Abstract
Patient: Female, 55-year-old
Final Diagnosis: Spontaneous coronary artery dissection
Symptoms: Chest pain
Clinical Procedure: —
Specialty: Cardiology
Objective:
Unusual or unexpected effect of treatment
Background:
Spontaneous coronary artery dissection can present with acute coronary syndrome, ventricular arrhythmias, or sudden cardiac death. Implantable cardioverter-defibrillator placement in patients with spontaneous coronary artery dissection is controversial. The purpose of publishing this case is to inform physicians of potential benefits of implantable cardioverter-defibrillator implantation in patients with spontaneous coronary artery dissection.
Case Report:
A 55-year-old woman presented with chest pain, with an electrocardiogram revealing anterior ST-elevation myocardial infarction and troponin peak of 53.8 ng/mL. Coronary angiography revealed mid-left anterior descending artery occlusion, with appearance of spontaneous coronary artery dissection that was not amenable to revascularization. The decision was made to treat medically. In recovery, the patient experienced ventricular fibrillation arrest. The patient was defibrillated once with achievement of return of spontaneous circulation. An Impella CP was placed to stabilize the patient. After the patient was stabilized, an implantable cardioverter-defibrillator was placed.
Conclusions:
Data on potential benefits of implantable cardioverter-defibrillator placement in patients with spontaneous coronary artery dissection are limited. Most patients with spontaneous coronary artery dissection recover normal coronary architecture; however, there are no guidelines for implantable cardioverter-defibrillator placement in patients with spontaneous coronary artery dissection. Patients with spontaneous coronary artery dissection with high-risk features may benefit from implantable cardioverter-defibrillator for secondary prevention of ventricular arrhythmia and sudden cardiac death, as shown with this case.
Keywords: Cardiopulmonary Resuscitation; Coronary Artery Dissection, Spontaneous; Death, Sudden, Cardiac; Defibrillators, Implantable; Ventricular Fibrillation
Introduction
Spontaneous coronary artery dissection (SCAD) is a rare cause of myocardial ischemia and acute myocardial infarction. Women are more likely to develop SCAD and account for 87% to 95% of SCAD presentations [1]. SCAD mimics atherosclerotic acute coronary syndrome, and most patients present with chest pain or an anginal equivalent. However, 3% to 11% of patients with SCAD present with ventricular arrhythmia [2]. The indications for implantable cardioverter defibrillator (ICD) placement for secondary prevention of sudden cardiac death (SCD) in the SCAD population are controversial [2,3]. We present a case of SCAD and ST-elevation myocardial infarction (STEMI) complicated by ventricular fibrillation and cardiogenic shock and discuss the potential benefits of ICD placement in patients with SCAD.
Case Report
A 55-year-old woman presented with chest pain. The patient had a past medical history of hypertension and hypothyroidism. The initial electrocardiogram revealed anterior STEMI, with ST-elevations in leads I, aVL, and V2–V3. The QTc interval was normal. The patient’s initial troponin result was negative, but 6 h later, the troponin level significantly increased and peaked at 53.800 ng/mL. After the initial electrocardiogram was seen, the patient was loaded with oral aspirin 324 mg and oral prasugrel 60 mg, with plan for emergent revascularization. She also received 4000 units of intravenous unfractionated heparin followed by an intravenous heparin drip and sublingual nitroglycerin 0.4 mg. Transthoracic echocardiogram revealed an ejection fraction of 40% to 45%, with grade 2 diastolic dys-function, with severe hypokinesis of the mid to apical anteroseptal and anterior walls and the apex, and no severe valvular heart disease. The patient underwent emergent coronary angiography, which revealed mid-left anterior descending artery occlusion, with a tapered long segment with severe tortuosity and appearance of SCAD (Figure 1). This occlusion was unable to be crossed, despite the use of multiple wires. The patient’s left main, left circumflex, and right coronary arteries were patent, and there were no collaterals to the distal left anterior descending artery.
Figure 1.
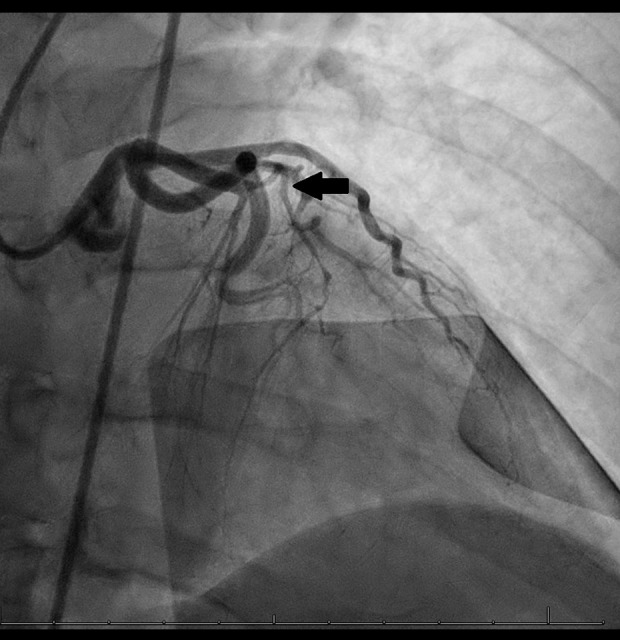
Mid-left anterior descending artery occlusion (arrow) with a tapered long segment with severe tortuosity.
A discussion was held among the interventional cardiologists and cardiothoracic surgeons, and the decision was made to treat medically with dual antiplatelet therapy and heparin drip. In recovery, the patient experienced ventricular fibrillation (VF) arrest. The patient received defibrillation once at 200 J and cardiopulmonary resuscitation for 2 min, with successful return of spontaneous circulation without administration of any medication. The interventional cardiologists and cardiothoracic surgeons again discussed, and the decision was made to place a mechanical circulatory support device to reduce her myocardial oxygen demand. An Impella CP was placed, with initial setting at P-4, with adequate cardiac power output, and a Swan-Ganz catheter was placed to monitor cardiac index. The patient also had close monitoring of her mean arterial pressure with an arterial line. The patient’s cardiac index was at least 2.37 L/min/m2 for 3 days on P-4. Her cardiac power output was at least 1.18 W. Since the patient’s cardiac index remained stable, the Impella was removed. The patient also underwent femoral angiography, which revealed bilateral external iliac artery fibromuscular dysplasia, which could cause her to be susceptible to SCAD. The electrophysiology team was consulted to evaluate for ICD placement. Since the VF arrest occurred in recovery and not during a procedure in which intracardiac instrumentation can induce VF, the electrophysiology team decided to place an ICD for secondary prevention of SCD. SCAD was the suspected etiology of this patient’s VF arrest. The patient had multiple device checks through 10 months after ICD placement, with no stored events of ventricular arrhythmias.
Discussion
SCAD is increasingly recognized as a cause of acute coronary syndrome and ventricular arrhythmias and is diagnosed via coronary angiography [1]. There are clear guidelines that outline the management of coronary intervention in SCAD; however, there is limited evidence about secondary prevention of ventricular arrhythmias. Current guidelines do not recommend routine placement of ICD in patients with SCAD; however, there is some emerging evidence that some patients can benefit from ICD placement [3,4].
Once SCAD is diagnosed, the goal of management in the acute phase is to restore and/or preserve cardiac function and myocardial perfusion. Thrombolysis is not recommended, owing to adverse outcomes. Percutaneous coronary intervention has more complications and poorer outcomes in patients with SCAD than in patients with acute coronary syndrome, such as iatrogenic injury [1]. Twenty-one percent of the patients who experienced SCD in the Massachusetts General Hospital SCAD registry had SCD either during or shortly after percutaneous coronary intervention [2]. Because of the higher risk of complications for percutaneous coronary intervention in SCAD patients, SCAD is managed conservatively, and most patients with SCAD recover normal coronary architecture [1].
Some patients with SCAD possess certain features that increase the risk of SCD, such as STEMI on presentation, a history of VF, and SCAD recurrence [2,5] (Table 1). Complications from SCAD are not uncommon, with a 47% rate of major adverse cardiovascular events and about 30% rate of SCAD recurrence within 10 years. A total of 14 out of the 102 patients in the Massachusetts General Hospital SCAD registry had SCD, with 7 cases occurring outside of the hospital [2]. Seven of those 14 patients had an ICD inserted, and 5 of those 7 patients had ICD inserted due to either inducible ventricular tachycardia or recurrent non-sustained ventricular tachycardia (VT). In an average follow-up of 3.5 years after ICD insertion, there was 1 patient whose ICD discharged for supraventricular tachycardia. Another patient had complications from the ICD. Since 50% of the patients who had SCD had SCD occur outside of the hospital, inserting an ICD in patients with SCAD makes sense, as this can be life-saving. Procedure-related complications are a valid concern, but only 1 patient out of 7 who had an ICD inserted had complications from the ICD [2]. A life vest could be a reasonable alternative option in this patient population, as patients can wear it temporarily until the SCAD lesion heals, but more studies need to be conducted.
Table 1.
Features in patients with spontaneous coronary artery dissection (SCAD) that increase the risk of sudden cardiac death.
Ueda et al discussed 2 cases in which the patients presented with acute VF and were found to have SCAD on further imaging studies. A subcutaneous ICD was implanted in both patients [5].
There is a Japanese retrospective study that showed a 2% incidence of SCD and 36% incidence of non-fatal MI during a median follow-up of 50 months after SCAD onset. For the patients who had non-fatal MI, 25% had potential progression of primary SCAD, and 75% had recurrent SCAD [5,6]. The authors of the PACIFIC registry studied Japanese patients with acute coronary syndrome. They discovered that in patients who presented with STEMI, there is a 4.2% recurrence rate of non-fatal MI and/or unstable angina at 2 years. Since SCAD has a high recurrence rate, it can be postulated that the recurrence rate of non-fatal MI and/or unstable angina after STEMI would be higher in patients with SCAD [5,7]. The Japanese Circulation Guidelines have a class 2b recommendation to implant an ICD in a patient who has VT/VF within 48 h of an MI that is due to a cause that has a high risk of re-exposure [5]. Thus, it can be reasoned that the Japanese Circulation Guidelines would recommend ICD implantation in a patient with VT/VF due to SCAD, since SCAD has a high recurrence rate, making the patient susceptible to SCD. SCAD can also cause VF due to abrupt ischemia with no collateral blood flow. Studies have shown that SCAD patients with a history of VT/VF and ejection fraction of <50% are at a higher risk of VT/VF recurrence. Patients with SCAD who possess features that increase the risk of VT/VF or SCD can benefit from ICD implantation, as these events can be unpredictable and fatal [5].
SCAD can occasionally predispose patients to cardiogenic shock. A case series by Sharma et al highlighted the role of mechanical circulatory support, such as the Impella, as a means of hemodynamic support in cases of patients with SCAD complicated by cardiogenic shock. The case report of Tarantini et al further built on this by identifying the use of the Impella device in a hemodynamically unstable patient with extensive left anterior descending artery SCAD in which medical management and revascularization were not appropriate options. In this case, the use of the Impella improved the patient’s symptoms and hemodynamics through increased coronary perfusion pressure, ultimately reducing oxygen demand in the context of decreased coronary flow [8]. While there were no major adverse events in the report by Tarantini et al, the use of Impella in SCAD poses potential risk of intramural hematoma propagation and increased risk of peripheral dissection [9]. Sharma et al further expanded on this by exploring the use of the Impella for supporting the myocardium, while allowing the SCAD vessel to heal spontaneously. Their series identified 2 cases of cardiogenic shock that occurred after revascularization without ongoing ischemia and draws ties to stress cardiomyopathy, namely, Takotsubo. Left ventricular dysfunction in SCAD without ischemia is hypothesized to be secondary to catecholamine surges or coronary wall stress and a hyperdynamic myocardium [8]. Current guidelines propose the use of mechanical circulatory support for management of Takotsubo, with studies supporting early initiation to avoid negative effects from inotropes [8,10,11]. Despite scant data on mechanical circulatory support in SCAD, the parallels in physiology with stress cardiomyopathy support the use of the Impella during resolution of SCAD, with or without revascularization.
Conclusions
Limited data exist on potential benefits of ICD placement in patients with SCAD. Most patients with SCAD recover normal coronary architecture, and ICD procedures can have complications. Thus, there are currently no guidelines in the United States on this topic. However, some patients with SCAD possess features that increase the risk of SCD and/or VT/VF, and these patients may benefit from ICD implantation.
Abbreviations
- SCAD
spontaneous coronary artery dissection;
- ICD
implantable cardioverter defibrillator;
- SCD
sudden cardiac death;
- STEMI
ST-elevation myocardial infarction;
- VF
ventricular fibrillation;
- PCI
percutaneous coronary intervention;
- VT
ventricular tachycardia
Footnotes
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
References:
- 1.Hayes SN, Tweet MS, Adlam D, et al. Spontaneous coronary artery dissection: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76(8):961–84. doi: 10.1016/j.jacc.2020.05.084. [DOI] [PubMed] [Google Scholar]
- 2.Sharma S, Rozen G, Duran J, et al. Sudden cardiac death in patients with spontaneous coronary artery dissection. J Am Coll Cardiol. 2017;70(1):114–15. doi: 10.1016/j.jacc.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Scagliola R, Brunelli C, Balbi M. Implantable cardioverter defibrillator therapy for secondary prevention in spontaneous coronary artery dissection: To place or not to place? This is the matter. Anatol J Cardiol. 2020;23(4):240. doi: 10.14744/AnatolJCardiol.2020.57870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS Guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. J Am Coll Cardiol. 2018;72(14):e91–220. doi: 10.1016/j.jacc.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 5.Ueda T, Osada J, Kowase S, Asano S, Yumoto K. Subcutaneous implantable cardioverter defibrillator for spontaneous coronary artery dissection with ventricular fibrillation: A report of two cases. J Cardiol Cases. 2022;26(4):260–63. doi: 10.1016/j.jccase.2022.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakashima T, Noguchi T, Haruta S, et al. Prognostic impact of spontaneous coronary artery dissection in young female patients with acute myocardial infarction: A report from the angina pectoris-myocardial infarction multicenter investigators in Japan. Int J Cardiol. 2016;207:341–48. doi: 10.1016/j.ijcard.2016.01.188. [DOI] [PubMed] [Google Scholar]
- 7.Daida H, Miyauchi K, Ogawa H, et al. Management and two-year long-term clinical outcome of acute coronary syndrome in Japan – Prevention of AtherothrombotiC Incidents Following Ischemic Coronary Attack (PACIFIC) registry. Circ J. 2013;77(4):934–43. doi: 10.1253/circj.cj-13-0174. [DOI] [PubMed] [Google Scholar]
- 8.Sharma S, Polak S, George Z, et al. Management of spontaneous coronary artery dissection complicated by cardiogenic shock using mechanical circulatory support with the Impella device. Catheter Cardiovasc Interv. 2021;97(1):74–77. doi: 10.1002/ccd.28677. [DOI] [PubMed] [Google Scholar]
- 9.Tarantini G, Fabris T, Rodinò G, Fraccaro C. Improvement of symptoms and coronary perfusion gradient with mechanical left ventricular unloading in flow-limiting complex spontaneous coronary artery dissection, without revascularization. Catheter Cardiovasc Interv. 2021;98(4):E581–E85. doi: 10.1002/ccd.29836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghadri JR, Wittstein IS, Prasad A, et al. International expert consensus document on takotsubo syndrome (Part II): Diagnostic workup, outcome, and management. Eur Heart J. 2018;39(22):2047–62. doi: 10.1093/eurheartj/ehy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beneduce A, Fausta Bertoldi L, Melillo F, et al. Mechanical circulatory support with impella percutaneous ventricular assist device as a bridge to recovery in takotsubo syndrome complicated by cardiogenic shock and left ventricular outflow tract obstruction. JACC Cardiovasc Interv. 2019;12(4):e31–e32. doi: 10.1016/j.jcin.2018.10.046. [DOI] [PubMed] [Google Scholar]


