Abstract
INTRODUCTION:
There is an extensive body of research regarding neurological outcomes following neonatal hypoxic-ischemic encephalopathy (HIE) treated with therapeutic hypothermia (TH), with limited data on growth outcomes. We examined perinatal characteristics associated with postnatal growth in this population.
METHODS:
Convenience cohort of 66 infants with HIE who underwent TH and participated in follow-up at 24 months of age were included. Regression modeling including perinatal anthropomorphics, markers of HIE, and systemic injury was used to evaluate growth at 24 months.
RESULTS:
Birth head circumference was associated with weight (p = 0.036). MRI severity, pH at admission and birth head circumference were associated with height (p = 0.043, p = 0.015 and p = 0.043 respectively). MRI severity and length of intubation were associated with head circumference (p = 0.038 and p < 0.001 respectively).
CONCLUSION:
There was a significant association between specific early factors and growth at 24 months among infants with HIE treated with TH.
INTRODUCTION
Neonatal hypoxic-ischemic encephalopathy (HIE) is one of the most common causes of perinatal brain injury and affects between 1.5 and 6 per 1000 live births [1, 2]. Since therapeutic hypothermia (TH) became standard of care for moderate to severe HIE [3], the majority of available literature has focused on neurological and developmental outcomes in this population. Few studies have described growth outcomes in this population despite risk for feeding problems and systemic injury that could negatively impact growth and potentially be a mediating factor of long-term developmental outcomes. The Eunice Kennedy Shriver National Institute for Child Health and Human Development (NICHD) trial reported slow growth (weight, length and head circumference) at 18–22 months and 6–7 years age in a cohort of term moderate to severe HIE survivors with moderate to severe cerebral palsy (CP) compared to those without CP [4]. Although Preeti et al. described anthropometric measures at one year of age following HIE, the study included neonates who had not undergone TH [5]. To our knowledge, there is no literature describing the association between neonatal characteristics and follow-up growth at 2 years of age in neonates who have been treated with TH.
The potential for systemic injury following perinatal hypoxic-ischemic insults has been well-described, with involvement of at least one system (renal, cardiac, hepatic or pulmonary) in up to nearly 100% of participants in some studies [6, 7]. The relationship between markers of systemic injury and neurodevelopmental outcomes following HIE has been explored. Acute kidney injury (AKI) in asphyxiated newborns has been shown to be a predictor of abnormal brain MRI at 7 to 10 days of age in some studies [8, 9] while hepatic, pulmonary and cardiac involvement have not been shown to be significant predictors of neuroimaging findings [6]. However, the association of systemic injury with anthropometric outcomes in neonates with HIE treated with hypothermia has not been well studied.
The goal of this study was to examine whether there are associations between neonatal variables and anthropometric measurements at twenty-four months of age in neonates who had undergone TH for HIE. Given the high prevalence of multisystem involvement, we evaluated neonatal variables including birth anthropometrics, early markers of HIE, and markers potentially reflecting systemic injury while controlling for sex and socioeconomic factors.
MATERIALS AND METHODS
Study design and participants
A prospective convenience sample of neonates with moderate to severe HIE who underwent treatment with TH in the neonatal intensive care unit at Johns Hopkins Hospital between 2010 and 2015 was utilized. All study participants completed a comprehensive follow-up evaluation at 24 months of age.
Institutional clinical protocol was followed during TH. Eligibility for hypothermia was based on clinical exam using the modified Sarnat criteria [10, 11] and blood gas obtained from the umbilical cord or during the first hour of life with pH <7.00 or a base deficit > 16 mmol/L. In the absence of available blood gases or pH < 7.15 or base deficit > 10 mmol/L, additional criteria were used to facilitate the diagnosis of HIE including an Apgar score of less than 5 at 10 min of life, requirement of ventilator assistance at or greater than 10 min of life, and history of an acute perinatal event. Infants born at 35 weeks or greater and those with a birth weight of 1.8 kg or greater were included in the study, as they met criteria for TH. Exclusion criteria comprise neonates with genetic abnormalities, in-utero drug exposure or prenatal infections, neonates ineligible for TH or those in foster care. Approval was obtained from the institutional research board at the home institution.
Independent variables
Neonatal characteristics collected included birth weight (grams), length (cm) and head circumference (cm), gestational age (weeks) and sex. Factors relating to HIE included pH (obtained from cord blood or within the first hour of life), base excess (obtained from cord blood or within the first hour of life), Apgar scores at one and five minutes of life and brain MRI at 10 to 16 days of life, categorized using the NICHD classification. Per NICHD criteria, categories included 0-normal; 1A-minimal cerebral lesions only with no involvement of basal ganglia (BG), thalamus (T), anterior limb of the internal capsule (ALIC) or posterior limb of the internal capsule (PLIC), and no area of watershed infarction; 1B-more extensive cerebral lesions without basal ganglia and thalamic (BGT), PLIC or ALIC involvement or infarction; 2A-any BGT, ALIC or PLIC involvement or watershed infarction noted without any other cerebral lesions; 2B-involvement of either BGT, ALIC or PLIC or area of infarction and additional cerebral lesions; and 3-cerebral hemispheric devastation [12, 13]. Additional factors related to more systemic injury were also recorded, including total duration of intubation (in days) required for respiratory support excluding intubation used for the purpose of a procedure, peak creatinine and alanine transaminase levels during the neonate’s NICU stay, and mode of feeding at discharge classified as oral, gavage feeds, gastric tube (G-tube) feeds, or combined feeds. Additionally, type of feeds during the NICU admission including breastmilk, formula or combined feeds were recorded. Lastly, two well-established proxies that included family insurance status (private vs. medical assistance) and census tract median income using the family’s address at time of the delivery [14, 15] were used to estimate socioeconomic status.
Dependent variables
Beginning in 2010, standard of care for neonates undergoing TH for HIE began including referral for neurodevelopmental follow-up upon discharge. These neonates were followed serially and included a comprehensive evaluation between 18–30 months. Outcomes included in this study are anthropometrics collected at the 24-month visit and included weight, height and head circumference recorded as percentiles and Z-scores.
Statistical analysis
Statistical analysis was completed using the software R Version 4.0. Descriptive statistics for neonatal characteristics were summarized using mean and standard deviation, median and interquartile range as appropriate. Since percentiles are bounded by 0 and 1 and likely not normally distributed, we used Z scores to estimate predictors of growth outcomes instead of percentiles. Assumptions for linear regression using Z scores for anthropometric outcomes were checked using the White test for heteroscedasticity, the Anderson Darling test to check normality and visual examination of the residuals to gauge linearity of the data. We used linear regression to explore the relationship between potential predictors and anthropometric outcomes at 24 months of age. These results were adjusted for birth anthropometric Z scores in order to investigate post-natal growth that would be more related to HIE. Results were also adjusted for sex and census-tract median income that might also impact post-natal growth. p value < 0.05 was considered statistically significant.
RESULTS
Sample characteristics
42 infants of an initial cohort of 66 neonates who underwent TH completed follow-up evaluation at 24 months of age. 9 (13.6%) out of 66 were deceased at the time of follow-up. Mean gestational age for our cohort was 38.9 weeks with a mean birth weight of 3273.8 g, mean birth length of 50 cm and mean head circumference of 34 cm. While 45% of our participants had a normal MRI (Grade 0), 17% had either NICHD Grade 2 A or 2B and 10% had NICHD Grade 3. Participant characteristics are summarized in Table 1.
Table 1.
Participant characteristics.
| Characteristics | Total | Mean | SD | N (%) |
|---|---|---|---|---|
| Base Deficit | 57 | −16.2 | 6.6 | |
| pH | 64 | 6.9 | 0.2 | |
| Apgar score ≤ 5 (1 min) | 66 | 63 (95%) | ||
| Apgar score ≤ 5 (5 min) | 66 | 49 (74%) | ||
| Apgar score ≤ 5 (10 min) | 59 | 31 (53%) | ||
| Peak ALT | 66 | 254.7 | 788.0 | |
| Peak creatinine | 66 | 1.0 | 0.5 | |
| Gestational age (weeks) | 66 | 38.9 | 1.7 | |
| Birth weight (g) | 66 | 3273.8 | 554.2 | |
| Birth head circumference (cm) | 66 | 34.0 | 1.8 | |
| Birth length (cm) | 66 | 50.0 | 3.4 | |
| Duration of intubation (days) | 66 | 6.0 | 9.6 | |
| Mode of nutrition | 58 | |||
| Breastmilk (1) | 29 (50%) | |||
| Formula (2) | 10 (17%) | |||
| Combined (3) | 19 (33%) | |||
| Census tract median income (thousands) | 66 | 68.2 | 30.3 | |
| Feeding | 58 | |||
| By mouth (1) | 46 (79%) | |||
| NG or NJ Tube (2) | 6 (10%) | |||
| G-Tube (3) | 3 (5%) | |||
| Combined (4) | 3 (5%) | |||
| MRI | 58 | |||
| 0 | 26 (45%) | |||
| 1A | 4 (7%) | |||
| 1B | 12 (21%) | |||
| 2A | 3 (5%) | |||
| 2B | 7 (12%) | |||
| 3 | 6 (10%) | |||
| Sex | 66 | |||
| Males | 37 (56%) | |||
| Females | 29 (44%) | |||
| Growth outcomes at 24 months | ||||
| G-Tube | 39 | 4 (10%) | ||
| Head circumference (Z score) | 40 | −0.17 | 1.28 | |
| Weight (Z score) | 42 | −0.07 | 1.04 | |
| Height (Z score) | 41 | −0.37 | 1.12 |
Predictors of head circumference at 24 months
Mean head circumference Z score at 24 months of age was −0.17. Birth anthropometrics and gestational age were not significant predictors of head circumference at 24 months. MRI NICHD grade was significantly associated with head circumference outcomes (p < 0.001), with increasing severity of MRI predicting lower head circumference Z scores (Fig. 1). This association remained significant after adjusting for birth head circumference Z score as well as sex and census-tract median income (p < 0.001). pH, base deficit and Apgar scores were not predictive of head circumference outcomes. Duration of intubation in the NICU was also a significant predictor of head circumference at 24 months (p = 0.022), with an increase in the number of days of intubation predicting lower head circumference Z scores at 24 months. This association remained significant even after adjusting for birth head circumference Z scores as well as sex and census-tract median income (p = 0.025). Duration of intubation was significantly correlated with MRI with a multicollinearity value of 0.476. Interestingly, neither marker of systemic injury, peak creatinine nor alanine transaminase levels in the first 24 h of life predicted head circumference outcomes. Mode of feeding during the NICU admission and type of feeding at discharge also did not predict head circumference outcomes. These results are summarized in Table 2.
Fig. 1. MRI NICHD grade is significantly associated with head circumference at 24 months.
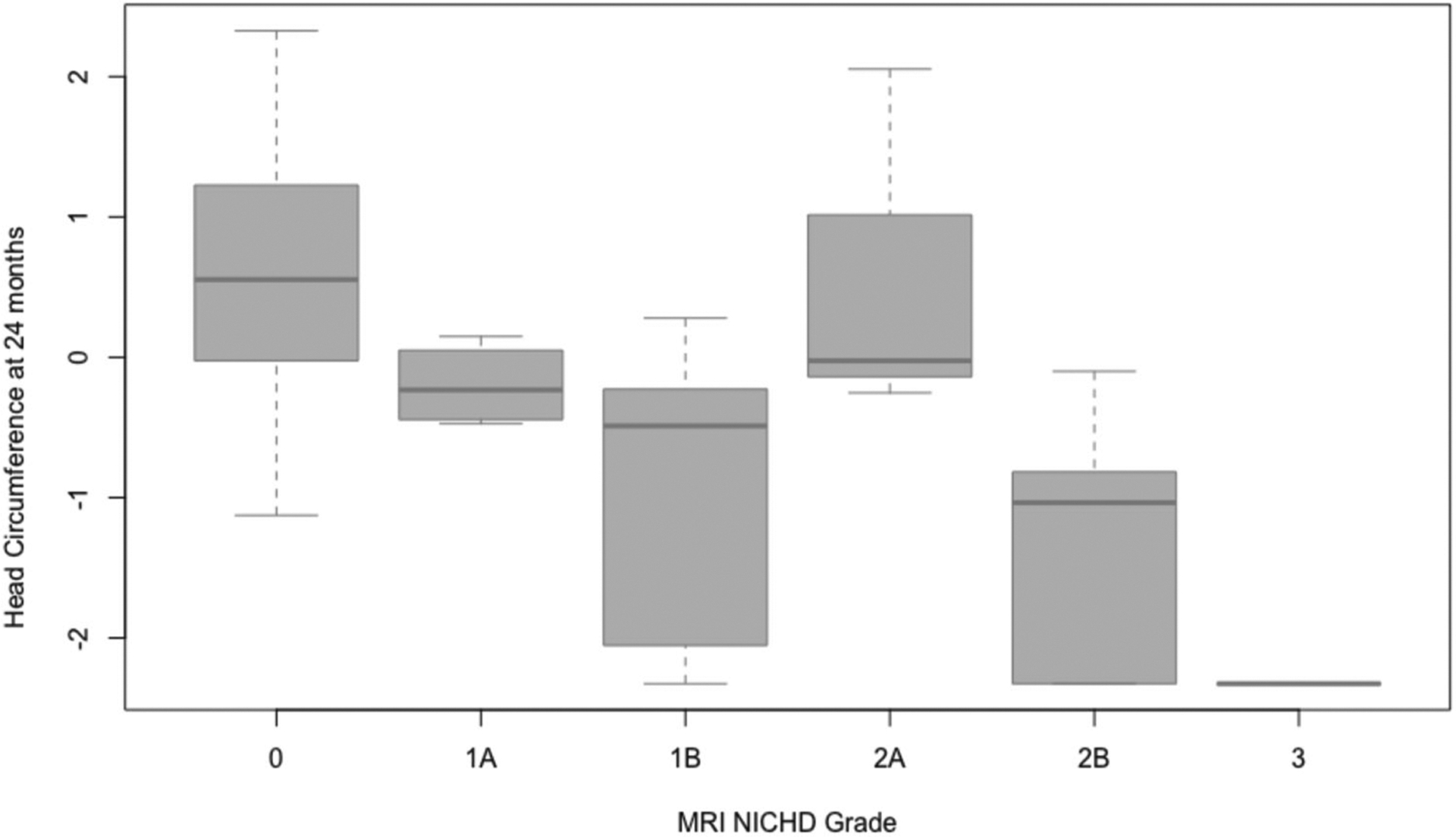
Figure 1 includes box and whisker plots showing the association between MRI NICHD grade obtained at 10–16 days of life following therapeutic hypothermia (TH) and head circumference at 24 months. MRI NICHD grade was significantly associated with head circumference outcomes (p < 0.001), with increasing severity of MRI predicting lower head circumference Z scores, which persisted after adjusting for sex and census-tract median income.
Table 2.
Factors associated with head circumference at 24 months.
| Unadjusted | Adjusted for sex, median income and baseline head circumference Z score | ||||||
|---|---|---|---|---|---|---|---|
| Beta | 95% CI | P | Beta | 95% CI | p | ||
| Formula | 40 | −0.46 | (−1.67, 0.74) | 0.453 | −0.41 | (−1.61, 0.79) | 0.511 |
| Combined | 40 | 0.12 | (−0.76, 1.00) | 0.790 | 0.43 | (−0.46, 1.33) | 0.349 |
| Duration of intubation | 40 | −0.05 | (−0.08, −0.01) | 0.022 | −0.04 | (−0.08, 0.00) | 0.038 |
| Birth head circumference (cm) | 40 | 0.12 | (−0.10, 0.33) | 0.305 | 0.11 | (−0.15, 0.37) | 0.400 |
| Birth length (cm) | 40 | −0.04 | (−0.15, 0.07) | 0.477 | −0.06 | (−0.19, 0.07) | 0.365 |
| MRI NICHD Grade | 38 | −0.48 | (−0.66, −0.30) | <0.001 | −0.47 | (−0.67, −0.28) | <0.001 |
| Mode of feeding at discharge | 40 | −0.22 | (−0.69, 0.25) | 0.365 | −0.26 | (−0.73, 0.21) | 0.291 |
| Birth weight (g) | 40 | 0.00 | (0.00, 0.00) | 0.874 | 0.00 | (0.00, 0.00) | 0.577 |
| Gestational age | 40 | −0.06 | (−0.36, 0.23) | 0.677 | −0.14 | (−0.45, 0.17) | 0.381 |
| Peak creatinine | 40 | 0.08 | (−0.57, 0.73) | 0.806 | 0.06 | (−0.60, 0.72) | 0.854 |
| Peak ALT | 40 | 0.00 | (0.00, 0.00) | 0.618 | 0.00 | (0.00, 0.00) | 0.300 |
| Apgar score (1 min) | 40 | 0.21 | (−0.02, 0.43) | 0.080 | 0.23 | (−0.01, 0.46) | 0.066 |
| Apgar score (5 min) | 40 | 0.12 | (−0.06, 0.29) | 0.216 | 0.18 | (−0.01, 0.36) | 0.077 |
| Apgar score (10 min) | 36 | 0.02 | (−0.21, 0.24) | 0.894 | 0.08 | (−0.17, 0.32) | 0.552 |
| pH | 40 | −0.64 | (−3.38, 2.10) | 0.650 | −0.58 | (−3.38, 2.22) | 0.686 |
| Base deficit | 35 | 0.00 | (−0.06, 0.06) | 0.977 | 0.00 | (−0.06, 0.06) | 0.968 |
Predictors of weight at 24 months
While birth weight and birth length were not significantly associated with weight at 24 months, birth head circumference was found to be a significant predictor of weight Z scores at 24 months of age (p = 0.039), with each centimeter increase in head circumference associated with an increase in the weight Z score (Fig. 2). This significance persisted after adjusting for sex and census-tract median income (p = 0.036). Gestational age did not predict weight Z scores in this cohort of term infants. Factors related to hypoxic injury including pH, base deficit, Apgar scores and MRI NICHD grades were not significant predictors of weight outcomes at 24 months. Duration of intubation in the NICU was not related to weight at 24 months. Importantly, markers of systemic injury including peak creatinine and alanine transaminase levels did not correlate with weight outcomes. Additionally, neither the type of feeding during admission nor mode of feeding at discharge predicted weight outcomes at 24 months of age. These results are summarized in Table 3.
Fig. 2. Birth head circumference in neonates with HIE significantly predicts weight at 24 months.
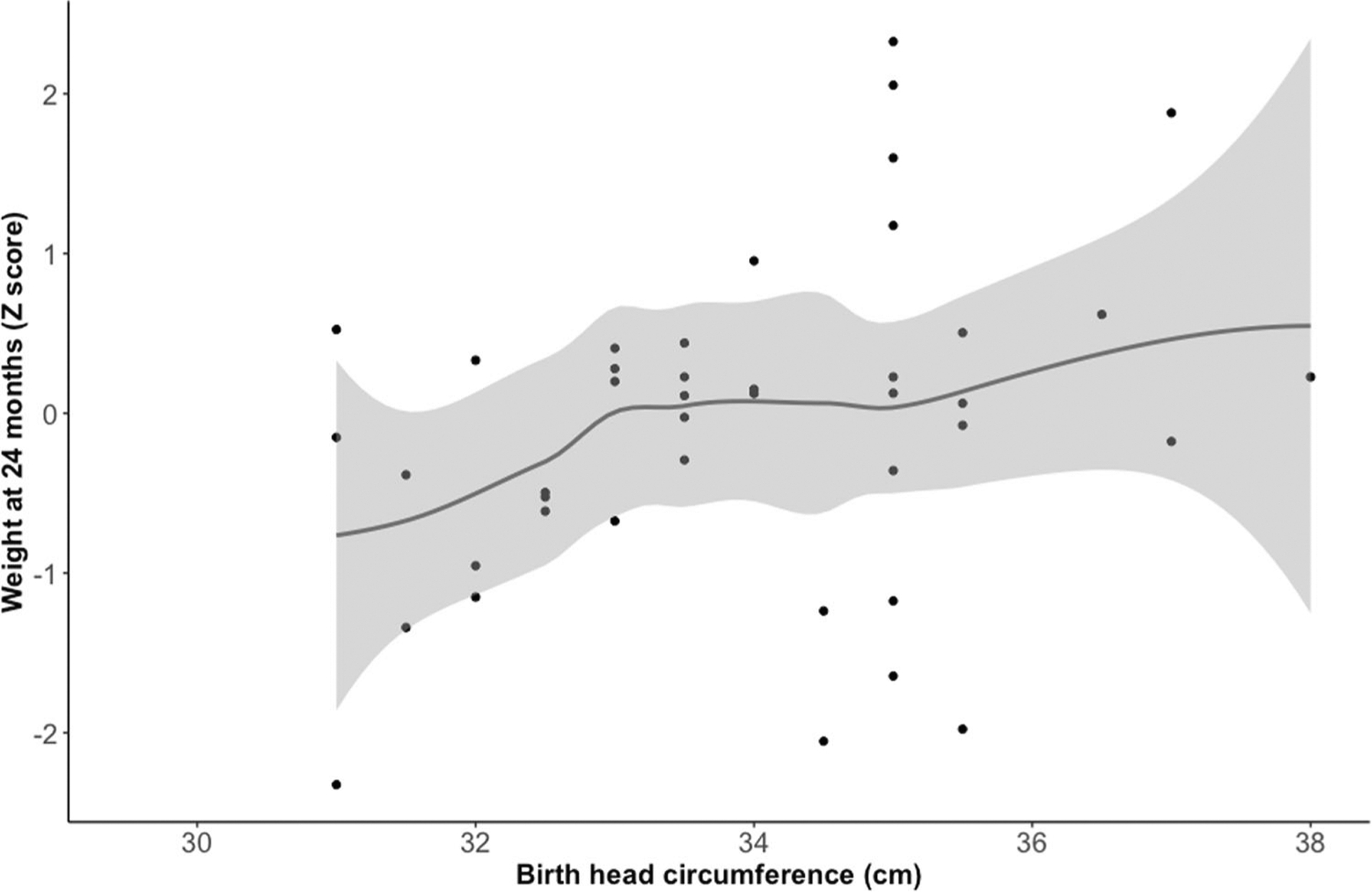
Figure 2 includes a scatterplot showing the relationship between birth head circumference and weight Z scores at 24 months of age. Birth head circumference was found to significantly predict weight Z scores at 24 months (p = 0.039), which persisted after adjusting for sex and census-tract median income. Each centimeter increase in head circumference associated with an increase in the weight Z score.
Table 3.
Factors associated with weight at 24 months.
| Unadjusted | Adjusted for sex, median income and baseline weight Z score | ||||||
|---|---|---|---|---|---|---|---|
| Beta | 95% CI | P | Beta | 95% CI | P | ||
| Formula | 42 | −0.60 | (−1.50, 0.31) | 0.203 | −0.38 | (−1.33, 0.57) | 0.437 |
| Combined | 42 | −0.16 | (−0.86, 0.54) | 0.654 | −0.09 | (−0.82, 0.63) | 0.802 |
| Duration of intubation | 42 | −0.02 | (−0.05, 0.01) | 0.316 | −0.02 | (−0.05, 0.01) | 0.311 |
| Birth head circumference (cm) | 42 | 0.19 | (0.02, 0.37) | 0.039 | 0.23 | (0.02, 0.44) | 0.036 |
| Birth length (cm) | 42 | 0.06 | (−0.04, 0.15) | 0.244 | 0.06 | (−0.05, 0.17) | 0.298 |
| MRI NICHD Grade | 40 | −0.11 | (−0.28, 0.06) | 0.200 | −0.11 | (−0.28, 0.06) | 0.214 |
| Mode of feeding at discharge | 42 | −0.01 | (−0.42, 0.41) | 0.980 | 0.02 | (−0.39, 0.44) | 0.909 |
| Birth weight (g) | 42 | 0.00 | (0.00, 0.00) | 0.056 | 0.00 | (0.00, 0.00) | 0.064 |
| Gestational age | 42 | 0.04 | (−0.19, 0.26) | 0.744 | −0.12 | (−0.39, 0.15) | 0.380 |
| Peak creatinine | 42 | 0.06 | (−0.47, 0.58) | 0.837 | 0.10 | (−0.43, 0.64) | 0.702 |
| Peak ALT | 42 | 0.00 | (0.00, 0.00) | 0.898 | 0.00 | (0.00, 0.00) | 0.859 |
| Apgar score (1 min) | 42 | 0.03 | (−0.15, 0.21) | 0.756 | 0.04 | (−0.14, 0.23) | 0.653 |
| Apgar score (5 min) | 42 | 0.02 | (−0.13, 0.18) | 0.772 | 0.04 | (−0.12, 0.20) | 0.652 |
| Apgar score (10 min) | 38 | −0.07 | (−0.25, 0.12) | 0.477 | −0.07 | (−0.26, 0.12) | 0.472 |
| pH | 42 | 0.34 | (−1.79, 2.48) | 0.756 | 0.32 | (−1.85, 2.49) | 0.772 |
| Base deficit | 37 | 0.00 | (−0.05, 0.05) | 0.936 | 0.01 | (−0.04, 0.07) | 0.670 |
Predictors of height at 24 months
Birth head circumference was a significant predictor of height Z scores at 24 months after adjusting for sex and census-tract median income (p = 0.043), with increase in birth head circumference predicting higher height Z scores. MRI NICHD grades reflecting greater anatomic involvement were significantly associated with lower height Z scores (p = 0.017), which remained statistically significant after adjusting for birth length Z score as well as sex and median income (p = 0.043) (Fig. 3). pH (obtained from cord blood or during the first hour of life) was also a significant predictor of height outcomes (p = 0.017), which persisted after adjusting for birth length Z score, sex, and median income (p = 0.015). Specifically, a greater degree of acidosis was associated with lower height Z scores at 24 months. Apgar scores and base deficit did not appear to be significant predictors of height. Additionally, markers of systemic injury, mode of feeding in the NICU and type of feeding at discharge did not significantly predict height, nor did the duration of intubation in the neonatal period. These results are summarized in Table 4.
Fig. 3. MRI NICHD grade significantly predicts height at 24 months.
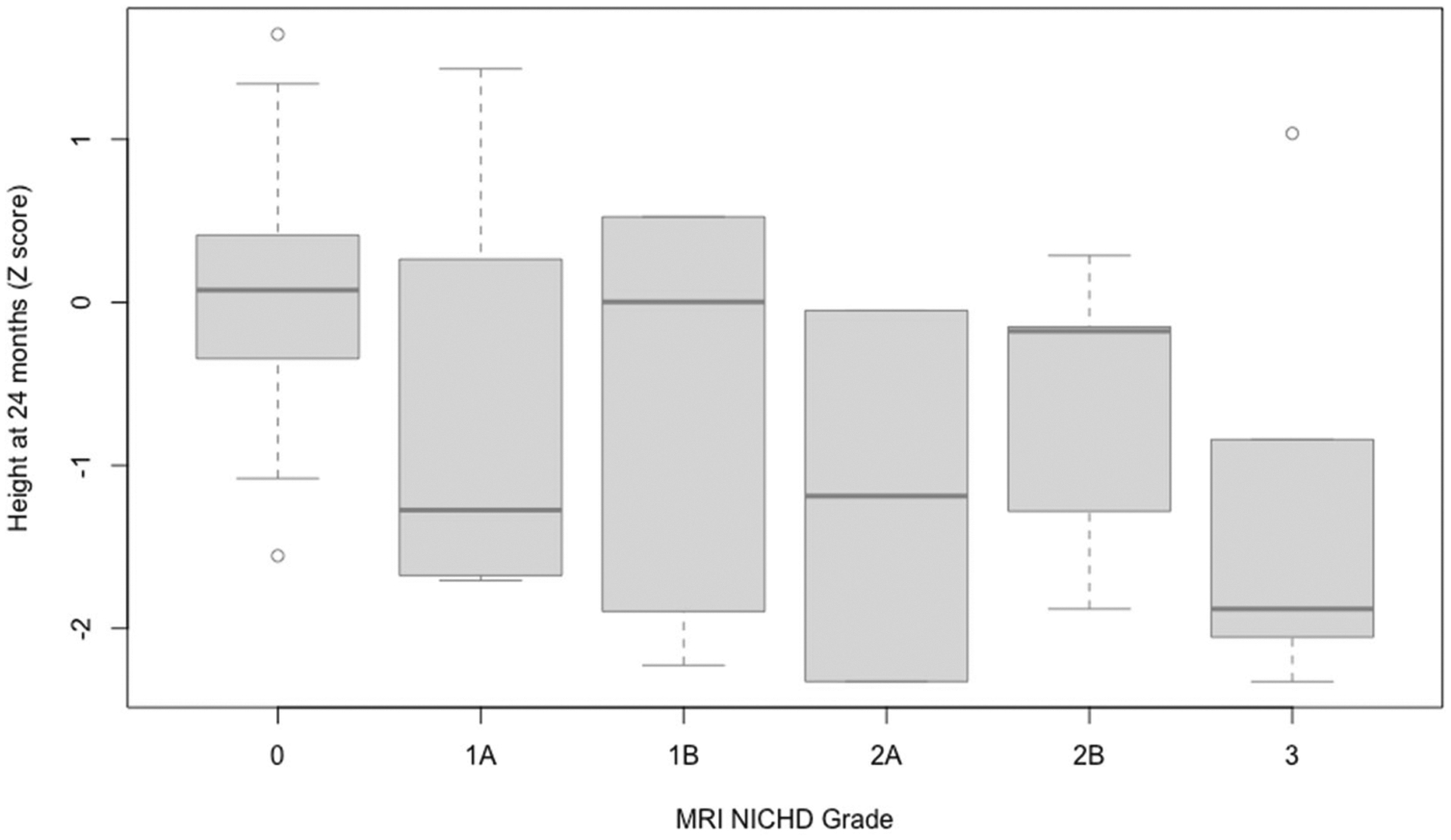
Figure 3 includes box and whicker plots showing the association between MRI NICHD grades and height Z scores at 24 months of age. MRI NICHD grades reflecting greater anatomic involvement were significantly associated with lower height Z scores (p = 0.017), which remained statistically significant after adjusting for birth length Z score as well as sex and median income (p = 0.043).
Table 4.
Factors associated with height at 24 months.
| Unadjusted | Adjusted for sex, median income and baseline length Z score | ||||||
|---|---|---|---|---|---|---|---|
| Beta | 95% CI | P | Beta | 95% CI | P | ||
| Formula | 41 | −0.86 | (−1.79, 0.08) | 0.080 | −0.87 | (−1.86, 0.13) | 0.096 |
| Combined | 41 | −0.56 | (−1.30, 0.18) | 0.145 | −0.48 | (−1.28, 0.33) | 0.253 |
| Duration of Intubation | 41 | −0.03 | (−0.06, 0.01) | 0.105 | −0.03 | (−0.06, 0.00) | 0.099 |
| Birth head circumference (cm) | 41 | 0.13 | (−0.06, 0.32) | 0.184 | 0.23 | (0.01, 0.44) | 0.043 |
| Birth length (cm) | 41 | 0.01 | (−0.09, 0.11) | 0.871 | 0.04 | (−0.07, 0.15) | 0.449 |
| MRI NICHD grade | 39 | −0.22 | (−0.40, −0.05) | 0.017 | −0.19 | (−0.37, −0.01) | 0.043 |
| Mode of feeding at discharge | 41 | −0.11 | (−0.50, 0.29) | 0.598 | −0.10 | (−0.50, 0.29) | 0.618 |
| Birth weight (g) | 41 | 0.00 | (0.00, 0.00) | 0.466 | 0.00 | (0.00, 0.00) | 0.259 |
| Gestational age | 41 | 0.06 | (−0.18, 0.30) | 0.620 | −0.01 | (−0.29, 0.27) | 0.971 |
| Peak creatinine | 41 | 0.15 | (−0.42, 0.71) | 0.610 | 0.05 | (−0.54, 0.63) | 0.879 |
| Peak ALT | 41 | 0.00 | (0.00, 0.00) | 0.826 | 0.00 | (0.00, 0.00) | 0.703 |
| Apgar score (1 min) | 41 | 0.07 | (−0.12, 0.26) | 0.476 | 0.11 | (−0.08, 0.31) | 0.271 |
| Apgar score (5 min) | 41 | 0.00 | (−0.16, 0.16) | 0.997 | 0.05 | (−0.11, 0.22) | 0.548 |
| Apgar score (10 min) | 37 | −0.12 | (−0.31, 0.07) | 0.237 | −0.09 | (−0.28, 0.11) | 0.398 |
| pH | 41 | 2.62 | (0.56, 4.69) | 0.017 | 2.74 | (0.63, 4.85) | 0.015 |
| Base deficit | 37 | 0.04 | (−0.02, 0.09) | 0.185 | 0.03 | (−0.02, 0.09) | 0.234 |
DISCUSSION/CONCLUSION
Feeding and growth are factors closely monitored in the neonate after the acute phases of HIE and treatment with TH and are considered when determining when to discharge from the NICU. Even after discharge from the NICU, feeding and growth are closely monitored as a marker of overall health, and factors that are important for neurodevelopment. This study aimed to identify factors during the neonatal course following HIE treated with TH that relate to later growth outcomes. Specifically, we examined early anthropometric measurements, markers of HIE, and biomarkers of systemic injury and the associations with later anthropometric outcomes at age 24 months.
Neonatal growth characteristics, specifically birth anthropometric measurements including head circumference, weight and length, did not generally predict later growth at age 24 months. This is not completely unexpected given that birth weight and height only have mild correlations with later growth in the general population [16]. In contrast, we observed that birth head circumference was associated with weight and height at age 24 months in our study.
Previously, suboptimal head growth in the first year of life was found to be associated with the pattern of brain lesions on neonatal brain MRI that included severe white matter, basal ganglia and thalamic involvement [16, 17]. Consistent with this existing body of literature [16, 17], we observed that worse MRI NICHD grade predicted smaller head circumference at age 24 months. This is likely related to a combination of reduced brain volumes secondary to early neurologic injury and subsequent atypical neurodevelopment, leading to a smaller head circumference by age 24 months. Of note, approximately 10% of our sample with moderate to severe HIE treated with TH met criteria of microcephaly by age 24 months, while Mercuri and colleagues observed that 50% of children who had HIE at birth and did not undergo TH, developed microcephaly by age 12 months. Therefore, TH mitigates the overall risk for severe neurologic injury resulting in microcephaly, as has been reported previously [18]. Building on these findings, we observed that longer duration of intubation was also associated with significantly smaller head circumference by age 24 months. Interestingly, our analyses revealed that our predictors of head circumference at age 24 months, MRI NICHD scores and days of intubation are moderately correlated (0.48). Therefore, it is possible that more severe neurologic injury leads to worse pulmonary function (i.e., central causes of respiratory problems) and/or worse pulmonary injury (i.e., persistent pulmonary hypertension). Given the evidence that fetal hypoxemia associated with severe HIE exacerbates pulmonary vasoconstriction [19], we speculate that increased length of intubation reflects more extensive cerebral injury and subsequent poor head growth; however, additional work is needed to delineate the mechanism involved in this association.
Our study did not reveal associations between markers of systemic injury, including renal and hepatic injury, and growth outcomes following HIE treated with hypothermia. Biomarkers including acidic pH and higher MRI NICHD grades predicted smaller height, in line with the themes described in the literature [4, 20].
Strengths
This study is one of the very few in the literature which describes comprehensive anthropometric outcomes at 24 months of age in a population who has undergone TH, in addition to reviewing the impact of neonatal characteristics on multiple outcomes. This study is amongst the first to describe the association of three a priori domains, including systemic injury, with anthropometric outcomes.
Limitations
One limitation of this study is that the prospective study cohort was a convenience sample of neonates who were able to follow-up within a 2-year period, which can introduce selection bias and may affect our findings. Another limitation is the non-availability of data regarding parental anthropometric measurements, which was not recorded in the electronic medical record. This represents an important area for exploration in future research. Additionally, the peak creatinine values in this study reflect the highest values during the neonate’s NICU stay, including the first 24 h. Thus, peak creatinine may have been impacted by maternal levels and not provided the most precise measure of systemic injury following HIE. Future directions may involve exploring the duration of this peak after the second day of life and its association with growth outcomes.
Conclusion
This study found that like in general populations, birth anthropometrics did not correlate well with growth at 2 years of age. The exception was head circumference, where a smaller head circumference was associated with lighter weight and shorter height. In this HIE cohort, both, more severe MRI NICHD grades and longer duration of intubation predicted lower head circumference at 24 months of age. More work is needed to determine if longer duration of intubation is related to severity of illness associated with worse brain injury or as a marker of systemic injury as other markers of systemic injury evaluated were not associated with growth at 2 years in this study. More acidic pH and worse MRI NICHD scores, reflecting increased severity of HIE, were associated with lower height at 24 months of age. This information can aid clinicians in counseling parents regarding later growth outcomes in infants with HIE who have undergone TH. This data is amongst the first of its kind and adds important evidence to the limited literature on long-term growth outcomes in this population.
ACKNOWLEDGEMENTS
We acknowledge the members of the Johns Hopkins Neurosciences Intensive Care Nursery whose collaboration has provided a platform for this research. We are particularly grateful to the participants and their families without whom this research would not be possible.
FUNDING
Vera Joanna Burton was supported by the NINDS/NIH K12-NS001696 during data collection for this project. Vera Joanna Burton, Gwendolyn Gerner, Frances Northington and Raul Chavez-Valdez were all supported in part by NIH/NICHD 1 R01 HD086058-01A1 during the preparation of this manuscript.
Footnotes
COMPETING INTERESTS
The authors declare no competing interests.
STATEMENT OF ETHICS
Study approval statement: The study was approved by the Institutional Review Board at Johns Hopkins University School of Medicine (IRB00133750). Written consent was obtained for the use of study participant data. This is not a clinical trial nor did it contain any experiments with animals.
DATA AVAILABILITY
The dataset of which this paper is based is a small pilot sample and is not retained or publicly archived.
REFERENCES
- 1.Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010;86:329–38. 10.1016/j.earlhumdev.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 2.de Vries LS, Jongmans MJ. Long-term outcome after neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2010;95:F220–4. [DOI] [PubMed] [Google Scholar]
- 3.Kattwinkel J, Perlman JM, Aziz K, Colby C, Fairchild K, Gallagher J, et al. Part 15: Neonatal Resuscitation: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122. Available from: https://www.ahajournals.org/doi/10.1161/CIRCULATIONAHA.110.971119 [DOI] [PubMed] [Google Scholar]
- 4.Vohr BR, Stephens BE, McDonald SA, Ehrenkranz RA, Laptook AR, Pappas A, et al. Cerebral palsy and growth failure at 6 to 7 years. Pediatrics. 2013;132:e905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Preeti S, Kadam A, Kadam S, Vaidya U, Kumar P, Bhagat I, et al. Anthropometric measures as biomarkers of neurodevelopmental outcomes of newborns with moderate to severe hypoxic ischemic encephalopathy. J Neonatal Perinat Med. 2019;12:127–34. [DOI] [PubMed] [Google Scholar]
- 6.Shah P Multiorgan dysfunction in infants with post-asphyxial hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2004;89:152F–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hankins G Neonatal organ system injury in acute birth asphyxia sufficient to result in neonatal encephalopathy. Obstet Gynecol. 2002;99:688–91. [DOI] [PubMed] [Google Scholar]
- 8.Sarkar S, Askenazi DJ, Jordan BK, Bhagat I, Bapuraj JR, Dechert RE, et al. Relationship between acute kidney injury and brain MRI findings in asphyxiated newborns after therapeutic hypothermia. Pediatr Res. 2014;75:431–5. [DOI] [PubMed] [Google Scholar]
- 9.Shellhaas RA, Kushwaha JS, Plegue MA, Selewski DT, Barks JDE. An evaluation of cerebral and systemic predictors of 18-month outcomes for neonates with hypoxic ischemic encephalopathy. J Child Neurol. 2015;30:1526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarnat HB. Neonatal encephalopathy following fetal distress: a clinical and electroencephalographic study. Arch Neurol. 1976;33:696. [DOI] [PubMed] [Google Scholar]
- 11.Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, et al. Whole-body hypothermia for neonates with hypoxic–ischemic encephalopathy. N Engl J Med. 2005;353:1574–84. [DOI] [PubMed] [Google Scholar]
- 12.Shankaran S, Barnes PD, Hintz SR, Laptook AR, Zaterka-Baxter KM, McDonald SA, et al. Brain injury following trial of hypothermia for neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2012;97:F398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chin EM, Jayakumar S, Ramos E, Gerner G, Soares BP, Cristofalo E, et al. Preschool language outcomes following perinatal hypoxic-ischemic encephalopathy in the age of therapeutic hypothermia. Dev Neurosci. 2018;40:627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas AJ, Eberly LE, Davey Smith G, Neaton JD. ZIP-code-based versus tract-based income measures as long-term risk-adjusted mortality predictors. Am J Epidemiol. 2006;164:586–90. [DOI] [PubMed] [Google Scholar]
- 15.Roux AVD, Kiefe CI, Jacobs DR, Haan M, Jackson SA, Nieto FJ, et al. Area characteristics and individual-level socioeconomic position indicators in three population-based epidemiologic studies. Ann Epidemiol. 2001;11:395–405. [DOI] [PubMed] [Google Scholar]
- 16.Mercuri E, Ricci D, Cowan FM, Lessing D, Frisone MF, Haataja L, et al. Head growth in infants with hypoxic–ischemic encephalopathy: correlation with neonatal magnetic resonance imaging. Pediatrics. 2000;106:235–43. [DOI] [PubMed] [Google Scholar]
- 17.Martinez-Biarge M, Bregant T, Wusthoff CJ, Chew ATM, Diez-Sebastian J, Rutherford MA, et al. White Matter and cortical injury in hypoxic-ischemic encephalopathy: antecedent factors and 2-year outcome. J Pediatr. 2012;161:799–807. [DOI] [PubMed] [Google Scholar]
- 18.Gancia P, Pomero G. Therapeutic hypothermia in the prevention of hypoxic-ischaemic encephalopathy: new categories to be enrolled. J Matern Fetal Neonatal Med. 2012;25:94–6. [DOI] [PubMed] [Google Scholar]
- 19.Peeters LLH, Sheldon RE, Jones MD, Makowski EL, Meschia G. Blood flow to fetal organs as a function of arterial oxygen content. Am J Obstet Gynecol. 1979;135:637–46. [DOI] [PubMed] [Google Scholar]
- 20.Natarajan G, Pappas A, Shankaran S. Outcomes in childhood following therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy (HIE). Semin Perinatol. 2016;40:549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset of which this paper is based is a small pilot sample and is not retained or publicly archived.


