Abstract
The erythroleukemia-inducing Friend spleen focus-forming virus (SFFV) encodes a unique envelope glycoprotein which allows erythroid cells to proliferate and differentiate in the absence of erythropoietin (Epo). In an effort to understand how SFFV causes Epo independence, we have been examining erythroid cells rendered factor independent by SFFV infection for constitutive activation of signal-transducing molecules. Previous studies from our laboratory showed that various signal-transducing molecules known to be activated by Epo, including Stat proteins and components of the Raf-1/MAP kinase pathway, are constitutively activated in SFFV-infected erythroid cells in the absence of Epo. Since another signal transduction pathway involving activation of phosphatidylinositol 3-kinase (PI 3-kinase) after Epo stimulation plays an important role in erythroid cell proliferation and differentiation, we carried out studies to determine if this pathway was also activated in SFFV-infected cells in the absence of Epo. Our studies show that PI 3-kinase is constitutively activated in erythroid cells rendered factor independent by infection with SFFV and that PI 3-kinase activity, but not Epo receptor tyrosine phosphorylation, is required for the proliferation of these cells in the absence of Epo. We further show that in SFFV-infected erythroid cells grown in the absence of Epo, PI 3-kinase associates with the insulin receptor substrate (IRS)-related adapter molecules IRS-2, Gab1, and Gab2, which are constitutively tyrosine phosphorylated in SFFV-infected cells. Finally, Akt, a protein kinase that is one of the downstream effectors of PI 3-kinase, and SHIP, a lipid phosphatase that is important for Akt activation through PI 3-kinase, are both tyrosine phosphorylated in SFFV-infected cells grown in the absence of Epo. Our results indicate that induction of Epo independence by SFFV requires the activation of PI 3-kinase and suggest that constitutive activation of this kinase in SFFV-infected cells may occur primarily through interaction of PI 3-kinase with constitutively phosphorylated IRS-related adapter molecules.
The proliferation and differentiation of erythroid cells are controlled by the binding of erythropoietin (Epo) to its cell surface receptor, resulting in the activation of various signal transduction pathways. The major pathways known to be activated through the Epo receptor (EpoR) are the Jak-Stat and the Ras/Raf-1/mitogen-activated protein kinase (MAPK) pathways. When Epo binds to the EpoR, the receptor-bound tyrosine kinase Jak2 becomes rapidly activated (41, 68), most likely through receptor dimerization (66), and is thought to phosphorylate itself and tyrosine residues located in the cytoplasmic region of the EpoR (17, 39). Specific phosphotyrosine residues on the receptor then serve as docking sites for Stat proteins, in particular Stat5, which become phosphorylated and translocated to the nucleus (11, 21, 22, 30, 48, 49, 52, 65). The tyrosine-phosphorylated sites on the Epo receptor also serve as docking sites for adapter molecules Shc and Grb2 (3, 9), which link the receptor to the Ras/Raf-1/MAPK pathway (5, 42, 62). Epo stimulation also leads to the activation of a third signal transduction pathway involving the lipid kinase phosphatidylinositol 3-kinase (PI 3-kinase). PI 3-kinase can be activated either through direct binding of its 85-kDa regulatory subunit to a specific tyrosine-phosphorylated site on the EpoR (10, 12, 26, 38, 40) or through binding to insulin receptor substrate (IRS)-related adapter molecules that are tyrosine phosphorylated after Epo stimulation (35, 63, 67). Activation of PI 3-kinase leads to the activation of the serine/threonine kinase Akt (2, 4, 18, 19) and various isoforms of protein kinase C (1, 44, 46, 61), and recent studies indicate that this pathway plays an important role in the proliferation, differentiation, and survival of erythroid progenitor cells (25, 31).
The Friend spleen focus-forming virus (SFFV) encodes a unique envelope glycoprotein which allows erythroid cells to proliferate in the absence of Epo, resulting in the development of erythroleukemia (for a review, see reference 53). In an attempt to understand how SFFV alters the growth and differentiation of erythroid cells, we have been studying signal transduction pathways known to be activated by Epo to determine if SFFV exerts its biological effects by activating any of these pathways. We previously showed that SFFV infection leads to the Epo-independent activation of Stat proteins (48) as well as the downstream components of the Raf-1/MAPK pathway (45). In this study, we have focused our investigation on determining the effects of SFFV infection on the activation of PI 3-kinase and its downstream effectors. Our results indicate that both PI 3-kinase and Akt kinase are constitutively activated in SFFV-infected cells growing in the absence of Epo and that activation of PI 3-kinase, but not EpoR tyrosine phosphorylation, is required for the Epo-independent proliferation of SFFV-infected cells. Further studies suggest that PI 3-kinase is constitutively activated in SFFV-infected erythroid cells, primarily by interaction with the IRS-related adapter molecules IRS-2, Gab1, and Gab2, all of which are constitutively phosphorylated in these cells.
MATERIALS AND METHODS
Cell lines.
HCD-57 cells, an Epo-dependent erythroleukemia cell line (54), were maintained in Iscove's modified Dulbecco minimal essential medium (IMDM) supplemented with 30% fetal calf serum (FCS), 5 × 10−5 M 2-mercaptoethanol, and Epo (0.3 U/ml). HCD-57 cells in which Epo dependence had been abrogated by infection with either SFFV-P or SFFV-A, two different variants of SFFV (54), were maintained in the same medium without Epo. Ba/F3 cells expressing the wild-type EpoR (BaF3/WT-ER) or a mutant EpoR devoid of intracellular tyrosine residues (BaF3/ZERO-ER) (20), a generous gift from Patrick Mayeux (Université Rene Descartes, Paris, France), were maintained in RPMI 1640 medium supplemented with 10% FCS and Epo (1 U/ml). They were infected with an amphotropic murine leukemia virus pseudotype of SFFV-P and selected for Epo independence as previously described (54).
Cell lysates and immunoprecipitation.
To prepare cell lysates, uninfected and SFFV-infected HCD-57 cells were starved in IMDM plus 1.5% fetal calf serum for 12 h, while uninfected and SFFV-infected BaF3/WT-ER and BaF3/ZERO-ER cells were starved in RPMI 1640 plus 0.5% bovine serum albumin for 7 h. Cells were then stimulated for various periods of time with 10 U of Epo/ml (or the concentrations indicated in Fig. 9) or left unstimulated. Cells were then washed and resuspended in lysis buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 10% glycerol, 1% Triton X-100, 2 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4, 1 μg each of aprotinin and leupeptin/ml). Immunoprecipitations were performed at 4°C overnight with the following antibodies: anti-IRS-2 (06-506), anti-Gab1 (06-579), anti-Gab2 (06-967), anti-PI 3-kinase p85 (06-195 and 05-212), and antiphosphotyrosine (05-321) (Upstate Biotechnology, Lake Placid, N.Y.); anti-EpoR (SC-697), anti-SH-PTP2 (SC-280), and anti-SHIP (SC-1964) (Santa Cruz Biotechnology, Santa Cruz, Calif.); and anti-Shc monoclonal antibody (S14630) (Transduction Laboratories, Lexington, Ky.). The immune complexes were collected with protein A-agarose (Upstate Biotechnology) or protein A/G-agarose beads (Santa Cruz Biotechnology).
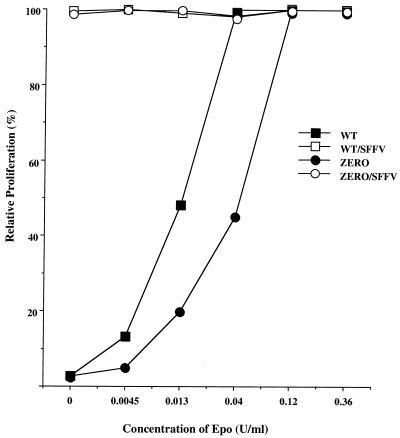
FIG. 9.
Induction of Epo independence by SFFV does not require EpoR tyrosine phosphorylation. Ba/F3 cells expressing wild-type EpoR (WT) or a mutant EpoR that contains no intracellular tyrosine residues (ZERO) were infected with SFFV-P, and Epo-independent clones were selected and designated WT/SFFV and ZERO/SFFV. Cells were grown without Epo or with various concentrations of Epo for 72 h, and the level of proliferation was measured as described in Materials and Methods. Relative proliferation was determined as a percentage of the absorbance at each point relative to the absorbance when the cells were grown in 0.36 U of Epo/ml.
Western blot analysis.
Immunoprecipitates were washed three times with phosphate-buffered saline containing 1% Triton X-100, 0.5% deoxycholic acid, and 0.1% sodium dodecyl sulfate, resuspended 1:2 in Laemmli sample buffer, and boiled for 5 min at 100°C. Immunoprecipitated proteins were separated by electrophoresis on 8% Tris-glycine minigels (Novex, San Diego, Calif.) and then transferred electrophoretically to nitrocellulose filters. The filters were incubated at room temperature for 1 h in blocking buffer (2% bovine serum albumin in Tris-buffered saline containing 0.2% Tween 20 [TBS-T]), followed by the addition of antiphosphotyrosine antibody (4G10; 05-321; Upstate Biotechnology) or specific antibodies to Akt (9272) or to Akt phosphorylated on serine 473 (9270; New England BioLab, Inc., Beverly, Mass.). After incubation at 4°C overnight, the filters were washed three times with TBS-T and then incubated for 30 min at room temperature with anti-mouse, anti-rabbit, or anti-goat immunoglobulin G antibody conjugated to horseradish peroxidase (Amersham Corp. Arlington Heights, Ill.). Blots were washed three times with TBS-T, and protein bands bound to the antibody were then detected by enhanced chemiluminescence using the ECL Western blotting analysis system (Amersham Corp.). In some cases, the filters were stripped and reprobed with another antibody.
PI 3-kinase assay.
PI 3-kinase activity was assayed as follows. Immunoprecipitates from cell lysates were washed once in buffer containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 0.5 mM phenylmethylsulfonyl fluoride, and 1 mM Na3VO4; once in 20 mM Tris-HCl (pH 7.5) containing 0.5 M LiCl; and twice in PI 3-kinase buffer (20 mM Tris-HCl [pH 7.5], 100 mM NaCl, 0.5 mM EGTA). Immunoprecipitates were then resuspended in PI 3-kinase buffer. Sonicated phosphoinositides (PI) (Sigma Chemical Corp., St. Louis, Mo.) were added to a final concentration of 0.2 mg/ml, and phosphorylation was initiated by the addition of 10 μCi of [γ-32P]ATP plus cold ATP and MgCl2 to final concentrations of 20 μM and 20 mM, respectively. After incubation at room temperature for 20 min, the lipids were extracted into chloroform and then methanol–1 N HCl (1:1). The extracts were then separated on thin-layer chromatography plates (silica gel 60; Merck, Darmstadt, Germany) in an atmosphere saturated with chloroform-methanol-NH4OH (28%)-water (215:190:25:35) for 2 h and then visualized by autoradiography. Quantitation of signals after autoradiography was performed by densitometry utilizing NIH-Image software.
Cell proliferation assay.
Uninfected or SFFV-infected HCD-57 cells were starved in IMDM containing 1.5% FCS for 12 h and then resuspended in medium containing 20% FCS with or without Epo (0.3 U/ml) and with or without PI 3-kinase inhibitor LY294002 (various concentrations; Sigma). Cells were then plated at a concentration of 5 × 104 cells/well in 96-well microtiter plates and incubated for 24 h before the addition of the cell proliferation reagent WST-1 (Boehringer GmbH, Mannheim, Germany). After incubation for 4 h in a humidified atmosphere, the absorbances of the samples were measured at 450 nm against background controls using an enzyme-linked immunosorbent assay reader. Relative proliferation was determined as a percentage by dividing the absorbance in the presence of inhibitor by the absorbance in the absence of inhibitor and multiplying by 100. Uninfected and SFFV-infected BaF3/WT-ER and BaF3/ZERO-ER cells were plated at a concentration of 104 cells/well in RPMI 1640 medium plus 10% FCS with or without Epo at various concentrations. After incubation for 72 h, WST-1 was added for 4 h and the absorbance was measured as described above. Relative proliferation of each line was determined as a percentage by dividing the absorbance for each point by the absorbance obtained when the cells were grown in 0.36 U of Epo/ml and multiplying by 100.
RESULTS
PI 3-kinase is constitutively activated in SFFV-infected erythroid cells.
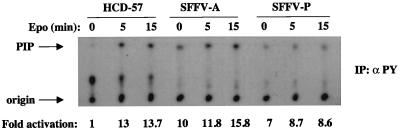
It has previously been demonstrated that PI 3-kinase is activated in erythroid cells in response to Epo. In order to determine if PI 3-kinase is constitutively activated in SFFV-infected cells, Epo-dependent HCD-57 cells or HCD-57 cells that had been rendered factor independent following infection with SFFV were left unstimulated or were stimulated with Epo for various amounts of time. Cell lysates were then immunoprecipitated with antiphosphotyrosine antibody, and PI 3-kinase activity was determined by an in vitro kinase assay using PI as a substrate. As shown in Fig. 1, there is a very low level of PI 3-kinase activity in unstimulated HCD-57 cells and it is greatly increased (13-fold) within 5 min after Epo stimulation. In contrast, HCD-57 cells infected with SFFV showed a high level of PI 3-kinase activation even in the absence of Epo stimulation.
FIG. 1.
PI 3-kinase is constitutively activated in SFFV-infected erythroid cells. Uninfected or SFFV-infected HCD-57 cells were left unstimulated or were stimulated with Epo for the indicated periods of time. Cell lysates were then immunoprecipitated (IP) with antiphosphotyrosine (αPY) antibody. PI 3-kinase activity was determined by an in vitro kinase assay using PI as a substrate. PI phosphate (PIP) was separated by thin-layer chromatography, and the plate was exposed to film for 24 h. PI 3-kinase activity was quantitated, and the results were expressed as fold activation relative to that for unstimulated HCD-57 cells.
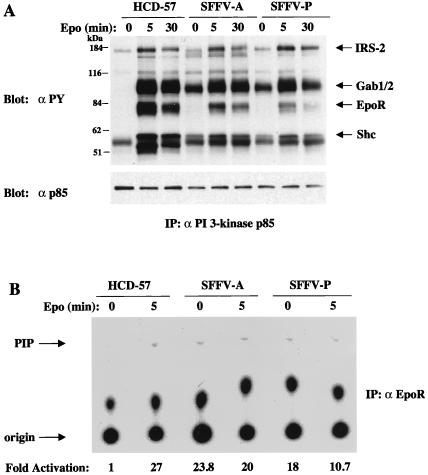
Association of the 85-kDa regulatory subunit of PI 3-kinase with tyrosine-phosphorylated proteins in SFFV-infected cells grown in the absence of Epo.
PI 3-kinase can be activated after Epo stimulation by direct association of its 85-kDa regulatory unit with tyrosine-phosphorylated EpoR molecules (10, 12, 26, 38, 40) or by association with one of the tyrosine-phosphorylated IRS adapter molecules IRS-2 (63), Gab1 (35, 67), and Gab2 (67). In order to examine the tyrosine-phosphorylated proteins associated with constitutively activated PI 3-kinase in SFFV-infected cells, we precipitated extracts of these cells with antiserum to PI 3-kinase p85 and then carried out Western blotting with antiphosphotyrosine antibodies. As shown in Fig. 2, Epo stimulation of both uninfected and SFFV-infected HCD-57 cells resulted in the association of PI 3-kinase with a number of tyrosine-phosphorylated proteins, including those with molecular masses of 180, 97, 78, and 57 kDa. These proteins were subsequently identified as IRS-2, Gab1 or -2, EpoR, and Shc, respectively (data not shown). In SFFV-infected cells grown in the absence of Epo, the p85 regulatory subunit of PI 3-kinase specifically associated with several of these tyrosine-phosphorylated proteins, but not detectably with the 78-kDa protein corresponding to the EpoR. We failed to detect tyrosine phosphorylation of the EpoR in SFFV-infected cells unless they were stimulated with Epo (data not shown), and that may explain our failure to detect its association with the p85 subunit of PI 3-kinase in the absence of Epo. Although PI 3-kinase does not appear to directly bind to tyrosine-phosphorylated EpoR molecules in SFFV-infected cells grown in the absence of Epo, it does appear to be associated with the EpoR complexes in these cells. As shown in Fig. 2B, we were able to detect constitutive PI 3-kinase activity in lysates from SFFV-infected HCD-57 cells, but not from uninfected HCD-57 cells, after immunoprecipitation with antiserum to the EpoR.
FIG. 2.
Association of PI 3-kinase p85 with tyrosine-phosphorylated proteins and detection of PI 3-kinase activity in the EpoR complex in SFFV-infected HCD-57 cells in the absence of Epo. (A) Uninfected and SFFV-infected HCD-57 cells were left unstimulated or were stimulated with Epo for the indicated periods of time. PI 3-kinase p85-associated proteins were detected by immunoprecipitation (IP) with anti-p85 antiserum, followed by immunoblotting with antiphosphotyrosine (αPY) or anti-p85 antiserum. (B) Anti-EpoR immunoprecipitates were tested for PI 3-kinase activity by an in vitro PI 3-kinase assay using PI as a substrate. PI phosphate (PIP) was separated by thin-layer chromatography, and the plate was exposed to film for 24 h. PI 3-kinase activity was quantitated, and the results were expressed as fold activation relative to that for unstimulated HCD-57 cells.
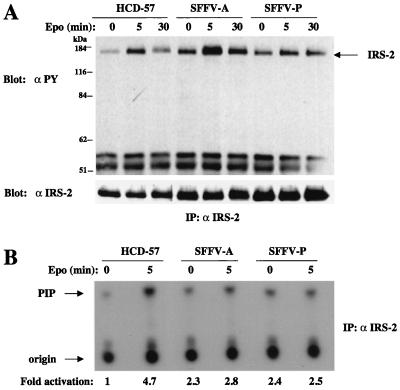
IRS-2, Gab1, and Gab2 are constitutively phosphorylated in SFFV-infected cells and associate with PI 3-kinase activity.
Since our data indicated that tyrosine-phosphorylated proteins corresponding in molecular weight to the IRS-related adapter proteins IRS-2, Gab1, and Gab2 were constitutively associated with the regulatory subunit of PI 3-kinase in SFFV-infected cells, we carried out further studies with specific antisera to these adapter molecules to determine if the activation of PI 3-kinase that occurs in SFFV-infected cells in the absence of Epo is primarily mediated through these adapter molecules. As shown in Fig. 3A, uninfected HCD-57 cells show a low level of constitutive IRS-2 tyrosine phosphorylation, perhaps due to the presence of insulin or insulin-like growth factors in the medium, and this level is greatly increased after Epo stimulation. The level of IRS-2 tyrosine phosphorylation was not increased by stimulation of HCD-57 cells with stem cell factor (data not shown). However, HCD-57 cells infected with SFFV show a significantly higher level of IRS-2 tyrosine phosphorylation in the absence of Epo than uninfected HCD-57 cells. To determine if the tyrosine-phosphorylated IRS-2 present in SFFV-infected cells in the absence of Epo was involved in the constitutive activation of PI 3-kinase in these cells, we carried out an in vitro PI 3-kinase assay using immunoprecipitates from cell lysates treated with an antiserum specific for IRS-2. As shown in Fig. 3B, a low level of PI 3-kinase activity associates with IRS-2 in uninfected HCD-57 cells before Epo stimulation and this level is greatly increased (approximately fivefold) after the cells are exposed to Epo for 5 min. In contrast, SFFV-infected HCD-57 cells showed high levels of IRS-2-associated PI 3-kinase activity in the absence of Epo stimulation.
FIG. 3.
IRS-2 is tyrosine phosphorylated in SFFV-infected erythroid cells in the absence of Epo and constitutively associates with PI 3-kinase. Uninfected and SFFV-infected HCD-57 cells were left unstimulated or were stimulated with Epo for the indicated periods of time. (A) IRS-2 was detected by immunoprecipitation (IP) with anti-IRS-2 antiserum followed by blotting with antiphosphotyrosine (αPY) or anti-IRS-2 antibodies. (B) Anti-IRS-2 immunoprecipitates were tested for PI 3-kinase activity by an in vitro PI 3-kinase assay using PI as a substrate. PI phosphate (PIP) was separated by thin-layer chromatography, and the plate was exposed to film for 24 h. PI 3-kinase activity was quantitated, and the results were expressed as fold activation relative to that for unstimulated HCD-57 cells.
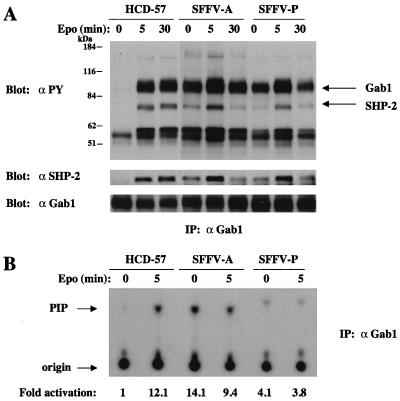
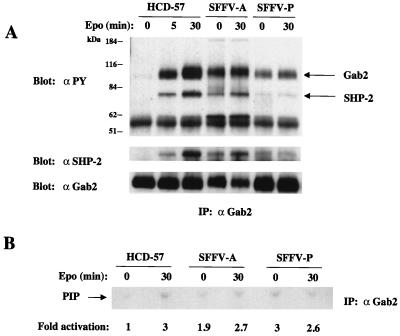
SFFV-infected cells show constitutive phosphorylation of, in addition to IRS-2, the related adapter molecules Gab1 and Gab2. As shown in Fig. 4A, Gab1 is tyrosine phosphorylated in uninfected HCD-57 cells only after Epo stimulation but is constitutively phosphorylated in SFFV-infected cells in the absence of Epo. Furthermore, as shown in Fig. 4B, Gab1 immunoprecipitates from SFFV-infected cells grown in the absence of Epo contained significantly more PI 3-kinase activity (4- to 14-fold) than immunoprecipitates from uninfected HCD-57 cells, where PI 3-kinase activity was negligible in the absence of Epo. Gab2 is also tyrosine phosphorylated in uninfected HCD-57 cells after Epo stimulation (Fig. 5A), but, like Gab1, it is constitutively phosphorylated in SFFV-infected HCD-57 cells. Furthermore, as shown in Fig. 5B, Gab2 constitutively associates with PI 3-kinase activity in SFFV-infected HCD-57 cells, whereas it does not significantly associate with PI 3-kinase in uninfected HCD-57 cells unless the cells are stimulated with Epo.
FIG. 4.
Gab1 is tyrosine phosphorylated in SFFV-infected erythroid cells in the absence of Epo and constitutively associates with PI 3-kinase activity. Uninfected and SFFV-infected HCD-57 cells were left unstimulated or were stimulated with Epo for the indicated periods of time. (A) Tyrosine-phosphorylated Gab1, total Gab1, and Gab1-associated SHP-2 were detected by immunoprecipitation (IP) of cell lysates with antiserum to Gab1, followed by immunoblotting with either antiphosphotyrosine (αPY), anti-Gab1, or anti-SHP-2 antibodies. (B) Anti-Gab1 immunoprecipitates were tested for PI 3-kinase activity by an in vitro PI 3-kinase assay using PI as a substrate. PI phosphate (PIP) was separated by thin-layer chromatography, and the plate was exposed to film for 24 h. PI 3-kinase activity was quantitated, and the results were expressed as fold activation relative to that for unstimulated HCD-57 cells.
FIG. 5.
Gab2 is tyrosine phosphorylated in SFFV-infected erythroid cells in the absence of Epo and constitutively associates with PI 3-kinase activity. Uninfected and SFFV-infected HCD-57 cells were left unstimulated or were stimulated with Epo for the indicated periods of time. (A) Tyrosine-phosphorylated Gab2, total Gab2, and Gab2-associated SHP-2 were detected by immunoprecipitation (IP) of cell lysates with antiserum to Gab2, followed by immunoblotting with either antiphosphotyrosine (αPY), anti-Gab2, or anti-SHP-2 antibodies. (B) Anti-Gab2 immunoprecipitates were tested for PI 3-kinase activity by an in vitro PI 3-kinase assay using PI as a substrate. PI phosphate (PIP) was separated by thin-layer chromatography, and the plate was exposed to film for 48 h. PI 3-kinase activity was quantitated, and the results were expressed as fold activation relative to that for unstimulated HCD-57 cells.
Previous studies have shown that the protein tyrosine phosphatase SHP-2 associates with tyrosine-phosphorylated Gab1 and Gab2 after stimulation with certain growth factors, resulting in the tyrosine phosphorylation of SHP-2 (35, 47, 58, 67). As shown in Fig. 4A and 5A, SHP-2 becomes tyrosine phosphorylated and associates with Gab1 and Gab2 in both uninfected and SFFV-infected HCD-57 cells after Epo stimulation. However, Epo-independent tyrosine phosphorylation of SHP-2 and its association with Gab1 and Gab2 can only be detected in SFFV-infected HCD-57 cells. The same results were obtained when anti-SHP-2 immunoprecipitates were examined (data not shown). In addition to SHP-2, Shc is also coprecipitated with Gab1 and Gab2, both in response to Epo and constitutively in SFFV-infected cells (note the 57-kDa tyrosine-phosphorylated proteins in Fig. 4A and 5A).
Inhibition of PI 3-kinase activity blocks Epo-independent proliferation of SFFV-infected cells.
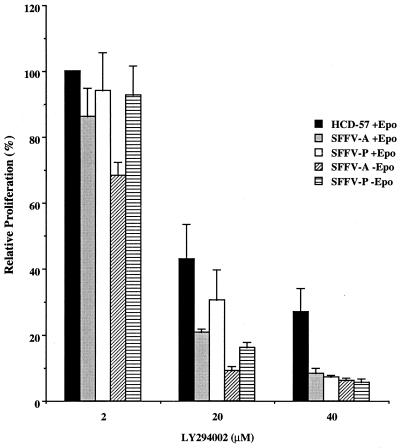
In order to determine if activation of PI 3-kinase is required for the Epo-independent proliferation of SFFV-infected cells, we studied the effect of the specific PI 3-kinase inhibitor LY294002 (64) on the proliferation of uninfected and SFFV-infected HCD-57 cells. As shown in Fig. 6, treatment with LY294002 of both uninfected and SFFV-infected HCD-57 cells growing in the presence of Epo resulted in a dose-dependent inhibition of proliferation at doses that are known to block PI 3-kinase activity. Importantly, the Epo-independent proliferation of SFFV-infected cells was severely inhibited by the drug (reduced by 94 to 95% at 40 μM), even more than was the Epo-dependent proliferation of uninfected HCD-57 cells (reduced by 72% at 40 μM). These results demonstrate that the Epo-independent proliferation of SFFV-infected cells is dependent on PI 3-kinase activation.
FIG. 6.
PI 3-kinase activity is required for the Epo-independent proliferation of SFFV-infected erythroid cells. Uninfected or SFFV-infected HCD-57 cells were grown with or without Epo for 24 h in medium containing various concentrations of the PI 3-kinase inhibitor LY294002. The level of cell proliferation was measured as described in Materials and Methods. Relative proliferation was determined as a percentage by comparing proliferation of the cells in the presence of the drug with proliferation in its absence. The means ± standard deviations of triplicate measurements are shown. The levels of proliferation of the cell lines in the absence of the drug were similar (relative proliferation levels were 1 for uninfected HCD-57 cells, 1.2 for SFFV-A-infected HCD-57 cells, and 1.5 for SFFV-P-infected HCD-57 cells). Uninfected HCD-57 cells, in the absence as well as in the presence of inhibitor, failed to proliferate unless Epo was added (data not shown).
Akt kinase and the lipid phosphatase SHIP are tyrosine phosphorylated after Epo stimulation and SFFV infection.
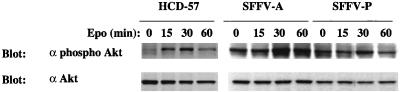
The protein kinase Akt is a downstream effector of activated PI 3-kinase in many systems, and it was recently shown to be activated in response to Epo (2). In order to determine if this kinase is constitutively activated in SFFV-infected cells, cell lysates were immunoblotted with an antiserum that specifically detects Akt phosphorylated on serine 473. As shown in Fig. 7, a very low level of phosphorylated Akt is detected in uninfected HCD-57 in the absence of Epo but the addition of Epo leads to a significant increase, with maximum levels of phosphorylated protein detected after a 30-min stimulation. In contrast, HCD-57 cells infected with SFFV express high levels of phosphorylated Akt in the absence of Epo, indicating that Akt is constitutively phosphorylated in SFFV-infected cells.
FIG. 7.
Akt kinase is constitutively phosphorylated in SFFV-infected erythroid cells in the absence of Epo. Uninfected or SFFV-infected HCD-57 cells were left unstimulated or stimulated with 10 U of Epo/ml for the indicated periods of time. Cell lysates were then immunoblotted with either anti-phospho-Akt (Ser 473) or anti-Akt antiserum.
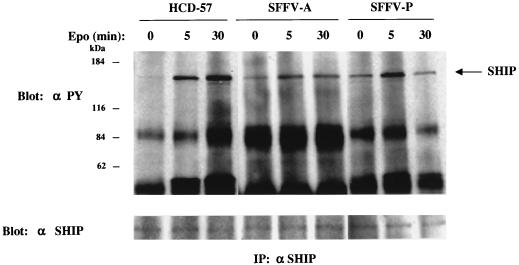
It has previously been shown that Akt is activated by only one of the three lipid products of PI 3-kinase, phosphatidylinositol 3,4-bisphosphate (19, 32). The levels of this product are thought to be regulated by specific inositol polyphosphate 5′-phosphatases such as SIP and SHIP (13, 29). Since SHIP is tyrosine phosphorylated in response to Epo (13), we examined HCD-57 cells and their virus-infected counterparts for the tyrosine phosphorylation of SHIP in the presence or absence of Epo. As shown in Fig. 8, SHIP is activated by tyrosine phosphorylation in uninfected HCD-57 cells only after Epo stimulation but is phosphorylated in SFFV-infected HCD-57 cells in the absence of Epo. Furthermore, SHIP can be coimmunoprecipitated with PI 3-kinase and Gab1 both after Epo stimulation and in SFFV-infected cells grown in the absence of Epo (data not shown).
FIG. 8.
SHIP is constitutively phosphorylated in SFFV-infected cells. Uninfected and SFFV-infected HCD-57 cells were left unstimulated or stimulated with Epo for the indicated periods of time. Cell lysates were then immunoprecipitated (IP) with antiserum to SHIP, followed by immunoblotting with antiphosphotyrosine (αPY) or anti-SHIP antiserum.
Induction of Epo independence by SFFV does not require EpoR tyrosine phosphorylation.
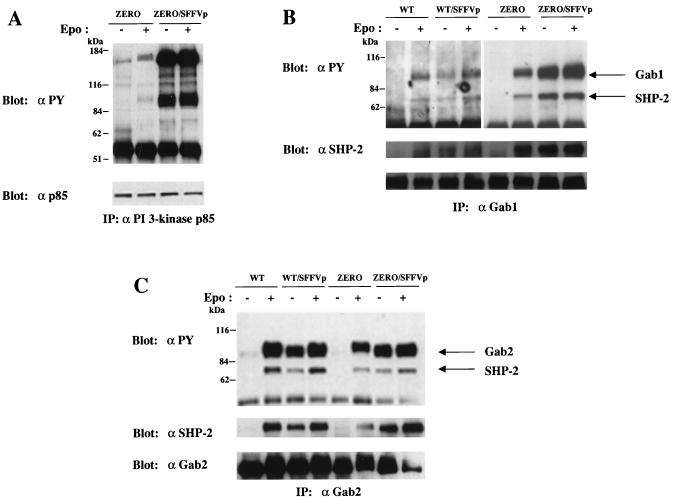
Activation of PI 3-kinase in erythroid cells can occur either by the direct binding of PI 3-kinase to phosphorylated tyrosine sites on the EpoR or by its binding to tyrosine-phosphorylated IRS-related adapter molecules, and our data suggest that activation of PI 3-kinase by SFFV occurs primarily by its binding to IRS adapter molecules. In order to determine if SFFV could render cells factor independent in the absence of EpoR tyrosine phosphorylation, we took advantage of a cell line expressing a mutant EpoR devoid of intracellular tyrosines. This line, designated BaF3/ZERO-ER (20), expresses an EpoR that lacks the last 109 amino acids and that contains a mutation of Tyr343 to Phe. As shown in Fig. 9, BaF3/ZERO-ER cells proliferate in response to Epo, but less efficiently than a line expressing the wild-type EpoR (BaF3/WT-ER). When BaF3/WT-ER and BaF3/ZERO-ER cells were infected with SFFV-P, they were both rendered factor independent and proliferated to high levels in the absence of Epo (Fig. 9). Further analysis of the Epo-independent SFFV-infected BaF3/ZERO-ER cells indicated that PI 3-kinase was constitutively associated in these cells with high levels of tyrosine-phosphorylated proteins corresponding to Gab proteins and IRS-2 (Fig. 10A). Like SFFV-infected HCD-57 cells, BaF3/WT-ER and BaF3/ZERO-ER cells rendered factor independent by SFFV showed constitutive tyrosine phosphorylation of both Gab1 (Fig. 10B) and Gab2 (Fig. 10C) and the constitutive association of SHP-2 with these adapter molecules. Thus, SFFV can induce Epo independence as well as the constitutive activation of IRS adapter proteins and their association with PI 3-kinase in cells expressing truncated EpoR molecules lacking intracellular tyrosine residues.
FIG. 10.
Phosphorylation of IRS adapter molecules and their association with PI 3-kinase do not require EpoR tyrosine phosphorylation. Uninfected or SFFV-infected BaF3/WT-ER or BaF3/ZERO-ER cells were left unstimulated or stimulated with Epo for 20 min. (A) PI 3-kinase p85-associated proteins were detected by immunoprecipitation (IP) of cell lysates with anti-p85 antiserum followed by immunoblotting with antiphosphotyrosine (αPY) or anti-p85 antiserum. ZERO, uninfected BaF3/ZERO-ER cells; ZERO/SFFVp, BaF3/ZERO-ER cells infected with SFFV-P. (B) Tyrosine-phosphorylated Gab1, total Gab1, and Gab1-associated SHP-2 were detected by immunoprecipitation of cell lysates with antiserum to Gab1, followed by immunoblotting with either antiphosphotyrosine, anti-Gab1, or anti-SHP-2 antibodies. WT, uninfected BaF3/WT-ER cells; WT/SFFVp, BaF3/WT-ER cells infected with SFFV-P. (C) Tyrosine-phosphorylated Gab2, total Gab2, and Gab2-associated SHP-2 were detected by immunoprecipitation of cell lysates with antiserum to Gab2, followed by immunoblotting with either antiphosphotyrosine, anti-Gab2, or anti-SHP-2 antibodies.
DISCUSSION
Previous studies from our laboratory have shown that infection of erythroid cells with the erythroleukemia-inducing Friend SFFV leads to the Epo-independent activation of components of the Jak-Stat and the Raf-1/MAPK pathways. In this study we show that PI 3-kinase is also constitutively activated in SFFV-infected erythroid cells and that its activity is required for the Epo-independent proliferation of these cells. We further show that both the Epo-independent proliferation of SFFV-infected cells and the activation of PI 3-kinase in these cells can occur in the absence of EpoR tyrosine phosphorylation and involves the activation of IRS-related adapter molecules. Finally, we demonstrate that Akt kinase, one of the downstream targets of PI 3-kinase, is also constitutively activated in SFFV-infected cells.
PI 3-kinase, which is rapidly activated in response to Epo, plays an essential role in the proliferation and differentiation of normal erythroid cells (10, 12, 25, 26, 28, 31, 38, 40). PI 3-kinase can be activated by Epo in one of several ways. The regulatory subunit of the kinase, p85, can bind directly to tyrosine 479 in the carboxyl terminus of the EpoR after it has been tyrosine phosphorylated in response to Epo (12). Alternatively, p85 can bind to adapter molecules, such as IRS-2 (63), Gab1 (35, 67), and Gab2 (67), which become tyrosine phosphorylated after Epo stimulation. It is not known which of these mechanisms is primarily used by erythroid cells to activate PI 3-kinase in response to Epo, although it is likely that maximum proliferation occurs when both of these mechanisms are intact. In contrast to Epo-induced activation of PI 3-kinase, Epo-independent activation of PI 3-kinase in SFFV-infected erythroid cells appears to occur primarily through activation of IRS-related adapter molecules. When we examined SFFV-infected erythroid cells, we were unable to detect the association of PI 3-kinase with tyrosine-phosphorylated EpoR proteins unless Epo was present, although PI 3-kinase activity could be immunoprecipitated with antiserum to the EpoR, indicating that PI 3-kinase activity was associated with the EpoR complex. In contrast, we could clearly detect the association of PI 3-kinase with the tyrosine-phosphorylated adapter molecules IRS-2, Gab1, and Gab2 in SFFV-infected erythroid cells grown in the absence of Epo. These IRS-related adapter molecules, which are tyrosine phosphorylated in normal erythroid cells only after Epo stimulation, are constitutively tyrosine phosphorylated in SFFV-infected erythroid cells, and PI 3-kinase activity could be immunoprecipitated with antisera specific to each of these adapter molecules. Thus, the Epo-independent activation of PI 3-kinase in SFFV-infected cells appears to be mediated preferentially through the binding of PI 3-kinase to tyrosine-phosphorylated IRS adapter molecules associated with the EpoR complex rather than through its direct binding to tyrosine-phosphorylated sites on the EpoR. This idea is supported by the fact that a hematopoietic cell line that expresses mutant EpoRs devoid of intracellular tyrosine residues, including the PI 3-kinase binding site, could be rendered factor independent after infection with SFFV. Importantly, PI 3-kinase constitutively associates with IRS adapter molecules in these cells, and Gab1 and Gab2 are constitutively phosphorylated. Thus, induction of Epo independence and activation of the PI 3-kinase pathway by SFFV do not require the phosphorylation of intracellular tyrosine residues in the EpoR.
The physiological relevance of constitutive activation of PI 3-kinase in SFFV-infected cells is demonstrated by the fact that we can completely inhibit the Epo-independent proliferation of these cells using low concentrations of LY294002, a specific inhibitor of PI 3-kinase. Interestingly, the Epo-independent proliferation of SFFV-infected HCD-57 cells was more sensitive to low doses of the PI 3-kinase inhibitor than that of uninfected HCD-57 cells or of SFFV-infected HCD-57 cells grown in the presence of Epo. This suggests that the Epo-independent growth of SFFV-infected cells is more dependent on activation of the PI 3-kinase pathway than the Epo-dependent growth of normal erythroid cells. Thus, it may be possible to use low concentrations of PI 3-kinase inhibitors to specifically block the proliferation of SFFV-infected erythroid cells in vivo without inhibiting the growth of normal cells.
For these studies, we examined HCD-57 cells infected with two different variants of SFFV: SFFV-P and SFFV-A. Although infection of HCD-57 cells with these variants does not cause any obvious difference in biological phenotype, infection of primary erythroid cells demonstrates that SFFV-P and SFFV-A have different effects on the growth and differentiation of erythroid cells (for a review, see reference 53). Cells infected with SFFV-P can both proliferate and differentiate in the absence of Epo, while those infected with SFFV-A still require Epo for differentiation and are hypersensitive to Epo for proliferation. It is, therefore, interesting to note that HCD-57 cells infected with SFFV-A, either in the absence or presence of Epo, consistently show higher levels of tyrosine-phosphorylated IRS-related adapter molecules and constitutive PI 3-kinase activity than cells infected with SFFV-P. Furthermore, SFFV-A-infected HCD-57 cells are more sensitive than SFFV-P-infected cells to growth inhibition by low doses of the PI 3-kinase inhibitor LY294002. The biological differences between these two variants are attributed to a subtle difference in the transmembrane regions of their envelope glycoproteins (7, 8), and this may alter how the viral proteins interact with the EpoR and, consequently, the signals that emanate from the receptor to control cell growth and differentiation. For example, interaction of the SFFV-A envelope glycoprotein, but not that of SFFV-P, with the EpoR may block the binding of a negative regulatory factor, allowing the cells to become hypersensitive to both Epo and SFFV and leading to higher levels of PI 3-kinase activity and higher levels of other signal-transducing molecules. Higher levels of PI 3-kinase may be required for the Epo-independent activation of cell proliferation by SFFV-A-infected cells because interaction of the SFFV-A envelope glycoprotein with the EpoR may be unable to activate a complementary signal transduction pathway activated by SFFV-P. This may explain why SFFV-A-infected HCD-57 cells are more sensitive to growth inhibition by low doses of the PI 3-kinase inhibitor LY294002.
Our previous studies showed that components of the Raf-1/MAPK pathway are constitutively activated in SFFV-infected cells (45), and this may be mediated by the activation of the IRS/PI 3-kinase pathway. In SFFV-infected erythroid cells, we observed the constitutive association of Gab1 and Gab2 with SHP-2, Shc, and SHIP, and formation of such multimolecular complexes could lead to the activation of Ras and the Raf-1/MAPK pathway (3, 47, 58, 59). Alternatively, MEK or MAPK may be directly phosphorylated in SFFV-infected cells by one of the isoforms of protein kinase C (16, 23, 31), another downstream effector of PI 3-kinase that we find constitutively activated in SFFV-infected cells (unpublished data). We also detect constitutive activation of Stat proteins in SFFV-infected erythroid cells (48), but it is unclear whether this is also mediated by activation of the IRS/PI 3-kinase pathway. In normal erythroid cells, Stat proteins are primarily activated after binding to specific tyrosine-phosphorylated sites on the EpoR, but they can also be activated at low levels in the absence of EpoR tyrosine phosphorylation (11, 21, 30), and this may occur by the direct binding of Stat proteins to phosphorylated IRS-related adapter molecules. However, we have been unable to detect the association of IRS-related adapter proteins with Stat proteins in SFFV-infected cells either in the absence or presence of Epo (data not shown).
It is not known how IRS-2, Gab1, and Gab2 become tyrosine phosphorylated in SFFV-infected HCD-57 cells grown in the absence of Epo. Epo stimulation leads to the activation of the tyrosine kinase Jak2 (41, 68), and it has been suggested that Jak kinases may phosphorylate IRS adapter molecules in other systems (58, 70). However, we have been unable to detect the constitutive phosphorylation of Jak2 or of any other Epo-induced kinase, including Fes, Lyn, and Tec (6, 24, 37, 60), in SFFV-infected cells (data not shown). Since interaction of the SFFV envelope glycoprotein with the EpoR may lead to activation of a tyrosine kinase not activated by Epo, we are also examining SFFV-infected cells for the constitutive activation of other tyrosine kinases. We have failed to detect the constitutive activation of Jak1, Jak3, or Tyk-2 (data not shown) and are currently examining SFFV-infected erythroid cells for the constitutive activation of Stk, a receptor tyrosine kinase that was recently implicated in susceptibility to SFFV-induced erythroleukemia (50).
In addition to demonstrating that PI 3-kinase is constitutively activated in SFFV-infected cells, we also show that one of its downstream targets, the serine/threonine kinase Akt (4, 18, 19), is also constitutively activated in SFFV-infected cells grown in the absence of Epo. Our studies further suggest that activation of Akt by PI 3-kinase is promoted by the constitutive activation in these virus-infected cells of the lipid phosphatase SHIP, whose activity leads to the generation of phospholipid products known to specifically activate Akt (19, 32). Activation of Akt is thought to promote cell survival by blocking apoptosis, primarily thorough phosphorylation of BAD (14, 15), a Bcl-2 family member whose death-promoting function is inactivated by phosphorylation. It is not known whether any Bcl-2 family members are phosphorylated in response to Epo. However, the levels of Bcl-2 and Bcl-XL dramatically drop after Epo withdrawal (2, 27, 56), suggesting that these Bcl-2 family members are the primary repressors of apoptosis in erythroid cells. Studies are, therefore, in progress to determine if BAD or any other Bcl-2 family members are phosphorylated or altered in expression level in SFFV-infected cells grown in the absence of Epo.
This study provides strong evidence that induction of Epo independence in vitro by SFFV is mediated by the constitutive activation of the PI 3-kinase/Akt pathway and suggests that deregulation of this pathway in vivo may be a critical step in the development of SFFV-induced erythroleukemia in mice. Recent studies have suggested that constitutive activation of the PI 3-kinase/Akt pathway may also play an important role in the development of hematopoietic malignancies and solid tumors in humans. Chronic myelogeneous leukemia and acute lymphoblastic leukemia associated with activation of the Bcr/Abl oncogene are associated with activation of the PI 3-kinase/Akt pathway (57), and PI 3-kinase or Akt or both have been shown to be constitutively activated, or their levels enhanced, in a number of solid human tumors (33, 43, 51, 55). IRS-2 has also been shown to be overexpressed in human pancreatic cancer cell lines (34), and this may lead to deregulation of the PI 3-kinase/Akt pathway in these cells. In many of these studies, inhibitors of PI 3-kinase have been shown to block the proliferation of the malignant cells, suggesting that constitutive activation of the PI 3-kinase pathway is critical for tumor cell growth. Consistent with this idea are recent studies showing that the tumor suppressor gene PTEN functions as a negative regulator of the PI 3-kinase/Akt pathway and that mutations in this gene can increase susceptibility to cancer (36, 69). Thus, constitutive activation of the PI 3-kinase/Akt pathway may be a common event leading to leukemia and cancer, making it an attractive target for therapeutic intervention.
ACKNOWLEDGMENTS
We thank Karen Cannon and Angelo Spadaccini for helpful assistance in the preparation of the manuscript and Patrick Mayeux for generously providing the BaF3/ZERO-ER cell line.
REFERENCES
- 1.Akimoto K, Takahashi R, Moriya S, Nishioka N, Takayanagi J, Kimura K, Fukui Y, Osada S, Mizuno K, Hirai S, Kazlauskas A, Ohno S. EGF or PDGF receptors activate atypical PKCλ through phosphatidylinositol 3-kinase. EMBO J. 1996;15:788–798. [PMC free article] [PubMed] [Google Scholar]
- 2.Bao H, Jacobs-Helber S M, Lawson A E, Penta K, Wickrema A, Sawyer S T. Protein kinase B (c-Akt), phosphatidylinositol 3-kinase, and STAT5 are activated by erythropoietin (EPO) in HCD57 erythroid cells but are constitutively active in an EPO-independent, apoptosis-resistant subclone (HCD57-SREI cells) Blood. 1999;93:3757–3773. [PubMed] [Google Scholar]
- 3.Barber D L, Corless C N, Xia K, Roberts T M, D'Andrea A D. Erythropoietin activates Raf1 by an Shc-independent pathway in CTLL-EPO-R cells. Blood. 1997;89:55–64. [PubMed] [Google Scholar]
- 4.Boudewijn B M, Coffer P J. Protein kinase B (c-Akt) in phosphatidylinositol 3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 5.Carroll M P, Spivak J L, McMahon M, Weich N, Rapp U F. Erythropoietin induces Raf-1 activation and Raf-1 is required for erythropoietin-mediated proliferation. J Biol Chem. 1991;266:14964–14969. [PubMed] [Google Scholar]
- 6.Chin H, Arai A, Wakao H, Kamiyama R, Miyasaka N, Miura O. Lyn physically associates with the erythropoietin receptor and may play a role in activation of the Stat5 pathway. Blood. 1998;91:3734–3745. [PubMed] [Google Scholar]
- 7.Chung S W, Wolff L, Ruscetti S. Transmembrane domain of the envelope gene of a polycythemia-inducing retrovirus determines erythropoietin-independent growth. Proc Natl Acad Sci USA. 1989;86:7957–7960. doi: 10.1073/pnas.86.20.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Constanthinescu S, Liu X, Beyer W, Fallon A, Shekar S, Henis Y I, Smith S O, Lodish H F. Activation of the erythropoietin receptor by the gp55-P viral envelope protein is determined by a single amino acid in its transmembrane domain. EMBO J. 1999;18:3334–3347. doi: 10.1093/emboj/18.12.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damen J E, Liu L, Cutler R L, Krystal G. Erythropoietin stimulates the tyrosine phosphorylation of Shc and its association with Grb2 and a 145-Kd tyrosine phosphorylated protein. Blood. 1993;82:2296–2303. [PubMed] [Google Scholar]
- 10.Damen J E, Mui A L-F, Puil L, Pawson T, Krystal G. Phosphatidylinositol 3-kinase associates, via its Src homology 2 domains, with the activated erythropoietin receptor. Blood. 1993;81:3204–3210. [PubMed] [Google Scholar]
- 11.Damen J E, Wakao H, Miyajima A, Krosl J, Humphries R K, Cutler R L, Krystal G. Tyrosine 343 in the erythropoietin receptor positively regulates erythropoietin-induced cell proliferation and Stat5 activation. EMBO J. 1995;14:5557–5568. doi: 10.1002/j.1460-2075.1995.tb00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damen J E, Cutler R L, Jiao H, Yi T, Krystal G. Phosphorylation of tyrosine 503 in the erythropoietin receptor (EpR) is essential for binding the P85 subunit of phosphatidylinositol (PI) 3-kinase and for EpR-associated PI 3-kinase activity. J Biol Chem. 1995;270:23402–23408. doi: 10.1074/jbc.270.40.23402. [DOI] [PubMed] [Google Scholar]
- 13.Damen J E, Liu L, Rosten P, Humphries R K, Jefferson A B, Majerus P W, Krystal G. The 145-kDa protein induced to associate with Shc by multiple cytokines is an inositol tetraphosphate and phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase. Proc Natl Acad Sci USA. 1996;93:1689–1693. doi: 10.1073/pnas.93.4.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 15.Del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997;278:687–689. doi: 10.1126/science.278.5338.687. [DOI] [PubMed] [Google Scholar]
- 16.Devemy E, Billat C, Haye B. Activation of Raf-1 and mitogen-activated proteins kinases by erythropoietin and inositolphosphate-glycan in normal and erythroid progenitor cells: involvement of protein kinase C. Cell Signal. 1997;9:41–46. doi: 10.1016/s0898-6568(96)00095-2. [DOI] [PubMed] [Google Scholar]
- 17.Dusanter-Fourt I N C, Lacombe C, Muller O, Billat C, Fischer S, Mayeux P. Erythropoietin induces the tyrosine phosphorylation of its own receptor in human erythropoietin-responsive cells. J Biol Chem. 1992;267:10670–10675. [PubMed] [Google Scholar]
- 18.Franke T F, Yang S I, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 19.Franke T F, Kaplan D R, Cantley L C, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 20.Gobert S, Porteu F, Pallu S, Muller O, Sabbah M, Dusanter-Fourt I, Courtois G, Lacombe C, Gisselbrecht S, Mayeux P. Tyrosine phosphorylation of the erythropoietin receptor: role for differentiation and mitogenic signal transduction. Blood. 1995;86:598–606. [PubMed] [Google Scholar]
- 21.Gobert S, Chretien S, Gouilleux F, Muller O, Pallard C, Dusanter-Fourt I, Groner B, Lacombe C, Gisselbrecht S, Mayeux P. Identification of tyrosine residues within the intracellular domain of the erythropoietin receptor crucial for STAT5 activation. EMBO J. 1996;15:2434–2441. [PMC free article] [PubMed] [Google Scholar]
- 22.Gouilleux F, Pallard C, Dusanter-Fourt I, Wakao H, Haldosen L-A, Norstedt G, Levy D, Groner B. Prolactin, growth hormone, erythropoietin and granulocyte-macrophage colony stimulating factor induce MGF-Stat5 DNA binding activity. EMBO J. 1995;14:2005–2013. doi: 10.1002/j.1460-2075.1995.tb07192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grammar T C, Blenis J. Evidence for MEK-independent pathways regulating the prolonged activation of the ERK-MAP kinases. Oncogene. 1997;14:1635–1642. doi: 10.1038/sj.onc.1201000. [DOI] [PubMed] [Google Scholar]
- 24.Hanazono Y, Chiba S, Sasaki K, Mano H, Yazaki Y, Hirai H. Erythropoietin induces tyrosine phosphorylation and kinase activity of the c-fps/fes proto-oncogene product in human erythropoietin-responsive cells. Blood. 1993;81:3193–3196. [PubMed] [Google Scholar]
- 25.Haseyama Y, Sawada K, Oda A, Koizumi K, Takano H, Tarumi T, Nishio M, Handa M, Ikeda Y, Koike T. Phosphatidylinositol 3-kinase is involved in the protection of primary cultured human erythroid precursor cells from apoptosis. Blood. 1999;94:1568–1577. [PubMed] [Google Scholar]
- 26.He T C, Zhuang H, Jiang N, Waterfield M D, Wojchowski D M. Association of the p85 regulatory subunit of phosphatidylinositol 3-kinase with an essential erythropoietin receptor subdomain. Blood. 1993;82:3530–3538. [PubMed] [Google Scholar]
- 27.Jacobs-Helber S M, Wickrema A, Birrer M J, Sawyer S T. AP1 regulation of proliferation and initiation of apoptosis in erythropoietin-dependent erythroid cells. Mol Cell Biol. 1998;18:3699–3707. doi: 10.1128/mcb.18.7.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaster R, Bittorf T, Brock J. Involvement of phosphatidylinositol 3-kinase in the mediation of erythropoietin-induced activation of p70S6k. Cell Signal. 1997;9:175–179. doi: 10.1016/s0898-6568(96)00138-6. [DOI] [PubMed] [Google Scholar]
- 29.Kavanaugh W, Pot M D A, Chin S M, Deuter-Reinhard M, Jefferson A B, Norris F A, Masiarz F R, Cousens L S, Majerus P W, Williams L T. Multiple forms of an inositol polyphosphate-5-phosphatase form signaling complexes with Shc and Grb2. Curr Biol. 1996;6:438–445. doi: 10.1016/s0960-9822(02)00511-0. [DOI] [PubMed] [Google Scholar]
- 30.Klingmüller U, Bergelson S, Hsiao J G, Lodish H F. Multiple tyrosine residues in the cytosolic domain of the erythropoietin receptor promote activation of STAT5. Proc Natl Acad Sci USA. 1996;93:8324–8328. doi: 10.1073/pnas.93.16.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klingmüller U, Wu H, Hsiao J G, Toker A, Duckworth B C, Cantley L C, Lodish H F. Identification of a novel pathway important for proliferation and differentiation of primary erythroid progenitors. Proc Natl Acad Sci USA. 1997;94:3016–3021. doi: 10.1073/pnas.94.7.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klippel A, Kavanaugh W M, Pot D, Williams L T. A specific product of phosphatidylinositol 3-kinase directly activates the protein kinase Akt through its pleckstrin homology domain. Mol Cell Biol. 1997;17:338–344. doi: 10.1128/mcb.17.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi M, Nagata S, Iwasaki T, Yanagihara K, Saitoh I, Karouji Y, Ihara S, Fukui Y. Dedifferentiation of adenocarcinomas by activation of phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1999;96:4874–4879. doi: 10.1073/pnas.96.9.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kornmann M, Maruyama H, Bergmann U, Tangvoranuntakul P, Beger H G, White M F, Korc M. Enhanced expression of the insulin receptor substrate-2 docking protein in human pancreatic cancer. Cancer Res. 1998;58:4250–4254. [PubMed] [Google Scholar]
- 35.Lecoq-Lafon C, Verdier F, Fichelson S, Chretien S, Gisselbrecht S, Lacombe C, Mayeux P. Erythropoietin induces the tyrosine phosphorylation of GAB1 and its association with SHC, SHP2 and phosphatidylinositol 3-kinase. Blood. 1999;93:2578–2585. [PubMed] [Google Scholar]
- 36.Li D M, Sun H. PTEN/MMAC1/TEP1 suppresses the tumorigenicity and induces G1 cell cycle arrest in human glioblastoma cells. Proc Natl Acad Sci USA. 1998;95:15406–15411. doi: 10.1073/pnas.95.26.15406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Machide M, Mano H, Todokoro K. Interleukin 3 and erythropoietin induce association of Vav with Tec kinase through Tec homology domain. Oncogene. 1995;11:619–625. [PubMed] [Google Scholar]
- 38.Mayeux P, Dusanter-Fourt I, Muller O, Mauduit P, Sabbah M, Druker B, Vainchenker W, Fischer S, Lacombe C, Gisselbrecht S. Erythropoietin induces the association of phosphatidylinositol 3′-kinase with a tyrosine-phosphorylated complex containing the erythropoietin receptor. Eur J Biochem. 1993;216:821–828. doi: 10.1111/j.1432-1033.1993.tb18203.x. [DOI] [PubMed] [Google Scholar]
- 39.Miura O, D'Andrea A, Kabat D, Ihle J N. Induction of tyrosine phosphorylation by the erythropoietin receptor correlates with mitogenesis. Mol Cell Biol. 1991;11:4895–4902. doi: 10.1128/mcb.11.10.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miura O, Nakamura N, Ihle J N, Aoki N. Erythropoietin-dependent association of phosphatidylinositol 3-kinase with tyrosine-phosphorylated erythropoietin receptor. J Biol Chem. 1994;269:614–620. [PubMed] [Google Scholar]
- 41.Miura O, Nakamura N, Quelle F W, Witthuhn B A, Ihle J N, Aoki N. Erythropoietin induces association of the JAK2 protein tyrosine kinase with the erythropoietin receptor in vivo. Blood. 1994;84:1501–1507. [PubMed] [Google Scholar]
- 42.Miura Y, Miura O, Ihle J N, Aoki N. Activation of the mitogen-activated protein kinase pathway by the erythropoietin receptor. J Biol Chem. 1994;269:29962–29969. [PubMed] [Google Scholar]
- 43.Moore S M, Rintoul R C, Walker T R, Chilvers E R, Haslett C, Sethi T. The presence of a constitutively active phosphoinositide 3-kinase in small cell lung cancer cells mediates anchorage-independent proliferation via a protein kinase B and p70s6k-dependent pathway. Cancer Res. 1998;15:5239–5247. [PubMed] [Google Scholar]
- 44.Moriya S, Kazlauskas A, Akimoto K, Hirai S-I, Mizuno K, Takenawa T, Fukui Y, Watanabe Y, Ozaki S, Ohno S. Platelet-derived growth factor activates protein kinase Cɛ through redundant and independent signaling pathways involving phospholipase Cγ or phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1996;93:151–155. doi: 10.1073/pnas.93.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muszynski K W, Ohashi T, Hanson C, Ruscetti S K. Both the polycythemia- and anemia-inducing strains of Friend spleen focus-forming virus induce constitutive activation of the Raf-1 mitogen-activated protein kinase signal transduction pathway. J Virol. 1998;72:919–925. doi: 10.1128/jvi.72.2.919-925.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakanishi H, Brewer K A, Exton J H. Activation of the ζ enzyme of protein kinase C by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1993;268:13–16. [PubMed] [Google Scholar]
- 47.Nishida K, Yoshida Y, Itoh M, Fukada T, Ohtani T, Shirogane T, Atsumi T, Takahashi-Tezuka M, Ishihara K, Hibi M, Hirano T. Gab-family adapter proteins act downstream of cytokine and growth factor receptors and T- and B-cell antigen receptors. Blood. 1999;93:1809–1816. [PubMed] [Google Scholar]
- 48.Ohashi T, Masuda M, Ruscetti S K. Induction of sequence-specific DNA-binding factors by erythropoietin and the spleen focus-forming virus. Blood. 1995;85:1454–1462. [PubMed] [Google Scholar]
- 49.Penta K, Sawyer S T. Erythropoietin induces the tyrosine phosphorylation, nuclear translocation, and DNA binding of STAT1 and STAT5 in erythroid cells. J Biol Chem. 1995;270:31282–31287. doi: 10.1074/jbc.270.52.31282. [DOI] [PubMed] [Google Scholar]
- 50.Persons D A, Paulson R F, Loyd M R, Herley M T, Bodner S M, Bernstein A, Correll P H, Ney P A. Fv2 encodes a truncated form of the Stk receptor tyrosine kinase. Nat Genet. 1999;23:159–165. doi: 10.1038/13787. [DOI] [PubMed] [Google Scholar]
- 51.Phillips W A, St. Clair F, Munday A D, Thomas R J, Mitchell C A. Increased levels of phosphatidylinositol 3-kinase activity in colorectal tumors. Cancer. 1998;1:41–47. doi: 10.1002/(sici)1097-0142(19980701)83:1<41::aid-cncr6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 52.Quelle F W, Wang D, Nosaka T, Thierfelder W E, Stravopodis D, Weinstein Y, Ihle J N. Erythropoietin induces the activation of STAT5 through association with specific tyrosines on the receptor that are not required for a mitogenic response. Mol Cell Biol. 1996;16:1622–1631. doi: 10.1128/mcb.16.4.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruscetti S K. Deregulation of erythropoiesis by the Friend spleen focus-forming virus. Int J Biochem Cell Biol. 1999;31:10–35. doi: 10.1016/s1357-2725(99)00074-6. [DOI] [PubMed] [Google Scholar]
- 54.Ruscetti S K, Janesch N J, Chakraborti A, Sawyer S T, Hankins W D. Friend spleen focus-forming virus induces factor independence in an erythropoietin-dependent erythroleukemia cell line. J Virol. 1990;64:1057–1062. doi: 10.1128/jvi.64.3.1057-1062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shayesteh L, Lu Y, Kuo W L, Baldocchi R, Godfrey T, Collins C, Pinkel D, Powell B, Mills G B, Gray J W. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999;21:64–65. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- 56.Silva M, Grillot D, Benito A, Richard C, Nuñez G, Fernández-Luna J L. Erythropoietin can promote erythroid progenitor survival by repressing apoptosis through Bcl-X1 and Bcl-2. Blood. 1996;88:1576–1582. [PubMed] [Google Scholar]
- 57.Skorski T, Bellacosa A, Nieborowska-Skorska M, Majewski M, Martinez R, Choi J K, Trotta R, Wlodarski P, Perrotti D, Chan T O, Wasik M A, Tsichlis P N, Calabretta B. Transformation of hematopoietic cells by BCR/ABL requires activation of a PI-3k/Akt-dependent pathway. EMBO. 1997;16:6151–6161. doi: 10.1093/emboj/16.20.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takahashi-Tezuka M, Yoshida Y, Fukada T, Ohtani T, Yamanaka Y, Nishida K, Nakajima K, Hibi M, Hirano T. Gab1 acts as an adapter molecule linking the cytokine receptor gp130 to ERK mitogen-activated protein kinase. Mol Cell Biol. 1998;18:4109–4117. doi: 10.1128/mcb.18.7.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tauchi T, Feng G-S, Shen R, Hoatlin M, Bagby G C, Jr, Kabat D, Lu L, Broxmeyer H E. Involvement of SH2-containing phosphotyrosine phosphatase Syp in erythropoietin receptor signal transduction pathways. J Biol Chem. 1995;270:5631–5635. doi: 10.1074/jbc.270.10.5631. [DOI] [PubMed] [Google Scholar]
- 60.Tilbrook P A, Ingley E, Williams J H, Hibbs M L, Klinken S P. Lyn tyrosine kinase is essential for erythropoietin-induced differentiation of J2E erythroid cells. EMBO J. 1997;16:1610–1619. doi: 10.1093/emboj/16.7.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Toker A, Meyer M, Reddy K K, Falck J R, Aneja R, Aneja S, Parra A, Burns D J, Ballas L M, Cantley L C. Activation of protein kinase C family members by the novel polyphosphoinositides PtdIns-3,4-P2 and PtdIns-3,4,5-P3. J Biol Chem. 1994;269:32358–32367. [PubMed] [Google Scholar]
- 62.Torti M, Marti K B, Altshuler D, Yamamoto K, Lapetina E G. Erythropoietin induces p21ras activation and p120GAP tyrosine phosphorylation in human erythroleukemia cells. J Biol Chem. 1992;267:8293–8298. [PubMed] [Google Scholar]
- 63.Verdier F, Chrétien S, Billat C, Gisselbrecht S, Lacombe C, Mayeux P. Erythropoietin induces the tyrosine phosphorylation of insulin receptor substrate-2. J Biol Chem. 1997;272:26173–26178. doi: 10.1074/jbc.272.42.26173. [DOI] [PubMed] [Google Scholar]
- 64.Vlahos C J, Matter W F, Hui K Y, Brown R F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 65.Wakao H, Harada N, Kitamura T, Mui A L-F, Miyajima A. Interleukin 2 and erythropoietin activate STAT5/MGF via distinct pathways. EMBO J. 1995;11:2527–2535. doi: 10.1002/j.1460-2075.1995.tb07250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watowich S S, Hilton D J, Lodish H F. Activation and inhibition of erythropoietin receptor function: role of receptor dimerization. Mol Cell Biol. 1994;14:3535–3549. doi: 10.1128/mcb.14.6.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wickrema A, Uddin S, Sharma A, Chen F, Alsayed Y, Ahmad S, Sawyer S T, Krystal G, Yi T, Nishada K, Hibi M, Hirano T, Platanias L C. Engagement of Gab1 and Gab2 in erythropoietin signaling. J Biol Chem. 1999;274:24469–24474. doi: 10.1074/jbc.274.35.24469. [DOI] [PubMed] [Google Scholar]
- 68.Witthuhn B A, Quelle F W, Silvennoinen O, Yi T, Tang B, Miura O, Ihle J N. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993;74:227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- 69.Wu X, Senechal K, Neshat M S, Whang Y E, Sawyers C L. The PTEN/MMAC1 tumor suppressor phosphatase functions as a negative regulator of the phosphoinositide 3-kinase/Akt pathway. Proc Natl Acad Sci USA. 1998;95:15587–15591. doi: 10.1073/pnas.95.26.15587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamauchi T, Kaburagi Y, Ueki K, Tsuji Y, Stark G R, Kerr I M, Tsushima T, Akanuma Y, Komuro I, Tobe K, Yazaki Y, Kadowaki T. Growth hormone and prolactin stimulate tyrosine phosphorylation of insulin receptor substrate-1, -2, and -3, their association with p85 phosphatidylinositol 3-kinase (PI 3-kinase), and concomitantly PI 3-kinase activation via JAK2 kinase. J Biol Chem. 1998;273:15719–15726. doi: 10.1074/jbc.273.25.15719. [DOI] [PubMed] [Google Scholar]