Abstract
Background:
As a key treatment goal for patients with symptomatic peripheral artery disease (PAD), improving health status has also become an important end point for clinical trials and performance-based care. An understanding of patient factors associated with 1-year PAD health status is lacking in patients with PAD.
Methods:
The health status of 1073 consecutive patients with symptomatic PAD in the international multicenter PORTRAIT (Patient-Centered Outcomes Related to Treatment Practices in Peripheral Arterial Disease: Investigating Trajectories) registry was measured at baseline and 1 year with the Peripheral Artery Questionnaire (PAQ). The association of 47 patient characteristics with 1-year PAQ scores was assessed using a random forest algorithm. Variables of clinical significance were retained and included in a hierarchical multivariable linear regression model predicting 1-year PAQ summary scores.
Results:
The mean age of patients was 67.7 ± 9.3 years, and 37% were female. Variables with the highest importance ranking in predicting 1-year PAQ summary score were baseline PAQ summary score, Patient Health Questionnaire-8 depression score, Generalized Anxiety Disorder-2 anxiety score, new onset symptom presentation, insurance status, current or prior diagnosis of depression, low social support, initial invasive treatment, duration of symptoms, and race. The addition of 19 clinical variables in an extended model marginally improved the explained variance in 1-year health status (from R2 0.312 to 0.335).
Conclusions:
Patients’ 1-year PAD-specific health status, as measured by the PAQ, can be predicted from 10 mostly psychosocial and socioeconomic patient characteristics including depression, anxiety, insurance status, social support, and symptoms. These characteristics should be validated and tested in other PAD cohorts so that this model can inform risk adjustment and prediction of PAD health status in comparative effectiveness research and performance-based care.
Keywords: Health status, Outcomes research, Peripheral artery disease
Patients with peripheral artery disease (PAD) experience significant functional limitations due to their disease.1 As a result, a key objective in PAD management is to improve health status, including symptoms, functioning, and quality of life. Measuring these patient-reported outcomes has become a primary focus for end points of clinical trials,2-4 supporting comparative effectiveness research, and more recently, as a metric for the quality of clinical care.4-7 To reliably quantify PAD treatment effects, we need to standardize and validate approaches for risk adjustment and prediction, as patients’ individual responses to PAD treatments are variable and impacted by their individual patient characteristics.8,9
A critical gap in PAD research is the development of standardized prediction models that can holistically capture demographics, socioeconomic factors, clinical characteristics, and psychosocial determinants of patients’ health status experience across different PAD treatment modalities. To address this gap, we aimed to develop a 1-year health status prediction model using longitudinal data from an international, prospective registry of patients with PAD who present with new-onset or an exacerbation of claudication. By identifying which patient characteristics have the largest impact on health status, we can identify high-risk patients, develop risk-adjustment strategies for quality performance bench-marking, and design targeted interventions to improve patients’ outcomes.
METHODS
Study design and participants.
The PORTRAIT (Patient-Centered Outcomes Related to Treatment Practices in Peripheral Arterial Disease: Investigating Trajectories) (NCT01419080) registry was an international, prospective registry of patients with symptomatic PAD presenting to vascular outpatient clinics in the United States, the Netherlands, and Australia. The study design for PORTRAIT has been previously described.10 In brief, consecutive patients with objective evidence of symptomatic claudication (Rutherford class I, II, or III with a resting ankle brachial index [ABI] of ≤0.90 or a significant drop in the postexercise ankle pressure of ≥20 mm Hg) were enrolled between June 2011 and December 2015. Patients with critical limb ischemia were excluded. Trained data collectors conducted a baseline interview and used structured case report forms to collect demographics, socioeconomic factors, and medical history. After the initial evaluation, the primary treatment strategy employed within the first 3 months of presentation was categorized as invasive (with endovascular or surgical peripheral vascular intervention) or medical management (including pharmacotherapy, supervised exercise therapy, or other). Standardized case report forms were used to abstract follow-up clinical information (medical history and medications) from the patients’ medical records over 1 year of follow-up. At 3 months, 6 months, and 1 year, telephone interviews were used to obtain information about patients’ health status. This study complies with the Declaration of Helsinki, and all participating centers obtained institutional review board approval. All patients provided informed consent.
Candidate predictor variables.
Forty-seven variables were considered in this analysis. Demographic variables included age, sex (male or female), race (White or non-White), marital status (married or unmarried), and country (United States or non-United States). Socioeconomic status was assessed by collecting education level (greater than high school education or not), current employment status (yes or no), insurance status (insured or uninsured), and avoidance of care due to cost (yes or no). Psychosocial factors included the Patient Health Questionnaire-2 (PHQ-2) score to screen for depressive symptoms, the Perceived Stress Scale-4 (PSS-4) score to assess perceived stress levels, and the Generalized Anxiety Disorder-2 (GAD-2) score to screen for generalized anxiety symptoms.11-13 These scores were modeled as continuous variables. The ENRICHD Social Support Instrument was used to assess for the presence of social support (low or normal/high social support).14 Comorbidities included a history of alcohol use, atrial fibrillation, body mass index, cancer, chronic back pain, chronic kidney disease, chronic lung disease, congestive heart failure, depression, diabetes, dyslipidemia, hypertension, osteoarthritis, prior coronary artery disease, stroke, transient ischemic attack, sleep apnea, and tobacco smoking status. PAD-related characteristics included duration of PAD symptoms (1-6 months or more than 6 months), legs involved (single or both), primary treatment strategy (invasive or noninvasive medical management), history of peripheral surgical bypass, history of endovascular treatment, PAD presentation type (new PAD symptoms or exacerbation of established PAD symptoms), Rutherford category (mild/moderate or severe),15 ABI (analyzed continuously), and symptom type (typical or atypical symptoms derived from the San Diego Claudication Questionnaire16). Postoperative complications were only captured in the United States sites and were uncommon (n = 32 events, 4.8%) and too low to include as a factor in the model. PAD care metrics included referral to supervised exercise therapy, statin use, antiplatelet use (aspirin or clopidogrel), and claudication medications (cilostazol/pentoxifylline). Additional variables included leisure time physical activity (high vs low),17 baseline PAD-specific health status measured with the PAQ summary score, and generic health status measured with the EuroQoL Five Dimensions Questionnaire visual analog scale score.18
Outcome measures.
The main outcome in this study was 1-year PAD-specific health status after initial PAD workup. Health status was measured using the PAQ, which is a 20-item questionnaire disease-specific instrument measuring the following domains: physical limitation, symptoms, quality of life, social limitation, and treatment satisfaction. A summary score is calculated from the physical limitation, symptom, social limitation, and quality of life domains. The PAQ domains and summary scores have a range of 0 to 100, with higher scores indicating better health status. The PAQ is a commonly used disease-specific instrument to quantify the health status impact of PAD19 and has been used in randomized trials testing the efficacy of PAD treatments, as well as in observational PAD registries.10,20-22 The PAQ has been shown to be sensitive to changes in clinical improvement in patients with symptomatic PAD undergoing elective revascularization with internally reliability (Cronbach’s α = 0.80-0.90) and correlation to other measures of patient health status (including the EuroQoL Five Dimensions Questionnaire).19,23 Lower PAQ scores in patients with claudication have also been shown to correlate, albeit weakly, to level of anatomic disease,24 lower ABI values,25 and an increased risk of poor clinical outcomes including major adverse cardiovascular events, major adverse limb events, and lower extremity revascularization.26
Statistical analysis.
Patient characteristics were summarized as means (standard deviation) or frequencies (%) and compared using standardized mean differences. Standardized differences below 10% or below 20% were considered negligible or small, respectively.27 Participants with missing 1-year PAQ summary scores were excluded from the analysis, and missing covariate data were imputed using Multiple Imputation in Chained Equations.
A random forest regression was applied to generate importance weights for all 47 candidate variables, with 1-year PAQ summary score as the outcome. Variables with the highest importance weights before a change in the regression slope were used to build a multivariable linear regression (base model). The random forest model was used to improve the classification of variables according to importance and enhance the factor selection for a linear regression model. Although random forest models provide excellent information on which variables are important, they do not provide information on the directionality of the association. Linear models provide risk estimates in the form of mean change in PAQ scores as well as confidence intervals (CIs) that can be readily used by clinicians.
Next, interactions were tested between treatment type (invasive vs noninvasive) and each variable in the linear regression model, and only significant main and interaction effects (P < .05) were retained in this base model. A correlation matrix using Pearson correlation coefficients was calculated for all variables in the linear regression model, with a correlation of >0.7 among two or more predictors indicating multicollinearity. The variance inflation factor (VIF) was also used to assess the correlation between independent variables and other predictors, with a VIF ≥5 indicating high multicollinearity. The base model was stratified by country (United States vs non-United States). We explored whether reintroducing 19 variables previously excluded from the base model (that are used clinically and have associations with health status in the literature) would dramatically improve model performance in an extended model. The additional variables in the extended model included age, sex, education, avoidance of care due to cost, body mass index, ABI, history of back pain, coronary artery disease, cancer, chronic kidney disease, diabetes, lung disease, osteoarthritis, prior peripheral vascular intervention, sleep apnea, stroke, tobacco use, PAD symptom type (typical or atypical), and leisure activity level.
Model fit statistics (R-squared, Akaike information criterion or AIC, and Bayesian information criterion or BIC28) were assessed for the base and extended models. Models were compared using a likelihood ratio test. A P value of less than .05 was considered statistically significant, and all tests were two-tailed. All statistical analyses were performed using Stata Statistical Software: Release 16 (StataCorp LLC).
RESULTS
Patient cohort.
Of the 1275 patients enrolled in the PORTRAIT registry, 202 were excluded because of missing 1-year PAQ scores (15.8%, Supplementary Table I, online only). Table I shows the baseline characteristics of the 1073 patients included in this analysis. The mean age was 67.7 ± 9.3 years, and 37.0% were female. Most patients were enrolled from the United States (62.1%) and of White race (82.4% overall, with 72.5% in the United States and 98.5% outside of the United States). Patients from the United States were less likely to be married (54.8% vs 67.3%), more likely to have a high school or higher education level (86.5% vs 43.4%), and had higher baseline PSS-4 stress scores (all standardized mean difference >0.2). The most common comorbidities in the full cohort were tobacco use (89.4%), hypertension (80.6%), dyslipidemia (79.9%), prior percutaneous coronary intervention or coronary artery bypass graft (35.6%), and diabetes (32.6%). The mean ABI was 0.67 ± 0.19; 28.5% of patients had severe claudication symptoms according to the Rutherford classification, and 52.5% were presenting with new onset symptoms, with most (67.8%) lasting more than 6 months. The mean PAQ summary score at baseline was 49.8 ± 21.7. An invasive primary treatment strategy was used in 281 patients (26.2%).
Table I.
Baseline characteristics of included participants from the PORTRAIT registry
| Characteristic | Total | Non-United States | United States | P value | SMD |
|---|---|---|---|---|---|
| Demographic | |||||
| Age, yearsa | 67.65 (9.27) | 65.74 (8.79) | 68.81 (9.37) | <.001 | −0.337 |
| Sex | |||||
| Male | 676 (63.0) | 282 (69.3) | 394 (59.2) | <.001 | 0.212 |
| Female | 397 (37.0) | 125 (30.7) | 272 (40.8) | ||
| Race | |||||
| Non-White | 189 (17.6) | 6 (1.5) | 183 (27.5) | <.001 | 0.795 |
| White | 884 (82.4) | 401 (98.5) | 483 (72.5) | ||
| Married | 636 (59.6) | 274 (67.3) | 362 (54.8) | <.001 | 0.258 |
| Country | |||||
| The United States | 666 (62.1) | 0 (0.0) | 666 (100.0) | <.001 | 19.096 |
| The Netherlands | 324 (30.2) | 324 (79.6) | 0 (0.0) | ||
| Australia | 83 (7.7) | 83 (20.4) | 0 (0.0) | ||
| Socioeconomic | |||||
| Education—high school or above | 749 (70.3) | 174 (43.4) | 575 (86.5) | <.001 | 1.012 |
| Insured | 1064 (99.2) | 406 (99.8) | 658 (98.8) | .165a | 0.113 |
| Working for pay | 260 (24.3) | 110 (27.0) | 150 (22.7) | .109 | 0.1 |
| Avoidance of care due to cost | 148 (13.9) | 43 (10.6) | 105 (15.9) | .016 | 0.156 |
| Psychosocial | |||||
| PHQ-8 depression scorea | 4.46 (4.80) | 4.33 (4.35) | 4.54 (5.05) | .498 | −0.044 |
| PSS-4 stress scorea | 3.81 (3.30) | 3.31 (2.94) | 4.11 (3.47) | <.001 | −0.25 |
| GAD-2 anxiety scorea | 0.95 (1.51) | 0.90 (1.43) | 0.98 (1.56) | .353 | −0.059 |
| Low social support (ESSI) | 167 (15.7) | 67 (16.7) | 100 (15.1) | .504 | 0.042 |
| Comorbidities | |||||
| Alcohol abuse | 63 (5.9) | 34 (8.4) | 29 (4.4) | .007 | 0.165 |
| Atrial fibrillation | 125 (11.6) | 34 (8.4) | 91 (13.7) | .009 | 0.17 |
| Cancer | 109 (10.2) | 42 (10.3) | 67 (10.1) | .891 | 0.009 |
| Chronic back pain | 149 (13.9) | 62 (15.2) | 87 (13.1) | .318 | 0.062 |
| Chronic kidney disease | 115 (10.7) | 17 (4.2) | 98 (14.7) | <.001 | 0.366 |
| Chronic lung disease | 176 (16.4) | 78 (19.2) | 98 (14.7) | .056 | 0.119 |
| Congestive heart failure | 104 (9.7) | 11 (2.7) | 93 (14.0) | <.001 | 0.416 |
| Depression, current | 113 (10.5) | 23 (5.7) | 90 (13.5) | <.001 | 0.27 |
| Diabetes | 350 (32.6) | 99 (24.3) | 251 (37.7) | <.001 | 0.292 |
| Dyslipidemia | 857 (79.9) | 263 (64.6) | 594 (89.2) | <.001 | 0.609 |
| Hypertension | 865 (80.6) | 268 (65.8) | 597 (89.6) | <.001 | 0.597 |
| Osteoarthritis (hip or knee) | 98 (9.1) | 27 (6.6) | 71 (10.7) | .026 | 0.144 |
| Prior coronary intervention | 382 (35.6) | 81 (19.9) | 301 (45.2) | <.001 | 0.561 |
| Prior CVA | 80 (7.5) | 27 (6.6) | 53 (8.0) | .423 | 0.051 |
| Prior TIA | 55 (5.1) | 28 (6.9) | 27 (4.1) | .042 | 0.125 |
| Sleep apnea | 90 (8.4) | 14 (3.4) | 76 (11.4) | <.001 | 0.308 |
| Smoking status | 957 (89.4) | 379 (93.3) | 578 (86.9) | <.001 | 0.217 |
| BMI, m2/kga | 29.10 (6.05) | 27.35 (4.88) | 29.64 (6.26) | <.001 | −0.408 |
| PAD characteristics | |||||
| Duration of symptoms—>6 months | 626 (67.8) | 187 (60.5) | 439 (71.5) | <.001 | 0.233 |
| Symptomatic leg(s)—single | 530 (49.4) | 239 (58.7) | 291 (43.7) | <.001 | 0.304 |
| Prior surgical bypass | 83 (7.7) | 15 (3.7) | 68 (10.2) | <.001 | 0.259 |
| Prior endovascular intervention | 291 (27.1) | 66 (16.2) | 225 (33.8) | <.001 | 0.414 |
| Symptom presentation—new onset | 560 (52.2) | 293 (72.0) | 267 (40.1) | <.001 | 0.679 |
| Rutherford symptom classification | |||||
| Mild/moderate | 758 (71.5) | 294 (73.9) | 464 (70.1) | .187 | 0.084 |
| Severe | 302 (28.5) | 104 (26.1) | 198 (29.9) | ||
| ABIa | 0.67 (0.19) | 0.65 (0.18) | 0.68 (0.19) | .027 | −0.14 |
| Symptom type—typical | 643 (84.9) | 298 (84.2) | 345 (85.6) | .584 | 0.04 |
| Initial invasive treatment | 281 (26.2) | 78 (19.2) | 203 (30.5) | <.001 | 0.264 |
| PAD care metrics | |||||
| Referral to SET | 229 (22.7) | 219 (59.0) | 10 (1.6) | <.001 | 1.602 |
| Statin prescribed | 864 (81.0) | 324 (80.6) | 540 (81.2) | .807 | 0.015 |
| Antiplatelet prescribed | 824 (77.2) | 224 (55.7) | 600 (90.2) | <.001 | 0.843 |
| Cilostazol prescribed | 129 (12.1) | 0 (0.0) | 129 (19.4) | <.001 | 0.68 |
| Physical activity level—sedentary | 414 (39.2) | 133 (34.0) | 281 (42.3) | .008 | 0.17 |
| Baseline health status | |||||
| EQ-5D-VAS scorea | 66.66 (19.00) | 66.37 (16.65) | 66.82 (20.17) | .721 | −0.024 |
| PAQ summary scorea | 49.75 (21.68) | 53.00 (20.46) | 47.77 (22.17) | <.001 | 0.245 |
ABI, Ankle brachial index; BMI, body mass index; CVA, cerebrovascular accident; EQ-5D-VAS, EuroQoL Five Dimensions Questionnaire visual analog scale; ESSI, ENRICHD Social Support Inventory; GAD-2, Generalized Anxiety Disorder-2 Item; PAD, peripheral artery disease; PAQ, Peripheral Artery Questionnaire; PHQ-8, Patient Health Questionnaire-8 Item; PORTRAIT, Patient-Centered Outcomes Related to Treatment Practices in Peripheral Arterial Disease: Investigating Trajectories; PSS-4, Perceived Stress Scale-4 Item; SET, supervised exercise therapy; SMD, standardized mean difference; TIA, transient ischemic attack.
Analyzed continuously as mean (standard deviation); all other variables shown as number (%).
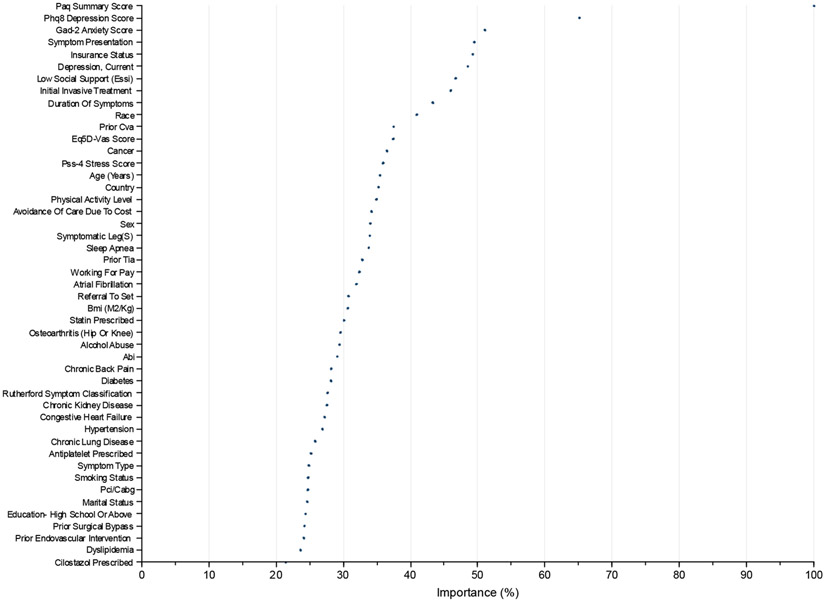
Predictors of 1-year health status.
Variables with the highest importance in the initial random forest model were baseline PAQ summary score, PHQ-8 depression score, GAD-2 anxiety score, new onset symptom presentation, insurance status (in the United States), current or prior diagnosis of depression, low social support, initial invasive treatment, duration of symptoms, and race (Fig 1). These variables were retained in the linear regression model. Baseline PAQ summary score and GAD-2 anxiety score had a significant interaction with primary treatment type (P value <.05), and this interaction term was additionally retained in the model. The correlation matrix (Supplementary Table II, online only) showed no significant multicollinearity between predictor variables (all Pearson correlation coefficients <0.7; mean VIF of 1.26 with no values ≥5).
Fig 1.
Random forest model importance weights for the 1-year Peripheral Artery Questionnaire (PAQ) summary score. ABI, Ankle brachial index; BMI, body mass index; CABG, coronary artery bypass graft; CVA, cerebrovascular accident; EQ-5D-VAS, EuroQoL Five Dimensions Questionnaire visual analog scale; ESSI, ENRICHD Social Support Inventory; GAD-2, Generalized Anxiety Disorder-2 Item; PCI, percutaneous coronary intervention; PHQ-8, Patient Health Questionnaire-8 Item; PSS-4, Perceived Stress Scale-4 Item; SET, supervised exercise therapy; TIA, transient ischemic attack.
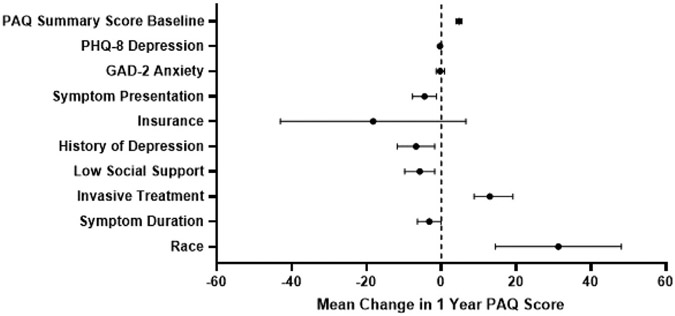
In the linear regression model, the following variables were significantly associated with 1-year PAQ summary score: baseline PAQ summary score, new onset symptom presentation, current or prior diagnosis of depression, low social support, initial invasive treatment, duration of symptoms, and race (Fig 2, Supplementary Table III, online only). Higher baseline PAQ summary score (4.74; 95% CI: 3.93, 5.54; P < .001; per 10-point increase), undergoing initial invasive treatment (12.93; 95% CI: 8.75, 19.11; P < .001), and White race (31.29; 95% CI: 14.45, 48.12; P < .001) were all associated with higher 1-year PAQ summary scores. Presenting with a symptom exacerbation as opposed to new onset of symptoms (−4.51; 95% CI: −7.71, −1.31; P = .006), history or current diagnosis of depression (−6.77; 95% CI: −11.75, −1.77; P = .008), low social support (−5.77; 95% CI: −9.79, −1.76; P = .005), and symptom duration of more than 6 months (−3.23; 95% CI: −6.37, −0.09; P = .044) were all associated with lower 1-year PAQ summary scores.
Fig 2.
Forest plot with the base model of factors predicting 1-year Peripheral Artery Questionnaire (PAQ) summary scores for patients with symptomatic peripheral artery disease (PAD) in all countries (the United States, Australia, and the Netherlands). Variables are shown on the y-axis, with the mean change in PAQ score on the x-axis. The mean change in PAQ score for each variable is represented by the dot, with the confidence interval shown with the bars. A dotted line is shown at a mean PAQ score of 0, with the values on the left indicating a worse health status score and those on the right indicating a better health status score. GAD-2, Generalized Anxiety Disorder-2 Item scale; PHQ-8, Patient Health Questionnaire-8 Item.
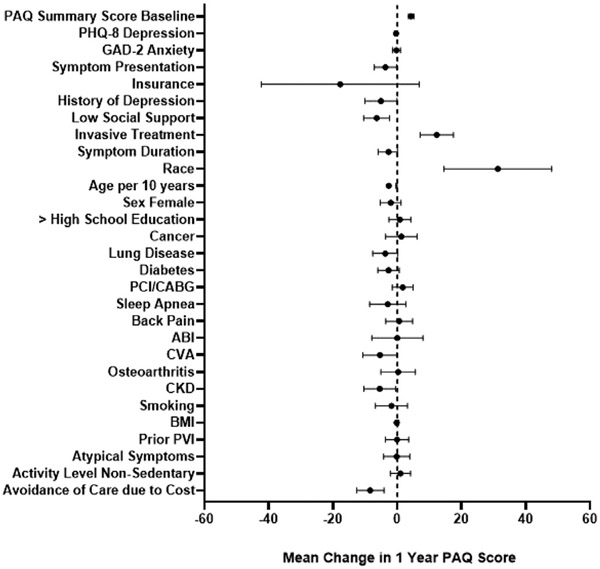
The base model was expanded to include 19 additional variables of potential relevance to PAD health status and outcomes (Fig 3, Supplementary Table III, online only). There was a very small (2%) absolute difference in variance of 1-year PAQ summary score explained between the extended model (R2 = 0.335, 29 variables) and the base model (R2 = 0.312, 10 variables) despite containing three times more variables. Model fit statistics and performance for both models are summarized in Table II.
Fig 3.
Forest plot with the extended model of factors predicting 1-year Peripheral Artery Questionnaire (PAQ) summary scores for patients with symptomatic peripheral artery disease (PAD) in all countries (the United States, Australia, and the Netherlands). Variables are shown on the y-axis, with the mean change in PAQ score on the x-axis. The mean change in PAQ score for each variable is represented by the dot, with the confidence interval shown with the bars. A dotted line is shown at a mean PAQ score of 0, with the values on the left indicating a worse health status score and those on the right indicating a better health status score. ABI, Ankle brachial index; BMI, body mass index; CABG, coronary artery bypass graft; CKD, chronic kidney disease; CVA, cerebrovascular accident; GAD-2, Generalized Anxiety Disorder-2 Item; PCI, percutaneous coronary intervention; PHQ-8, Patient Health Questionnaire-8 Item; PVI, peripheral vascular intervention.
Table II.
Model fit statistics for the base and extended models
| Base model | Extended model | |
|---|---|---|
| R2 | 0.313 | 0.334 |
| AIC (smaller is better) | 7352.0 | 7344.2 |
| BIC (smaller is better) | 7441.6 | 7523.4 |
| Likelihood ratio test | P < .001 | |
AIC, Akaike information criteria; BIC, Bayesian information criteria.
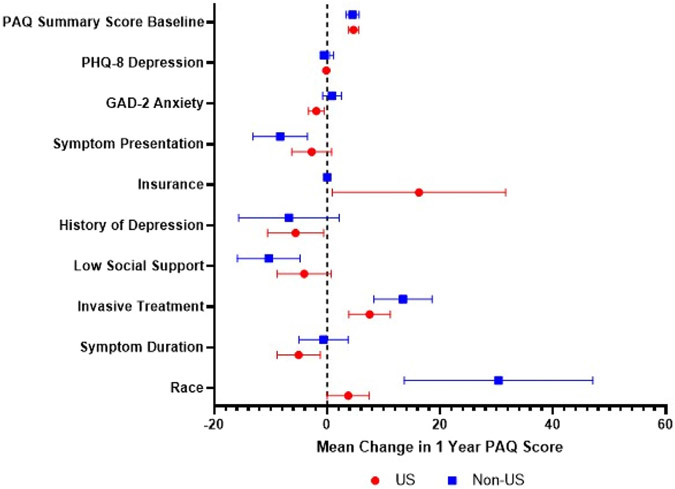
When stratified by country, the mean differences of insurance and race were different between patients within the United States and outside the United States (Australia/the Netherlands) (Fig 4, Supplementary Table IV, online only). Having insurance in the United States was associated with higher 1-year PAQ summary scores (16.3; 95% CI: 0.90, 31.6; P = .038) but was unable to be assessed outside the United States as all patients were insured. White race outside of the United States was associated with higher PAQ summary scores (30.35; 95% CI: 13.64, 47.07; P < .001) compared with within the United States (3.74; 95% CI: 0.02, 7.48; P = .049).
Fig 4.
Forest plot with the base model of factors predicting 1-year Peripheral Artery Questionnaire (PAQ) summary scores for patients with symptomatic peripheral artery disease (PAD) stratified by the United States vs non-United States (the Netherlands/Australia). Variables are shown on the y-axis, with the mean change in PAQ score on the x-axis. The mean change in PAQ score for each variable is represented by the dot, with the confidence interval shown with the bars. A dotted line is shown at a mean PAQ score of 0, with the values on the left indicating a worse health status score and those on the right indicating a better health status score. GAD-2, Generalized Anxiety Disorder-2 Item scale; PHQ-8, Patient Health Questionnaire-8 Item.
DISCUSSION
In this study, we have identified 10 relatively easily obtainable patient characteristics that help to explain the variation in 1-year health status (as measured by the PAQ) for patients presenting with symptomatic PAD, which corresponds with the expected performance of models predicting human behavior and functioning.29,30 This novel model includes baseline health status in combination with a range of other variables, including demographics (race), psychosocial (depression and low social support), and PAD-related characteristics (initial invasive treatment, symptom presentation, and current exacerbation of symptoms). Expanding the model to include additional variables did not substantially improve the model’s prediction performance, suggesting that these 10 characteristics measured in PORTRAIT are important in the prediction of a patient’s 1-year health status. In addition to key comorbidities and symptoms, socioeconomic and psychosocial factors need to be considered during the evaluation of patients with symptomatic PAD. Assessment of these factors should be feasible from a clinical standpoint, as variables were retained that are mostly routinely available as part of a patient’s clinical evaluation.
Our work provides novel information on which patient characteristics may best predict health status. Our results are also unique as we were able to incorporate a wider range of patient and treatment characteristics to provide for a more holistic assessment as to which factors may impact their PAD-specific health status.19 While previously published work from the PORTRAIT registry has identified factors like early invasive treatment as associated with better health status,31 most other data sources do not have access to factors that fit the biopsychosocial model of disease,32 or used only generic or one-dimensional instruments of functioning that do not capture all relevant domains of PAD-specific health status.33,34 When considering which factors impact outcomes for patients, we need a broader assessment of risk that include these biopsychosocial variables and the social determinants of health that impact patients’ functional status and quality of life.
As clinical trials and comparative effectiveness research are increasingly focusing on patient-reported outcomes as primary end points of PAD treatment evaluation,35,36 it is important that we understand potential heterogeneity of treatment effects and factors that may impact patients’ health status functioning. Traditional biomedical factors have a limited ability to explain health status outcomes in PAD,25 and broader assessments of risk are needed. Psychosocial factors (including depression,37,38 stress,39 and socioeconomic status40-42) have been linked with poor health status and clinical outcomes in patients with PAD. This has consequences for the nature of care delivered that may impact PAD-specific health status outcomes.
Health status and other patient-reported outcomes are also becoming of increasing importance as indicators of the quality of care delivered to patients, impacting novel reimbursement models.7,41 We need to be able to appropriately risk-adjust models and benchmarking criteria according to patients’ health status in order to set quality of care standards in PAD similar to other clinical quality benchmarks (eg, 30-day readmission rates for heart failure).6 Expected treatment benefits may differ depending on patients’ baseline health status, other medical conditions, and broader determinants of health (including patients’ mental health or access to care issues). These factors may moderate expected treatment gains and/or may need additional resources to address them. Apart from risk adjustment, our model also identified modifiable factors that can prompt further evaluation and targeted interventions (such as behavioral health and care management), which may improve the health status of patients with PAD.43,44 Implementing programs to address these factors would require a population health management strategy with infrastructure to monitor and address psychosocial comorbidities in the clinic. Implementation science methods and a more rigorous study of the best practices to integrate psychosocial screening and potentially mental health care in the care of vascular patients would be prudent.
The importance of validation and replication of prediction models is underscored by the interactions we found, depending on the context patients with PAD are receiving their care. Being able to leverage the international nature of the PORTRAIT registry highlighted that health care contexts matter. The Netherlands/Australia have a different health care system characterized by broader and affordable access and coverage for health services, as compared with the United States.45,46 Future validation work across other registries, settings, and patient cohorts to further refine risk adjustment modeling and generate a risk score for health status to be used in a clinical setting would be beneficial.
Our study has the following limitations. First, patients excluded due to missing 12-month PAQ scores had higher baseline PHQ-8 depression scores, PSS-4 stress scores, and GAD-2 anxiety scores, and were more likely to have a history of depression and to be sedentary (Supplementary Table I, online only), which may limit the generalizability of the results. Second, the initial and full models predicting 1-year health status only explained around 30% of the variation in 1-year health status. This is in line with other prediction models for health status and highlights the challenge of achieving high variance explanation for health status outcomes.33,47,48 Third, we cannot make causal inferences between predictors and health status outcomes due to the observational nature of this study. Finally, despite internal validation analyses, further work is needed to extend generalizability of our findings to patients seen outside the PORTRAIT study consortium.
CONCLUSIONS
A simple model consisting of 10 relatively easy obtainable characteristics was able to predict in health status at 1 year among patients with symptomatic PAD undergoing routine clinical care. The model can be used to identify sources of heterogeneity of health status responses among patients from a complete biopsychosocial perspective and can be used as a model for risk adjustment for performance-based care.
Supplementary Material
ARTICLE HIGHLIGHTS.
Type of Research: Retrospective review of prospectively collected multicenter cohort study data
Key Findings: In 1073 patients with symptomatic peripheral artery disease, the patient characteristics most predictive of 1-year health status (as measured by the Peripheral Artery Questionnaire) were baseline health status, depression, anxiety, low social support, new onset symptoms, duration of symptoms, initial invasive treatment, insurance status, and race.
Take Home Message: Patients’ 1-year peripheral artery disease-specific health status can be predicted from 10 mostly psychosocial and socioeconomic patient characteristics.
Acknowledgments
The research reported in this paper was partially funded through a Patient-Centered Outcomes Research Institute (PCORI) award (IP2 PI000753-01; CE-1304-6677), the Netherlands Organization for Scientific Research (VENI grant no. 916.11.179), and an unrestricted grant from W. L. Gore & Associates, Inc. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH). The statements in this paper are solely the responsibility of the authors and do not necessarily represent the views of the PCORI, its Board of Governors or Methodology Committee. All manuscripts for the PORTRAIT study are prepared by independent authors who are not governed by the funding sponsors and are reviewed by an academic publications committee before submission. The funding organizations and sponsors of the study had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
DISCLOSURES
J.A.S. is a consultant to Bayer, Janssen, Merck, MyoKardia, Novartis, Terumo, and United Healthcare. He receives grant support from Janssen and MyoKardia. He holds the copyright to the Peripheral Artery Questionnaire, Kansas City Cardiomyopathy Questionnaires, and the Seattle Angina Questionnaire. He also serves on the Board of Blue Cross/Blue Shield of Kansas City. E.S. is supported by NIH/National Heart, Lung, and Blood Institute (K23HL150290), Food & Drug Administration, BD, Boston Scientific, Cook, CSI, Laminate Medical, Medtronic, and Philips. He is a consultant for Abbott, Bayer, BD, Boston Scientific, Cook, CSI, Inari, Infraredx, Janssen, Medtronic, Philips, Shockwave, and VentureMed. K.G.S. reports unrestricted research grants from Cardiva Medical Inc, Cook Medical, Inc, Merck & Co, Inc, Shockwave Medical Inc, and Janssen Pharmaceutical Companies of Johnson & Johnson. She is a consultant for Optum Labs, Inc, and Abbott Laboratories. C.M-H. reports grant funding from Shockwave Medical, Inc and is a consultant for Abbott Laboratories, Cook Medical, Inc, and Optum Labs, Inc. The other authors do not have conflict of interest or financial ties to disclose.
Footnotes
Additional material for this article may be found online at www.jvascsurg.org.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
REFERENCES
- 1.McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–1606. [DOI] [PubMed] [Google Scholar]
- 2.Guidance for Industry. Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. U.S. Department of Health and Human Services Food and Drug Administration; 2009. [Google Scholar]
- 3.Bradley SM. The routine clinical capture of patient-reported outcomes: how competition on value will lead to change. Circ Cardiovasc Qual Outcomes. 2014;7:635–636. [DOI] [PubMed] [Google Scholar]
- 4.Wright CV, Goodheart C, Bard D, et al. Promoting measurement-based care and quality measure development: the APA mental and behavioral health registry initiative. Psychol Serv. 2020;17:262. [DOI] [PubMed] [Google Scholar]
- 5.Glied SA, Stein BD, McGuire TG, et al. Measuring performance in psychiatry: a call to action. Psychiatr Serv. 2015;66:872–878. [DOI] [PubMed] [Google Scholar]
- 6.Basch E, Torda P, Adams K. Standards for patient-reported outcome—based performance measures. JAMA. 2013;310:139–140. [DOI] [PubMed] [Google Scholar]
- 7.Van Der Wees PJ, Nijhuis-Van Der Sanden MW, Ayanian JZ, Black N, Westert GP, Schneider EC. Integrating the use of patient-reported outcomes for both clinical practice and performance measurement: views of experts from 3 countries. Milbank Q. 2014;92:754–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenfield S, Kravitz R, Duan N, Kaplan SH. Heterogeneity of treatment effects: implications for guidelines, payment, and quality assessment. Am J Med. 2007;120(Suppl 1):S3–S9. [DOI] [PubMed] [Google Scholar]
- 9.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;69:1465–1508. [DOI] [PubMed] [Google Scholar]
- 10.Smolderen KG, Gosch K, Patel M, et al. PORTRAIT (Patient-Centered Outcomes Related to Treatment Practices in Peripheral Arterial Disease: Investigating Trajectories): overview of design and rationale of an international prospective peripheral arterial disease study. Circ Cardiovasc Qual Outcomes. 2018;11:e003860. [DOI] [PubMed] [Google Scholar]
- 11.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. [DOI] [PubMed] [Google Scholar]
- 12.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284–1292. [DOI] [PubMed] [Google Scholar]
- 13.Warttig SL, Forshaw MJ, South J, White AK. New, normative, English-sample data for the Short Form Perceived Stress Scale (PSS-4). J Health Psychol. 2013;18:1617–1628. [DOI] [PubMed] [Google Scholar]
- 14.Vaglio J Jr, Conard M, Poston WS, et al. Testing the performance of the ENRICHD Social Support Instrument in cardiac patients. Health Qual Life Outcomes. 2004;2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517–538. [DOI] [PubMed] [Google Scholar]
- 16.Criqui MH, Denenberg JO, Bird CE, Fronek A, Klauber MR, Langer RD. The correlation between symptoms and non-invasive test results in patients referred for peripheral arterial disease testing. Vasc Med. 1996;1:65–71. [DOI] [PubMed] [Google Scholar]
- 17.Held C, Iqbal R, Lear SA, et al. Physical activity levels, ownership of goods promoting sedentary behaviour and risk of myocardial infarction: results of the INTERHEART study. Eur Heart J. 2012;33:452–466. [DOI] [PubMed] [Google Scholar]
- 18.EuroQol—a new facility for the measurement of health-related quality of life. Health Pol. 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 19.Spertus J, Jones P, Poler S, Rocha-Singh K. The peripheral artery questionnaire: a new disease-specific health status measure for patients with peripheral arterial disease. Am Heart J. 2004;147:301–308. [DOI] [PubMed] [Google Scholar]
- 20.Jones WS, Baumgartner I, Hiatt WR, et al. Ticagrelor compared with clopidogrel in patients with prior lower extremity revascularization for peripheral artery disease. Circulation. 2017;135:241–250. [DOI] [PubMed] [Google Scholar]
- 21.Wu A, Coresh J, Selvin E, et al. Lower extremity peripheral artery disease and quality of life among older individuals in the community. J Am Heart Assoc. 2017;6:e004519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bunte MC, Cohen DJ, Jaff MR, et al. Long-term clinical and quality of life outcomes after stenting of femoropopliteal artery stenosis: 3-year results from the STROLL study. Catheter Cardiovasc Interv. 2018;92:106–114. [DOI] [PubMed] [Google Scholar]
- 23.Smolderen KG, Hoeks SE, Aquarius AE, et al. Further validation of the peripheral artery questionnaire: results from a peripheral vascular surgery survey in the Netherlands. Eur J Vasc Endovasc Surg. 2008;36:582–591. [DOI] [PubMed] [Google Scholar]
- 24.Vogel TR, Braet DJ, Kruse RL, et al. Level of disease and association with health status in patients presenting with claudication from the PORTRAIT registry. J Vasc Surg. 2020;72:2017–2026. [DOI] [PubMed] [Google Scholar]
- 25.Johnston AL, Vemulapalli S, Gosch KL, et al. Ankle-brachial index in patients with intermittent claudication is a poor indicator of patient-centered and clinician-based evaluations of functional status. J Vasc Surg. 2019;69:906–912. [DOI] [PubMed] [Google Scholar]
- 26.Rymer JA, Mulder H, Smolderen KG, et al. Association of health status scores with cardiovascular and limb outcomes in patients with symptomatic peripheral artery disease: insights from the EUCLID (Examining Use of Ticagrelor in Symptomatic Peripheral Artery Disease) trial. J Am Heart Assoc. 2020;9:e016573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Routledge; 2013. [Google Scholar]
- 28.McElreath R. Statistical Rethinking: A Bayesian Course with Examples in R and Stan. Chapman and Hall/CRC; 2018. [Google Scholar]
- 29.Hair JF, Ringle CM, Sarstedt M. Partial least squares structural equation modeling: rigorous applications, better results and higher acceptance. Long Range Plan. 2013;46:1–12. [Google Scholar]
- 30.Falk RF, Miller NB. A Primer for Soft Modeling. University of Akron Press; 1992. [Google Scholar]
- 31.Angraal S, Hejjaji V, Tang Y, et al. One-year health status outcomes following early invasive and noninvasive treatment in symptomatic peripheral artery disease. Circ Cardiovasc Interv. 2022;15:e011506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engel GL. The clinical application of the biopsychosocial model. J Med Philos. 1981;6:101–124. [DOI] [PubMed] [Google Scholar]
- 33.Gardner AW, Montgomery PS, Wang M, Xu C. Predictors of health-related quality of life in patients with symptomatic peripheral artery disease. J Vasc Surg. 2018;68:1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McDermott MM, Mehta S, Liu K, et al. Leg symptoms, the ankle-brachial index, and walking ability in patients with peripheral arterial disease. J Gen Intern Med. 1999;14:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Low Wang CC, Blomster JI, Heizer G, et al. Cardiovascular and limb outcomes in patients with diabetes and peripheral artery disease: the EUCLID trial. J Am Coll Cardiol. 2018;72:3274–3284. [DOI] [PubMed] [Google Scholar]
- 36.Anand SS, Eikelboom JW, Dyal L, et al. Rivaroxaban plus aspirin versus aspirin in relation to vascular risk in the COMPASS trial. J Am Coll Cardiol. 2019;73:3271–3280. [DOI] [PubMed] [Google Scholar]
- 37.Jelani QU, Mena-Hurtado C, Burg M, et al. Relationship between depressive symptoms and health status in peripheral artery disease: role of sex differences. J Am Heart Assoc. 2020;9:e014583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arya S, Lee S, Zahner GJ, et al. The association of comorbid depression with mortality and amputation in veterans with peripheral artery disease. J Vasc Surg. 2018;68:536–545.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malik AO, Peri-Okonny P, Gosch K, et al. Association of perceived stress levels with long-term mortality in patients with peripheral artery disease. JAMA Netw Open. 2020;3:e208741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pande RL, Creager MA. Socioeconomic inequality and peripheral artery disease prevalence in US adults. Circ Cardiovasc Qual Outcomes. 2014;7:532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vart P, Coresh J, Kwak L, Ballew SH, Heiss G, Matsushita K. Socioeconomic status and incidence of hospitalization with lower-extremity peripheral artery disease: atherosclerosis risk in communities study. J Am Heart Assoc. 2017;6:e004995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ultee KH, Bastos Goncalves F, Hoeks SE, et al. Low socioeconomic status is an independent risk factor for survival after abdominal aortic aneurysm repair and open surgery for peripheral artery disease. Eur J Vasc Endovasc Surg. 2015;50:615–622. [DOI] [PubMed] [Google Scholar]
- 43.Rollman BL, Belnap BH, LeMenager MS, Mazumdar S, Schulberg HC, Reynolds CF III. The Bypassing the Blues treatment protocol: stepped collaborative care for treating post-CABG depression. Psychosom Med. 2009;71:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jelani Q-u-a, Mena-Hurtado C, Gosch K, et al. Association of sleep apnea with outcomes in peripheral artery disease: insights from the PORTRAIT study. PLoS One. 2021;16:e0256933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van de Ven WP, Schut FT. Universal mandatory health insurance in the Netherlands: a model for the United States? Health Aff (Millwood). 2008;27:771–781. [DOI] [PubMed] [Google Scholar]
- 46.Dixit SK, Sambasivan M. A review of the Australian healthcare system: a policy perspective. SAGE Open Med. 2018;6:2050312118769211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spertus JA, Salisbury AC, Jones PG, Conaway DG, Thompson RC. Predictors of quality-of-life benefit after percutaneous coronary intervention. Circulation. 2004;110:3789–3794. [DOI] [PubMed] [Google Scholar]
- 48.Thomas S, Mehrholz J. Health-related quality of life, participation, and physical and cognitive function of patients with intensive care unit-acquired muscle weakness 1 year after rehabilitation in Germany: the GymNAST cohort study. BMJ Open. 2018;8:e020163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.