Abstract
Introduction and importance
Ovarian fibromas are benign tumours arising from the connective tissue of the ovarian cortex, classified into three pathological subtypes: fibroma, thecoma, and fibrothecoma. Their diagnosis is complicated by their solid nature and potential association with ascites and pleural effusion, resembling Meigs syndrome. Elevated serum CA125 levels can further complicate differentiation from malignant ovarian epithelial tumours.
Case presentation
A 37-year-old female from a rural area presented with a distended abdomen and weight loss lasting 2 months. Clinical examinations revealed a solid pelvic mass and diagnostic tests showed significantly elevated CA125 levels. Imaging suggested a large ovarian mass and surgical intervention confirmed a fibrothecoma of the left ovary. The postoperative course was uneventful, with subsequent resolution of ascites and pleurisy.
Clinical discussion
The diagnosis of ovarian fibromas/fibrothecomas poses challenges due to their asymptomatic nature, solid appearance, and occasional association with the Meigs syndrome. Elevated CA125 levels can mislead the diagnosis of epithelial ovarian carcinoma. The case underscores the importance of considering ovarian fibromas/fibrothecomas in the differential diagnosis of ovarian tumours with elevated CA125 levels, especially in women of reproductive age. The benign nature of these tumours necessitates a conservative surgical approach, emphasizing the importance of intraoperative frozen section analysis.
Conclusion
Ovarian fibrothecomas associated with elevated serum CA125 levels are rare. Their presentation can mimic malignant ovarian neoplasms, leading to potential diagnostic confusion. Surgical removal remains the treatment of choice, with a favorable prognosis post-surgery.
Keywords: Demons Meigs syndrome, Tumour markers, CA125, Fibrothecoma, Case report
Highlights
-
•
Ovarian fibromas/fibrothecomas are rare benign tumors that can mimic malignant ovarian neoplasms due to elevated serum CA125 levels, presenting diagnostic challenges in distinguishing them from epithelial ovarian carcinoma, especially in women of reproductive age.
-
•
The case illustrates the successful identification and surgical removal of an ovarian fibrothecoma in a 37-year-old female, leading to the resolution of associated ascites and pleurisy, highlighting the importance of surgical intervention in managing these tumors.
-
•
Emphasizes the necessity for gynecologists to consider ovarian fibromas/fibrothecomas in the differential diagnosis when encountering ovarian tumors with elevated CA125 levels, to ensure accurate diagnosis and appropriate, conservative surgical management.
1. Introduction
Ovarian fibromas are tumours that develop from the connective tissue within the ovarian cortex, encompassing three distinct pathological subtypes. Young and Scully provided the initial description of ovarian fibromas in 1983 [1]. An immune assay for detecting serum CA125 levels has been developed to facilitate the differential diagnosis between benign and malignant ovarian epithelial tumours and to monitor the progression of epithelial ovarian carcinoma. However, its sensitivity is considered suboptimal as a biomarker for the screening or early diagnosis of epithelial ovarian carcinoma [2]. Ovarian fibroma/fibrothecoma falls into the category of sex cord-stromal tumours, constituting approximately 5 % to 8 % of all ovarian tumours. Most ovarian fibromas/fibrothecomas manifest in adolescents and young women, presenting as solid pelvic or adnexal masses with a generally benign biobehavioral [3,4]. Approximately 10 % to 15 % of ovarian fibromas/fibrothecomas may be associated with ascites. We present this case report according to the SCARE guidelines [5] of ovarian fibromas/fibrothecomas that can mimic malignant ovarian neoplasms due to elevated serum CA125 levels.
2. Case presentation
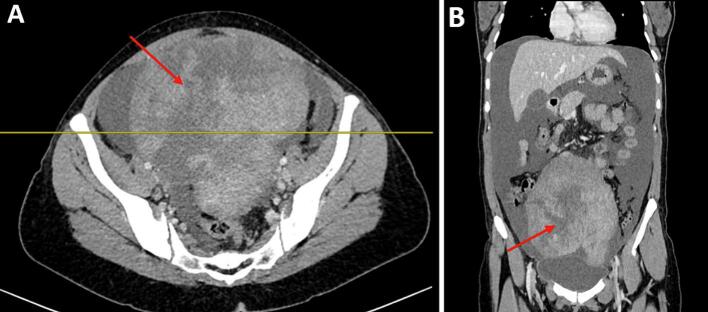
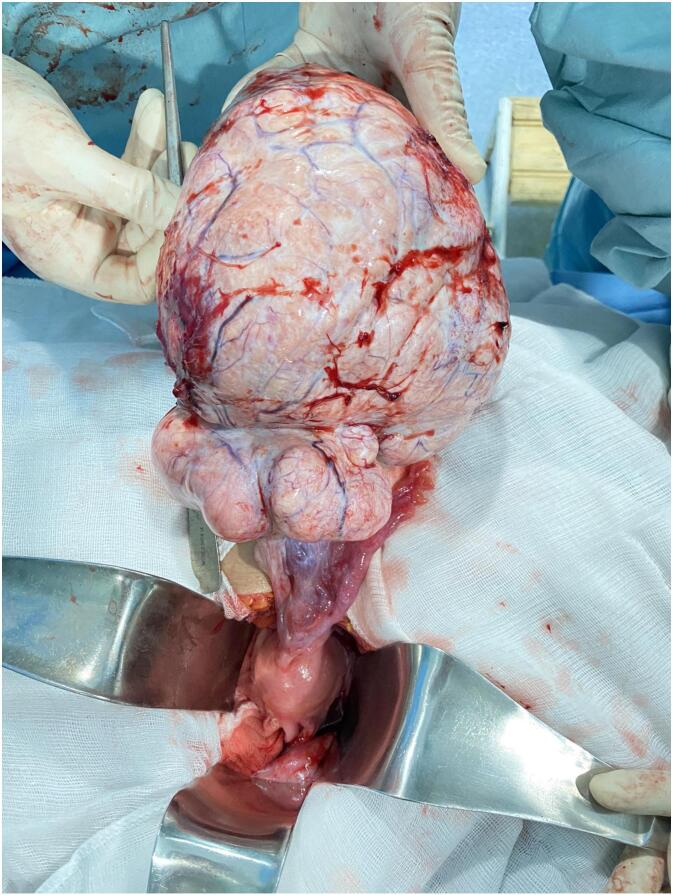
A 37-year-old female G2P1A1 with one vaginal delivery in her obstetric history, from a rural area, presented with a distended abdomen and weight loss that had lasted 2 months. She had menarche at 12 years old and has had a regular menstrual cycle. On physical examination: she weighed 60 kg with a body mass index of 20 kg/m2. Blood pressure was 120/60mmhg, pulse rate of 90 beats per minute and peripheral oxygen saturation of 98 % in room air. Abdominal examinations revealed a distended abdomen and a non-mobile mass with slight tenderness in the lower quadrants. The percussion revealed shifting dullness. On biological examination: BHCG was negative. Haemoglobin was 9,4 g/dl, white blood cell count: 5.990 × 109/μl, red blood cell count: 4230 × 109/μl, platelet count: 355000/mm3, AST: 12u/l, ALT: 14u/l, creatinine: 44 mg/l. The coagulation profiles were within normal limits, CA 125 level was 663,3 IU/ml (Positive), CA19–9 level was 8,05 IU/mml (negative), ACE level was 0,685 ng/ml (negative) and AFP S level was 7,41 IU/ml (negative). On radiological examination: transabdominal and transvaginal ultrasound revealed the presence of a large hypo-echogenic heterogeneous left latero-uterine mass vascularised on colour Doppler measuring 14.7 cm long. The CT scan showed a multolocated heterogenous intraperitoneal mass widely vascularised of 14.7 * 14.5 * 16.8 cm associated with ascites and pleural fluid (Fig. 1). Magnetic resonance imaging confirmed the ultrasound findings, but could not rule out ovarian malignancy. It revealed a voluminous abdominopelvic tissue mass, most probably left ovarian, measuring 17 * 15.3 * 10.3 cm with a polylobed outline; this mass has a T1 hypo signal T1, an intermediate signal and is heterogeneous in T2, has a diffusion hyper signal with ADC restriction (0.60). This mass shows progressive enhancement with a plateau according to a type 2 curve after injection of the paramagnetic product (Fig. 2). This mass is classified as ORADS 5. After the pre-operative evaluation, it was decided to treat the patient surgically with laparotomy. Intraoperatively, a solid mass was found in the left ovary, without necrosis and not confluent with the adjacent tissues (Fig. 3). Moderate serous ascites. A left adnexectomy and multiple peritoneal and epiploic biopsies were performed. Peritoneal cytology was performed. The postoperative course was straightforward. Histological and cytological results: A fibrothecoma of the left ovary measuring 21 cm long. Left tubal wall without histological lesion. Peritoneal and epiploic biopsies with no histological lesions. The ascites fluid is free of any cells suspected of malignancy. The postoperative course was uneventful. A follow-up CT scan was performed one month after surgery and showed resolution of ascites and pleurisy.
Fig. 1.
Coronal (A) and axial (B) view of the intraperitoneal mass (red arrow)
Fig. 2.
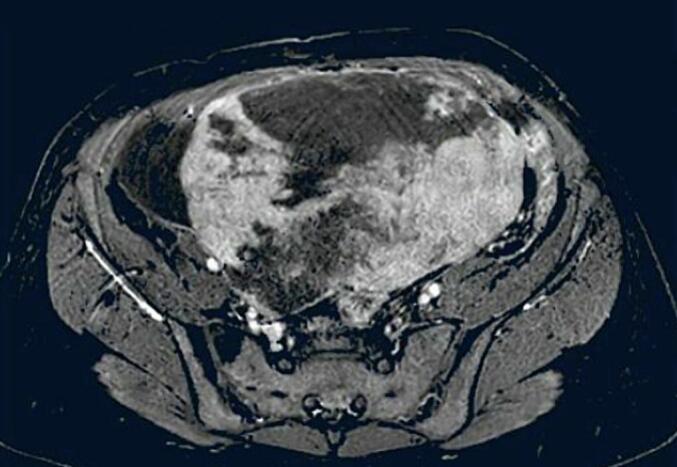
Magnetic resonance imaging of left ovarian fibrothecoma.
Fig. 3.
Surgical specimen of left ovarian fibrothecoma.
3. Discussion
Diagnosis of ovarian fibromas through clinical and laboratory means presents challenges. While these fibromas are typically asymptomatic and diagnosed incidentally, approximately 43.5 % of cases present with mild abdominal pain as the primary symptom [6]. Instances of acute abdominal pain necessitating immediate intervention occur when the tumour undergoes torsion and necrosis [7]. Further diagnostic exploration is essential due to the solid nature of ovarian fibromas, their correlation with ascites and pleural effusion (known as Meigs syndrome), and elevated serum CA125 levels, which may indicate the need to rule out ovarian malignancy [8]. CA125 antigen serves as a tumour marker generated by various clinical tissues, encompassing the epithelium of fallopian tubes, endometrium, endocervix, ovaries, and mesothelial cells found in the pleura, pericardium, and peritoneum [9]. Elevated serum CA125 levels are notably linked to enlarged ovarian fibromas (diameter ≥ 10 cm) or the presence of Meigs syndrome [4]. Ovarian fibromas generally manifest as large tumours, with an average size of 140 mm. The largest documented ovarian fibroma reached dimensions of 300 mm × 200 mm × 100 mm [10]. These tumours exhibit a variable macroscopic appearance, commonly presenting as solid stromal growths (as observed in our case) and less frequently as cystic tumours referred to as cystadenofibromas. The clinical differentiation between ovarian fibromas and other solid ovarian tumours poses a significant challenge [10]. In contrast to the thecoma, the fibrothecoma lacks functional activity, displaying increased collagen deposits and fewer cells with cytoplasmic vacuolation. The fibrothecoma exhibits higher cellularity with more tightly packed cells, characterized by a paler cytoplasm than fibroma. Identifying these tumours is crucial, given their benign nature, and complete removal ensures a curative outcome [11]. However, it is crucial to consistently consider Meigs syndrome in cases where an ovarian tumour is associated with ascites and pleural effusion [10]. Meigs syndrome is more commonly encountered in individuals in their fifth decade of life. Patients with Meigs' syndrome typically present with symptoms such as fatigue, shortness of breath, increased abdominal girth, fluctuations in weight, non-productive cough, bloating, and, in pre-menopausal women, amenorrhea or menstrual irregularities [12]. Due to technological advancements, high-resolution ultrasonography and contrast-enhanced ultrasound imaging have emerged as crucial modalities for clinically diagnosing and distinguishing uterine and ovarian neoplasms [3]. A review of the literature indicates different contrasts in contrast-enhanced ultrasound imaging between ovarian fibromas and subserous myomas. Ovarian fibromas showed hypoenhancement with delayed perfusion compared to the uterus, whereas subserous myomas exhibited homogeneous isoenhancement with synchronous enhancement that mirrors the myometrium in the early phase [3]. The origin of ascitic fluid in Meigs' syndrome seems to stem from a generalized secretion by the tumour itself [12]. Furthermore, tumour release of vascular endothelial growth factor (VEGF), IL-1, IL-6, IL-8 and TNF-α stimulates the mesothelial cells, serving as another factor in the fluid exudation process. It is worth noting that only large tumours are commonly associated with ascites, which may not be readily apparent during physical examination, and the amount of ascetic fluid does not correlate with the development of pleural effusion [12]. Our examination of the existing literature has provided an initial understanding of the origins of effusions related to this syndrome [13]. It suggests that migration of peritoneal fluid to the pleural cavity occurs through transdiaphragmatic lymphatic pathways. In particular, this network is more extensive on the right side, influenced by hepatic mass, resulting in a predominant occurrence of pleural effusion on the right [13]. The development of ascites is attributed to the release of fluid due to tumour oedema resulting from compression or torsion of the fibroid on its axis [13]. The treatment of Demons-Meigs syndrome involves a surgical approach through exploratory laparotomy, which involves the extraction of the ovarian tumour. In cases where the patient is in the prepubertal period, options such as lumpectomy, partial oophorectomy, or unilateral salpingectomy may be recommended. Women of childbearing age may undergo surgical treatment in the form of a unilateral adnexectomy [14]. Postmenopausal patients may undergo total hysterectomy with bilateral adnexectomy as part of their treatment. It should be noted that in one-third of cases, the contralateral ovary remains unaffected [14]. Demons-Meigs syndrome is characterized by the resolution of ascites and pleural fluid following the surgical removal of the tumour. Rapid recurrence of effusions consistently occurs after repeated punctures in the absence of surgery, an observation initially documented by Albert Jean Octave Demons in 1903 [14]. Demons-Meigs syndrome is a benign condition characterized by a favorable prognosis when promptly addressed. Permanent resolution of pleural effusion and ascites occurs following tumour removal. The postoperative life expectancy for individuals with this syndrome aligns with that of the general population after undergoing surgery [15]. In summary, ovarian fibromas/fibrothecomas can sporadically present with an elevated serum CA125 level, leading to potential misdiagnosis as ovarian epithelial carcinoma. Factors such as substantial tumour size and the presence of ascites are independently associated with this condition. It is important to note that elevated serum CA125 is not directly attributable to tumour cells. Gynecologists should be vigilant regarding the distinctive clinical characteristics of this uncommon ovarian tumour. Conservative surgery and intraoperative frozen section are recommended, particularly for patients of reproductive age.
Conclusions Ovarian fibrothecomas associated with elevated serum CA125 levels are exceptionally uncommon. Despite their infrequent occurrence, it is suggested that ovarian fibromas accompanied by elevated CA125 levels should be considered in the differential diagnosis of epithelial ovarian carcinoma, not only in postmenopausal women but also in women of reproductive age. However, for patients in their reproductive years, such as in our case, the suitable course of action appears to be surgical removal of the fibroma or adnexectomy, depending on the surgical conditions and capabilities. This is because elevated serum CA-125 levels associated with ovarian neoplasia do not necessarily indicate a malignant neoplasm. Consequently, the consideration of minimally invasive surgery for biopsy collection becomes imperative.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The local Research Ethics Committee has confirmed that no ethical approval is required for case reports.
Funding
This research did not receive specific grants from the public, commercial or not-for-profit sectors.
Author contribution
All the authors participated in the manuscript and validated the final version of the manuscript.
Guarantor
Mohamed Ali Chaouch.
Registration of research studies
N/A.
Consent
Written informed consent was obtained from the patient to publish this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Declaration of competing interest
No conflict of interest to disclose.
Acknowledgements
None.
References
- 1.Thanasa A, Thanasa E, Kamaretsos E, Paraoulakis I, Ziogas AC, Kontogeorgis G, et al. Surgical treatment of a rare case of ovarian fibroma associated with elevated CA125 levels in a patient of reproductive age: a case report. Cureus. 15[1]:e34097. [DOI] [PMC free article] [PubMed]
- 2.Bottoni P., Scatena R. In: Advances in Cancer Biomarkers: From Biochemistry to Clinic for a Critical Revision (Internet) Scatena R., editor. Springer Netherlands; Dordrecht: 2015. The role of CA 125 as tumor marker: biochemical and clinical aspects; pp. 229–244. (Advances in Experimental Medicine and Biology). [cité 21 janv 2024] Disponible sur: [DOI] [PubMed] [Google Scholar]
- 3.Chen H., Liu Y., Shen L. fei, Jiang M. jiao, Yang Z. fang, Fang G. ping. Ovarian thecoma-fibroma groups: clinical and sonographic features with pathological comparison. J. Ovarian Res. 22 Nov 2016;9(1):81. doi: 10.1186/s13048-016-0291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ali A.A., Jeannot B.M., Olasinde A.A., Abdirizak Y., Amin A.O., Muhumuza J., et al. A rare case of Demons-Meigs’ syndrome with a 7.5 kg giant ovarian fibroma associated with severe dyspnea: case report. Ann. Med. Surg. Dec 2023;85(12):6243. doi: 10.1097/MS9.0000000000001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sohrabi C., Mathew G., Maria N., Kerwan A., Franchi T., Agha R.A. The SCARE 2023 guideline: updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. Lond. Engl. 2023;109(5):1136. doi: 10.1097/JS9.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leung S.W., Yuen P.M. Ovarian fibroma: a review on the clinical characteristics, diagnostic difficulties, and management options of 23 cases. Gynecol. Obstet. Investig. 2006;62(1):1–6. doi: 10.1159/000091679. (22 févr) [DOI] [PubMed] [Google Scholar]
- 7.Boujoual M., Hakimi I., Kouach J., Oukabli M., Moussaoui D.R., Dehayni M. Large twisted ovarian fibroma in menopausal women: a case report. Pan Afr. Med. J. [Internet] 2015;20 doi: 10.11604/pamj.2015.20.322.5998. http://www.panafrican-med-journal.com/content/article/20/322/full/ [cité 21 janv 2024] Disponible sur: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Numanoglu C., Kuru O., Sakinci M., Akbayır O., Ulker V. Ovarian fibroma/fibrothecoma: retrospective cohort study shows limited value of risk of malignancy index score. Aust. N. Z. J. Obstet. Gynaecol. 2013;53(3):287–292. doi: 10.1111/ajo.12090. [DOI] [PubMed] [Google Scholar]
- 9.Yazdani S., Alijanpoor A., Sharbatdaran M., Bouzari Z., Abedisamakoosh M., Lakaieandi F., et al. Meigs’ syndrome with elevated serum CA125 in a case of ovarian fibroma/thecoma. Caspian J. Intern. Med. 2014;5(1):43–45. [PMC free article] [PubMed] [Google Scholar]
- 10.Meigs syndrome: about an uncommon case report. Clin. J. Obstet. Gynecol. 2023;6(1):010–013. (6 janv) [Google Scholar]
- 11.K M., B Z.T., K S.D., K A., S B., Togo M.A., et al. Ovarian fibrothecoma: a case report. Saudi J. Med. Pharm. Sci. 2023;9(04):237–239. (9 avr) [Google Scholar]
- 12.A case report on Meigs syndrome and elevated serum CA-125: a rare case report. J. Pulmonol. Respir. Res. 2021;5(1):031–033. (24 mars) [Google Scholar]
- 13.K D., Keita S., Traore M., Kone O., S Y., S B., et al. A case of Demons-Meigs syndrome observed at the Markala Reference Health Center. East Afr. Scholars J. Med. Sci. 2023;6:286–289. (23 juin) [Google Scholar]
- 14.Oumaima D.W. 2023. Demons Meigs Syndrome: About Two Cases. [Google Scholar]
- 15.Elisabeth R.H., Joëlle R.V., Nalisoa R.F., Lucas R.Z., Ranaliarinosy R.M., Soa R.N. Demons-Meigs syndrome with a high CA 125 level: a case report at Soavinandriana Center Hospital Antananarivo. OJPathology. 2023;13(02):73–78. [Google Scholar]