Abstract
Background
The number of obese patients requiring general anaesthesia is likely to increase in coming years, and obese patients pose considerable challenges to the anaesthetic team. Tracheal intubation may be more difficult and risk of aspiration of gastric contents into the lungs is increased in obese patients. Supraglottic airway devices (SADs) offer an alternative airway to traditional tracheal intubation with potential benefits, including ease of fit and less airway disturbance. Although SADs are now widely used, clinical concerns remain that their use for airway management in obese patients may increase the risk of serious complications.
Objectives
We wished to examine whether supraglottic airway devices can be used as a safe and effective alternative to tracheal intubation in securing the airway during general anaesthesia in obese patients (with a body mass index (BMI) > 30 kg/m2).
Search methods
We searched for eligible trials in the following databases: Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, Issue 8, 2012), MEDLINE via Ovid (from 1985 to 9 September 2012) and EMBASE via Ovid (from 1985 to 9 September 2012). The Cochrane highly sensitive filter for randomized controlled trials was applied in MEDLINE and EMBASE. We also searched trial registers such as www.clinicaltrials.gov and the Current Controlled Clinical Trials Website (http://www.controlled‐trials.com/) for ongoing trials. The start date of these searches was limited to 1985, shortly before the first SAD was introduced, in 1988. We undertook forward and backward citation tracing for key review articles and eligible articles identified through the electronic resources.
Selection criteria
We considered all randomized controlled trials of participants aged 16 years and older with a BMI > 30 kg/m2 undergoing general anaesthesia. We compared the use of any model of SAD with the use of tracheal tubes (TTs) of any design.
Data collection and analysis
We used standard methodological procedures expected by The Cochrane Collaboration. Two review authors independently assessed trial quality and extracted data, including information on adverse events. We contacted study authors for additional information. If sufficient data were available, results were presented as pooled risk ratios (RRs) with 95% confidence intervals (CIs) based on random‐effects models (inverse variance method). We employed the Chi2 test and calculated the I2 statistic to investigate study heterogeneity.
Main results
We identified two eligible studies, both comparing the use of one model of SAD, the ProSeal laryngeal mask airway (PLMA) with a TT, with a total study population of 232. One study population underwent laparoscopic surgery. The included studies were generally of high quality, but there was an unavoidable high risk of bias in the main airway variables, such as change of device or laryngospasm, as the intubator could not be blinded. Many outcomes included data from one study only.
A total of 5/118 (4.2%) participants randomly assigned to PLMA across both studies were changed to TT insertion because of failed or unsatisfactory placement of the device. Postoperative episodes of hypoxaemia (oxygen saturation < 92% whilst breathing air) were less common in the PLMA groups (RR 0.27, 95% CI 0.10 to 0.72). We found a significant postoperative difference in mean oxygen saturation, with saturation 2.54% higher in the PLMA group (95% CI 1.09% to 4.00%). This analysis showed high levels of heterogeneity between results (I2 = 71%). The leak fraction was significantly higher in the PLMA group, with the largest difference seen during abdominal insufflation-a 6.4% increase in the PLMA group (95% CI 3.07% to 9.73%).
No cases of pulmonary aspiration of gastric contents, mortality or serious respiratory complications were reported in either study. We are therefore unable to present effect estimates for these outcomes.
In all, 2/118 participants with a PLMA suffered laryngospam or bronchospasm compared with 4/114 participants with a TT. The pooled estimate shows a non‐significant reduction in laryngospasm in the PLMA group (RR 0.48, 95% CI 0.09 to 2.59).
Postoperative coughing was less common in the PLMA group (RR 0.10, 95% CI 0.03 to 0.31), and there was no significant difference in the risk of sore throat or dysphonia (RR 0.25, 95% CI 0.03 to 2.13). On average, PLMA placement took 5.9 seconds longer than TT placement (95% CI 3 seconds to 8.8 seconds). There was no significant difference in the proportion of successful first placements of a device, with 33/35 (94.2%) first‐time successes in the PLMA group and 32/35 (91.4%) in the TT group.
Authors' conclusions
We have inadequate information to draw conclusions about safety, and we can only comment on one design of SAD (the PLMA) in obese patients. We conclude that during routine and laparoscopic surgery, PLMAs may take a few seconds longer to insert, but this is unlikely to be a matter of clinical importance. A failure rate of 3% to 5% can be anticipated in obese patients. However, once fitted, PLMAs provide at least as good oxygenation, with the caveat that the leak fraction may increase, although in the included studies, this did not affect ventilation. We found significant improvement in oxygenation during and after surgery, indicating better pulmonary performance of the PLMA, and reduced postoperative coughing, suggesting better recovery for patients.
Keywords: Humans; Laryngeal Masks; Airway Management; Airway Management/instrumentation; Airway Management/methods; Anesthesia, General; Anesthesia, General/instrumentation; Intubation, Intratracheal; Intubation, Intratracheal/instrumentation; Laparoscopy; Obesity; Obesity/complications; Randomized Controlled Trials as Topic
Plain language summary
Different devices for airway management in obese patients during general anaesthesia
Patients undergoing general anaesthesia need to have their airway protected as they lose their normal reflexes. This is most commonly achieved by placing a tube down through the mouth and larynx into the trachea (a tracheal tube (TT)) and using this to ventilate the lungs. Supraglottic airway devices (SADs) offer an alternative; they extend from the mouth into the throat but sit above the larynx. The number of obese patients requiring general anaesthesia is likely to increase because obesity is becoming more common and is a risk factor for many chronic health conditions, such as diabetes, cancers and cardiovascular disease. Securing the airway in obese patients may be difficult, and obese patients are at increased risk for complications during anaesthesia, such as difficulty passing an airway and aspiration of stomach contents into the lungs. SADs offer potential benefits, but concerns remain that they may increase the risk of these serious complications.
We searched the databases to September 2012, to find controlled trials that had randomly assigned obese participants (with body mass index (BMI) greater than 30 kg/m2) undergoing general anaesthesia to TT or SAD for airway management. We wanted to investigate the effect of airway type on risk of failed placement; serious complications and death; oxygenation of the blood during and after surgery; coughing, sore throat or hoarseness during or after placement; and time taken and number of attempts needed to fit the airway.
We found two randomized studies with a total of 232 obese participants, both of which studied one model of SAD-the ProSeal laryngeal mask airway (PLMA). No relevant outcomes for death or other serious complications occurred in these studies.We found that in 3% to 5% of obese participants, it was not possible to fit a PLMA, and a change of device to a TT was required. The proportion of successful first attempts at airway placement did not differ between PLMA and TT, although it took approximately six seconds longer to place an SAD than a TT. We found significant postoperative reduction of almost 75% in episodes of low oxygen saturation and an improvement in mean oxygen saturation of 2.5% during recovery in the PLMA group. Postoperative cough was less common among participants in the PLMA group. Our findings are consistent with both increased and decreased risks of both sore throat and hoarseness in the PLMA group.
Identifying optimal anaesthetic techniques for obese patients is a priority for research. We could not establish the safety of SAD use in obese patients. Large databases created from medical records may be needed to clarify this issue.
Summary of findings
Summary of findings for the main comparison. PLMA versus tracheal tube for airway management during general anaesthesia in obese participants.
| PLMA versus tracheal tube for airway management during general anaesthesia in obese participants | ||||||
| Patient or population: airway management during general anaesthesia in obese participants Settings: Intervention: PLMA versus tracheal tube | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | PLMA versus tracheal tube | |||||
| Failed placement/change of device | Not estimable | 118 (2 studies) | ⊕⊕⊝⊝ lowa | 5/118 participants (4.2%) with PLMA required change to tracheal tube (TT) because of failed insertion. No equivalent outcome identified for the TT group | ||
| Episodes of hypoxaemia in PACU < 92% oxygen saturation | 429 per 1000 | 116 per 1000 (43 to 309) | RR 0.27 (0.1 to 0.72) | 70 (1 study) | ⊕⊕⊕⊝ moderateb | |
| Oxygen saturation of peripheral blood (%) in PACU | The mean oxygen saturation of peripheral blood (%) in PACU ranged across control groups from 90.3% to 94.7% | The mean oxygen saturation of peripheral blood (%) in PACU in the intervention groups was 2.54 higher (1.09 to 4 higher) | 204 (2 studies) | ⊕⊕⊝⊝ lowa,c | ||
| Pulmonary aspiration of gastric contents | See comment | See comment | Not estimable | 232 (2 studies) | See comment | No cases of pulmonary aspiration occurred in study populations |
| Serious respiratory complications and mortality-not reported | See comment | See comment | Not estimable | ‐ | See comment | No cases of serious respiratory complications or mortality within 30 days of anaesthesia reported in study populations |
| Laryngospasm/bronchoconstriction between induction and recovery | 35 per 1000 | 18 per 1000 (3 to 100) | RR 0.5 (0.09 to 2.84) | 232 (2 studies) | ⊕⊝⊝⊝ very lowd,e,f | |
| Time to secure airway | The mean time to secure airway in the control groups was 20 seconds | The mean time to secure airway in the intervention groups was 5.9 higher (3 to 8.8 higher) | 70 (1 study) | ⊕⊕⊝⊝ lowb,d | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence: High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aSubstantial differences between study populations. Heterogeneity (I2 = 71%). bBased on one study only. cUnclear clinical importance of this difference in oxygenation. dImpossible for intubator/assessor to be blinded to airway device used. eBased on 5 events only. fConfidence interval crosses no effect and is consistent with increased and decreased risk.
Background
Description of the condition
The prevalence of obesity is increasing. The World Health Organization (WHO) estimated that more than half a billion adults were obese in 2008, with a body mass index (BMI) > 30 kg/m2 (WHO 2012). Obesity rates vary greatly between countries, with prevalence rates ranging from USA 33%, Canada 24%, UK 23% Greece 18% to Norway 10% and Japan and Korea both 4%, all in 2009 (OECD 2010; OECD 2012). Obesity is becoming more common in countries in which the current prevalence is lower than in the developed world, such as India and China. If these trends continue, projections are that the global prevalence of obesity may be 38% in 2030, with highest prevalences in China and Latin America (Kelly 2008).
Obesity is a risk factor for many chronic health conditions such as diabetes, cancers and cardiovascular disease. The number of obese patients requiring general anaesthesia for surgery can be expected to increase, reflecting the rising prevalence of obesity, frequent co‐morbidities in these patients and the use of bariatric (gastric band or bypass) surgery to treat obesity. Patients undergoing general anaesthesia need to have their airway protected as they lose their normal reflexes. Sometimes this can be achieved by supporting the jaw with a combination of a facemask and simple airway manoeuvres and adjuncts. Ventilation may be spontaneous (patients breathing for themselves), but mechanical positive‐pressure ventilation (PPV) may also be used. The latter is essential if the surgical procedure requires muscle relaxation. During PPV, an airway device that seals the airway must be used.
Obese patients pose considerable challenges to the anaesthetic team. Tracheal intubation may be more difficult than in non‐obese patients (Juvin 2003; Karkouti 2000; Lundstrom 2009). In the UK, the Fourth National Audit of the Royal College of Anaesthetists' and Difficult Airway Society (NAP4) showed that obese patients accounted for 42% of individuals who experienced a major airways complication during anaesthesia (leading to death, brain damage, an emergency surgical airway or intensive care unit (ICU) admission) (Cook 2011a). Risk of aspiration of gastric contents into the lungs is increased in obese patients because of the presence of hiatus hernia and increased intra‐abdominal pressure. The heavy chest wall, the splinted diaphragm and reduced chest wall compliance alter lung volumes and gas exchange and increase the work of breathing. This means that higher inflation pressures are required to ventilate the lungs of obese patients. Obese patients have a decreased respiratory reserve, making them vulnerable to hypoxia if an airway is lost, and increasing the risk of postoperative chest infection and other complications (Adams 2000; Malhotra 2008; Marley 2005).
Description of the intervention
A cuffed tracheal tube (TT), running from the mouth, through the larynx and into the trachea, has been considered the most reliable device for securing a patient’s airway during general anaesthesia. Because an inflatable cuff lies within the trachea, a TT may achieve the best protection against aspiration and can enable increased pressures during ventilation. Passing a TT requires the use of a laryngoscope and can be a difficult procedure. Use of the laryngoscope to pass the TT is associated with alterations in the patient's cardiovascular system, such as changes in pulse rate and blood pressure, during insertion. There is also a risk of damage to oropharyngeal structures, including dental trauma and mucosal bleeding. Failure to intubate after induction of anaesthesia, especially if the patient's muscles have been paralyzed by the use of a muscle relaxant, requires immediate action and rescue by another means of ventilation.
Supraglottic airway devices (SADs) offer an alternative airway to traditional tracheal intubation or a face mask. The classic laryngeal mask airway (cLMA, Intavent Direct, Maidenhead, UK) was introduced in the 1980s as an alternative to the face mask and the TT (Brain 1985). This consists of a 'mask', which sits in the pharynx, encircling the larynx, and a tube leading through the mouth to the anaesthetic circuit. The mask has a ventral cuff, which is inflated in situ, forming an oval seal around the laryngeal inlet. The resulting seal is a low‐pressure one, and use with PPV above 20 cm water pressure (cm H2O) leads to risk of leakage of gases around the cuff. This can result in loss of ventilating gas with associated risks of hypoventilation and the possibility of leaking gas entering the oesophagus and the stomach (gastric inflation). Gastric inflation in turn increases the chances of regurgitation and aspiration of gastric contents into the airway and lungs. Obesity, gastro‐oesophageal reflux and laparoscopic surgery have therefore been considered contraindications to the use of SADs for airway management.
A group of SADs with features intended to decrease the risk of aspiration are now available. Most of these newer designs include an additional drainage tube, which provides access to the gastro‐intestinal tract. In several models, the seal with the airway has been improved, thereby enabling the use of higher airway pressures during PPV without leakage of ventilating gases. The newer models have been labelled second‐generation SADs to distinguish them from SADs without these features (Cook 2011). Second‐generation devices include the ProSeal Laryngeal Mask Airway (PLMA, Intavent Direct, Maidenhead, UK & Teleflex, Westmeath, Ireland) (Brain 2000), i‐gel (Intersurgical Limited, Berkshire, UK), the Supreme Laryngeal Mask Airway (SLMA, Teleflex, Westmeath, Ireland), the Laryngeal Suction Mark II (LTS II, VBM Medizintechnik GmbH, Sulz am Neckar, Germany), the Streamlined Liner of the Pharynx Airway (SLIPA, CurveAir Ltd, London UK), the Baska Mask (Baska Mask, Strathfield, Australia), the GuardianCPV (Umedaes Ltd, Kowloon, Hong Kong) and the AES Guardian CPV (Anesthesia Service Inc, Oklahoma City, Oklahoma, USA).
Design developments, research findings and clinical experience have resulted in expansion of the use of SADs beyond their original indication in low‐risk patients. It has been shown that SADs are now used in 56% of anaesthetics. Of this 56%, most are standard laryngeal masks, and only 10% are second‐generation devices (Woodall 2011). A large body of literature, including descriptive, observational and interventional studies, describes the efficacy of various designs of SAD (Verghese 1996). Reviews of randomized trials of first‐ and second‐generation devices compared with a TT have reported advantages in ease of fitting, sore throat, postoperative pain, lower risk of damage and less haemodynamic disturbance (Cook 2005; Hohlrieder 2007; Hohlrieder 2007a; Zhang 2009). An ongoing Cochrane review is comparing the effectiveness of the PLMA and the cLMA in unselected surgical patients (Qamarul 2011).
The potential increased risk of serious complications, such as aspiration, when an SAD is used remains unresolved in the literature, as these are, fortunately, rare events (Brimacombe 1995a). In the UK, NAP4 estimated the incidence of a serious adverse airway event (death, brain damage, unplanned ICU admission) as 1 in 46,000 general anaesthetics (95% CI 35,000 to 69,000), and of death and brain damage as 1 in 202,000 (95% CI 119,000 to 657,000) (Cook 2011a). Surrogate measures such as gas leak, seal pressure and gastric regurgitation can be used as markers of airway safety, and evidence is conflicting regarding increased risk of these markers when SADs are used (Brimacombe 1995; Yu 2010). An improved seal has been noted in newer second‐generation designs, with indications but not conclusive evidence that the risk of aspiration might be lower (Cook 2005). Concerns about airway complications in SADs are even greater for obese patients, who are already at increased risk of aspiration and hypoventilation whilst an airway is secured.
Why it is important to do this review
The number of obese patients requiring general anaesthesia will increase both in the developed and the developing world, and SADs offer potential benefits in the management of these patients. Although SADs are now widely used, clinical concerns remain that their use for airway management in obese patients may increase the risk of serious complications. It is important to resolve the issue of the safety of SADs. Existing reviews consider predominantly or exclusively non‐obese patient populations. The safety and effectiveness of SADs in obese patients have not been systematically reviewed. This review will compare the safety and effectiveness of tracheal tubes with SADs of any design when used in obese patients.
Objectives
We wished to examine whether supraglottic airway devices can be used as a safe and effective alternative to tracheal intubation to secure the airway during general anaesthesia in obese patients (with a BMI > 30 kg/m2).
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomized controlled trials (RCTs), including quasi‐randomized studies and cluster‐randomized studies. We did not include simulation studies in this review. We would have included cross‐over trials if the order of insertion of devices had been randomly assigned. We included both trials that have been designed as equivalence or non‐inferiority trials and the more usual superiority trials.
Types of participants
We included studies with participants aged 16 years and over with a BMI > 30 kg/m2 undergoing general anaesthesia with spontaneous or positive‐pressure ventilation. We excluded studies of children because of different anaesthetic considerations in obese children. We included trials that consisted of a mixed participant population, such as some participants younger than 16 years or non‐obese participants, if the results from obese participants aged 16 years and over were reported separately.
Types of interventions
We considered studies that compared the use of any model of SAD versus tracheal tubes of any design. Because of differences in design, we planned to divide the intervention into two groups.
First‐generation SADs such as cLMA.
Second‐generation SADs such as ProSeal LMA (PLMA), i‐gel, Supreme LMA (SLMA), LTS II and SLIPA.
We aimed to compare the use of each group (first or second generation) with the use of a tracheal tube. We planned to consider individual SAD designs, such as PLMA or SLMA, versus TT in subgroup analyses if sufficient studies were identified. We did not include any studies that made a comparison between types of SAD, such as comparing the cLMA with the PLMA. We included any method of insertion of the airway and any mode of ventilation. We included single‐use and reusable devices. We did not include as an intervention group intubating SADs, such as LMA FasTrach (Teleflex, Westmeath, Ireland), the Intubating Laryngeal Mask Airway (ILMA, Intavent Direct, Maidenhead, UK) or the LMA CTrach (Intavent Direct, Maidenhead, UK).
Types of outcome measures
Our primary outcomes were the serious complications that underpin clinical uncertainty about whether or not to use SADs in obese patients. However, we anticipated that these outcomes may not be available in many eligible studies. Secondary outcomes included surrogate process markers for airway problems, such as leak fraction, and seal pressures, which might indicate problems with sealing the airway and achieving good ventilation. Other secondary outcomes such as trauma, cough or laryngospasm may reflect irritation to the vocal cords and are important, as they can increase the risk of postoperative complications. We studied first attempt success rates as this is an important potential advantage of SADs. We also aimed to assess the impact on patient‐reported measures of sore throat or hoarseness after surgery.
Outcomes did not form part of the study eligibility assessment. Studies that met participant, intervention and comparison criteria were included in the review, even if they did not report relevant outcomes. We planned to attempt to contact authors to find out whether outcome data had been collected, but if this information was not available, these studies would have been recorded in a separate category of 'eligible but no outcome data available'.
Primary outcomes
Failed placement, or change of airway device required.
Hypoxia between induction and full recovery-episodes of arterial oxygen saturation < 90% or lowest or mean arterial oxygen saturation.
Serious respiratory complications (including lower respiratory tract infection) within 30 days of anaesthesia.
Pulmonary aspiration of gastric contents anytime between induction of anaesthesia and leaving recovery.
Mortality within 30 days of anaesthesia.
Secondary outcomes
Leak fraction, defined as ratio of expired to inspired tidal volume or minute volume on a scale of 0 to 1.
Airway seal pressure achieved, measured in cm H2O.
Laryngospasm or bronchospasm between induction and leaving recovery.
Coughing between induction and leaving recovery.
Laryngeal or airway trauma, including any one of damage to vocal cords, bleeding or dental injury.
Patient‐reported sore throat or hoarseness: early (within 1 to 2 hours after surgery) or early and late (within 48 hours of surgery).
Placement: proportion with successful first placement of airway device.
Placement: total time required for securing airway device and commencing ventilation.
Search methods for identification of studies
Electronic searches
We searched for eligible trials in the following databases: the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 8 2012, see Appendix 1), MEDLINE via Ovid (from 1985 to 9 September 2012; see Appendix 2) and EMBASE via Ovid (from 1985 to 9 September 2012; see Appendix 3). The Cochrane highly sensitive filter for randomized controlled trials was applied in MEDLINE and EMBASE. We also searched trial registers in June 2012, such as www.clinicaltrials.gov and the Current Controlled Clinical Trials Website (http://www.controlled‐trials.com/), to identify ongoing trials. The start date of the searches was limited to 1985, shortly before the first SAD was introduced, in 1988.
Searching other resources
We undertook forward (June 2012) and backward (October 2012) citation tracing for key review articles and eligible articles identified through the electronic resources.
We used six key articles for forward citation tracking (Frappier 2003; Maltby 2002; Maltby 2003; Natalini 2003; Weber 2011; Zoremba 2009). These key articles were selected after discussion between investigators and had a specific focus on the use of SAD in obese populations. Web of Science was used to identify all articles that had cited these articles, and the titles were compiled. We used seven articles for backward citation (Carron 2012; Frappier 2003; Goldmann 2011; Kristensen 2010, Maltby 2002; Zoremba 2009). These articles were read by one investigator (AN) and their references reviewed for any studies referring to SAD use in obese participants.
We contacted investigators known to be involved in previous studies to enquire about ongoing and unpublished studies.
Data collection and analysis
Selection of studies
Results of the searches were collated and duplicates removed.
The selection of eligible articles took place in two stages. First, all titles and abstracts were screened by AN and AFS to remove studies that are very unlikely to be eligible. A pilot study of 100 titles was performed before all titles were reviewed to clarify criteria for discarding articles at this stage. If no abstract was available but the title was possibly relevant, the full text of the article was obtained. Later, when all titles and abstracts had been screened, the full text of potentially relevant titles was reviewed and recorded on the study eligibility form by AN and AFS(draft is included in Appendix 4),who then compared results. No differences required referral to TMC.
The search filters used for electronic databases had included a requirement for obesity, and hence search results were restricted to those with explicit mention of obese participants in the study population. Forward citation results were not restricted in this way, as they included all articles that had cited one of the key papers. We were concerned that some studies that did not meet the obese filter on our database searches might actually include some obese participants, and that we would miss these studies. We therefore selected from the forward citation search for full text review all RCTs that had compared an SAD with TTs, regardless of whether the study population was described as including obese participants. Studies that stated in the abstract that participants with BMI > 30 kg/m2 had been excluded were not selected for full text review.
Data extraction and management
Data were extracted from eligible studies by two investigators (AN and AFS), who used a paper‐based extraction form (see Appendix 4). This form was reviewed after data from the first three papers had been entered and modified as required. If duplicate publications from the same study were identified, we created a composite data set from all eligible publications. If relevant information or data were not available in the paper, we contacted the lead author to request the additional details. We resolved disagreements by discussion and, if necessary, by consultation with TMC or SSR.
Assessment of risk of bias in included studies
We used the Cochrane 'Risk of bias tool' to assess the quality of study design and the extent of potential bias (Higgins 2011) We considered the following domains.
Sequence generation.
Allocation concealment.
Blinding of participants, personnel and outcomes assessors.
Incomplete outcome data.
Selective outcomes reporting.
Other sources of bias.
It was not possible for the anaesthetist or the intubator to be blinded to the intervention in this research question. Similarly, it was difficult for assessors of outcomes during airway placement to be unaware of the allocation of the participant. Outcomes assessed during or after the operation, such as airway trauma or respiratory complications, could be assessed by staff other than the anaesthetist who were unaware of which device was being used. It was feasible for participants to not know their device allocation, which may be important for patient‐reported outcomes such as sore throat. We considered the likely impact of any detection bias on our results.
For other sources of bias, we aimed to review the original protocol of the trial, if this was available, to identify any changes to the procedure that may indicate bias. Cluster designs could be used in this topic, with the anaesthetist, operating theatre or hospital being the unit of randomization. For any cluster‐randomized trials included, we would have paid particular attention to baseline characteristics of participants and the expertise of the anaesthetist or intubator. The skill of the anaesthetist or intubator is an important confounder and needs to be addressed by randomization.
Cross‐over trials would have been included for certain outcomes only. Irreversible outcomes, such as complications or aspiration, cannot be assessed by using this design. Outcomes such as time to placement, seal pressure and failure rate could be assessed in a cross‐over design with a single anaesthetist using both airways sequentially. It is important that the order of insertion was randomly assigned in any cross‐over studies identified, so that outcome measures that may be subject to carry‐over can be considered.
A 'Risk of bias table' was completed for each included study as part of the data extraction form (see Appendix 4). For each outcome, risk of bias assessments were summarized for each domain on risk of bias graphs and figures and across all domains in a 'Table 1'.
Measures of treatment effect
Outcomes in this review include dichotomous outcomes, such asmortality, complications and successful first attempt, and continuous outcomes, such as time for placement and oxygen saturation. Some outcomes could have been recorded on short ordinal scales, such as pain ratings for sore throat. We planned to convert these to dichotomous outcomes, where appropriate, with the cut‐point determined by the clinical context. For dichotomous outcomes, we entered totals and numbers of events within each randomization group, calculated risk ratios with 95% confidence intervals and entered the data into RevMan 5.2 (RevMan 5.2). If the data had been presented in other forms such as odds or hazard ratios and we were unable to obtain the raw data from the authors, we would have used the generic variance option in RevMan 5.2 but would not have combined different effect measures (odds, risk or hazard ratios) in the same model. For continuous outcomes that had been measured on the same scale in different studies, such as time for placement, we calculated mean differences.
Unit of analysis issues
For any cluster trials included in the review, we planned to extract data directly from the publication only if the analysis accounted for the cluster design with a method such as multi‐level modelling or generalized estimating equations. If these adjustments had not been made within the report, we planned to undertake approximate analyses by recalculating standard errors or sample sizes on the basis of design effect. We would then analyse the resulting effect estimates and their standard errors using the generic inverse variance method in RevMan.
Included studies may have reported more than one comparison, for example, participants may have been allocated to a first‐ or second‐generation SAD and both groups compared with participants allocated to a TT group. This would not have posed unit of analysis issues, as these comparisons would appear in different meta‐analyses. However, if a study had used two different designs of second‐generation SADs, this might have raised unit of analysis issues. We planned to check that the same control group had not been used for both comparisons. If the same control group had been used, we planned to divide the control group, if data allowed, or combine the groups into a single pair‐wise comparison (Section 16.5.4, Higgins 2011).
Dealing with missing data
We contacted authors to request missing outcome data. If this was unsuccessful, we planned to perform sensitivity analyses to compare the effects of complete case analysis and worst case scenario and last observation carried forward options on the results of individual studies and any meta‐analyses. This is particularly important, as the research question concerns the equivalence or non‐inferiority of SADs to TT. Intention‐to‐treat (ITT) analysis may not be the most appropriate method for some outcomes, as it tends to bias results toward no difference (Section 16.2.1, Higgins 2011). Changing an airway during anaesthesia was an important outcome; we performed ITT for this outcome and included participants who had been excluded from the main analyses of the studies.
Assessment of heterogeneity
We expected that the findings for any given outcome may differ between studies included in the review. This heterogeneity may be due to the following.
Individual model or design of SAD.
Degree of obesity (e.g. BMI 30 to 34, 35 to 40, > 40 kg/m2).
Method of insertion.
Training and expertise of intubator.
Type of operation and type of anaesthetic given.
If we identified sufficient studies, we planned to assess heterogeneity using the Chi2 test and the I2 statistic. Important heterogeneity (Chi2 P < 0.1 or I2 > 50%) was investigated by using subgroup analyses.
Assessment of reporting biases
We planned to examine funnel plots to assess the potential for publication bias if we were able to identify sufficient studies (at least 10) reporting on a particular outcome. We also planned to use visual assessment supplemented by Egger’s test for asymmetry.
In addition to studies with no published results, reporting bias may be present within a study if data on some outcomes were collected but not reported. We contacted the study author to request outcome data if a report or the study protocol suggested that eligible outcomes had not been reported.
Data synthesis
We attempted meta‐analysis of outcomes for which we had comparable effect measures and for which measures of heterogeneity indicate that pooling of results was appropriate. We planned to combine data from non‐inferiority and superiority trials in a single meta‐analysis only if we had totals and numbers of events or comparable effect estimates, and if the studies had used comparable methods.
An I2 value > 80% would argue against presentation of an overall estimate. Our choice of a fixed‐effect or random‐effects statistical model for any meta‐analysis was influenced by study characteristics, such as patient group and degree of obesity, type of SAD, expertise of intubator and study size. We aimed to use Mantel-Haenszel models, where possible, for most dichotomous outcomes.
Subgroup analysis and investigation of heterogeneity
If we identified sufficient studies, we planned to attempt subgroup analyses to investigate potential sources of heterogeneity.
Individual model or design of SAD.
Degree of obesity (e.g. BMI 30 to 34, 35 to 40, > 40 kg/m2).
Method of insertion.
Training and expertise of intubator.
Type of operation and type of anaesthetic given.
Design of SAD and degree of obesity were of particular interest. We will assess the difference in effect size between subgroups using the I2 statistic (Higgins 2011).
Sensitivity analysis
We planned to undertake sensitivity analyses to explore the potential impact of missing data, as described in the Dealing with missing data section. We also planned to carry out analyses stratified by risk of bias and to model the potential impact of unpublished data on the overall effect estimates obtained.
Summary of findings
We used the principles of the GRADE system to conduct an overall assessment of the evidence related to each of the following outcomes (Guyatt 2008).
Failed placement, or change of airway device required.
Hypoxia between induction and full recovery.
Serious respiratory complications (including lower respiratory tract infection) within 30 days of anaesthesia.
Aspiration of gastric contents at any time between induction of anaesthesia and leaving recovery.
Mortality within 30 days of anaesthesia.
Airway seal pressure achieved.
Placement: proportion with successful first placement, or total time for securing airway device and commencing ventilation.
The GRADE approach incorporates risk of bias, directness of evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias to yield an overall measure of how confident we can be that our estimate of effect is correct. AFS and AN each independently used GRADEPRO software to create a 'Summary of findings' table for each outcome. Any discrepancies were discussed and, if needed, were referred to TMC for a final decision.
Results
Description of studies
Results of the search
1.
Study flow diagram.
We found 986 records through database searches and a further 106 records by forward citation tracking and 11 records from backward citation tracking. We found three potentially relevant clinical trials on the trial database websites. We found publications from two of these trials (Carron 2012; Hohlrieder 2007) and contacted the lead investigator of another (Olsen 2012). A total of 969 titles/abstracts were reviewed, and 14 articles were selected for full text review.
Included studies
We found only two eligible studies (Carron 2012; Zoremba 2009). These studies are summarized in Characteristics of included studies. Both studies are RCTs in which individual participants were randomly assigned. We found no cluster‐randomized or cross‐over trials. but both studies were powered and designed to address differences in physiological measurements between SAD and TT groups (blood pressure and plasma noradrenaline levels in Carron 2012 and oxygen saturation in Zoremba 2009). Neither study was designed as a non‐inferiority or equivalence trial.
Study population and interventions
Both of these studies compared the use of PLMA with use of a TT. One study reported using size 5 SAD for all participants (Carron 2012), when many intubators would use size 4 in women, and the other study did not specify size (Zoremba 2009). In Carron 2012, the study population consisted of 75 participants who were undergoing laparoscopic bariatric surgery, all with BMI > 35 kg/m2. The study population in Zoremba 2009 included 157 participants with BMI 30 to 35 kg/m2 who were undergoing various routine procedures that did not include abdominal surgery. Both studies excluded patients with predicted difficult airways. Carron 2012 used two experienced intubators, but Zoremba 2009 did not specify the number of experience intubators used.
Outcomes reported
Both studies reported cases in which a change of PLMA airway device was needed, although this was often reported as an exclusion from the study. We were not able to identify an equivalent outcome for the TT group. Other outcomes reported included episodes of hypoxaemia in the postanaesthesia care unit (PACU) (Carron 2012) and oxygen saturation. Times of measurement of oxygen saturation differed, with Carron 2012 reporting oxygen saturation during surgery and in the PACU, and Zoremba 2009 reporting times in the PACU and 30 minutes, 2 hours and 24 hours after surgery.
No cases of gastric aspiration were reported in either study. Carron 2012 reported mortality and serious respiratory complications (no cases), but Zoremba 2009 did not report these outcomes. No cases of laryngeal trauma were reported in either study, and we did not include data on blood staining on devices as provided in Carron 2012. Carron 2012 reported peak inspiratory pressure and leak fraction for both groups during surgery, presence/absence of cough and sore throat/dysphonia between end of surgery and discharge and laryngospasm after device removal. Zoremba 2009 reported cases of laryngospasm/bronchospasm after/during LMA placement or after TT removal. Carron 2012 reported the proportion of successful first insertions and time to secure the airway.
Excluded studies
Details of papers that were excluded after full text review are given in Characteristics of excluded studies. We found five studies that were RCTs with obese participants, but they either compared different models of SAD or used intubating SADs that we had excluded (Arslan 2012; Dhonneur 2006; Mann 2012; Weber 2011; Ydemann 2012). Two studies with the correct intervention and comparison, which had specifically included obese participants, did not report outcomes separately in the papers on these participants and study investigators were unable to provide these data on request (Maltby 2002; Maltby 2003). Two studies were commentaries (Larsson 2009; Li 2013), one was a non‐randomized study (Uppal 2009) and one had been withdrawn by the journal (Piper 2004). The remaining randomized studies, which had not mentioned obese participants, had excluded them (Lim 2007), had allowed BMI up to 35 or did not mention any eligibility based on BMI (Abdi 2010; Borkowski 2005; Hohlrieder 2007; Khazin 2008; Miller 2006). These studies were often small, and none reported obese participants separately. Furthermore, mean BMI or weight and height indicated that very few study participants had BMI > 30 kg /m2. We did however attempt to contact these authors by email to ask whether data on obese participants were available. We received replies from only one (Khazin 2008), and this study had included no obese participants. On the basis of these enquiries and our estimate of likely numbers of cases from these studies, we decided that it was not necessary to revise the database search strategy to remove the obese filter from the database searches.
A third clinical trial found via trial websites (Olsen 2012) included a participant population with BMI up to 35 kg/m2. By contacting the investigators, we established that data on obese participants were available. However, we excluded this study because the LMA had been inserted in the prone position, and we concluded that this complicated comparisons with TT insertion in the supine position.
In Characteristics of excluded studies, we have summarized the studies excluded after full text review..
Risk of bias in included studies
The risk of bias for the included studies is summarized in Figure 2 and Figure 3.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
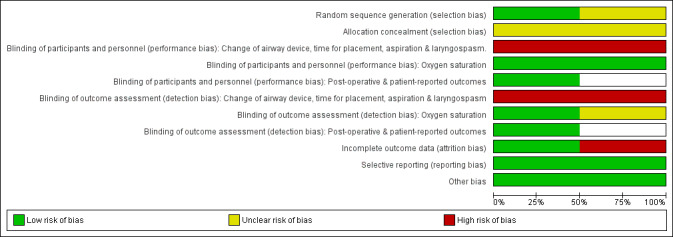
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Both studies were described as randomized with allocation via sealed envelopes. Risk was classified as unclear, as precise details on the numbering of envelopes and, for Zoremba 2009, the method of random number generation, are lacking. However, neither study has important baseline imbalances, which suggests that allocation was randomly assigned effectively.
Blinding
It was clearly not possible in either study for the anaesthetist to be unaware of the airway device used. Carron 2012 aimed to have the surgeon blinded to allocation, but this may have been difficult to maintain. Carron 2012 described a standardized protocol for insertion and criteria for failed placement, whereas this information was not given in Zoremba 2009. Despite standardized protocols for ventilation, an unavoidable high risk of performance and detection bias was identified for outcomes assessed during anaesthesia and surgery, including change of airway device, time for insertion, success rate and laryngospasm.
In both included studies, the anaesthetic protocol differed slightly between groups. For example, participants randomly assigned to the TT groups received a greater quantity of neuromuscular blocking agents compared with participants in the PLMA group in both studies. In Zoremba 2009, peak and cuff pressures differed between TT and SAD groups (Characteristics of included studies). The intervention therefore included more than just the airway device, but also other elements of the anaesthetic, such as airway pressure.
Oxygen saturation is an automated measurement, and measurements in the PACU and on the ward before discharge were recorded by blinded investigators in both included studies. In Zoremba 2009, both groups were given opioids postoperatively as required to keep participants pain free. This could potentially have affected participants' breathing and ventilation and hence assessment of oxygen saturation, but the study authors state that consumption was similar in both groups. Intraoperative oxygen saturation and leak fraction measurements reported in Carron 2012 may have been made by unblinded observers; therefore, we have marked these as unclear risk.
In Carron 2012, both participants and investigators were blinded for participant‐reported outcomes, such as cough and sore throat; therefore these outcomes were deemed to be at low risk of detection bias.
Incomplete outcome data
We used ITT analyses wherever possible, and for our review, participants who had been excluded from the main analyses in each paper were used for outcomes such as change of airway device and laryngospasm. Two participants were excluded from the TT group in Carron 2012 because of difficult TT placement or prolonged surgery. In Zoremba 2009, 9/80 participants in the PLMA group and 7/77 in the TT group were omitted from the oxygen saturation records because of low fast track scores, which included low oxygen saturation, pain during coughing and dyspnoea. These excluded cases may therefore have had lower oxygen saturation results, but the proportion missing (approximately 10%) is similar in the two groups. Because of these exclusions, the number of participants in each group varies for different outcomes.
Selective reporting
The included studies focused on surrogate proxy measures such as oxygen saturation, which they had the statistical power to address.
Other potential sources of bias
Funding sources were not stated for either study. It is not apparent whether any commercial sponsors were involved.
Effects of interventions
See: Table 1
Comparison: PLMA versus tracheal tube
Failed intubation or change of airway device
A total of 2/38 (5.2%) participants in the PLMA group in Carron 2012 and 3/80 participants (3.8%) in the PLMA group in Zoremba 2009 were changed to TT insertion because of failed or unsatisfactory placement of the device. This yields an overall 4.2% risk for change of device. We were not able to identify a reliable comparison group for the TT group. Two TT participants were excluded from Carron 2012 because of difficult intubation and prolonged surgery. Therefore, we were unable to provide an effect estimate for this outcome.
Hypoxia
Carron 2012 reported episodes of hypoxaemia (oxygen saturation < 92% whilst breathing air) in the PACU (Table 2) with a significant reduction in risk of hypoxaemia in the PLMA group (risk ratio 0.27, 95% confidence interval (CI) 0.10 to 0.72).
1. Dichotomous outcomes from Carron 2012.
| Dichotomous outcomes | PLMA | TT | Effect estimate | ||
| Events | Total | Events | Total | MH risk ratio (95% CI) |
|
| Episodes of hypoxaemia (< 92% saturation) | 4 | 35 | 15 | 35 | 0.27 (0.10 to 0.72) |
| Coughing between induction and recovery | 3 | 35 | 29 | 35 | 0.10 (0.03 to 0.31) |
| Patient‐reported sore throat/dysphonia | 1 | 35 | 4 | 35 | 0.25 (0.03 to 2.13) |
| Successful placement at first attempt | 33 | 35 | 32 | 35 | 1.03 (0.91 to 1.17) |
Oxygen saturation was reported by both studies with little overlap in times measured. Data from Carron 2012 obtained during surgery showed no evidence of a difference in oxygen saturation between the two groups. Data obtained in the PACU and postoperatively from Zoremba 2009 show improved oxygen saturation among participants in the PLMA group at all postoperative time points. Records from the PACU-the one time‐point for which both studies contributed gave a significant mean difference, with oxygen saturation 2.54% higher in the PLMA group (95% CI 1.09% to 4.00%). This analysis shows high levels of heterogeneity between results (I2 = 71%), with Carron 2012 reporting a non‐significant increase in oxygen saturation in the PLMA group. We used a random‐effects model because of differences between participant groups.
Pulmonary aspiration of gastric contents, mortality or serious respiratory complication
No cases of pulmonary aspiration of gastric contents were reported in either study. Carron 2012 reported mortality and serious respiratory complications (no cases of either), but Zoremba 2009 did not report these outcomes. We are therefore unable to present any effect estimates for these outcomes.
Leak fraction and airway seal pressure
Carron 2012 reported leak fraction (defined as the difference between inspiratory and expiratory tidal volume as a percentage of inspiratory tidal volume) during surgery (Table 3). The leak fraction was significantly higher in the PLMA group before, during and after carboperitoneum, with the largest difference seen during carboperitoneum-a 6.4% increase in the PLMA group (95% CI 3.07% to 9.73%).
2. Continuous outcomes from Carron 2012.
| Continuous outcomes | PLMA | TT | Effect estimate | ||||
| Mean | SD | Total | Mean | SD | Total | Mean difference (95% CI) | |
| Leak fraction % ((Vt insp‐Vt exp)/Vt insp) | |||||||
| · Before carboperitoneum | 4.4 | 7.2 | 35 | 0.2 | 0.5 | 35 | 4.20 (1.81 to 6.59) |
| · During carboperitoneum | 6.9 | 10 | 35 | 0.5 | 1 | 35 | 6.40 (3.07 to 9.73) |
| · After carboperitoneum | 2.9 | 3.8 | 35 | 0.3 | 0.6 | 35 | 2.60 (1.33 to 3.87) |
| Time to secure airway (seconds) | 25.8 | 6.9 | 35 | 19.9 | 5.4 | 35 | 5.9 (3.00 to 8.80) |
Mean oropharyngeal leak pressure was reported in Carron 2012 for the PLMA group as 29.6 (SD 4.2) cm H2O but was not reported for the TT group. Zoremba 2009 states, "a maximum peak pressure of 30 cm H2O was set to be tolerable (25 cm H2O in the PLMA group)". We are not able to report an effect estimate for this outcome.
Laryngospasm
Participants with this outcome were (with one exception) excluded from the main analyses of both reports but are relevant for this review. Carron 2012 reported one participant in the PLMA group who was excluded because of laryngospasm on emergence and one participant in the TT group who suffered bronchoconstriction postoperatively. Zoremba 2009 excluded four participants because of laryngospasm or bronchospasm after/during PLMA placement or after TT removal (Analysis 1.2). We combined these outcomes, and the pooled estimate shows a non‐significant reduction in laryngospasm in the PLMA group (risk ratio 0.50, 95% CI 0.09 to 2.84). Heterogenity was low (I2 = 0%).
1.2. Analysis.

Comparison 1 PLMA versus tracheal tube, Outcome 2 Laryngospasm/bronchoconstriction between induction and recovery.
Coughing, laryngeal trauma, sore throat or hoarseness
All of these outcomes were reported by Carron 2012 only. No cases of laryngeal trauma were reported. We did not include blood staining on the device as trauma. Postoperative coughing was less common in the PLMA group (Table 2; risk ratio 0.10, 95% CI 0.03 to 0.31), and no significant difference in the risk of sore throat or dysphonia was reported in the PLMA group (Table 2; risk ratio 0.25, 95% CI 0.03 to 2.13).
Airway placement time and success rate
These outcomes were reported by Carron 2012 only. PLMA placement took on average 5.9 seconds longer than TT placement (95% CI 3 seconds to 8.8 seconds; Table 3). There was no significant difference in proportions of successful first placement of the device, with 33/35 (94.2%) first time successes in the PLMA group and 32/35 (91.4%) in the TT group (Table 2).
Subgroup analyses and investigation of heterogeneity
We were not able to undertake any of our planned subgroup analyses because of the small number of included studies. The two included study populations differed in degree of obesity-with Carron 2012 describing a morbidly obese population compared with the mildly obese participants described in Zoremba 2009-and in type of operation-laparoscopic abdominal (Carron 2012) versus peripheral (Zoremba 2009). Both studies contributed to only three outcomes: change of airway device, oxygen saturation in the PACU and laryngo/bronchopsasm. The high degree of heterogeneity in oxygen saturation observed in the PACU may reflect these underlying differences between studies.
Discussion
Summary of main results
We found only two eligible studies, with a total of 232 participants, both of which had compared a second‐generation SAD (ProSeal LMA) with TT. The two study populations differed in degree of obesity (morbid (Carron 2012) vs mild (Zoremba 2009)) and type of surgery (bariatric surgery with abdominal insufflation (Carron 2012) vs various surgical procedures without abdominal insufflation (Zoremba 2009)).
We were not able to answer the question of whether use of an SAD rather than a TT for airway management in obese participants increased the risk of mortality or other serious complications. As anticipated, no relevant outcomes were reported in the included RCTs, and the studies were not designed to address this topic. Because these are rare events (Brimacombe 1995a; Cook 2011a), RCTs are highly unlikely to provide data to resolve this issue. Our review found that in 3% to 5% of obese participants, it was not be possible to fit an SAD, and a change of device to TT was required. Although no participant harm may have resulted from these incidents, they are high‐risk events with the potential for serious adverse consequences.
The proportion of successful first attempts at airway placement did not differ between SAD and TT, although it took approximately six seconds longer to place an SAD than a TT. We do not consider this increase in placement time to have clinical relevance.
We found a significant reduction of almost 75% in hypoxaemic episodes in the PACU (Carron 2012) and a statistically significant improvement in oxygen saturation of 2.5% in PACU in the SAD group (Zoremba 2009). This is a wider gap than has been found in manyanaesthesia studies, and although this small difference would not be important to many individuals, we consider that it is indicative of a significantly different pulmonary performance with the PLMA.
The leak fraction was higher in participants with the SAD, and, as expected, this difference was greatest during the carboperitoneum. The clinical importance of the observed 6.4% increase is unclear.
Our findings are consistent with both increased and decreased risk of both sore throat/dysphonia and laryngospasm in the SAD group. Postoperative cough was less common among participants in the SAD group.
Overall completeness and applicability of evidence
The generalizability of our results is limited because we obtained data on only one model of SAD-the ProSeal LMA. Use of this model in the studies may not have reflected routine use with different sizes (Included studies). Our total study population is small, and, although one study included participants with morbid obesity, both studies excluded patients with anticipated difficult airways. For many reported outcomes, we were able to obtain data from only one study. Important differences between the studies in population, anaesthetic protocol and experience of intubators is reflected in high heterogeneity for oxygen saturation in the PACU. We were not able to access data on obese participants within unselected study populations, but we estimated that inclusion of these participants would not have substantially increased our sample size.
Quality of the evidence
Included studies were of generally high quality with low risk of bias (Figure 2; Figure 3). An unavoidable high risk of bias was seen in the main airway variables, such as change of device or laryngospasm, as the intubator could not be blinded. Later observations of oxygen saturation and participant‐reported variables outside the operating theatre were blinded in both studies.
Potential biases in the review process
We are confident that we have identified all published studies that report specifically on the use of SAD versus TT in obese participants. Numerous other studies that we were not able to include consisted of mixed populations, which may include some obese participants that we were not able to include because we could not obtain specific data on this group. We have no reason to expect that the results for these obese participants would differ from those derived from the two included studies.
Agreements and disagreements with other studies or reviews
No other reviews deal specifically with use of an SAD versus a TT in obese participants. Other reviews on unselected patients using both RCTs and observational studies have shown benefit for SADs with lower risk of laryngospasm, postoperative cough and sore throat among participants for whom SADs were used (Brimacombe 1995; Cook 2005, Yu 2010; Zhang 2009). Brimacombe 1995 reported improved oxygen saturation during emergence as a benefit of SADs. These findings are consistent with this review. Zoremba 2009 suggested that observed improvement in oxygenation was related to a greater need for neuromuscular blockade agents (NMBAs) in the TT group, resulting in atelectasis rather than an effect of residual blockade. Because SADs routinely require less use of NMBAs than TTs, this effect can be anticipated in other populations. Apart from the use of NMBAs, other possible mechanisms include less interference with airway clearance mechanisms of the lungs, less pain and therefore better coughing and airway clearance (Hohlrieder 2007; Hohlrieder 2007a; Tanaka 2003) and less bronchoconstriction (Natalini 2002). Yu 2010 reported no difference in percentages of first attempt success between SADs and TTs, and Zhang 2009 described no difference in ease of insertion, again consistent with our results in obese participants.
Observational studies of SAD use in large populations of unselected patients (Bernardini 2009; Brimacombe 1995a; Verghese 1996; Voyagis 1996) have confirmed the low risk of aspiration, estimated as 2/10,000 in Brimacombe 1995a. However, no data are available to confirm that aspiration is this low in obese participants. These studies rely on routine data, and although detailed descriptions of participants are not available, it is likely that SAD use was avoided in obese participants. In one study of 11,910 participants, LMA use was abandoned in only 0.19% of cases because of inadequate seal or failed placement (Verghese 1996). This is considerably lower than the 3% to 5% failure rate among RCTs included in this review and is consistent with higher risk in obese patients. However this study used first‐generation SADs, making direct comparison with our results impossible. One study of 1000 consecutive uses of PLMA in a mixed population reported a 0.6% failure rate (Cook 2007), which was also lower than the failure rate reported amongobese participants in these studies.
Several additional RCTs with study populations of obese participants were identified, some of which compare different models of SAD, for example, i‐gel with the LMA, or unique (Weber 2011) or intubating LMA with CTrach (Arslan 2012). Others compare direct or indirect laryngoscopy with intubating SADs (Dhonneur 2006; Ydemann 2012). These comparisons are too diverse to allow overall conclusions to be drawn, but with a total study population of 384, no cases of aspiration or serious complications were recorded. Dhonneur 2006, while studying use of CTrach with direct laryngoscopy in 100 morbidly obese participants undergoing bariatric surgery, noted better oxygenation in the CTrach group.
Authors' conclusions
Implications for practice.
This is clearly an important clinical question, but we have inadequate information to draw conclusions about safety and can comment on the efficacy and use of only one design (the PLMA) in obese patients. We conclude that during routine and laparoscopic surgery, PLMAs may take a few seconds longer to insert, which may be clinically important. A failure rate of 3% to 5% can be anticipated in obese patients. However, once fitted, they provide at least as good oxygenation, with the caveat that leak fraction may increase, although in the included studies, this did not appear to affect ventilation. We found significant improvement in oxygenation during and after surgery and reduced postoperative coughing, suggesting better recovery for patients.
Implications for research.
Optimal anaesthetic techniques for obese patients are a priority for current research. Our conclusions have been limited by lack of data on diverse models of SAD, and additional trials of different designs of SAD compared with TT in obese patients are needed. Such trials will contribute important broader outcome data on efficacy, improving generalizability. However, concerns about safety and the potentially increased risk of serious complications such as aspiration may make anaesthetic providers reluctant to use any SAD in obese patients. We were not able to address these concerns in this review. An individual patient data meta‐analysis, accessing data on all obese patients, including those within unselected study populations, is one option, but this may still result in an inadequate total sample size. Observational analysis of routine data sets may be needed for adequate power, but at present these data sets lack granularity and patient detail. There is a need for routine anaesthetic data sets to have more comprehensive data on body weight and for detailed analysis to be performed to overcome the biases inherent in the use of routine data.
Acknowledgements
We would like to thank Mathew Zacharias (content editor: protocol and review), Nathan Pace (statistical editor: review), Marialena Trivella (statistical editor: protocol), Federico Bilotta (peer reviewer: review), Georgina Imberger (peer reviewer: protocol) and Anil Patel (peer reviewer: protocol and review) for their help and editorial advice.
We would also like to thank the following investigators for their helpful replies to email enquiries: Dr Ulderico Freo (regarding Carron 2012), Dr M Zoremba (regarding Zoremba 2009), Dr Roger Maltby (regarding Maltby 2002; Maltby 2003), Dr Karsten Olsen (regarding Olsen 2012) and Dr Dan Benhamou for suggestions on extra titles.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Intubation, Intratracheal explode all trees #2 MeSH descriptor Laryngeal Masks explode all trees #3 MeSH descriptor Airway Management explode all trees #4 (LMA or (laryng*adj3 mask) or intub* or supraglottic or (airway adj3 manag*)) or (CLMA or PLMA or SLMA or i‐gel):ti,ab #5 (#1 OR #2 OR #3 OR #4) #6 MeSH descriptor Overweight explode all trees #7 MeSH descriptor Obesity explode all trees #8 MeSH descriptor Bariatric Surgery explode all trees #9 (obes* or overweight* or bariatric or BMI or body mass index) or (gastric near3 band*) #10 (#6 OR #7 OR #8 OR #9) #11 (#5 AND #10)
Appendix 2. Ovid MEDLINE search strategy
1. exp intubation, intratracheal/ or exp laryngeal masks/ or airway management/ or exp intubation, intratracheal/ or (LMA or (laryng*adj3 mask) or intub* or supraglottic or (airway adj3 manag*)).af. or (CLMA or PLMA or SLMA or i‐gel).ti,ab. 2. exp overweight/ or exp obesity/ or exp bariatric surgery/ or (obes* or overweight* or bariatric or BMI or body mass index).af or (gastric adj3 band*).mp. 3. 1 and 2 4. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. 5. 4 and 3
Appendix 3. Ovid EMBASE search strategy
1. exp respiratory tract intubation/ or exp laryngeal mask/ or exp respiration control/ or (LMA or laryng*adj3 mask or intub* or supraglottic or (airway adj3 manag*)).af. or (CLMA or PLMA or SLMA or i‐gel).ti,ab. 2. exp obesity/ or exp bariatric surgery/ or (obes* or overweight* or bariatric or BMI or body mass index).af.or (gastric adj3 band*).mp. 3. 1 and 2 4. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 4. Draft data extraction form
1. General information
| Date form completed(dd/mm/yyyy) | |
| Name/ID of person extracting data | |
|
Report title (title of paper/abstract/report that data are extracted from) |
|
|
Report ID (ID for this paper/abstract/report) |
|
|
Study ID (surname of first author and year first full report of study was published, e.g. Smith 2001) |
|
|
Report IDs of other reports of this study (e.g. duplicate publications, follow‐up studies) |
|
| Reference details | |
| Report author contact details | |
|
Publication type (e.g. full report, abstract, letter) |
|
|
Study funding sources (including role of funders) |
|
|
Possible conflicts of interest (for study authors) |
|
2. Study eligibility
| Study characteristics |
Eligibility criteria (Insert eligibility criteria for each characteristic as defined in the Protocol) |
Yes/No/Unclear | Details of outcomes & location in text |
| Type of study | Randomized controlled trial | ||
| Controlled clinical trial (quasi‐randomized trial and cluster‐randomized) |
|||
| Cross‐over trial (both interventions in participants‐order randomized) |
|||
|
Participants |
Adults > 16 years with BMI > 25 kg/m2 undergoing GA |
||
| Types of intervention and comparison |
Comparison of either |
||
| First‐generation SAD (e.g. cLMA) | |||
| Second‐generation SAD (e.g. PLMA, SLMA) | |||
| With | |||
| Tracheal tube | |||
| Failed intubation or change of airway device required | |||
| Hypoxia between 1 minute before induction and full recovery | |||
| Serious respiratory complications (including lower respiratory tract infection) within 30 days of anaesthetic | |||
| Aspiration of gastric contents at any time between induction of anaesthetic and leaving recovery | |||
| Outcomes are not part of the eligibility criteria, so a study that meets design, participant and intervention criteria is included | |||
| INCLUDE EXCLUDE UNCLEAR | |||
|
Reason for exclusion |
|||
DO NOT PROCEED IF STUDY EXCLUDED FROM REVIEW
3. Population and setting
| Description |
Location in text |
|
|
Population description (types of surgical procedures included) |
||
|
Definition of obesity (BMI ranges and means, medians) |
||
|
Setting (including location and social context) |
||
| Inclusion criteria | ||
| Exclusion criteria | ||
|
Method/s of recruitment of participants |
||
| Informed consent obtained |
4. Methods
|
Descriptions as stated in report/paper |
Location in text |
|
| Aim of study | ||
| Design(e.g. parallel, cross‐over, cluster) | ||
|
Unit of allocation (by individuals, cluster/groups or body parts) |
||
| Start date | |
|
| End date | |
|
| Total study duration | ||
| Ethical approval needed/obtained for study |
5. Participants
Provide overall data and, if available, comparative data for each intervention or comparison group.
|
Description as stated in report/paper |
Location in text | |
|
Total no. randomly assigned (or total pop. at start of study for NRCTs) |
||
|
Clusters (if applicable, no., type, no. people per cluster) |
||
| Baseline imbalances | ||
|
Withdrawals and exclusions (if not provided below by outcome) |
||
| Age | ||
| Sex | ||
| Race/Ethnicity | ||
|
Type and duration of surgery (e.g. peripheral or abdominal) |
||
|
Type of ventilation (spontaneous or mechanical, airway pressures used) |
||
|
Details of anaesthetic given (including position, premed, preoxygenation, induction and maintenance agents ) |
||
|
Neuromuscular blockade given (agents used) |
||
| Training and seniority of intubator | ||
|
Other relevant sociodemographics |
||
| Subgroups measured | ||
| Subgroups reported |
6. Intervention groups
6.1 Intervention group-repeated as required
|
Description as stated in report/paper |
Location in text |
|
|
Group name (first‐generation SAD, second‐generation SAD) |
First‐ or second‐generation SAD | |
| No. randomly assigned to group | ||
|
Description of device (name and manufacturer) |
||
| Method of insertion | ||
| Co‐interventions |
6.2 Comparison group
|
Description as stated in report/paper |
Location in text | |
|
Group name |
Tracheal tube | |
|
No. randomly assigned to group |
||
|
Description of device (name and manufacturer) |
||
| Method of insertion | ||
| Co‐interventions |
7. Outcomes
| TYPES OF OUTCOME MEASURES | MEASURED | REPORTED | FORM COMPLETED |
| Primary outcomes | |||
| Failed intubation or change of airway device required | |||
| Hypoxia between 1 minute before induction and full recovery | |||
| Serious respiratory complications (including lower respiratory tract infection) within 30 days of anaesthetic | |||
| Aspiration of gastric contents at any time between induction of anaesthetic and leaving recovery | |||
| Mortality within 30 days of anaesthetic | |||
| Secondary outcomes | |||
| Leak fraction | |||
| Airway seal pressure achieved | |||
| Laryngospasm on emergence | |||
| Laryngeal/airway trauma | |||
| Participant‐reported sore throat or hoarseness | |||
| Placement-proportion successful 1st | |||
| Placement-total time for securing airway device and commencing ventilation |
For each outcome ticked, please complete a separate outcome form.
|
Description as stated in report/paper |
Location in text |
|
|
Outcome name (number of attempts, pain) |
||
| Time points measured | ||
| Time points reported | ||
| Outcome definition(with diagnostic criteria if relevant) | ||
| Person measuring/reporting | ||
|
Unit of measurement (if relevant) |
||
| Scales: levels, upper and lower limits(indicate whether high or low score is good) | ||
| Is outcome/tool validated? | ||
| Imputation of missing data (e.g. assumptions made for ITT analysis) | ||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
||
| Power | ||
| RESULTS |
Description as stated in report/paper |
Location in text |
| Comparison | ||
| Outcome | ||
| Subgroup | ||
| Time point (specify whether from start or end of intervention) | ||
| Post‐intervention or change from baseline? | ||
| Results: Intervention* | ||
| Results: Comparison* | ||
| No. missing participants and reasons | ||
| No. participants moved from other group and reasons | ||
| Any other results reported | ||
|
Unit of analysis (individuals, cluster/groups or body parts) |
||
| Statistical methods used and appropriateness of these methods(e.g. adjustment for correlation) | ||
| Reanalysis required?(specify) | ||
| Reanalysed results |
*Results for continuous outcome: mean: SD (or other variance): total number of participants.
Results for dichotomous outcome: number of participants with outcome: total number of participants.
8. Risk of bias assessment
| Domain |
Risk of bias : high/low/unclear |
Support for judgement |
Location in text |
|
Random sequence generation (selection bias) |
|||
|
Allocation concealment (selection bias) |
|||
|
Blinding of participants and personnel (performance bias) |
|||
|
Blinding of outcome assessment (detection bias) |
|||
|
Incomplete outcome data (attrition bias) |
|||
|
Selective outcome reporting? (reporting bias) |
|||
|
Other bias (baseline characteristics for cluster‐randomized, carryover for cross‐over trials) |
9. Applicability
| Yes/No/Unclear | Support for judgment | |
| Have important populations been excluded from the study?(consider disadvantaged populations and possible differences in the intervention effect) | ||
| Is the intervention likely to be aimed at disadvantaged groups?(e.g .lower socioeconomic groups) | ||
|
Does the study directly address the review question? (any issues of partial or indirect applicability) |
10. Other information
|
Description as stated in report/paper |
Location in text |
|
| Key conclusions of study authors | ||
| References to other relevant studies | ||
| Correspondence required for further study information(from whom, what and when) | |
|
Data and analyses
Comparison 1. PLMA versus tracheal tube.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oxygen saturation of peripheral blood (SpO2) (%) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 During surgery-before carboperitoneum | 1 | 70 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐0.11, 1.11] |
| 1.2 During surgery-during carboperitoneum | 1 | 70 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.58, 0.78] |
| 1.3 During surgery-after carboperitoneum | 1 | 70 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.54, 1.34] |
| 1.4 in PACU | 2 | 204 | Mean Difference (IV, Random, 95% CI) | 2.54 [1.09, 4.00] |
| 1.5 After surgery-30 minutes | 1 | 134 | Mean Difference (IV, Random, 95% CI) | 2.30 [1.57, 3.03] |
| 1.6 After surgery-2 hours | 1 | 134 | Mean Difference (IV, Random, 95% CI) | 1.60 [0.85, 2.35] |
| 1.7 After surgery-24 hours | 1 | 134 | Mean Difference (IV, Random, 95% CI) | 1.5 [0.91, 2.09] |
| 2 Laryngospasm/bronchoconstriction between induction and recovery | 2 | 232 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.09, 2.84] |
1.1. Analysis.
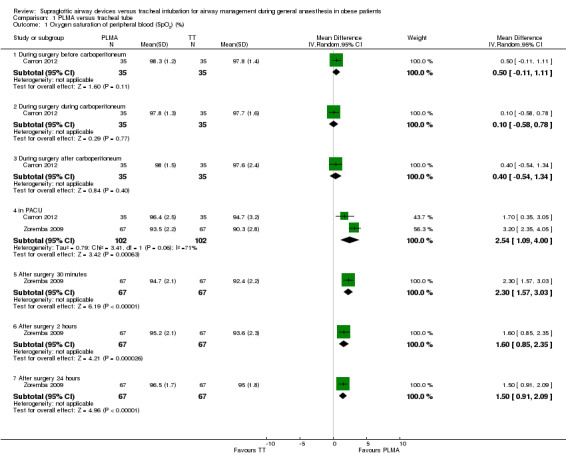
Comparison 1 PLMA versus tracheal tube, Outcome 1 Oxygen saturation of peripheral blood (SpO2) (%).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Carron 2012.
| Methods | Single‐centre RCT. Padua, Italy | |
| Participants | 75 participants with BMI > 30 kg/m2 undergoing laparoscopic bariatric surgery. Excluded patients with symptoms of reflux, gastric ulcer or predicted difficult airway Mean age (SD): PLMA group 43.2 years (12.3); TT group 42.4 years (11.5) Mean BMI (SD): PLMA group 43.9 kg/m2(6.1); TT group 45.4 kg/m2 (4.3) % female: PLMA 83% TT 71% |
|
| Interventions | 38 participants randomly assigned to the PLMA group and 37 to the TT group. 5 participants withdrawn from analysis, 35 in each group reported PLMA laryngeal mask airway company. Size 5. Positioned using digital technique ‐ standardized 3 excluded from PLMA group ‐ 2 failure of SAD placement and fibreoptic intubation, 1 laryngospasm on emergence No model of TT given. ID 7.5/8 mm. No details given of intubation technique. 2 excluded from TT group because of difficult intubation and prolonged surgery |
|
| Outcomes | Change of airway device Hypoxic episodes in PACU (oxygen saturation < 92%) Oxygen saturation during operation (before, during and after carboperitoneum) and in the PACU Pulmonary aspiration of gastric contents Leak fraction and peak inspiratory pressure (before, during and after carboperitoneum) Laryngospasm on emergence Participant‐reported sore throat and hoarseness between end of surgery and discharge (8 to 9 hours after surgery) Placement-time and % successful first attempt |
|
| Details of anaesthetic & ventilation | Premed midazolam 0.5 mg/kg and ranitidine 50 mg Preoxygenated for 2 minutes Anaesthesia induced with fentanyl 1 mcg/kg and propofol 2 to 3 mg/kg Maintenance: sevoflurane. 35/65 oxygen/air mixture, PEEP 5 cm H2O and PIP variable to obtain Vt exp of 10 mL/kg, respiratory rate of 12 breaths/min and inspiratory: expiratory ratio of 1:1 End of surgery: sevoflurane stopped and fresh gas flow increased to 10 L/min. Just before airway removal: lung ventilation manually assisted with continuous positive airway pressure during inspiration Airway removed when train‐of‐four ratio (TOFR) was 0.9 and the participant was awake, able to respond to simple commands, to sustain hand grip and move arms Breathing air in PACU |
|
| Neuromuscular blockade & reversal given | Succinylcholine 1 mg/kg given to TT participants to facilitate intubation ‐ not to PLMA group After induction, single IV bolus of cisatracurium 0.15 mg/kg for NMB in TT group and 0.05 mg/kg in PLMA group. Additional IV cisatracurium 2 mg given if TOFR > 0.5 Muscle relaxation at end of surgery reversed with IV atropine 0.01 mg/kg IBW and neostigmine methylsulfate 0.04 mg/kg IBW if TOFR ≤ 0.9 |
|
| Training and seniority of intubator | Two individuals with extensive experience (> 5000 uses with PLMA) randomly performed airway management and anaesthesia maintenance | |
| Notes | No funding sources named Authors emailed for additional information, and reply received 3/12/12 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer–generated numbers" |
| Allocation concealment (selection bias) | Unclear risk | "Opaque sealed envelopes" used. Not described as consecutively numbered |
| Blinding of participants and personnel (performance bias) Change of airway device, time for placement, aspiration & laryngospasm. | High risk | Anaesthetist unblinded. Aim was to blind surgeon, but personal communication from authors indicates that this may have been difficult to maintain at all times |
| Blinding of participants and personnel (performance bias) Oxygen saturation | Low risk | Standardized ventilation protocol |
| Blinding of participants and personnel (performance bias) Post‐operative & patient‐reported outcomes | Low risk | Participants and nurses in PACU and surgical ward were blinded to allocation |
| Blinding of outcome assessment (detection bias) Change of airway device, time for placement, aspiration & laryngospasm | High risk | Standardized protocol for deciding failure of insertion of PLMA. but impossible to blind to allocation group |
| Blinding of outcome assessment (detection bias) Oxygen saturation | Unclear risk | Automatic measurements. Nurses in PACU blinded, but measurements in OR made by unblinded observers |
| Blinding of outcome assessment (detection bias) Post‐operative & patient‐reported outcomes | Low risk | Participants and observer were blinded to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analyses with participants excluded because of failed/difficult placement or laryngospasm included in relevant outcomes |
| Selective reporting (reporting bias) | Low risk | Outcomes outlined in Methods section of paper. Outcome of time to independent walking not reported on trial website |
| Other bias | Low risk | Participants in both groups similar at baseline |
Zoremba 2009.
| Methods | Single‐centre parallel‐group RCT. University hospital, Marburg, Germany | |
| Participants | 162 participants undergoing "peripheral minor surgery" (breast surgery, knee arthroscopy, TURP, hand surgery). BMI between 30 and 35 kg/m2. No operations requiring abdominal insufflation or head‐down tilt. Excluded patients in whom airway examination suggested difficult intubation. 5 patients (3 in TT group and 2 in PLMA group) declined to continue with protocol, leaving 157 randomly assigned participants Mean age (SD): PLMA 49 years (12); TT 53 years (12) Mean BMI (SD): PLMA group 30 kg/m2(3.2); TT 32 kg/m2 (3.7) |
|
| Interventions | 80 participants randomly assigned to PLMA. Method of insertion not standardized. No size given. 13 then excluded for some analyses. 3 excluded because of unsatisfactory placement of PLMA. 1 excluded because of laryngo/bronchospasm during/after airway placement. 9 excluded, as measurement unsatisfactory because of fast track score < 10 within 20 minutes after surgery. This score measures pain, N&V, level of consciousness and oxygen saturation 77 participants randomly assigned to TT. 10 then excluded for some analyses. 7 excluded, as measurement unsatisfactory because of fast track score < 10 within 20 minutes after surgery. This score measures pain, N&V, level of consciousness and oxygen saturation. 1 excluded because of laryngo/bronchospasm during/after airway placement |
|
| Outcomes | Unsatisfactory PLMA placement Oxygen saturation in recovery and postoperatively (30 minutes, 2 hours and 24 hours) Pulmonary aspiration of gastric contents Layrngo/bronchospasm between induction and recovery |
|
| Details of anaesthetic & ventilation | Premed: oral chlorazepat 20 mg 24 hours before surgery. 3 minutes preoxygenation with 100% O2 via face mask Induction: fentanyl 2 to 3 mcg/kg and propofol 2 mg/kg, followed by continuous infusion of propofol 5 to 10 mg/kg/h Subsequent dosages of remifentanil (0.1 to 0.2 mcg/kg/min) and propofol adjusted according to haemodynamic variables; BIS values within range between 40 and 60 Mechanical ventilation: tidal volume of 8 mL/kg. Maximum peak pressure 30 cm H2O for TT and 25 cm H2O for PLMA. PEEP of 10 cm H2O used in TT group. Cuff pressure continuously adjusted to 30 cm H2O in TT group and 50 cm H2O in PLMA group TOFR of 0.9 before extubation. When participants awake and breathing spontaneously, trachea extubated without previous suction in head‐up position with positive pressure of 10 cm H2O and breathing 100% O2 Breathing air during transfer to PACU Postoperative pain relief given to both groups-1 g paracetamol and 1 g metmizol IV-followed by intermittent piritramide IV as needed to keep pain VAS score < 4 |
|
| Neuromuscular blockade & reversal given | Single dose of rocuronium (0.5 mg/kg ideal body weight) to facilitate orotracheal intubation-only in TT group. No other NMB agent given | |
| Training and seniority of intubator | Not given | |
| Notes | No funding sources named Authors emailed for additional information, and reply received 25/10/2012 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Patients were allocated on a random basis”. Personal communication from author "based on adaptive randomization" |
| Allocation concealment (selection bias) | Unclear risk | Communication from authors: "The respective anaesthesiologist (not involved in study) has randomly chosen a closed envelope containing the study group allocation" |
| Blinding of participants and personnel (performance bias) Change of airway device, time for placement, aspiration & laryngospasm. | High risk | Method of placement and definition of failure were not standardized. Ventilation settings were standardized. No details of how many different intubators |
| Blinding of participants and personnel (performance bias) Oxygen saturation | Low risk | Nurses in PACU blinded |
| Blinding of outcome assessment (detection bias) Change of airway device, time for placement, aspiration & laryngospasm | High risk | Assessed by anaesthetist, who was unblinded |
| Blinding of outcome assessment (detection bias) Oxygen saturation | Low risk | Assessed by nurse blinded to allocation. Standardized protocol: participant in 30‐degree head‐up position breathing air for 5 minutes. All participants free from pain. Text states that opioid consumption in both groups was similar |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9/80 in PLMA group and 7/77 in TT group missing from oxygen saturation results because of low fast‐track score. These cases may have lower oxygen saturation results, but % missing is similar in the two groups. For other analyses, only 5 missing across both groups |
| Selective reporting (reporting bias) | Low risk | All measured outcomes reported, except oxygen saturation during operation |
| Other bias | Low risk | Groups similar at baseline |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdi 2010 | RCT comparing PLMA with TT in participants undergoing laparoscopic gynaecological surgery. No mention of obese participants |
| Arslan 2012 | RCT comparing use of intubating LMA with LMA CTrach in morbidly obese participants (BMI > 40 kg/m2) |
| Borkowski 2005 | RCT comparing PLMA with TT in participants undergoing laparotomy. Included participants up to BMI 35 kg/m2 |
| Dhonneur 2006 | RCT comparing use of LMA CTrach with direct laryngoscopy in morbidly obese participants (BMI > 35 kg/m2) |
| Hohlrieder 2007 | RCT comparing PLMA with TT in participants undergoing laparoscopic gynaecological surgery. Included participants up to BMI 35 kg/m2 |
| Khazin 2008 | RCT comparing LMA classic and PLMA with TT in participants undergoing various types of surgery. Included participants up to BMI 35 kg/m2. Contact with authors confirmed no participants with BMI > 30 were in fact included |
| Larsson 2009 | Commentary on Zoremba 2009 |
| Li 2013 | Commentary on Ydemann 2012 |
| Lim 2007 | RCT comparing PLMA with TT in participants undergoing laparotomy. Participants with BMI > 30 excluded |
| Maltby 2002 | RCT comparing PLMA with TT in participants undergoing laparoscopic cholecystectomy. Separate randomisation stratum for participants with BMI > 30 kg/m2, but outcome data not available for obese participants |
| Maltby 2003 | RCT comparing PLMA with TT in participants undergoing laparoscopic gynaecological surgery. Separate randomisation stratum for participants with BMI > 30 kg/m2 but outcome data not available for obese participants |
| Mann 2012 | RCT comparing two models of SAD‐LTS (laryngeal tube suction) with PLMA |
| Miller 2006 | RCT comparing PLMA and SLIPA with TT in participants undergoing laparoscopic gynaecological surgery. Excluded morbid obese participants |
| Olsen 2012 | Trial comparing PLMA and TT for back surgery in the prone position. Data obtained from authors showed that 9 participants in the PLMA group were obese, but the study was excluded as PLMA was placed whilst participant was in the prone position, whereas TT placed in usual supine position, making comparison difficult |
| Uppal 2009 | Non‐randomized study comparing i‐gel with TT |
| Weber 2011 | RCT comparing use of i‐gel with LMA unique in mild‐moderate obese participants (BMI 25 to 35 kg/m2) |
| Ydemann 2012 | RCT comparing use of Intubating LMA with Glidescope in morbidly obese participants (BMI > 35 kg/m2) |
Differences between protocol and review
We made the following changes to the published protocol (Nicholson 2012).
Outcomes-change of airway and airway seal/leak pressure. No obvious comparison was noted in the TT group, and so effect estimates were not possible. We have presented as %/pressure in the SAD group.
In this review, we made a minor alteration to the outcome from the protocol Laryngospasm on emergence to Laryngospasm/bronchospasm between induction and recovery. This decision was based on presentation of important data in the studies, which, despite contact with investigators, were not available for emergence only, and we believed it was more important to include the data than to omit the outcome.
Contributions of authors
Conceiving of the review: Andrew F Smith (AFS)
Co‐ordinating the review: Amanda Nicholson (AN)
Undertaking manual searches: AN with support from CARG
Screening search results: AN and AFS
Organizing retrieval of papers: Sharon R Lewis (SRL), AN
Screening retrieved papers against inclusion criteria: AN, AFS, Tim M Cook (TMC)
Appraising quality of papers: AN, AS. Additional advice from TMC and Stephanie S Reed (SSR)
Abstracting data from papers: any two authors, with additional advice from TMC and SSR
Writing to authors of papers for additional information: AN
Providing additional data about papers: AN
Obtaining and screening data on unpublished studies: AN, TMC
Providing data management for the review: AN
Entering data into Review Manager (RevMan 5.2): SL, AN
Handling RevMan statistical data: AN
Peforming other statistical analysis not using RevMan: AN
Interpreting data: SL, AN, TC, AS
Making statistical inferences: AN, TC, AS
Writing the review: all authors
Securing funding for the review: AS
Performing previous work that served as the foundation of the present study: N/A
Serving as guarantor for the review (one author): AS
Taking responsibility for reading and checking the review before submission: AN
Sources of support
Internal sources
No sources of support supplied
External sources
-
NIHR Cochrane Collaboration Programme Grant. Enhancing the safety, quality and productivity of perioperative care. Project Ref: 10/4001/04, UK.
This grant funds the work of AN, AFS and SRL on this review.
Declarations of interest
From March to August 2011, AN worked for the Cardiff Research Consortium, which provides research and consultancy services to the pharmaceutical industry. The Cardiff Research Consortium has no connection with AN's work with The Cochrane Collaboration. AN’s husband has small direct holdings in several drug and biotech companies as part of a wider balanced share portfolio.
TMC was previously paid by Intavent Orthofix and the LMA company, several years ago, for lecturing. This company manufactures and distributes several supraglottic airway devices. His department has been given free or at cost airway equipment for evaluation or research from numerous airway companies. TMC has never had any financial involvement in any such company.
All other authors: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Carron 2012 {published and unpublished data}
Zoremba 2009 {published and unpublished data}
References to studies excluded from this review
Abdi 2010 {published data only}
Arslan 2012 {published data only}
- Arslan ZI, Ozdamar D, Yildiz TS, Solak ZM, Toker K. Tracheal intubation in morbidly obese patients: a comparison of the Intubating Laryngeal Mask Airway and Laryngeal Mask Airway CTrach. Anaesthesia 2012;67(3):261‐5. [PUBMED: 22321082] [DOI] [PubMed] [Google Scholar]
Borkowski 2005 {published data only}
Dhonneur 2006 {published data only}
- Dhonneur G, Ndoko SK, Yavchitz A, Foucrier A, Fessenmeyer C, Pollian C, et al. Tracheal intubation of morbidly obese patients: LMA CTrach vs direct laryngoscopy. British Journal of Anaesthesia 2006;97(5):742‐5. [PUBMED: 16997840] [DOI] [PubMed] [Google Scholar]
Hohlrieder 2007 {published data only}
- Hohlrieder M, Brimacombe J, Eschertzhuber S, Ulmer H, Keller C. A study of airway management using the ProSeal LMA laryngeal mask airway compared with the tracheal tube on postoperative analgesia requirements following gynaecological laparoscopic surgery. Anaesthesia. 2007/08/19 2007; Vol. 62, issue 9:913‐8. [ NCT00402870] [DOI] [PubMed]
Khazin 2008 {published data only}
Larsson 2009 {published data only}
Li 2013 {published data only}
Lim 2007 {published data only}
Maltby 2002 {published data only (unpublished sought but not used)}
Maltby 2003 {published data only (unpublished sought but not used)}
Mann 2012 {published data only}
- Mann V, Mann STW, Rupp D, Rohrig R, Weigand MA, Muller M. Influence of head position and neuromuscular block on the clinical efficacy of supraglottic airway devices. A prospective randomized study to compare the laryngeal tube suction (LT(TM)) with the ProSeal((TM)) laryngeal mask. Notfall and Rettungsmedizin 2012; Vol. 15, issue 2:136‐41. [WOS:000304153300007]
Miller 2006 {published data only}
Olsen 2012 {unpublished data only}
- Olsen KS. Laryngeal mask or endotracheal tube for back surgery in the prone position. http://clinicaltrials.gov/show/NCT01041352. 2012.
Uppal 2009 {published data only}
Weber 2011 {published data only}
Ydemann 2012 {published data only}
- Ydemann M, Rovsing L, Lindekaer AL, Olsen KS. Intubation of the morbidly obese patient: GlideScope((R)) vs. Fastrach. Acta anaesthesiologica Scandinavica 2012;56(6):755‐61. [PUBMED: 22524487] [DOI] [PubMed] [Google Scholar]
Additional references
Adams 2000
- Adams JP, Murphy PG. Obesity in anaesthesia and intensive care. British Journal of Anaesthesia 2000;85(1):91‐108. [PUBMED: 10927998] [DOI] [PubMed] [Google Scholar]
Bernardini 2009
- Bernardini A, Natalini G. Risk of pulmonary aspiration with laryngeal mask airway and tracheal tube: analysis on 65 712 procedures with positive pressure ventilation. Anaesthesia 2009;64(12):1289‐94. [PUBMED: 19860753] [DOI] [PubMed] [Google Scholar]
Brain 1985
- Brain AI, McGhee TD, McAteer EJ, Thomas A, Abu‐Saad MA, Bushman JA. The laryngeal mask airway. Development and preliminary trials of a new type of airway. Anaesthesia. 1985/04/01 1985; Vol. 40, issue 4:356‐61. [PUBMED: 4003736] [PubMed]
Brain 2000
Brimacombe 1995
Brimacombe 1995a
Cook 2005
Cook 2007
- Cook TM, Gibbison B. Analysis of 1000 consecutive uses of the ProSeal laryngeal mask airway by one anaesthetist at a district general hospital. British Journal of Anaesthesia 2007;99(3):436‐9. [PUBMED: 17604305] [DOI] [PubMed] [Google Scholar]
Cook 2011
- Cook TM, Howes B. Supraglottic airway devices: recent advances. Continuing Education in Anaesthesia, Critical Care and Pain 2011; Vol. 11, issue 2:56‐61.
Cook 2011a
- Cook TM, Woodall N, Frerk C. Major complications of airway management in the UK: results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: anaesthesia. British Journal of Anaesthesia. 2011/03/31 2011; Vol. 106, issue 5:617‐31. [PUBMED: 21447488] [DOI] [PubMed]
Frappier 2003
Goldmann 2011
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians?. BMJ. 2008/05/06 2008; Vol. 336, issue 7651:995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [updated March 2011] . The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Hohlrieder 2007a
- Hohlrieder M, Brimacombe J, Goedecke A, Keller C. Postoperative nausea, vomiting, airway morbidity, and analgesic requirements are lower for the ProSeal laryngeal mask airway than the tracheal tube in females undergoing breast and gynaecological surgery. British Journal of Anaesthesia. 2007/07/10 2007; Vol. 99, issue 4:576‐80. [17617554] [DOI] [PubMed]
Juvin 2003
Karkouti 2000
Kelly 2008
- Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. International Journal of Obesity (2005) 2008;32(9):1431‐7. [PUBMED: 18607383] [DOI] [PubMed] [Google Scholar]
Kristensen 2010
Lundstrom 2009
- Lundstrom LH, Moller AM, Rosenstock C, Astrup G, Wetterslev J. High body mass index is a weak predictor for difficult and failed tracheal intubation: a cohort study of 91,332 consecutive patients scheduled for direct laryngoscopy registered in the Danish Anesthesia Database. Anesthesiology. 2009/02/06 2009; Vol. 110, issue 2:266‐74. [PUBMED: 19194154] [DOI] [PubMed]
Malhotra 2008
- Malhotra A, Hillman D. Obesity and the lung: 3. Obesity, respiration and intensive care. Thorax 2008;63(10):925‐31. [PUBMED: 18820119] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marley 2005
- Marley RA, Hoyle B, Ries C. Perianesthesia respiratory care of the bariatric patient. Journal of Perianesthesia Nursing 2005;20(6):404‐31; quiz 432‐4. [PUBMED: 16387272] [DOI] [PubMed] [Google Scholar]
Natalini 2002
- Natalini G, Franceschetti ME, Pletti C, Recupero D, Lanza G, Bernardini A. Impact of laryngeal mask airway and tracheal tube on pulmonary function during the early postoperative period. Acta Anaesthesiologica Scandinavica 2002;46(5):525‐8. [PUBMED: 12027846] [DOI] [PubMed] [Google Scholar]
Natalini 2003
OECD 2010
- Obesity and the economics of prevention: Fit not fat. http://www.oecd.org/health/healthpoliciesanddata/obesityandtheeconomicsofpreventionfitnotfat.htm (accessed 9 October 2012)..
OECD 2012
- OECD. Obesity update. http://www.oecd.org/health/49716427.pdf (accessed 22 May 2013).
Piper 2004
- Piper SN, Triem JG, Rohm KD, Maleck WH, Schollhorn TAH, Boldt J. ProSeal (TM) laryngeal mask versus endotracheal intubation in patients undergoing gynaecologic laparoscopy (Retracted article. See vol. 46, pg. 490, 2011). Anasthesiologie Intensivmedizin Notfallmedizin Schmerztherapie 2004; Vol. 39, issue 3:132‐7. [WOS:000220327200003] [DOI] [PubMed]
Qamarul 2011
- Qamarul Hoda M, Samad K, Ullah H. ProSeal versus classic laryngeal mask airway (LMA) for positive pressure ventilation in adult patients undergoing elective surgery. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD009026] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 5.2 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Tanaka 2003
- Tanaka A, Isono S, Ishikawa T, Sato J, Nishino T. Laryngeal resistance before and after minor surgery: endotracheal tube versus laryngeal mask airway. Anesthesiology 2003;99(2):252‐8. [PUBMED: 12883396] [DOI] [PubMed] [Google Scholar]
Verghese 1996
Voyagis 1996
- Voyagis GS, Photakis D, Kellari A, Kostanti E, Kaklis S, Secha‐Dousaitou PN, et al. The laryngeal mask airway: a survey of its usage in 1,096 patients. Minerva Anestesiologica 1996;62(9):277‐80. [PUBMED: 9038036] [PubMed] [Google Scholar]
WHO 2012
- World Health Organisation. 10 facts on obesity. http://www.who.int/features/factfiles/obesity/facts/en/index1.html. Accessed on 2 September 2013.
Woodall 2011
Yu 2010
Zhang 2009
- Zhang YJ, Liu XZ, Liu B. Proseal laryngeal mask airway versus endotracheal tubes for intraoperative airway management during laparoscopic biliary tract surgery: a systematic review (Provisional abstract). Chinese Journal of Evidence‐Based Medicine 2009; Vol. 9, issue 5:552‐7. [CENTRAL: DARE‐12009105697]
References to other published versions of this review
Nicholson 2012
- Nicholson A, Cook TM, Lewis SR, Reed SS, Smith AF. Supraglottic airway devices versus tracheal intubation for airway management during general anaesthesia in obese patients. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD010105] [DOI] [PMC free article] [PubMed] [Google Scholar]


