
| 12 months after index emergency department encounter | |||||
|
| |||||
| Intervention (n = 157) | Control (n = 86) | Test statistic | p-value | Odds Ratio (OR)(95% CI) | |
|
| |||||
| n (%) | n (%) | ||||
|
| |||||
| Overdose related ED re-presentation | 26 (16.6%) | 15 (17.4%) | 0.03 | 0.86 | 0.94 (0.47, 1.89) |
| MOUD linkage | 77 (49.0%) | 38 (44.2%) | 0.53 | 0.47 | 1.22 (0.72, 2.06) |
| All-cause ED re-presentation | 102 (65.0%) | 56 (65.1%) | 0.00 | 0.98 | 0.99 (0.57, 1.72) |
| All-cause inpatient admission | 71 (45.2%) | 36 (41.9%) | 0.25 | 0.61 | 1.15 (0.67, 1.95) |
| Medicaid enrollment (among baseline uninsured) | 16 (53.3%) | 15 (75.0%) | 2.39 | 0.12 | 1.79 (0.62, 2.26) |
| Overdose mortality | 3 (1.9%) | 6 (7.0%) | 0.04 | 0.07 | 3.85 (0.94, 15.80) |
| All-cause mortality | 7 (4.5%) | 9 (10.5%) | 0.04 | 0.10 | 2.50 (0.90, 7.00) |
| Median (IQR) | Median (IQR) | ||||
| Duration of MOUD engagement (days)* | 84.0 (30.0, 168.0) | 53.5 (22.0, 207.3) | −0.18 | 0.86 | 1.07 (0.54, 2.09) |
Note: Forty-one patients died in the post-intervention observation window (POINT = 25 [15.92%] and Control = 16 [18.60%]): patients were excluded from 1-, 3-, or 12-month analysis if they died within the observation window. Test statistics and p-values are based on Pearson chi-square test for binary variables and Wilcoxon rank-sum test for continuous variables.
For participants with MOUD engagement only.
Lastly, Table 4 presents results of the post hoc analysis conducted to identify whether motivation and readiness to treatment varied according to patients’ presenting concerns (e.g., overdose or other opioid-related issue—e.g., intoxication, withdrawal, abscess, endocarditis, etc.). While no differences were observed related to motivation, participants who presented due to an overdose had significantly lower treatment readiness scores compared to the other groups (3.7 vs. 4.2, p=0.005).
Table 4.
Motivation and readiness by presenting problem (N = 243)
| Presented due to an opioid overdose (n = 132) | Presented due to other opioid-related issue (n = 111) | |||
|---|---|---|---|---|
|
| ||||
| M (SD) | M (SD) | t-statistic | p-value | |
| Confident can start treatment if want | 4.2 (0.96) | 4.3 (0.93) | −0.58 | 0.561 |
| Important to stop using drugs | 4.3 (1.08) | 4.4 (1.02) | −0.61 | 0.536 |
| Treatment would work | 3.8 (1.02) | 3.9 (0.95) | −0.60 | 0.547 |
| Willing to enter treatment soon | 3.7 (1.32) | 4.2 (1.06) | −2.81 | 0.005 |
| Total | 16.0 (3.38) | 16.7 (3.03) | −1.55 | 0.123 |
DISCUSSION
While underpowered, trial results suggest no benefits of the POINT intervention above those observed in the control arm. These results contribute to the growing body of evidence related to the effectiveness of ED-based PRSS interventions for OUD, with most prior research of similar interventions focusing on relatively short-term service-related outcomes (e.g., naloxone dispensation, time to MOUD initiation), being retrospective or cross-sectional, or lacking a control group.21,23,34 The findings of this trial are similar to that of another randomized study conducted by Beaudoin et al.9 that also did not support an effect of PRSSs on post-ED treatment engagement. However, this prior work focused on short-term MOUD linkage, making the POINT study the first randomized trial of such an intervention to consider longer-term overdose outcomes. Though, given recruitment difficulties encountered, more fully powered trials assessing longer-term outcomes for PRSS-ED interventions are called for.
Participants in the current study had high overdose-related ED re-presentation rates compared to prior studies using national data35,36—7% vs. 3% for 90-day and 17% vs. 9%% for 12 months—which may reflect Indiana’s position as one of the primary contributors to the overdose epidemic since at least 2015.37 Given that ED performance indicators and resulting reimbursement are driven heavily by patient re-presentations, adoption and sustainability of PRSS and other ED-based OUD interventions will remain low unless the ability to improve these rates is demonstrated. This reinforces the need for such programs to focus on linkage to MOUD versus other treatment options, as it is the only treatment pathway that is demonstrated to prevent overdose re-presentation.38 While not significant, POINT arm participants did have 5% greater MOUD linkage, and a prior quasi-experimental evaluation of POINT in its original setting did demonstrate a significantly higher rate of post-intervention MOUD initiation.10 One key difference between the interventions implemented in these two POINT studies is that the ED setting for the latter had external provider agreements to accelerate methadone and buprenorphine treatment intake. Similar arrangements were not able to be established for the current study. While PRSSs were encouraged to explore external linkage, they informed researchers that they were making more referrals to an intensive outpatient treatment program within their hospital system that was just beginning to prescribe buprenorphine and had X-waiver limitations regarding how many patients to whom its providers could prescribe. As detailed in another publication exploring POINT implementation issues,39 PRSSs faced greater MOUD linkage challenges due to this limited treatment supply. This emphasizes that a community’s MOUD treatment infrastructure is a contextual factor that must be considered when planning, implementing, and evaluating treatment linkage interventions.
While Beaudoin et al.’s9 study and the current one both demonstrate no effect of PRSS-delivered services, deeper consideration of the control arms provides insights worth noting. Beaudoin et al.’s control intervention was delivered by licensed social workers for whom treatment linkage did not differ compared to PRSS. Prior to the POINT trial, the two participating EDs were not providing specific services for patients with OUD, as physicians had neither the resources nor time to deliver them and social workers were often unavailable. Therefore, it is possible that the minimal level of intervention POINT research assistants provided—list of community resources and naloxone—to the control arm had some effect. This complicates the ability to decipher whether a difference would have been observed if the hospital sites had more established ED practices for OUD presentations. Results from these studies suggest some level of intervention is likely beneficial for ED patients with OUD and prior work assessing implementation of general substance misuse screening and referral services suggest potentially overburdened ED staff could benefit from support of interventionists with expertise in this area.40,41 Indeed, PRSSs’ unique scope of practice can fill gaps by assisting patients to develop harm reduction knowledge/sills and connect with local recovery communities.12,15
The rationale supporting POINT and many other similar interventions9,16,42,43 is that patients are more receptive to treatment linkage in the period immediately following an overdose. However, the immediate post-overdose period might not be a time when patients are as receptive to treatment linkage as some have suggested, and this is supported by the current study’s ad hoc analysis of motivation as related to patient’s reasons for ED presentation. While all patients had similar views of treatment importance and effectiveness as well as the ability to engage with treatment, overdose survivors were less likely to endorse immediately starting treatment. Compatible findings for overdose survivors have been reported in prior observational and qualitative studies.44,45 This apparent lack of connection between motivational factors and willingness for overdose survivors may be the result of additional factors affecting behavior change which were not adequately addressed by POINT. For instance, the unpleasant physiological and psychological effects of overdose and naloxone revival46 may result in more difficulties in listening, focusing, and considering service opportunities than experienced by ED patients with OUD who present for other reasons. It is possible these motivational differences might have affected the outcomes observed considering control patients’ significantly higher rate of overdose at index ED presentation. Moreover, individuals with OUD presenting to EDs are a heterogeneous group in terms of patterns of use and routes of administration47 and these differences likely translate to differences in intervention efficacy.48 Therefore, it is possible that adjusting PRSS approaches to better engage overdose patients (e.g., enhancing harm reduction services, longer-term and more intensive follow-up) could yield better results,45,47 as could broadening PRSS engagement to include all patients with OUD, not just those post-overdose. Future studies should also investigate the effectiveness of PRSS interventions in serving patients who use substances other than opioids.
Integrating robust PRSS services within evidence-based ED buprenorphine induction programs has the potential to better serve overdose survivors. Immediate buprenorphine induction is demonstrated to improve outcomes for patients presenting with OUD.49,50 However, and likely related to lower motivation, overdose survivors’ uptake of buprenorphine induction services or prescription is less frequent than those presenting for withdrawal or other concerns.51 PRSSs are well suited to provide such services,23,34 as their scope of work includes supporting a patient’s choices while helping mitigate substance use risks through harm reduction education and service linkage.13 Future research comparing the effect of PRSS-enhanced buprenorphine induction to standard care buprenorphine induction on outcomes for overdose survivors other than immediate treatment linkage would benefit the field.
Limitations
The most considerable limitation of this study is that it was underpowered in relation to the primary outcome. Additionally, the generalizability of the results is limited by the study’s focus on two hospitals within a single state. The study’s pragmatic trial design enhances external validity;26 however, it also resulted in limitations that likely impacted the results. For instance, multiple changes in ED leadership and PRSS supervisors respectively weakened relationships between ED staff and PRSSs and consistency of clinical supervision that PRSSs received. 39 A lower number of control patients were enrolled due to differences in patients presenting for each arm during the allocation phase, as depicted in Figure 1. This difference could reflect an enhanced ability for PRSSs vs. research assistants to identify eligible patients since they had real-time access to patient medical records as hospital employees. In contrast, research assistants were limited to information provided through alerts viewable on the EHR’s ED tracking board and could not dig more deeply into patient records. This assumption is supported by the discrepancy in patients assigned to arms in the secondary analysis (331 POINT vs. 303 control). This might also account for the significantly higher rate of control patients with overdose as a baseline presenting concern, as overdose was the easiest study eligibility criteria to identify with the more limited information available to research assistants.
Regarding the study’s measures, two of the four questions used to assess treatment motivation and readiness are limited in that they were developed for this study and have not been validated. The unknown level of accuracy related to administrative data utilized presents a limitation; however, this is outweighed by the collection of robust data from multiple state-level systems, as these data were likely more accurate than patient self-report. While the study did not meet the original recruitment goal due to larger contextual issues, results of the intention-to-treat analysis of the larger secondary dataset support the results observed. Other limitations related to POINT’s pragmatic design are described elsewhere.39
Conclusion
This cluster randomized trial found no difference between patients with OUD who received an ED-based PRSS intervention compared to a list of resources provided by research staff. Limitations include being underpowered in relation to the primary outcome and the focus on a limited geographical region. However, results are consistent with prior research and triangulate with the secondary intent-to-treat analysis presented. PRSS may have additional benefits to patients beyond those assessed given their primary function is non-clinical, and more harm reduction- or recovery-oriented measures should be used in future work. Future research should also consider the investigation of PRSS as an integrated component of multi-modal ED-based OUD interventions. From an ethical perspective, EDs should provide specialized assistance to support high-utilizing patients who use opioids or other substances, and PRSSs provide a potential solution for the many EDs without such services to fill this gap.
Supplementary Material
Acknowledgments:
Krista Brucker, MD, is the developer of the POINT intervention. We acknowledge Regenstrief Institute Inc.’s sourcing of data for this project. We would also like to thank Indiana’s Division of Mental Health and Addiction (DMHA), Management Performance Hub (MPH), the Indiana Professional Licensing Agency (IPLA), and the Indiana Department of Health for providing access to data for this study. This work would not have been possible without the support of Brad Bennett and Paula Tyler of Indiana
Primary Funding:
This work was supported by the National Institute on Drug Abuse (R21DA045850; R33DA045850). The content is solely the authors’ responsibility and does not necessarily represent the official views of their institutions or the funder.
Footnotes
Declaration of competing interests: None
Trial registration: NCT03336268
References
- 1.Centers for Disease Control and Prevention. Provisional drug overdose death counts. Published April 10, 2023. Accessed April 16, 2023. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm
- 2.World Health Organization. Opioid overdose. Published August 4, 2021. Accessed July 17, 2023. https://www.who.int/news-room/fact-sheets/detail/opioid-overdose
- 3.Houry DE, Haegerich TM, Vivolo-Kantor A. Opportunities for prevention and intervention of opioid overdose in the emergency department. Ann Emerg Med. 2018;71(6):688–690. doi: 10.1016/j.annemergmed.2018.01.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langabeer JR, Stotts AL, Bobrow BJ, et al. Prevalence and charges of opioid-related visits to U.S. emergency departments. Drug Alcohol Depend. 2021;221:108568. doi: 10.1016/j.drugalcdep.2021.108568 [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Wang Y, Nielsen S, Kuhn L, Lam T. A systematic review of opioid overdose interventions delivered within emergency departments. Drug Alcohol Depend. 2020;213:108009. doi: 10.1016/j.drugalcdep.2020.108009 [DOI] [PubMed] [Google Scholar]
- 6.Busch SH, Fiellin DA, Chawarski MC, et al. Cost-effectiveness of emergency department-initiated treatment for opioid dependence. Addict Abingdon Engl. Published online August 16, 2017. doi: 10.1111/add.13900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opioid dependence: a randomized clinical trial. JAMA. 2015;313(16):1636–1644. doi: 10.1001/jama.2015.3474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawk K, McCormack R, Edelman EJ, et al. Perspectives about emergency department care encounters among adults with opioid use disorder. JAMA Netw Open. 2022;5(1):e2144955. doi: 10.1001/jamanetworkopen.2021.44955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beaudoin FL, Jacka BP, Li Y, et al. Effect of a peer-led behavioral intervention for emergency department patients at high risk of fatal opioid overdose: A randomized clinical trial. JAMA Netw Open. 2022;5(8):e2225582. doi: 10.1001/jamanetworkopen.2022.25582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watson DP, Weathers T, McGuire A, et al. Evaluation of an emergency department-based opioid overdose survivor intervention: Difference-in-difference analysis of electronic health record data to assess key outcomes. Drug Alcohol Depend. 2021;221:108595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jack HE, Oller D, Kelly J, Magidson JF, Wakeman SE. Addressing substance use disorder in primary care: The role, integration, and impact of recovery coaches. Subst Abuse. 2018;39(3):307–314. doi: 10.1080/08897077.2017.1389802 [DOI] [PubMed] [Google Scholar]
- 12.Reif S, Braude L, Lyman DR, et al. Peer recovery support for individuals with substance use disorders: assessing the evidence. Psychiatr Serv Wash DC. 2014;65(7):853–861. doi: 10.1176/appi.ps.201400047 [DOI] [PubMed] [Google Scholar]
- 13.SAMHSA. Peer support workers for those in recovery. Published August 23, 2017. Accessed April 22, 2023. https://www.samhsa.gov/brss-tacs/recovery-support-tools/peers
- 14.Bardwell G, Kerr T, Boyd J, McNeil R. Characterizing peer roles in an overdose crisis: Preferences for peer workers in overdose response programs in emergency shelters. Drug Alcohol Depend. 2018;190:6–8. doi: 10.1016/j.drugalcdep.2018.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faulkner-Gurstein R The social logic of naloxone: Peer administration, harm reduction, and the transformation of social policy. Soc Sci Med. 2017;180:20–27. doi: 10.1016/j.socscimed.2017.03.013 [DOI] [PubMed] [Google Scholar]
- 16.Powell KG, Treitler P, Peterson NA, Borys S, Hallcom D. Promoting opioid overdose prevention and recovery: An exploratory study of an innovative intervention model to address opioid abuse. Int J Drug Policy. 2019;64:21–29. doi: 10.1016/j.drugpo.2018.12.004 [DOI] [PubMed] [Google Scholar]
- 17.Wagner KD, Oman RF, Smith KP, et al. “Another tool for the tool box? I’ll take it!”: Feasibility and acceptability of mobile recovery outreach teams (MROT) for opioid overdose patients in the emergency room. J Subst Abuse Treat. Published online May 1, 2019. doi: 10.1016/j.jsat.2019.04.011 [DOI] [PubMed] [Google Scholar]
- 18.Im DD, Chary A, Condella AL, et al. Emergency department clinicians’ attitudes toward opioid use disorder and emergency department-initiated buprenorphine treatment: A mixed-methods study. West J Emerg Med. 2020;21(2):261–271. doi: 10.5811/westjem.2019.11.44382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowenstein M, Kilaru A, Perrone J, et al. Barriers and facilitators for emergency department initiation of buprenorphine: A physician survey. Am J Emerg Med. 2019;37(9):1787–1790. doi: 10.1016/j.ajem.2019.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watson DP, Staton MD, Gastala N. Identifying unique barriers to implementing rural emergency department-based peer services for opioid use disorder through qualitative comparison with urban sites. Addict Sci Clin Pract. 2022;17(1):41. doi: 10.1186/s13722-022-00324-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watson DP, Phalen P, Medcalf S, Messmer S, McGuire A. Evaluation of post-discharge engagement for emergency department patients with opioid use history who received telehealth recovery coaching services. Subst Abuse Treat Prev Policy. 2023;18(1):9. doi: 10.1186/s13011-023-00523-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramdin C, Guo M, Fabricant S, Santos C, Nelson L. The impact of a peer-navigator program on naloxone distribution and buprenorphine utilization in the emergency department. Subst Use Misuse. 2021;0(0):1–7. doi: 10.1080/10826084.2021.2023187 [DOI] [PubMed] [Google Scholar]
- 23.Samuels EA, Bernstein SL, Marshall BDL, Krieger M, Baird J, Mello MJ. Peer navigation and take-home naloxone for opioid overdose emergency department patients: Preliminary patient outcomes. J Subst Abuse Treat. 2018;94:29–34. doi: 10.1016/j.jsat.2018.07.013 [DOI] [PubMed] [Google Scholar]
- 24.Staton MD, Watson DP, Thorpe D. Implementation of peer recovery coach services for opioid overdose patients in emergency departments in Indiana: findings from an informal learning collaborative of stakeholders. Transl Behav Med. Published online April 17, 2021:ibab031. doi: 10.1093/tbm/ibab031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watson DP, Brucker K, McGuire A, et al. Replication of an emergency department-based recovery coaching intervention and pilot testing of pragmatic trial protocols within the context of Indiana’s Opioid State Targeted Response plan. J Subst Abuse Treat. Published online June 6, 2019. doi: 10.1016/j.jsat.2019.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ford I, Norrie J. Pragmatic Trials. N Engl J Med. 2016;375(5):454–463. doi: 10.1056/NEJMra1510059 [DOI] [PubMed] [Google Scholar]
- 27.Watson DP, Andraka-Christou B, Clarke T, Wiegandt J. Introduction to the special issue on innovative interventions and approaches to expand medication assisted treatment: Seizing research opportunities made available by the opioid STR program. J Subst Abuse Treat. Published online October 23, 2019. doi: 10.1016/j.jsat.2019.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ICAADA. CAPRC I Certification, Certified Peer Addiction Recovery Coach I. Published 2018. Accessed December 31, 2018. https://icaada.org/CAPRC-I.php
- 29.Regenstrief Institute. RDS Data. RDS Data. Accessed June 17, 2023. https://www.regenstrief.org/rds/data/
- 30.Pharmacy Connections and Pharmacy Network | Surescripts. Accessed December 9, 2020. https://surescripts.com/network-alliance/eprescribing-connected-pharmacies/ [Google Scholar]
- 31.Bonell C, Fletcher A, Morton M, Lorenc T, Moore L. Realist randomised controlled trials: A new approach to evaluating complex public health interventions. Soc Sci Med. 2012;75(12):2299–2306. doi: 10.1016/j.socscimed.2012.08.032 [DOI] [PubMed] [Google Scholar]
- 32.Plaisance P, Lurie KG, Vicaut E, et al. A comparison of standard cardiopulmonary resuscitation and active compression–decompression resuscitation for out-of-hospital cardiac arrest. N Engl J Med. 1999;341(8):569–575. doi: 10.1056/NEJM199908193410804 [DOI] [PubMed] [Google Scholar]
- 33.Leon GD, Melnick G, Kressel D, Jainchill N. Circumstances, motivation, readiness, and suitability (the CMRS Scales): Predicting retention in therapeutic community treatment. Am J Drug Alcohol Abuse. 1994;20(4):495–515. doi: 10.3109/00952999409109186 [DOI] [PubMed] [Google Scholar]
- 34.Samuels E Emergency department naloxone distribution: a Rhode Island department of health, recovery community, and emergency department partnership to reduce opioid overdose deaths. R I Med J 2013. 2014;97(10):38–39. [PubMed] [Google Scholar]
- 35.Grzebinski S, Stein L, Dhamoon MS. Characteristics and outcomes of hospitalizations and readmissions for opioid dependence and overdose: Nationally representative data. Subst Abuse. 2021;42(4):654–661. doi: 10.1080/08897077.2020.1823548 [DOI] [PubMed] [Google Scholar]
- 36.Peterson C, Liu Y, Xu L, Nataraj N, Zhang K, Mikosz CA. U.S. National 90-day readmissions after opioid overdose discharge. Am J Prev Med. 2019;56(6):875–881. doi: 10.1016/j.amepre.2018.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. Drug overdose mortality by state. Published March 1, 2022. Accessed July 25, 2023. https://www.cdc.gov/nchs/pressroom/sosmap/drug_poisoning_mortality/drug_poisoning.htm
- 38.Morgan JR, Barocas JA, Murphy SM, et al. Comparison of rates of overdose and hospitalization after initiation of medication for opioid use disorder in the inpatient vs outpatient setting. JAMA Netw Open. 2020;3(12):e2029676. doi: 10.1001/jamanetworkopen.2020.29676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dir AL, Watson DP, Zhiss M, Taylor L, Bray BC, McGuire A. Barriers impacting the POINT pragmatic trial: the unavoidable overlap between research and intervention procedures in “real-world” research. Trials. 2021;22(1):114. doi: 10.1186/s13063-021-05065-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Babor TF, McRee BG, Kassebaum PA, Grimaldi PL, Ahmed K, Bray J. Screening, brief intervention, and referral to treatment (SBIRT): Toward a public health approach to the management of substance abuse. Subst Abuse. 2007;28(3):7–30. doi: 10.1300/J465v28n03_03 [DOI] [PubMed] [Google Scholar]
- 41.Bernstein E, Bernstein JA, Stein JB, Saitz R. SBIRT in emergency care settings: Are we ready to take it to scale? Acad Emerg Med. 2009;16(11):1072–1077. doi: 10.1111/j.1553-2712.2009.00549.x [DOI] [PubMed] [Google Scholar]
- 42.Essien UR, Sileanu FE, Zhao X, et al. Racial/Ethnic differences in the medical treatment of opioid use disorders within the VA healthcare system following non-fatal opioid overdose. J Gen Intern Med. 2020;35(5):1537–1544. doi: 10.1007/s11606-020-05645-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wagner KD, Mittal ML, Harding RW, et al. “It’s Gonna be a lifeline”: Findings from focus group research to investigate what people who use opioids want from peer-based postoverdose interventions in the emergency department. Ann Emerg Med. 2020;76(6):717–727. doi: 10.1016/j.annemergmed.2020.06.003 [DOI] [PubMed] [Google Scholar]
- 44.Coupet E, D’Onofrio G, Chawarski M, et al. Emergency department patients with untreated opioid use disorder: A comparison of those seeking versus not seeking referral to substance use treatment. Drug Alcohol Depend. 2021;219:108428. doi: 10.1016/j.drugalcdep.2020.108428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hawk K, Grau LE, Fiellin DA, et al. A qualitative study of emergency department patients who survived an opioid overdose: Perspectives on treatment and unmet needs. Acad Emerg Med Off J Soc Acad Emerg Med. 2021;28(5):542–552. doi: 10.1111/acem.14197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ataiants J, Mazzella S, Roth AM, Sell RL, Robinson LF, Lankenau SE. Overdose response among trained and untrained women with a history of illicit drug use: a mixed-methods examination. Drugs Abingdon Engl. 2021;28(4):328–339. doi: 10.1080/09687637.2020.1818691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bray BC, Watson DP, Salisbury-Afshar E, Taylor L, McGuire A. Patterns of opioid use behaviors among patients seen in the emergency department: Latent class analysis of baseline data from the POINT pragmatic trial. J Subst Use Addict Treat. 2023;146:208979. doi: 10.1016/j.josat.2023.208979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cushman PA, Liebschutz JM, Anderson BJ, Moreau MR, Stein MD. Buprenorphine initiation and linkage to outpatient buprenorphine do not reduce frequency of injection opiate use following hospitalization. J Subst Abuse Treat. 2016;68:68–73. doi: 10.1016/j.jsat.2016.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hawk K, Hoppe J, Ketcham E, et al. Consensus recommendations on the treatment of opioid use disorder in the emergency department. Ann Emerg Med. Published online June 2021:S0196064421003061. doi: 10.1016/j.annemergmed.2021.04.023 [DOI] [PubMed] [Google Scholar]
- 50.Hawk K, D’Onofrio G. Emergency department screening and interventions for substance use disorders. Addict Sci Clin Pract. 2018;13(1):18. doi: 10.1186/s13722-018-0117-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faude S, Delgado MK, Perrone J, et al. Variability in opioid use disorder clinical presentations and treatment in the emergency department: A mixed-methods study. Am J Emerg Med. Published online January 7, 2023. doi: 10.1016/j.ajem.2023.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


