Abstract
Glucocorticoids are widely prescribed as anti-inflammatory and immunosuppressive agents. This results in at least 1% of the population using chronic glucocorticoid therapy, being at risk for glucocorticoid-induced adrenal insufficiency. This risk is dependent on the dose, duration and potency of the glucocorticoid, route of administration, and individual susceptibility. Once glucocorticoid-induced adrenal insufficiency develops or is suspected, it necessitates careful education and management of affected patients. Tapering glucocorticoids can be challenging when symptoms of glucocorticoid withdrawal develop, which overlap with those of adrenal insufficiency. In general, tapering of glucocorticoids can be more rapidly within a supraphysiological range, followed by a slower taper when on physiological glucocorticoid dosing. The degree and persistence of HPA axis suppression after cessation of glucocorticoid therapy are dependent on overall exposure and recovery of adrenal function varies greatly amongst individuals. This first European Society of Endocrinology/Endocrine Society joint clinical practice guideline provides guidance on this clinically relevant condition to aid clinicians involved in the care of patients on chronic glucocorticoid therapy.
Keywords: adrenal insufficiency, glucocorticoids, steroids, adrenal crisis, substitution therapy, glucocorticoid withdrawal
Summary of Recommendations
The recommendations are worded as recommend (strong recommendation) and suggest (weak recommendation). The quality of evidence behind the recommendations is classified as very low (⊕○○○), low (⊕⊕○○), moderate (⊕⊕⊕○), and strong (⊕⊕⊕⊕). Recommendations that were based on good clinical practice and experience of the working group members are not formally graded but acknowledged in the guideline as “good clinical practice”. Recommendations that were neither based on evidence or good clinical practice, are not graded at all (also see “Methods” section).
-
1. General recommendations for glucocorticoid therapy of non-endocrine conditions and recommendations regarding patient education
R 1.1—We recommend that, in general, patients on, or tapering off glucocorticoids for non-endocrine conditions do not need to be evaluated by an endocrinology specialist.
R 1.2—We recommend that clinicians who implement treatment with glucocorticoids educate patients about various endocrine aspects of glucocorticoid therapy. (Good clinical practice)
R 1.3—We recommend that patients on glucocorticoid therapy have access to current up-to-date and appropriate information about different endocrine aspects of glucocorticoid therapy. (Good clinical practice)
-
2. Recommendations regarding taper of systemic glucocorticoid therapy for non-endocrine conditions, diagnosis and approach to glucocorticoid-induced adrenal insufficiency, and glucocorticoid withdrawal syndrome
R 2.1—We suggest not to taper glucocorticoids in patients on short-term glucocorticoid therapy of <3-4 weeks, irrespective of the dose. In these cases, glucocorticoids can be stopped without testing due to low concern for HPA axis suppression. (⊕○○○)
R 2.2—Glucocorticoid taper for patients on long-term glucocorticoid therapy should only be attempted if the underlying disease for which glucocorticoids were prescribed is controlled, and glucocorticoids are no longer required. In these cases, glucocorticoids are tapered until approaching the physiologic daily dose equivalent is achieved (eg, 4-6 mg prednisone). (Good clinical practice)
R 2.3—We recommend consideration of glucocorticoid withdrawal syndrome that may occur during glucocorticoid taper. When glucocorticoid withdrawal syndrome is severe, glucocorticoid dose can be temporarily increased to the most recent one that was tolerated, and the duration of glucocorticoid taper could be increased. (Good clinical practice)
R 2.4—We recommend against routine testing for adrenal insufficiency in patients on supraphysiologic doses of glucocorticoids, or if they are still in need of glucocorticoid treatment for the underlying disease. (Good clinical practice)
R 2.5—We suggest that patients taking long-acting glucocorticoids (eg, dexamethasone or betamethasone) should be switched to shorter-acting glucocorticoids (eg, hydrocortisone or prednisone) when long-acting glucocorticoids are no longer needed. (⊕○○○)
-
R 2.6—We suggest that patients on a physiologic daily dose equivalent, and aiming to discontinue glucocorticoid therapy, either:
1. continue to gradually taper the glucocorticoid dose, while being monitored clinically for signs and symptoms of adrenal insufficiency, or
2. be tested with a morning serum cortisol. (⊕○○○)
-
R 2.7—If confirmation of recovery of the HPA axis is desired, we recommend morning serum cortisol as the first test. The value of morning serum cortisol should be considered as a continuum, with higher values more indicative of HPA axis recovery. (⊕○○○)
As a guide:
1. we suggest that the test indicates recovery of the HPA axis if cortisol is >300 nmol/L or 10 μg/dL and glucocorticoids can be stopped safely;
2. we suggest that if the result is between 150 nmol/L or 5 μg/dL and 300 nmol/L or 10 μg/dL, the physiologic glucocorticoid dose should be continued, and the morning cortisol repeated after an appropriate time period (usually weeks to months);
3. we suggest that if the result is <150 nmol/L or 5 μg/dL, the physiologic glucocorticoid dose should be continued, and the morning cortisol repeated after a few months.
R 2.8—We suggest against routinely performing a dynamic test for diagnosing adrenal insufficiency in patients tapering or stopping glucocorticoid therapy. (⊕○○○)
-
R 2.9—We suggest awareness of possible glucocorticoid-induced adrenal insufficiency in patients:
1. with current or recent use of non-oral glucocorticoid formulations presenting with signs and symptoms indicative of adrenal insufficiency, or
2. using multiple glucocorticoid formulations simultaneously, or
3. using high-dose inhaled or topical glucocorticoids, or
4. using inhaled or topical glucocorticoids for >1 year, or
5. who received intra-articular glucocorticoid injections in the previous 2 months, or
6. receiving concomitant treatment with strong cytochrome P450 3A4 inhibitors.
R 2.10—We suggest that patients with current or previous glucocorticoid treatment presenting with signs and symptoms of exogenous Cushing syndrome are assumed to have glucocorticoid-induced adrenal insufficiency. (Good clinical practice)
R 2.11—We suggest that patients aiming to discontinue glucocorticoids, but without recovery of HPA axis in one year while on physiologic daily dose equivalent, should be evaluated by an endocrinology specialist. We suggest that patients on glucocorticoids and history of adrenal crisis should also be evaluated by an endocrinology specialist. (Good clinical practice)
R 2.12—We recommend against the use of fludrocortisone in patients with glucocorticoid-induced adrenal insufficiency.
-
3. Recommendations on diagnosis and therapy of adrenal crisis in patients with glucocorticoid-induced adrenal insufficiency
-
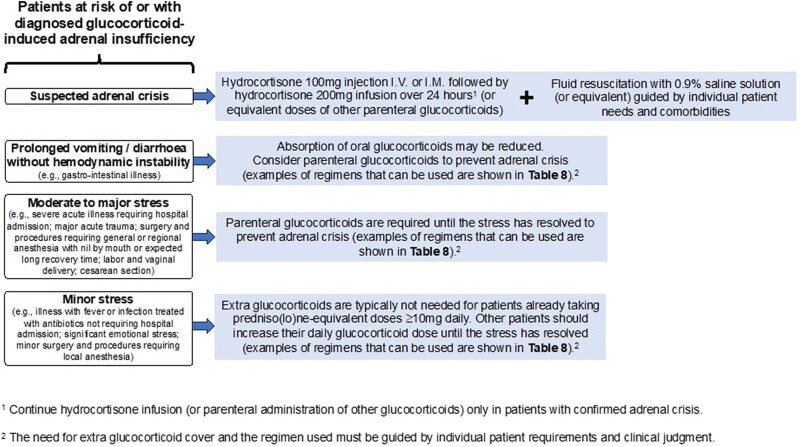
R 3.1—We recommend that patients with current or recent glucocorticoid use who did not undergo biochemical testing to rule out glucocorticoid-induced adrenal insufficiency should receive stress dose coverage when they are exposed to stress. (Good clinical practice)
R3.1A—Oral glucocorticoids should be used in case of minor stress and when there are no signs of hemodynamic instability or prolonged vomiting or diarrhea.
R3.1B—Parenteral glucocorticoids should be used in case of moderate to major stress, procedures under general or regional anesthesia, procedures requiring prolonged avoidance or inability of oral intake, or when there are signs of hemodynamic instability or prolonged vomiting or diarrhea.
R 3.2—We suggest that in patients with current or recent glucocorticoid use who did not undergo biochemical testing to rule out glucocorticoid-induced adrenal insufficiency and present with hemodynamic instability, vomiting, or diarrhea, the diagnosis of adrenal crisis should be considered irrespective of the glucocorticoid type, mode of administration, and dose; patients with suspected adrenal crisis should be treated with parenteral glucocorticoids and fluid resuscitation. (Good clinical practice)
-
Introduction
At least 1% of the population use chronic glucocorticoid therapy as anti-inflammatory and immunosuppressive agents (1-3). Virtually every discipline of medicine applies glucocorticoids via multiple modes of administration (including oral, inhaled, intranasal, intra-articular, topical, and intravenous), and frequently for prolonged duration. Suppression of the hypothalamic-pituitary-adrenal (HPA) axis is an inevitable effect of chronic exogenous glucocorticoid therapy and recovery of adrenal function varies greatly amongst individuals. Glucocorticoid-induced adrenal insufficiency necessitates careful education and management, and in the rare cases of adrenal crisis, prompt diagnosis and therapy (4). Considering the widespread use of glucocorticoids and the risk for glucocorticoid-induced adrenal insufficiency, the present clinical practice guideline provides guidance on this clinically relevant condition to aid the endocrinology specialists, as well as general practitioners and other specialists involved in the care of these patients.
Epidemiology of Glucocorticoid Therapy
Since their first description in the late 1940s (5), glucocorticoids have remained cornerstone agents in treating a wide array of medical conditions, ranging from autoimmune diseases, inflammatory disorders and severe allergic reactions to the prevention of transplant rejection and as antineoplastic agents for hematologic neoplasias. Earlier studies estimated that the prevalence of oral glucocorticoid use was approximately 1% in the United Kingdom and the United States adult populations (1-3). Based on a population of more than 65 000 patients registered with general practitioners in 1995 in the United Kingdom, continuous (> 3 months) oral glucocorticoids were prescribed for 0.5% of the total population and 1.4% of patients age 55 years or older (6). Additional data from the United Kingdom showed an increase of long-term glucocorticoid prescriptions between 1989 and 2008 from 0.59% to 0.79% of adult patients (1). In a population-based study from Denmark, the annual prevalence of systemic glucocorticoid prescription in primary care was found to be 3% with a remarkably high rate among the elderly of up to 10% during 1999-2015 (7).
Adverse Effects of Long-term Glucocorticoid Therapy
While glucocorticoids are highly effective agents in the treatment of autoimmune and inflammatory disorders, they can cause adverse reactions, particularly when administered at high doses and/or for a prolonged period. However, even relatively low dose (in the range of physiologic daily dose equivalent), long-term glucocorticoid therapy is linked to a range of adverse outcomes. For instance, a British cohort study involving 9387 patients with rheumatoid arthritis observed over a median of 8 years (with an average prednisone dosage of 5.8 mg/day for approximately 9.5 months) exhibited elevated rates of conditions such as diabetes, osteoporosis, fractures, hypertension, thrombotic events, gastrointestinal complications, and increased mortality, compared to those not treated with glucocorticoids (8). Of note, these observations may be confounded by the underlying disease severity. Additional studies have corroborated these findings, linking even low dose glucocorticoid use (prednisone 2.5-7.5 mg/day) to increased risks of cardiovascular disease (9), severe infections (10), hypertension (11), diabetes (12), osteoporosis and fractures (13, 14), and increased overall mortality with concurrent type 2 diabetes mellitus (15). While the absolute risk elevations were relatively modest, the implications are significant given the extensive patient population exposed to low dose glucocorticoids (15).
Pathophysiology of Glucocorticoid-induced Adrenal Insufficiency
Glucocorticoids suppress HPA axis activity by inhibiting the production of corticotropin-releasing hormone (CRH) by the hypothalamus and adrenocorticotropic hormone (ACTH) by the pituitary. Inhibition of CRH and ACTH induced by exogenous glucocorticoids is similar to the mechanisms involved in the physiologic cortisol negative feedback (16). Prolonged duration of supraphysiologic glucocorticoid therapy often leads to a reduction in the overall responsiveness of the anterior pituitary gland. In rodent models, glucocorticoids exert pro-apoptotic effects on the pituitary gland (17) and promote protein degradation as represented by Crooke's hyaline in corticotroph cells (18). This ultimately results in atrophy of the adrenal cortex. Conversely, following withdrawal of glucocorticoids, there is resurgence of ACTH stimulation of the adrenal cortex. In most instances, the adrenal cortex will recover and produce adequate levels of cortisol. Despite these adaptive responses, the time to full biochemical and clinical restitution of the HPA axis is highly variable.
Any glucocorticoid dose above the physiologic daily dose equivalent can potentially lead to suppression of the HPA axis. The degree and persistence of HPA axis suppression after cessation of glucocorticoid therapy are dependent on overall exposure, which, amongst other factors, is determined by potency of the glucocorticoid (Table 1), glucocorticoid dose, length of therapy, and individual susceptibility. Notably, any route of administration has the potential of HPA axis suppression, including oral, topical, inhaled, intra-nasal, intravenous and intra-articular administration.
Table 1.
Pharmacologic characteristics of commonly prescribed systemic glucocorticoids (19-23)
| Glucocorticoids | Approximate equivalent dosea | Glucocorticoid potency (relative to hydrocortisone)a, b | Plasma half-life (min)a, c | Biological half-life (hours)a | Therapeutic indications |
|---|---|---|---|---|---|
| Short-acting glucocorticoids with lower potency | |||||
| Hydrocortisone | 20 mg | 1.0 | 90-120 | 8-12 | Adrenal insufficiency replacement |
| Cortisone acetate | 25 mg | 0.8 | 80-120 | 8-12 | Adrenal insufficiency replacement |
| Deflazacort | 7.5 mg | 1.0 | 70-120 | Not defined | Duchenne muscular dystrophy |
| Intermediate-acting glucocorticoids with moderate potency | |||||
| Prednisone | 5 mg | 4.0 | 60 | 12-36 | Anti-inflammatory, immunosuppressant; Adrenal insufficiency replacement |
| Prednisolone | 5 mg | 4.0 | 115-200 | 12-36 | Anti-inflammatory, immunosuppressant; Adrenal insufficiency replacement |
| Triamcinolone | 4 mg | 5.0 | 30 | 12-36 | Anti-inflammatory, immunosuppressant |
| Methylprednisolone | 4 mg | 5.0 | 180 | 12-36 | Anti-inflammatory, immunosuppressant |
| Long-acting glucocorticoids with highest potency | |||||
| Dexamethasone | 0.5 mg | 30-60 | 200 | 36-72 | Anti-inflammatory, immunosuppressant; Usually reserved for short-term use in severe, acute conditions |
| Betamethasone | 0.5 mg | 25-40 | 300 | 36-72 | Anti-inflammatory, immunosuppressant; Usually reserved for short-term use in severe, acute conditions |
a These are estimates based on historically accepted conversion factors and should be seen as a guide only. There can be considerable variation depending on factors such as route of administration, the individual patient's metabolism and susceptibility.
b Glucocorticoid potency equivalences apply to oral and/or intravenous administration. Mineralocorticoid effects are not considered.
c Plasma half-life does not reflect the biological half-life.
With regards to glucocorticoid therapy, immunosuppressive and anti-inflammatory doses considerably exceed the equivalent of endogenous cortisol production and, therefore, invariably result in HPA axis suppression. While tapering glucocorticoids within the supraphysiologic dose range, patients can develop glucocorticoid withdrawal syndrome, which manifests with clinical features similar to those of adrenal insufficiency. However, symptoms due to adrenal insufficiency are much more likely to develop when overall total daily glucocorticoid dose is below physiologic levels, or levels required for an adequate stress response.
Epidemiology of Glucocorticoid-induced Adrenal Insufficiency and Associated Morbidity and Mortality
A meta-analysis of the risk of developing biochemical glucocorticoid-induced adrenal insufficiency stratified by glucocorticoid route of administration showed pooled percentages of 4.2% (95% CI 0.5-28.9) for nasal administration, 48.7% (95% CI 36.9-60.6) for oral use, and 52.2% (95% CI 40.5-63.6) for intra-articular administration (24). The risk also varied when stratified for the underlying disease and increased with higher dose (low dose 2.4% (95% CI 0.6-9.3) to high dose 21.5% (95% CI 12.0-35.5)) and longer treatment duration (1.4% (95% CI 0.3-7.4) (<28 days) to 27.4% (95% CI 17.7-39.8) (>1 year)) in patients with asthma. Since an estimated minimum of 1% of adult populations (United States and United Kingdom) use oral glucocorticoids at any given time (1-3), this would imply several million people are at risk of developing glucocorticoid-induced adrenal insufficiency in these countries alone.
It must be taken into consideration that in most of the studies the diagnosis of glucocorticoid-induced adrenal insufficiency was based on biochemical testing, whereas the clinical relevance of this biochemical glucocorticoid-induced adrenal insufficiency was not established. In the above-mentioned meta-analysis, ten of the 74 included studies also assessed symptoms of adrenal insufficiency (although not systematically scored) in a total of 521 patients (24). Of these 521 patients, 98 patients had biochemical evidence of adrenal insufficiency. Ten of them (10%) reported symptoms. However, 88 (90%) did not report any symptoms indicating that clinical symptoms are not specific and do not correlate well with biochemical findings.
A Danish self-controlled case series including 286 680 persons who discontinued prolonged (≥3 months) oral glucocorticoid treatment, assessed the presence of clinical consequences of glucocorticoid-induced adrenal insufficiency after glucocorticoid cessation (25). Comparing the discontinuation period with the reference period (the period before treatment started), increased incidence rate ratios of clinical indicators of adrenal insufficiency were found: 2.5 (95% CI 1.4-4.3) for hypotension, 1.7 (95% CI 1.6-1.9) for gastrointestinal symptoms, 2.2 (95% CI 0.7-7.3) for hypoglycemia, and 1.5 (95% CI 1.1-2.0) for hyponatremia.
Only a few studies report on the incidence of adrenal crisis in patients with glucocorticoid-induced adrenal insufficiency. In a United States survey reporting on self-perceived determinants of health in patients with adrenal insufficiency, a median of 0 (IQR 0-0.33) adrenal crises per person-year since diagnosis were reported in glucocorticoid-induced adrenal insufficiency, compared to 0.07 (IQR 0-0.25) in primary adrenal insufficiency and 0 (IQR 0-0.14) in secondary adrenal insufficiency (26). A Dutch study found an incidence rate of 15.1 (95% CI 11.0-19.9) per 100 person-years in 28 patients with glucocorticoid-induced adrenal insufficiency, compared to 5.2 (95% CI 4.3-6.3) in 111 patients with primary adrenal insufficiency and 3.6 (95% CI 3.1-4.1) in 319 patients with secondary adrenal insufficiency (27). These outcomes must be interpreted cautiously however because of the few subjects with glucocorticoid-induced adrenal insufficiency and possible selection bias. In this study, the presence of comorbidities (including neurologic, cardiac and malignant diseases) was the most important risk factor for developing adrenal crisis. Of note, in six patients with glucocorticoid-induced adrenal insufficiency, adrenal crisis was precipitated by a reduction in glucocorticoid dose. There were 20 deaths in the total cohort, but none was reported as related to adrenal crisis.
In the European Adrenal Insufficiency Registry which included 1233 patients with secondary adrenal insufficiency followed for 5 years, 18 deaths were reported (28). The Registry included various etiologies of adrenal insufficiency and the percentage of patients with their condition attributed to exogenous glucocorticoids could not be ascertained [personal communication with the author]. Only one of the 26 deaths was clearly attributed to an adrenal crisis and this death occurred in a patient with glucocorticoid-induced adrenal insufficiency [data retrieved after contacting the author] (28). A retrospective cohort study from the United Kingdom including 70 638 oral glucocorticoid users found a sharp increase in the incidence of mortality during the first 2 months after glucocorticoid cessation, which then rapidly decreased after the first 3 months. Whilst only 13 subjects had their cause of death recorded as adrenal insufficiency, the relationship with glucocorticoid cessation raises the suspicion of possible undiagnosed adrenal crises (29).
The use of supraphysiologic glucocorticoids (prednisone equivalent dose > 5 mg daily) has been associated with a higher risk of all-cause mortality (adjusted hazard ratio of 1.97 (95% CI 1.81-2.15) in rheumatoid arthritis patients (30), with increasing risk with higher current daily and cumulative doses (29, 31). This association was not observed with daily glucocorticoid doses below 5 mg prednisone equivalent (30, 32). Estimates from these studies have to be interpreted cautiously because of potential underlying confounding factors such as underlying disease and disease severity (30).
Definitions
We recognize that there is great inter-individual variation in responses to glucocorticoids, likely affecting the risk for glucocorticoid-induced adrenal insufficiency. Consequently, glucocorticoid exposure should be considered as a multidimensional risk factor, including dose and frequency, administration mode, duration of therapy, potency of glucocorticoid, and individual susceptibility. Glucocorticoid exposure via oral administration that poses risk for adrenal insufficiency, is expected to at least exceed both of the following thresholds:
Duration of glucocorticoid therapy to pose risk for adrenal insufficiency —3-4 weeks, or greater
Dose of glucocorticoid therapy to pose risk for adrenal insufficiency —any dose greater than daily hydrocortisone equivalent of 15-25 mg (4-6 mg prednisone or prednisolone, 3-5 mg methylprednisone, 0.25-0.5 mg dexamethasone)
The following defined terms will be used in the remainder of these guidelines:
Physiologic daily dose equivalent : Daily glucocorticoid dose equivalent to average daily cortisol production (15-25 mg hydrocortisone, 4-6 mg prednisone or prednisolone, 3-5 mg methylprednisone, 0.25-0.5 mg dexamethasone). Endogenous production of cortisol is estimated to be 9-10 mg/day. The above mentioned doses are based on an estimate of bioavailability
Supraphysiologic glucocorticoid therapy : Any dose greater than physiologic daily dose equivalent (see above)
Short-term glucocorticoid therapy : Any glucocorticoid therapy of less than 3-4 weeks duration
Long-term glucocorticoid therapy : Glucocorticoid therapy greater than 3-4 weeks duration with glucocorticoid doses greater than physiologic daily dose equivalent of hydrocortisone (15-25 mg hydrocortisone, 4-6 mg prednisone or prednisolone, 3-5 mg methylprednisone, 0.25-0.5 mg dexamethasone)
Glucocorticoid taper : Taper of glucocorticoid therapy dose, initially guided by the management of the underlying disease (= therapeutic taper), and later by the management of glucocorticoid withdrawal and adrenal insufficiency (= endocrine taper)
Glucocorticoid withdrawal syndrome: Symptoms experienced when lowering glucocorticoid dose within the supraphysiologic glucocorticoid dose range, that are not due to the underlying disease for which the glucocorticoids were initially prescribed for and per definition not due to untreated adrenal insufficiency, as the total glucocorticoid daily dose is still supraphysiologic
Glucocorticoid doses vary based on glucocorticoid agent and are defined as physiologic within the lower and upper ranges to illustrate the inter-individual differences. In the recommendations, prednisone and prednisolone are used interchangeably.
Methods
Guideline Working Group
This joint clinical guideline was initiated and developed on behalf of The European Society of Endocrinology (ESE) and The Endocrine Society (ES). The chairs of the working group, Felix Beuschlein (ESE) and Tobias Else (ES), were appointed by the ESE Clinical Committee and ES Clinical Guidelines Subcommittee, respectively. Olaf Dekkers served as the methodology lead, Christine Yedinak as Endocrine Nurses Society Representative and Alessandro Prete as ESE Young Endocrinologists and Scientists representative. The other members were suggested by the chairs and approved by the ESE Clinical Committee and ES Clinical Guidelines Subcommittee, including Irina Bancos, Stefanie Hahner, Oksana Hamidi, Eystein S. Husebye, Niki Karavitaki and Anand Vaidya. Leonie van Hulsteijn joined the guideline working group for methodology support. Prior to the process, all participants completed conflict of interest forms (Supplementary Table S1; see section on Supplementary Material). The process was approved by the ESE Executive Committee and ES Society Board of Directors.
There were several virtual working group meetings and one in-person meeting, and the working group communicated by email in between meetings.
Target Groups
This guideline was developed for health care professionals who see adult patients with long-term supraphysiologic glucocorticoid exposure and who seek guidance for glucocorticoid taper and evaluation of these patients” adrenal function. The guideline served as a source document for the preparation of a patient information leaflet and educational material published on the ESE and ES websites, to empower patients and glucocorticoid prescribing clinicians.
Aims
The overall purpose of this guideline is to provide clinicians with practical guidance on the evaluation of adrenal function of adult patients with long-term supraphysiologic glucocorticoid therapy and for supplementation therapy in case of glucocorticoid-induced adrenal insufficiency. In clinical practice, both the recommendations and the clinical judgment of treating physicians should be taken into account. Recommendations are not meant to replace clinical acumen and may need adaptation to local circumstances.
Summary of Methods Used for Guideline Development
The methods used for establishing the guideline have been described in detail previously (33, 34). In short, Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) was used as a methodological basis. The first step was to define the clinical questions (see below) followed by systematic literature searches. We estimated an average effect for specific outcomes where possible and rated the quality of the evidence behind the recommendations as very low (⊕○○○), low (⊕⊕○○), moderate (⊕⊕⊕○), or strong (⊕⊕⊕⊕). Not all recommendations were formally graded (see below).
Considered for the recommendations were the quality of the evidence, the balance of desirable and undesirable outcomes, and individual values and preferences (patient preferences, goals for health, costs, management inconvenience, feasibility of implementation) (33, 35). The recommendations are worded as “recommend” (strong recommendation) or “suggest” (weak recommendation). The meaning of a strong recommendation is that all reasonably informed persons (clinicians, policy makers and patients) would want the management in accordance with the recommendation, while for a weak recommendation, most persons would still act in accordance with the guideline, but a substantial number would not (36). Formal evidence syntheses were performed and graded only for recommendations addressing our initial clinical questions (see “Clinical questions, eligibility criteria, and definition of endpoints” section). Recommendations that were based on good clinical practice and experience of the working group members are not formally graded (37), but acknowledged in the guideline as “good clinical practice”. Recommendations that were neither based on evidence or good clinical practice, are not graded at all. Consensus was reached upon discussion; minority positions were considered in the rationale behind recommendations.
Review Process
A draft of the guideline was reviewed by four experts in the field (see “Acknowledgments” section) and was distributed to all ESE and ES members for commenting. All comments and suggestions were then discussed and implemented as thought appropriate by the guideline working group (Supplementary Table S9; see section on Supplementary Material).
Results of the Systematic Reviews
Clinical Questions, Eligibility Criteria, and Definition of Endpoints
At the start of the guideline process, the working group formulated clinical questions regarding evaluation of adrenal function and treatment of patients after long-term supraphysiologic glucocorticoid exposure. The clinical questions that formed the basis for the systematic reviews are summarized in Supplementary Table S2 (see section on Supplementary Material).
Eligible articles were required to present data on adult patients (≥18 years). Articles presenting data on glucocorticoid-induced adrenal insufficiency based on biochemical testing were included based on the use of the high-dose (250 μg) short ACTH (1-24)-test (also referred by brand names as Synacthen or cosyntropin test), since these tests are widely used in clinical practice. During this test, 250 μg of synthetic ACTH (ACTH (1-24), or another corticotropic agent), is administered intravenously. To determine adrenal response to synthetic ACTH, serum cortisol levels are measured 30 and 60 minutes after administration. In primary and secondary adrenal insufficiency, peak cortisol levels < 500 nmol/L (< 18.1 μg/dL), depending on assay, are indicative of adrenal insufficiency (38, 39). Since the population under study was assessed for glucocorticoid insufficiency, the definition of a positive test was based on cut-off values provided in the individual articles. For clinical question I (incidence and predictors of recovery of HPA axis function in patients with glucocorticoid-induced adrenal insufficiency), the number of persons with recovery of HPA axis at re-testing (numerator) and the total number of persons with glucocorticoid-induced adrenal insufficiency tested at baseline (denominator) were used to estimate the incidence of recovery.
We did not include case reports or case series, which are more prone to selection and publication bias; only studies reporting a population of ten or more patients were eligible. In case of multiple studies describing the same cohort, the study comprising the highest number of subjects was included. Eligible studies were restricted to languages familiar to the authors (English, French, German, Dutch and Spanish). Authors were contacted for clarification when reported data were not sufficient for accurate data extraction.
Description of Search and Selection of Literature
PubMed, MEDLINE, Embase, Web of Science, and Cochrane Library were searched with the help of a specialized librarian to identify potentially relevant studies. The literature searches for questions I-Ia, II and III were performed in January 2023, February 2023 and March 2023, respectively. Searches can be found in Supplementary Appendix S1 (see section on Supplementary material).
All studies obtained from the searches were entered into reference manager software (EndNote X20, Clarivate Analytics, Philadelphia, PA) and title and abstract were screened. Potentially relevant studies were retrieved for detailed assessment. References of included studies were assessed for additional relevant articles.
For question I and sub-question Ia (incidence and predictors of recovery of HPA axis function in patients with glucocorticoid-induced adrenal insufficiency), we used data from the study by Broersen et al (24). In this systematic review published in 2015, the risk of adrenal insufficiency following use of various types of glucocorticoids for several underlying diseases was reported. This systematic review included 17 publications in which patients had been retested for adrenal insufficiency. Given this existing review, an original search as described above was performed from February 2014 onwards, identifying 373 additional papers. After detailed assessment, three manuscripts were included reporting data on recovery of the HPA axis.
For clinical question II (optimal tapering scheme in patients no longer requiring chronic glucocorticoid treatment), 873 papers were identified, of which five were included. For clinical question III (diagnostic accuracy of morning cortisol vs 250 μg ACTH(1-24)-test), three of the 843 identified papers were included.
Summary and Interpretation of Evidence From the Systematic Reviews
Clinical Question I: What Is the Incidence of Recovery of HPA Axis Function in Patients With Glucocorticoid-induced Adrenal Insufficiency?
Broersen et al performed a meta-analysis on eleven out of seventeen studies re-testing patients for biochemical adrenal insufficiency for which results could be categorized in short-term (defined as less than 4 weeks) high-dose glucocorticoid therapy re-testing after 4 weeks (six studies), and long-term (>1 year) medium-dose glucocorticoid therapy re-testing after 6 months (five studies) (24). Included articles had to use a cutoff value for serum cortisol of ≤500 nmol/L (18 μg/dL) or higher (eg, ≤550 nmol/L or 19.8 μg/dL) or 11-deoxycortisol of ≤200 nmol/L (7.2 μg/dL) after the metyrapone test to diagnose adrenal insufficiency. Pooled analysis of studies in the first group (141 patients), demonstrated a decrease in adrenal insufficiency from 38.7% after cessation of glucocorticoid therapy to 14.9% after 4 weeks. Pooled analysis of studies in the second group (174 patients) indicated a decrease in adrenal insufficiency from 56.4% at baseline to 25.3% after 6 months.
Three additional studies assessing recovery of HPA axis function in a total of 202 patients with glucocorticoid-induced adrenal insufficiency were included based on the search from February 2014 onwards (40-42). The description of the GRADE evidence can be found in Supplementary Table S3 and details of included studies in Supplementary Table S4 (see section on Supplementary Material). The Menzies-Gow study assessed adrenal function in patients with severe eosinophilic asthma after achieving a stable predniso(lo)ne dosage of 5 mg/day for four weeks. In the studies by Baek and Leong, included patients displayed large clinical variability with respect to underlying disease and mean glucocorticoid treatment dose and duration before diagnosis of glucocorticoid-induced adrenal insufficiency were not described. Adrenal function was assessed using the 250 μg ACTH (1-24)-test in all studies. Except for the Menzies-Gow study, timing of re-testing was not standardized. In the study by Baek et al, in 58.8% of patients adrenal function recovered after a median of 16 months (40). In the study by Leong et al, 60.6% of patients showed recovered adrenal function, with a median recovery time of 24 months (41). In the study by Menzies-Gow et al, 10% of patients showed recovered adrenal function after 3 months (42). Although these data are based on a limited number of patients with a low quality of evidence (ie, certainty in these estimates) due to heterogeneity and a serious risk of bias, the data suggest that adrenal function can recover in a time frame from a few months to up to 4 years in some cases. It must be emphasized that the diagnosis of glucocorticoid-induced adrenal insufficiency in the studies by Baek and Leong was based on results of biochemical testing, while signs and symptoms of adrenal insufficiency were not reported. It is thus uncertain whether this biochemical glucocorticoid-induced adrenal insufficiency was of clinical relevance. The study by Menzies-Gow reported no adrenal crises, or (serious) adverse events related to adrenal insufficiency.
Studies assessing recovery of HPA axis function through measurement of morning cortisol or low-dose 1 µg ACTH (1-24)-test were not formally included in the systematic review (see “Clinical questions, eligibility criteria, and definition of endpoints’), but reported recovery incidence rates of 17% to 100% within a range of 4 days to 3 years (43-50). It is plausible that in studies reporting recovery at re-testing already after a couple of days, initial cortisol levels may have represented adrenal suppression due to remaining circulating long-acting exogenous glucocorticoids rather than true adrenal insufficiency.
Clinical Sub-question Ia: Which Clinical/Biochemical Parameters Predict Recovery of HPA Axis Function in Patients With Glucocorticoid-induced Adrenal Insufficiency?
Both studies included for clinical question I also assessed predictors of recovery of adrenal function (40, 41). In the study by Baek et al, patients recovering adrenal function had higher cortisol increments during the first ACTH (1-24)-test than patients without recovery (219 vs 99 nmol/L (10.3 vs 6.7 μg/dL), OR 1.58 per μg/dL increase in cortisol, 95%CI 1.02-2.46) when adjusting for confounders, basal cortisol concentration and basal ACTH levels (40). In the study by Leong et al, patients recovering adrenal function had higher ambulatory morning cortisol values in between retesting with ACTH (1-24)-test than patients not recovering (286 vs 186 nmol/L (7.9 vs 3.6 μg/dL), OR 1.02 per μg/dL increase in cortisol, 95%CI 1.01-1.04) (41). There were no studies assessing clinical parameters predicting HPA axis recovery.
Clinical Question II: What Is the Optimal Tapering Scheme in Patients no Longer Requiring Chronic Glucocorticoid Treatment for the Underlying Condition?
Four randomized-controlled trials were included (51-54), and one single-arm study (42). The GRADE table is shown in Supplementary Table S5, and details of the studies are shown in Supplementary Table S6 (see section on Supplementary Material). Three studies compared the effects of a tapering scheme of glucocorticoids vs placebo after short-term use of high dose glucocorticoids in a total of 135 patients with multiple sclerosis, asthma, or chronic obstructive pulmonary disease exacerbation (51, 53, 54). One study compared the effects of tapering vs continuing glucocorticoids after long-term use in patients with rheumatoid arthritis who achieved remission or low disease activity (52), so only data of the patient group tapering glucocorticoids (n = 131) were considered. The last study evaluated the effectiveness and safety of a rapid, individualized steroid-reduction algorithm after beralizumab initiation in 598 patients with severe, eosinophilic asthma (42). Although adrenal function was not the primary endpoint of included studies, Burmester et al predefined symptomatic adrenal insufficiency as one of their secondary outcomes, and Menzies-Gow et al as a safety outcome, and from the three other studies data on (serious) adverse events and hospital readmission were used as a proxy for symptomatic adrenal insufficiency/adrenal crisis. The data showed no symptomatic adrenal insufficiency and no clinical events related to potential adrenal insufficiency during follow-up in all five studies.
Although the total number of included patients is relatively small and there is heterogeneity due to various underlying diseases, results from the included studies suggest that it is often safe to stop glucocorticoids abruptly after short-term use of high dose glucocorticoids. After long-term use of glucocorticoids, more rapid tapering of glucocorticoids when on a supraphysiologic dosing, followed by a slower taper when on physiologic glucocorticoid dosing, appears to be a safe strategy (42, 52). There were no studies identified comparing different tapering schemes.
Clinical Question III: What Is the Diagnostic Accuracy of a Morning Cortisol Value vs 250 μg ACTH (1-24)-test in Diagnosing Glucocorticoid-induced Adrenal Insufficiency?
Three studies were included (55-57). The GRADE evidence table is shown in Supplementary Table S7, and details of the studies are shown in Supplementary Table S8 (see section on Supplementary material). All studies assessed the diagnostic performance of a morning serum cortisol value vs 250 µg ACTH (1-24)-test. Of note, in the studies of Sagar et al and Sbardella et al ACTH (1-24) was administered intramuscularly or intravenously, and results could not be stratified for intravenous ACTH (1-24) only. Both studies measured cortisol by immunoassay. In the study by Sagar et al, 100% of patients with morning cortisol < 100 nmol/L (< 3.6 μg/dL) failed ACTH (1-24)-test, while all patients with morning cortisol >350 nmol/L (>12.6 μg/dL) passed ACTH (1-24)-test (56) (see Supplementary Table S8 for cut-off values for ACTH (1-24)-testing in included studies). The results of the study by Sbardella et al showed that morning cortisol ≥336 nmol/L (≥12.1 μg/dL) had a specificity of 100% for predicting a normal ACTH (1-24)-test, and morning cortisol ≤124 nmol/L (≤4.5 μg/dL) was 100% sensitive for predicting failure (57). Positive and negative predictive values were not reported. Debono et al found that a baseline serum cortisol >310 nmol/L (>11.2 μg/dL) measured by immunoassay excluded glucocorticoid-induced adrenal insufficiency with a sensitivity of 98% and a negative predictive value of 97% (data retrieved after contacting the authors) (55). A baseline serum cortisol < 152 nmol/L (< 5.5 μg/dL) confirmed glucocorticoid-induced adrenal insufficiency with a specificity of 97% and a positive predictive value of 95%.
For serum cortisol measured by LC-MS/MS, a value > 327 nmol/L (>11.8 μg/dL) resulted in a sensitivity of 98% and a negative predictive value of 99% for excluding glucocorticoid-induced adrenal insufficiency, and a value <152 nmol/L (<5.5 μg/dL) resulted in a specificity of 98% and a positive predictive value of 99% for confirming glucocorticoid-induced adrenal insufficiency.
The quality of evidence was moderate due to applicability concerns and the numbers were too small to draw firm conclusions on the value of morning cortisol as stand-alone test to diagnose glucocorticoid-induced adrenal insufficiency. Importantly, test results of both serum cortisol and 250 µg ACTH(1-24)-test were not related to clinical endpoints such as adrenal crisis.
Recommendations
-
1. General recommendations for glucocorticoid therapy of non-endocrine conditions and recommendations regarding patient education
R 1.1—We recommend that, in general, patients on, or tapering off glucocorticoids for non-endocrine conditions do not need to be evaluated by an endocrinology specialist.
Rationale
Despite their efficacy as anti-inflammatory and immunosuppressive agents, chronic use of glucocorticoids can induce manifestations of Cushing syndrome, along with concomitant central and later permanent adrenal insufficiency (suppression of the entire HPA axis) (58). For this reason, clinicians prescribing glucocorticoids for non-endocrine reasons are advised to employ the lowest effective dose and duration of therapy and consider tapering glucocorticoid doses when treatment is no longer necessary for the underlying condition.
Given the widespread use of glucocorticoids, it is imperative that treating physicians of any discipline be well-versed in the clinical consequences of long-term supraphysiologic glucocorticoid therapy and the prevention, diagnosis, and treatment of glucocorticoid-induced adrenal insufficiency. It is equally critical to recognize signs and symptoms of adrenal insufficiency and be experienced in methods to taper and/or stop glucocorticoids once their pharmacologic effects are no longer required.
The management of glucocorticoid therapy is considered a general medical skill that should be managed by the prescribing clinician, also taking into consideration the underlying disease as a determinant of tapering speed. Furthermore, the affected number of patients (at least 1% of the general population) is too large with too few endocrinology providers to perform consultations for each instance of glucocorticoid tapering. When prescribing clinicians decide that glucocorticoid therapy is no longer required, they should educate their patient on methods to taper the dose, symptoms of adrenal insufficiency and appropriate responses, and proceed to wean the dose (Table 2). In the vast majority of cases, glucocorticoid taper does not cause any clinical endocrine concerns. In rare cases, however, when long-term supraphysiologic glucocorticoid therapy has resulted in prolonged suppression of HPA axis (greater than 1 year), or when patients experience adrenal crises, referral to or consultation with an endocrine specialist should be considered (see recommendation 2.11). However, it should be recognized that endocrinology providers have no specialized diagnostic approaches or therapies to facilitate unique care of glucocorticoid tapering. In this regard, the education and approach to stopping glucocorticoid therapy is a general medical process that every clinician who prescribes glucocorticoids should be familiar with.
Table 2.
Overview of topics prescribing clinicians should discuss with patients when prescribing oral glucocorticoids
| Considerations | Eligible patients | Timing | Comments |
|---|---|---|---|
| Risk for developing exogenous Cushing syndrome | All patients on long-term supraphysiologic glucocorticoid therapy | At the time of initiation | There are many sequelae of exogenous Cushing syndrome. Patients should be educated on the most common and clinically significant, including weight gain, sarcopenia, hyperglycemia, hypertension, bone demineralization |
| Risk for developing chronic adrenal insufficiency | Even transient adrenal insufficiency requires education to raise awareness for the need to stress dose when appropriate | ||
| Education on stress dosing strategies | Patients on long-term supraphysiologic glucocorticoid therapy who have reduced dosing to physiologic, or subphysiologic, levels | At least at the time when dosing approaches a physiologic range | Dedicated education should be provided to prepare patients with confirmed, or likely, adrenal insufficiency for routine and emergent stress dosing |
| Education on injectable emergency glucocorticoid administration | |||
| Glucocorticoid withdrawal syndrome | Patients on long-term supraphysiologic glucocorticoid therapy who are ready to begin tapering the dose | At the time glucocorticoid tapering begins | Some patients on long term supraphysiologic glucocorticoid therapy experience symptoms as the doses are tapered |
R 1.2—We recommend that clinicians who implement treatment with glucocorticoids educate patients about various endocrine aspects of glucocorticoid therapy. (Good clinical practice)
Rationale
Clinicians prescribing long-term supraphysiologic glucocorticoid therapy should actively educate their patients about the potential development of adverse manifestations associated with exogenous Cushing syndrome during extended use. Furthermore, patients need to be informed about the risks of adrenal insufficiency, especially when tapering glucocorticoid medication below the physiologic daily dose equivalent (see Definitions section). Clinicians should also provide comprehensive guidance on the importance of stress dosing with glucocorticoids. (see recommendation 3.1). Informing patients of the adverse effects of glucocorticoids and methods to monitor and mitigate these outcomes is crucial to enhancing the beneficial aspects of glucocorticoid therapy while minimizing the undesired adverse events and risks thereof. Education on stress and emergency dosing can prevent symptoms of adrenal insufficiency and hospitalizations for adrenal crises. Lastly, all patients initiating a glucocorticoid taper should be educated on the possibility of glucocorticoid withdrawal syndrome (58). The symptoms of glucocorticoid withdrawal have substantial overlap with symptoms of adrenal insufficiency and in some cases a disease flare, and can impede the tapering of glucocorticoids (see recommendation 2.3). Anticipation of these potential symptoms can increase awareness and minimize the need for urgent care.
R 1.3—We recommend that patients on glucocorticoid therapy have access to current up-to-date and appropriate information about different endocrine aspects of glucocorticoid therapy. (Good clinical practice)
Rationale
Empowering patients with knowledge of the benefits and risks of glucocorticoid therapy is critical (59). Patients require information in an age, education level, and learning style-appropriate format, along with access to supportive social resources such as family members or care providers and disease-oriented support groups. We recommend the inclusion of at least one family member or primary caregiver in all education sessions (60).
Patient education and empowerment to adjust glucocorticoid doses according to stressors are essential to prevent severe symptoms of adrenal insufficiency and adrenal crisis (61). Confidence in self-management to prevent adrenal crisis was demonstrated to be low in a large study that surveyed patients with adrenal insufficiency, including patients with glucocorticoid-induced adrenal insufficiency (26). Poor disease knowledge and lack of awareness of adrenal insufficiency subtype diagnosis were associated with higher rates of adrenal crisis. Standardized patient education programs for patients and their relatives proved to be useful for sustainably improving the level of knowledge regarding the prevention of adrenal crisis, as well as self-confidence in dealing with the disease (62, 63).
The risk for developing adrenal insufficiency and the potential for adrenal crisis during glucocorticoid treatment and taper is low but increases with the cumulative number of risk factors including glucocorticoid potency, administration route, dose and treatment duration (Table 3).
Table 3.
Risk factors for developing adrenal insufficiency, and susceptibility to adrenal crisis, during glucocorticoid therapy and withdrawal from therapy
| Factors | Risk for adrenal insufficiency and crisis | ||
|---|---|---|---|
| Low | Moderate | High | |
| Glucocorticoid potency | Hydrocortisone Cortisone acetate Deflazacort |
Prednisone Prednisolone Methylprednisolone Triamcinolone |
Dexamethasone Betamethasone Fluticasone |
| Administration Route | Nasal Topical Ophthalmic |
Inhaled | Systemic (oral, intramuscular, I intravenous) Intra-articular Concurrent use of differently aadministered glucocorticoid |
| Dose | Low | Medium | High |
| Duration of use | <3-4 weeks | 3-4 weeks-3 months | >3 months |
| Body Mass Index (64) | Normal | Overweight | Obese |
| Age (65) | Younger adults | Older adults | |
The educational content and timing of education delivery should be individualized to each patient. This relates to adverse effects of glucocorticoid therapy, symptoms of withdrawal and adrenal crisis and means to prevent and treat adrenal crisis. Patients at low risk for developing adrenal insufficiency or adrenal crisis may not require substantial education when initiated on glucocorticoid therapy. In contrast, patients with a moderate-to-high number of risk factors should receive more intensive education to minimize the risk of adverse outcomes. They may require multiple, well-timed trainings that should be reinforced until their glucocorticoid therapy is discontinued (Table 2).
-
2. Recommendations regarding taper of systemic glucocorticoid therapy for non-endocrine conditions, diagnosis and approach to glucocorticoid-induced adrenal insufficiency, and glucocorticoid withdrawal syndrome
R 2.1—We suggest not to taper glucocorticoids in patients on short-term glucocorticoid therapy of <3-4 weeks, irrespective of the dose. In these cases, glucocorticoids can be stopped without testing due to low concern for HPA axis suppression. (⊕○○○)
Rationale
Short-term glucocorticoid therapy is commonly used for conditions such as exacerbation of asthma, chronic obstructive lung disease, inflammatory bowel disease, allergic skin reactions, and rheumatoid arthritis. In a United States insurance database study of 1.5 million adults, 21% had received at least one course of oral glucocorticoids during the last three years, with a median dose of 20 mg prednisone equivalent and a median duration of 6 days (66). A starting dose of 50 mg of prednisone tapering to zero within 5-7 or 10-14 days are typical treatment regimens for exacerbation of asthma (Global Strategy for Asthma Management and Prevention. www.ginasthma.org/2023-gina-main-report).
There is no evidence that such short treatment periods lead to clinically relevant suppression of HPA axis, although there is lack of large high-quality studies. Suppression as evaluated by a 1 µg ACTH (1-24)-test has been reported (46). However, this test is less validated than a 250 µg ACTH (1-24)-test and should be interpreted with caution (67). While adrenal insufficiency is unlikely after short-term glucocorticoid therapy, clinicians should be aware that even short-term glucocorticoid treatment can lead to complications such as increased incidence of sepsis, gastrointestinal bleeding, thromboembolism, and fractures (66, 68).
R 2.2—Glucocorticoid taper for patients on long-term glucocorticoid therapy should only be attempted if the underlying disease for which glucocorticoids were prescribed is controlled, and glucocorticoids are no longer required. In these cases, glucocorticoids are tapered until approaching the physiologic daily dose equivalent is achieved (eg, 4-6 mg prednisone). (Good clinical practice)
Rationale
Glucocorticoids should only be tapered if the underlying disease no longer requires glucocorticoid therapy. In general, glucocorticoid taper can be faster and in larger decrements if the total daily glucocorticoid dose is high (eg, greater than 30 mg of prednisone). As the total daily glucocorticoid dose is approaching the physiologic daily dose equivalent (greater than equivalent of 15-25 mg hydrocortisone, 4-6 mg prednisone, see Table 1), the taper should be slower and with smaller decrements (Table 4). In certain patients with glucocorticoid-induced complications, such as uncontrolled hypertension and hyperglycemia, glucocorticoid-induced psychosis, or herpetic keratitis, a more rapid glucocorticoid taper towards physiologic daily dose equivalent may be required. The pre-test probability of adrenal atrophy and concurrent adrenal insufficiency is high for patients taking long-term supraphysiologic glucocorticoid doses; adrenal function testing is unnecessary until a physiologic glucocorticoid dose is achieved.
Table 4.
Suggested tapering regimen depending on glucocorticoid dose
| Patient's current daily prednisone equivalent dose | Suggested prednisone decrements | Time interval |
|---|---|---|
| >40 mg | 5-10 mg decrease | Every week |
| 20-40 mg | 5 mg decrease | Every week |
| 10-20 mg | 2.5 mg decrease | Every 1-4 weeks |
| 5-10 mg | 1 mg decrease | Every 1-4 weeks |
| 5 mg | In absence of clinical symptoms or negative testing for adrenal insufficiency continue 1 mg decrease (if low dosage prednisolone preparations are not available, alternative: 20 mg hydrocortisone with 5 mg decrease) | Every 4 weeks |
HPA recovery is possible once the glucocorticoid therapy has been tapered to a near-physiologic daily dose (eg, 4-6 mg prednisone). At this time, taper or assessment for HPA recovery could be performed unless glucocorticoids at this dose are required for control of the underlying condition (for example transplant, or polymyalgia rheumatica).
It is helpful to consider the likelihood of adrenal insufficiency and the risk of underlying disease flare before planning further tapering. It is also important to consider the underlying comorbidities and evaluate concurrent drugs that could impact glucocorticoid metabolism and overall glucocorticoid exposure. Although lacking systematic evidence, empirically, the patient's previous history of success or failure of glucocorticoid taper may also help design the most effective glucocorticoid taper. Additional factors that may impact the risk of adrenal insufficiency include inter-individual variability of glucocorticoid pharmacodynamics and pharmacokinetics. A study examining oral and intravenous methylprednisolone found that 20% of individuals demonstrated increased clearance of methylprednisolone (69). In general, older individuals have reduced drug clearance (65), despite a small sample size in these studies, data suggest a considerable and multifactorial inter-individual variability in what would be considered a physiological glucocorticoid dose.
R 2.3—We recommend consideration of glucocorticoid withdrawal syndrome that may occur during glucocorticoid taper. When glucocorticoid withdrawal syndrome is severe, glucocorticoid dose can be temporarily increased to the most recent one that was tolerated, and the duration of glucocorticoid taper could be increased. (Good clinical practice)
Rationale
Glucocorticoid withdrawal syndrome occurs due to dependence on supraphysiologic glucocorticoids while decreasing the dose of glucocorticoids (70-72). Patients should be informed that glucocorticoid withdrawal symptoms are expected to occur during the glucocorticoid dose reduction and what the differences are between glucocorticoid withdrawal syndrome, adrenal insufficiency, and underlying disease flare. It should be emphasized that an insufficient glucocorticoid supply is not expected to occur when the glucocorticoid dose is greater than the physiologic daily dose equivalent. As exceptions, it should be noted that the glucocorticoid requirement may be significantly higher in the case of critical illness or that glucocorticoid absorption is not guaranteed in gastroenteritis. Many of the symptoms of the withdrawal syndrome are nonspecific and overlap with symptoms of the underlying disease, especially in inflammatory musculoskeletal disorders. Managing glucocorticoid withdrawal syndrome and glucocorticoid taper in these patients may be especially challenging. Patients should be educated on symptoms of glucocorticoid withdrawal to avoid anxiety related to unexpected symptoms or reactive, unnecessary, or excessive increase in glucocorticoids.
Glucocorticoid withdrawal syndrome is reported to occur in 40-67% of patients tapering glucocorticoids following curative adrenalectomy in adrenal Cushing syndrome (72). Duration of exogenous glucocorticoid use, glucocorticoid dose and type, and individual susceptibility likely impact the severity and duration of glucocorticoid withdrawal, but systematic studies are lacking. In a recent study investigating glucocorticoid withdrawal syndrome in patients following curative surgery for endogenous hypercortisolism, symptoms of glucocorticoid withdrawal syndrome included arthralgias, myalgias, weakness, fatigue, sleep disturbances, and mood changes in up to 50% of patients (71). Symptoms are thought to occur due to an abrupt decrease in glucocorticoid exposure leading to an increase in inflammatory cytokines (73). Symptoms of glucocorticoid withdrawal syndrome overlap with those seen in patients with untreated or not optimally treated adrenal insufficiency (Table 5) (26), and most patients with glucocorticoid withdrawal syndrome do have concomitant adrenal insufficiency (72). Since symptoms of adrenal insufficiency and glucocorticoid withdrawal significantly overlap, good clinical guidance to differentiate between those is to consider the total daily dose of glucocorticoids with high doses making adrenal insufficiency less likely. For example, a patient treated for several months with prednisone 20-40 mg might experience glucocorticoid withdrawal symptoms, but concerns for spontaneous symptoms and signs of adrenal insufficiency are only a concern once the taper reaches 5-7.5 mg.
Table 5.
Clinical features of adrenal insufficiency, glucocorticoid withdrawal syndrome and common underlying conditions
| Glucocorticoid withdrawal syndrome | Adrenal insufficiency | Underlying condition for which glucocorticoids were initially prescribed | |
|---|---|---|---|
| Symptoms | General malaise, fatigue, nausea, muscle and joint pain, sleep disturbances, mood change | General malaise, fatigue, nausea, muscle and joint pain | Depending on condition (eg, joint pain in rheumatoid arthritis). Common overlapping symptoms (general malaise, fatigue) |
| Signs | Cushingoid features common, especially earlier in the glucocorticoid taper | Weight lossa, hypotension, orthostasis | Disease-specific signs reappear |
| Timing of symptoms and signs occurrence | At any point during glucocorticoid taper, usually when prednisone is decreased <15 mg/day Higher risk with long-term supraphysiologic glucocorticoid therapy |
Only when not treated with optimal glucocorticoid therapy (subphysiologic glucocorticoid dose, increased glucocorticoid requirements due to sickness) | At any point during glucocorticoid taper if the underlying condition is sub-optimally controlled with a non-glucocorticoid agent |
| Biochemistry | Normal electrolytes Glucocorticoid-induced hyperglycemia may be present | Hyponatremia, hypoglycemia | Biomarkers of disease activity (sedimentation rate, disease-specific biomarkers) |
| HPA axis | Testing is not recommended If tested, ACTH and cortisol are usually undetectable |
Initially, low ACTH and cortisol Later in recovery: normal-elevated ACTH, low cortisol |
Not applicable |
| Risk of adrenal crisis | Unlikely, if glucocorticoids are administered (as patients with glucocorticoid withdrawal syndrome also have adrenal insufficiency) | Yes, if not optimally treated with glucocorticoid therapy | Not applicable |
General remarks: Patients with glucocorticoid-induced adrenal insufficiency may be asymptomatic at baseline conditions but can develop symptoms—from mild to life-threatening adrenal crisis—when exposed to potential triggers (see Table 9). When present, symptoms of adrenal insufficiency are often non-specific and can overlap with those of the disease for which glucocorticoids are prescribed. Recurrence of underlying autoimmune diseases can occur during tapering of exogenous glucocorticoids. Signs and symptoms of adrenal insufficiency can overlap with those of glucocorticoid withdrawal syndrome, which arises from the discontinuation of rapid tapering of glucocorticoid therapy in patients who developed a tolerance to supraphysiologic glucocorticoid levels. In patients on glucocorticoids close to the physiological range, adrenal insufficiency and glucocorticoid withdrawal syndrome cannot be distinguished with complete accuracy.
a weight loss due to resolving GC induced Cushing syndrome should be considered.
The overall duration, type, and daily dose of glucocorticoid used should be considered when designing a glucocorticoid taper. Patients treated with higher glucocorticoid doses, long-acting glucocorticoids, and for a longer duration of time are likely to have more glucocorticoid withdrawal symptoms. Patients with features of exogenous Cushing syndrome are more likely to have a challenging glucocorticoid taper course because of glucocorticoid withdrawal syndrome (Table 5).
Slow decrease in glucocorticoid dose is the only known intervention that may help prevent severe glucocorticoid withdrawal symptoms. In patients following a curative surgery for endogenous hypercortisolism (71) baseline clinical severity score was associated with the severity of glucocorticoid withdrawal, and symptoms worsened once total daily glucocorticoid dose reached below 30 to 35 mg of hydrocortisone equivalent (eg, 7.5 prednisone). Clinical severity was calculated based on the presence of physical features and comorbidities potentially related to glucocorticoid excess, and may also be applied in patients treated with supraphysiologic glucocorticoids when deciding on the rapidity of glucocorticoid taper, with slower taper in patients with high clinical severity score, and a more rapid taper in patients with lower clinical severity score. In a patient with severe glucocorticoid withdrawal syndrome despite a slower glucocorticoid taper, increasing the glucocorticoid dose temporarily to the most recent dose prior to onset of glucocorticoid withdrawal syndrome will usually alleviate the symptoms.
R 2.4—We recommend against routine testing for adrenal insufficiency in patients on supraphysiologic doses of glucocorticoids, or if they are still in need of glucocorticoid treatment for the underlying disease. (Good clinical practice)
Rationale
If the glucocorticoid dose is in the supraphysiologic range, suppression of the HPA axis is expected and it is unnecessary to test adrenal function. Similarly, testing is unnecessary in patients unable to stop glucocorticoid treatment, for example patients with organ transplants and in cases of polymyalgia rheumatica. These patients should be educated on management of glucocorticoid-induced adrenal insufficiency (see section R.3).
R 2.5—We suggest that patients taking long-acting glucocorticoids (eg, dexamethasone or betamethasone) should be switched to shorter-acting glucocorticoids (eg, hydrocortisone or prednisone) when long-acting glucocorticoids are no longer needed. (⊕○○○)
Rationale
The use of long-acting glucocorticoids with higher glucocorticoid potency predisposes to a more pronounced suppression of HPA axis and subsequent adrenocortical function impairment. This is due to the continuous and non-circadian glucocorticoid effect of these drugs, especially when administered systemically (Table 1).
Long-acting glucocorticoids such as dexamethasone or betamethasone, even in physiologic daily dose equivalent, are more likely to cause HPA axis suppression, exogenous Cushing syndrome, and glucocorticoid withdrawal syndrome when being tapered (24, 74-78). HPA axis recovery is impossible in the setting of continuous administration of long-acting glucocorticoids. In contrast, intermediate- or short-acting glucocorticoids—which have both a shorter biological half-life and lower glucocorticoid potency—are more likely to allow HPA recovery, provided that they are not administered at nighttime, when they can more pronouncedly inhibit ACTH production and the early-morning rise of endogenous cortisol (19, 79).
If treatment with long-acting glucocorticoids is no longer needed, we recommend changing to shorter-acting formulations such as prednisone, prednisolone, hydrocortisone, or cortisone acetate to promote recovery of the HPA axis. For patients on non-oral glucocorticoids, eg, inhaled steroids, in whom there is a concern for glucocorticoid-induced adrenal insufficiency, a switch to short-acting oral glucocorticoids would be appropriate when non-oral glucocorticoids or no longer needed. Prednisone and hydrocortisone have a wider variety of available doses and allow for a more gradual taper in smaller decrements, thus potentially enabling HPA axis to recover (19, 80). For replacement of adrenal insufficiency, prednisone is usually provided as single morning dose, whereas due to shorter half-life hydrocortisone and cortisone acetate are divided into 2-3 doses with higher doses given in the morning and subsequent doses given at lunch and late afternoon if applicable (38).
Currently, the optimal type and dose of glucocorticoids to use during the taper has not been established. There is also a lack of reliable data comparing different strategies and tapering regimens vary widely in clinical practice. Moreover, there is no compelling evidence to switch intermediate-acting glucocorticoids such as prednisone to hydrocortisone or cortisone acetate to further promote the recovery of the HPA axis. The evidence of the effect of different types and dosages of glucocorticoid taper on the timing of HPA axis recovery and possible symptoms of glucocorticoid withdrawal remain limited and inconclusive (72, 79, 81-83). Consequently, an individualized approach to glucocorticoid taper is possible and necessary.
-
R 2.6—We suggest that patients on a physiologic daily dose equivalent, and aiming to discontinue glucocorticoid therapy, either:
1. continue to gradually taper the glucocorticoid dose, while being monitored clinically for signs and symptoms of adrenal insufficiency, or
-
2. be tested with a morning serum cortisol.
(⊕○○○)
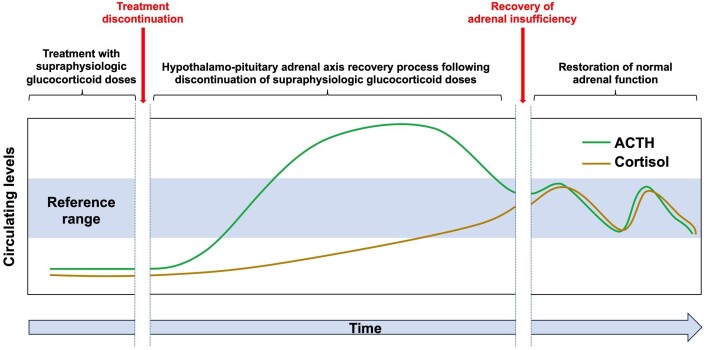
During the initial glucocorticoid tapering, ACTH and cortisol levels remain suppressed. When the dose of glucocorticoid therapy is lowered, the hypothalamus and pituitary gland start to recover, resulting in increased production of ACTH. ACTH increase can promote the recovery of adrenal function leading to an increase and recovery in cortisol. Complete recovery of cortisol production can remain impaired in a minority of patients (58, 84-86) (Fig. 1).
Figure 1.
Schematic representation of HPA axis recovery following discontinuation of supraphysiologic glucocorticoid therapy (adapted from: Prete and Bancos 2021 (58)).
There is no compelling evidence to guide optimal tapering. Discontinuation of long-term glucocorticoid therapy necessitates a cautious approach due to an increased risk of adrenal insufficiency, though the risk of clinically relevant adrenal crisis is generally low. Although glucocorticoid dose and treatment duration are associated with the development of adrenal insufficiency, predicting the risk of adrenal insufficiency remains challenging. A uniform approach to tapering the glucocorticoid dose has not yet been established and there is a lack of sufficient data on this topic (see Clinical question II). While some authors recommend a rapid reduction of the glucocorticoid dose to slightly above physiologic daily dose equivalent (eg, 7.5 mg prednisone), followed by a further reduction in smaller steps, others prefer testing of HPA axis to guide further tapering or immediate discontinuation, if normal adrenocortical function is demonstrated. An ongoing randomized controlled clinical trial (TOASST) is testing abrupt cessation vs gradual tapering once a dose of prednisone 7.5 mg is achieved (87).
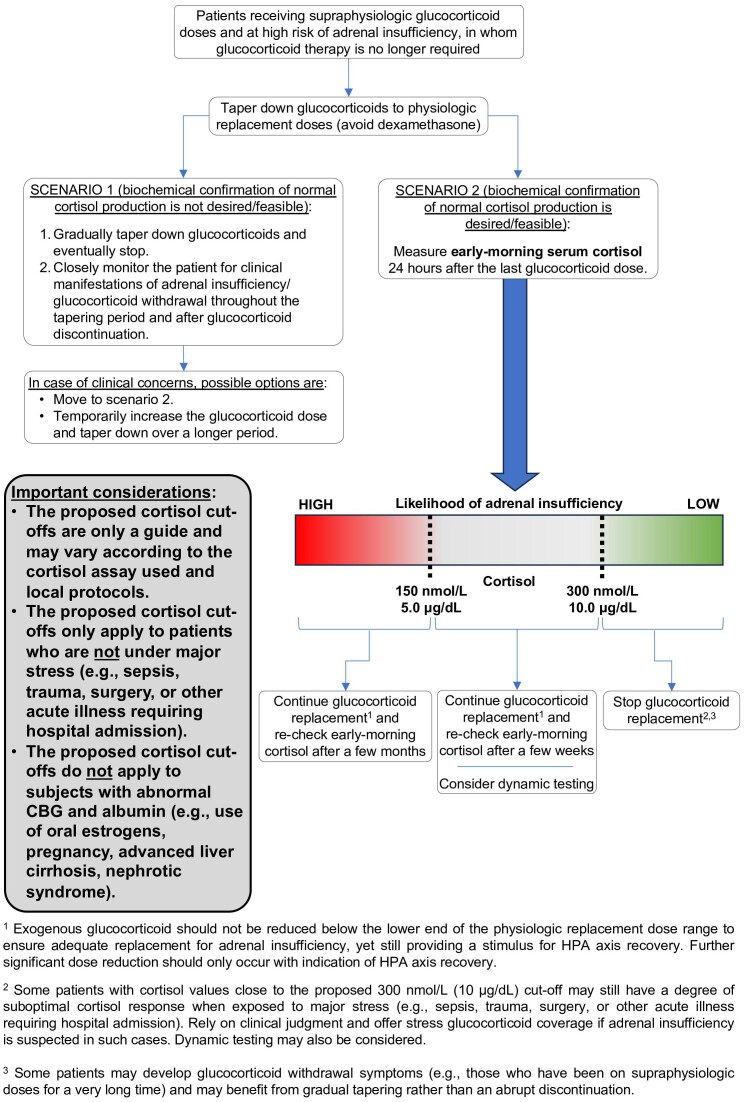
Once glucocorticoids are tapered down to physiologic replacement doses, the panel suggests two possible approaches for the discontinuation of glucocorticoid therapy (Fig. 2). Selecting one approach over the other might be driven by patient-related aspects including co-morbidities, co-medication, age and pre-test probability for adrenal insufficiency or by the medical context such as training and experience of the treating clinician or accessibility to laboratory diagnostics. There are no studies showing the superiority of any of these approaches in terms of clinical outcomes or cost-benefit.
Figure 2.
Proposed approach to systemic glucocorticoid discontinuation.
Patients may gradually taper glucocorticoids while being cautiously monitored for clinical manifestations of adrenal insufficiency. If the patient experiences signs and symptoms of adrenal insufficiency, glucocorticoid regimen should be restarted and not discontinued until recovery of HPA axis is documented. If the patient does not experience any symptoms, the tapering proceeds until glucocorticoid discontinuation.
Alternatively, patients may undergo testing with a morning serum cortisol (sample collected between 8:00 and 9:00 Am) for the determination of HPA axis recovery (R 2.7). If adrenal insufficiency is documented, exogenous glucocorticoid should not be reduced below the lower end of physiologic replacement dose ranges to ensure adequate replacement for adrenal insufficiency, yet still providing a stimulus for HPA-axis recovery (25). Patients should be retested according to recommendations in 2.7. and further significant dose reduction should only occur with indication of HPA-axis recovery.
R 2.7—If confirmation of recovery of the HPA axis is desired, we recommend morning serum cortisol as the first test. The value of morning serum cortisol should be considered as a continuum (Considering this continuum, suggested cut-offs in nmol/l and μg/dL are not exact conversions but have been rounded to improve clinical applicability in an international context.), with higher values more indicative of HPA axis recovery. (⊕○○○)
As a guide:
we suggest that the test indicates recovery of the HPA axis if cortisol is >300 nmol/L or 10 μg/dL and glucocorticoids can be stopped safely;
we suggest that if the result is between 150 nmol/L or 5 μg/dL and 300 nmol/L or 10 μg/dL, the physiologic glucocorticoid dose should be continued, and the morning cortisol repeated after an appropriate time period (usually weeks to months);
we suggest that if the result is <150 nmol/L or 5 μg/dL, the physiologic glucocorticoid dose should be continued, and the morning cortisol repeated after a few months.
Rationale
Due to the ease/convenience of testing, experience and validation, a morning serum cortisol level (measured between 8:00 and 9:00 Am, after holding glucocorticoid dose for at least 24 hours) is the recommended test to examine for recovery of HPA axis following glucocorticoid therapy (see also results of Clinical Question III). The test should be done only after reaching the range of a physiologic equivalent daily dose (eg, prednisone 4-6 mg daily or hydrocortisone 15-25 mg total daily dose, see Definitions). Several other approaches to HPA axis assessment exist, including measurement of waking salivary cortisone, morning DHEA-S measurement, 250 µg ACTH (1-24)-test, overnight metyrapone test and insulin tolerance test (39). However, the literature comparing different tests for adrenal insufficiency in the context of glucocorticoid use is very limited; importantly, test results are hardly related to clinically relevant outcomes (see section 3). Assessment should be done at least 24 hours after the last dose of glucocorticoids (excluding dexamethasone). It should be emphasized that biochemical testing for adrenal insufficiency is sensitive, but not specific. Persistence of biochemical suppression or insufficient recovery of HPA axis is a prerequisite for clinical adrenal insufficiency, yet even amongst those patients with biochemical insufficiency, the risk for clinically meaningful adrenal insufficiency and adrenal crisis remains very low. Due to the low prevalence of clinically relevant adrenal insufficiency despite the high prevalence of biochemical adrenal insufficiency following a glucocorticoid taper, testing can provide a safeguard in identifying those less at risk but is not a prerequisite for continued tapering.
Although proposing a serum cortisol cut-off of 300 nmol/L (10 μg/dL) as a guide, the panel suggests that the value of serum cortisol is considered as a continuum, rather than an arbitrary cut-off, with higher values more likely to indicate HPA axis recovery. Patients with very low morning cortisol levels (as a guide: <150 nmol/L (5 μg/dL)) are very likely to have persistent adrenal insufficiency (88). In such cases, dynamic testing is unlikely to be useful. We recommend that these patients continue with physiologic daily dose equivalent glucocorticoid replacement aiming for the lowest safe dose and undergo repeat morning cortisol testing until recovery occurs. As a general guide, the glucocorticoid dose should provide sufficient replacement, but also a sufficient stimulus for recovery (meaning avoiding any over-replacement). Frequency of repeat measuring may range between 1 to 6 months, depending on the dose and length of glucocorticoid therapy and the prior trajectory of cortisol values.
In patients with higher serum cortisol levels but below 300 nmol/L (10 μg/dL), HPA axis recovery is possible. In such cases, we suggest that the most cost-effective and practical strategy is that these patients continue with physiologic daily dose equivalent glucocorticoid replacement and have morning serum cortisol re-checked every few weeks until recovery occurs. If cortisol levels remain between 150 nmol/L (5 μg/dL) and 300 nmol/L (10 μg/dL), dynamic testing can be considered.
In a study of patients with suspected primary or secondary adrenal insufficiency, morning cortisol ≥354 nmol/L (12.8 μg/dL) predicted normal adrenal function with 100% sensitivity (89). One might also extrapolate some of the cut-off values from experiences with therapy of endogenous Cushing syndrome. In patients recovering from endogenous hypercortisolism, morning cortisol ≥276 nmol/L (10.0 μg/dL) was associated with no reported symptoms of glucocorticoid withdrawal syndrome or instances of adrenal crisis (72). Given these considerations, and the fact that there is substantial variability in the calibration between different cortisol assays, we consider cortisol values greater than 300 nmol/L (10 μg/dL) as a reasonable threshold to indicate recovery of HPA function following glucocorticoid-induced adrenal insufficiency.
When interpreting the values of morning cortisol measurement, it has to be taken into account that several factors can affect the results. Cortisol production is affected by the sleep-awake cycle, with cortisol secretion reaching its peak just minutes before waking up. Thus, morning serum cortisol can appear falsely low in individuals with disrupted circadian rhythm (eg, night shift workers, jet lag, and severe insomnia) (38). In addition, serum cortisol concentrations can be elevated in patients with elevated cortisol-binding globulin, such as seen during pregnancy and in women on oral estrogens (90, 91). By contrast, serum cortisol concentrations can be decreased in patients with low albumin and cortisol binding globulin, as in hypoalbuminemic states (such as advanced cirrhosis, nephrotic syndrome, and malnutrition), and prolonged critical illness (92, 93).
The interpretation of serum cortisol varies depending on the assays used. Available techniques for measuring serum cortisol listed from least to most accurate methods are immunoassays using polyclonal antibodies, immunoassays using more specific monoclonal antibody to cortisol, and liquid chromatography-tandem mass spectrometry (57, 94, 95). For example, in a large study of patients undergoing 250 µg ACTH (1-24)-test, baseline cortisol that excluded adrenal insufficiency varied between 336 (12.2 μg/dL) and 506 nmol/L (18.3 μg/dL) when measured by three different immunoassays (57). Most prior studies utilized different forms of immunoassays, rather than mass spectrometry-based assays. Therefore, it is important to point out that, ideally, physicians should be familiar with cut-off values used in their laboratories.
Concomitant measurement of baseline DHEAS was reported to have a good diagnostic accuracy in making a diagnosis of secondary adrenal insufficiency, including those with glucocorticoid-induced adrenal insufficiency (96, 97). Data on DHEAS use to diagnose recovery from glucocorticoid-induced adrenal insufficiency are scarce, but suggest that normalization of cortisol secretion occurs prior to normalization of DHEAS, making it a less favorable laboratory value to detect adrenal axis recovery. In one study of 32 patients with history of endogenous Cushing syndrome, patients with ACTH-independent Cushing syndrome had lower DHEAS than patients with ACTH-dependent Cushing syndrome at the time of HPA axis recovery, suggesting that duration of adrenal cortex atrophy impacts androgen recovery (98).
A promising alternative is waking salivary cortisone or cortisol (55). This non-invasive and practical ambulatory test holds the promise of replacing in-hospital assessments to test for adrenal insufficiency, but is currently not widely available.
R 2.8—We suggest against routinely performing a dynamic test for diagnosing adrenal insufficiency in patients tapering or stopping glucocorticoid therapy. (⊕○○○)
Rationale
Morning cortisol measurement can serve as a simple approach to HPA axis assessment, obviating the need for other tests in many patients (see recommendation 2.7) (99-101). However, if cortisol remains indeterminate (see 2.7), dynamic testing can be considered. The decision to carry out dynamic testing should consider the test's availability, feasibility, costs and regional accessibility. There is no evidence that a specific test in the context of glucocorticoid treatment is superior. Dynamic testing options include 250 µg ACTH (1-24) and, less commonly, overnight metyrapone (102) and insulin tolerance tests. The 250 µg ACTH (1-24) test only examines the direct response of the adrenal gland to supraphysiologic ACTH stimulation. In primary and secondary adrenal insufficiency, a peak cortisol level <500 nmol/L (<18.1 μg/dL), depending on assay, at 30 or 60 minutes is indicative of adrenal insufficiency (38, 39). As suppression of the HPA axis subsequently results in adrenocortical atrophy with impaired cortisol response, the test may yield less reliable results in patients on shorter duration of glucocorticoid therapy (39). The overnight metyrapone stimulation test and insulin tolerance test are more labor-intensive and can be associated with significant adverse effects. They assess the entire HPA axis, but head-to-head studies comparing different dynamic tests in this patient population are lacking. Furthermore, most of the published studies using dynamic testing to diagnose glucocorticoid-induced adrenal insufficiency rely on ACTH (1-24) stimulation. The panel suggests against the use of the 1 µg ACTH (1-24) test since it does not provide better diagnostic accuracy than the standard 250 µg and there are no commercially available preparations of 1 µg ACTH (1-24) (38, 103). If dynamic testing is employed, it should be done after holding any glucocorticoid therapy for at least 24 hours to avoid interference in steroid measurements.
-
R 2.9—We suggest awareness of possible glucocorticoid-induced adrenal insufficiency in patients:
1. with current or recent use of non-oral glucocorticoid formulations presenting with signs and symptoms indicative of adrenal insufficiency, or
2. using multiple glucocorticoid formulations simultaneously, or
3. using high dose inhaled or topical glucocorticoids, or
4. using inhaled or topical glucocorticoids for >1 year, or
5. who received intra-articular glucocorticoid injections in the previous 2 months, or
6. receiving concomitant treatment with strong cytochrome P450 3A4 inhibitors.
Rationale
Glucocorticoid-induced adrenal insufficiency can occur with any glucocorticoid formulation (104) (Table 6) and there is no established safe level of dose exposure (24). Published studies provide some guidance on the overall degree of risk in patients treated with glucocorticoids. However, establishing the risk on an individual basis is challenging and relies on clinical judgment. We suggest that some groups of non-oral glucocorticoid users carry a higher risk, although evidence is limited.
Table 6.
Non-oral glucocorticoid formulations and risk of glucocorticoid-induced adrenal insufficiency
| Prevalence of glucocorticoid-induced adrenal insufficiencya | Factors increasing the risk of glucocorticoid-induced adrenal insufficiency | Strategies to mitigate the risk of glucocorticoid-induced adrenal insufficiencyb | |
|---|---|---|---|
| Inhaled glucocorticoids |
|
|
|
| Intra-articular glucocorticoids | 52.2% (40.5-63.6) |
|
|
| Percutaneous (topical) glucocorticoids | 4.7% (CI 1.1-18.5) |
|
|
| Intra-nasal glucocorticoids | 4.2% (CI 0.5-28.9) |
|
|
These doses are expressed as total daily doses and should be seen as a guide only. Doses are based on information from manufactures” summaries of product characteristics, Global Initiative for Asthma (2023), and the British National Formulary.
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus.
a Based on a systematic review and meta-analysis of studies assessing the prevalence of biochemical impairment of the HPA axis, regardless of clinical correlates (24). Systematic data on the prevalence of signs and symptoms of adrenal insufficiency are lacking.
b Suggested strategies include consideration of reduced doses, frequencies, and alternative treatments, but sufficient control of the underlying glucocorticoid dependent disease remains paramount.
- Fluticasone propionate >500 μg/day
- Beclometasone dipropionate (standard particle inhalers) > 1000 μg/day
- Beclometasone dipropionate (extra fine particle inhalers) > 400 μg/day
- Budesonide >800 μg/day
- Ciclesonide >320 μg/day
- Fluticasone furoate >200 μg/day
- Mometasone furoate standard particle >400 μg/day
d Strong inhibitors include boceprevir, ceritinib, clarithromycin, cobicistat, darunavir, idelalisib, indinavir, itraconazole, ketoconazole, lopinavir, mifepristone, nefazodone, nelfinavir, posaconazole, ritonavir, saquinavir, telaprevir, telithromycin, and voriconazole.
We suggest that glucocorticoid-induced adrenal insufficiency should be suspected in patients with current or recent use of non-oral glucocorticoid formulations presenting with signs and symptoms indicative of adrenal insufficiency (Table 5). Manifestations of adrenal insufficiency are often non-specific and can overlap with other conditions including those for which glucocorticoids were prescribed. It is therefore imperative that healthcare professionals maintain a high degree of suspicion for the presence of adrenal insufficiency.
Patients receiving multiple types of glucocorticoids (eg, oral and inhaled) are more susceptible to developing glucocorticoid-induced adrenal insufficiency, reflecting the cumulative risk of systemic absorption and impact on the HPA axis. Pooled data from 11 studies on 354 patients found a risk of 42.7% (95%CI 28.6-58.0) (24).
In patients treated with inhaled glucocorticoids, the risk correlates directly with treatment dose and duration. A total of 21.5% (95%CI 12.0-35.5) of patients using high doses of inhaled glucocorticoids (58) and 27.4% (95%CI 17.7-39.8) of those treated for more than 1 year were found to have biochemical evidence of glucocorticoid-induced adrenal insufficiency (24) (Table 6). A Canadian study found only 392 hospital admissions due to glucocorticoid-induced adrenal insufficiency over a 15-year period among adults receiving inhaled glucocorticoids (105). Patients using higher daily doses and cumulative yearly doses had an almost twofold higher risk of hospital admission than those with lower exposure (105). A study focusing on the general practice records of 2.4 million people in the UK identified only 31 cases of established glucocorticoid-induced adrenal insufficiency linked to inhaled glucocorticoids (106). However, the same study also found a very low prevalence of glucocorticoid-induced adrenal insufficiency in patients on oral glucocorticoids, suggesting that this problem is largely unrecognized or under-reported (106). Of note, among all inhaled glucocorticoids fluticasone propionate is most frequently associated with the development of symptomatic glucocorticoid-induced adrenal insufficiency and exogenous Cushing syndrome (100, 104, 107-111). This is potentially linked to its pharmacokinetics (long half-life of 14.4 hours) and pharmacodynamics (binding affinity to the glucocorticoid receptors 18 times that of dexamethasone) (112). Regarding intranasal glucocorticoid use, the risk of glucocorticoid-induced adrenal insufficiency is low for short-term use at the recommended doses (Table 6). However, several intra-nasal glucocorticoids have high bioavailability and glucocorticoid receptor binding affinity, which can result in significant systemic exposure after prolonged use (113).
Robust evidence about the impact of intra-articular glucocorticoid injections on the HPA axis is lacking. Glucocorticoids can be detected in the urine for months after injections (114, 115) suggesting prolonged systemic absorption (24). We suggest that patients are monitored for signs and symptoms of adrenal insufficiency and that healthcare professionals have a low threshold for testing especially within 2 months of injections and in patients who receive simultaneous or multiple injections over a short period. Commonly used intra-articular glucocorticoid preparations often lead to a suppression of 4 weeks and therefore low morning cortisol values are expected during that time frame, and recovery can be confirmed in the following 4 weeks. Evidence regarding epidural glucocorticoid injections is also very limited but patients receiving multiple injections and higher doses appear to carry a higher risk of glucocorticoid-induced adrenal insufficiency (116-119).
Most glucocorticoids are metabolized by the hepatic cytochrome P450 3A4 (CYP3A4). Strong CYP3A4 inhibitors—which include food ingredients such as grapefruit juice, several antibiotics, antifungals, and the protease inhibitor ritonavir among others—have been shown to significantly increase circulating concentrations of glucocorticoids and hence substantially increase the risk of suppressing HPA axis. Several cases of exogenous Cushing syndrome linked to oral and non-oral glucocorticoid formulations in patients using strong CYP3A4 inhibitors have been published (108, 109). Ritonavir is the most commonly reported offending medication, used as part of antiviral combinations to treat HIV infection, hepatitis C infection, and COVID-19.
R 2.10—We suggest that patients with current or previous glucocorticoid treatment presenting with signs and symptoms of exogenous Cushing syndrome are assumed to have glucocorticoid-induced adrenal insufficiency. (Good clinical practice)
Rationale
Patients with a history of glucocorticoid treatment/exposure presenting with manifestations of Cushing syndrome (Table 7) should be assumed to have a fully suppressed HPA axis and managed accordingly. Exogenous Cushing syndrome can occur with any glucocorticoid formulation and can take several months to resolve after the glucocorticoid daily dose is decreased to physiological range (120, 121). Morning cortisol measurement can differentiate those with adrenal insufficiency (suppressed cortisol level) or identify those with recovering adrenal axis, but persistence of cushingoid features.
Table 7.
Signs and symptoms of glucocorticoid-induced (exogenous) cushing syndrome
| Symptoms | Muscle weakness Sleep disturbances (insomnia) Increased appetite Mood and cognitive disturbances (irritability, impaired memory, depression) |
| Signs | Proximal muscle weakness and wasting Excess weight gain and central obesity Supraclavicular and dorsocervical fat accumulation Facial and upper neck plethora with facial rounding Skin atrophy with easy bruising, red stretchmarks, and poor wound healing Acne Menstrual irregularities in women |
| Other manifestations | Cardiometabolic risk factors (hypertension, dysglycemia, dyslipidemia, hypercoagulability) Osteoporosis and fragility fractures Hypogonadism, reduced libido, and reduced fertility |
R 2.11—We suggest that patients aiming to discontinue glucocorticoids, but without recovery of HPA axis in one year while on physiologic daily dose equivalent, should be evaluated by an endocrinology specialist. We suggest that patients on glucocorticoids and history of adrenal crisis should also be evaluated by an endocrinology specialist. (Good clinical practice)
Rationale
Prior studies have shown that adrenal insufficiency may last even up to 2-4 years after glucocorticoid cessation, owing to slow recovery of adrenal cortisol production (40, 47, 122-124). Persistent impairment of cortisol secretion beyond four years suggests that recovery of adrenal function is very unlikely and long-term glucocorticoid replacement should be continued (99, 124). Additional regular testing beyond four years may not be helpful but can be considered on a case-by-case basis.
The panel suggests that patients with persistent adrenal insufficiency while on physiologic daily dose equivalent of glucocorticoids for longer than one year should be evaluated by an endocrinology specialist to assess for underlying causes of adrenal insufficiency other than glucocorticoid-induced adrenal insufficiency (eg, pituitary causes). The panel suggests that patients who experience an adrenal crisis while on glucocorticoids should be evaluated by an endocrinology specialist. Patients with adrenal insufficiency for more than one year should be treated with standard replacement doses of hydrocortisone or prednisone (Table 1). Furthermore, it is necessary to provide education to these patients regarding the adjustment of glucocorticoid substitution therapy doses during stressful situations to prevent adrenal crises or to manage them (38) (see Section 3).
R 2.12—We recommend against the use of fludrocortisone in patients with glucocorticoid-induced adrenal insufficiency.
Rationale
Secretion of the mineralocorticoid aldosterone is largely regulated by the renin-angiotensin system and potassium levels. Accordingly, mineralocorticoid function is expected to be preserved in glucocorticoid-induced adrenal insufficiency, as in other forms of secondary or tertiary adrenal insufficiency. Substitution therapy with fludrocortisone is not indicated.
-
3. Recommendations on diagnosis and therapy of adrenal crisis in patients with glucocorticoid-induced adrenal insufficiency
-
R 3.1—We recommend that patients with current or recent glucocorticoid use who did not undergo biochemical testing to rule out glucocorticoid-induced adrenal insufficiency should receive stress dose coverage when they are exposed to stress. (Good clinical practice)
R3.1A—Oral glucocorticoids should be used in case of minor stress and when there are no signs of hemodynamic instability or prolonged vomiting or diarrhea.
R3.1B—Parenteral glucocorticoids should be used in case of moderate to major stress, procedures under general or regional anesthesia, procedures requiring prolonged avoidance or inability of oral intake, or when there are signs of hemodynamic instability or prolonged vomiting or diarrhea.
-
Rationale
As discussed in sections R1.2, R1.3, and R3.2, patients need to be educated on stress dosing of glucocorticoids aiming to prevent adrenal crises and their negative sequelae (Fig. 3). Potential adrenal insufficiency and the need for stress dosing should be considered even after discontinuation of glucocorticoid therapy and replacement, particularly in those patients that had not undergone biochemical testing to confirm recovery of the HPA-axis. Based on a retrospective analysis mortality is increased particularly in the immediate period following the cessation of glucocorticoid therapy (29). Therefore, patients with current or recent glucocorticoid use who did not undergo biochemical testing to rule out glucocorticoid-induced adrenal insufficiency who are under minor stress (eg, fever, infection requiring antibiotics, physical trauma, significant emotional stress) not leading to hemodynamic instability and with no evidence of oral glucocorticoid malabsorption (vomiting, diarrhea) or are undergoing a surgical procedure under local anesthesia will require coverage with stress dose of oral glucocorticoids (as a general guide, see Table 8). The recommended stress dose of hydrocortisone is the same as for patients with primary or secondary adrenal insufficiency of other etiology: patients should receive double the physiologic replacement dose (ie, hydrocortisone 40 mg daily, usually split in three doses 20 mg on rising, 10 mg 12 midday, 10 mg 5Pm) (125). In patients using other glucocorticoid formulations, a dose equivalent to 40 mg hydrocortisone is suggested and this regime needs to be offered for the duration of the stress period eg, prednisone 10 mg total daily dose to be given in one or two divided doses (as a general guide, see Table 8). Particularly for patients undergoing surgery under general or regional anesthesia associated with long recovery time, parenteral stress doses of hydrocortisone or equivalent doses of other glucocorticoids such as methylprednisolone or dexamethasone are recommended (as a general guide, see Table 8). We base our suggested stress-dose glucocorticoid regimens on clinical practice and the guidelines from the Association of Anaesthetists, the Royal College of Physicians, the Endocrine Society and the Society for Endocrinology UK (38, 126). However, we acknowledge that in the absence of robust evidence and head-to-head comparison of different glucocorticoid regimens, practices vary considerably among centers and lower doses are also routinely used in patients under moderate or major stress (127).
Figure 3.
Management of patients at risk of or with diagnosed glucocorticoid-induced adrenal insufficiency with suspected adrenal crisis or during exposure to stress.
Table 8.
Suggested glucocorticoid regimens in patients at risk of or with diagnosed glucocorticoid-induced adrenal insufficiency during exposure to stress
| General considerations | Examples | Suggested regimen | |
|---|---|---|---|
| Minor stress | If the patient is already taking hydrocortisone ≥40 mg daily prednisone ≥10 mg daily, or dexamethasone ≥1 mg daily, there is typically no need to increase the dose unless there are signs of hemodynamic instability |
|
If not on daily glucocorticoids: give hydrocortisone 40 mg total daily dose, to be given in three divided doses (eg, 20 mg on rising, 10 mg 12 midday, 10 mg 5pm). Continue for 2-5 days until well (or for the duration of antibiotic treatment). If on hydrocortisone <40 mg total daily dose: increase to 40 mg total daily dose, to be given in three divided doses (eg, 20 mg on rising, 10 mg 12 midday, 10 mg 5pm). Continue for 2-5 days until well (or for the duration of antibiotic treatment). If on prednisone <10 mg total daily dose: increase to 10 mg total daily dose, to be given in one or two divided doses. Continue for 2-5 days until well (or for the duration of antibiotic treatment). If on dexamethasone <1 mg total daily dose: increase to 1 mg once daily. Continue for 2-5 days until well. |
| Minor surgery including any procedure requiring local anesthesia |
If not on daily glucocorticoids: give oral hydrocortisone 40 mg total daily dose, to be given in three divided doses (eg, 20 mg one hour prior to the procedure, 10 mg six hours after the procedure, 10 mg after a further six hours). Continue glucocorticoids in patients who remain unwell after the procedure until clinically stable. If on hydrocortisone <40 mg total daily dose: increase to 40 mg total daily dose, to be given in three divided doses (eg, 20 mg one hour prior to the procedure, 10 mg six hours after the procedure, 10 mg after a further six hours). Continue increased dose in patients who remain unwell after the procedure until clinically stable. If on prednisone <10 mg total daily dose: increase to 10 mg total daily dose, to be given one hour prior to the procedure. Continue increased dose in patients who remain unwell after the procedure until clinically stable. If on dexamethasone <1 mg total daily dose: increase to 1 mg total daily dose, to be given one hour prior to the procedure. Continue increased dose in patients who remain unwell after the procedure until clinically stable. |
||
| Bowel procedures not carried out under general anesthesia |
If not on daily glucocorticoids: give hydrocortisone 20 mg total daily dose, to be given in three divided doses (eg, 10 mg one hour prior to the procedure, 5 mg six hours after the procedure, 5 mg after a further six hours). If on daily glucocorticoids: continue normal glucocorticoid dose. Give an equivalent I.V. dose if prolonged nil by mouth. |
||
| Moderate and major stress | If the patient is already taking hydrocortisone ≥200 mg daily, prednisone ≥50 mg daily, or dexamethasone ≥6-8 mg daily, there is typically no need to increase the dose In patients with suspected reduced absorption (persistent vomiting or diarrhea), nil by mouth, or unable to take tablets, give stress-dose glucocorticoids I.V. or I.M. High body weight can be taken into consideration as a factor indicating higher dosage requirements |
Severe intercurrent illness, for example:
|
For patients with persistent vomiting or diarrhea who are well enough to remain out of hospital: Hydrocortisone 100 mg I.M. injection immediately, which can be repeated after 6 hours if needed. If symptoms do not resolve or hemodynamic instability develops, admit to hospital for I.V. urgent glucocorticoid and fluid administration. Patients requiring hospital admission: Hydrocortisone 100 mg I.V. bolus or I.M. injection immediately, followed by immediate initiation of a continuous infusion of hydrocortisone 200 mg over 24 hours. If a continuous infusion is not feasible, give hydrocortisone 50 mg I.V. boluses every 6 hours. The duration and dose of the glucocorticoid regimen thereafter must be individualized based on the stressor type and the patient's clinical status. |
| Surgery or any procedure requiring general or regional anesthesia with anticipated short recovery time and no nil by mouth |
Intra-operative regimen: Hydrocortisone 100 mg I.V. bolus at induction, followed by immediate initiation of a continuous infusion of hydrocortisone 200 mg over 24 hours. If a continuous infusion is not feasible, give hydrocortisone 50 mg I.V. boluses every 6 hours. Postoperative regimen: Resume oral glucocorticoids at an increased dose for 48 hours (eg, hydrocortisone 40 mg/daily in three divided doses; prednisone 10 mg/daily in one or two divided doses; dexamethasone 1 mg once daily) and then resume the pre-surgical dose. In case of post-operative complications (eg, significant pain, infections), maintain an increased oral dose or give stress-dose glucocorticoids I.V. as clinically appropriate. |
||
| Surgery (including cesarean section) or any procedure requiring general or regional anesthesia with nil by mouth or expected long recovery time |
Intra-operative regimen: Hydrocortisone 100 mg I.V. bolus at induction, followed by immediate initiation of a continuous infusion of hydrocortisone 200 mg over 24 hours. If a continuous infusion is not feasible, give hydrocortisone 50 mg I.V. boluses every 6 hours. Postoperative regimen: Continuous infusion of hydrocortisone 200 mg over 24 hours while the patient is nil by mouth. If a continuous infusion is not feasible, give hydrocortisone 50 mg I.V. boluses every 6 hours. If the post-operative period is uncomplicated and once the patient can eat, resume oral glucocorticoids at an increased dose for 48 hours (eg, hydrocortisone 40 mg/daily in three divided doses; prednisone 10 mg/daily in one or two divided doses; dexamethasone 1 mg once daily) and then resume the pre-surgical dose. In case of post-operative complications (eg, significant pain, infections), maintain an increased oral dose or give stress-dose glucocorticoids I.V. as clinically appropriate. |
||
| Labor and vaginal delivery | Hydrocortisone 100 mg I.V. bolus at onset of labor, followed by immediate initiation of a continuous infusion of hydrocortisone 200 mg over 24 hours. If a continuous infusion is not feasible, give hydrocortisone 50 mg I.V. boluses every 6 hours. |
R 3.2—We suggest that in patients with current or recent glucocorticoid use who did not undergo biochemical testing to rule out glucocorticoid-induced adrenal insufficiency and present with hemodynamic instability, vomiting, or diarrhea, the diagnosis of adrenal crisis should be considered irrespective of the glucocorticoid type, mode of administration, and dose; patients with suspected adrenal crisis should be treated with parenteral glucocorticoids and fluid resuscitation. (Good clinical practice)
Rationale
Adrenal crisis (also known as acute adrenal insufficiency or Addisonian crisis) can occur in patients taking oral supraphysiologic doses of glucocorticoids, if drug availability suddenly decreases (eg, missed doses, gastroenteritis). It is a life-threatening emergency that must be promptly recognized and treated. Therefore, timely therapy is essential and takes precedence over the evaluation for other causes of symptoms that are in accordance with adrenal crisis. Adrenal crisis is characterized by the inability of the adrenal cortex to produce enough cortisol to respond appropriately to stressors such as infections, trauma, and surgery (Table 9). The pathophysiology of adrenal crisis is complex and not fully understood, but it is invariably characterized by volume depletion and vasoplegia resulting in hypotension and—if left untreated—shock and eventually death (128, 129). Adrenal crisis can occur not only in patients treated with oral glucocorticoids but also in patients receiving only inhaled (110, 130), topical (131), intra-articular (132), or other glucocorticoid formulations (133). This highlights the potential clinical risks associated with the untoward systemic absorption of non-oral glucocorticoids. As mentioned above, adrenal crisis should be considered in patients in the period immediately following cessation of glucocorticoid therapy.
Table 9.
Signs and symptoms of adrenal crisis and potential precipitating factors
| General considerations |
|
| Diagnosis | Hypotension or hypovolemic shock plus at least one of the following:
|
| Possible laboratory abnormalities (not required for the diagnosis) |
|
| Factors that can trigger an adrenal crisis or elicit symptoms of adrenal insufficiency |
Common to all patients with adrenal insufficiency:
|
Adrenal crisis is a clinical diagnosis and should be suspected in patients with current or recent use of any glucocorticoid formulation presenting with hypotension, collapse, or acute abdominal symptoms (Table 9). Hyponatremia may also be associated. A high degree of clinical suspicion is paramount, as patients may not have been tested for suspected glucocorticoid-induced adrenal insufficiency prior to the acute event and adrenal crisis may be the first manifestation of the disease. Treatment must not be delayed by laboratory or imaging investigations. If an established or impending adrenal crisis is suspected, the patient should immediately receive an injection of 100 mg hydrocortisone intravenously or intramuscularly followed by rapid volume resuscitation with intravenous administration of 0.9% saline solution (or equivalent) (38). Patients with confirmed adrenal crisis should be maintained on hydrocortisone at a dose of 200 mg hydrocortisone per 24 hours (preferably by continuous intravenous infusion, alternatively by intravenous or intramuscular injection of 50 mg hydrocortisone every 6 hours) until clinical recovery and further guidance by an endocrinology specialist (38, 134). In patients with very high body weight, higher doses might be considered. Some centers use equivalent parenteral doses of other glucocorticoids such as methylprednisolone or dexamethasone; head-to-head comparison data of different treatment strategies for adrenal crisis are lacking. Any identifiable potential triggers (eg, infections, trauma) should be treated where possible. Short-term administration of parenteral glucocorticoids at the recommended doses is safe; hence, treatment should be initiated even if an adrenal crisis diagnosis is eventually ruled out.
Evidence regarding the incidence of adrenal crisis in patients with glucocorticoid-induced adrenal insufficiency is limited (see introduction). Some data suggest that the risk is low considering the relatively small number of hospital admissions for adrenal crisis recorded in patients on long-term glucocorticoids (129). The preserved aldosterone production and some residual cortisol production in glucocorticoid-induced adrenal insufficiency may explain these observations. One study found a higher incidence of adrenal crisis—precipitated by infections in about half of cases—in patients with established glucocorticoid-induced adrenal insufficiency compared to other causes of adrenal insufficiency (27), but this was not observed in other studies (26).
A significant proportion of patients with glucocorticoid-induced adrenal insufficiency may be undiagnosed; as such, adrenal insufficiency symptoms and adrenal crisis can be missed. A population-based study found an increased incidence of potential manifestations of untreated adrenal insufficiency (hypotension, gastrointestinal symptoms, hypoglycemia, and hyponatremia) after discontinuation of long-term oral glucocorticoids (25). Individuals exposed to infections—common triggers of adrenal insufficiency symptoms—and older individuals taking higher glucocorticoid doses for longer periods prior to discontinuation carried a higher risk of developing these manifestations (25). Another study found a sharp mortality increase in the first 3-6 months after cessation of long-term oral glucocorticoids (29). Whilst it is not possible to establish how many deaths were due to unrecognized adrenal crisis, these data highlight the need for close clinical monitoring of patients coming off long-term glucocorticoid therapy (29, 130).
Adrenal crisis prevention is one of the main goals of the management of any patient with adrenal insufficiency and is achieved through regular patient education about its signs and symptoms, possible precipitating factors (see recommendations 1.2 and 1.3, Table 9), when and how to increase glucocorticoid dose (sick day rules), and the provision of patient-held prompts to healthcare professionals should they become seriously ill or unconscious (eg, ESE steroid emergency card: A Standardised European Emergency Card for Patients with Adrenal Insufficiency | ESE (ese-hormones.org)) (125, 135). When compared to other adrenal insufficiency etiologies, patients with an established diagnosis of glucocorticoid-induced adrenal insufficiency were found to be less aware of their diagnosis, to engage less with preventative strategies (possession of emergency injectable hydrocortisone, wearing medical alert gear), experienced considerably more delays during emergency treatment for adrenal crises, and generally had more difficulty in managing their condition with poorer self-perceived health (26). These observations highlight the need for prevention strategies and education of patients and healthcare professionals alike.
Future Research
Evidence for the majority of above recommendations regarding glucocorticoid-induced adrenal insufficiency is low or very low. Therefore, future epidemiology research needs to define the true risk of clinical adrenal crisis and adrenal insufficiency. Additional data regarding morbidity and mortality of glucocorticoid-induced adrenal insufficiency is required to understand the associated health risk, which will ultimately define the approach to care for patients tapering long-term glucocorticoid therapy.
There is a need for further definition of risk factors contributing to the development and susceptibility of adrenal insufficiency, such as genetic predisposition, environmental influences, concurrent medication and underlying disease for which glucocorticoid therapy is initiated for.
Biomedical and psychosocial research into understanding of glucocorticoid withdrawal is warranted, ideally providing clinical scoring systems or biomarkers, in order to better differentiate glucocorticoid withdrawal from glucocorticoid-induced adrenal insufficiency.
Established dynamic tests for glucocorticoid-induced adrenal insufficiency identify a relatively large proportion of patients with biochemical HPA axis insufficiency following glucocorticoid therapy, yet there is only a very low reported number of patients that develop clinical evidence of adrenal insufficiency and only an exceedingly low number of patients develop adrenal crisis. Therefore, more specific and predictive tests and follow-up parameters (including salivary cortisol and potentially continuous monitoring of interstitial cortisol) are needed to identify at-risk patients who would benefit from dedicated preventive intervention.
More research is needed aiming to identify glucocorticoids retaining immunosuppressive and anti-inflammatory properties, but having less effect on HPA axis suppression and an improved adverse effect profile. In addition, the exploration of other therapeutic strategies, such as concurrent HPA axis stimulation to prevent suppression should also be entertained.
There is a need for a harmonization of cortisol assays. While most cut-off values were established using different immunoassays, usually overestimating true cortisol values due to varying degrees of cross-reactivity with other steroid metabolites, the advent of mass spectrometry allows for a specific measurement of cortisol. Future research needs to establish cut-off values using mass spectrometry and clinical care needs to adapt this measurement for routine cortisol measurements.
Acknowledgments
The authors of the guideline would like to thank and acknowledge Johannes Bijlsma (rheumatologist, The Netherlands), Maria Fleseriu (MD, FACE, Oregon Health & Science University, Portland, Oregon, United States), Jens Otto Lunde Jørgensen (endocrinologist, Aarhus University, Denmark) and John Newell-Price (endocrinologist, University of Sheffield, United Kingdom) for their expert review. We are also grateful for all the valuable and critical comments of members of the European Society of Endocrinology and the Endocrine Society. The comments of the reviewers as well as our responses are available in Supplementary Table S9 (see section on Supplementary material).
In addition, the authors would like to thank J.W. Schoones for his help in conducting the literature searches.
Contributor Information
Felix Beuschlein, Department of Endocrinology, Diabetology and Clinical Nutrition, University of Zürich (USZ) and University of Zürich (UZH), 8091 Zürich, Switzerland; Medizinische Klinik und Poliklinik IV, Klinikum der Universität, Ludwig-Maximilians-Universität, 81377 Munich, Germany; The LOOP Zurich Medical Research Center, 8044 Zurich, Switzerland.
Tobias Else, Department of Internal Medicine, Division of Metabolism, Endocrinology and Diabetes, University of Michigan, Ann Arbor, MI 48109, USA.
Irina Bancos, Division of Endocrinology, Metabolism, and Nutrition, Mayo Clinic, Rochester, MN 55905, USA; Joint appointment in Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN 55905, USA.
Stefanie Hahner, Department of Internal Medicine I, Division of Endocrinology and Diabetes, University Hospital, University of Würzburg, 97080 Wuerzburg, Germany.
Oksana Hamidi, Division of Endocrinology and Metabolism, University of Texas Southwestern Medical Center, Dallas, TX 75390-8857, USA.
Leonie van Hulsteijn, European Society of Endocrinology, Bristol BS32 4QW, UK; Department of Clinical Epidemiology, Leiden University Medical Center, 2333 ZA Leiden, The Netherlands.
Eystein S Husebye, Department of Clinical Science, University of Bergen, N-5021 Bergen, Norway; Department of Medicine, Haukeland University Hospital, N-5021 Bergen, Norway.
Niki Karavitaki, Institute of Metabolism and Systems Research, College of Medical and Dental Sciences, University of Birmingham, Birmingham B15 2TT, UK; Centre for Endocrinology, Diabetes and Metabolism, Birmingham Health Partners, Birmingham B15 2TT, UK; Department of Endocrinology, Queen Elizabeth Hospital, University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2TH, UK.
Alessandro Prete, Institute of Metabolism and Systems Research, College of Medical and Dental Sciences, University of Birmingham, Birmingham B15 2TT, UK; Centre for Endocrinology, Diabetes and Metabolism, Birmingham Health Partners, Birmingham B15 2TT, UK; NIHR Birmingham Biomedical Research Centre, University of Birmingham and University Hospitals Birmingham NHS Foundation Trust, Birmingham B15 2TH, UK.
Anand Vaidya, Center for Adrenal Disorders, Division of Endocrinology, Diabetes, and Hypertension, Brigham and Women's Hospital, Harvard Medical School, Boston, MA 02115, USA.
Christine Yedinak, Department of Neurological Surgery, Oregon Health & Sciences University, Portland, OR 97239-3098, USA.
Olaf M Dekkers, Department of Clinical Epidemiology, Leiden University Medical Center, 2333 ZA Leiden, The Netherlands; Department of Endocrinology and Metabolism, Leiden University Medical Center, 2333 ZA Leiden, The Netherlands; Department of Clinical Epidemiology, Aarhus University, 8200 Aarhus, Denmark.
Supplementary Material
The supplementary material of this guideline can be found at DOI 10.5281/zenodo.10890177.
Funding
This guideline was sponsored by the European Society of Endocrinology.
References
- 1. Fardet L, Petersen I, Nazareth I. Prevalence of long-term oral glucocorticoid prescriptions in the UK over the past 20 years. Rheumatology (Oxford). 2011;50(11):1982‐1990. [DOI] [PubMed] [Google Scholar]
- 2. Overman RA, Yeh JY, Deal CL. Prevalence of oral glucocorticoid usage in the United States: a general population perspective. Arthritis Care Res (Hoboken). 2013;65(2):294‐298. [DOI] [PubMed] [Google Scholar]
- 3. van Staa TP, Leufkens HG, Abenhaim L, Begaud B, Zhang B, Cooper C. Use of oral corticosteroids in the United Kingdom. QJM. 2000;93(2):105‐111. [DOI] [PubMed] [Google Scholar]
- 4. Baker EH. Is there a safe and effective way to wean patients off long-term glucocorticoids? Br J Clin Pharmacol. 2021;87(1):12‐22. [DOI] [PubMed] [Google Scholar]
- 5. Hench PS, Kendall EC, Slocumb CH, Polley HF. The effect of a hormone of the adrenal cortex (17-hydroxy-11-dehydrocorticosterone: compound E) and of pituitary adrenocortical hormone in arthritis: preliminary report. Ann Rheum Dis. 1949;8(2):97‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walsh LJ, Wong CA, Pringle M, Tattersfield AE. Use of oral corticosteroids in the community and the prevention of secondary osteoporosis: a cross sectional study. BMJ. 1996;313(7053):344‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Laugesen K, Jørgensen JOL, Sørensen HT, Petersen I. Systemic glucocorticoid use in Denmark: a population-based prevalence study. BMJ Open. 2017;7(5):e015237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wilson JC, Sarsour K, Gale S, Pethö-Schramm A, Jick SS, Meier CR. Incidence and risk of glucocorticoid-associated adverse effects in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2019;71(4):498‐511. [DOI] [PubMed] [Google Scholar]
- 9. Spivey CA, Griffith J, Kaplan C, Postlethwaite A, Ganguli A, Wang J. A retrospective analysis of corticosteroid utilization before initiation of biologic DMARDs among patients with rheumatoid arthritis in the United States. Rheumatol Ther. 2018;5(1):255‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. George MD, Baker JF, Winthrop K, et al. Risk for serious infection with low-dose glucocorticoids in patients with rheumatoid arthritis: a cohort study. Ann Intern Med. 2020;173(11):870‐878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Costello RE, Yimer BB, Roads P, Dixon JM, G W. Glucocorticoid use is associated with an increased risk of hypertension. Rheumatology (Oxford). 2021;60(1):132‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lillegraven S, Greenberg JD, Reed GW, et al. Immunosuppressive treatment and the risk of diabetes in rheumatoid arthritis. PLoS One. 2019;14(1):e0210459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim D, Cho SK, Park B, Jang EJ, Bae SC, Sung YK. Glucocorticoids are associated with an increased risk for vertebral fracture in patients with rheumatoid arthritis. J Rheumatol. 2018;45(5):612‐620. [DOI] [PubMed] [Google Scholar]
- 14. Cheng TT, Lai HM, Yu SF, et al. The impact of low-dose glucocorticoids on disease activity, bone mineral density, fragility fractures, and 10-year probability of fractures in patients with rheumatoid arthritis. J Investig Med. 2018;66(6):1004‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Costello RE, Marsden A, Movahedi M, et al. The effect of glucocorticoid therapy on mortality in patients with rheumatoid arthritis and concomitant type II diabetes: a retrospective cohort study. BMC Rheumatol. 2020;4(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Drouin J, Trifiro MA, Plante RK, Nemer M, Eriksson P, Wrange O. Glucocorticoid receptor binding to a specific DNA sequence is required for hormone-dependent repression of pro-opiomelanocortin gene transcription. Mol Cell Biol. 1989;9(12):5305‐5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nolan LA, Levy A. Anterior pituitary trophic responses to dexamethasone withdrawal and repeated dexamethasone exposures. J Endocrinol. 2001;169(2):263‐270. [DOI] [PubMed] [Google Scholar]
- 18. Marin F, Cheng Z, Kovacs K. Ubiquitin immunoreactivity in corticotrophs following glucocorticoid treatment and in pituitary adenomas. Arch Pathol Lab Med. 1993;117(3):254‐258. [PubMed] [Google Scholar]
- 19. Meikle AW, Tyler FH. Potency and duration of action of glucocorticoids. Effects of hydrocortisone, prednisone and dexamethasone on human pituitary-adrenal function. Am J Med. 1977;63(2):200‐207. [DOI] [PubMed] [Google Scholar]
- 20. Bledsoe RK, Montana VG, Stanley TB, et al. Crystal structure of the glucocorticoid receptor ligand binding domain reveals a novel mode of receptor dimerization and coactivator recognition. Cell. 2002;110(1):93‐105. [DOI] [PubMed] [Google Scholar]
- 21. Czock D, Keller F, Rasche FM, Häussler U. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet. 2005;44(1):61‐98. [DOI] [PubMed] [Google Scholar]
- 22. Daley-Yates PT. Inhaled corticosteroids: potency, dose equivalence and therapeutic index. Br J Clin Pharmacol. 2015;80(3):372‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nicolaides NC, Pavlaki AN, Maria Alexandra MA, Chrousos GP. Glucocorticoid therapy and adrenal suppression. In: Feingold KR, Anawalt B, Blackman MR, et al., eds. Endotext. MDText.com, Inc; 2000:4-5. [Google Scholar]
- 24. Broersen LH, Pereira AM, Jørgensen JO, Dekkers OM. Adrenal insufficiency in corticosteroids use: systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100(6):2171‐2180. [DOI] [PubMed] [Google Scholar]
- 25. Laugesen K, Petersen I, Sørensen HT, Jørgensen JOL. Clinical indicators of adrenal insufficiency following discontinuation of oral glucocorticoid therapy: a Danish population-based self-controlled case series analysis. PLoS One. 2019;14(2):e0212259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li D, Genere N, Behnken E, et al. Determinants of self-reported health outcomes in adrenal insufficiency: a multisite survey study. J Clin Endocrinol Metab. 2021;106(3):e1408‐e1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smans LC, Van der Valk ES, Hermus AR, Zelissen PM. Incidence of adrenal crisis in patients with adrenal insufficiency. Clin Endocrinol (Oxf). 2016;84(1):17‐22. [DOI] [PubMed] [Google Scholar]
- 28. Quinkler M, Ekman B, Zhang P, Isidori AM, Murray RD. Mortality data from the European adrenal insufficiency registry-patient characterization and associations. Clin Endocrinol (Oxf). 2018;89(1):30‐35. [DOI] [PubMed] [Google Scholar]
- 29. Mebrahtu TF, Morgan AW, Keeley A, Baxter PD, Stewart PM, Pujades-Rodriguez M. Dose dependency of iatrogenic glucocorticoid excess and adrenal insufficiency and mortality: a cohort study in England. J Clin Endocrinol Metab. 2019;104(9):3757‐3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Movahedi M, Costello R, Lunt M, Pye SR, Sergeant JC, Dixon WG. Oral glucocorticoid therapy and all-cause and cause-specific mortality in patients with rheumatoid arthritis: a retrospective cohort study. Eur J Epidemiol. 2016;31(10):1045‐1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. del Rincón I, Battafarano DF, Restrepo JF, Erikson JM, Escalante A. Glucocorticoid dose thresholds associated with all-cause and cardiovascular mortality in rheumatoid arthritis. Arthritis Rheumatol. 2014;66(2):264‐272. [DOI] [PubMed] [Google Scholar]
- 32. Listing J, Kekow J, Manger B, et al. Mortality in rheumatoid arthritis: the impact of disease activity, treatment with glucocorticoids, TNFα inhibitors and rituximab. Ann Rheum Dis. 2015;74(2):415‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dekkers OM, Burman P. ESE guidelines, why and how. Eur J Endocrinol. 2015;173(2):E1‐E2. [DOI] [PubMed] [Google Scholar]
- 34. Bollerslev J, Rejnmark L, Marcocci C, et al. European society of endocrinology clinical guideline: treatment of chronic hypoparathyroidism in adults. Eur J Endocrinol. 2015;173(2):G1‐20. [DOI] [PubMed] [Google Scholar]
- 35. Langer G, Meerpohl JJ, Perleth M, Gartlehner G, Kaminski-Hartenthaler A, Schünemann H. GRADE guidelines: 1. Introduction—gRADE evidence profiles and summary of findings tables. Z Evid Fortbild Qual Gesundhwes. 2012;106(5):357‐368. [DOI] [PubMed] [Google Scholar]
- 36. Andrews JC, Schünemann HJ, Oxman AD, et al. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation's direction and strength. J Clin Epidemiol. 2013;66(7):726‐735. [DOI] [PubMed] [Google Scholar]
- 37. Guyatt GH, Schünemann HJ, Djulbegovic B, Akl EA. Guideline panels should not GRADE good practice statements. J Clin Epidemiol. 2015;68(5):597‐600. [DOI] [PubMed] [Google Scholar]
- 38. Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(2):364‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fleseriu M, Hashim IA, Karavitaki N, et al. Hormonal replacement in hypopituitarism in adults: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(11):3888‐3921. [DOI] [PubMed] [Google Scholar]
- 40. Baek JH, Kim SK, Jung JH, Hahm JR, Jung J. Recovery of adrenal function in patients with glucocorticoids induced secondary adrenal insufficiency. Endocrinol Metab (Seoul). 2016;31(1):153‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leong SH, Shander S, Ratnasingam J. Predicting recovery of the hypothalamic-pituitary-adrenal axis after prolonged glucocorticoid use. Endocr Pract. 2018;24(1):14‐20. [DOI] [PubMed] [Google Scholar]
- 42. Menzies-Gow A, Gurnell M, Heaney LG, et al. Oral corticosteroid elimination via a personalised reduction algorithm in adults with severe, eosinophilic asthma treated with benralizumab (PONENTE): a multicentre, open-label, single-arm study. Lancet Respir Med. 2022;10(1):47‐58. [DOI] [PubMed] [Google Scholar]
- 43. Abdul AJ, Ghai B, Bansal D, Sachdeva N, Bhansali A, Dhatt SS. Hypothalamic pituitary adrenocortical axis suppression following a single epidural injection of methylprednisolone acetate. Pain Physician. 2017;20(7):E991‐E1001. [PubMed] [Google Scholar]
- 44. Baz-Hecht M, Osher E, Yachnin T, et al. The low-dose (1 microg) adrenocorticotropin stimulation test in kidney and kidney-pancreas transplant patients: a potential guideline for steroid withdrawal. Clin Transplant. 2006;20(1):72‐77. [DOI] [PubMed] [Google Scholar]
- 45. Habib G, Khazin F, Jabbour A, et al. Simultaneous bilateral knee injection of methylprednisolone acetate and the hypothalamic-pituitary adrenal axis: a single-blind case-control study. J Investig Med. 2014;62(3):621‐626. [DOI] [PubMed] [Google Scholar]
- 46. Henzen C, Suter A, Lerch E, Urbinelli R, Schorno XH, Briner VA. Suppression and recovery of adrenal response after short-term, high-dose glucocorticoid treatment. Lancet. 2000;355(9203):542‐545. [DOI] [PubMed] [Google Scholar]
- 47. Jamilloux Y, Liozon E, Pugnet G, et al. Recovery of adrenal function after long-term glucocorticoid therapy for giant cell arteritis: a cohort study. PLoS One. 2013;8(7):e68713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mader R, Lavi I, Luboshitzky R. Evaluation of the pituitary-adrenal axis function following single intraarticular injection of methylprednisolone. Arthritis Rheum. 2005;52(3):924‐928. [DOI] [PubMed] [Google Scholar]
- 49. Nguyen KL, Lauver D, Kim I, Aresery M. The effect of a steroid “burst” and long-term, inhaled fluticasone propionate on adrenal reserve. Ann Allergy Asthma Immunol. 2003;91(1):38‐43. [DOI] [PubMed] [Google Scholar]
- 50. Schuetz P, Leuppi JD, Bingisser R, et al. Prospective analysis of adrenal function in patients with acute exacerbations of COPD: the reduction in the use of corticosteroids in exacerbated COPD (REDUCE) trial. Eur J Endocrinol. 2015;173(1):19‐27. [DOI] [PubMed] [Google Scholar]
- 51. Bazi A, Baghbanian SM, Ghazaeian M, Saeedi M, Hendoiee N. Efficacy and safety of oral prednisolone tapering following intravenous methyl prednisolone in patients with multiple sclerosis relapses: a randomized, double-blind, placebo-controlled trial. Mult Scler Relat Disord. 2021;47:102640. [DOI] [PubMed] [Google Scholar]
- 52. Burmester GR, Buttgereit F, Bernasconi C, et al. Continuing versus tapering glucocorticoids after achievement of low disease activity or remission in rheumatoid arthritis (SEMIRA): a double-blind, multicentre, randomised controlled trial. Lancet. 2020;396(10246):267‐276. [DOI] [PubMed] [Google Scholar]
- 53. O'Driscoll BR, Kalra S, Wilson M, Pickering CA, Carroll KB, Woodcock AA. Double-blind trial of steroid tapering in acute asthma. Lancet. 1993;341(8841):324‐327. [DOI] [PubMed] [Google Scholar]
- 54. Sayiner A, Aytemur ZA, Cirit M, Unsal I. Systemic glucocorticoids in severe exacerbations of COPD. Chest. 2001;119(3):726‐730. [DOI] [PubMed] [Google Scholar]
- 55. Debono M, Elder CJ, Lewis J, et al. Home waking salivary cortisone to screen for adrenal insufficiency. NEJM Evid. 2023;2(2):EVIDoa2200182. [DOI] [PubMed] [Google Scholar]
- 56. Sagar R, Mackie S, A WM, Stewart P, Abbas A. Evaluating tertiary adrenal insufficiency in rheumatology patients on long-term systemic glucocorticoid treatment. Clin Endocrinol (Oxf). 2021;94(3):361‐370. [DOI] [PubMed] [Google Scholar]
- 57. Sbardella E, Isidori AM, Woods CP, et al. Baseline morning cortisol level as a predictor of pituitary-adrenal reserve: a comparison across three assays. Clin Endocrinol (Oxf). 2017;86(2):177‐184. [DOI] [PubMed] [Google Scholar]
- 58. Prete A, Bancos I. Glucocorticoid induced adrenal insufficiency. BMJ. 2021;374:n1380. [DOI] [PubMed] [Google Scholar]
- 59. Shearer NB. Health empowerment theory as a guide for practice. Geriatr Nurs. 2009;30(2):4‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Weiss-Laxer NS, Crandall A, Hughes ME, Riley AW. Families as a cornerstone in 21st century public health: recommendations for research, education, policy, and practice. Front Public Health. 2020;8:503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dineen R, Thompson CJ, Sherlock M. Adrenal crisis: prevention and management in adult patients. Ther Adv Endocrinol Metab. 2019;10:2042018819848218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Repping-Wuts HJ, Stikkelbroeck NM, Noordzij A, Kerstens M, Hermus AR. A glucocorticoid education group meeting: an effective strategy for improving self-management to prevent adrenal crisis. Eur J Endocrinol. 2013;169(1):17‐22. [DOI] [PubMed] [Google Scholar]
- 63. Burger-Stritt S, Eff A, Quinkler M, et al. Standardised patient education in adrenal insufficiency: a prospective multi-centre evaluation. Eur J Endocrinol. 2020;183(2):119‐127. [DOI] [PubMed] [Google Scholar]
- 64. Akalestou E, Genser L, Rutter GA. Glucocorticoid metabolism in obesity and following weight loss. Front Endocrinol (Lausanne). 2020;11:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tornatore KM, Logue G, Venuto RC, Davis PJ. Pharmacokinetics of methylprednisolone in elderly and young healthy males. J Am Geriatr Soc. 1994;42(10):1118‐1122. [DOI] [PubMed] [Google Scholar]
- 66. Waljee AK, Rogers MA, Lin P, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ. 2017;357:j1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cross AS, Helen Kemp E, White A, et al. International survey on high- and low-dose synacthen test and assessment of accuracy in preparing low-dose synacthen. Clin Endocrinol (Oxf). 2018;88(5):744‐751. [DOI] [PubMed] [Google Scholar]
- 68. Yao TC, Huang YW, Chang SM, Tsai SY, Wu AC, Tsai HJ. Association between oral corticosteroid bursts and severe adverse events: a nationwide population-based cohort study. Ann Intern Med. 2020;173(5):325‐330. [DOI] [PubMed] [Google Scholar]
- 69. Hill MR, Szefler SJ, Ball BD, Bartoszek M, Brenner AM. Monitoring glucocorticoid therapy: a pharmacokinetic approach. Clin Pharmacol Ther. 1990;48(4):390‐398. [DOI] [PubMed] [Google Scholar]
- 70. Hochberg Z, Pacak K, Chrousos GP. Endocrine withdrawal syndromes. Endocr Rev. 2003;24(4):523‐538. [DOI] [PubMed] [Google Scholar]
- 71. Zhang CD, Li D, Singh S, et al. Glucocorticoid withdrawal syndrome following surgical remission of endogenous hypercortisolism: a longitudinal observational study. Eur J Endocrinol. 2023;188(7):592‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hurtado MD, Cortes T, Natt N, Young WF Jr, Bancos I. Extensive clinical experience: hypothalamic-pituitary-adrenal axis recovery after adrenalectomy for corticotropin-independent cortisol excess. Clin Endocrinol (Oxf). 2018;89(6):721‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Vogel F, Braun L, Zopp S, et al. Low-grade inflammation during the glucocorticoid withdrawal phase in patients with Cushing's syndrome. Eur J Endocrinol. 2023;188(4):375‐384. [DOI] [PubMed] [Google Scholar]
- 74. Charmandari E, Nicolaides NC, Chrousos GP. Adrenal insufficiency. Lancet. 2014;383(9935):2152‐2167. [DOI] [PubMed] [Google Scholar]
- 75. Crowley RK, Argese N, Tomlinson JW, Stewart PM. Central hypoadrenalism. J Clin Endocrinol Metab. 2014;99(11):4027‐4036. [DOI] [PubMed] [Google Scholar]
- 76. Han HS, Park JC, Park SY, et al. A prospective multicenter study evaluating secondary adrenal suppression after antiemetic dexamethasone therapy in cancer patients receiving chemotherapy: a Korean south west oncology group study. Oncologist. 2015;20(12):1432‐1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jasani MK, Boyle JA, Greig WR, et al. Corticosteroid-induced suppression of the hypothalamo-pituitary-adrenal axis: observations on patients given oral corticosteroids for rheumatoid arthritis. Q J Med. 1967;36(143):261‐276. [PubMed] [Google Scholar]
- 78. Nichols T, Nugent CA, Tyler FH. Diurnal variation in suppression of adrenal function by glucocorticoids. J Clin Endocrinol Metab. 1965;25(3):343‐349. [DOI] [PubMed] [Google Scholar]
- 79. Arshad MF, Elder C, Newell-Price J, Ross R, Debono M. A retrospective study on weaning glucocorticoids and recovery of the hypothalamic-pituitary-adrenal axis. J Clin Endocrinol Metab. Published online January 31, 2024. doi: 10.1210/clinem/dgae059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Li Y, Lu L, Androulakis IP. The physiological and pharmacological significance of the circadian timing of the HPA axis: a mathematical modeling approach. J Pharm Sci. 2024;113(1):33‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Berr CM, Di Dalmazi G, Osswald A, et al. Time to recovery of adrenal function after curative surgery for Cushing's syndrome depends on etiology. J Clin Endocrinol Metab. 2015;100(4):1300‐1308. [DOI] [PubMed] [Google Scholar]
- 82. Prete A, Paragliola RM, Bottiglieri F, et al. Factors predicting the duration of adrenal insufficiency in patients successfully treated for cushing disease and nonmalignant primary adrenal Cushing syndrome. Endocrine. 2017;55(3):969‐980. [DOI] [PubMed] [Google Scholar]
- 83. Richter B, Neises G, Clar C. Glucocorticoid withdrawal schemes in chronic medical disorders. A systematic review. Endocrinol Metab Clin North Am. 2002;31(3):751‐778. [DOI] [PubMed] [Google Scholar]
- 84. Brigell DF, Fang VS, Rosenfield RL. Recovery of responses to ovine corticotropin-releasing hormone after withdrawal of a short course of glucocorticoid. J Clin Endocrinol Metab. 1992;74(5):1036‐1039. [DOI] [PubMed] [Google Scholar]
- 85. Graber AL, Ney RL, Nicholson WE, Island DP, Liddle GW. Natural history of pituitary-adrenal recovery following long-term suppression with corticosteroids. J Clin Endocrinol Metab. 1965;25(1):11‐16. [DOI] [PubMed] [Google Scholar]
- 86. Raff H, Sharma ST, Nieman LK. Physiological basis for the etiology, diagnosis, and treatment of adrenal disorders: Cushing's syndrome, adrenal insufficiency, and congenital adrenal hyperplasia. Compr Physiol. 2014;4:739‐769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Komminoth M, Donath MY, Hepprich M, et al. Glucocorticoid withdrawal and glucocorticoid-induced adrenal insufficiency: study protocol of the randomized controlled «TOASST” (Taper Or Abrupt Steroid STop) multicenter trial. PLoS One. 2023;18(4):e0281585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kazlauskaite R, Evans AT, Villabona CV, et al. Corticotropin tests for hypothalamic-pituitary- adrenal insufficiency: a metaanalysis. J Clin Endocrinol Metab. 2008;93(11):4245‐4253. [DOI] [PubMed] [Google Scholar]
- 89. Kumar R, Carr P, Wassif W. Diagnostic performance of morning serum cortisol as an alternative to short synacthen test for the assessment of adrenal reserve; a retrospective study. Postgrad Med J. 2022;98(1156):113‐118. [DOI] [PubMed] [Google Scholar]
- 90. Bancos I, Erickson D, Bryant S, et al. Performance of free versus total cortisol following cosyntropin stimulation testing in an outpatient setting. Endocr Pract. 2015;21(12):1353‐1363. [DOI] [PubMed] [Google Scholar]
- 91. Kalaria T, Buch H, Agarwal M, et al. Morning serum cortisol is superior to salivary cortisone and cortisol in predicting normal adrenal function in suspected adrenal insufficiency. Clin Endocrinol (Oxf). 2022;96(6):916‐918. [DOI] [PubMed] [Google Scholar]
- 92. Hamrahian AH, Oseni TS, Arafah BM. Measurements of serum free cortisol in critically ill patients. N Engl J Med. 2004;350(16):1629‐1638. [DOI] [PubMed] [Google Scholar]
- 93. Rauschecker M, Abraham SB, Abel BS, et al. Cosyntropin-stimulated serum free cortisol in healthy, adrenally insufficient, and mildly cirrhotic populations. J Clin Endocrinol Metab. 2016;101(3):1075‐1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Manosroi W, Phimphilai M, Khorana J, Atthakomol P. Diagnostic performance of basal cortisol level at 0900-1300h in adrenal insufficiency. PLoS One. 2019;14(11):e0225255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ravindran R, Carter JL, Kumar A, et al. Pre-test cortisol levels in predicting short synacthen test outcome: a retrospective analysis. Clin Med Insights Endocrinol Diabetes. 2022;15:11795514221093316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Charoensri S, Chailurkit L, Muntham D, Bunnag P. Serum dehydroepiandrosterone sulfate in assessing the integrity of the hypothalamic-pituitary-adrenal axis. J Clin Transl Endocrinol. 2017;7:42‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Edo N, Morita K, Ishiwata C, et al. Diagnostic value of standard deviation score of log-transformed serum dehydroepiandrosterone sulfate in patients with hypothalamic-pituitary-adrenal axis insufficiency. Endocr J. 2021;68(11):1337‐1345. [DOI] [PubMed] [Google Scholar]
- 98. Klose M, Jørgensen K, Kristensen L. Characteristics of recovery of adrenocortical function after treatment for Cushing's syndrome due to pituitary or adrenal adenomas. Clin Endocrinol (Oxf). 2004;61(3):394‐399. [DOI] [PubMed] [Google Scholar]
- 99. Pofi R, Feliciano C, Sbardella E, et al. The short synacthen (corticotropin) test can be used to predict recovery of hypothalamo-pituitary-adrenal axis function. J Clin Endocrinol Metab. 2018;103(8):3050‐3059. [DOI] [PubMed] [Google Scholar]
- 100. Woods CP, Argese N, Chapman M, et al. Adrenal suppression in patients taking inhaled glucocorticoids is highly prevalent and management can be guided by morning cortisol. Eur J Endocrinol. 2015;173(5):633‐642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yo WS, Toh LM, Brown SJ, Howe WD, Henley DE, Lim EM. How good is a morning cortisol in predicting an adequate response to intramuscular synacthen stimulation? Clin Endocrinol (Oxf). 2014;81(1):19‐24. [DOI] [PubMed] [Google Scholar]
- 102. Saini J, Garcia RG, Herndon J, Erickson D, Gruber L, Bancos I. Use of overnight metyrapone test in suspected secondary adrenal insufficiency: a retrospective single centre-study. Clin Endocrinol (Oxf). 2023;100(3):203‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ospina NS, Nofal A, Bancos A, et al. ACTH stimulation tests for the diagnosis of adrenal insufficiency: systematic review and meta-analysis. J Clin Endocrinol Metab. 2016;101(2):427‐434. [DOI] [PubMed] [Google Scholar]
- 104. Raschi E, Fusaroli M, Massari F, et al. The changing face of drug-induced adrenal insufficiency in the food and drug administration adverse event reporting system. J Clin Endocrinol Metab. 2022;107(8):e3107‐e3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lapi F, Kezouh A, Suissa S, Ernst P. The use of inhaled corticosteroids and the risk of adrenal insufficiency. Eur Respir J. 2013;42(1):79‐86. [DOI] [PubMed] [Google Scholar]
- 106. Mortimer KJ, Tata LJ, Smith CJ, et al. Oral and inhaled corticosteroids and adrenal insufficiency: a case-control study. Thorax. 2006;61(5):405‐408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. VR A. Inhalational steroids and iatrogenic Cushing's syndrome. Open Respir Med J. 2014;8(1):74‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ahmet A, Kim H, Spier S. Adrenal suppression: a practical guide to the screening and management of this under-recognized complication of inhaled corticosteroid therapy. Allergy Asthma Clin Immunol. 2011;7(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Foisy MM, Yakiwchuk EM, Chiu I, Singh AE. Adrenal suppression and Cushing's syndrome secondary to an interaction between ritonavir and fluticasone: a review of the literature. HIV Med. 2008;9(6):389‐396. [DOI] [PubMed] [Google Scholar]
- 110. Sannarangappa V, Jalleh R. Inhaled corticosteroids and secondary adrenal insufficiency. Open Respir Med J. 2014;8(1):93‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Todd GR, Acerini CL, Buck JJ, et al. Acute adrenal crisis in asthmatics treated with high-dose fluticasone propionate. Eur Respir J. 2002;19(6):1207‐1209. [DOI] [PubMed] [Google Scholar]
- 112. Paragliola RM, Papi G, Pontecorvi A, Corsello SM. Treatment with synthetic glucocorticoids and the hypothalamus-pituitary-adrenal axis. Int J Mol Sci. 2017;18(10):2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Daley-Yates PT, Larenas-Linnemann D, Bhargave C, Verma M. Intranasal corticosteroids: topical potency, systemic activity and therapeutic index. J Asthma Allergy. 2021;14:1093‐1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Guaraldi F, Gori D, Calderoni P, et al. Comparative assessment of hypothalamic-pituitary-adrenal axis suppression secondary to intrabursal injection of different glucocorticoids: a pilot study. J Endocrinol Invest. 2019;42(9):1117‐1124. [DOI] [PubMed] [Google Scholar]
- 115. Lansang MC, Farmer T, Kennedy L. Diagnosing the unrecognized systemic absorption of intra-articular and epidural steroid injections. Endocr Pract. 2009;15(3):225‐228. [DOI] [PubMed] [Google Scholar]
- 116. Habib G, Jabbour A, Salman J, Hakim G, Haddad H. The effect of epidural methylprednisolone acetate injection on the hypothalamic-pituitary-adrenal axis. J Clin Anesth. 2013;25(8):629‐633. [DOI] [PubMed] [Google Scholar]
- 117. Iranmanesh A, Gullapalli D, Singh R, Veldhuis JD. Hypothalamo-pituitary-adrenal axis after a single epidural triamcinolone injection. Endocrine. 2017;57(2):308‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Jacobs S, Pullan PT, Potter JM, Shenfield GM. Adrenal suppression following extradural steroids. Anaesthesia. 1983;38(10):953‐956. [DOI] [PubMed] [Google Scholar]
- 119. Kay J, Findling JW, Raff H. Epidural triamcinolone suppresses the pituitary-adrenal axis in human subjects. Anesth Analg. 1994;79(3):501‐505. [DOI] [PubMed] [Google Scholar]
- 120. Leary J, Swislocki A. Hypothalamic-Pituitary-adrenal suppression and iatrogenic Cushing's syndrome as a complication of epidural steroid injections. Case Rep Endocrinol. 2013;2013:617042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Psomadakis C, Tweddell R, Lewis F. Too much of a good thing? Iatrogenic Cushing syndrome secondary to excessive topical steroid use in lichen sclerosus. Clin Exp Dermatol. 2023;48(4):429‐430. [DOI] [PubMed] [Google Scholar]
- 122. Dinsen S, Baslund B, Klose M, et al. Why glucocorticoid withdrawal may sometimes be as dangerous as the treatment itself. Eur J Intern Med. 2013;24(8):714‐720. [DOI] [PubMed] [Google Scholar]
- 123. Joseph RM, Hunter AL, Ray DW, Dixon WG. Systemic glucocorticoid therapy and adrenal insufficiency in adults: a systematic review. Semin Arthritis Rheum. 2016;46(1):133‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Pelewicz K, Miśkiewicz P. Glucocorticoid withdrawal-an overview on when and how to diagnose adrenal insufficiency in clinical practice. Diagnostics (Basel). 2021;11(4):728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Simpson H, Tomlinson J, Wass J, Dean J, Arlt W. Guidance for the prevention and emergency management of adult patients with adrenal insufficiency. Clin Med (Lond). 2020;20(4):371‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Woodcock T, Barker P, Daniel S, et al. Guidelines for the management of glucocorticoids during the peri-operative period for patients with adrenal insufficiency: guidelines from the Association of Anaesthetists, the Royal College of Physicians and the Society for Endocrinology UK. Anaesthesia. 2020;75(5):654‐663. [DOI] [PubMed] [Google Scholar]
- 127. Chen Cardenas SM, Santhanam P, Morris-Wiseman L, Salvatori R, Hamrahian AH. Perioperative evaluation and management of patients on glucocorticoids. J Endocr Soc. 2022;7(2):bvac185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Hahner S, Ross RJ, Arlt W, et al. Adrenal insufficiency. Nat Rev Dis Primers. 2021;7(1):19. [DOI] [PubMed] [Google Scholar]
- 129. Rushworth RL, Torpy DJ, Falhammar H. Adrenal crisis. N Engl J Med. 2019;381(9):852‐861. [DOI] [PubMed] [Google Scholar]
- 130. Iwasaku M, Shinzawa M, Tanaka S, Kimachi K, Kawakami K. Clinical characteristics of adrenal crisis in adult population with and without predisposing chronic adrenal insufficiency: a retrospective cohort study. BMC Endocr Disord. 2017;17(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Nathan AW, Rose GL. Fatal iatrogenic Cushing's syndrome. Lancet. 1979;1(8109):207. [DOI] [PubMed] [Google Scholar]
- 132. Jansen TL, Van Roon EN. Four cases of a secondary Cushingoid state following local triamcinolone acetonide (Kenacort) injection. Neth J Med. 2002;60(3):151‐153. [PubMed] [Google Scholar]
- 133. Barlow AD, Clarke GA, Kelly MJ. Acute adrenal crisis in a patient treated with rectal steroids. Colorectal Dis. 2004;6(1):62‐64. [DOI] [PubMed] [Google Scholar]
- 134. Prete A, Taylor AE, Bancos I, et al. Prevention of adrenal crisis: cortisol responses to major stress compared to stress dose hydrocortisone delivery. J Clin Endocrinol Metab. 2020;105(7):2262‐2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Quinkler M, Dahlqvist P, Husebye ES, Kämpe O. A European emergency card for adrenal insufficiency can save lives. Eur J Intern Med. 2015;26(1):75‐76. [DOI] [PubMed] [Google Scholar]