Abstract
Context
Individuals with type 2 diabetes (T2D) have an increased risk of bone fractures despite normal or increased bone mineral density. The underlying causes are not well understood but may include disturbances in the gut-bone axis, in which both glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-2 (GLP-2) are regulators of bone turnover. Thus, in healthy fasting participants, both exogenous GIP and GLP-2 acutely reduce bone resorption.
Objective
The objective of this study was to investigate the acute effects of subcutaneously administered GIP and GLP-2 on bone turnover in individuals with T2D.
Methods
We included 10 men with T2D. Participants met fasting in the morning on 3 separate test days and were injected subcutaneously with GIP, GLP-2, or placebo in a randomized crossover design. Blood samples were drawn at baseline and regularly after injections. Bone turnover was estimated by circulating levels of collagen type 1 C-terminal telopeptide (CTX), procollagen type 1 N-terminal propeptide (P1NP), sclerostin, and PTH.
Results
GIP and GLP-2 significantly reduced CTX to (mean ± SEM) 66 ± 7.8% and 74 ± 5.9% of baseline, respectively, compared with after placebo (P = .001). In addition, P1NP and sclerostin increased acutely after GIP whereas a decrease in P1NP was seen after GLP-2. PTH levels decreased to 67 ± 2.5% of baseline after GLP-2 and to only 86 ± 3.4% after GIP.
Conclusion
Subcutaneous GIP and GLP-2 affect CTX and P1NP in individuals with T2D to the same extent as previously demonstrated in healthy individuals.
Keywords: glucose-dependent insulinotropic polypeptide (GIP), glucagon-like peptide-2 (GLP-2), bone turnover, gut-bone axis, CTX, P1NP
Individuals with type 2 diabetes (T2D) have an increased risk of bone fractures despite having a normal to increased bone mineral density (BMD) (1-4). Several factors may influence bone metabolism and the risk of fractures in T2D, including disturbances in the gut-bone axis; however, the exact underlying mechanisms are not well understood (5-9).
The intestinal hormones glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-2 (GLP-2) usually regulate postprandial bone turnover (10-12).
Upon meal ingestion, GIP is released into the circulation from K-cells in the proximal small intestine and, together with glucagon-like peptide-1 (GLP-1), potentiates the glucose-dependent insulin secretion from the pancreatic beta cells and regulates postprandial blood glucose (13). GIP plays an important role in the pathogenesis of T2D since its insulinotropic effect is severely diminished in these patients (14, 15). Regarding its bone effects, studies have shown that GIP receptors (GIPRs) are expressed on bone cells (16-18) and that GIP regulates bone turnover (12, 19-21).
Bone turnover can be estimated by measuring circulating levels of bone turnover markers. The International Osteoporosis Foundation and the International Federation of Clinical Chemistry and Laboratory Medicine recommend that C-terminal cross-linking telopeptide of type I collagen (CTX) and procollagen type I N-terminal propeptide (P1NP) are used as markers for bone resorption and bone formation, respectively (22, 23). GIP induces an acute inhibition of the bone resorption marker CTX (12, 19-21) and acutely increases the bone formation marker P1NP (20, 21) in healthy humans without diabetes; conversely, blocking the GIPR using a selective GIPR antagonist (GIP(3-30)NH2) diminishes GIP-induced changes in bone turnover (24). Furthermore, it has been found that perimenopausal women with a homozygous GIPR polymorphism have decreased BMD and a 50% increased risk of nonvertebral fractures (25). Since the insulinotropic effect of GIP is reduced in individuals with T2D, the effects of GIP on bone turnover could also be altered and lead to an increased fracture risk in these patients.
GLP-2 is another intestinal hormone, which is cosecreted with GLP-1 from L-cells in the gastrointestinal tract upon meal ingestion and has intestinotrophic effects (26). Like GIP, GLP-2 acutely reduces the bone resorption marker CTX in healthy humans after subcutaneous injection (20, 21, 27-30), and 4 months of daily GLP-2 treatment significantly and dose dependently increased BMD in postmenopausal women (29). It is unknown whether GLP-2 also decreases bone resorption in T2D, but the effect may be altered (5).
Aim
The aim of this study was to investigate the effects of subcutaneous GIP and GLP-2 injections on CTX and P1NP serum concentrations in individuals with T2D.
Methods
Ethics
The study was approved by the Scientific Ethical Committee of the Capital Region of Denmark (H-16047626) and registered at the Danish Data Protection Agency (SUND-2017-21) and at ClinicalTrials.gov (NCT03867656). All study participants gave oral and written consent before inclusion.
Study Participants
We included men with T2D on metformin treatment (see later data). Exclusion criteria were use of antidiabetic treatment other than metformin, diagnosed with osteoporosis or gastrointestinal disease, previous gastrointestinal surgery, smoking, steroid treatment, or weight changes exceeding 3 kg within the last 3 months. At a screening visit, residual beta cell function was tested using a glucagon stimulation test (31). Participants had a dual-energy X-ray absorptiometry scan after completing the test days.
Experimental Design
The study was designed as a randomized, placebo-controlled, crossover study with 3 test days (separated by at least 1 week) with subcutaneous injections of GIP, GLP-2, or placebo, respectively. Sequences were generated at http://www.randomization.com. Participants had to refrain from alcohol ingestion and strenuous physical activity for 2 days before each test day, and they had to fast from 10 Pm the evening before the test day. On test days, participants met fasting in the morning (at around 8 Am) at the research facility at Hvidovre University Hospital and were placed in a hospital bed and had a catheter inserted into a cubital vein for blood sampling. After a resting period of 20 minutes, baseline blood samples were drawn (at t = −10 and −5 minutes). Then the peptide [200 µg GIP, 800 µg GLP-2, or placebo (saline)] was injected subcutaneously as a bolus in the umbilical region as 2 1 mL injections at t = 0 minutes. Blood samples were then drawn at t = 7, 15, 30, 45, 60, 90, 120, 150, 180, 210, and 240 minutes relative to the peptide injection.
Peptide Injections
Human GIP(1-42) was synthesized by PolyPeptide Group (Strasbourg, France), and human GLP-2(1-33) was synthesized by Bachem (Bubendorf, Switzerland). Both had a purity above 97%. Peptides were dissolved in a 50 nM sodium hydrogen carbonate buffer, pH 8.5, containing in addition 0.5% human serum albumin and NaCl 9 mg/mL. Solutions were prepared by the Capital Region Pharmacy (Herlev, Denmark) to a final concentration of 100 µg/mL for GIP and 400 µg/mL for GLP-2. Vials were stored at −20 °C. Placebo was saline (NaCl 9 mg/mL).
Laboratory Methods
Blood for plasma was collected into chilled EDTA tubes to which were added dipeptidyl peptidase-4 inhibitor (valine pyrrolidide, a gift from Novo Nordisk) to a final concentration of 0.01 mmol/L. Tubes were centrifuged for 10 minutes at 1200 × g at 4 °C. Blood for serum was collected into clot activator tubes and left for coagulation 30 minutes at room temperature before centrifugation 10 minutes at 1200 × g at 4 °C.
During test days, blood pressure and heart rate were monitored (Omron M6, Intelli Sense, Omron Healthcare Europe B.V., Hoofddorp, The Netherlands), and glucose concentrations were measured using an YSI model 2300D STAT plus analyzer (YSI Incorporated, Yellow Springs, OH, USA).
CTX, P1NP, and PTH were measured in serum samples using an automated immunoassay and chemiluminescence method on an IDS-iSYS Multi-Discipline Automated System (ImmunoDiagnosticSystems, Frankfurt, Germany). Limit of detection (LoD) for CTX is 0.034 ng/mL, while P1NP has a LoD of < 1.0 ng/mL and PTH has a LoD of 2.5 pg/mL, and all assays have a coefficient of variation of < 10%. Sclerostin was measured using the human Sclerostin HS EIA Kit (TECOmedical, Quidel Corporation, San Diego, CA, USA).
Insulin and C-peptide were measured by sandwich immunoassays using the chemiluminescence technology (Advia Centaur XP, Siemens). Plasma concentrations of GIP and GLP-2 were measured by in house radioimmunoassays after extraction at a final concentrations of 70% ethanol for GIP measurements and 75% ethanol for GLP-2 measurements. Intact GIP was measured with an antibody directed toward the N-terminal of intact GIP (code no. 98171; RRID:AB_2943621) (32). Human GIP (Bachem cat. no. H5645) was used as standard and 125I-labeled human GIP (Perkin Elmer, cat. no. Nex402) was used as tracer. Intact GLP-2 was measured using an antibody specific for the N-terminal of intact GLP-2 (code no. 92160; RRID:AB_2943622) with human GLP-2 as standard and 125I-labeled rat GLP-2 with an Asp33 to Tyr33 substitution as tracer (33). Sensitivity for all assays is below 5 pmol/L, and intra-assay coefficient of variation is below 10% (32, 33).
Statistical Analysis
Based on previous studies (11, 20), we calculated that a minimum of 8 participants would be necessary to detect a difference of 20% in CTX with a power of 90%, two-sided 5% significance level, and a SD of 13%. Results of CTX, P1NP, PTH, and sclerostin are expressed as percentage of baseline values (mean of t = −10 and t = −5 minutes). We performed a mixed model analysis with Geisser–Greenhouse correction followed by Holm–Sidak multiple comparisons tests with interventions compared to placebo. Baseline values of CTX, P1NP, PTH, sclerostin, insulin, C-peptide, glucose, heart rate, and blood pressure were compared between test days by repeated measures one-way ANOVA with Geisser–Greenhouse correction and Holm–Sidak multiple comparisons test. All analyses were performed using GraphPad Prism version 9 for Windows (GraphPad Software, San Diego, CA, USA, www.graphpad.com). Results are presented as mean ± SEM. Characteristics of study participants are presented as median and range.
Results
Study Participants
Ten nonsmoking Caucasian men with only metformin-treated T2D were included. Age was 60.5 (49.0-68.0) years, body mass index was 28.2 (23.6-39.5) kg/m2, and hemoglobin A1c was 58 (45-69) mmol/mol. Glucagon-stimulated C-peptide was 1820 (1000-2280) pmol/L. Five participants were receiving pharmacological treatment for hypertension, 7 were receiving statin treatment for hypercholesterolemia, 1 was treated for hypothyroidism, and 1 received vitamin D supplement (10 µg/day). For further characteristics of study participants, see Table 1.
Table 1.
Characteristics of study participants
| (n = 10) | Median | Range |
|---|---|---|
| Age (years) | 60.5 | 49.0-68.0 |
| Height (m) | 1.78 | 1.66-1.85 |
| Weight (kg) | 83.7 | 77.0-129 |
| BMI (kg/m2) | 28.2 | 23.6-39.5 |
| Systolic blood pressure (mmHg) | 151 | 119-161 |
| Diastolic blood pressure (mmHg) | 88.0 | 72.0-104 |
| Heart rate (bpm) | 68 | 43-83 |
| Hemoglobin (mmol/L) | 8.70 | 7.20-10.7 |
| HbA1c (mmol/mol) | 58 | 45-69 |
| Duration of T2D (years) | 6.0 | 0.50-21 |
| Stimulated C-peptide (pmol/L)a | 1820 | 1000-2280 |
| eGFR (mL/min/1.73m2) | > 90 | 72 – > 90 |
| Calcium total (mmol/L) | 2.32 | 2.15-2.56 |
| PTH intact (pg/mL) | 42.4 | 21.7-54.7 |
| 25-OH-vitamin D (nmol/L) | 66 | 31-86 |
| Fat %b | 27.6 | 21.6-37.5 |
| BMD t score spineb | −0.50 | −2.7-3.1 |
| BMD t score hipb | 0.2 | −1.7-2.3 |
| BMD totalb | 0.5 | −1.1-2.5 |
Abbreviations: BMI, body mass index; BMD, bone mineral density; eGFR, estimated glomerular filtration rate; HbA1C, hemoglobin A1C; T2D, type 2 diabetes.
a Stimulated C-peptide was determined in a glucagon test at a screening visit.
b Results from dual-energy X-ray absorptiometry scan.
GIP and GLP-2 Concentrations
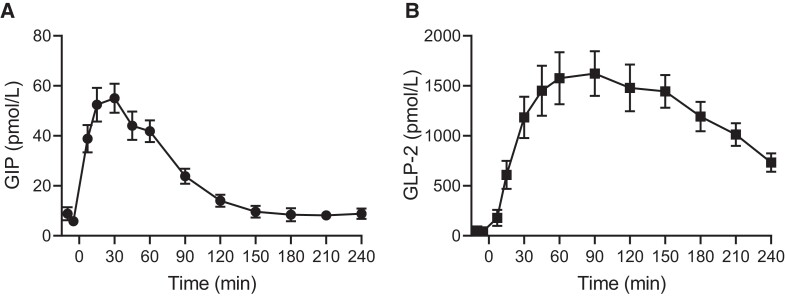
After subcutaneous injection of 200 µg GIP (t = 0 minutes), intact GIP in plasma rose from a basal level of 7.3 ± 1.4 to a peak of 55 ± 5.8 pmol/L at t = 30 minutes and returned to basal level after about 150 minutes (Fig. 1A). After subcutaneous injection of 800 µg GLP-2 (t = 0 minutes), intact GLP-2 rose from 48.3 ± 16.9 to reach a peak in plasma levels of 1623 ± 224 pmol/L at t = 90 minutes and was at 240 minutes still increased over basal levels (Fig. 1B).
Figure 1.
Plasma concentrations of GIP and GLP-2. Plasma concentrations of intact GIP (A) and intact GLP-2 (B) from t = −10 to t = 240 minutes during test days with subcutaneous injection of 200 µg GIP (A) and 800 µg GLP-2 (B), respectively. Data are shown as mean ± SEM.
Abbreviations: GIP, glucose-dependent insulinotropic polypeptide; GLP-2, glucagon-like peptide-2.
CTX, P1NP, PTH, and Sclerostin
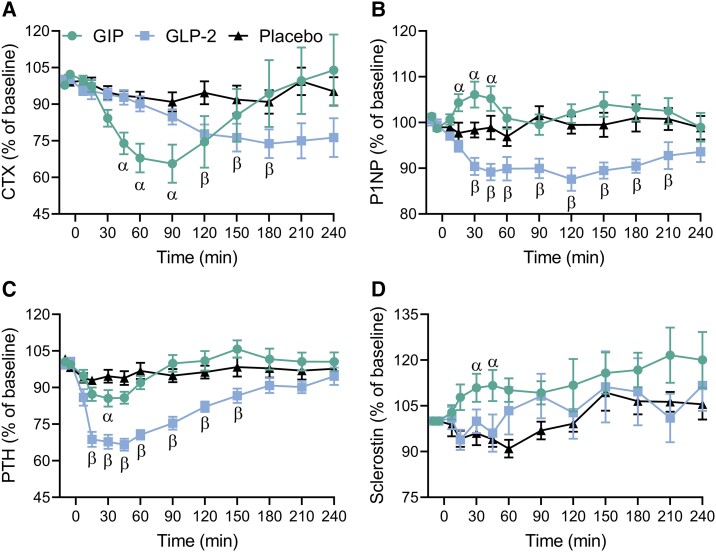
At baseline, levels of the bone resorption marker CTX were comparable between test days (Table 2). Both GIP and GLP-2 suppressed CTX compared with placebo (Fig. 2A). GIP rapidly decreased CTX whereas GLP-2 decreased it more slowly. Thus, GIP significantly suppressed CTX in the period from t = 45 minutes to t = 90 minutes, with a maximal suppression of CTX to 66 ± 7.8% of baseline levels at t = 90 minutes compared with 91 ± 4.0% of baseline levels after placebo (P = .001). GLP-2 significantly suppressed CTX in the period from t = 120 minutes to t = 180 minutes, with maximal suppression of CTX to 74 ± 5.9% of baseline levels at t = 180 minutes compared with 91 ± 4.8% of baseline levels after placebo (P = .01) (Fig. 2A).
Table 2.
Baseline levels on test days. Baseline levels (mean of t = −10 and t = −5 minutes) of CTX, P1NP, PTH, sclerostin, insulin, C-peptide, glucose, heart rate, systolic and diastolic blood pressure
| Placebo | GIP | GLP-2 | P-value of rm one-way ANOVA | |
|---|---|---|---|---|
| CTX (ng/mL) | 0.19 ± 0.096 | 0.19 ± 0.097 | 0.19 ± 0.10 | 0.90 |
| P1NP (ng/mL) | 37 ± 3.4 | 36 ± 3.2 | 37 ± 3.2 | 0.13 |
| PTH (pg/mL) | 41 ± 4.2 | 37 ± 2.7 | 35 ± 2.8 | 0.23 |
| Sclerostin (ng/mL) | 0.78 ± 0.088 | 0.79 ± 0.10 | 0.77 ± 0.11 | 0.94 |
| Insulin (pmol/L) | 81 ± 7.4 | 79 ± 9.7 | 78 ± 10.2 | 0.86 |
| C-peptide (pmol/L) | 670 ± 39 | 637 ± 46 | 624 ± 42 | 0.06 |
| Glucose (mmol/L) | 8.5 ± 0.36 | 8.7 ± 0.37 | 8.4 ± 0.35 | 0.67 |
| Heart rate (bmp) | 63 ± 3.6 | 65 ± 3.5 | 66 ± 4.5 | 0.38 |
| Systolic blood pressure (mmHg) | 140 ± 4.80 | 136 ± 5.73 | 139 ± 5.87 | 0.54 |
| Diastolic blood pressure (mmHg) | 85.9 ± 2.41 | 83.0 ± 2.49 | 84.5 ± 3.01 | 0.25 |
Abbreviations: CTX, C-terminal telopeptide; GIP, glucose-dependent insulinotropic polypeptide; GLP-2, glucagon-like peptide-2; P1NP, procollagen type 1 N-terminal propeptide; rm, repeated measures.
Data are shown as mean ± SEM. Differences between groups are analyzed by rm one-way ANOVA with Geisser–Greenhouse correction and Holm–Sidak multiple comparison tests between all test days.
Figure 2.
Serum concentrations of CTX, P1NP, PTH, and sclerostin. CTX (A), P1NP (B), PTH (C), and sclerostin (D) presented as percentage of baseline (mean of t = −10 and t = −5 minutes) on test days with subcutaneous injection of GIP (circle), GLP-2 (square), or placebo (triangle). Data are shown as mean ± SEM. Data were analyzed by a mixed-effects model for the period from t = −10 to t = 240 minutes with Geisser–Greenhouse correction and Holm–Sidak multiple comparison tests with interventions compared to placebo. Symbols indicate a statistically significant difference (P < .05) between placebo and GIP (α) and between placebo and GLP-2 (β).
Abbreviations: CTX, C-terminal telopeptide; GIP, glucose-dependent insulinotropic polypeptide; GLP-2, glucagon-like peptide-2; P1NP, procollagen type 1 N-terminal propeptide.
Baseline levels of the bone formation marker P1NP were comparable between test days (Table 2). Compared with placebo, P1NP was significantly increased by GIP from t = 15 minutes to t = 45 minutes, with a maximal increase in P1NP to 106 ± 2.8% of baseline levels reached at t = 30 minutes compared with 98 ± 1.7% of baseline levels after placebo (P = .01) (Fig. 2B). GLP-2 significantly decreased P1NP from t = 30 minutes to t = 210 minutes, with P1NP reaching a minimum of 87.6 ± 2.5% of baseline levels at t = 120 minutes compared with 99.5 ± 1.9% of baseline levels after placebo (P = .01) (Fig. 2B).
At baseline, levels of PTH were comparable between test days (Table 2). Both GIP and GLP-2 reduced PTH compared with placebo (Fig. 2C). However, GIP resulted in a significantly decreased PTH level only at t = 30 minutes [86 ± 3.4% of baseline levels compared with 95 ± 2.6% after placebo (P = .03)]. GLP-2 resulted in decreased PTH levels from t = 15 minutes to t = 150 minutes, with PTH reaching a minimum of 67 ± 2.5% of baseline levels at t = 45 minutes compared with 94 ± 2.8% of baseline levels after placebo (P < .0001) (Fig. 2C).
Sclerostin levels at baseline were comparable between test days (Table 2). The sclerostin level was significantly increased by GIP at t = 30 and t = 45 minutes, with a maximal increase to 112 ± 5.12% of baseline levels at t = 45 minutes, which was significantly higher compared with placebo (P = .04) (Fig. 2D). Neither GLP-2 nor placebo affected sclerostin levels.
Other Measurements
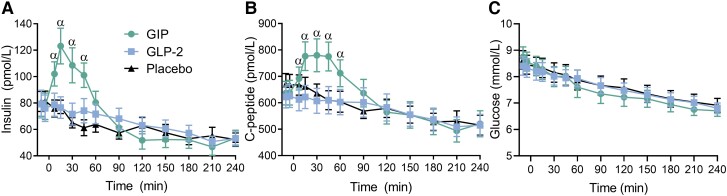
Baseline levels of insulin, C-peptide, and glucose are given in Table 2. After subcutaneous injection of placebo, the levels of insulin, C-peptide, and glucose declined during the test period (Fig. 3A and 3C). Insulin and C-peptide levels increased during the first hour after GIP injection but were comparable to placebo after GLP-2 (Fig. 3A and 3B). Glucose levels were comparable to placebo after subcutaneous injection of GIP or GLP-2 (Fig. 3C).
Figure 3.
Serum concentrations of insulin, C-peptide, and glucose. Insulin (A), C-peptide (B), and glucose (C) presented as absolute values during test days with subcutaneous injection of GIP (circle), GLP-2 (square), or placebo (triangle). Data are shown as mean ± SEM. Data were analyzed using a mixed-effects model for the period t = −10 to t = 240 minutes with Geisser–Greenhouse correction and Holm–Sidak multiple comparison tests with interventions compared to placebo. Symbols indicate a significant difference (P < .05) between placebo and GIP (α).
Abbreviations: GIP, glucose-dependent insulinotropic polypeptide; GLP-2, glucagon-like peptide-2.
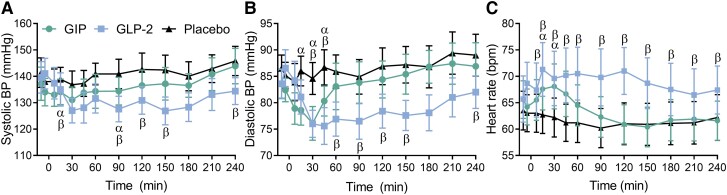
Heart rate and systolic and diastolic blood pressure were comparable at baseline (Table 2). Blood pressure, especially diastolic blood pressure, decreased and heart rate increased after both GIP and GLP-2 with the effect lasting less than 1 hour after the GIP injection and longer after the GLP-2 injection (Fig. 4A-C).
Figure 4.
Blood pressure and heart rate. Systolic blood pressure (A), diastolic blood pressure (B), and heart rate (C) presented as absolute values during test days with subcutaneous injection of GIP (circle), GLP-2 (square), or placebo (triangle). Data are shown as mean ± SEM. Data were analyzed using a mixed-effects model for the period t = −10 to t = 240 minutes with Geisser–Greenhouse correction and Holm–Sidak multiple comparison tests with interventions compared to placebo. Symbols indicate significant differences (P < .05) between placebo and GIP (α), and between placebo and GLP-2 (β).
Abbreviations: GIP, glucose-dependent insulinotropic polypeptide; GLP-2, glucagon-like peptide-2.
Discussion
GIP and GLP-2 are known to regulate bone turnover in healthy individuals (11, 12, 20, 21, 34). In this study, we found that the acute effects of exogenous GIP and GLP-2 on bone turnover are also present in individuals with T2D.
GIP and GLP-2 Have Preserved Bone Effects in T2D
We found that a subcutaneous bolus injections of GIP as well as GLP-2 reduced the bone resorption marker CTX in individuals with T2D. In addition, GIP acutely increased the bone formation marker P1NP. Our findings demonstrate that subcutaneous bolus injection of GIP and GLP-2 in individuals with T2D elicit the same characteristic sequence of effects as in healthy individuals without diabetes with a rapid effect after GIP injection and a more slowly developing suppression after GLP-2 injection (20, 21). A continuous 90-minute GIP infusion has previously been demonstrated to reduce CTX in T2D (35). However, the increase in GIP plasma levels with the current protocol closely mimic meal-induced excursions, suggesting that meal-stimulated endogenous GIP would have a similar effect. Furthermore, an infusion of a selective GIPR antagonist (GIP(3-30)NH2) was found to inhibit the postprandial reduction in CTX by ∼ 50% in T2D (36) [corresponding to previous findings in healthy individuals (24)]. Thus, the severe reduction in the insulinotropic effect of GIP observed in individuals with T2D (15) does not seem to be coupled to a corresponding reduction in the bone effects of GIP. In the present study there was an early small effect of the GIP injection on insulin and C-peptide levels, which was without effect on plasma glucose levels (14, 15). The insulin response corresponds to the small “early” insulin response generally seen in patients upon GIP infusions, whereas an effect on “late phase” insulin secretion is typically completely lost (15). The inhibitory effect of GIP on CTX has been found to be independent of endogenous insulin since CTX is also inhibited in individuals with type 1 diabetes (37). In a recent study we showed more pronounced decreases in CTX after oral glucose compared to isoglycemic intravenous glucose infusion both in healthy individuals and in C-peptide-negative individuals with type 1 diabetes, suggesting that suppression of bone resorption after nutrient intake may at least partly occur via a gut-bone axis working independently of changes in insulin and glucose levels (38). Regarding GLP-2, the present study is, to our knowledge, the first to investigate the effects of exogenous GLP-2 on bone turnover in patients with T2D. GLP-2R antagonist studies revealing the contribution of endogenous GLP-2 to postprandial bone turnover regulation in healthy individuals as well as in individuals with T2D have not yet been conducted.
Low Bone Turnover in T2D
We found that baseline (fasting) levels of CTX were markedly lower in our study participants of individuals with T2D (0.19 ng/mL) compared with our previous observations in healthy young men (0.73-0.89 ng/mL) (20, 21). Also, baseline levels of P1NP were lower in the individuals with T2D (∼ 37 ng/mL) compared with observations in healthy young men (70-86 ng/mL) (20, 21). Many factors may influence the level of bone turnover markers including age, sex, and morbidities. In agreement with our findings, the bone turnover markers are generally found to be decreased in T2D (36, 39-41). It has also been reported that the postprandial suppression in CTX is attenuated in individuals with T2D (5, 7) and that these patients lack the normal glucose-stimulated reduction in CTX (35), whereas others report a normal postprandial suppression of CTX (36). Interestingly, despite low basal levels of CTX and P1NP, we found that both exogenous GIP and GLP-2 were able to induce acute changes in CTX and P1NP levels that were very similar to our previous observations in healthy participants (in percentage of baseline) (20, 21). However, since we did not include a control group, we cannot exclude that the effects of GIP and GLP-2 on bone turnover markers may be different in individuals with T2D compared with matched healthy controls. But overall, the effects of exogenous GIP and GLP-2 on CTX and P1NP seem to be preserved in T2D.
In people with T2D, it has been reported that the low bone turnover is accompanied by an increased sclerostin level (42). Sclerostin is secreted from osteocytes and is a negative regulator of the Wnt pathway resulting in anti-anabolic bone effects. In the present study, the lack of a healthy control group makes it difficult to draw any conclusions from the sclerostin data obtained.
GIP and GLP-2 Decrease Blood Pressure and Increase Heart Rate in T2D
In the present study in individuals with T2D, GIP and GLP-2 decreased blood pressure and increased heart rate similar to what has previously been observed in healthy individuals (20, 34). This might be explained by a GIP- and GLP-2-induced redistribution of blood flow eg, to the intestines and to adipose tissue that in turn decreases blood pressure and results in a compensatory increase in heart rate (43, 44).
In Individuals With T2D, GLP-2 Reduces PTH, Whereas the GIP-induced Reduction in PTH is Diminished
In healthy participants, both GIP and GLP-2 have been found to acutely reduce fasting PTH levels to ∼ 60% to 70% of baseline with GIP having a short-lasting effect of less than 1 hour and GLP-2 having a more prolonged effect lasting for hours (20, 21). Interestingly, in the present study in individuals with T2D, GLP-2 induced a marked reduction in PTH (nadir ∼65% of baseline), whereas the GIP-induced suppression of PTH was smaller (nadir ∼85% of baseline). In agreement with our findings, another study also reports that GIP only modestly suppresses PTH in individuals with T2D (35). It has also been found that during an oral glucose tolerance test, PTH secretion is less pronounced in individuals with type 1 diabetes as compared to in healthy individuals, and the insulin levels correlate inversely with PTH levels suggesting that insulin may be involved in the acute regulation of PTH secretion (45). So far, we are unable to explain the minor decrease in PTH after GIP injection in T2D, but the insulin sensitivity on the parathyroid chief cells may be decreased. However, the acute decrease in PTH after GIP injection does not seem to be important for the GIP-mediated effects on bone turnover since GIP has also been found to decrease bone resorption in patients with hypoparathyroidism (46). In contrast, the GLP-2 effect is lost in patients with hypoparathyroidism (46). Thus, the underlying mechanisms of GIP and GLP-2 are probably separate with GIP inducing its bone effects independently of changes in PTH, whereas the effect of GLP-2 may involve changes in PTH.
T2D and Osteoporosis
Individuals with T2D have an increased risk of bone fractures compared to healthy controls despite having a normal to increased BMD (1-4). Several factors may influence bone metabolism and the risk of fractures in T2D, but the exact underlying mechanisms are unclear. Suggested factors include antidiabetic medication (47-49), diabetes-related comorbidities that may increase fall tendency (50), as well as age and time with T2D (50, 51). Since T2D is also associated with low bone turnover (40) and alterations in the microarchitecture of the bone tissue (40, 52), it has been suggested that osteocyte dysfunction with increased levels of sclerostin (49) or altered function of osteoblasts and osteoclasts due to accumulation of advanced glycation end products (53) could play a role. Finally, disturbances in the gut-bone axis have been proposed as an explanatory factor (5-9). Although studies have found that existing antiosteoporotic agents increase BMD and reduce fracture risk in T2D, the state of low bone turnover in T2D has raised concerns regarding the use of antiresorptive agents that result in long-term reductions in both CTX and P1NP (54).
Our findings show that the acute effects of both exogenous GIP and GLP-2 on bone turnover are preserved in individuals with T2D. Therefore, GIP and/or GLP-2 that acutely reduce CTX and have a more neutral effect on P1NP could be a potential novel treatment of T2D-associated osteoporosis. Future studies include testing the effects of repeated dosing/chronic treatment with GIP and/or GLP-2 on bone turnover markers, bone tissue, and fracture risk in T2D.
Strengths and Limitations
A limitation of the present study is that we did not include a control group, which would clearly have supported the conclusions. However, we have already conducted studies in healthy participants (20, 21). Another limitation is that only men with T2D and no women were included, limiting the generalizability of our findings. However, a main strength of the study is the crossover design, where each participant served as his own control.
Subcutaneous injections of GIP and GLP-2 resulted in lower peak concentrations and the plasma exposure was extended compared with previous observations in healthy individuals (20). This may be explained by more subcutaneous fat and/or less blood flow to the injection site, which may affect the pharmacokinetic profile. However, despite the moderately different plasma profiles of GIP and GLP-2 in the present study as compared with observations in healthy individuals, the effects on bone turnover markers were very similar in timing (20).
Conclusion
In T2D, both GIP and GLP-2 injected subcutaneously reduce the bone resorption marker CTX. In addition, GIP increases the bone formation marker P1NP, whereas GLP-2 decreases it. Whether chronic GIP and/or GLP-2 treatments are associated with a decreased fracture risk in individuals with T2D is unexplored but could potentially be a novel treatment option for T2D-associated fracture risk.
Contributor Information
Kirsa Skov-Jeppesen, Department of Biomedical Sciences, University of Copenhagen, DK-2200 Copenhagen, Denmark; Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen, DK-2200 Copenhagen, Denmark.
Charlotte B Christiansen, Department of Biomedical Sciences, University of Copenhagen, DK-2200 Copenhagen, Denmark; Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen, DK-2200 Copenhagen, Denmark.
Laura S Hansen, Center for Clinical Metabolic Research, Gentofte Hospital, University of Copenhagen, DK-2900 Hellerup, Denmark.
Johanne A Windeløv, Department of Biomedical Sciences, University of Copenhagen, DK-2200 Copenhagen, Denmark; Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen, DK-2200 Copenhagen, Denmark.
Nora Hedbäck, Department of Endocrinology, Hvidovre University Hospital, DK-2650 Hvidovre, Denmark.
Lærke S Gasbjerg, Department of Biomedical Sciences, University of Copenhagen, DK-2200 Copenhagen, Denmark.
Morten Hindsø, Department of Endocrinology, Hvidovre University Hospital, DK-2650 Hvidovre, Denmark.
Maria S Svane, Department of Endocrinology, Hvidovre University Hospital, DK-2650 Hvidovre, Denmark.
Sten Madsbad, Department of Endocrinology, Hvidovre University Hospital, DK-2650 Hvidovre, Denmark.
Jens J Holst, Department of Biomedical Sciences, University of Copenhagen, DK-2200 Copenhagen, Denmark; Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen, DK-2200 Copenhagen, Denmark.
Mette M Rosenkilde, Department of Biomedical Sciences, University of Copenhagen, DK-2200 Copenhagen, Denmark.
Bolette Hartmann, Department of Biomedical Sciences, University of Copenhagen, DK-2200 Copenhagen, Denmark; Novo Nordisk Foundation Center for Basic Metabolic Research, University of Copenhagen, DK-2200 Copenhagen, Denmark.
Funding
This work was supported by Dagmar Marshalls Fond, number 500020 (K.S.) and Aase og Ejnar Danielsens Fond, number 19-10-0467 (K.S.) and by a research grant from the Danish Diabetes Academy, which is funded by the Novo Nordisk Foundation, grant number NNF17SA0031406 (C.B.C.).
Author Contributions
K.S., M.S.S., S.M., L.S.G., J.J.H., M.M.R., and B.H. conceptualized and designed the study. K.S., L.S.H., C.B.C., N.H., M.H., and M.S.S. recruited participants and performed the test days. K.S. and C.B.C. measured bone turnover markers. J.A.W. and B.H. measured plasma concentrations of GIP and GLP-2. K.S. drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors have provided approval of the final version to be published.
Disclosures
K.S., C.B.C., L.S.H., J.A.W., N.H., M.H., M.S.S., and S.M. have nothing to disclose. L.S.G. is a minority shareholder and cofounder of Antag Therapeutics. J.J.H. has within the last year (2022) served on scientific advisory panels and/or speaker for Novo Nordisk, Eli Lilly, Zealand Pharma. He has given lectures and received financial support for travel from Novo Nordisk, Novo Nordisk Pharma, Novo Nordisk Scandinavia AB, and Mayo Clinic. He has served as a consultant for Alphasights, Eli Lilly, Shouti/Structure TX, Zealand Pharma. He is currently consulting for GV Management L.L.C. He is cofounder of Antag Therapeutics and Bainan Biotech and sits on the board of directors of Antag Therapeutics and Bainan Biotech, which is unpaid. He is supported by grants from Arla Foods, ERC Advanced Grants, and the Novo Nordisk Foundation Center for Basic Metabolic Research Faculty of Health and Medical Sciences University of Copenhagen Denmark. He serves as an investigator for Boehringer Ingelheim and Scohia. M.M.R. is a minority shareholder and cofounder of Antag Therapeutics and Bainan Biotech and chair of the board of directors of Bainan Biotech and has served on scientific advisory panels for and/or has received speaker honoraria from AlphaSights. B.H. is a board member and cofounder of Bainan Biotech.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes-a meta-analysis. Osteoporos Int. 2007;18(4):427‐444. [DOI] [PubMed] [Google Scholar]
- 2. Schwartz AV, Vittinghoff E, Bauer DC, et al. Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA. 2011;305(21):2184‐2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schwartz AV, Sellmeyer DE, Ensrud KE, et al. Older women with diabetes have an increased risk of fracture: a prospective study. J Clin Endocrinol Metab. 2001;86(1):32‐38. [DOI] [PubMed] [Google Scholar]
- 4. Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol. 2007;166(5):495‐505. [DOI] [PubMed] [Google Scholar]
- 5. Lopes LS, Schwartz RP, Ferraz-de-Souza B, da Silva MER, Corrêa PHS, Nery M. The role of enteric hormone GLP-2 in the response of bone markers to a mixed meal in postmenopausal women with type 2 diabetes mellitus. Diabetol Metab Syndr. 2015;7(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sherk VD, Schauer I, Shah VN. Update on the acute effects of glucose, insulin, and incretins on bone turnover in vivo. Curr Osteoporos Rep. 2020;18(4):371‐377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chailurkit LO, Chanprasertyothin S, Rajatanavin R, Ongphiphadhanakul B. Reduced attenuation of bone resorption after oral glucose in type 2 diabetes. Clin Endocrinol (Oxf). 2008;68(6):858‐862. [DOI] [PubMed] [Google Scholar]
- 8. Hygum K, Starup-Linde J, Langdahl BL. Diabetes and bone. Osteoporos Sarcopenia. 2019;5(2):29‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mabilleau G, Gobron B, Bouvard B, Chappard D. Incretin-based therapy for the treatment of bone fragility in diabetes mellitus. Peptides. 2018;100:108‐113. [DOI] [PubMed] [Google Scholar]
- 10. Clowes JA, Allen HC, Prentis DM, Eastell R, Blumsohn A. Octreotide abolishes the acute decrease in bone turnover in response to oral glucose. J Clin Endocrinol Metab. 2003;88(10):4867‐4873. [DOI] [PubMed] [Google Scholar]
- 11. Henriksen DB, Alexandersen P, Bjarnason NH, et al. Role of gastrointestinal hormones in postprandial reduction of bone resorption. J Bone Miner Res. 2003;18(12):2180‐2189. [DOI] [PubMed] [Google Scholar]
- 12. Helsted MM, Gasbjerg LS, Lanng AR, et al. The role of endogenous GIP and GLP-1 in postprandial bone homeostasis. Bone. 2020;140:115553. [DOI] [PubMed] [Google Scholar]
- 13. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131‐2157. [DOI] [PubMed] [Google Scholar]
- 14. Holst JJ, Knop FK, Vilsbøll T, Krarup T, Madsbad S. Loss of incretin effect is a specific, important, and early characteristic of type 2 diabetes. Diabetes Care. 2011;34 Suppl 2(Supplement_2):S251‐S257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vilsboll T, Krarup T, Madsbad S, Holst JJ. Defective amplification of the late phase insulin response to glucose by GIP in obese type II diabetic patients. Diabetologia. 2002;45(8):1111‐1119. [DOI] [PubMed] [Google Scholar]
- 16. Bollag RJ, Zhong Q, Phillips P, et al. Osteoblast-derived cells express functional glucose-dependent insulinotropic peptide receptors. Endocrinology. 2000;141(3):1228‐1235. [DOI] [PubMed] [Google Scholar]
- 17. Zhong Q, Itokawa T, Sridhar S, et al. Effects of glucose-dependent insulinotropic peptide on osteoclast function. Am J Physiol Endocrinol Metab. 2007;292(2):E543‐E548. [DOI] [PubMed] [Google Scholar]
- 18. Pacheco-Pantoja EL, Ranganath LR, Gallagher JA, Wilson PJ, Fraser WD. Receptors and effects of gut hormones in three osteoblastic cell lines. BMC Physiol. 2011;11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nissen A, Christensen M, Knop FK., Vilsbøll T, Holst JJ, Hartmann B. Glucose-dependent insulinotropic polypeptide inhibits bone resorption in humans. J Clin Endocrinol Metab. 2014;99(11):E2325‐E2329. [DOI] [PubMed] [Google Scholar]
- 20. Skov-Jeppesen K, Svane MS, Martinussen C, et al. GLP-2 and GIP exert separate effects on bone turnover: a randomized, placebo-controlled, crossover study in healthy young men. Bone. 2019;125:178‐185. [DOI] [PubMed] [Google Scholar]
- 21. Gabe MBN, Skov-Jeppesen K, Gasbjerg LS, et al. GIP and GLP-2 together improve bone turnover in humans supporting GIPR-GLP-2R co-agonists as future osteoporosis treatment. Pharmacol Res. 2022;176:106058. [DOI] [PubMed] [Google Scholar]
- 22. Vasikaran S, Cooper C, Eastell R, et al. International osteoporosis foundation and international federation of clinical chemistry and laboratory medicine position on bone marker standards in osteoporosis. Clin Chem Lab Med. 2011;49(8):1271‐1274. [DOI] [PubMed] [Google Scholar]
- 23. Vasikaran S, Eastell R, Bruyère O, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22(2):391‐420. [DOI] [PubMed] [Google Scholar]
- 24. Gasbjerg LS, Hartmann B, Christensen MB, et al. GIP’s effect on bone metabolism is reduced by the selective GIP receptor antagonist GIP(3-30)NH2. Bone. 2020;130:115079. [DOI] [PubMed] [Google Scholar]
- 25. Torekov SS, Harsløf T, Rejnmark L, et al. A functional amino acid substitution in the glucose-dependent insulinotropic polypeptide receptor (GIPR) gene is associated with lower bone mineral density and increased fracture risk. J Clin Endocrinol Metab. 2014;99(4):E729‐E733. [DOI] [PubMed] [Google Scholar]
- 26. Estall JL, Drucker DJ. Glucagon-like peptide-2. Annu Rev Nutr. 2006;26(1):391‐411. [DOI] [PubMed] [Google Scholar]
- 27. Henriksen DB, Alexandersen P, Byrjalsen I, et al. Reduction of nocturnal rise in bone resorption by subcutaneous GLP-2. Bone. 2004;34(1):140‐147. [DOI] [PubMed] [Google Scholar]
- 28. Henriksen DB, Alexandersen P, Hartmann B, et al. Disassociation of bone resorption and formation by GLP-2: a 14-day study in healthy postmenopausal women. Bone. 2007;40(3):723‐729. [DOI] [PubMed] [Google Scholar]
- 29. Henriksen DB, Alexandersen P, Hartmann B, et al. Four-month treatment with GLP-2 significantly increases hip BMD: a randomized, placebo-controlled, dose-ranging study in postmenopausal women with low BMD. Bone. 2009;45(5):833‐842. [DOI] [PubMed] [Google Scholar]
- 30. Askov-Hansen C, Jeppesen PB, Lund P, Hartmann B, Holst JJ, Henriksen DB. Effect of glucagon-like peptide-2 exposure on bone resorption: effectiveness of high concentration versus prolonged exposure. Regul Pept. 2013;181:4‐8. [DOI] [PubMed] [Google Scholar]
- 31. Scheen AJ, Castillo MJ, Lefèbvre PJ. Assessment of residual insulin secretion in diabetic patients using the intravenous glucagon stimulatory test: methodological aspects and clinical applications. Diabetes Metab. 1996;22(6):397‐406. [PubMed] [Google Scholar]
- 32. Deacon CF, Nauck MA, Meier J, Hücking K, Holst JJ. Degradation of endogenous and exogenous gastric inhibitory polypeptide in healthy and in type 2 diabetic subjects as revealed using a new assay for the intact peptide. J Clin Endocrinol Metab. 2000;85(10):3575‐3581. [DOI] [PubMed] [Google Scholar]
- 33. Hartmann B, Johnsen AH, Ørskov C, Adelhorst K, Thim L, Holst JJ. Structure, measurement, and secretion of human glucagon-like peptide-2. Peptides. 2000;21(1):73‐80. [DOI] [PubMed] [Google Scholar]
- 34. Skov-Jeppesen K, Veedfald S, Madsbad S, Holst JJ, Rosenkilde MM, Hartmann B. Subcutaneous GIP and GLP-2 inhibit nightly bone resorption in postmenopausal women: a preliminary study. Bone. 2021;152:116065. [DOI] [PubMed] [Google Scholar]
- 35. Christensen MB, Lund AB, Jorgensen NR, Holst JJ, Vilsbøll T, Knop FK. Glucose-Dependent insulinotropic polypeptide (GIP) reduces bone resorption in patients with type 2 diabetes. J Endocr Soc. 2020;4(9):bvaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stensen S, Gasbjerg LS, Krogh LL, et al. Effects of endogenous GIP in patients with type 2 diabetes. Eur J Endocrinol. 2021;185:33‐45. [DOI] [PubMed] [Google Scholar]
- 37. Christensen MB, Lund A, Calanna S, et al. Glucose-Dependent insulinotropic polypeptide (GIP) inhibits bone resorption independently of insulin and glycemia. J Clin Endocrinol Metab. 2018;103(1):288‐294. [DOI] [PubMed] [Google Scholar]
- 38. Hartmann B, Longo M, Mathiesen DS, et al. Signs of a glucose and insulin-independent gut-bone axis and aberrant bone homeostasis in type 1 diabetes. J Clin Endocrinol Metab. 2023;109:e259‐e265. [DOI] [PubMed] [Google Scholar]
- 39. Purnamasari D, Puspitasari MD, Setiyohadi B, Nugroho P, Isbagio H. Low bone turnover in premenopausal women with type 2 diabetes mellitus as an early process of diabetes-associated bone alterations: a cross-sectional study. BMC Endocr Disord. 2017;17(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hygum K, Starup-Linde J, Harsløf T, Vestergaard P, Langdahl BL. MECHANISMS IN ENDOCRINOLOGY: diabetes mellitus, a state of low bone turnover—a systematic review and meta-analysis. Eur J Endocrinol. 2017;176(3):R137‐R157. [DOI] [PubMed] [Google Scholar]
- 41. Starup-Linde J, Vestergaard P. Biochemical bone turnover markers in diabetes mellitus—a systematic review. Bone. 2016;82:69‐78. [DOI] [PubMed] [Google Scholar]
- 42. García-Martín A, Rozas-Moreno P, Reyes-García R, et al. Circulating levels of sclerostin are increased in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012;97(1):234‐241. [DOI] [PubMed] [Google Scholar]
- 43. Asmar M, Asmar A, Simonsen L, et al. The gluco- and liporegulatory and vasodilatory effects of glucose-dependent insulinotropic polypeptide (GIP) are abolished by an antagonist of the human GIP receptor. Diabetes. 2017;66(9):2363‐2371. [DOI] [PubMed] [Google Scholar]
- 44. Bremholm L, Hornum M, Andersen UB, Holst JJ. The effect of glucagon-like peptide-2 on arterial blood flow and cardiac parameters. Regul Pept. 2010;159(1-3):67‐71. [DOI] [PubMed] [Google Scholar]
- 45. Sass MR, Wewer Albrechtsen NJ, Pedersen J, et al. Secretion of parathyroid hormone may be coupled to insulin secretion in humans. Endocr Connect. 2020;9(7):747‐754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Skov-Jeppesen K, Hepp N, Oeke J, et al. The antiresorptive effect of GIP, but not GLP-2, is preserved in patients with hypoparathyroidism- a randomized crossover study. J Bone Miner Res. 2021;36:1448‐1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vestergaard P, Rejnmark L, Mosekilde L. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia. 2005;48(7):1292‐1299. [DOI] [PubMed] [Google Scholar]
- 48. Ferrari SL, Abrahamsen B, Napoli N, et al. Diagnosis and management of bone fragility in diabetes: an emerging challenge. Osteoporos Int. 2018;29(12):2585‐2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Starup-Linde J, Hygum K, Langdahl BL. Skeletal fragility in type 2 diabetes Mellitus. Endocrinol Metab (Seoul). 2018;33(3):339‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ivers RQ, Cumming RG, Mitchell P, Peduto AJ. Diabetes and risk of fracture: the blue mountains eye study. Diabetes Care. 2001;24(7):1198‐1203. [DOI] [PubMed] [Google Scholar]
- 51. Leslie WD, Lix LM, Prior HJ, et al. Biphasic fracture risk in diabetes: a population-based study. Bone. 2007;40(6):1595‐1601. [DOI] [PubMed] [Google Scholar]
- 52. Osima M, Kral R, Borgen TT, et al. Women with type 2 diabetes mellitus have lower cortical porosity of the proximal femoral shaft using low-resolution CT than nondiabetic women, and increasing glucose is associated with reduced cortical porosity. Bone. 2017;97:252‐260. [DOI] [PubMed] [Google Scholar]
- 53. Kurra S, Siris E. Diabetes and bone health: the relationship between diabetes and osteoporosis-associated fractures. Diabetes Metab Res Rev. 2011;27(5):430‐435. [DOI] [PubMed] [Google Scholar]
- 54. Sheu A, Greenfield JR, White CP, Center JR. Assessment and treatment of osteoporosis and fractures in type 2 diabetes. Trends Endocrinol Metab. 2022;33(5):333‐344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.