Abstract
Context
Sugar alcohols (also called polyols) are regarded as a “healthy” sugar substitute. One of the possible reasons for their safe use in pregnant women is their natural origin and the presence of polyols in maternal and fetal samples during normal human gestation. But little is known about the association between circulating sugar alcohols levels and maternal metabolic disorders during pregnancy.
Objective
We aimed to detect the concentration of the polyols in participants with and without gestational diabetes mellitus (GDM), and to investigate the association between maternal serum levels of polyols and GDM, as well as newborn outcomes.
Methods
A nested population-based case–control study was conducted in 109 women with and without GDM. Maternal concentrations of serum erythritol, sorbitol, and xylitol in the fasting state were quantified using a time of flight mass spectrometry system.
Result
In women with GDM, serum concentrations of erythritol and sorbitol were higher, but serum concentrations of xylitol were lower than those in women without GDM. Per 1-SD increment of Box–Cox-transformed concentrations of erythritol and sorbitol were associated with the increased odds of GDM by 43% and 155% (95% CI 1.07-1.92 and 95% CI 1.77-3.69), while decreased odds were found for xylitol by 25% (95% CI 0.57-1.00). Additionally, per 1-SD increase of Box–Cox-transformed concentrations of serum sorbitol was associated with a 52% increased odds of large for gestational age newborns controlling for possible confounders (95% CI 1.00-2.30).
Conclusion
Maternal circulating sugar alcohols levels during pregnancy were significantly associated with GDM. These findings provide the potential roles of polyols on maternal metabolic health during pregnancy.
Keywords: polyols, gestational diabetes mellitus, newborn outcomes
Sugar substitutes are widely used in the food industry to reduce caloric intake, including non-nutritive sweeteners (NNSs), sugar alcohols, and others. These sugar substitutes were generally believed to have beneficial effects on metabolism (1, 2). Therefore, they were recommended for use in different metabolic states (3, 4). Notably, a new evidence-based guideline released by the World Health Organization discourages the use of NNSs for managing body weight in individuals without diabetes (5). Unfortunately, the recommendations for the use of sugar substitutes in pregnant women are currently quite limited.
The association between sugar substitute intake and maternal metabolism during pregnancy has not been extensively studied in humans. It has been reported that pregnant women with daily consumption of NNS beverages tend to gain more body weight (6). Previously, our research team found positive associations between maternal circulating aspartame levels and abnormal lipid/glucose metabolism during pregnancy (7). Little is known on the links between sugar alcohols and maternal metabolism at gestation.
Sugar alcohols (also called polyols) are regarded as a healthy sugar substitute in individuals with and without gestation. This is mainly due to their natural origin and their metabolic benefits (8). For instance, xylitol consumption could increase beta cell numbers and improve glucose tolerability in a type 2 diabetes model of rats (8, 9). Erythritol exposure could lower glucose levels, stimulate gut hormones, and exert antioxidative properties (10-12). However, an association between polyols and adverse health outcomes was observed. Four weeks of sorbitol consumption may induce glucose intolerance by changing the composition of the gut microbiome (13). Another possible reason ensuring safe use of sugar alcohols in pregnant women is their presence in maternal and fetal samples during normal human gestation (14). To date, little is known about the association between plasma sugar alcohols levels and glucose regulation during gestation.
Sorbitol and xylitol are more widely used in China. Recently, erythritol has attracted more attention, given the considerable controversy regarding its correlation with human metabolism (15, 16). We aimed to quantitatively evaluate the associations between maternal serum levels of polyols during pregnancy and gestational diabetes mellitus (GDM), as well as newborn outcomes.
Materials and Methods
Participants and Procedure
A population-based nested case–control study was conducted on 109 pregnant women with GDM and 109 age and prepregnancy body mass index (BMI)–matched participants without GDM, as previously described (7). In brief, we performed an observational study at Chongming Hospital, affiliated to Shanghai University of Health and Medicine Sciences, from September 2019 to May 2021. We calculated the necessary sample size of 385 participants to achieve 80% power and a 95% CI and a prevalence of GDM, using PASS 14 Power Analysis and Sample Size Software (NCSS, LLC. Kaysville, Utah). A random sample of 670 participants achieved an effect size of 2. A total of 632 pregnant women were enrolled in the final study. Those who met the following criteria were excluded: (1) uncertain date of last menstrual period; (2) multiple pregnancies; (3) previous diagnosis of diabetes requiring treatment with medication before pregnancy; (4) secondary diabetes; (5) induction of pregnancy with the assistance of advanced reproductive technology. Eventually, 113 out of 632 women were diagnosed with GDM. Excluding 4 women missing blood samples, all women with GDM were selected as cases to achieve statistical power. Group sample sizes of 109 cases and 109 controls achieved 100% power to show no difference in serum aspartame levels between the GDM and non-GDM groups using PASS 14 Power Analysis and Sample Size Software (NCSS, LLC, Kaysville, Utah). This study was approved by the ethics committee of Xinhua Hospital, affiliated to Shanghai Jiao Tong University School of Medicine (project number XHEC-D-2017-035). All participants gave informed written consent.
Information during pregnancy was collected through 3 face to face interviews at 9 to 14, 24 to 32, and 34 to 36 weeks; the self-reported pregnancy outcomes were obtained after the due date via a phone view. At 9 to 14 weeks’ gestation, information was obtained during the first visit, including name, age, prepregnancy weight, gravidity and parity times, family history of diabetes, and history of chronic diseases. The enrolled women are scheduled to have a 75-g oral glucose tolerance test (OGTT) at a prenatal visit as close to 28 weeks of gestation as possible and always within 24 to 32 weeks (17). At that visit, the OGTT was obtained, and height, weight, and blood pressure were measured. Maternal blood pressure, weight body, and waist circumference were measured at 34 to 36 weeks.
Laboratory Measurements
Fasting, 1-hour, and 2-hour plasma glucose levels after glucose load were tested using the glucose oxidase method (ADVIA-1650 Chemistry System, Bayer, Leverkusen, Germany). HbA1c was tested via high-performance liquid chromatography (BIO-RAD, Laboratories, Hercules, CA, USA). Fasting, 1-hour, and 2-hour insulin levels were determined by chemiluminescent microparticle immunoassay, using the Architect Insulin Reagent Kit (catalog #8K41-74, RRID:AB_3075437) on an ARCHITECT ci16200 analyzer (Abbott Laboratories, Chicago, IL, USA). Homeostasis model assessment for insulin resistance (HOMA-IR) was calculated as follows: fasting plasma glucose (FPG) (mmol/L) × fasting insulin (FINS) (mU/L)/22.5. The Matsuda insulin sensitivity index (ISI) derived from OGTT was calculated as (10 000/sqrt [FPG × FINS × mean glucose × mean insulin]) (18-20). HOMA-β was calculated as (FINS × 20/[FPG – 3.5]). Fasting triglycerides, total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol concentrations were measured using an automatic biochemical analyzer (AU5800; Beckman Coulter, Brea, CA, USA).
Serum Polyols Levels Measurements
Sample preparation
Fasting serum specimens were collected during OGTT and stored at −80 °C until analyzed. Samples were thawed on ice at 4 °C before preparation. Quality control samples were prepared by aspirating equal amounts of each serum sample and mixing them. Twenty-five–microliter samples of serum were added to 1.5-mL Eppendorf tubes with 170 μL of precooled methanol, and were shaken at 1450 rpm for 15 minutes at 10 °C (MSC-100, Allsheng Instruments, Co., Ltd., Hangzhou, China). The mixture was kept at −20 °C for 20 minutes and then centrifuged at 18 000g for 20 minutes at 4 °C (Microfuge 20R, Beckman Coulter, Inc., Indianapolis, IN, USA); 160 μL of supernatant was lyophilized in a lyophilizer (Labconco, Kansas City, USA). Next, 50 μL of methoxamine solution was added; the reaction was carried out at 37 °C for 2 hours. Following that, 50 μL of MSTFA was added and the reaction was carried out at 37 °C for 1 hour, awaiting analysis. All the standards were obtained from (Sigma-Aldrich, St. Louis, MO, USA). Methanolic pyridine and MSFTA (with 1% TMCS) were purchased from Thermo-Fisher Scientific (FairLawn, NJ, USA). All standards were accurately weighed and prepared in water to obtain the individual stock solutions at a concentration of 5.0 mg/mL. The appropriate amount of each stock solution was mixed to create stock calibration solutions.
Targeted metabolite measurement
Serum polyols quantitation was performed using gas chromatography coupled with time of flight and mass spectrometry (GC-TOF/MS) (Pegasus HT, Leco Corp., St. Joseph, MO, USA) with an Agilent 7890 B gas chromatography and a Gerstel multipurpose sample MPS 2 with dual heads (Gerstel, Muchlheim, Germany) (21, 22). A Rxi-5Sil MS capillary column (30 m × 0.25 mm × 0.25 µm i.d., 0.25-μm film thickness; Restek corporation, Bellefonte, PA, USA) was used for separation. Helium was used as the carrier gas at a constant flow rate of 1.0 mL/minute. Derivatized samples (1 μL each) were injected into the GC/MS system in the splitless injection mode. A programmed column temperature was optimized for successful separation (Table S1 (23)). The temperature of the injection and transfer interface were both set to 270 °C. The source temperature was 220 °C. The measurements were made using electron impact ionization (70 eV) in the full scan mode (m/z 50-500). The acquisition rate was set to 15 spectra/second. Instrument optimization was performed every 48 hours.
The raw data generated by GC-TOF/MS were processed using Chroma TOF software (v5.51, Leco Corp., USA) for peak integration, calibration, and quantification of each metabolite. The self-developed platform iMAP (v1.0, Metabo-Profile, Shanghai, China) was used for statistical analyses.
Primary and Secondary Outcomes
The primary maternal outcome was GDM, which was defined by the International Association of Diabetes and Pregnancy Study Groups criteria (24) as ≥1 of the following results: fasting ≥5.1 mmol/L; 1 hour ≥10.0 mmol/L; and 2 hours ≥ 8.5 mmol/L. A second outcome was large for gestational age (LGA) newborns. LGA newborns weigh about 4 kg or more at birth (25).
Statistical Analysis
Continuous variables are shown as means ± SD or medians (interquartile range) for skewed variables. Categorized variables are presented as numbers (proportions). For a comparison of continuous variables, Student's t-tests and Wilcoxon rank-sum tests were used. For a comparison of categorical data, chi-square tests or Fisher's exact tests were used.
Associations between serum polyols and maternal traits
Spearman rank correlations were used to assess the relationships between serum polyol levels and maternal traits at OGTT. Maternal traits included age at OGTT, gestational age at OGTT, prepregnancy BMI, mean arterial pressure (MAP) at OGTT, fasting, 1 hour, and 2 hour plasma glucose, fasting, 1 hour and 2 hour insulin, HbA1c, Matsuda ISI, HOMA-IR, HOMA-β, triglycerides, total cholesterol, LDL cholesterol, HDL cholesterol, and GDM.
Multiple logistic regression models were used to identify the odds of GDM according to 1-SD increment and tertiles of maternal serum polyols during pregnancy. Serum polyols levels were Box–Cox transformed in multiple logistic regression models. Adjusted cofactors included maternal age at OGTT, family history of diabetes (yes = 1, no = 0), MAP during OGTT, prepregnancy BMI, and parity (0, 1+). We reported odds ratios (ORs) and 95% CIs as measures of association.
Prediction of LGA
Spearman rank correlations found an association between serum sorbitol and birth weight. Next, we tried to identify the odds of LGA according to a 1-SD increase of Box–Cox-transformed serum sorbitol during pregnancy using multiple logistic regression models. Regression models included an unadjusted model (model 1); model 1 + age at OGTT, family history of diabetes, and parity (model 2); model 2 + prepregnancy BMI (model 3); model 3 + MAP (model 4); and model 3 + GDM (model 5).
The area under the receiver operating characteristic curve (ROC) was used to evaluate the predictive performance of maternal clinical factors and fasting Box–Cox-transformed levels of serum sorbitol during late pregnancy using the pROC R package, version 1.18.0 (https://www.rdocumentation.org/packages/pROC/versions/1.18.0) and rms package, version 6.3-0 (https://www.rdocumentation.org/packages/rms/versions/6.3-0) on R (version 4.1.0, R Foundation for Statistical Computing). DeLong's test was applied to compare areas under the curve of 2 models using the pROC package. Two types of variable setups were used to perform this classification using clinical traits, separately and together with fasting Box–Cox-transformed serum sorbitol.
A 2-sided P ≤ .05 was considered to be statistically significant. In multiple logistic regression models, results were considered significant associations if the 95% CIs did not cross 0. Comparisons of participant characteristics were carried out using SAS version 9.3 (SAS Institute, Cary, NC, USA).
Results
Participants’ Characteristics in GDM and Non-GDM Groups
Demographic data are shown elsewhere for the 218 women (109 GDM and 109 non-GDM) who participated in the study and for whom serum polyols levels were available (Table S2 (23)).
Women with GDM had higher levels of fasting, 1 hour, and 2 hour plasma glucose, fasting and 2 hour insulin, HbA1c, HOMA-IR, and triglycerides, and lower levels of Matsuda ISI (all P < .05). The birth timing, birth weight and height, and the proportions of full-term delivery, and cesarean section did not differ between GDM and non-GDM groups.
Comparisons of Serum Polyols in Participants With and Without GDM
The distribution of serum polyols levels at fasting state was skewed. The median levels of serum erythritol, sorbitol, and xylitol were 3.05 (2.75-3.45) µmol/L, 1.49 (1.24-1.89) µmol/L, 1.30 (1.5-1.90) µmol/L, respectively (Fig. S1 (23)).
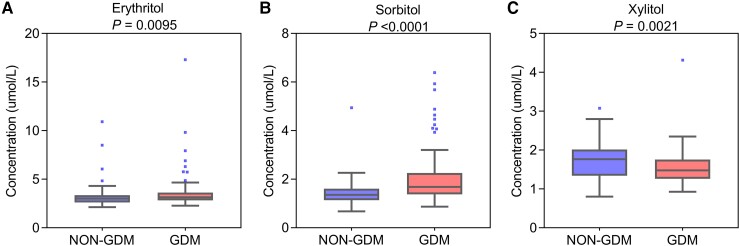
Significant differences of serum polyols levels were available in women with and without GDM (Fig. 1). Women with GDM had significantly higher serum levels of erythritol and sorbitol, but lower levels of xylitol (3.14 [2.83-3.61] vs 3.01 [2.61-3.35] µmol/L, 1.69 [1.38-2.24] vs 1.35 [1.14-1.6] µmol/L, 1.48 [1.26-1.75] vs 1.77 [1.34-2.01]; P = .0095, P < .001, and P = .0021, respectively).
Figure 1.
Box plots comparing serum polyols in women with and without GDM. (A) Erythritol. (B) Sorbitol. (C) Xylitol. Data are given as median (interquartile range). The top of the box indicates the 75th percentile and the bottom of the box indicates the 25th percentile. The top and bottom whiskers represent the 97.5th and 2.5th percentiles, respectively. Dots mark outlier values. Wilcoxon rank-sum tests were used for the comparisons of serum polyols levels. P < .05 was considered to be statistically significant. Abbreviation: GDM, gestational diabetes mellitus.
Participants’ Characteristics According to Serum Polyols Tertiles
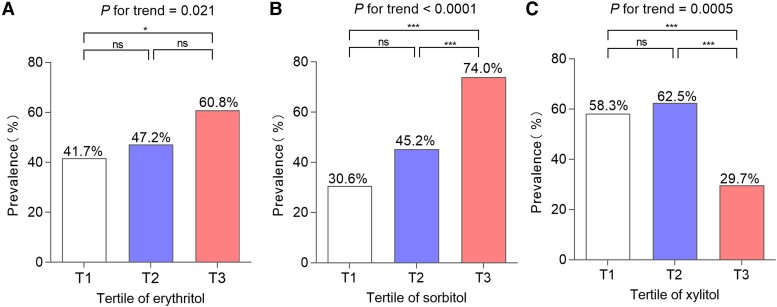
Figure 2 shows that the prevalence of GDM was significantly different across the tertiles of serum polyol levels. For erythritol, the prevalence of GDM in the first tertile was significantly lower than that in the third tertile (41.7% vs 60.8%, P = .021), whereas no significances were found between the first/third and second tertile. For sorbitol, the prevalence of GDM in the third tertile was significantly higher than those in the first and second tertiles, respectively (74.0% vs 30.6% and 74.0% vs 45.2%, P < .0001 and P < .0004), whereas the prevalence in the second tertile showed no significance from that in the first tertile. Unexpectedly, the prevalence of GDM in the third tertile of serum xylitol levels was much lower than those in the first and second tertiles, respectively (29.7% vs 58.3%, 29.7% vs 62.5%, P = .0005 and P < .0001). Whereas, the prevalence in the second tertile was not different from those in the first and third tertiles.
Figure 2.
The prevalence of GDM according to serum polyols tertiles. (A) Erythritol: T1, <2.86 µmol/L; T2, 2.86-3.32 µmol/L; T3, ≥3.31 µmol/L. (B) Sorbitol: T1, <1.32 µmol/L; T2, 1.32-1.69 µmol/L; T3, ≥1.69 µmol/L. (C) Xylitol: T1, <1.39 µmol/L; T2, 1.39-1.81 µmol/L; T3, ≥1.81 µmol/L. The chi-square test and chi-square test for trend were used to compare the prevalence of GDM and calculate P for trend and P values. P < .05 was considered to be statistically significant. nsP > .05, ***P < .001, and *P < .05. Abbreviation: T, tertile.
The comparisons of other metabolic traits across serum polyols tertiles are shown elsewhere (Tables S3-5 (23)). Age at OGTT, 1 hour and 2 hour plasma glucose, 2 hour insulin, Matsuda's ISI, and triglycerides showed significant differences across tertiles of serum erythritol levels (all P < .05). More traits showed significant differences across tertiles of serum sorbitol levels, including fasting, 1 hour and 2 hour plasma glucose, 1 hour and 2 hour insulin HbA1c, HOMA-IR, and Matsuda's ISI. Across serum xylitol tiles, the levels of 2 hour plasma glucose, fasting insulin, HOMA-IR, triglycerides, HDL cholesterol, and LDL cholesterol showed significant differences (all P < .05).
Factors Associated With Maternal Serum Polyols Levels
Spearman correlation analysis was used to identify the participants’ traits associated with maternal serum polyol levels at OGTT during pregnancy (Table S6 (23)). Maternal serum polyol levels were associated with GDM (erythritol: Spearman's rho = 0.1762, P = .0091; sorbitol: Spearman's rho = 0.3908, P ≤ .0001; xylitol: Spearman's rho = −0.2090; P = .0019).
Serum erythritol levels were positively associated with fasting, 1 hour, and 2 hour plasma glucose, 2 hour insulin, HOMA-IR, triglycerides, and LDL cholesterol. For serum sorbitol levels, positive associations were found for fasting, 1 hour, and 2 hour plasma glucose, 1 hour and 2 hour insulin, HbA1c, HOMA-IR, alanine transaminase, birth height, and weight of children. Serum xylitol levels were negatively associated with fasting insulin, HOMA-IR, triglycerides, and LDL cholesterol. Serum erythritol and sorbitol levels showed negative associations with Matsuda's ISI, while serum xylitol levels showed a positive association.
Associations Between Maternal Serum Polyols Levels and GDM
Multiple logistic regression models were used to identify the odds of GDM per 1-SD increment of Box–Cox-transformed and tertiles of maternal serum polyols during pregnancy, respectively (Table 1). Maternal serum polyol levels during pregnancy were significantly associated with GDM at OGTT.
Table 1.
The associations between maternal serum polyols at fasting and GDM during pregnancy
| Event/Participants | Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|---|
| OR (95 CI%) | OR (95 CI%) | OR (95 CI%) | OR (95 CI%) | ||
| Erythritol | |||||
| 1SD | — | 1.51 (1.14-2.01)a | 1.52 (1.14-2.03)a | 1.52 (1.14-2.03)a | 1.43 (1.07-1.92)a |
| Tertiles | — | 1.48 (1.06-2.05)a | 1.48(1.06-2.07)a | 1.48 (1.06-2.08)a | 1.34 (0.94-1.89) |
| Tertile 1 (ref.) | 30/72 | — | — | — | — |
| Tertile 2 | 34/72 | 1.25 (0.65-2.42) | 1.27 (0.64-2.50) | 1.27 (0.64-2.51) | 1.27 (0.64-2.51) |
| Tertile 3 | 45/74 | 2.17 (1.12-4.21)a | 2.18 (1.12-4.27)a | 2.19 (1.12-4.29)a | 2.19 (1.12-4.29) |
| Sorbitol | |||||
| 1SD | — | 2.76 (1.92-3.96)a | 2.76 (1.91-3.97)a | 2.76 (1.91-3.98)a | 2.55 (1.77-3.69)a |
| Tertiles | — | 2.54 (1.77-3.64)a | 2.52 (1.75-3.63)a | 2.53 (1.76-3.64)a | 2.30 (1.58-3.35)a |
| Tertile 1 (ref.) | 22/72 | — | — | — | — |
| Tertile 2 | 33/73 | 1.88 (0.95-3.71) | 1.83 (0.92-3.63) | 1.83 (0.92-3.65) | 1.73 (0.85-3.50) |
| Tertile 3 | 54/73 | 6.46 (3.13-13.33)a | 6.40 (3.08-13.26)a | 6.40 (3.09-13.28)a | 5.34 (2.52-11.33)a |
| Xylitol | |||||
| 1SD | — | 0.71 (0.54-0.94)a | 0.71 (0.54-0.94)a | 0.71 (0.54-0.94)a | 0.75 (0.57-1.00)a |
| Tertiles | — | 0.55 (0.39-0.78)a | 0.55 (0.39-0.73)a | 0.55 (0.39-0.77)a | 0.59 (0.40-0.84)a |
| Tertile 1 | 42/72 | 3.31 (1.67-6.56)a | 3.34 (1.68-6.66)a | 3.38 (1.69-6.74)a | 2.89 (1.43-5.85)a |
| Tertile 2 | 45/72 | 3.94 (1.98-7.85)a | 4.07 (2.03-8.18)a | 4.09 (2.03-8.23)a | 3.74 (1.80-7.78)a |
| Tertile 3 (ref.) | 22/74 | — | — | — | — |
Multiple logistic regression models were used to identify the odds of GDM according to 1-SD increment and tertiles of maternal serum polyols during pregnancy, respectively. Serum polyols levels were Box–Cox-transformed in multiple logistic regression models. Model 1, unadjusted. Model 2, model 1 + age at OGTT, family history of diabetes, and parity. Model 3, model 2 + prepregnancy BMI. Model 4, model 3 + mean arterial pressure.
a Statistical significance: CI does not cross 1.
The unadjusted logistic regression model showed that a 1-SD increment of Box–Cox-transformed serum erythritol levels was associated with increased odds of GDM (OR 1.51, 95% CI 1.14-2.01). Full adjustment for possible confounders in model 4 showed little effect (OR 1.43, 95% CI 1.07-1.92). Additionally, women in the third tertile had increased odds of GDM compared with those in the first tertile in the unadjusted logistic regression models (OR 2.17, 95% CI 1.12-4.21). After full adjustment in model 4, the association persisted (OR 2.19, 95% CI 1.12-4.29). The associations were not available for women in the second tertile in unadjusted and fully adjusted models.
Per 1-SD increment of Box–Cox-transformed serum sorbitol level was associated with increased odds of GDM in the unadjusted and fully adjusted logistic regression models (OR 2.76, 95% CI 1.92-3.96 and OR 2.55, 95% CI 1.77-3.69, respectively). Compared with women in the first tertile, those in the third tertile had increased odds of GDM in the unadjusted model (OR 5.46, 95% CI 3.13-13.33). The full adjustment had minimal attenuation (OR 5.34, 95% CI 2.52-11.33). The associations were not found for women in the second tertile from model 1 to model 4.
Negative associations were found between 1-SD increment of Box–Cox-transformed serum xylitol levels and GDM in the unadjusted and fully adjusted logistic regression models (OR 0.71, 95% CI 0.54-0.94 and OR 0.75, 95% CI 0.57-1.00, respectively). Women in the first and second tertile showed increased odds of GDM compared with those in the third tertile in unadjusted logistic regression models (OR 3.31 95% CI 1.67-6.56 and OR 3.94, 95% CI 1.98-7.85). The additional adjustment made minimal attenuation in model 4 (OR 2.89, 95% CI 1.43-5.85 for first tertile and OR 3.74, 95% CI 1.80-7.78 for second tertile).
Associations Between Serum Polyols Levels and Child Outcome
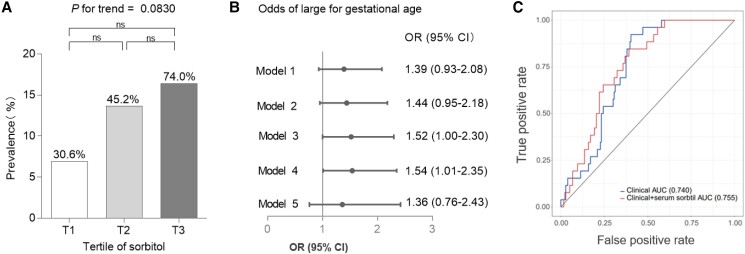
Serum sorbitol levels were positively associated with newborn birth weight in Spearman correlation analysis. Next, we tried to evaluate the associations between serum sorbitol levels and LGA in multiple logistic regression models. The prevalence of LGA in the groups of serum sorbitol tertiles was 30.6% (5/72), 45.2% (10/73), and 74% (12/73), respectively (Fig. 3A). No significant differences of the rate of LGA were found across the titles of serum sorbitol levels.
Figure 3.
Associations between serum polyols and large for gestational age. (A) The prevalence of large for gestational age according to serum polyols tertiles. The chi-square test and chi-square test for trend were used to calculate P for trend and P values. P < .05 was considered to be statistically significant. ns: P > .05. (B) Forest plot of the association between 1-SD increase of Box–Cox-transformed serum sorbitol and the odds of large for gestational age. Multiple logistic regression models were used to identify the odds of large for gestational age according to 1-SD increase of Box–Cox-transformed serum sorbitol during pregnancy. Large for gestational age was defined as birthweight greater than 4000 g (missing data = 14). Statistical significance: CI does not cross 1. Model 1, unadjusted. Model 2, model 1 + age at OGTT, family history of diabetes, and parity. Model 3, model 2 + prepregnancy BMI. Model 4, model 3 + mean arterial pressure. Model 5, model 4 + GDM. (C) Area under the ROC curve for newborn large for gestational age. The models included (1) maternal clinical factors alone (age at OGTT, family history of diabetes, parity, pregnancy BMI, mean arterial pressure, and GDM); (2) maternal clinical factors + fasting Box–Cox-transformed serum sorbitol. Abbreviations: ROC, receiver operating characteristic; AUC, area under the ROC.
Figure 3B shows that per 1-SD increase of Box–Cox-transformed levels of serum sorbitol showed no significant association with the odds of LGA in the unadjusted logistic regression model (OR 1.39, 95% CI 0.93-2.08). Additional adjustment for age at OGTT, family history of diabetes, parity, and prepregnancy BMI in model 3 made the association significant (OR 1.52, 95% CI 1.00-2.30). The association persisted after further adjustment for MAP in model 4, whereas the adjustment for GDM attenuated the association in model 5 (OR 1.36, 95% CI 0.76-2.43).
ROC curves for predicting LGA are shown in Fig. 3C. The models included (1) maternal clinical factors alone (age at OGTT, family history of diabetes, parity, pregnancy BMI, MAP, and GDM), (2) maternal clinical factors + fasting Box–Cox-transformed serum sorbitol. The area under the ROC curve for the model that included clinical factors alone was 0.74. The addition of fasting Box–Cox-transformed serum sorbitol to the clinical factors resulted in minimal improvement in the prediction of LGA newborn with areas under the curve of 0.739 (P = .56 compared with the area under the ROC curve for clinical factors alone), respectively.
Discussion
This nested case–control study demonstrated that fasting serum xylitol levels during pregnancy were negatively associated with the odds of GDM. But serum sorbitol and erythritol concentrations were positively associated with the increased odds of GDM. Notably, we reported that serum sorbitol levels were positively associated with an increased risk of LGA newborns.
Xylitol is used as a sugar substitute in various manufactured products, such as drugs, dietary supplements, and toothpaste. Approximately 50% to 95% of eaten xylitol is slowly absorbed into the gastrointestinal tract (26). In vivo, xylitol is metabolized in the liver and serves as an intermediate of the uronic acid cycle (27, 28). Oral administration of xylitol causes only minor fluctuations in blood xylitol concentrations (26, 28, 29). Besides, it took 24 hours for circulating xylitol levels to return to baseline after xylitol consumption by patients in the intensive care unit (30). Accordingly, we speculated that serum xylitol at fasting in our study reflects endogenous xylitol levels.
In the present study, we have demonstrated a negative association between serum xylitol levels and blood glucose levels, as well as fasting insulin and HOMA-IR. The role of xylitol in glucose metabolism is still largely unknown. The previous report indicates that xylitol infusion in rat liver can effectively inhibit gluconeogenesis (31) and reduce hepatic redox (32). Moreover, xylitol in rodent liver is converted into xylose, which is then transformed into D-xylulose-5-P, an entry point into the pentose phosphate cycle (27). The pentose phosphate pathway has been reportedly associated with insulin resistance in type 2 diabetes (33). Besides, an increase in insulin secretion was observed in isolated islets during incubation with xylitol (34). In addition, our study showed that serum xylitol levels were negatively associated with serum triglycerides, cholesterol, and LDL cholesterol levels. These above findings indicated that xylitol might play important roles in the pathological process of GDM and dyslipidemia during pregnancy.
After ingestion, 90% of erythritol is rapidly absorbed by the gastrointestinal tract (35) and is largely excreted unchanged in urine (36). In 1996, Bornet et al found that plasma erythritol levels increased proportionally to the amount of erythritol intake in 2 hours (37). Subsequently, circulating erythritol levels were detected in a dose-dependent absorption 3 hours after erythritol intake (22). Consistently, postprandial erythritol plasma levels in healthy participants following consumption of an erythritol-sweetened drink (30 g) remained substantially elevated for over 2 days (16). Furthermore, erythritol is produced endogenously by the pentose phosphate in the human body (38, 39). Published data to date suggest that fasting erythritol potentially reflects both endogenous and postprandial levels (16).
Adverse associations between circulating erythritol and metabolic disease were reported during gestation. The Hyperglycemia and Adverse Pregnancy Outcome Study group demonstrated a positive association between maternal erythritol and insulin resistance in pregnant women (15). We confirmed the association in the present study. In the first trimester of pregnancy, erythritol concentrations were detected in maternal serum, intervillous fluid, coelomic fluid, and amniotic fluid from normal human pregnancies (40). Collectively, a thorough understanding of the specific pathophysiological link between erythritol and maternal glucose metabolism during pregnancy is necessary.
Published data have reported increased circulating sorbitol levels in women with GDM (41), as well as in nonpregnant individuals with type 2 diabetes (42). We made a novel discovery in the present study by identifying a positive association between serum sorbitol levels and newborn birth weight. It is well-established that GDM is associated with an increased risk of LGA (43). Our findings suggest that higher circulating sorbitol may act as a mediator between GDM and the increased risk of LGA. This hypothesis gains support as further adjustment for GDM significantly attenuated the association between serum sorbitol levels and LGA.
The study had several limitations. Firstly, the sample size in this present study was relatively small. A larger population is necessary to confirm our findings in the future. Secondly, the information of dietary habits of these women was not collected. This, therefore, led to difficulties in linking the amount of dietary sugar alcohol use with circulating levels in our study. Thirdly, the observational study cannot confirm causality.
Conclusions
The present case–control study suggested that maternal serum xylitol levels during pregnancy were negatively associated with the odds of GDM. However, maternal serum sorbitol and erythritol showed positive associations with GDM. In offspring, serum sorbitol levels were associated with newborn birth weight. These findings provide the potential roles of polyols on maternal and neonatal metabolic health during pregnancy.
Abbreviations
- BMI
body mass index
- FINS
fasting insulin
- FPG
fasting plasma glucose
- GC-TOF/MS
gas chromatography coupled with time of flight and mass spectrometry
- GDM
gestational diabetes mellitus
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- HOMA-IR
homeostasis model assessment for insulin resistance
- ISI
insulin sensitivity index
- LGA
large for gestational age
- MAP
mean arterial pressure
- NNS
non-nutritive sweetener
- OGTT
oral glucose tolerance test
- OR
odds ratio
- ROC
receiver operating characteristic curve
Contributor Information
Xiaoyong Li, Department of Endocrinology and Metabolism, Xinhua Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200092, China.
Yu Liu, Department of Endocrinology and Metabolism, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200127, China.
Yicheng Qi, Department of Endocrinology and Metabolism, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200127, China.
Yiming Wu, Department of Endocrinology, Chongming Hospital Affiliated to Shanghai University of Health & Medicine Sciences (Chongming Branch of Xinhua Hospital), Shanghai 202150, China.
Meng Wang, Department of Endocrinology and Metabolism, Xinhua Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200092, China.
Jing Gao, Department of Traditional Chinese Medicine, Pujiang Community Health Service Center, Minhang District, Shanghai 201112, China.
Qing Su, Department of Endocrinology and Metabolism, Xinhua Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200092, China.
Jing Ma, Department of Endocrinology and Metabolism, Renji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200127, China.
Li Qin, Department of Endocrinology and Metabolism, Xinhua Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200092, China; Department of Endocrinology, Chongming Hospital Affiliated to Shanghai University of Health & Medicine Sciences (Chongming Branch of Xinhua Hospital), Shanghai 202150, China.
Funding
This study was founded by DMRFP_I_06 from Shanghai Health and Medical Development Foundation, Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant (20181807), Science and Technology Commission of Shanghai Municipality-Science and Technology Program (20DZ2201500), Joint Research Project of Pudong Health (PW2023-D13), and the Young Scientists Fund of the National Natural Science Foundation of China (82100864). This study was founded Shanghai Municipal Health Commission (20204Y0294 and 202240107). This study was also founded by Shanghai “Rising Stars of Medical Talents” Youth Development Program-Youth Medical Talents-Specialist Program (2022) and the Natural Science Foundation of Minhang District, Shanghai, China (2021MHZ2020).
Author Contributions
X.Y.L and Y.L. contributed to data interpretation and manuscript writing. Y.L. and Y.C.Q. led the data analysis. Y.C.Q, Y.M.W., M.W., Q.S., and J.G. contributed to the acquisition and interpretation of data. L.Q. and J.M. were involved in all aspects of the study, including study design and data collection, analysis, and interpretation. All authors made critical intellectual contributions to drafting and/or revising the manuscript and all approved the final version. L.Q. and J.M. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures
The authors declare that there is nothing to disclose.
Data Availability
Data and codes used for analyses will be made available by the corresponding authors upon request.
References
- 1. Sowa PM, Keller E, Stormon N, Lalloo R, Ford PJ. The impact of a sugar-sweetened beverages tax on oral health and costs of dental care in Australia. Eur J Public Health. 2019;29(1):173‐177. [DOI] [PubMed] [Google Scholar]
- 2. de Cock P. Erythritol functional roles in oral-systemic health. Adv Dent Res. 2018;29(1):104‐109. [DOI] [PubMed] [Google Scholar]
- 3. High-Intensity Sweeteners . FDA May 19, 2014. https://www.fda.gov/food/food-additives-petitions/high-intensity-sweeteners
- 4. ElSayed NA, Aleppo G, Aroda VR, et al. 5. Facilitating positive health behaviors and well-being to improve health outcomes: standards of care in diabetes-2023. Diabetes Care. 2023;46(Supplement 1):S68‐S96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Use of non-Sugar Sweeteners: WHO Guideline. World Health Organization; 2023. [PubMed] [Google Scholar]
- 6. Azad MB, Sharma AK, de Souza RJ, et al. Association between artificially sweetened beverage consumption during pregnancy and infant body mass index. JAMA Pediatr. 2016;170(7):662‐670. [DOI] [PubMed] [Google Scholar]
- 7. Liu Y, Li X, Wu Y, Su Q, Qin L, Ma J. The associations between maternal serum aspartame and sucralose and metabolic health during pregnancy. Nutrients. 2022;14(23):5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wölnerhanssen BK, Meyer-Gerspach AC, Beglinger C, Islam MS. Metabolic effects of the natural sweeteners xylitol and erythritol: a comprehensive review. Crit Rev Food Sci Nutr. 2020;60(12):1986‐1998. [DOI] [PubMed] [Google Scholar]
- 9. Msomi NZ, Erukainure OL, Salau VF, Olofinsan KA, Islam MS. Xylitol improves antioxidant, purinergic and cholinergic dysfunction, and lipid metabolic homeostasis in hepatic injury in type 2 diabetic rats. J Food Biochem. 2022;46(4):e14040. [DOI] [PubMed] [Google Scholar]
- 10. Meyer-Gerspach AC, Wingrove JO, Beglinger C, et al. Erythritol and xylitol differentially impact brain networks involved in appetite regulation in healthy volunteers. Nutr Neurosci. 2022;25(11):2344‐2358. [DOI] [PubMed] [Google Scholar]
- 11. Wen H, Tang B, Stewart AJ, et al. Erythritol attenuates postprandial blood glucose by inhibiting α-glucosidase. J Agric Food Chem. 2018;66(6):1401‐1407. [DOI] [PubMed] [Google Scholar]
- 12. Wölnerhanssen BK, Cajacob L, Keller N, et al. Gut hormone secretion, gastric emptying, and glycemic responses to erythritol and xylitol in lean and obese subjects. Am J Physiol Endocrinol Metab. 2016;310(11):E1053‐E1061. [DOI] [PubMed] [Google Scholar]
- 13. Li CH, Wang CT, Lin YJ, et al. Long-term consumption of the sugar substitute sorbitol alters gut microbiome and induces glucose intolerance in mice. Life Sci. 2022;305:120770. [DOI] [PubMed] [Google Scholar]
- 14. Brusati V, Józwik M, Józwik M, et al. Fetal and maternal non-glucose carbohydrates and polyols concentrations in normal human pregnancies at term. Pediatr Res. 2005;58(4):700‐704. [DOI] [PubMed] [Google Scholar]
- 15. Liu Y, Kuang A, Talbot O, et al. Metabolomic and genetic associations with insulin resistance in pregnancy. Diabetologia. 2020;63(9):1783‐1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Witkowski M, Nemet I, Alamri H, et al. The artificial sweetener erythritol and cardiovascular event risk. Nat Med. 2023;29(3):710‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. HAPO Study Cooperative Research Group . The hyperglycemia and adverse pregnancy outcome (HAPO) study. Int J Gynaecol Obstet. 2002;78(1):69‐77. [DOI] [PubMed] [Google Scholar]
- 18. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462‐1470. [DOI] [PubMed] [Google Scholar]
- 19. Kirwan JP, Huston-Presley L, Kalhan SC, Catalano PM. Clinically useful estimates of insulin sensitivity during pregnancy: validation studies in women with normal glucose tolerance and gestational diabetes mellitus. Diabetes Care. 2001;24(9):1602‐1607. [DOI] [PubMed] [Google Scholar]
- 20. Alves JM, Zink J, Chow T, et al. Contributions of prenatal exposures and child lifestyle to insulin sensitivity. J Clin Endocrinol Metab. 2020;105(7):2413‐2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang JH, Chen WL, Li JM, et al. Prognostic significance of 2-hydroxyglutarate levels in acute myeloid leukemia in China. Proc Natl Acad Sci U S A. 2013;110(42):17017‐17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen WL, Wang JH, Zhao AH, et al. A distinct glucose metabolism signature of acute myeloid leukemia with prognostic value. Blood. 2014;124(10):1645‐1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li X, Liu Y, Qi Y, et al. Supplementary material for “Maternal serum polyols and its link to gestational diabetes mellitus: a population-based nested case-control study.” github. Deposited November 21, 2023. https://github.com/Lisaliu2023/Yu-Liu_Renji.git [DOI] [PMC free article] [PubMed]
- 24. International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(7):676‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ricart W, López J, Mozas J, et al. Maternal glucose tolerance status influences the risk of macrosomia in male but not in female fetuses. J Epidemiol Community Health. 2009;63(1):64‐68. [DOI] [PubMed] [Google Scholar]
- 26. Asano T, Levitt MD, Goetz FC. Xylitol absorption in healthy men. Diabetes. 1973;22(4):279‐281. [DOI] [PubMed] [Google Scholar]
- 27. Jakob A, Williamson JR, Asakura T. Xylitol metabolism in perfused rat liver. Interactions with gluconeogenesis and ketogenesis. J Biol Chem. 1971;246(24):7623‐7631. [PubMed] [Google Scholar]
- 28. Ylikahri R. Metabolic and nutritional aspects of xylitol. Adv Food Res. 1979;25:159‐180. [DOI] [PubMed] [Google Scholar]
- 29. Bordier V, Teysseire F, Senner F, et al. Absorption and metabolism of the natural sweeteners erythritol and Xylitol in humans: a dose-ranging study. Int J Mol Sci. 2022;23(17):9867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schneider AS, Schettler A, Markowski A, et al. Assessment of xylitol serum levels during the course of parenteral nutrition including xylitol in intensive care patients: a case control study. Clin Nutr. 2014;33(3):483‐488. [DOI] [PubMed] [Google Scholar]
- 31. Williamson JR, Scholz R, Browning ET, Thurman RG, Fukami MH. Metabolic effects of ethanol in perfused rat liver. J Biol Chem. 1969;244(18):5044‐5054. [PubMed] [Google Scholar]
- 32. Handler JA, Thurman RG. Redox interactions between catalase and alcohol dehydrogenase pathways of ethanol metabolism in the perfused rat liver. J Biol Chem. 1990;265(3):1510‐1515. [PubMed] [Google Scholar]
- 33. Ge T, Yang J, Zhou S, Wang Y, Li Y, Tong X. The role of the pentose phosphate pathway in diabetes and cancer. Front Endocrinol (Lausanne). 2020;11:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Montague W, Taylor KW. Pentitols and insulin release by isolated rat islets of langerhans. Biochem J. 1968;109(3):333‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Munro IC, Berndt WO, Borzelleca JF, et al. Erythritol: an interpretive summary of biochemical, metabolic, toxicological and clinical data. Food Chem Toxicol. 1998;36(12):1139‐1174. [DOI] [PubMed] [Google Scholar]
- 36. Bornet FR, Blayo A, Dauchy F, Slama G. Plasma and urine kinetics of erythritol after oral ingestion by healthy humans. Regul Toxicol Pharmacol. 1996;24(2):S280‐S285. [DOI] [PubMed] [Google Scholar]
- 37. Bornet FR, Blayo A, Dauchy F, Slama G. Gastrointestinal response and plasma and urine determinations in human subjects given erythritol. Regul Toxicol Pharmacol. 1996;24(2):S296‐S302. [DOI] [PubMed] [Google Scholar]
- 38. Hootman KC, Trezzi JP, Kraemer L, et al. Erythritol is a pentose-phosphate pathway metabolite and associated with adiposity gain in young adults. Proc Natl Acad Sci U S A. 2017;114(21):E4233‐E4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schlicker L, Szebenyi DME, Ortiz SR, Heinz A, Hiller K, Field MS. Unexpected roles for ADH1 and SORD in catalyzing the final step of erythritol biosynthesis. J Biol Chem. 2019;294(44):16095‐16108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jauniaux E, Hempstock J, Teng C, Battaglia FC, Burton GJ. Polyol concentrations in the fluid compartments of the human conceptus during the first trimester of pregnancy: maintenance of redox potential in a low oxygen environment. J Clin Endocrinol Metab. 2005;90(2):1171‐1175. [DOI] [PubMed] [Google Scholar]
- 41. Enquobahrie DA, Denis M, Tadesse MG, Gelaye B, Ressom HW, Williams MA. Maternal early pregnancy Serum metabolites and risk of gestational diabetes mellitus. J Clin Endocrinol Metab. 2015;100(11):4348‐4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Preston GM, Calle RA. Elevated serum sorbitol and not fructose in type 2 diabetic patients. Biomark Insights. 2010;5:33‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lowe WL Jr, Scholtens DM, Lowe LP, et al. Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA. 2018;320(10):1005‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and codes used for analyses will be made available by the corresponding authors upon request.