Abstract
Plant architecture is an important feature for agronomic performance in crops. In maize, which is a monoecious plant, separation of floral organs to produce specific gametes has been studied from different perspectives including genetic, biochemical and physiological. Maize mutants affected in floral organ development have been key to identifying genes, hormones and other factors like miRNAs important for sex determination. In this review, we describe floral organ formation in maize, representative mutants and genes identified with a function in establishing sexual identity either classified as feminizing or masculinizing, and its relationship with hormones associated with sexual organ identity as jasmonic acid, brassinosteroid and gibberellin. Finally, we discuss the challenges and scopes of future research in maize sex determination.
Keywords: Floral organs, Sex identity, Hormones, Feminized, Maize
Introduction
Reproduction is the process by which all the species may preserve, copy and transmit genes; hence, it may be considered one of the most important aspects in the life of most organisms. In plants, the different forms of reproduction have favored an adequate genetic variability to achieve their survival and evolution. Between the different kinds of evolutionary strategies to increase reproduction efficiency is the development of sexual organs (De Craene 2018).
The discovery of reproductive organs in plants is attributed to the German physician and botanist Rudolf Jakob Camerarius, who wrote a letter about sex in plants which was published in 1694 (Žárský and Tupý 1995) starting a scientific revolution to explain how mating in plants works. For reproduction in the plant kingdom different molecular mechanisms have arisen naturally, leading to anatomical diversity. This diversity is intended to promote “outcrossing” which will consequently lead to heterogeneity as well as to generate genetic variability, assuming the adaptability of organisms (Dellaporta and Calderon-Urrea 1993).
There is a broad diversity of modalities for reproduction in plants. For instance, a plant can be bisexual, unisexual, monoecious, or dioecious, among others. Bisexual flowers are the most common in plants; according to Yampolsky and Yampolsky (1922), 75% of the flowering species are bisexual, while having unisexual flowers is not so common in nature; since only around 4% are monoecious, producing flowers considered “incomplete” because they are only male or female but those flowers are both in the same plant; finally, around 7% are dioecious, namely unisexual organisms producing only female or male flowers (Ashton 1969). Sexual determination in plants has evolved independently in many different species throughout history (Chuck 2010), so, it cannot be considered that there is a single gene network that determines sex identity in flowers, but that there are a whole set of factors involved, such as the environment, hormones and genes (Dellaporta and Calderon-Urrea 1994).
The maize flower
Zea mays is considered a monoecious species, since it produces female inflorescences called “ears” and male inflorescences called “tassels”, in separate positions in the same plant (Cheng et al. 1983). Unisexuality in maize is caused by the abortion that occurs selectively in both floral organs, male and female (Fig. 1); in the male flower carpel development is suppressed, while in the female flower anthers are suppressed; in addition, in female inflorescences a half of all florets are aborted (Yang et al. 2021).
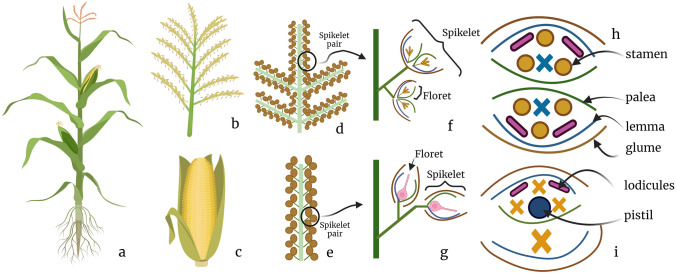
Fig. 1.
Floral organ development in a maize plant. a Adult maize plant in reproductive stage. b Mature tassel. c Mature ear. d, e Male and female spikelet pairs. f, g Male and female florets, respectively; the first ones are double and the second ones are single. h Male flower development with pistil suppression (labeled with blue “x”). i Female flower development with anther suppression (labeled with yellow “x”)
Female floral organs in maize are found on ears produced in the axil of leaves along the stem, distributed in an interleaved way, while male flowers are located at the apex of the plant (Dellaporta and Calderon-Urrea 1994). This growth pattern and characteristic morphology to each one makes each type of flower easily distinguishable. Some maize inbred lines form “tillers” (smaller plants originating from the same main stem) that may follow the mother plant pattern and produce tassel and ears, tillers are dependents on genetics, hormones and photoperiod (Cheng et al. 1983). At genetic level it is well known the function of the transcription factor Tb1 (Teosinte branched 1) negatively regulating outgrowth of tillers by activation of gt1 (grassy tillers 1) and positively regulating hormones like abscisic acid and jasmonic acid, so, tb1 and gt1 mutants have more tillers (Dong et al. 2019; Whipple et al. 2011). However, the molecular basis to form tillers depending of photoperiod has been little explored. Some reports relating tillering with light quality (red/far red ratio) showed that high ratio of far red light perceived by a PHYTOCHROME B (PHYB) activates Tb1 expression, consequently affecting the downstream pathways leading to tiller formation and so increasing apical dominance (Kebrom et al. 2006; Dong et al. 2019).
Despite the fact that there is an important anatomical difference in tassels and ears at the adult stage of development, both male and female floral meristems are very similar in morphology showing initially bisexual flowers, but it is not identical, since it has been reported a difference in meristem growth rate and branching (Cheng et al. 1983; Irish and Nelson 1989). Throughout development at the morphological level, meristems gradually enlarge to shape the inflorescence. Unlike Arabidopsis or other model species where inflorescence meristem (IM) directly produces floral meristems (FM), in grasses IM first produces higher-order meristems in this order: IM forms spikelet pair meristems (SPM), which produce two spikelet meristems (SM), then, SM give rise to lower and upper floral meristems (LFM/PFM) (Yang et al. 2021). In male inflorescence, elongation produces branching to give place the tassel architecture, which does not occur in ears. Both male and female flowers start forming two subtended glumes which protect floral meristems, and then a variety of organs are formed from floral meristems including lemma, palea, stamens and gynoecium (Fig. 1h, i). At the early stage of development, both flowers are considered bisexual; however, as development progresses at the tassel primordia, gynoecium cells become highly vacuolated and end up degenerating, while anthers cells continue cell division until reaching sexual maturity; also in some genetic backgrounds red and purple flavonoids are synthesized and stored at this tissue (Dellaporta and Calderon-Urrea 1994). Reciprocally, at the ears primordia, anther cells arrest and abort, while carpel cells division continues to form mature female organs (Yang et al. 2021). It has been proposed that sex determination by programmed cell death (PCD) in maize occurs at a late stage, probably after expression of homeotic genes that specify floral organ identity (Chuck 2010). In this way, upon reaching reproductive maturity there will be a clear distinction between the male and female flowers.
Maize mutants affected in sexual determination and their relationship with hormones
Over nearly a century, a number of maize mutants have been described, which made it possible to recapitulate the molecular history of the genes that have participated in sex flower determination. These mutants have been identified because they present phenotypes in which there is no carpel or anther abortion. We have divided them in two sections: feminized and masculinized.
Feminized mutants
A considerable number of mutants have been described as feminized, namely, that do not present carpel abortion in tassels. Broadly studied feminized mutants are the ones affected in genes related to Jasmonic acid (JA) pathway. Representative examples are tasselseed (ts) mutants, which have been extensively studied for the last 3 decades, the classic phenotype in ts mutants is a transformation of male to female flowers. By using double ts mutants, they could be classified in two classes: I and II, in function of the observed genetic interactions (Irish et al. 1994). Class I mutants show a feminized phenotype, while class II shows a more complex phenotype, where in addition to feminization they have an irregular branching pattern in inflorescences. As a first approach to describe genetic interactions, it was proposed that mutations were acting epistatically; however, then double-mutant phenotypes suggested a synergy between both classes (Irish 1997). These phenotypes are shown as sterile and highly branched inflorescences, so, in addition to carpel suppression in tassels, ts genes may have a role either directly or indirectly in inflorescence organogenesis and branching regulation (Irish 1997).
Class I
This class includes the recessive ts1 and ts2 mutants, and the dominant Ts3 and Ts5 mutants, which do not suppress carpels in the tassels; as a consequence, they show an abnormal feminized phenotype, in some genetic backgrounds being able to form fertile bisexual flowers that self-pollinate producing seeds in tassels (Emerson 1920; Nickerson and Dale 1955; Irish et al. 1994; Lunde et al. 2019).
The ts1 gene encodes a lipoxygenase called ZmLOX8, which functions directly in the jasmonic acid (JA) biosynthesis pathway (Acosta et al. 2009). It was suggested that this enzyme is involved in JA biosynthesis, based on observations by Acosta et al. about the drastic low level of endogenous JA in ts1 mutants. Accordingly, when JA is exogenously applied to ts1 tassels, the wild-type phenotype is recovered. Based on those experiments, a relationship between JA and male flower development in maize was proposed. ts2 encodes an alcohol dehydrogenase enzyme, which seems to be involved in metabolism of steroidal molecules or in metabolism of GA-like compounds (DeLong et al. 1993). To test the ability of the TS2 protein to bind hormones, Wu et al. (2007) explored the binding between TS2 and JA or GA; however, no binding was detected. Apparently dwarf mutants (affected in GA biosynthesis) and Class I tasselseed mutants function in separate pathways, but it is still questionable how the GA exogenous application rescues the ts2 mutant (Wu et al. 2007).
Other Class I ts mutants include some dominants like Ts3 and Ts5, which show similar phenotypes to ts1 and ts2, but not so severe (Nickerson and Dale 1955; Neuffer et al. 1997). The Ts5 mutant is considered a loss-of-function dominant mutant affected in the JA biosynthetic pathway, Ts5 overexpresses the gene CYP94B1 which is wound inducible and inactivates the JA precursor jasmonoyl-L-isoleucine (JA-Ile), so Ts5 tassels show lower JA and JA-Ile than tassels in wild-type plants. Based on those observations it was proposed that the Ts5 phenotype results from JA signaling pathway disruption, and that this hormonal imbalance affects maize monoecious development (Lunde et al. 2019). Finally, the dominant Ts3 mutant is waiting for a better characterization at the phenotypic and molecular level; its feminized phenotype was described by Nickerson and Dale (1955); however, detailed analyses are still needed.
Class II
In this class, the ts4 and Ts6 mutants are included; mutants in this class besides being feminized show an atypical branching pattern both in ears and tassels. This kind of mutations affects the fate of reproductive meristems; ts4 mutants show an indeterminate spikelet meristem, pistils are not suppressed in tassels, and male flowers do not develop, resulting in complete feminization (Chuck et al. 2007). Ts6 mutants are delayed in the conversion of reproductive meristems; in addition, the atypical branching is suppressed at the base of tassels allowing forming normal spikelets (Irish 1997). On the other hand, the dominant mutant Ts6 shows indeterminate floral meristem (Irish 1997), and consistently with this phenotype it mis-expresses the Knotted1 homeobox gene (Jackson et al. 1994), indicating that there is a delay in meristem differentiation. The Ts6 mutation site affects the ts4 binding site, and ts4 encodes a microRNA (miR172), subsequently affecting transcription factors binding APETALA2, necessary for floral meristem determination (Chuck et al. 2007; Banks 2008). Since Ts6 is a dominant mutant, it has been hypothesized that Ts6 functions mis-expressing a factor which causes prolonged meristem activity (Chuck 2010).
On the other hand, the nana plant 1 (na1) and the required to maintain repression 6 (rmr6) mutants have a phenotype similar to the tasselseed group, with carpel growth in male flowers (Parkinson et al. 2007; Hartwig et al. 2011; Li and Liu 2016). na1 has a very low endogenous brassinosteroid (BR) level; analysis of na1 mutant identified a loss-of-function mutation in a DET2 homolog gene, which functions in BR biosynthesis; in consequence, na1 shows an accumulation of the DET2 substrate (24R)-24-methyl- cholest-4-en-3-one and a decrease in BR metabolites. Accordingly, exogenous application of BR inhibitors in wild-type maize plants produces the phenotype of na1 (Hartwig et al. 2011); these experiments showed the relationship of NA1 with BR biosynthesis. The rmr6 mutant possesses a tassel with female characters, forming bisexual fertile flowers and seed production, unlike the tasselseed and na1 mutants (Hollick et al. 2005; Parkinson et al. 2007). rmr6 is also affected in internode elongation with reduced apical internodes, genetic analysis and association with factors involved in transition from vegetative to reproductive stage of development has related rmr6 with GA and Auxin pathways (Li et al. 2023). However, additional analyses are required to decipher the precise molecular function of Rmr6.
Another recently reported feminized maize mutant is gt1;ra3 (Klein et al. 2022); this double mutant was originated from a gt1 (grassy tillers 1) mutant enhancer screening to identify genes that together with the gt1 transcription factor are involved in carpel suppression in tassels; from this screen, ra3 (ramosa 3) was identified. gt1;ra3 is affected in carpel suppression due to a mutation in the transcription factor GRASSY TILLERS1 (GT1) and in the trehalose-6-phosphate phosphatase (TPP) gene encoding the RAMOSA 3 protein, producing carpel growth in tassels and therefore feminized male flowers (Klein et al. 2022). gt1 mutant itself has a weak carpel suppression phenotype and it was identified by tiller formation and its genetic relationship with Tb1 (Whipple et al. 2011; Dong et al. 2019). In addition, the function of GT1 has been related to light; it was reported to be induced by shade conditions, controlling pistil growth inhibition by acting downstream of the PCD signal from TS2 (Whipple et al. 2011; Bartlett et al. 2015). Transcriptomic analysis of gt1;ra3 in feminized tassel development showed 73 genes with function related to carpel suppression, for example, PCD, ROS, proteolysis, sugar metabolism and ABA and GA response related genes (Klein et al. 2022). All together these findings suggest a key role of hormones and light in floral organ identity; however, in maize there is still a long way to go to understand the relationship between light quality, hormonal balance and sex determination.
Masculinized mutants
In addition to JA and BR, GA (Gibberellin) is another plant hormone that has been related to sex determination in maize, the clearest example is the exogenous application of GA in wild-type tassels causing lack of carpel suppression in male flowers (Nickerson 1959). A number of reports have shown a direct relationship of high GA level with feminization and low GA level with masculinization. There is a group of mutants affected in genes encoding enzymes of the gibberellin biosynthesis pathway; they are the dwarf (d) d1, d2, d3, d5 recessive mutants and the Dwarf (D) D8, D9 dominant mutants (Winkler and Freeling 1994; Fujioka et al. 1988). These mutants have been classified as GA-sensitive and GA-insensitive, as the name suggests, GA-sensitive mutants are able to recover the wild-type phenotype by exogenous application of GA, but GA-insensitive mutants do not (Phinney 1984). Some of these mutants have a low level of GA due to defects in different steps of the synthesis, for instance, d3 which encodes the GA 3-oxidase enzyme that catalyzes the final step of the GA synthesis (Winkler and Helentjaris 1995; Chen et al. 2014).
On the other hand, D8 and D9 mutants, categorized as GA-insensitive, are mutants affected in genes encoding DELLA (aspartic acid–glutamic acid–leucine–leucine–alanine) domain transcription factors (Peng et al. 1999; Lawit et al. 2010), which act as master repressors of the GA signaling pathway. The D8 mutant does not respond to GA and has been proposed that the dwarf plant 8 (d8) may be a receptor of GA (Fujioka et al. 1988). Peng et al. (1999) showed that D8 has a deletion that removes four amino acids from the DELLA domain, suggesting that this is the reason for its insensitivity to GA.
Unlike the D8 mutant, it took at least a decade before D9 began to be properly characterized. The first report of the mutant was by Winkler and Freeling (1994), who confirmed that it is a paralog of the affected gene in the D8 mutant, although both genes are located in different chromosomes, in D9 a DELLA protein is also affected, with point substitutions in a pair of amino acids. Dwarfism also occurs in D9 mutant, and accordingly to proposed function, late flowering is observed when this gene is overexpressed (Lawit et al. 2010).
Inflorescence phenotype in Dwarf mutants either recessives (d) or dominants (D) is related to reduction of GA level, and it shows different degrees of a masculinizing phenotype in ears with anther development in ear florets, which produces upper female ear florets with both anthers and pistils, showing bisexual flowers and lower ear florets with androecium identity (Best and Dilkes 2022). The phenotype in tassel anthers is mostly like in wild-type plants, with normal anther development but in some cases with compact tassels (Best et al. 2016).
Another masculinized mutant is the Anther-ear 1 (An1), which was first described by Emerson and Emerson (1922); it has an affected phenotype in plant height although not as severe as the GA-insensitive mutants; in addition, it presents delayed sexual maturity and development of bisexual flowers in the ears location, with anther development in the proximal and distal female spikelet florets (Bensen et al. 1995). This mutant is sensitive to GA, since the affected gene is involved in the synthesis of ent-kaurene, which is the first intermediate in the GA biosynthetic pathway. Like other previously described mutants, this one shows a positive and regenerative response to monoecious flowering with exogenous application of GA (Bensen et al. 1995). Overall, it can be assumed that GA biosynthesis and signaling are necessary for the proper formation of both male as female flowers in maize.
The last one mutant affected in floral organ development described here is the recessive silkless1 (sk1); it has normal tassel development, but completely sterile ear flowers by the absence of pistils and some anther persistence in ear florets (Hayward et al. 2016; Best and Dilkes 2023). The affected gene in sk1 encodes a UDP-glycosyltransferase that regulates endogenous JA level by inactivation of JA via conjugation; thus, sk1 mutant shows high level of JA (Hayward et al. 2016). According to this function, when the sk1 gene was constitutively expressed in transgenic plants, all pistils in female flowers were completely developed and even produced complete feminization in tassels demonstrating that sk1 gain of function blocks the accumulation of jasmonates and sk1 lack of function produces accumulation of JA in maize florets (Hayward et al. 2016). In addition, the androecious flower phenotype in sk1 is enhanced in the double mutant d5 sk1, showing an interaction of GA and JA pathways to establish floral organ identity in maize (Best and Dilkes 2023).
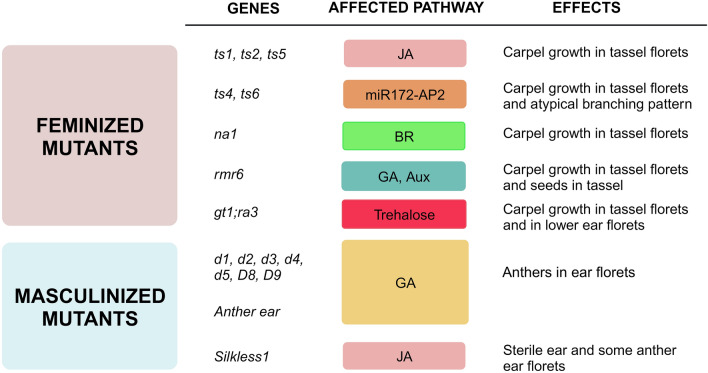
In summary, in maize there are multiple pathways relating genes and hormones converging in different developmental stages to determine floral identity and related phenotypes to this process; Fig. 2 shows representative mutants, genes and pathways where they are working as well as effects in phenotype when their function is disrupted.
Fig. 2.
Summary of feminized and masculinized mutants, affected genes, related pathways and effects on phenotype
Challenges and scopes of research in maize sex determination
Advances in genomics and transcriptomics either using organ tissues or single-cell ‘omics’ are giving light to understand biological processes under studied before the development of high resolution technologies in maize; thanks to such technologies, it is possible to have transcriptome atlas of the maize inflorescences through development (Satterlee et al. 2020; Xu et al. 2021). In addition, the development of new gene-editing technologies (Liu et al. 2021) is increasing exponentially the amount of allelic variants either in regulatory or in coding sequences for study of genetic determinants important in maize sex identity. On the other hand, metabolomic and proteomic approaches are still a challenge in grasses, since the amount of secondary metabolites in different tissues frequently interfere with getting reproducible results based on analyte quantification (Walley et al. 2016). However, with the development of technologies increasingly sensitive these tools will be as used as in model dicot species. With the increasing data from single-cell RNAseq (Satterlee et al. 2020; Xu et al. 2021) and interactomics (Wu et al. 2019; Abraham-Juárez et al. 2020; Han et al. 2023), it will be possible to correlate gene expression, co-expression pathways and interacting networks with specific biological functions such as feminizing or masculinizing gene functions in inflorescences and their relationship with hormonal pathways. Maize is a species of particular interest to understand and manipulate outcrossing on a large scale in the field, during production of hybrid lines in agriculture; also it is an excellent model to study gene evolution in grasses, so, understanding how organ identity is established to form floral structures is of huge interest in this species.
Conclusion and perspectives
Maize is an important model species to study outcrossing mechanisms in grasses. Thanks to the knowledge generated regarding developmental biology and reproduction in maize, identification and use of natural and induced mutants have been possible. In turn, characterization of developmental mutants has been key to identify genes and pathways with functions in sex determination. Very importantly, the growing genomic, transcriptomic, metabolomic and recently proteomic information in maize together with development of new gene-editing technologies is giving light in the understanding of molecular processes involved in sexual organ identity establishment. However, given the large number of factors involved in sex determination not only in maize, but in different species, elucidation of molecular mechanisms in this process remains as a challenge in plant biology. Environmental factors and their relationship with the genetic ones are among the least studied, and due to climate change, these are attracting more and more interest from researchers in the developmental biology field especially in species of agronomic interest. Therefore, multidisciplinary approaches are necessary to elucidate the molecular processes that are key to determine cell identity and that could be manipulated to improve the performance of cultivable plants such as maize.
Acknowledgements
The authors are grateful to the editors for the opportunity to contribute to this special issue and the anonymous reviewers for helping to improve the manuscript. This work was supported by a Collaborative grant from UC Mexus Conacyt (CN-19-149) to MJ-AJ, UGA-Langebio Cinvestav early career funds to MJ-AJ and a graduate fellowship by Conacyt to C-GM (1147672).
Author contribution statement
MJ-AJ and C-GM wrote the article and designed the figures. Both authors contributed to the article and approved the submitted version.
Declaration
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be taken as a potential conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abraham-Juárez MJ, Schrager-Lavelle A, Man J, Whipple C, Handakumbura P, Babbit C, Bartlett M. Evolutionary variation in MADS box dimerization affects floral development and protein abundance in maize. Plant Cell. 2020;32(11):3408–3424. doi: 10.1105/tpc.20.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta IF, Laparra H, Romero SP, Schmelz E, Hamberg M, Mottinger JP, Moreno MA, Dellaporta SL. tasselseed1 is a lipoxygenase affecting jasmonic acid signaling in sex determination of maize. Science. 2009;323(5911):262–265. doi: 10.1126/science.1164645. [DOI] [PubMed] [Google Scholar]
- Ashton PS. Speciation among tropical forest trees: some deductions in the light of recent evidence. Biol J Linn Soc. 1969;1:155–196. doi: 10.1093/genetics/7.3.203. [DOI] [Google Scholar]
- Banks JA. MicroRNA, sex determination and floral meristem determinacy in maize. Genome Biol. 2008;9:204. doi: 10.1186/gb-2008-9-1-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett ME, Williams SK, Taylor Z, DeBlasio S, Goldshmidt A, Hall DH, Schmidt RJ, Jackson DP, Whipple CJ. The maize PI/GLO ortholog Zmm16/sterile tassel silky ear1 interacts with the zygomorphy and sex determination pathways in flower development. Plant Cell. 2015;27:3081–3098. doi: 10.1105/tpc.15.00679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensen R, Johal G, Crane V, Tossverg J, Schnable P, Meeley R, Briggs S. Cloning and characterization of de Maize An1 gene. Plant Cell. 1995;7:75–84. doi: 10.1105/tpc.7.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best NB, Dilkes BP. Transcriptional responses to gibberellin in the maize tassel and control by DELLA domain proteins. Plant J. 2022;112(2):493–517. doi: 10.1111/tpj.15961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best N, Dilkes B. Genetic evidence that brassinosteroids suppress pistils in the maize tassel independent of the jasmonic acid pathway. Plant Direct. 2023;7(7):e501. doi: 10.1002/pld3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best N, Hartwig T, Budka J, Fujioka F, Johal G, Schulz B, Dilkes B. nana plant2 encodes a maize ortholog of the arabidopsis brassinosteroid biosynthesis gene DWARF1, identifying developmental interactions between brassinosteroids and gibberellins. Plant Physiol. 2016;171(4):2633–2647. doi: 10.1104/pp.16.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hou M, Liu L, Wu S, Shen Y, Ishiyama K, Kobayashi M, McCarty D, Tan B. The maize DWARF1 encodes a gibberellin 3-oxidase and is dual localized to the nucleus and cytosol. Plant Physiol. 2014;166(4):2028–2039. doi: 10.1104/pp.114.247486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng PC, Greyson RI, Walden DB. Organ initiation and the development of unisexual flowers in the tassel and ear of Zea mays. Am J Bot. 1983;70:450–462. doi: 10.2307/2443252. [DOI] [Google Scholar]
- Chuck G. Molecular mechanisms of sex determination in monoecious and dioecious plants. Adv Mol Res. 2010 doi: 10.1016/S0065-2296(10)54002-3. [DOI] [Google Scholar]
- Chuck G, Meeley R, Irish E, Sakai H, Hake S. The maize tasselseed4 controls sex determination and meristem cell fate by targeting Tasselseed6/indeterminate spikelet1. Nat Genet. 2007;39(12):1517–1521. doi: 10.1038/ng.2007.20. [DOI] [PubMed] [Google Scholar]
- De Craene LR. Understanding the role of floral development in the evolution of angiosperm flowers: clarifications from a historical and physico-dynamic perspective. J Plant Res. 2018;131:367–393. doi: 10.1007/s10265-018-1021-1. [DOI] [PubMed] [Google Scholar]
- Dellaporta S, Calderon-Urrea A. Sex determination in flowering plants. Plant Cell. 1993;5(10):1241–1251. doi: 10.1105/tpc.5.10.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellaporta S, Calderon-Urrea A. The sex determination process in maize. Science. 1994;266(5190):1501–1505. doi: 10.1126/science.7985019. [DOI] [PubMed] [Google Scholar]
- DeLong A, Calderon-Urrea A, Dellaporta SL. Sex determination gene TASSELSEED2 of maize encodes a short-chain alcohol dehydrogenase required for stage-specific floral organ abortion. Cell. 1993;74(4):757–768. doi: 10.1016/0092-8674(93)90522-r. [DOI] [PubMed] [Google Scholar]
- Dong Z, Xiao Y, Govindarajulu R, Feil R, Siddoway ML, Nielsen T, Lunn JE, Hawkins J, Whipple C, Chuck G. The regulatory landscape of a core maize domestication module controlling bud dormancy and growth repression. Nat Commun. 2019;10:3810. doi: 10.1038/s41467-019-11774-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson J. Heritable characters in maize. Heredity. 1920;11:65. doi: 10.1093/oxfordjournals.jhered.a101971. [DOI] [Google Scholar]
- Emerson RA, Emerson SE. Genetic interrelations of two andromonoecious types of maize. Genetics. 1922;7:203–227. doi: 10.1093/genetics/7.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka S, Yamane H, Spray CR. Qualitative and quantitative analyses of gibberellins in vegetative shoots of normal, dwarf-1, dwarf-2, dwarf-3, and dwarf-5 seedlings of Zea mays L. Plant Physiol. 1988;88:1367–1372. doi: 10.1104/pp.88.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L, Zhong W, Qian J, Jin M, Tian P, Zhu W, Zhang H, Sun Y, Feng J, Liu X, Chen G. A multi-omics integrative network map of maize. Nat Genet. 2023;55:144–153. doi: 10.1038/s41588-022-01262-1. [DOI] [PubMed] [Google Scholar]
- Hartwig T, Chuck G, Fujioka S, Klempiena A, Weizbauera R, Potlurid DP, Choee S, Johalf G, Schulz B. Brassinosteroid control of sex determination in maize. Proc Natl Acad Sci USA. 2011;108:19814–19819. doi: 10.1073/pnas.1108359108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward AP, Moreno MA, Howard TP, Hague J, Nelson K, Heffelfinger C, Romero S, Kausch AP, Glauser G, Acosta IF, Mottinger JP, Dellaporta SL. Control of sexuality by the sk1-encoded UDP-glycosyltransferase of maize. Sci Adv. 2016 doi: 10.1126/sciadv.1600991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollick JB, Kermicle JL, Parkinson SE. Rmr6 maintains meiotic inheritance of paramutant states in Zea mays. Genetics. 2005;171:725–740. doi: 10.1534/genetics.105.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish EE. Class II tassel seed mutations provide evidence for multiple types of inflorescence meristems in maize (Poaceae) Am J Bot. 1997;84:1502–1515. doi: 10.2307/2446611. [DOI] [PubMed] [Google Scholar]
- Irish EE, Nelson T. Sex determination in monoecious and dioecious plants. The Plant Cell. 1989. pp. 737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish EE, Langdale JA, Nelson TM. Interactions between tasselseed genes and other sex determining genes in maize. Dev Genet. 1994;15:155–171. doi: 10.1002/dvg.1020150206. [DOI] [Google Scholar]
- Jackson D, Veit B, Hake S. Expression of maize KNOTTED1 related homeobox genes in the shoot apical meristem predicts patterns of morphogenesis in the vegetative shoot. Development. 1994;120:405–413. doi: 10.1242/dev.120.2.405. [DOI] [Google Scholar]
- Kebrom TH, Burson BL, Finlayson SA. Phytochrome B represses Teosinte Branched1 expression and induces sorghum axillary bud outgrowth in response to light signals. Plant Physiol. 2006;140:1109–1117. doi: 10.1104/pp.105.074856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein H, Gallagher J, Demesa-Arevalo E, Abraham-Juárez MJ, Heeney M, Feil R, Lunn J, Xiao Y, Chuck G, Whipple C, Jackson D, Barlett M. Recruitment of an ancient branching program to suppress carpel development in maize flowers. PNAS. 2022;119:2. doi: 10.1073/pnas.2115871119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawit SJ, Wych HM, Deping X, Kundu S, Tomes DT. Maize DELLA proteins dwarf plant8 and dwarf plant9 as modulators of plant development. Plant Cell Physiol. 2010;5111:1854–1868. doi: 10.1093/pcp/pcq153. [DOI] [PubMed] [Google Scholar]
- Li Q, Liu B. Genetic regulation of maize flower development and sex determination. Planta. 2016;245:1–14. doi: 10.1007/s00425-016-2607-2. [DOI] [PubMed] [Google Scholar]
- Li Q, Liu N, Wu C. Novel insights into maize (Zea mays) development and organogenesis for agricultural optimization. Planta. 2023;257:94. doi: 10.1007/s00425-023-04126-y. [DOI] [PubMed] [Google Scholar]
- Liu L, Gallagher J, Arevalo ED, Chen R, Skopelitis T, Wu Q, Bartlett M, Jackson D. Enhancing grain-yield-related traits by CRISPR-Cas9 promoter editing of maize CLE genes. Nat Plants. 2021;7:287–294. doi: 10.1038/s41477-021-00858-5. [DOI] [PubMed] [Google Scholar]
- Lunde C, Kimberlin A, Leiboff S, Koo A, Hake S. Tasselseed5 overexpresses a wound-inducible enzyme, ZmCYP94B1, that affects jasmonate catabolism, sex determination, and plant architecture in maize. Commun Biol. 2019;2:114. doi: 10.1038/s42003-019-0354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuffer G, Coe E, Wessler S (1997) Mutants of maize. Cold Spring Harbor
- Nickerson NH. Sustained treatment with gibberellin acid of five different kinds of maize. Ann Mo Bot Gard. 1959;47:19–37. doi: 10.2307/2446574. [DOI] [Google Scholar]
- Nickerson NH, Dale EE. Tassel modifications in Zea mays. Ann Mo Bot Gard. 1955;42:195–211. doi: 10.2307/2394655. [DOI] [Google Scholar]
- Parkinson SE, Gross SM, Hollick JB. Maize sex determination and abaxial leaf fates are canalized by a factor that maintains repressed epigenetic states. Dev Biol. 2007;308:462–473. doi: 10.1016/j.ydbio.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Peng J, Richards DE, Hartley NM, Murphy GP, Devos KM, Flintham JE. ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature. 1999;400:256–261. doi: 10.1038/22307. [DOI] [PubMed] [Google Scholar]
- Phinney BO, Gibberellin A. Dwarfism and the control of shoot elongation in higher plants. Cambridge: Cambridge University Press; 1984. [Google Scholar]
- Satterlee JW, Strable J, Scanlon MJ. Plant stem-cell organization and differentiation at single-cell resolution. Proc Natl Acad Sci USA. 2020;117:33689–33699. doi: 10.1073/pnas.2018788117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walley JW, Sartor RC, Shen Z, Schmitz RJ, Wu KJ, Urich MA, Nery JR, Smith LG, Schnable JC, Ecker JR, Briggs SP. Integration of omic networks in a developmental atlas of maize. Science. 2016;353:6301. doi: 10.1126/science.aag1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipple CJ, Kebrom TH, Weber AL, Yang F, Hall D, Meeley R, Schmidt R, Doebley J, Brutnell TP, Jackson DP. grassy tillers1 promotes apical dominance in maize and responds to shade signals in the grasses. Proc Natl Acad Sci USA. 2011;108:506–512. doi: 10.1073/pnas.1102819108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler RG, Freeling M. Physiological genetics of the dominant gibberellin-nonresponsive maize dwarfs, Dwarf8 and Dwarf9. Planta. 1994;193:341–348. doi: 10.1007/BF00201811. [DOI] [Google Scholar]
- Winkler RG, Helentjaris T. The maize Dwarf3 gene encodes a cytochrome P450-mediated early step in Gibberellin biosynthesis. Plant Cell. 1995;7(8):1307–1317. doi: 10.1105/tpc.7.8.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Knapp S, Stamp A, Stammers DK, Jornval H, Dellaporta SL. Biochemical characterization of TASSELSEED 2, an essential plant short-chain dehydrogenase/reductase with broad spectrum activities. FEBS. 2007;274:1172–1182. doi: 10.1111/j.1742-4658.2007.05642.x. [DOI] [PubMed] [Google Scholar]
- Wu J, Lawit S, Weers B, Habben J. Overexpression of zmm28 increases maize grain yield in the field. PNAS. 2019;116(47):23850–23858. doi: 10.1073/pnas.1902593116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Crow M, Rice BR, Li F, Harris B, Liu L, Demesa-Arevalo E, Lu Z, Wang L, Fox N. Single-cell RNA sequencing of developing maize ears facilitates functional analysis and trait candidate gene discovery. Dev Cell. 2021;56:557–568.e6. doi: 10.1016/j.devcel.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yampolsky C, Yampolsky H. Distribution of sex forms in the phanerogamic flora. Bibliogr Genet. 1922;3:1–62. [Google Scholar]
- Yang H, Nukunya K, Ding Q, Thompson B. Tissue-specific transcriptomics reveal functional differences in floral development. Plant Physiol. 2021;188(2):1158–1173. doi: 10.1093/plphys/kiab557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Žárský V, Tupý J. A missed anniversary: 300 years after Rudolf Jacob Camerarius' “De sexu plantarum epistola’. Sexual Plant Reprod. 1995;8:375–376. doi: 10.1007/BF00243206. [DOI] [Google Scholar]