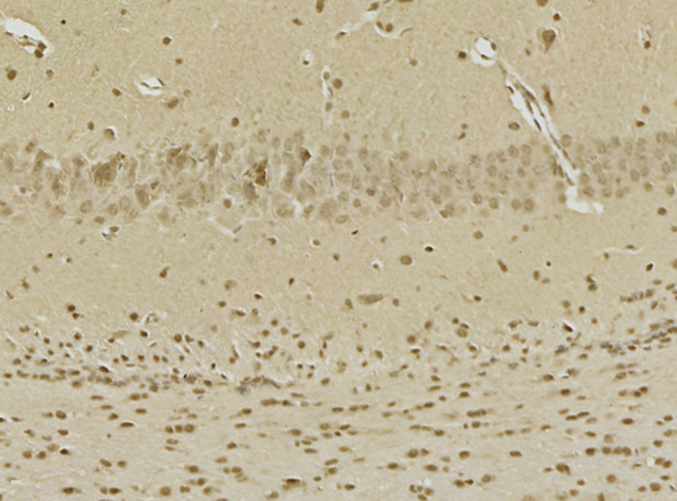
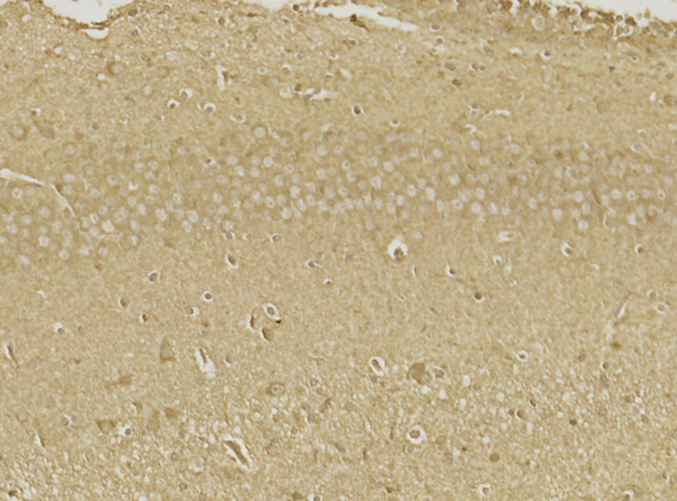
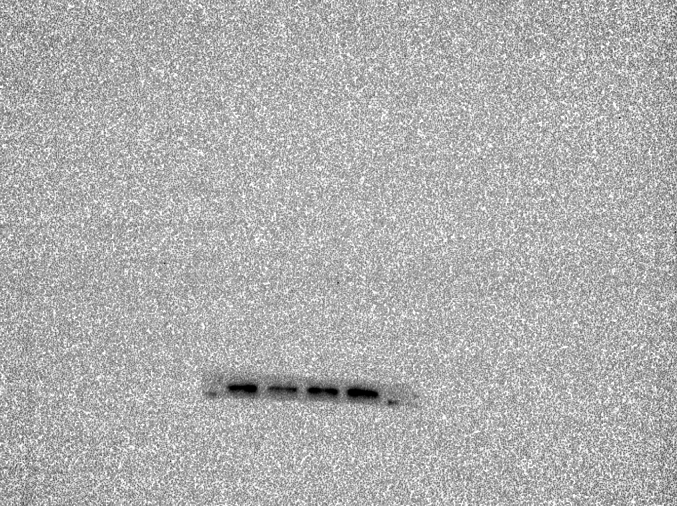
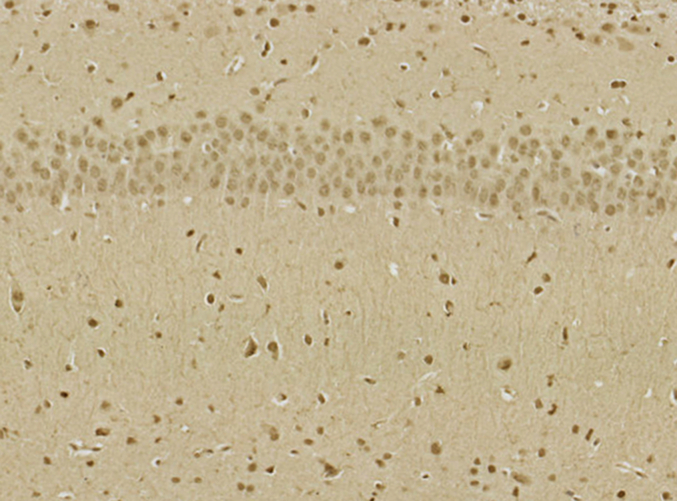
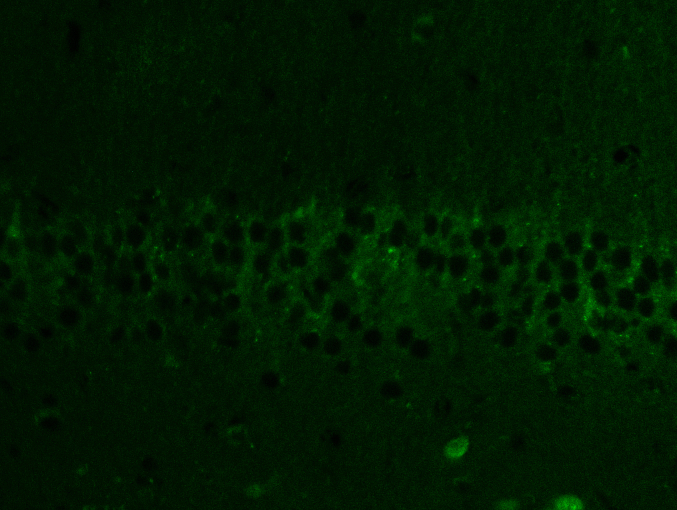
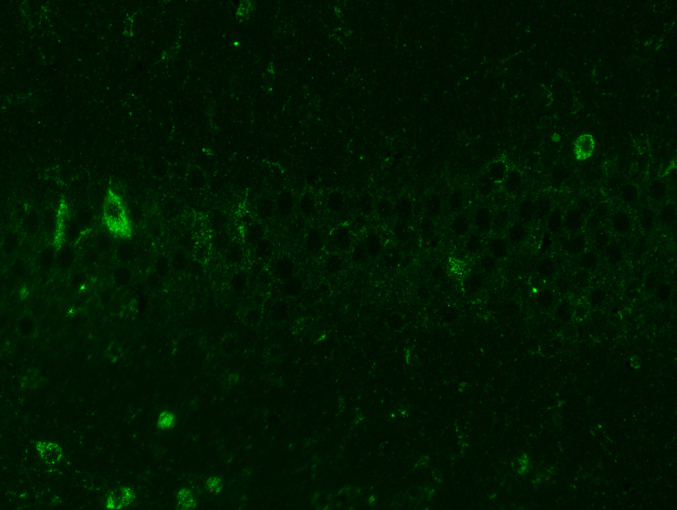
Abstract
Objective
The present study aimed to see if 20-Deoxyingenol(20-DOI) could protect hippocampus neurons from the neurotoxic effects of morphine and reduce memory loss in rats.
Method
Male Wistar rats were given morphine hydrochloride (45 mg/kg, sc, four weeks) and 20-DOI (10, 20 mg/kg, ip., coadministered with morphine) for the Morris Water Maze (MWM) test to investigate the effects of 20-DOI on spatial learning and memory. Western blotting was used to evaluate the expression of the hippocampal CA1 region of the cleaved caspase-3, Bax, and Bcl2 proteins and so on. Moreover, these assays were used to evaluate the expression of superoxide dismutase (SOD)2, heme oxygenase 1(HO1) protein, and glutathione peroxidase (GPx) activity within the hippocampus CA1 area.
Results
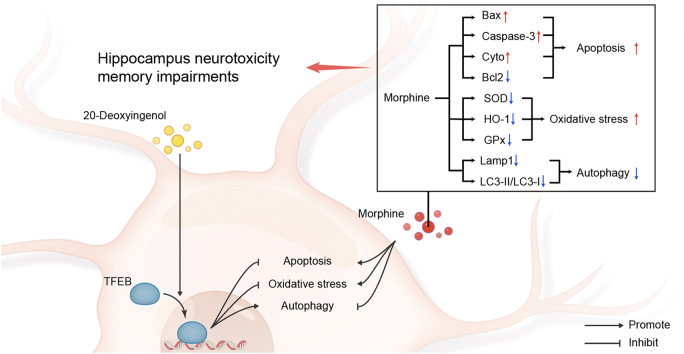
The administration of 20-DOI (10 and 20 mg/kg) to morphine-treated mice enhanced spatial learning and reduced memory deficits. Additionally, 20-DOI treatment reduced apoptosis and oxidative stress in the hippocampal CA1 region of morphine-treated rats. Moreover, 20-DOI improved the autophagy level of the hippocampal CA1 area of morphine-treated rats using Transcription factor EB (TFEB), and 20-DOI prevented spatial learning and memory impairment in morphine-treated rats. The current observation could be partially due to the inhibition of neuronal apoptosis and oxidative stress in the hippocampal CA1 region of rats treated with morphine and the improved autophagy in this region.
Conclusions
20-DOI attenuated morphine administration in rats with chronic disease caused spatial learning and memory dysfunction. These mechanistic effects could be partially related to 20-DOI protecting the CA1 region of rat hippocampal neurons from the morphine-induced oxidative stress, apoptosis, and autophagy through TFEB.
Keywords: 20-Deoxyingenol, Morphine, Hippocampus, Apoptosis, Oxidative stress, Autophagy
Graphical abstract
Highlights
-
•
20-DOI treatment reduced apoptosis and oxidative stress in the hippocampal CA1 region of morphine-treated rats.
-
•
20-DOI protecting the CA1 region of rat hippocampal neurons from the morphine-induced autophagy through TFEB.
-
•
20-DOI attenuated morphine administration in rats with chronic disease caused spatial learning and memory dysfunction.
1. Introduction
Previous research has shown the hippocampus to play a critical role in spatial learning and memory [1]. It also indicates that opioids can affect hippocampal-dependent memory [2]. Learning and memory disorders caused by morphine addiction are related to the imbalance of HCN1 and HCN2 subunits in CA1 region [3].CA1 neural network plays an integrated role in memory [4].Additionally, long-term use of opioids can lead to blocked nerve formation, decreased bone density, and changes in the synaptic transmission process [5,6]. Related studies reveal that chronic use of morphine causes oxidative stress, apoptosis, and changes in autophagy in different brain regions, like the hippocampus [7]. In addition, decreased antioxidant enzyme activity of enzymes like glutathione peroxidase (GPx) and superoxide dismutase (SOD) causes oxidative stress damage during morphine administration [8]. In vivo experiments in rats have established that oxidative stress results in neurodegeneration in multiple brain areas, including the hippocampus, nucleus accumbens, and cerebral cortex, related to morphine-induced apoptosis [7,9,10].
In recent times, the primary source of new drugs is natural and herbal substances with medicinal promise [11]. In addition, more and more natural therapeutic compounds and their components have been used as complementary or alternative therapies for neurodegenerative diseases [12]. Therefore, numerous investigations have evaluated the protective properties of natural herbs with unique effects on oxidative stress management, reducing apoptosis, and improving autophagy [13,14].In this case, 20-Deoxyingenol(20-DOI) is isolated from the dried roots of Euphorbiaceae [15]. It has various pharmacological properties, including anti-inflammatory, anti-aging, anti-apoptotic, anti-oxidative, and promoting autophagy. Furthermore, 20-DOI can improve the occurrence of autophagy by Transcription factor EB (TFEB) [16]. TFEB is the main regulator of the whole autophagy lysosomal pathway (ALP) and overexpression of TFEB can alleviate aging related memory deficits in mice [17].
Based on the critical role of the hippocampus in chronic opioid-influenced emotional behavior, the current research aimed to determine the effect of 20- Deoxyingenol on hippocampal-dependent learning and memory impairments due to morphine. In addition, we also evaluated the impact of 20-DOI on oxidative stress, autophagy, and neuronal apoptosis in the hippocampal CA1 region of the rat after chronic morphine treatment.
2. Material and methods
2.1. Ethics statement
The Animal Care and Use Committee of Wenzhou Medical University authorized all the surgical operations, therapies, and postoperative animal care protocols following the "Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health." [18]The Animal Care and Use Committee of Wenzhou Medical University approved this study(ethic code:wydw2022-0703).
2.2. Reagents and antibodies
The 20-DOI (purity>98 %) was obtained from MCE (shanghai, China). An anti-TFEB antibody was obtained from Proteintech (Rosemont, Illinois, USA). Anti-LC3, BAX, BCL2, cleaved-caspase3, and Cyto antibodies were obtained from the Cell Signaling Technology (Beverly, Massachusetts, USA). The Anti-β-actin, LAMP1, SOD2, HO1, p62, and Lamin B antibodies were obtained from Abcam (Cambridge, Massachusetts, USA). Superoxide dismutase (SOD) and Glutathione peroxidase (GPx) assay kits were purchased from the Solarbio Company (Beijing, China).
2.3. Animals
The male Wistar rats (220 ± 10 g) were purchased from the Shanghai Animal Center of the Chinese Academy of Sciences. Other than behavioral testing, the rats were kept in polycarbonate cages in a colony room with an alternating 12-h light/dark cycle at 22–24 ±°C (40–60 % humidity). The rats were provided specific pathogen free mice's feed and water after autoclaving available at will. Before the studies, the animals were provided at least one week for acclimatizing to the laboratory setting.
2.4. Experimental design
The animals were assigned randomly to one of the four experimental groups, with eight rats in each group.
The groups were as follows: (1) Sham group: rats were treated with normal saline (200 μl/rat, i.p.) for four weeks;
(2) Morphine + sham group: rats were treated with morphine (45 mg/kg, sc) and normal saline (200 μl/rat, i.p.) for four weeks; (3, 4) Morphine + 20-DOI groups: rats were given morphine (45 mg/kg, sc) and 20-DOI (i.p.) at a dosage of 10 and 20 mg/kg, respectively, for four weeks.
2.5. Morris water maze test
The Morris water maze (MWM) assessed the involvement of the hippocampus in spatial learning and memory. Approximately 140 cm in diameter and 60 cm high, the MWM was filled with water at 22 ± 1 °C and was enclosed entirely in black. Four sites were designated as the beginning conditions inside the water pool, separated into four conceptual quadrants (N, S, E, or W). A buried black platform (10 cm diameter) was installed in the middle of the southwest quadrant of the lake, 2 cm below the water surface. It was kept in the middle of the room, with four additional labyrinth visual clues placed on the walls surrounding each side of the equipment.
We conducted the MWM test after 21 days of morphine treatment.During the learning phase,the animals were subjected to four separate trials each day for four days. Each individual rat was randomly selected from the four-quadrant locations and put in the water with its back against the pool wall. The time limit for each test was 60 s. The rats were escorted and compelled to remain on the platform for 15 s to ensure that they could not reach the platform on their own within 60 s.Spatial probe tests were conducted 24 h after the last navigational experiment. The platform was removed, and the rats were dropped into the water from any location facing the pool wall. We recorded the escape latency and the swimming speed. The goal quadrant swim time and platform-site crossings were also recorded. In addition, the animal mobility was measured using an automated tracking system (Ethovision software; version 7.1, Noldus Information Technology, Netherlands).
2.6. Hippocampus tissue preparation
After completing the MMW test, mice were euthanized with CO2.An ice-cold saline solution was used to extract the brain from the skull to isolate the hippocampal tissue. The next step was to dissect and homogenize the CA1 region of the hippocampus using cold-lysis buffer (10 mM Tris–HCl, 1 mM EDTA, 0.1 % SDS, 0.1 % Na 20-DOIxycholate, 1 % NP-40; 2 μg each of the protease inhibitors leupeptin, aprotinin, and pepstatin A; and 0.5 μmol/l PMSF at pH 7.4). Next, the lysate was separated from the supernatant by centrifuging for 20 min at 4 °C. Then, the complete cell fraction was transferred from the supernatant to the new tubes. The Bradford technique helped determine the protein content in each fraction. For molecular analyses, the samples from the supernatants were kept at −80 °C.
2.7. Western blot analysis
2.7.1. Some molecular indicators in the hippocampal
region in morphine-dependent rats were studied using Western blotting (WB). Polyacrylamide gel(10 %) electrophoresis separated proteins with the same quantity and then electrically transferred them to a PVDF membrane. Tris-buffered saline with Tween20 (TBS-T) (150 mM NaCl, 20 mM Tris–HCl, 0.1 percent Tween20, at pH 7.4) was used to block the membranes overnight at 4 °C. Then, a primary antibody was incubated overnight on the PVDF membranes and washed off. The blocking buffer was used to dilute all the antibodies. After washing three times with TBST, the blots were incubated with the corresponding secondary antibody for 2h at room temperature. The chemiluminescence was used to detect the blots, and band densitometry was performed with ImageJ.
2.8. Immunofluorescence
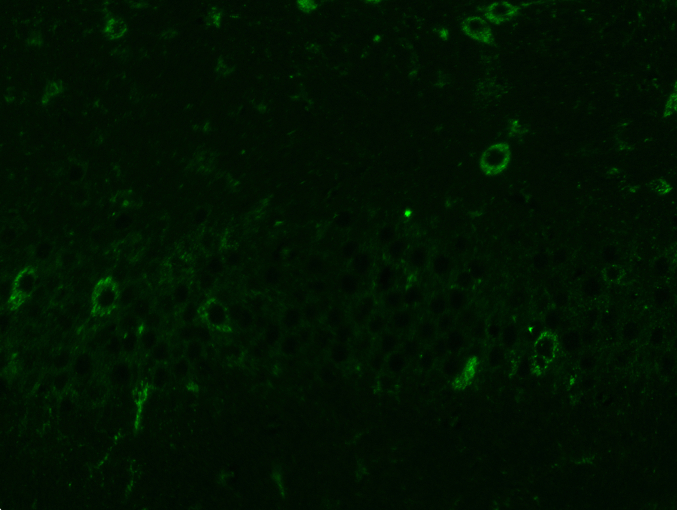
The specimens were all dehydrated, paraffin-embedded, and then sectioned at a thickness of 5 μm. Subsequently, the sections were deparaffinized in xylene, washed with ethanol, and hydrated for immunofluorescence. Then, the sections were treated with 10 % bovine serum albumin in PBS containing Triton X-100 and incubated overnight at 4 °C with the primary antibody. After incubation, Alexa Fluor 488 goat anti-rabbit secondary antibody was added for 1 h at room temperature to the sections that had been previously washed four times. After rewashing with PBS three times, the sections were rinsed for 10 min with 4,6-diamino-2-phenylindole (DAPI). Finally, the sections were rewashed with PBS and covered by a coverslip. In the experiment, an observer blindly counted the number of TFEB nuclear positive cells within each slice using an Olympus fluorescent microscope (Olympus, Tokyo, Japan). For quantitative evaluation, eight rats from each group were used.
2.9. Histopathological analysis
Each hippocampus tissue was stained with hematoxylin and eosin (HE) stain. The image was captured using an Olympus fluorescence microscope (Olympus, Tokyo, Japan). Another set of expert histology researchers analyzed the cells and morphology of the hippocampus using microscopy without any prior knowledge. The scoring mechanism for the platform was being assessed. Eight mice were utilized from each group for histomorphology scoring. The sections were blocked with 1 % (w/v) goat serum albumin for 1 h at 37 °C, followed by incubation with primary antibody (Neun, SOD, 1:200) at 4 °C for another 24 h. Sections from the negative sham group were treated with nonspecific IgG. Then, the sections were treated for 1 h at 37 °C with HRP-conjugated secondary antibodies. Therefore, it was determined that each specimen had at least three parts for viewing.
2.10. Molecular modeling
The 2D structure of one major compound was downloaded from the PubChem (https://pubchem.) database, and Chem3D was used for optimization. Then, a 3D map of the interacting target protein was searched and downloaded from the PDB (https://www.rcsb.org/) database. PyMoL 2.4.0 software was used to remove water and the original ligand from the target protein. Finally, the AutoDock visualization software was used for molecular docking, and mapping was done with PyMoL. The AutoDock software was used for docking. The lower the binding energy, the better the docking score and the higher the possibility of binding.
2.11. Statistical analysis
The statistical analyses were carried out using the SPSS 20.0 statistical software tool (IBM, Armonk, NY, USA). The data were analyzed using a one-way analysis of variance (ANOVA), followed by Tukey's test to see if there were any differences between the sham and treatment groups. The Kruskal-Wallis H test assessed non-parametric data (OARSI and synovitis scores). P value of <0.05 was used to indicate statistical significance.
3. Result
3.1. Effects of 20-DOI on spatial learning and memory impairments in morphine-treated animals
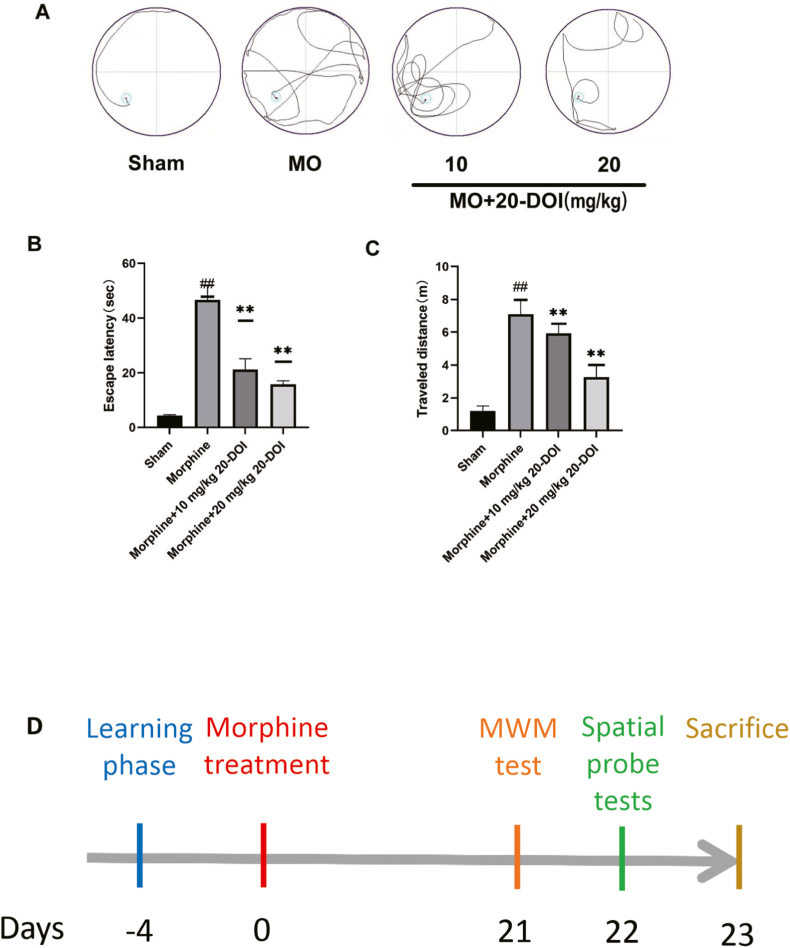
We completed a water maze test in response to the place navigation and spatial probing tests for investigating the relationship between 20-DOI and morphine-induced cognitive dysfunction. The place navigation test depicted a decrease in latency and the distance covered to locate and reach the concealed escape platform. Moreover, morphine-pretreated rats took longer than the sham group and had a higher latency time(Fig. 1A). Thus, morphine-pretreated rats had weaker spatial learning abilities. However, 20-DOI at 10 and 20 mg/kg decreased the time for morphine-treated rats to flee(Fig. 1B) and the distance traveled(Fig. 1C). In addition, the probe test in the MWM test revealed that after the platform was removed, the morphine-pretreated group showed a decreased memory capacity than the sham group. Therefore, chronic morphine treatment significantly impairs spatial memory. However, 20-DOI had a significant ameliorating effect on morphine-induced memory impairment. Hence, these results indicate that 20-DOI protects rats from morphine-induced impairments of learning and memory.
Fig. 1.
The effect of 20-Deoxyingenol(20-DOI) treatment on spatial learning function in morphine-treated rats. The animals were treated with morphine hydrochloride (45 mg/kg, sc, four weeks) except for the sham group.(A) Representative of swim route traces of rats from different groups. Each bar represents the mean of escape latency (B) distance traveled and (C) finding a hidden platform over four consecutive day trials in the Morris Water Maze (MWM) test. All the data were presented as mean ± SD (n = 5); ##P < 0.01 vs sham group, *P<0.05,**P < 0.01 vs morphine group.
3.2. Effect of 20-DOI on apoptosis in the hippocampus CA1 of morphine-treated animals
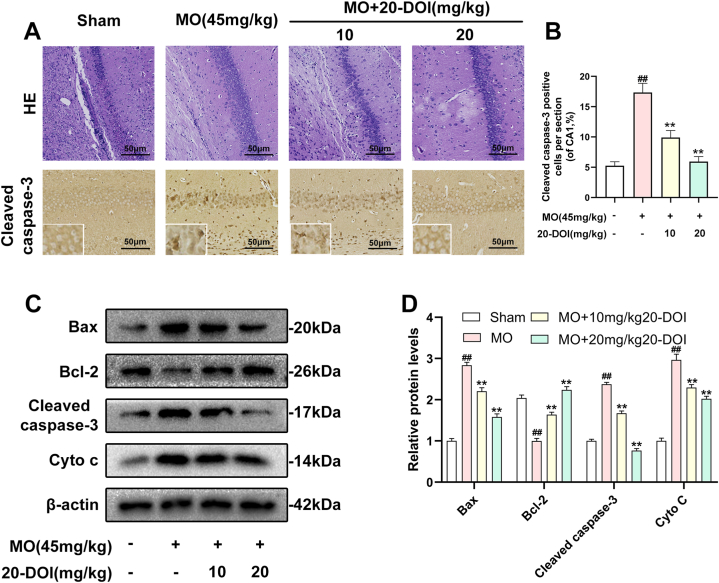
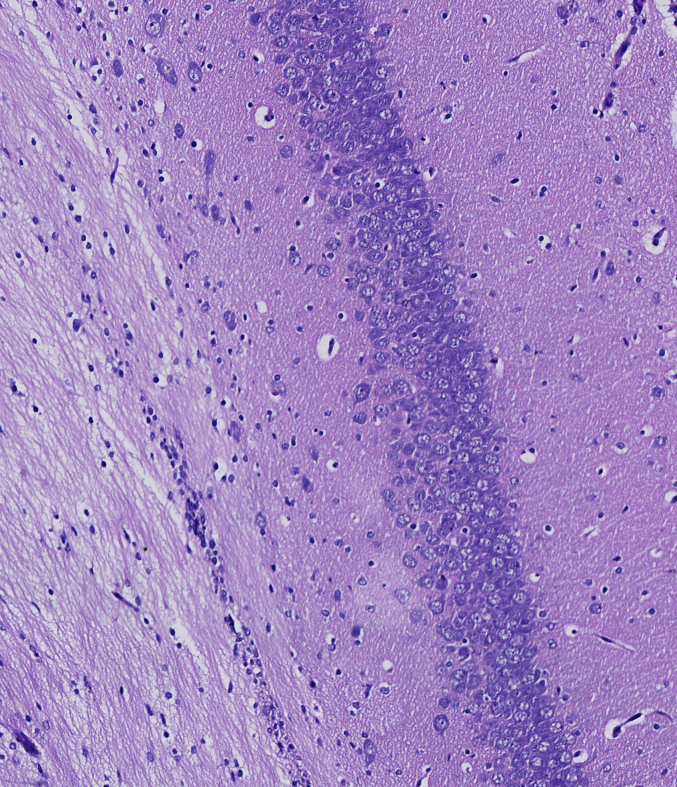
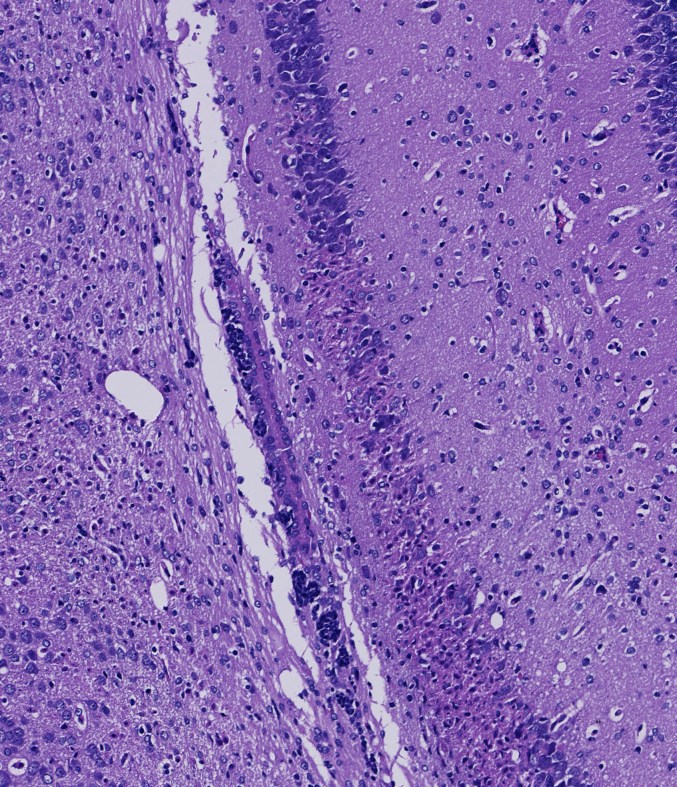
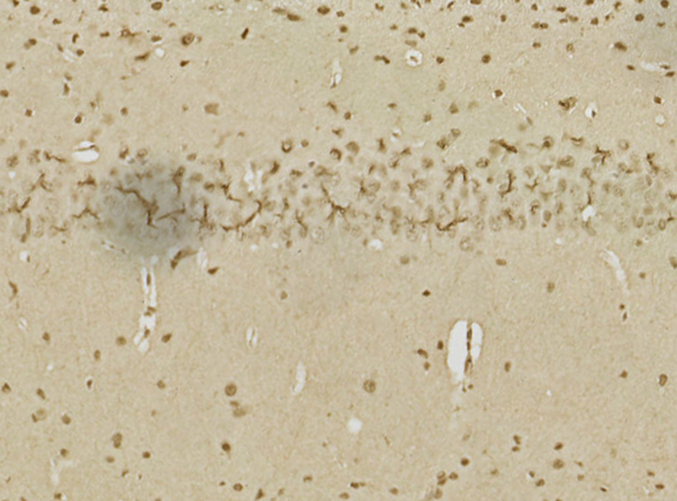
The impact of 20-DOI on apoptosis was assessed in morphine-pretreated rat hippocampus CA1. Immunohistochemistry analysis revealed that caspase-3 expression within morphine-pretreated hippocampus CA1 was higher than the unaffected counterparts. In addition, 20-DOI could improve the observed phenomenon(Fig. 2A). As 20-DOI dosage rises, 20-DOI may dramatically reduce caspase-3 expression, which is upregulated by morphine pretreatment(Fig. 2B).Western blot was used to determine the expression of Bax, Bcl2, caspase-3, and Cyto. The molecular expressions of Bax, caspase-3, and Cyto were dramatically enhanced than in the sham group, while the molecular expression of Bcl2 was significantly reduced in the morphine pretreatment group. However, 20-DOI could effectively reverse this change, causing a decrease in the molecular expression of Bax, caspase-3, and Cyto and an increase in the expression of Bcl2 in a dose-dependent manner(Fig. 2C).
Fig. 2.
20-Deoxyingenol (20-DOI) treatment inhibits morphine-induced apoptosis in the hippocampus. The animals were treated with morphine hydrochloride (45 mg/kg, sc, four weeks) except for the sham group. (A, B) Represented hematoxylin and eosin (HE) staining, the immunohistochemistry of cleaved caspase-3 of the hippocampus in the four groups at four weeks after morphine treatment. (C, D) Western blotting revealed the level of Bax, Bcl-2, cleaved caspase-3, and Cyto c in the hippocampus of treated animals. All the data were presented as mean ± SD (n = 5); ##P < 0.01 vs Sham group, *P<0.05,**P < 0.01 vs morphine group.
3.3. Effect of 20-DOI on oxidative stress in the hippocampus CA1 of morphine-treated animals
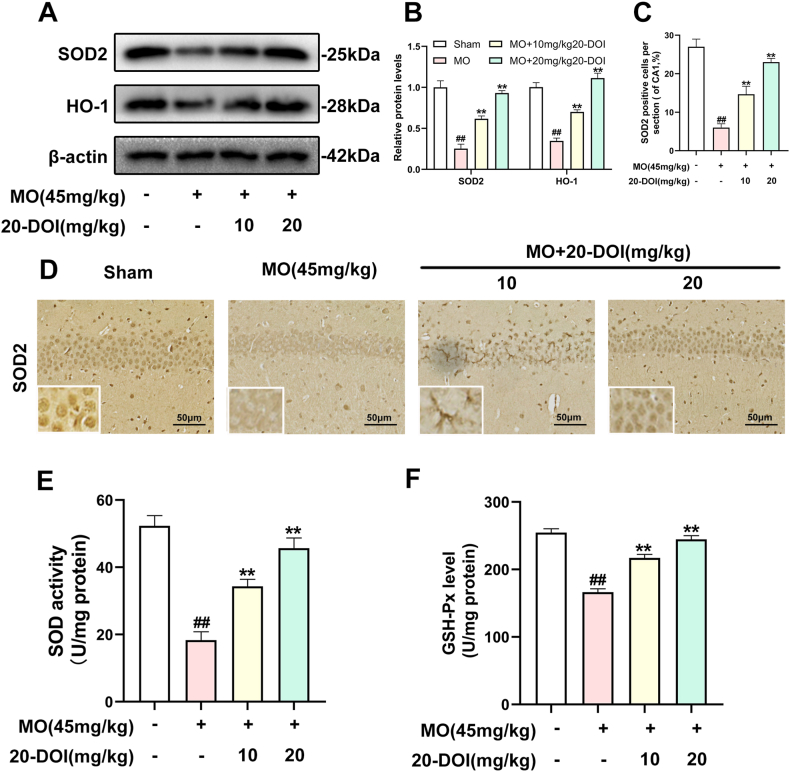
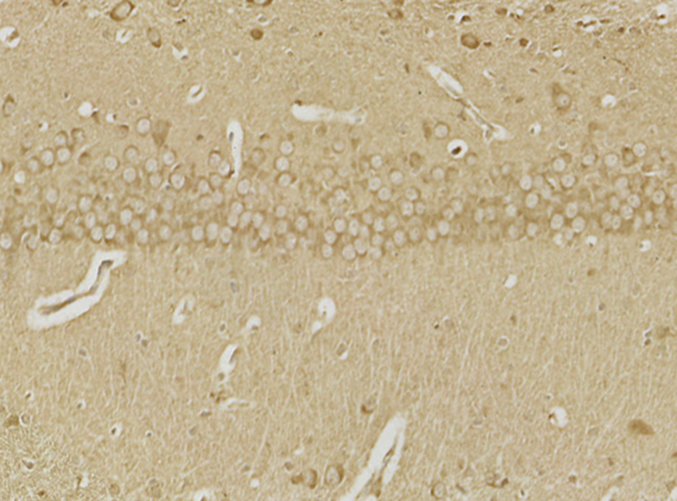
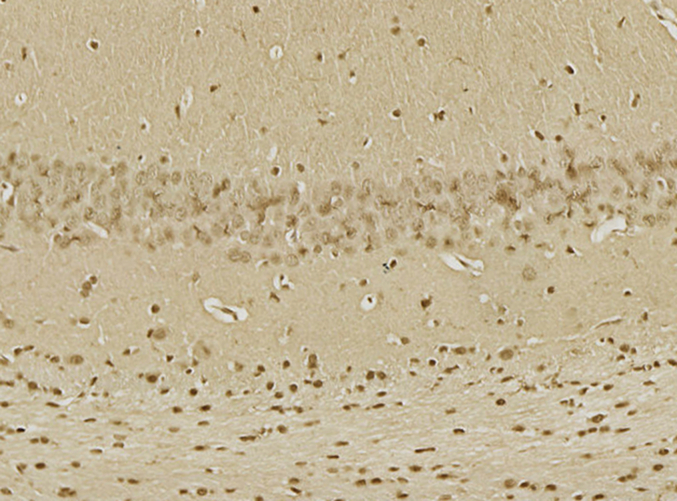
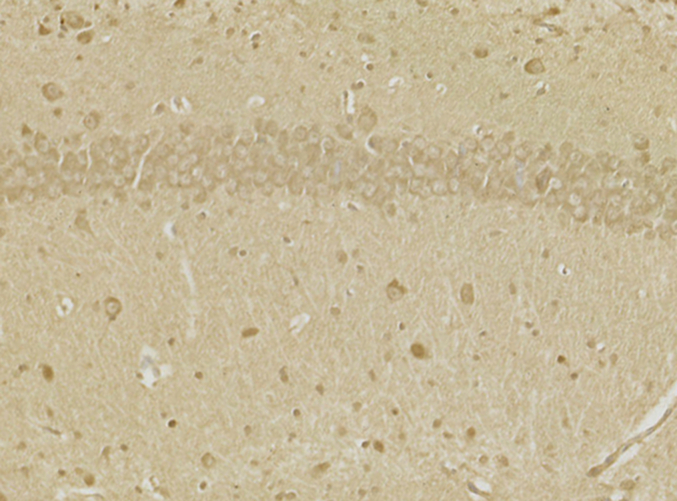
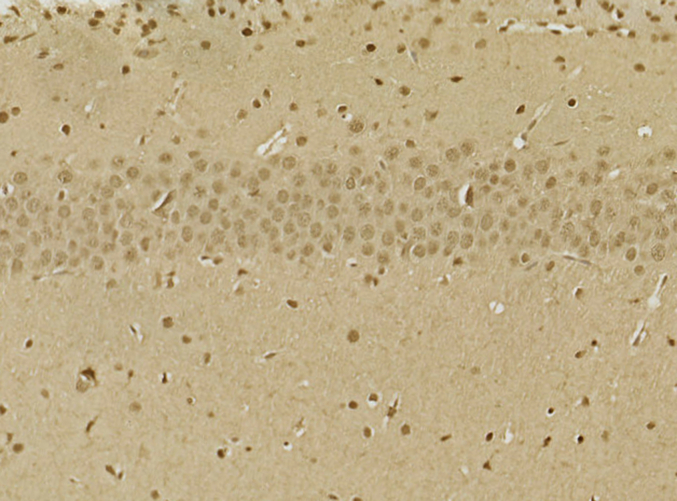
The SOD, HO-1, and Gpx were significantly lowered in the CA1 area of the hippocampal tissue of the morphine-treated rats than in saline-treated rats. Administration of 20-DOI dramatically enhanced the SOD, HO-1, and Gpx activity levels within the CA1 area of chronic morphine-treated rats(Fig. 3A–C). The immunohistochemistry findings were consistent with the above results and were dose-dependent(Fig. 3D). In the hippocampal regions of rats given morphine, 20-DOI dramatically increases the activities of glutathione peroxidase (GPx) and superoxide dismutase (SOD)(Fig. 3E and F).
Fig. 3.
20-Deoxyingenol (20-DOI) treatment inhibits morphine-induced oxidative stress in the hippocampus. The animals were treated with morphine hydrochloride (45 mg/kg, sc, four weeks) except for the Sham group. (A, B) Western blotting revealed the level of superoxide dismutase (SOD)2, heme oxygenase 1(HO-1) in the hippocampus of treated animals. (C, D) Representing the immunohistochemistry of SOD2 of the Hippocampus in the four groups at four weeks post-morphine treatment. (E, F) SOD and glutathione peroxidase (GPx) activity in the hippocampus of morphine-treated rats. All the data were presented as mean ± SD (n = 5); ##P < 0.01 vs Sham group, *P<0.05,**P < 0.01 vs morphine group.
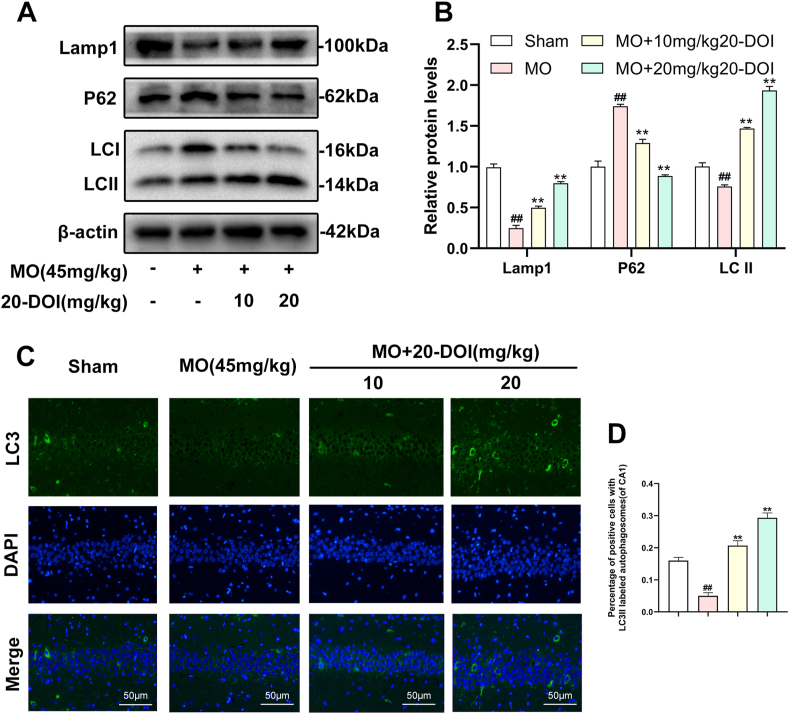
3.4. Effect of 20-DOI on autophagy in the hippocampus CA1 of morphine-treated animals
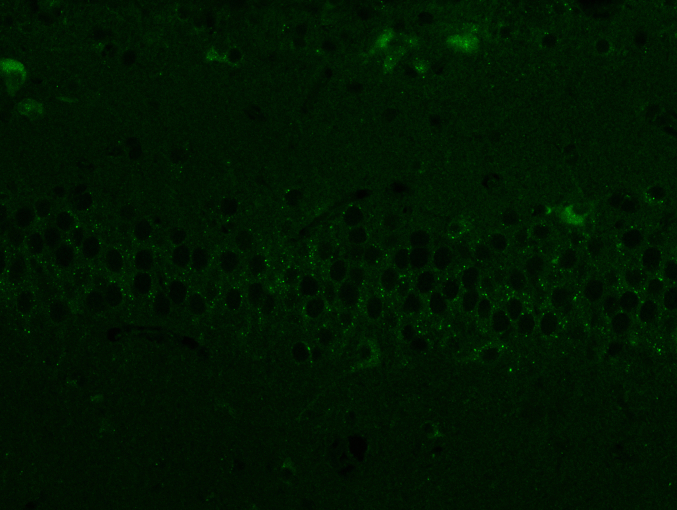
20-DOI recovered autophagic flux in morphine-treated rat hippocampal CA1 tissue. Western blotting was employed to identify LC3-II/ILC3-I and p62 as indications of autophagy flux and Lamp1 as lysosomal biogenesis indicators. Despite increased P62 expression, Lamp1 expression and the ratio of LC3-II/LC3-I within the hippocampal CA1 reduced after morphine pretreatment. It indicated that morphine-treated hippocampal CA1 impeded autophagic flow, inhibiting lysosome biogenesis. After treatment with 20-DOI, the Lamp1 and LC3-II/LC3-I ratio expressions were both up-regulated compared to morphine administration alone. In contrast, p62 expression was reduced, indicating restoration of the autophagy flux and lysosome biogenesis stimulation(Fig. 4A and B). In immunofluorescence detection, and the results indicated that the expression level of LC3II reduced after morphine administration. In contrast, treatment with 20-DOI resulted in an increase, consistent with the findings from Western blotting(Fig. 4C). After morphine therapy, our findings implied that 20-DOI could help restore autophagic flux in the hippocampus CA1.
Fig. 4.
20-Deoxyingenol (20-DOI) treatment induces autophagy in the hippocampus. All the animals were treated with morphine hydrochloride (45 mg/kg, sc, four weeks) except for the Sham group. (A, B) Western blotting revealed the level of Lamp1,p62, and LC3 in the hippocampus of treated animals. (C, D) Immunofluorescence staining of LC3 treated with morphine hydrochloride. All the data were presented as mean ± SD (n = 5); ##P < 0.01 vs Sham group, *P<0.05,**P < 0.01 vs morphine group.
3.5. 20-DOI may promote autophagic flux in the hippocampus CA1 via TFEB
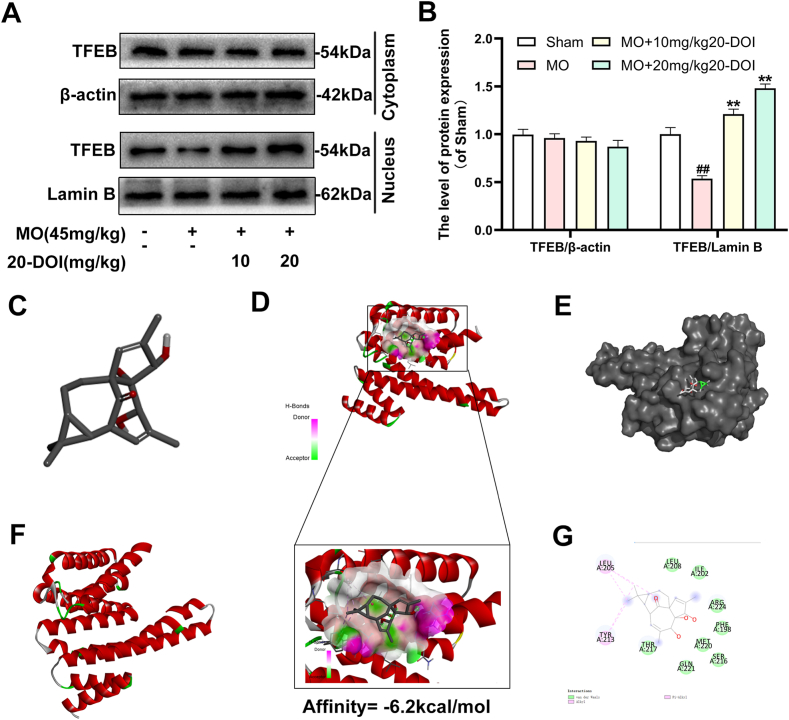
Western blotting examined the impact of 20-DOI on TFEB. After morphine therapy, there was a drop in the nuclear level of TFEB, while the nuclear level of TFEB elevated after 24h of 20-DOI treatment. Furthermore, the TFEB nuclear level increased when morphine and 20-DOI were administered to the hippocampus CA1 together(Fig. 5A and B). These findings indicated that 20-DOI could increase the nuclear level of TFEB in morphine-treated hippocampus CA1. Therefore, we speculate that 20-DOI can promote the autophagic flux in the CA1 hippocampus through TFEB.
Fig. 5.
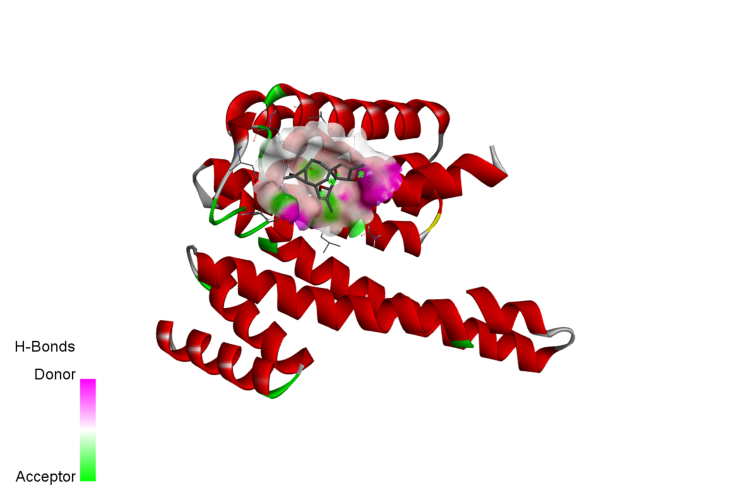
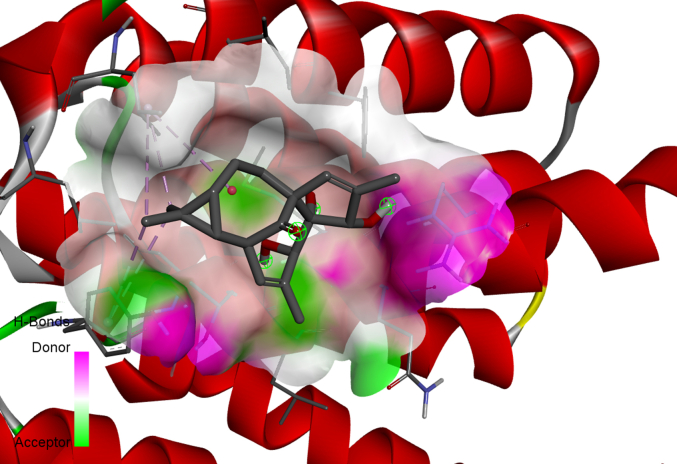
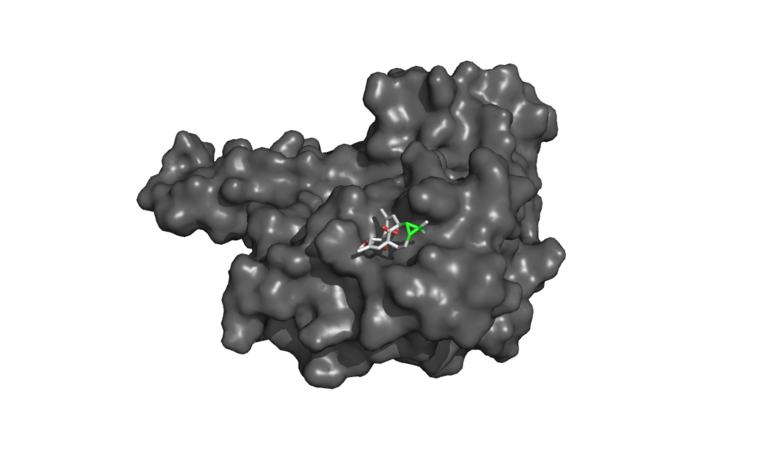
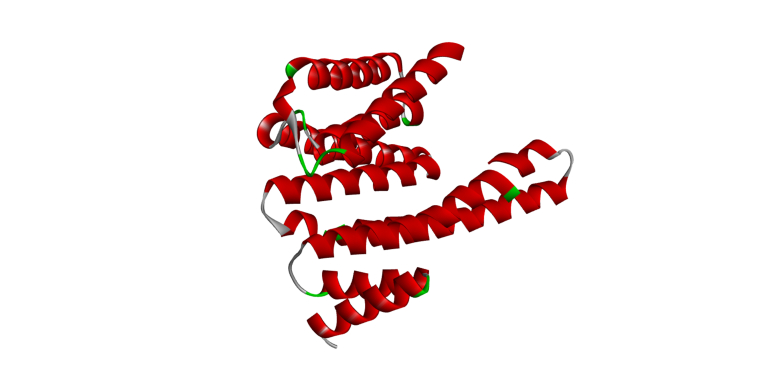
20-Deoxyingenol (20-DOI) treatment promotes the translocation of Transcription factor EB (TFEB) to nuclear and molecular docking between 20-DOI and TFEB. The animals were treated with morphine hydrochloride (45 mg/kg, sc, four weeks) except for the Sham group. (A, B) Western blotting revealed the levels of TFEB in the cytoplasm and nucleus in the hippocampus of treated animals. (C,D,E,F,G) The 3D docking analysis between 20-DOI and TFEB, and affinity energy is −6.2 kcal/mol. The 2D binding model between 20-DOI and TFEB and the molecules of
TFEB interacts with 20-DOI using eight van der Waals and two pi-alkyl bonds. All the data were presented as mean ± SD (n = 5); ##P < 0.01 vs Sham group, *P<0.05,**P < 0.01 vs morphine group.
3.6. Molecular docking determines the interaction between 20-DOI and TFEB
We used molecular docking analysis to search for potential proteins that could be controlled by 20-DOI to understand better the underlying mechanism of the effect of 20-DOI on autophagy. Before undergoing the molecular docking study, the 20-DOI structure was constructed in the Discovery Studio 2016(Fig. 5C). The possible targets of 20-DOI like TFEB were retrieved from the Protein Data Bank (PDB)(Fig. 5F).
After that, molecular docking was used to determine the affinity of the 20-DOI for several potential targets. The -CDOCKER energy score was the highest (6.2 kcal/mol) for 20-DOI versus the TFEB(Fig. 5D), indicating that the affinity of 20-DOI for TFEB is the strongest.
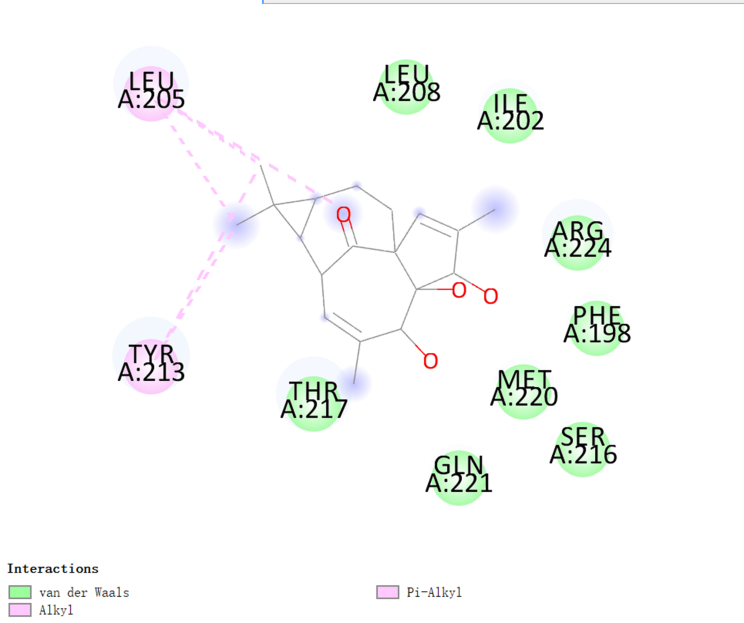
A two-dimensional binding model was used to visualize the binding sites of 20-DOI and TFEB to demonstrate the potential of 20-DOI in activating TFEB(Fig. 5E). The findings indicated that 20-DOI could interact with TFEB through van der Waals, salt bridge, alkyl, and hydrogen bonds, with three possible active sites (ARG224, LEU205, TYR213)(Fig. 5G). Moreover, 20-DOI may dissociate the TFEB and induce autophagy by engaging with these sites.
These findings indicate that 20-DOI may directly interact with TFEB and influence autophagy.
4. Discussion
The current work investigated the beneficial effects of 20-DOI on cognitive impairment, neuronal apoptosis, oxidative stress, and autophagy in the CA1 region of the hippocampus caused by enduring morphine administration in rats. Based on our findings, the neuroprotective effects of 20-DOI against morphine-induced neurotoxicity in the hippocampus are associated with oxidative stress prevention by enhancing the activities of SOD, HO-1, and Gpx, apoptosis attenuation by prohibiting the expression of cleaved caspase-3, Cyto and Bax/Bcl2 ratio protein and the amelioration of autophagy through TFEB.
Previous research has established that opioids affect various structures in the brain, including memory impairment with morphine [10,19]. However, the underlying mechanism of morphine-induced memory impairment is not fully elucidated. Studies have depicted that memory impairment due to morphine may be caused by any damage to the related areas of the hippocampus [20]. The hippocampus itself has irreplaceable functions concerning spatial learning and memory formation [21]. Evolution of hippocampal cortical aggregates during memory consolidation promoted by dentate gyrus CA3 inhibitory circuit [22]. CA3 axons innervated CA1 neurons in a highly topographical manner [23].In a study on the mGlu5 receptor protein, it was found that CA3 may play a role in short-term memory and CA1 may play a role in long-term memory consolidation [24]. The Morris water maze is one of the most potent pieces of evidence to measure hippocampal functions [25].
We found through the Morris water maze that morphine-pretreated rats had much higher latency and traveled farther than sham rats, consistent with the previous studies [26]. This indicates that chronic morphine treatment can damage the hippocampal function in rats and lead to defects in spatial learning and memory.
Although many studies have revealed that morphine has severe neurotoxic effects on the hippocampus [27], the mechanism is unclear. However, previous studies have shown that the damaging effects of opioids like morphine could be due to oxidative stress [28].
Apoptosis is a process involving caspase family members being activated through pro-apoptotic stimuli like endoplasmic reticulum stress and reactive oxygen species [29]. Changes in the intracellular environment, including endoplasmic reticulum stress, excess reactive oxygen species, and the pro-apoptotic B-cell lymphoma-2-related X protein, lead to incomplete mitochondrial membranes, release cytochrome C in the cytoplasm, and oxidative stress or Ca2+ overload. Thus, the permeability transition pore is opened, the mitochondria ruptured, apoptotic bodies formed, and finally, apoptosis happened [30,31]. Many studies have depicted that morphine can cause pathological brain tissue damage and is closely associated with brain neuron apoptosis [32]. At the same time, morphine treatment resulted in a significant increase in the expression of apoptotic proteins like caspase-3, Bax, and cyto, and relatively decreased Bcl-2 protein, stimulating hippocampal apoptosis [7,33,34].
Regulation of oxidative stress can alleviate excessive autophagy, apoptosis, and lipid metabolism disorders [35]. It has been depicted that morphine induces neuronal oxidative stress damage and neurodegeneration [36]. Moreover, the level of lipid peroxidation in the brain of animals was increased after morphine administration [37]. Furthermore, morphine can elevate dopamine concentration in the synapse, which is metabolized by monoamine oxidase-B in the human body for catalyzing the production of dihydroxyphenylacetic acid and hydrogen peroxide [[38], [39], [40]]. Thus, the endogenous hydrogen peroxide decomposition produces several cytotoxic reactive oxygen species like hydroxyl radicals [41]. Moreover, morphine can also be metabolized for generating free radicals, releasing excessive reactive oxygen species, and causing oxidative stress damage [7]. The levels of total thiol levels,SOD, and GPx in the hippocampus of animals injected with morphine decreased, indicating the central nervous system is vulnerable to oxidative stress [42]. Similarly, studies have shown that GSH levels were reduced after intraventricular injection of morphine in the cerebrospinal fluid of patients with intractable cancer pain. Additionally, morphine could deplete the antioxidant GSH predisposing the central nervous system to oxidative stress [43].
Studies have depicted that morphine treatment of rat pheochromocytoma cells can increase ROS, triggering autophagy and clearing damaged mitochondria [44]. Although morphine-induced memory impairment was attenuated when autophagy was activated in the hippocampal neurons, it also proved that the repeated injections of morphine activated the autophagic flux in the hippocampal CA1 region [45]. After inhibiting autophagy with 3-MA, it was observed that morphine-induced memory impairment in mice was significantly elevated, and the death of pyramidal layer cells within the hippocampal CA1, CA3 and DG regions was also considerably increased [46]. Morphine negatively regulates the maturation of autophagosomes, hindering the fusion of autophagosomes with autophagolysosome [47].TFEB is abnormally expressed in neurodegenerative disorders, including Alzheimer's and Parkinson's [48]. Recent studies have also revealed that this abnormality of TFEB is associated with either autophagy or lysosomal dysfunction [49].
In the present study, 20-DOI reversed neuronal apoptosis, oxidative stress response, abnormal autophagy, and inhibited neurogenesis, spatial learning, and memory impairment in chronic morphine-treated rats. Several studies have revealed that Euphorbiaceae and its extracts demonstrate anti-apoptotic effects on embryonic kidney cells [50]. Meanwhile, 20-DOI suppresses apoptosis in chondrocytes and improves autophagy by TFEB [16]. In addition, Euphorbiaceae reduces oxidative stress and lipotoxicity by concurrently modulating GLUT4 overexpression in the diabetic rat liver tissues [51].
This study suggests that 20-DOI can promote nuclear TFEB expression, inhibit apoptosis and oxidative stress in hippocampal CA1, and improve autophagy. Under chronic morphine conditions, the hippocampal CA1 apoptosis and oxidative stress are exacerbated, and autophagy becomes abnormal, which can be reversed with 20-DOI. Furthermore, studies have shown that autophagic subsection formation is decreased, and lysosomal activity is disrupted through chronic morphine treatment. Our study showed that 20-DOI administration promoted autophagosome production and increased TFEB expression in the nucleus of the hippocampal CA1 region. These findings indicate that 20-DOI could inhibit apoptosis, oxidative stress, and autophagy by modulating TFEB in hippocampal CA1. Therefore, 20-DOI inhibits chronic morphine-induced apoptosis, improves oxidative stress, enhances autophagic flux, restores lysosomal function, and improves morphine-induced hippocampus neurotoxicity and memory deficits in the rats.
Our study was limited to examining the role of 20-DOI with the oxidative stress, apoptosis, and autophagy. However, we have not explored specific molecules pathways. Secondly, our research only preliminarily verified the role of TFEB. Therefore, further research on the role of TFEB in morphine-induced hippocampus neurotoxicity is required.Furthermore, Previous studies have indicated that the aggravation of memory impairment caused by morphine was due to autophagy inhibition in hippocampal neurons, resulting in increased apoptosis of neurons, especially those located in CA1 region. Therefore, we only studied the hippocampal CA1 region, and further research is needed to investigate the effects of 20-DOI on other regions such as CA3 in the hippocampus.
5. Conclusion
We observed that 20-DOI attenuated morphine administration in rats with chronic disease caused spatial learning and memory dysfunction. These mechanistic effects could be partially related to 20-DOI protecting the CA1 region of rat hippocampal neurons from the morphine-induced oxidative stress, apoptosis, and autophagy through TFEB.
Funding sources
This work was supported by Department science and Technology of Zhejiang Province (LGD21H310004).
Data availability statement
Data will be made available on request.
The use and declaration of AI and AI-assisted technologies
None.
CRediT authorship contribution statement
Jianfeng Ma: Writing – review & editing, Writing – original draft, Project administration, Data curation. Linfang Zou: Software, Project administration, Investigation. Yani Lou: Validation, Methodology, Investigation. Yuanqu Lin: Visualization, Methodology, Investigation. Jiansong Zhou: Software, Methodology, Data curation. Nanbin Ju: Investigation, Data curation. Jun Pan: Supervision, Investigation. Xutong Zhang: Funding acquisition, Formal analysis. Dansi Qi: Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e31605.
Contributor Information
Jianfeng Ma, Email: majianfeng@wmu.edu.cn.
Jun Pan, Email: panjun@wmu.edu.cn.
Xutong Zhang, Email: zhangxutong@wmu.edu.cn.
Dansi Qi, Email: qidansi@hotmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
figs4.
figs5.
figs6.
figs7.
figs8.
figs9.
figs10.
figs11.
figs12.
figs13.
figs14.
figs15.
figs16.
figs17.
figs18.
figs19.
figs20.
figs21.
figs22.
figs23.
figs24.
figs25.
figs26.
figs27.
figs28.
figs29.
figs30.
figs31.
figs32.
figs33.
figs34.
figs35.
figs36.
figs37.
figs38.
figs39.
figs40.
figs41.
figs42.
References
- 1.Fujisawa S., Ouchi A. Replays of socially acquired information in the hippocampus. Neuron. 2022;110(5):744–745. doi: 10.1016/j.neuron.2022.02.005. [DOI] [PubMed] [Google Scholar]
- 2.McQuiston A.R. Mu opioid receptor activation normalizes temporo-ammonic pathway driven inhibition in hippocampal CA1. Neuropharmacology. 2011;60(2–3):472–479. doi: 10.1016/j.neuropharm.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou M., Lou P., Lu Y., Li C.J., Wang D.S., Lu Q., et al. Imbalance of HCN1 and HCN2 expression in hippocampal CA1 area impairs spatial learning and memory in rats with chronic morphine exposure. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2015;56:207–214. doi: 10.1016/j.pnpbp.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Eichenbaum H. On the integration of space, time, and memory. Neuron. 2017;95(5):1007–1018. doi: 10.1016/j.neuron.2017.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L., Huang L., Lu K.R., Liu Y.T., Tu G.H., Zhu M.J., et al. Cocaine-induced synaptic structural modification is differentially regulated by dopamine D1 and D3 receptors-mediated signaling pathways. Addiction Biol. 2017;22(6):1842–1855. doi: 10.1111/adb.12462. [DOI] [PubMed] [Google Scholar]
- 6.Ramli F.F., Hashim S.A.S., Effendy N.M. Factors associated with low bone density in opioid substitution therapy patients: a systematic review. Int. J. Med. Sci. 2021;18(2):575–581. doi: 10.7150/ijms.52201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osmanlıoğlu H.Ö., Yıldırım M.K., Akyuva Y., Yıldızhan K., Nazıroğlu M. Morphine induces apoptosis, inflammation, and mitochondrial oxidative stress via activation of TRPM2 channel and nitric oxide signaling pathways in the Hippocampus. Mol. Neurobiol. 2020;57(8):3376–3389. doi: 10.1007/s12035-020-01975-6. [DOI] [PubMed] [Google Scholar]
- 8.Pourhassanali N., Zarbakhsh S., Miladi-Gorji H. Morphine dependence and withdrawal-induced changes in mouse Sertoli cell (TM4) line: evaluation of apoptotic, inflammatory and oxidative stress biomarkers. Reprod. Toxicol. 2021;105:175–183. doi: 10.1016/j.reprotox.2021.09.004. [DOI] [PubMed] [Google Scholar]
- 9.otaghinejad M., Karaimian S.M., Motaghinejad O., Shabab B., Asadighaleni M., Fatima S. The effect of various morphine weaning regimens on the sequelae of opioid tolerance involving physical dependency, anxiety and hippocampus cell neurodegeneration in rats. Fundam. Clin. Pharmacol. 2015;29(3):299–309. doi: 10.1111/fcp.12121. [DOI] [PubMed] [Google Scholar]
- 10.Askari N., Mousavi A., Vaez-Mahdavi M.R. Maternal deprivation effect on morphine-induced CPP is related to changes in opioid receptors in selected rat brain regions (hippocampus, prefrontal cortex, and nucleus accumbens) Behav. Process. 2022;197 doi: 10.1016/j.beproc.2022.104607. [DOI] [PubMed] [Google Scholar]
- 11.Parvez M.K., Rishi V. Herb-drug interactions and hepatotoxicity. Curr. Drug Metabol. 2019;20(4):275–282. doi: 10.2174/1389200220666190325141422. [DOI] [PubMed] [Google Scholar]
- 12.Dhahri M., Alghrably M., Mohammed H.A., Badshah S.L., Noreen N., Mouffouk F., et al. Natural polysaccharides as preventive and therapeutic horizon for neurodegenerative diseases. Pharmaceutics. 2021;14(1):1. doi: 10.3390/pharmaceutics14010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu L.H., Chen L.Y., Gu G.Y., Wang Y., Xu Y.Z., Zhong Y.G. Natural products from traditional Chinese medicine for the prevention and treatment of heart failure: progress and perspectives. Rev. Cardiovasc. Med. 2022;23(2):60. doi: 10.31083/j.rcm2302060. [DOI] [PubMed] [Google Scholar]
- 14.Wang L., Zhang H.Y., Gao B., Shi J., Huang Q., Han Y.H., et al. Tetramethylpyrazine protects against glucocorticoid-induced apoptosis by promoting autophagy in mesenchymal stem cells and improves bone mass in glucocorticoid-induced osteoporosis rats. Stem Cell. Dev. 2017;26(6):419–430. doi: 10.1089/scd.2016.0233. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L., Gao L., Li Z.J., Yan X.J., Yang Y.J., Tang Y.P., et al. Bio-guided isolation of the cytotoxic terpenoids from the roots of Euphorbia kansui against human normal cell lines L-O2 and GES-1. Int. J. Mol. Sci. 2012;13(9):11247–11259. doi: 10.3390/ijms130911247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu M.B., Jin J., Ren C.H., Chen X.M., Pan Z.Y., Wu Y.S., et al. 20-Deoxyingenol alleviates osteoarthritis by activating TFEB in chondrocytes. Pharmacol. Res. 2021;165 doi: 10.1016/j.phrs.2020.105361. [DOI] [PubMed] [Google Scholar]
- 17.Wang H.J., Karuppan M.K.M., Devadoss D., Nair M., Chand H.S., Lakshmana M.K. TFEB protein expression is reduced in aged brains and its overexpression mitigates senescence-associated biomarkers and memory deficits in mice. Neurobiol. Aging. 2021;106:26–36. doi: 10.1016/j.neurobiolaging.2021.06.003. [DOI] [PubMed] [Google Scholar]
- 18.National Research Council (US) eighth ed. National Academies Press (US); Washington (DC): 2011. Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Guide for the Care and Use of Laboratory Animals.https://nap.nationalacademies.org/catalog/12910 [Google Scholar]
- 19.Withey S.L., Cao L., Moura F.B., Cayetano K.R., Rohan M.L., Bergman J., et al. Fentanyl-induced changes in brain activity in awake nonhuman primates at 9.4 Tesla. Brain Imaging Behav. 2022;16(4):1684–1694. doi: 10.1007/s11682-022-00639-4. [DOI] [PubMed] [Google Scholar]
- 20.Milanesi L.H., Rossato D.R., Rosa J.L.O., D'avila L.F., Metz V.G., Wolf J.F., et al. Topiramate-chitosan nanoparticles prevent morphine reinstatement with no memory impairment: dopaminergic and glutamatergic molecular aspects in rats. Neurochem. Int. 2021;150 doi: 10.1016/j.neuint.2021.105157. [DOI] [PubMed] [Google Scholar]
- 21.Zagrebelsky M., Lonnemann N., Fricke S., Kellner Y., Preuß E., Michaelsen-Preusse K., et al. Nogo-A regulates spatial learning as well as memory formation and modulates structural plasticity in the adult mouse hippocampus. Neurobiol. Learn. Mem. 2017;138:154–163. doi: 10.1016/j.nlm.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 22.Twarkowski H., Steininger V., Kim M.J., Sahay A. A dentate gyrus-CA3 inhibitory circuit promotes evolution of hippocampal-cortical ensembles during memory consolidation. Elife. 2022;11 doi: 10.7554/eLife.70586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hongo Y., Ogawa K., Takahara Y., Takasu K., Royer S., Hasegawa M., et al. Topological organization of CA3-to-CA1 excitation. Eur. J. Neurosci. 2015;42(5):2135–2143. doi: 10.1111/ejn.12969. [DOI] [PubMed] [Google Scholar]
- 24.Riedel G., Casabona G., Platt B., Macphail E.M., Nicoletti F. Fear conditioning-induced time- and subregion-specific increase in expression of mGlu5 receptor protein in rat hippocampus. Neuropharmacology. 2000;39(11):1943–1951. doi: 10.1016/s0028-3908(00)00037-x. [DOI] [PubMed] [Google Scholar]
- 25.Jung S.H., Brownlow M.L., Pellegrini M., Jankord R. Divergence in Morris water maze-based cognitive performance under chronic stress is associated with the hippocampal whole transcriptomic modification in mice. Front. Mol. Neurosci. 2017;10:275. doi: 10.3389/fnmol.2017.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saffar S., Fatemi I., Rahmani M., Hassanshahi J., Sahamsizadeh A., Allahtavakoli M., et al. The effect of epigallocatechin-3-gallate on morphine-induced memory impairments in rat: EGCG effects on morphine neurotoxicity. Hum. Exp. Toxicol. 2020;39(7):994–1002. doi: 10.1177/0960327120909540. [DOI] [PubMed] [Google Scholar]
- 27.Gorio A., Vergani L., Malosio M.L., Lesma E., Giulio A.M.D. Perinatal exposure to morphine: reactive changes in the brain after 6-hydroxydopamine. Eur. J. Pharmacol. 1996;303(1–2):21–26. doi: 10.1016/0014-2999(96)00054-4. [DOI] [PubMed] [Google Scholar]
- 28.Kasala S., Briyal S., Prazad P., Ranjan A.K., Stefanov G., Donovan R., et al. Exposure to morphine and caffeine induces apoptosis and mitochondrial dysfunction in a neonatal rat brain. Front Pediatr. 2020;8:593. doi: 10.3389/fped.2020.00593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heo J.R., Lee G.A., Kim G.S., Hwang K.A., Choi K.C. Phytochemical-induced reactive oxygen species and endoplasmic reticulum stress-mediated apoptosis and differentiation in malignant melanoma cells. Phytomedicine. 2018;39:100–110. doi: 10.1016/j.phymed.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Cui J.Y., Li Y.K., Zhang W.Q., Qian H.R., Zhang Z.Y., Xu K. Alginic acid induces oxidative stress-mediated hormone secretion disorder, apoptosis and autophagy in mouse granulosa cells and ovaries. Toxicology. 2022;467 doi: 10.1016/j.tox.2022.153099. [DOI] [PubMed] [Google Scholar]
- 31.Park J.H., Ku H.J., Lee J.H., Park J.W. Idh2 deficiency exacerbates acrolein-induced lung injury through mitochondrial redox environment deterioration. Oxid. Med. Cell. Longev. 2017 doi: 10.1155/2017/1595103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karkhah A., Ataee R., Ataie A. Morphine pre- and post-conditioning exacerbates apoptosis in rat hippocampus cells in a model of homocysteine-induced oxidative stress. Biomed Rep. 2017;7(4):309–313. doi: 10.3892/br.2017.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye G.H., Tao L.Y., Ma C.L., Wen D., Yu L.S., Fan Y.Y., et al. Influences of CCK-8 on expressions of apoptosis-related genes in prefrontal cortex neurons of morphine-relapse rats. Neurosci. Lett. 2016;631:115–121. doi: 10.1016/j.neulet.2016.08.028. [DOI] [PubMed] [Google Scholar]
- 34.Razavi Y., Alamdary S.Z., Katebi S.N., Khodagholi F., Haghparas A. Morphine-induced apoptosis in the ventral tegmental area and hippocampus after the development but not extinction of reward-related behaviors in rats. Cell. Mol. Neurobiol. 2014;34(2):235–245. doi: 10.1007/s10571-013-0007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu S.F., Yu W.L., Jiang X.X., Huang R.M., Zhang X.Y., Lan J., et al. Protective effects of curcumin on ATO-induced nephrotoxicity in ducks in relation to suppressed autophagy, apoptosis and dyslipidemia by regulating oxidative stress. Ecotoxicol. Environ. Saf. 2021;219 doi: 10.1016/j.ecoenv.2021.112350. [DOI] [PubMed] [Google Scholar]
- 36.Ferreira-Chamorro P., Redondo A., Riego G., Leánez S., Pol O. Sulforaphane inhibited the nociceptive responses, anxiety- and depressive-like behaviors associated with neuropathic pain and improved the anti-allodynic effects of morphine in mice. Front. Pharmacol. 2018;9:1332. doi: 10.3389/fphar.2018.01332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Motaghinejad M., Karimian M., Motaghinejad O., Shabab B., Yazdani I., Fatima S. Protective effects of various dosage of Curcumin against morphine induced apoptosis and oxidative stress in rat isolated hippocampus. Pharmacol. Rep. 2015;67(2):230–235. doi: 10.1016/j.pharep.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Chen Y.M., Fu Y., An Y.J., Cao J., Wang J.H., Zhang J.C. Interactive effects of morphine and dopamine receptor agonists on spatial recognition memory in mice. Clin. Exp. Pharmacol. Physiol. 2018;45(4):335–343. doi: 10.1111/1440-1681.12889. [DOI] [PubMed] [Google Scholar]
- 39.Faro L.R.F., Costas-Ferreira C., Pantoja A.A., Durán R. Protective effects of antioxidants on striatal dopamine release induced by organophosphorus pesticides. Pestic. Biochem. Physiol. 2022;182 doi: 10.1016/j.pestbp.2022.105035. [DOI] [PubMed] [Google Scholar]
- 40.Bagnoli E., Diviney T., FitzGerald U. Dysregulation of astrocytic mitochondrial function following exposure to a dopamine metabolite: implications for Parkinson's disease. Eur. J. Neurosci. 2021;53(9):2960–2972. doi: 10.1111/ejn.14764. [DOI] [PubMed] [Google Scholar]
- 41.Qi Y., Ren S.S., Ye J.W., Tian Y.M., Wang G.G., Zhang S.Q., et al. Infection microenvironment-activated core-shell nanoassemblies for photothermal/chemodynamic synergistic wound therapy and multimodal imaging. Acta Biomater. 2022;143:445–458. doi: 10.1016/j.actbio.2022.02.034. [DOI] [PubMed] [Google Scholar]
- 42.Rozisky J.R., Laste G., Macedo I.C., Santos V.S., Krolow R., Noschang C., et al. Neonatal morphine administration leads to changes in hippocampal BDNF levels and antioxidant enzyme activity in the adult life of rats. Neurochem. Res. 2013;38(3):494–503. doi: 10.1007/s11064-012-0941-8. [DOI] [PubMed] [Google Scholar]
- 43.Goudas L.C., Langlade A., Serrie A., Matson W., Milbury P., Thurel C., et al. Acute decreases in cerebrospinal fluid glutathione levels after intracerebroventricular morphine for cancer pain. Anesth. Analg. 1999;89(5):1209–1215. [PubMed] [Google Scholar]
- 44.Feng Y.M., Jia Y.F., Su L.Y., Wang D., Lv L., Xu L., et al. Decreased mitochondrial DNA copy number in the hippocampus and peripheral blood during opiate addiction is mediated by autophagy and can be salvaged by melatonin. Autophagy. 2013;9(9):1395–1406. doi: 10.4161/auto.25468. [DOI] [PubMed] [Google Scholar]
- 45.Liu L.T., Song Y.Q., Chen X.S., Liu Y., Zhu J.J., Zhou L.M., et al. Morphine-induced RACK1-dependent autophagy in immortalized neuronal cell lines. Br. J. Pharmacol. 2020;177(7):1609–1621. doi: 10.1111/bph.14922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan J.G., He L., Li X.P., Li M., Zhang X.N., Venesky J., et al. Activating autophagy in hippocampal cells alleviates the morphine-induced memory impairment. Mol. Neurobiol. 2017;54(3):1710–1724. doi: 10.1007/s12035-016-9735-3. [DOI] [PubMed] [Google Scholar]
- 47.Wan J., Ma J., Anand V., Ramakrishnan S., Roy S. Morphine potentiates LPS-induced autophagy initiation but inhibits autophagosomal maturation through distinct TLR4-dependent and independent pathways. Acta Physiol. 2015;214(2):189–199. doi: 10.1111/apha.12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baron O., Boudi A., Dias C., Schilling M., Nölle A., Vizcay-Barrena G., et al. Stall in canonical autophagy-lysosome pathways prompts nucleophagy-based nuclear breakdown in neurodegeneration. Curr. Biol. 2017;27(23):3626–3642. e6. doi: 10.1016/j.cub.2017.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rai S.N., Tiwari N., singh P., Mishra D., Singh A.K., Hooshmandi E., et al. Therapeutic potential of vital transcription factors in Alzheimer's and Parkinson's disease with particular emphasis on transcription factor EB mediated autophagy. Front. Neurosci. 2021;15 doi: 10.3389/fnins.2021.777347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Athmouni K., Belhaj D., Feki A.E., Ayadi H. Optimization, antioxidant potential, modulatory effect and anti-apoptotic action in of Euphorbia bivonae polysaccharides on hydrogen peroxide-induced toxicity in human embryonic kidney cells HEK293. Int. J. Biol. Macromol. 2018;116:482–491. doi: 10.1016/j.ijbiomac.2018.04.172. [DOI] [PubMed] [Google Scholar]
- 51.Oyebode O.A., Erukainure O.L., Chuturgoon A.A., Ghazi T., Naidoo P., Chukwuma C.I., et al. Bridelia ferruginea Benth. (Euphorbiaceae) mitigates oxidative imbalance and lipotoxicity, with concomitant modulation of insulin signaling pathways via GLUT4 upregulation in hepatic tissues of diabetic rats. J. Ethnopharmacol. 2022;284 doi: 10.1016/j.jep.2021.114816. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.