Abstract
Background and Hypothesis:
Hearing voices is a common and often distressing experience for people with psychosis, and many individuals experience medication-resistant auditory verbal hallucinations (AVH). Psychosocial interventions are often employed to address distress over hearing voices. However, although links have been made between adverse social experiences and psychosis broadly, no work has yet delineated the relationship between day-to-day social stress and hallucination severity. We aimed to define that relationship in both clinical and non-clinical voice-hearers.
Study Design:
A sample of 278 participants with a history of hearing voices was selected from the Yale Control Over Perceptual Experiences (COPE) Project. They were administered self-report measures of recent stress and recent auditory experiences within a cross-sectional design. Regression models were used to evaluate whether self-reported aspects of recent stress—and social stress in particular—were related to recent frequency of and distress over hearing voices. Related demographics and clinical characteristics were included as covariates.
Study Results:
A significant relationship was observed between recent social stress and both recent frequency of and distress over hearing voices. While other aspects of recent stress were also related to recent distress over voices, social stressors uniquely predicted distress over voice-hearing, beyond the influence of other stressors. Depressive symptom severity was also related to distress over voices.
Conclusions:
Results suggest that daily social stress may be an important consideration and a potential treatment target for individuals experiencing clinical distress over auditory hallucinations.
Keywords: adverse social experiences, auditory verbal hallucinations, social defeat, stress
1. Introduction
Approximately 60% of people with schizophrenia hear voices (Slade & Bentall, 1988), and about 25–30% will persist despite antipsychotic treatment (Kantrowitz et al., 2019; Meltzer, 1992; Shergill et al., 1998). While social support and networks are often important resources in treatment for people who are distressed by hearing voices (Thomas et al., 2014), no studies to date have identified a link between recent social stress and daily voice-hearing frequency, intensity, or distress.
Stressful life experiences likely contribute to heightened psychosis risk (Bahlinger et al., 2022; Gomes et al., 2019; van Os et al., 2010; Varese et al., 2012; Zubin & Spring, 1977) and there seems to be a complex and unique relationship between social stress and psychosis in particular. Social adversity has been shown to predict overall symptoms of psychosis across the psychosis spectrum (Jaya et al., 2016). In people with a schizophrenia spectrum diagnosis, a smaller social network size is associated with more severe psychiatric symptoms overall (Degnan et al., 2018), and people with schizophrenia tend to report more social stress and a preference to be alone when they are with others (Mote & Fulford, 2020). Further, both social networks and social support tend to diminish prior to the onset of a psychotic disorder (Gayer-Anderson & Morgan, 2013) and several studies have demonstrated that social withdrawal is a robust early risk factor for psychosis (Cornblatt et al., 2012; Matheson et al., 2013; Schiffman, 2004). Taken together, this evidence points to social experiences as a potential avenue to explore when trying to understand psychosis risk and symptom development.
Several models have attempted to explain the relationship between social experiences and psychosis. One in particular, the social defeat hypothesis (SDH), proposes that chronic experiences of social exclusion and related feelings of defeat increase the risk for psychosis; biologically, this may be mediated by increased sensitivity to dopamine in the mesolimbic pathway (Selten & Cantor-Graae, 2005). In a review of evidence for the SDH, Selten et al. (2013) summarized an array of studies showing that experiences of social defeat provide a plausible mechanism for increased risk of schizophrenia. Though the SDH explains one possible pathway to psychosis, it is important to note that the relationship between social experiences and psychosis is complex, and other models have also attempted to explain various facets of this relationship (e.g., Hoffman’s social deafferentation hypothesis, Dodell-Feder et al., 2020; Hoffman, 2007; Marschall et al., 2020; Michael & Park, 2016).
While the link between lifetime experiences of social adversity and psychosis risk is well established, whether ongoing social stress contributes to day-to-day fluctuations in auditory hallucination frequency or intensity remains unclear. According to the SDH, in addition to increasing one’s lifetime risk of developing psychosis, people who experience chronic social exclusion may also have an enhanced dopamine response to daily experiences of social defeat, leading to corresponding fluctuations in psychotic experiences (Schlier et al., 2018; Selten et al., 2013; Selten & Cantor-Graae, 2005). Consistent with this, Schlier et al. (2018) found that recent experiences of social exclusion presented a potential shorter-term trigger for perceptual abnormalities in a nonclinical community sample. They proposed that present exposure to social exclusion triggers perceptual abnormalities that are short-lived in healthy people, but that the same experiences lead to more severe or distressing hallucination-spectrum experiences in people with recurring social defeat. However, they noted that the presence of auditory hallucinations was scarce in their sample, and that future research was needed to examine the relationship between recent social experiences and hearing voices. Therefore, we aimed to examine whether recent social stress was related to hearing voices in people with and without a psychiatric diagnosis, and we hypothesized that heightened social stress would be related to increased distress over auditory hallucinations across the spectrum of voice-hearing experiences. We provide the first empirical evidence of a relationship between daily stress and severity of voice-hearing experiences in a large sample of clinical (i.e., with a psychiatric diagnosis) and non-clinical (without a psychiatric diagnosis or need for treatment) voice-hearers.
2. Materials and methods
2.1. Participants and Procedure
The data examined in the present study were originally collected as part of the online Yale Control Over Perceptual Experiences Project (https://www.spirit.research.yale.edu) (Kafadar et al., 2022; Mourgues et al., 2022). Participants ages 18 to 65 were recruited through advertisements with community partners who work with people with unusual perceptual experiences and/or beliefs, as well as through social media and mental health professionals. All procedures were approved by the Yale University Institutional Review Board/Human Interest Committee and all participants provided informed consent for participation. Exclusion criteria were self-reported cognitive, neurologic, or seizure disorders, or being under the influence of recreational substances at the time of participation. Participants were included in the present analyses if they identified a lifetime incidence of auditory hallucinations.
Measures
The Daily Stress Inventory (DSI) (Brantley et al., 1987) was used to measure recent stress and to extract a score reflecting recent social stress. The DSI asks about 58 different potentially stressful experiences, including whether they occurred in the past 24 hours and how stressful each was (0 = did not occur, 1 = occurred but was not very stressful, 2–7 = ranging from “caused very little stress” to “caused me to panic”). Three overall scores are generated: frequency (total number of events endorsed); impact (sum of the impact ratings); and average impact (impact divided by frequency).
A self-report version of the Chicago Hallucination Assessment Tool (CHAT; (Kern et al., 2015) was used to assess two measures of severity of recent voice-hearing experiences: frequency of and distress over voice-hearing. Participants were first oriented to recent experiences using the prompt, “Please answer the following about any auditory experiences you’ve experienced in the past two days.” Two specific items were selected. To measure recent frequency of voices, the item asking, “How often do these experiences happen on average?” was used. Response options ranged from 0 = not present to 3 = occur continuously or almost continuously. To measure recent distress over voices, the item asking, “How distressing are these experiences for you?” was used. Response options ranged from 1 = “not present” to 5 = “extremely distressing, feel the worst I could possibly feel”.
Additional measures were included to assess the clinical characteristics of the sample and to evaluate for potentially important covariates. The Patient Health Questionnaire (PHQ-9) (Kroenke et al., 2001) was used to measure recent depression symptom severity. The 9-item version of Raven’s Progressive Matrices (Bilker et al., 2012) was used to estimate nonverbal fluid intelligence. Additionally, participants answered self-report questions about their clinical status, including current psychiatric medication use, history of psychiatric treatment, and history of psychiatric diagnosis, all of which were treated as dichotomous variables (1 = yes, 0 = no).
2.2. Statistical Analysis
Data analysis was performed using IBM SPSS Statistics for Windows, version 29 (IBM Corp., Armonk, N.Y., USA). One-way analysis of variance (ANOVA) and Spearman’s correlations were first used to check for relationships between demographic and clinical characteristics with outcome variables. If any significant relationships were found, they were then explored as covariates in subsequent analyses.
Next, we tested whether social stress was specifically related to each of the voice-hearing severity measures. Exploratory factor analysis was conducted on the DSI impact scores, to determine whether a factor specifically related to recent social stress could be extracted from the measure. Specifically, the principal axis method of factor extraction with varimax rotation was used (Beavers et al., n.d.). Once a factor was identified, a variable for recent social stress was created by calculating the sum of the impact scores for the items that loaded onto the recent social stress factor (Supplementary Table 1). First, we used bivariate correlations to test whether recent stressful experiences (DSI) were related to voice-hearing experiences. A series of hierarchical regression models were then used to examine whether recent social stress was related to recent frequency of and distress over voices while accounting for potential covariates. Finally, regression models were used to explore whether the relationships between recent social stress and voice-hearing experiences were unique to social stress, or whether other stress factors extracted from the DSI showed similar relationships.
3. Results
3.1. Sample Characteristics
Table 1 reports the summary of demographic and clinical characteristics of the sample, as well as relationships with the two primary outcome measures (frequency of and distress over hearing voices). Frequencies for each of the outcome measures can be found in Supplementary Tables 1 and 2. Both recent voice-hearing frequency and distress related to voices were significantly negatively correlated with age, positively correlated with PHQ-9 scores, and were significantly different between groups on current psychiatric medication use and history of psychiatric treatment. Specifically, both current psychiatric medication use and history of psychiatric treatment were associated with greater frequency of and distress over hearing voices, compared to individuals not currently on medication or without a history of psychiatric treatment. Additionally, recent frequency of voices was positively correlated with nonverbal fluid intelligence, as estimated by Raven’s Progressive Matrices scores.
Table 1.
Sample demographic and clinical characteristics (N = 278)
| Demographics and Clinical Characteristics | Total | Recent Frequency of Voices ANOVA (F) or Spearman’s rho (ρ) | Recent Distress over Voices ANOVA (F) or Spearman’s rho (ρ) |
|---|---|---|---|
|
| |||
| Age, Years, Mean (SD) | 35.53 (13.43) | ρ = −0.14 (p < 0.02*) | ρ = −0.28 (p < 0.001***) |
| Sex, F, n (%) | 214 (77) | F = 1.15 (p = 0.29) | F = 3.71 (p = 0.54) |
| Gender, n (%) | F = 1.74 (p = 0.14) | F = 1.87 (p = 0.12) | |
| Male | 64 (23) | - | - |
| Female | 192 (69) | - | - |
| Nonbinary | 12 (4) | - | - |
| Genderfluid | 9 (3) | - | - |
| Other | 1 (<1) | - | - |
| Trans Identity, n (%) | 22 (8) | F = 1.96 (p = 0.16) | F = 1.77 (p = 0.18) |
| Race, n (%) | + | + | |
| American Indian/Alaskan Native | 1 (<1) | - | - |
| Asian | 26 (9) | - | - |
| Black | 9 (3) | - | - |
| More than one race | 25 (9) | - | - |
| Native Hawaiian/Other Pacific Islander | 1 (<1) | - | - |
| White | 201 (72) | - | - |
| Unknown/Prefer not to say | 15 (5) | - | - |
| Ethnicity | + | + | |
| Hispanic or Latino | 31 (11) | - | - |
| Not Hispanic or Latino | 235 (85) | - | - |
| Unknown/Prefer not to say | 12 (4) | - | - |
| Raven’s Progressive Matrix Score, 9-item version, Mean (SD) | 3.60 (142) | ρ = 0.14 (p = 0.02*) | ρ = −0.04 (p = 0.54) |
| Current Medication Use, n (%) | 108 (39) | F = 10.13 (p < 0.01**) | F = 18.14 (p < 0.001***) |
| Self-Reported Psychosis Spectrum Illness, n, (%) | 28 (10) | F = 0.30 (p = 0.58) | F = 0.50 (p = 0.48) |
| Self-Reported History of Mental Health Treatment, n (%) | 196 (71) | F = 7.57 (p <0.01**) | F = 14.90 (p < 0.001***) |
| PHQ-9 score, Mean (SD) | 10.33 (8.45) | ρ = 0.21 (p < 0.001***) | ρ = 0.41 (p < 0.001***) |
| Daily Stress Inventory, Impact Score, Mean (SD) | 72.63 (2.92) | ρ = 0.21 (p = 0.001***) | ρ = 0.30 (p < 0.001***) |
p < 0.05
p < 0.01
p < 0.001
Analysis of variance was not conducted due to low and/or unequal sample sizes
F, Female; PHQ-9, Patient Health Questionnaire
Significant correlations observed between daily stress and voice-hearing severity
As shown in Table 1, Spearman correlations indicated that recent stressful experiences on the DSI were significantly positively related to both recent voice-hearing frequency (ρ (278) = 0.21, p < 0.001) and distress (ρ (278) = 0.30, p < 0.001). We further asked what aspects of daily stress may be driving the observation. Given the proposed relationship between adverse social experiences and voice-hearing severity, we expected that social stress in particular may play an important role. We used exploratory factor analysis to identify factors present in the DSI. Results of principal axis factoring are shown in the Supplementary Material. One factor was identified as an indicator of recent social stress and was used in subsequent analyses.
3.2. Recent social stress significantly predicted recent voice-hearing severity
Hierarchical regression models were used to examine whether recent social stress is related to recent frequency and distress over voices, while also accounting for covariates. In the first model, recent frequency of voices was added in the first step, with covariates added in the second step. Results are shown in Figure 1. Recent social stress was found to significantly and independently predict recent frequency of hearing voices, accounting for 5% of the variance in frequency. When potential covariates were included (i.e., age, Raven’s progressive matrices total score, current medication use, history of psychiatric treatment, and PHQ-9 score), the Raven total score was a significant predictor and recent social stress remained a significant predictor of frequency of voices.
Figure 1.
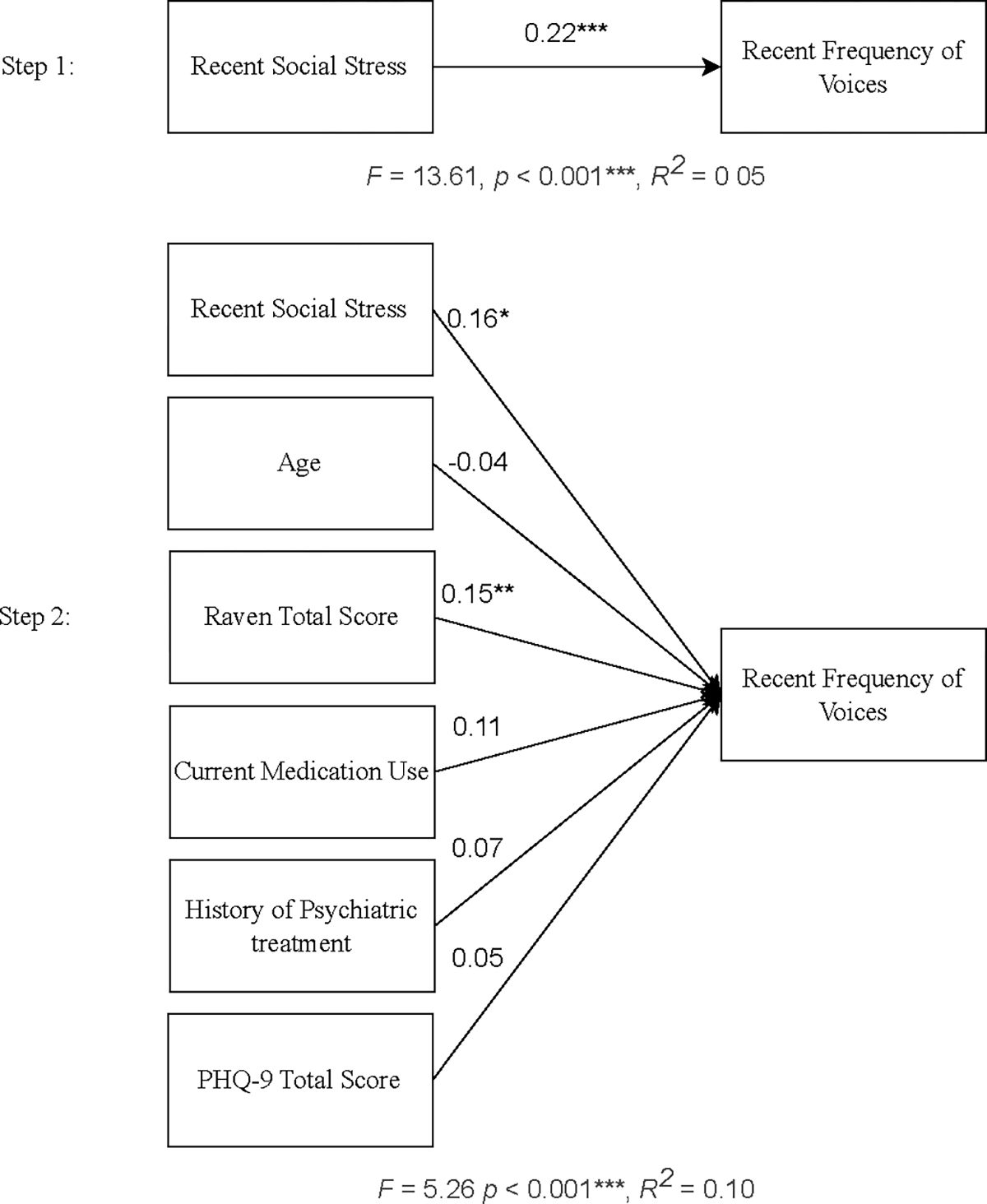
Hierarchical regression predicting recent frequency of voices, with covariates (n = 278)
*p < 0.05, **p < 0.01, ***p < 0.001
PHQ-9, Patient Health Questionnaire
β values are shown for each predictor
In the second model, recent distress over voices was added in the first step, with covariates added in the second step. Results are shown in Figure 2. Recent social stress was found to significantly predict recent distress over voices in the first step, accounting for 10% of the variance in distress. The model remained significant in the second step, and neither age, current medication use, nor history of psychiatric treatment significantly predicted distress over voices. However, PHQ-9 scores significantly predicted recent distress over voices and recent social stress was no longer a significant predictor. Given this finding, we conducted a follow-up Spearman’s rank correlation to explore the relationship between depressive symptoms and recent social stress; a significant positive correlation between depression symptom severity (PHQ-9 scores) and recent social stress was noted, with a relatively strong effect size (ρ = 0.42, p < 0.001) (Rea & Parker, 1992). This suggests that there may be a complex relationship between depression severity, recent social stress, and distress over voices that warrants future follow-up.
Figure 2.

Hierarchical regression predicting recent distress over voices, with covariates (n = 278)
*p < 0.05, **p < 0.01, ***p < 0.001
Raven, Raven Progressive Matrices total score; PHQ-9, Patient Health Questionnaire
β values are shown for each predictor
3.3. Recent social stress uniquely predicted recent distress over voices
Hierarchical regression models were further used to examine whether the relationships between recent social stress and voice-hearing experiences were unique to social stress, or whether other stress factors from the DSI exhibited similar relationships. In the first model, each of the factors extracted from the DSI were added as predictors, and recent frequency of voices was identified as the dependent variable. Results are shown in Table 2. The overall model was not significant, and recent social stress did not predict recent frequency of voices when controlling for other aspects of recent stress. In the second model, the outcome variable was identified as recent distress over voices. Results are shown in Table 3. The overall model was significant, and recent social stress significantly and independently predicted recent distress over voices, beyond the relationship explained by more general stress experiences.
Table 2.
Regression model with DSI. factors predicting recent frequency of voices (n = 252)
| Predictor | β | t | p | R2 | F | p |
|---|---|---|---|---|---|---|
|
| ||||||
| Overall Model | 0.07 | 1.84 | 0.054 | |||
| Factor 1 | 0.15 | 1.47 | 0.14 | |||
| Factor 2 (RSS) | 0.17 | 1.73 | 0.08 | |||
| Factor 3 | −0.10 | −1.20 | 0.23 | |||
| Factor 4 | 0.06 | 0.68 | 0.49 | |||
| Factor 5 | 0.04 | 0.51 | 0.61 | |||
| Factor 6 | 0.03 | 0.42 | 0.67 | |||
| Factor 7 | −0.01 | −0.06 | 0.95 | |||
| Factor 8 | −0.02 | −0.32 | 0.75 | |||
| Factor 9 | −0.11 | −1.29 | 0.20 | |||
| Factor 10 | −0.02 | −0.25 | 0.80 | |||
p < 0.05
p < 0.01
p < 0.001
RSS, Recent Social Stress
Table 3.
Regression model with DSf factors predicting recent distress over voices (n = 252)
| Predictor | β | t | p | R2 | F | p |
|---|---|---|---|---|---|---|
|
| ||||||
| Overall Model | 0.16 | 4.66 | <0.001*** | |||
| Factor 1 | 0.37 | 3.94 | <0.001*** | |||
| Factor 2 (RSS) | 0.19 | 2.11 | 0.04* | |||
| Factor 3 | −0.08 | −0.97 | 0.33 | |||
| Factor 4 | −0.04 | −0.51 | 0.61 | |||
| Factor 5 | 0.06 | 0.69 | 0.49 | |||
| Factor 6 | −0.04 | −0.54 | 0.59 | |||
| Factor 7 | −0.004 | −0.06 | 0.96 | |||
| Factor 8 | 0.004 | 0.05 | 0.96 | |||
| Factor 9 | −0.08 | −0.99 | 0.32 | |||
| Factor 10 | −0.11 | −1.55 | 0.12 | |||
p < 0.05
p < 0.01
p < 0.001
RSS, Recent Social Stress
4. Discussion
In a large sample of clinical and non-clinical voice-hearers, we observed a significant relationship between recent social stress and both recent frequency of and distress over hearing voices. While more general recent stress was also related to recent distress over voices, recent social stress was uniquely related to distress over voice-hearing experiences. This is consistent with prior research showing a broader relationship between adverse social experiences and psychosis (Delespaul et al., 2002; Gayer-Anderson & Morgan, 2013; Jaya et al., 2016; Kesting et al., 2013; van Os et al., 2010; Varese et al., 2012; Verdoux et al., 2003). However, this is the first study to explore the specific relationship between recent social stress and recent experiences of voices. This suggests that daily social stress may be a useful consideration for people who hear voices, and a potential treatment target for individuals experiencing clinical distress over auditory hallucinations.
The significant relationship between recent social stress and voice-hearing experiences has important implications for treatment and future research, although further examination of related moderators may be useful. For example, we also observed evidence suggesting that depression symptom severity may be related to this process. This finding is consistent with recent research showing a relationship between depressive symptoms and hearing voices (Scott et al., 2020; Siddi et al., 2019), and it can help to inform hypotheses for future study in this line of research. Future studies might explore moderators related to depressive symptoms, such as negative thoughts about social experiences, general anhedonia, social anhedonia, perceived locus of control, and negative affect, all of which may respond well to current evidence-based treatments, such as cognitive behavioral therapy (CBT) (Bighelli et al., 2018; Dickerson, 2000; Gallagher et al., 2014; Gautam et al., 2020; Rector & Beck, 2012; Reich & Infurna, 2016).
What is it exactly about recent stress that may make psychotic symptoms worse, and why social stress in particular? In their cognitive model of hallucinations, Slade and Bentall proposed that stressful events lead to emotional and neural changes that increase a person’s tendency to hallucinate above a critical threshold (Delespaul et al., 2002; Slade, 2019; Slade & Bentall, 1988). Further, from a computational psychiatry perspective, one of the most difficult things to infer is the internal state and intentions of other human beings (Frith & Singer, 2008; Lockwood & Klein-Flügge, 2020; Molapour et al., 2021). Since social situations are particularly ambiguous, negative social experiences likely trigger increased uncertainty stress (Peters et al., 2017). During this social stress, and related experiences of high uncertainty, the human brain likely engages in strategies to avoid surprise and minimize uncertainty, which may make them susceptible to interpret internal experiences as having an external cause (Adams et al., 2013; Sheldon et al., 2022). One potential mechanism is that social defeat may impact dopaminergic signaling in the salience network, leading to hyper-precise prediction errors and delusion formation at psychosis onset (Schimmelpfennig et al., 2023). Furthermore, individuals with psychosis exhibit abnormal connections between the amygdala (a brain region involved in stress responses) and neural areas implicated in hallucinations, including weakened connections with the medial prefrontal cortex and strengthened connections with auditory processing regions. This connection between social stress and sensory processing in the brain may result in reliance on hyper-precise priors, which are known to underlie day-to-day hallucination severity (Kafadar et al., 2022).
A strength of the present study was the inclusion of both clinical and nonclinical voice-hearers. While many people with psychotic illness experience distress due to hearing voices, some people hear voices and do not experience distress, and others may even find their voice hearing experiences to be pleasant or meaningful (Johns et al., 2014; Moseley et al., 2022; Powers et al., 2017). There is evidence to suggest that auditory experiences occur on a continuum, and healthy voice-hearers (without a psychiatric diagnosis or need for clinical care) may be instrumental in informing transdiagnostic approaches to auditory hallucinations (Alderson-Day et al., 2021; Baumeister et al., 2017). Greater insight into the social determinants of symptom-related distress across this spectrum can inform the development of interventions for people who are distressed by the experience of hearing voices. Our findings indicate that both current psychiatric medication use and history of psychiatric treatment are associated with a higher frequency of and distress over hearing voice. Identifying and addressing factors related to these aspects of symptom severity, such as recent social stress, may potentially reduce the need for clinical care in people who experience auditory hallucinations.
Several limitations should be considered. Due to the cross-sectional nature of the data, no causal interpretations of the results should be made. While recent social stress may play a role in more severe voice hearing experiences, there may be other variables that account for this relationship altogether. Further, the relationship between social experiences and psychosis may be bi-directional or cyclical, such that auditory hallucinations may in turn lead to increased stress and withdrawal. Regarding voice hearing frequency, we observed that the relationship with recent social stress was no longer significant when accounting for other types of recent stress. However, this may be due to correlations between the stress-related factors that were included in the model. In fact, recent social stress was significantly correlated with all of the nine other factors (see Supplementary Table 4) and including them together in one model likely reduced the power to detect an effect specific to social stress. Another limitation of the present study is that we utilized the DSI in a way that has not yet been validated. However, we showed preliminary evidence that the DSI may be further applied to explore different aspects of daily stress, and that those may have differential relationships with clinical characteristics and functioning. Additionally, we were not able to examine differences in patterns between race and ethnicity groups, due to small and unequal sample sizes. It will be crucial to examine these relationships across demographic groups, especially given the disproportionate impact of social defeat stress on people of color (Selten et al., 2013).
The relationship between social experiences and psychosis is a complex and promising avenue for exploration, especially for psychosocial intervention of psychiatric disorders. The current study provided a first step in exploring how daily stress, and social experiences in particular, may be related to daily variation in distressing perceptual experiences. It provides the foundation for future work that will aim to understand why social experiences and psychosis are related and how to address this relationship in treatment.
Supplementary Material
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams RA, Stephan KE, Brown HR, Frith CD, & Friston KJ (2013). The Computational Anatomy of Psychosis. Frontiers in Psychiatry, 4. 10.3389/fpsyt.2013.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderson-Day B, Woods A, Moseley P, Common S, Deamer F, Dodgson G, & Fernyhough C (2021). Voice-Hearing and Personification: Characterizing Social Qualities of Auditory Verbal Hallucinations in Early Psychosis. Schizophrenia Bulletin, 47(1), 228–236. 10.1093/schbul/sbaa095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahlinger K, Lincoln TM, & Clamor A (2022). Recovery After Stress—Autonomic and Subjective Arousal in Individuals With Psychosis Compared to Healthy Controls. Schizophrenia Bulletin, 48(6), 1373–1383. 10.1093/schbul/sbac097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister D, Sedgwick O, Howes O, & Peters E (2017). Auditory verbal hallucinations and continuum models of psychosis: A systematic review of the healthy voice-hearer literature. Clinical Psychology Review, 51, 125–141. 10.1016/j.cpr.2016.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavers AS, Lounsbury JW, Richards JK, Huck SW, Skolits GJ, & Esquivel SL (n.d.). Practical Considerations for Using Exploratory Factor Analysis in Educational Research. 10.7275/QV2Q-RK76 [DOI] [Google Scholar]
- Bighelli I, Huhn M, Schneider-Thoma J, Krause M, Reitmeir C, Wallis S, Schwermann F, Pitschel-Walz G, Barbui C, Furukawa TA, & Leucht S (2018). Response rates in patients with schizophrenia and positive symptoms receiving cognitive behavioural therapy: A systematic review and single-group meta-analysis. BMC Psychiatry, 18(1), 380. 10.1186/s12888-018-1964-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilker WB, Hansen JA, Brensinger CM, Richard J, Gur RE, & Gur RC (2012). Development of Abbreviated Nine-Item Forms of the Raven’s Standard Progressive Matrices Test. Assessment, 19(3), 354–369. 10.1177/1073191112446655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantley PJ, Waggoner CD, Jones GN, & Rappaport NB (1987). A daily stress inventory: Development, reliability, and validity. Journal of Behavioral Medicine, 10(1), 61–73. 10.1007/BF00845128 [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Carrion RE, Addington J, Seidman L, Walker EF, Cannon TD, Cadenhead KS, McGlashan TH, Perkins DO, Tsuang MT, Woods SW, Heinssen R, & Lencz T (2012). Risk Factors for Psychosis: Impaired Social and Role Functioning. Schizophrenia Bulletin, 38(6), 1247–1257. 10.1093/schbul/sbr136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnan A, Berry K, Sweet D, Abel K, Crossley N, & Edge D (2018). Social networks and symptomatic and functional outcomes in schizophrenia: A systematic review and meta-analysis. Social Psychiatry and Psychiatric Epidemiology, 53(9), 873–888. 10.1007/s00127-018-1552-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delespaul P, deVries M, & van Os J (2002). Determinants of occurrence and recovery from hallucinations in daily life. Social Psychiatry and Psychiatric Epidemiology, 37(3), 97–104. 10.1007/s001270200000 [DOI] [PubMed] [Google Scholar]
- Dickerson FB (2000). Cognitive behavioral psychotherapy for schizophrenia: A review of recent empirical studies. Schizophrenia Research, 43(2–3), 71–90. 10.1016/S0920-9964(99)00153-X [DOI] [PubMed] [Google Scholar]
- Dodell-Feder D, Shovestul B, Woodyatt J, Popov V, & Germine L (2020). Social anhedonia, social networks, and psychotic-like experiences: A test of social deafferentation. Psychiatry Research, 284, 112682. 10.1016/j.psychres.2019.112682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, & Singer T (2008). The role of social cognition in decision making. Philosophical Transactions of the Royal Society B: Biological Sciences, 363(1511), 3875–3886. 10.1098/rstb.2008.0156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher MW, Naragon-Gainey K, & Brown TA (2014). Perceived Control is a Transdiagnostic Predictor of Cognitive-Behavior Therapy Outcome for Anxiety Disorders. Cognitive Therapy and Research, 38(1), 10–22. 10.1007/s10608-013-9587-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam M, Tripathi A, Deshmukh D, & Gaur M (2020). Cognitive Behavioral Therapy for Depression. Indian Journal of Psychiatry, 62(8), 223. 10.4103/psychiatry.IndianJPsychiatry_772_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayer-Anderson C, & Morgan C (2013). Social networks, support and early psychosis: A systematic review. Epidemiology and Psychiatric Sciences, 22(2), 131–146. 10.1017/S2045796012000406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes FV, Zhu X, & Grace AA (2019). Stress during critical periods of development and risk for schizophrenia. Schizophrenia Research, 213, 107–113. 10.1016/j.schres.2019.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman RE (2007). A Social Deafferentation Hypothesis for Induction of Active Schizophrenia. Schizophrenia Bulletin, 33(5), 1066–1070. 10.1093/schbul/sbm079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaya ES, Ascone L, & Lincoln TM (2016). Social Adversity and Psychosis: The Mediating Role of Cognitive Vulnerability. Schizophrenia Bulletin, sbw104. 10.1093/schbul/sbw104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LC, Kompus K, Connell M, Humpston C, Lincoln TM, Longden E, Preti A, Alderson-Day B, Badcock JC, Cella M, Fernyhough C, McCarthy-Jones S, Peters E, Raballo A, Scott J, Siddi S, Sommer IE, & Larøi F (2014). Auditory Verbal Hallucinations in Persons With and Without a Need for Care. Schizophrenia Bulletin, 40(Suppl_4), S255–S264. 10.1093/schbul/sbu005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafadar E, Fisher VL, Quagan B, Hammer A, Jaeger H, Mourgues C, Thomas R, Chen L, Imtiaz A, Sibarium E, Negreira AM, Sarisik E, Polisetty V, Benrimoh D, Sheldon AD, Lim C, Mathys C, & Powers AR (2022). Conditioned Hallucinations and Prior Overweighting Are State-Sensitive Markers of Hallucination Susceptibility. Biological Psychiatry, 92(10), 772–780. 10.1016/j.biopsych.2022.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz JT, Sehatpour P, Avissar M, Horga G, Gwak A, Hoptman MJ, Beggel O, Girgis RR, Vail B, Silipo G, Carlson M, & Javitt DC (2019). Significant improvement in treatment resistant auditory verbal hallucinations after 5 days of double-blind, randomized, sham controlled, fronto-temporal, transcranial direct current stimulation (tDCS): A replication/extension study. Brain Stimulation, 12(4), 981–991. 10.1016/j.brs.2019.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern B, Axelrod J, Gao Y, & Keedy S (2015). Exchange the magnifying glass for a microscope: The Chicago Hallucination Assessment Tool (CHAT). Schizophrenia Bulletin, 41, S110–S110. [Google Scholar]
- Kesting M-L, Bredenpohl M, Klenke J, Westermann S, & Lincoln TM (2013). The impact of social stress on self-esteem and paranoid ideation. Journal of Behavior Therapy and Experimental Psychiatry, 44(1), 122–128. 10.1016/j.jbtep.2012.07.010 [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JB (2001). The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9), 606–613. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood PL, & Klein-Flügge MC (2020). Computational modelling of social cognition and behaviour—A reinforcement learning primer. Social Cognitive and Affective Neuroscience, nsaa040. 10.1093/scan/nsaa040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschall TM, Brederoo SG, Ćurčić-Blake B, & Sommer IEC (2020). Deafferentation as a cause of hallucinations: Current Opinion in Psychiatry, 33(3), 206–211. 10.1097/YCO.0000000000000586 [DOI] [PubMed] [Google Scholar]
- Matheson SL, Vijayan H, Dickson H, Shepherd AM, Carr VJ, & Laurens KR (2013). Systematic meta-analysis of childhood social withdrawal in schizophrenia, and comparison with data from at-risk children aged 9–14 years. Journal of Psychiatric Research, 47(8), 1061–1068. 10.1016/j.jpsychires.2013.03.013 [DOI] [PubMed] [Google Scholar]
- Meltzer HY (1992). Treatment of the Neuroleptic-Nonresponsive Schizophrenic Patient. Schizophrenia Bulletin, 18(3), 515–542. 10.1093/schbul/18.3.515 [DOI] [PubMed] [Google Scholar]
- Michael J, & Park S (2016). Anomalous bodily experiences and perceived social isolation in schizophrenia: An extension of the Social Deafferentation Hypothesis. Schizophrenia Research, 176(2–3), 392–397. 10.1016/j.schres.2016.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molapour T, Hagan CC, Silston B, Wu H, Ramstead M, Friston K, & Mobbs D (2021). Seven computations of the social brain. Social Cognitive and Affective Neuroscience, nsab024. 10.1093/scan/nsab024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley P, Powell A, Woods A, Fernyhough C, & Alderson-Day B (2022). Voice-Hearing Across The Continuum: A Phenomenology of Spiritual Voices. Schizophrenia Bulletin, 48(5), 1066–1074. 10.1093/schbul/sbac054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mote J, & Fulford D (2020). Ecological momentary assessment of everyday social experiences of people with schizophrenia: A systematic review. Schizophrenia Research, 216, 56–68. 10.1016/j.schres.2019.10.021 [DOI] [PubMed] [Google Scholar]
- Mourgues C, Hammer A, Fisher V, Kafadar E, Quagan B, Bien C, Jaeger H, Thomas R, Sibarium E, Negreira AM, Sarisik E, Polisetty V, Nur Eken H, Imtiaz A, Niles H, Sheldon AD, & Powers AR (2022). Measuring Voluntary Control Over Hallucinations: The Yale Control Over Perceptual Experiences (COPE) Scales. Schizophrenia Bulletin, 48(3), 673–683. 10.1093/schbul/sbab144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, McEwen BS, & Friston K (2017). Uncertainty and stress: Why it causes diseases and how it is mastered by the brain. Progress in Neurobiology, 156, 164–188. 10.1016/j.pneurobio.2017.05.004 [DOI] [PubMed] [Google Scholar]
- Powers AR, Kelley MS, & Corlett PR (2017). Varieties of Voice-Hearing: Psychics and the Psychosis Continuum. Schizophrenia Bulletin, 43(1), 84–98. 10.1093/schbul/sbw133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea LM, & Parker RA (1992). Designing and conducting survey research: A comprehensive guide. Jossey-Bass Publishers. [Google Scholar]
- Rector NA., & Beck AT. (2012). Cognitive Behavioral Therapy for Schizophrenia: An Empirical Review Rector Neil A.and Beck Aaron T.(2001). Reprinted from the J Nerv Ment Dis 189:278–287. Journal of Nervous & Mental Disease, 200(10), 832–839. 10.1097/NMD.0b013e31826dd9af [DOI] [PubMed] [Google Scholar]
- Reich JW., & Infurna FJ. (Eds.). (2016). Perceived control: Theory, research, and practice in the first 50 years. Oxford University Press. [Google Scholar]
- Schiffman J (2004). Childhood Videotaped Social and Neuromotor Precursors of Schizophrenia: A Prospective Investigation. American Journal of Psychiatry, 161(11), 2021–2027. 10.1176/appi.ajp.161.11.2021 [DOI] [PubMed] [Google Scholar]
- Schimmelpfennig J, Topczewski J, Zajkowski W, & Jankowiak-Siuda K (2023). The role of the salience network in cognitive and affective deficits. Frontiers in Human Neuroscience, 17, 1133367. 10.3389/fnhum.2023.1133367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlier B, Winkler K, Jaya ES, & Lincoln TM (2018). Fluctuations in Hallucination Spectrum Experiences Co-vary with Social Defeat but not with Social Deafferentation. A 3-Week Daily Assessment Study. Cognitive Therapy and Research, 42(1), 92–102. 10.1007/s10608-017-9871-8 [DOI] [Google Scholar]
- Scott M, Rossell SL, Toh WL, & Thomas N (2020). The relationship between anxiety, depression, and subtypes of negative auditory verbal hallucination (AVH) content in affective and non-affective psychosis. Psychiatry Research, 294, 113500. 10.1016/j.psychres.2020.113500 [DOI] [PubMed] [Google Scholar]
- Selten J-P, & Cantor-Graae E (2005). Social defeat: Risk factor for schizophrenia? The British Journal of Psychiatry: The Journal of Mental Science, 187, 101–102. 10.1192/bjp.187.2.101 [DOI] [PubMed] [Google Scholar]
- Selten J-P, van der Ven E, Rutten BPF, & Cantor-Graae E (2013). The social defeat hypothesis of schizophrenia: An update. Schizophrenia Bulletin, 39(6), 1180–1186. 10.1093/schbul/sbt134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon AD, Kafadar E, Fisher V, Greenwald MS, Aitken F, Negreira AM, Woods SW, & Powers AR (2022). Perceptual pathways to hallucinogenesis. Schizophrenia Research, 245, 77–89. 10.1016/j.schres.2022.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shergill SS, Murray RM, & McGuire PK (1998). Auditory hallucinations: A review of psychological treatments. Schizophrenia Research, 32(3), 137–150. 10.1016/S0920-9964(98)00052-8 [DOI] [PubMed] [Google Scholar]
- Siddi S, Nuñez C, Senior C, Preti A, Cuevas-Esteban J, Ochoa S, Brébion G, & Stephan-Otto C (2019). Depression, auditory-verbal hallucinations, and delusions in patients with schizophrenia: Different patterns of association with prefrontal gray and white matter volume. Psychiatry Research: Neuroimaging, 283, 55–63. 10.1016/j.pscychresns.2018.12.001 [DOI] [PubMed] [Google Scholar]
- Slade PD (2019). Models of Hallucination: From Theory to Practice. In David AS& Cutting JC(Eds.), The Neuropsychology of Schizophrenia (1st ed., pp. 245–253). Psychology Press. 10.4324/9781315785004-15 [DOI] [Google Scholar]
- Slade PD, & Bentall RP (1988). Sensory deception: A scientific analysis of hallucination. (pp. x, 275). Johns Hopkins University Press. [Google Scholar]
- Thomas N, Hayward M, Peters E, van der Gaag M, Bentall RP, Jenner J, Strauss C, Sommer IE, Johns LC, Varese F, Garcia-Montes JM, Waters F, Dodgson G, & McCarthy-Jones S (2014). Psychological Therapies for Auditory Hallucinations (Voices): Current Status and Key Directions for Future Research. Schizophrenia Bulletin, 40(Suppl 4), S202–S212. 10.1093/schbul/sbu037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os J, Kenis G, & Rutten BPF (2010). The environment and schizophrenia. Nature, 468(7321), 203–212. 10.1038/nature09563 [DOI] [PubMed] [Google Scholar]
- Varese F, Smeets F, Drukker M, Lieverse R, Lataster T, Viechtbauer W, Read J, van Os J, & Bentall RP (2012). Childhood Adversities Increase the Risk of Psychosis: A Meta-analysis of Patient-Control, Prospective- and Cross-sectional Cohort Studies. Schizophrenia Bulletin, 38(4), 661–671. 10.1093/schbul/sbs050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoux H, Husky M, Tournier M, Sorbara F, & Swendsen JD (2003). Social environments and daily life occurrence of psychotic symptoms. Social Psychiatry and Psychiatric Epidemiology, 38(11), 654–661. 10.1007/s00127-003-0702-8 [DOI] [PubMed] [Google Scholar]
- Zubin J, & Spring B (1977). Vulnerability: A new view of schizophrenia. Journal of Abnormal Psychology, 86(2), 103–126. 10.1037/0021-843X.86.2.103 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


