Abstract
Currently-used assessments for fibromyalgia require clinicians to suspect a fibromyalgia diagnosis, a process susceptible to unintentional bias. Automated assessments of standard patient-reported outcomes (PROs) could be used to prompt formal assessments, potentially reducing bias. We sought to determine whether hierarchical clustering of patient-reported pain distribution on digital body map drawings predicted fibromyalgia diagnosis. Using an observational cohort from the University of Pittsburgh’s Patient Outcomes Repository for Treatment registry, which contains PROs and electronic medical record data from 21,423 patients (3/17/2016–6/25/2019) presenting to pain management clinics, we tested the hypothesis that hierarchical clustering subgroup was associated with fibromyalgia diagnosis, as determined by ICD-10 code. Logistic regression revealed a significant relationship between body map cluster subgroup and fibromyalgia diagnosis. The cluster subgroup with the most body areas selected was the most likely to receive a diagnosis of fibromyalgia when controlling for age, gender, anxiety, and depression. Despite this, more than two-thirds of patients in this cluster lacked a clinical fibromyalgia diagnosis. In an exploratory analysis to better understand this apparent underdiagnosis, we developed and applied proxies of fibromyalgia diagnostic criteria. We found that proxy diagnoses were more common than ICD-10 diagnoses, which may be due to less frequent clinical fibromyalgia diagnosis in men. Overall, we find evidence of fibromyalgia underdiagnosis, likely due to gender bias. Coupling PROs that take seconds to complete, such as a digital pain body map, with machine learning is a promising strategy to reduce bias in fibromyalgia diagnosis and improve patient outcomes.
Keywords: Chronic pain, Pain Measurement, Fibromyalgia, Cluster Analysis, Machine Learning
Graphical Abstract

Introduction
The bodily distribution of pain is critical for diagnosis and can be quickly assessed in the clinic with pain drawings that prompt the patient to mark the corresponding areas of their pain on a body map23. By applying hierarchical clustering to digital pain body maps in a sample of 21,423 patients with chronic pain, we previously identified 9 unique subgroups of patients that differed significantly from each other in key, clinically-relevant characteristics including pain intensity, quality, physical function and three-month treatment outcomes1.
One subgroup, “Widespread – Heavy”, had the highest number of body regions selected, which is characteristic of fibromyalgia31, 40. The 2016 revision to the 2010/2011 American College of Rheumatology (ACR) fibromyalgia criteria set out new diagnostic guidelines, which include the Widespread Pain Index (WPI) and Symptom Severity Score (SSS)39. In these guidelines the degree of ‘widespreadedness’ of pain is the key defining characteristic of fibromyalgia. To capture the spectrum of fibromyalgia presentation, interrelated cutoffs between the two measures were identified38.
Establishing a diagnosis is important in fibromyalgia, as previous studies suggest missing a diagnosis leads to worse patient and system-level outcomes2, 11, 21, 37. Unfortunately, delayed diagnosis appears to be common. One study estimated the average time to diagnosis is 3 years from time of initial contact with a provider, with most diagnoses ultimately accredited to a secondary provider12.
The goal of the current study is to discern whether hierarchical clustering applied to the body map can aid in the diagnosis of fibromyalgia. Patients within the “Widespread-Heavy” cluster had significantly worse symptom severity and outcomes compared to all other clusters1. Given prior literature suggesting underdiagnosis of fibromyalgia particularly in men and older patients11, 18, 28, 35, 41, 43, we suspected that underdiagnosis and therefore undertreatment of fibromyalgia may contribute to poor outcomes in this cluster. To evaluate this in our large dataset, we applied an informatic approach by combining patient-reported outcomes and machine learning. We hypothesized that certain body map responses identified with hierarchical clustering are associated with fibromyalgia diagnosis.
Methods
An observational cohort study utilizing the University of Pittsburgh’s Patient Outcomes Repository for Treatment registry (PORT) was employed13. Patient-reported outcomes (PROs) collected with the Collaborative Health Outcomes Information Registry software (CHOIR)33, 34 were combined with electronic medical record (EMR) data from index appointments at the seven University of Pittsburgh Medical Center (UPMC) Pain Medicine clinics located around Western Pennsylvania. This research was authorized by the University of Pittsburgh Institutional Review Board and the UPMC Quality Improvement Committee with a waiver of individual informed consent.
The study cohort consists of 21,423 patients encountered from 3/17/2016 – 6/25/2019 at a UPMC Pain Medicine clinic13. The cohort was derived by merging a dataset used to identify body map clusters, described in our prior study1, with additional EMR data to allow for an analysis of pain diagnosis codes. A small number of patients lacking EMR data were not included (n = 235). Gender and racialized identity20, 26, 30 were derived from EMR records. Gender in the EMR was binary during the study period, reflecting medical intake questions, which is why gender is reported as such in the current study. Included patients completed a set of validated pain assessments on a tablet using CHOIR prior to their clinical evaluation33, 34. As part of their routine clinical care, patients were evaluated by fellowship-trained, Pain Medicine physicians with a thorough history and physical. Based on this evaluation, physicians entered ICD-10 codes directly into the EMR at the time of the encounter, limiting errors in medical coding.
PROs included pain location, pain intensity, pain radiation, pain interference with daily activities, neuropathic quality, physical functioning, symptoms of anxiety, symptoms of depression, and overall global/mental health, described in detail previously1. Patient-Reported Outcomes Measurement Information System (PROMIS) tools yield standardized T-scores normalized to a population of 21,133 US citizens (mean (M) = 50, standard deviation (SD) = 10, range = 0–100 )34. The CHOIR body map has been previously validated and consists of two side-by-side drawings with 74 different regions the patients can select, which correspond to their pain32.
Fibromyalgia clinical cases were identified using EMR data. ICD-10 diagnosis codes associated with the clinical index visit were queried for M79.1 - fibromyalgia. The index visit was defined as the earliest completed body map and CHOIR assessment. If this code was present, then cases were assigned as having a clinical diagnosis of fibromyalgia, termed “Index Fibro.” Additionally, we identified patients that received a diagnosis of fibromyalgia any time between 3/2016–11/2021, termed, “Total Fibro.” This date range was chosen based on EMR data availability.
We also identified informatic proxies of fibromyalgia by adapting the WPI and SSS to generate CHOIR WPI and CHOIR SS respectively. To generate the CHOIR WPI, the body map for the revised ACR WPI criteria was harmonized with the CHOIR body map using a graphically-determined conversion factor (See Supplemental Fig 1). CHOIR WPI was met if 21 / 60 WPI-matching regions were selected.
We generated CHOIR SS by combining PRO variables reflecting characteristic symptoms of fibromyalgia that overlap with SSS domains including fatigue, sleep disturbance, cognitive, and psychiatric symptoms4, 10, 24. This was informed by previous work identifying a relationship between PROMIS measures and Fibromyalgia Impact Questionnaire (FIQR)27. Specifically, to generate CHOIR SS we took clinically-meaningful cutoff values of variables associated with FIQR including PROMIS Physical Function, a mean of Physical and Mental Global Health, PROMIS Sleep Disturbance, PROMIS Anxiety, and PROMIS Depression based on Merriwether and colleagues27. The cutoff value for each variable was ½ of a standard deviation worse than 50, the normalized mean. This cutoff represents a minimally clinically important difference (MCID)29. A participant met CHOIR SS criteria if their T scores were worse than the MCID cutoff for all PRO variables.
A second proxy SSS was generated with PROMIS Physical Function alone, as this variable reflects perceived physical function, the key driver of fibromyalgia severity and global impact according to Merriwether and colleagues27. CHOIR PF was determined similarly to CHOIR SS. A score of ½ of standard deviation worse than mean (i.e. < 45) met criteria for CHOIR PF.
The two symptom severity proxies were combined with CHOIR WPI and are termed CHOIR WPI/SS (meets CHOIR WPI cutoff and CHOIR SS criteria above) and CHOIR WPI/PF (meets CHOIR WPI cutoff and CHOIR PF criteria above).
Data were organized, cleaned, and analyzed in Excel (Microsoft, Redmond, Washington), StataMP v17 (Statacorp, College Station, Texas), and MATLAB (The Mathworks Inc., Natick, Massachusetts). The distribution of fibromyalgia diagnoses across the nine clusters of pain distribution1 was examined using a multivariable logistic regression with diagnosis of fibromyalgia as the outcome of interest; cluster as the primary predictor; and covariates of age, gender, PROMIS anxiety, and PROMIS depression. Age, gender, anxiety, and depression were included in the regression analysis because of the previously identified association of each with a diagnosis of fibromyalgia6, 42. Predicted probabilities were calculated following logistic regression analysis. Proportions with Agresti-Coull 95% confidence intervals were calculated for clinical and proxy fibromyalgia diagnosis and for gender. Multivariable logistic regression with gender as the predictor of interest was used to query differences in gender representation across clinical and proxy diagnostic methods. Univariate tests across two groups included t-test or Chi2 test for continuous or categorical variables respectively. Sensitivity (sen) was calculated as: 100 * (informatic yes & clinical yes) / clinical yes. Specificity (spc) was calculated as: 100 * (informatic no & clinical no) / clinical no. Positive predictive value (PPV) was calculated as: 100 * (informatic yes & clinical yes) / informatic yes. Negative predictive value (NPV) was calculated as: 100 * (informatic no & clinical no) / informatic no.
Results
Clinical fibromyalgia diagnosis is more frequent in the Widespread-Heavy subgroup compared to all other subgroups
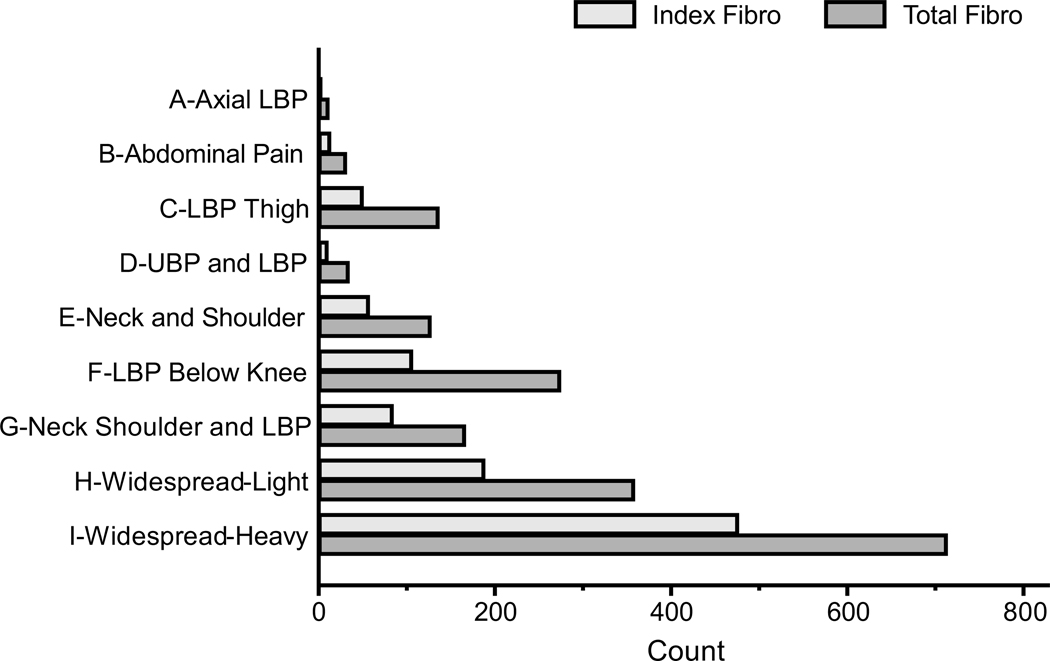
Following hierarchical clustering by body map data alone1, we examined the distribution of clinical fibromyalgia diagnosis in each cluster subgroup. In the total sample (N=21,423), there were 987 cases of fibromyalgia diagnosed at the index encounter (Table 1, “Index Fibro”). There were 1,850 total clinical fibromyalgia cases associated with any encounter (“Total Fibro”). 476 (48.2%) of all Index Fibro diagnoses occurred in Widespread – Heavy cluster (Fig. 1). Using Index Fibro as the outcome of interest, cluster subgroup as the primary predictor, and covariates of age, gender, PROMIS anxiety, and PROMIS depression, a multivariable logistic regression was calculated (N =21344, LR Chi2(12) = 1970.69, p<0.0001, R2 = 0.248) with cluster subgroup being a statistically significant predictor of Index Fibro (Wald test: Chi2(8) = 810.15, p<0.0001). Being in the Widespread-Heavy cluster increased the odds of fibromyalgia diagnosis at the index encounter compared to several other cluster groups: versus Axial Low Back Pain (LBP) OR(95%CI) 29.6 (9.4–93.0), versus LBP Thigh OR(95%CI) 14.1 (10.4–19.0), versus Upper and Lower Back Pain OR(95%CI) 14.9 (7.9–28.3), and versus LBP Below Knee OR(95%CI) 11.8 (9.5–14.8). The predicted probability of having a clinical fibromyalgia diagnosis adjusting for age, gender, anxiety, and depression in the Widespread-Heavy cluster was 0.159 (95% CI 0.146–0.173, Fig 2). This is the highest probability amongst all clusters with no overlap of 95% confidence intervals. Adding total number of body regions selected as a covariate, body map cluster remained a statistically significant predictor of Index Fibro (N =21344, LR Chi2(13) = 2158.48, p<0.0001, R2 = 0.271; Wald test: Chi2(8) = 141.97, p<0.0001).
Table 1.
Characteristics and Patient-Reported Outcomes by Fibromyalgia Criteria
| ICD-10 DIAGNOSIS |
PRO DIAGNOSIS |
||||||
|---|---|---|---|---|---|---|---|
| Total | Index Fibro | Total Fibro | Widespread-Heavy | CHOIR WPI | CHOIR WPI/SS | CHOIR WPI/PF | |
| Counts | 21,423 | 987 | 1850 | 2123 | 2036 | 1052 | 1999 |
| Age, years, mean ± SD | 60.19 ± 15.53 | 51.52 ± 13.36 | 53.39 ± 13.59 | 55.09 ± 13.98 | 53.43 ± 13.89 | 52.04 ± 13.18 | 53.45 ± 13.89 |
| Gender, male/total (%) | 40% | 7% | 8% | 29% | 27% | 26% | 27% |
| Racialized identity, n | 21,283 | 976 | 1836 | 2104 | 2018 | 1043 | 1981 |
| White, % yes | 83% | 77% | 78% | 81% | 81% | 80% | 81% |
| Black, % yes | 15% | 20% | 20% | 17% | 17% | 18% | 17% |
| Other, % yes | 2% | 3% | 2% | 2% | 2% | 2% | 2% |
| BMI, kg/m2, mean ± SD (n) | 30.61 ± 7.69 (n = 15,878) | 32.28 ± 9 (n = 693) | 31.97 ± 8.65 (n = 1309) | 31.81 ± 8.51 (n = 1473) | 31.94 ± 8.55 (n = 1418) | 32 ± 8.68 (n = 727) | 32.02 ± 8.58 (n = 1389) |
| Pain measures | |||||||
| Average Pain Intensity, mean ± SD | 6.49 ± 2.01 | 6.95 ± 1.75 | 6.83 ± 1.87 | 7.11 ± 1.78 | 7.27 ± 1.74 | 7.44 ± 1.67 | 7.28 ± 1.73 |
| Pain Interference, mean ± SD (n) | 66.36 ± 6.18 (n = 21,282) | 69.03 ± 5.59 (n = 977) | 68.4 ± 5.85 (n = 1835) | 69.33 ± 5.62 (n = 2097) | 70.16 ± 5.31 (n = 2019) | 71.45 ± 4.94 (n = 1049) | 70.32 ± 5.19 (n = 1982) |
| PainDetect total score, mean ± SD (n) | 16.5 ± 8.27 (n = 12,808) | 21.55 ± 7.2 (n = 637) | 20.98 ± 7.44 (n = 929) | 22.52 ± 7.26 (n = 1323) | 23.39 ± 7.18 (n = 1300) | 24.79 ± 6.68 (n = 598) | 23.55 ± 7.07 (n = 1275) |
| CHOIR body map regions selected, mean ± SD | 10.62 ± 11.03 | 27.6 ± 17.24 | 23.52 ± 16.78 | 34.2 ± 14.51 | 37.63 ± 12.13 | 38.5 ± 12.73 | 37.67 ± 12.13 |
| ACR body map regions selected, mean ± SD | 9.15 ± 9.16 | 22.98 ± 14.09 | 19.74 ± 13.75 | 28 ± 12.61 | 31.72 ± 9.75 | 32.33 ± 10.17 | 31.76 ± 9.75 |
| Overall health and function | |||||||
| Global mental health, mean ± SD (n) | 43.44 ± 9.43 (n = 18,520) | 37.33 ± 8.06 (n = 873) | 37.76 ± 8.36 (n = 1681) | 38.35 ± 8.94 (n = 1787) | 37.68 ± 8.71 (n = 1711) | 34.28 ± 7.35 | 37.53 ± 8.63 (n = 1681) |
| Global physical health, mean ± SD (n) | 35.6 ± 7.18 (n = 18,520) | 31.57 ± 5.67 (n = 873) | 32.22 ± 6.05 (n = 1681) | 31.19 ± 6.1 (n = 1787) | 30.51 ± 5.89 (n = 1711) | 28.96 ± 5.11 | 30.35 ± 5.72 (n = 1681) |
| Global health mean, mean ± SD (n) | 39.52 ± 7.41 (n = 18,520) | 34.45 ± 5.88 (n = 873) | 34.99 ± 6.28 (n = 1681) | 34.77 ± 6.49 (n = 1787) | 34.1 ± 6.26 (n = 1711) | 31.62 ± 5.13 | 33.94 ± 6.13 (n = 1681) |
| Physical Function, mean ± SD (n) | 35.28 ± 7.03 (n = 21,422) | 33.74 ± 5.82 (n = 987) | 33.9 ± 5.87 (n = 1850) | 32.51 ± 5.96 (n = 2123) | 31.97 ± 5.87 (n = 2036) | 30.79 ± 5.17 | 31.65 ± 5.42 |
| Sleep Disturbance, mean ± SD (n) | 59.68 ± 9.34 (n = 21,344) | 63.91 ± 8.29 (n = 980) | 63.03 ± 8.8 (n = 1842) | 64.01 ± 8.73 (n = 2107) | 65.06 ± 8.55 (n = 2025) | 68.02 ± 7.19 | 65.15 ± 8.55 (n = 1988) |
| Depression, mean ± SD (n) | 55.23 ± 10.43 (n = 21,344) | 60.76 ± 9.44 (n = 980) | 60.29 ± 9.45 (n = 1842) | 60.01 ± 10.1 (n = 2107) | 60.89 ± 9.99 (n = 2025) | 66.27 ± 6.56 | 61.04 ± 9.91 (n = 1988) |
| Anxiety, mean ± SD (n) | 56.27 ± 10.05 (n = 21,344) | 61.73 ± 8.95 (n = 980) | 61.29 ± 9.11 (n = 1842) | 60.79 ± 9.76 (n = 2107) | 61.76 ± 9.48 (n = 2025) | 66.76 ± 6.17 | 61.87 ± 9.45 (n = 1988) |
Abbreviations: PRO, Patient-Reported Outcome; Index Fibro, has ICD-10 diagnosis on index encounter; Total Fibro, has ICD-10 diagnosis associated with any encounter; Widespread-Heavy, hierarchical clustering subgroup based on pain body map alone; CHOIR WPI, proxy Widespread Pain Index derived from PROs; CHOIR WPI/SS, proxy combined WPI and Symptom Severity Score derived from PROs; CHOIR WPI/PF, proxy combined WPI and PROMIS Physical Function.
Fig 1. Clinical fibromyalgia diagnosis is most frequent in the Widespread-Heavy subgroup.
Bars reflect counts of ICD-10 fibromyalgia diagnosis present in each of the nine clusters identified by hierarchical clustering. Index Fibro means there was an ICD-10 diagnosis at the index encounter. Total Fibro reflects the presence of an ICD-10 diagnosis at any encounter in the dataset. LBP = Low Back Pain, UBP = Upper Back Pain
Fig 2. Adjusted probabilities of a clinical fibromyalgia diagnosis across body map cluster subgroups.
Logistic regression of Index Fibro on body map cluster membership adjusting for age, gender, anxiety, and depression was used to calculate marginal probabilities of having Index Fibro (error bars: 95% CI). A-Axial Low Back Pain (LBP), B-Abdominal Pain, C-LBP Thigh, D-Upper and Lower Back Pain, E-Neck and Shoulder, F-LBP Below Knee, G-Neck Shoulder and LBP, H-Widespread—Light, I-Widespread—Heavy.
Informatic and clinical diagnoses of fibromyalgia are significantly different, suggesting fibromyalgia is underdiagnosed
Despite being more likely that fibromyalgia would be diagnosed in patients assigned to the Widespread – Heavy cluster, fibromyalgia is not diagnosed for the majority of patients belonging to the Widespread – Heavy cluster. Of the 2123 patients in Widespread – Heavy cluster, only 476 received a fibromyalgia ICD-10 diagnosis at the index encounter (22.4%, Table 2). Patients with the ICD-10 diagnosis tended to be younger, female, and reported more body regions as painful than those without the ICD-10 diagnosis (p < 0.001). However, patients in the Widespread-Heavy cluster but without an ICD-10 diagnosis still reported a large number of painful body regions (mean ~32 out of 74 on the CHOIR body map) and had similar pain intensity and physical function as compared with those patients with an ICD-10 diagnosis (p = 0.884 and 0.217 respectively). This raises the possibility that patients in the Widespread-Heavy cluster lacking an ICD-10 fibromyalgia diagnosis have unrecognized fibromyalgia.
Table 2.
Characteristics and Patient-Reported Outcomes of Widespread-Heavy Patients With and Without an ICD-10 Fibromyalgia Diagnosis
| Patients classified as Widespread-Heavy |
||||
|---|---|---|---|---|
| Total | Has ICD-10 at index | No ICD-10 at index | P-valuea | |
| Counts | 2,123 | 476 | 1,647 | |
| Age, years, mean ± SD | 55.09 ± 13.98 | 49.81 ± 13.35 | 56.62 ± 13.79 | <.001 |
| Gender, male/total (%) | 29% | 7% | 35% | <.001 |
| Racialized identity, n | 2104 | 468 | 1636 | .481 |
| White, % yes | 81% | 79% | 81% | |
| Black, % yes | 17% | 19% | 17% | |
| Other, % yes | 2% | 3% | 2% | |
| BMI, kg/m2, mean ± SD (n) | 31.81 ± 8.51 (n = 1473) | 32.35 ± 8.87 (n = 333) | 31.66 ± 8.39 (n = 1140) | .190 |
| Pain measures | ||||
| Average Pain Intensity, mean ± SD | 7.11 ± 1.78 | 7.09 ± 1.65 | 7.11 ± 1.82 | .884 |
| Pain Interference, mean ± SD (n) | 69.33 ± 5.62 (n = 2097) | 70.09 ± 5.43 (n = 468) | 69.11 ± 5.65 (n = 1629) | .001 |
| PainDetect total score, mean ± SD (n) | 22.52 ± 7.26 (n = 1323) | 22.81 ± 6.7 (n = 315) | 22.42 ± 7.42 (n = 1008) | .410 |
| CHOIR body map regions, mean ± SD | 34.2 ± 14.51 | 40.5 ± 14.34 | 32.39 ± 14.04 | <.001 |
| ACR body map regions, mean ± SD | 28 ± 12.61 | 33.17 ± 12.22 | 26.51 ± 12.33 | <.001 |
| Overall health and function | ||||
| Global mental health, mean ± SD (n) | 38.35 ± 8.94 (n = 1787) | 36.35 ± 8.22 (n = 415) | 38.95 ± 9.06 (n = 1372) | <.001 |
| Global physical health, mean ± SD (n) | 31.19 ± 6.1 (n = 1787) | 30.1 ± 5.38 (n = 415) | 31.53 ± 6.27 (n = 1372) | <.001 |
| Global health mean, mean ± SD (n) | 34.77 ± 6.49 (n = 1787) | 33.22 ± 5.7 (n = 415) | 35.24 ± 6.64 (n = 1372) | <.001 |
| Physical Function, mean ± SD (n) | 32.51 ± 5.96 (n = 2123) | 32.81 ± 5.27 (n = 476) | 32.43 ± 6.15 (n = 1647) | .217 |
| Sleep Disturbance, mean ± SD (n) | 64.01 ± 8.73 (n = 2107) | 64.94 ± 8.15 (n = 470) | 63.75 ± 8.87 (n = 1637) | .009 |
| Depression, mean ± SD (n) | 60.01 ± 10.1 (n = 2107) | 62.16 ± 9.52 (n = 470) | 59.39 ± 10.18 (n = 1637) | <.001 |
| Anxiety, mean ± SD (n) | 60.79 ± 9.76 (n = 2107) | 62.93 ± 8.89 (n = 470) | 60.17 ± 9.91 (n = 1637) | <.001 |
NOTE. A—t-test or Chi2 test for continuous or categorical variables respectively. Bolded values are P < .05.
To address this possibility, we examined the prevalence of different informatic proxies of fibromyalgia diagnosis based on patient-reported outcomes (“PRO Diagnosis,” Table 1). Within the Widespread – Heavy cluster, PRO diagnosis proxies were more frequent than ICD-10 diagnosis (Fig 3). A significantly greater percentage of patients in the Widespread – Heavy cluster fulfilled CHOIR WPI/SS criteria (739/2123 = 34.8% Agresti-Coull 95% CI [32.8% - 36.9%]) compared to those who received a clinical diagnosis of fibromyalgia at the index encounter (476/2123 = 22.4% Agresti-Coull 95% CI [20.7% – 24.3%]). Further, patients meeting CHOIR WPI/PF criteria constituted a significantly greater proportion of the Widespread – Heavy cluster than the preceding measures of fibromyalgia diagnosis (1407/2123 = 66.3% Agresti-Coull 95% CI [64.2% - 68.3%]).
Fig 3. In patients with the most widespread pain, more patients meet informatic criteria for fibromyalgia than are diagnosed clinically.
Bars represent counts of patients within the Widespread-Heavy cluster having an ICD-10 diagnosis of fibromyalgia (Index Fibro) or meeting one of our two approximations of ACR fibromyalgia diagnostic criteria, CHOIR WPI/SS and CHOIR WPI/PF.
To compare PRO diagnosis proxies and ICD-10 cases more directly, we restricted our dataset to a sample with complete data for the calculation of all informatic criteria (N = 18,520). This is smaller than the entire dataset because PROMIS global mental and physical health, used in calculating CHOIR WPI/SS, were missing from 2,903 patients. Notably, a sensitivity analysis revealed similar findings as described above in the restricted dataset. Table 3 shows contingency tables for informatic criteria and ICD-10 cases, with sensitivity (sen), specificity (spc) positive predictive value (PPV), and negative predictive value (NPV) calculations relative to clinical ICD-10 diagnosis. Overlap among informatic and clinical diagnoses is also shown. Widespread-Heavy and CHOIR WPI/PF have similar sensitivity and specificity, with ~48% sensitivity and ~92% specificity for Fibro Index. CHOIR WPI/SS is less sensitive (31.5%) and more specific (95.6%) for Fibro Index. Examining the overlap between informatic criteria demonstrates that CHOIR WPI/SS represents a subset of CHOIR WPI/PF cases (Fibro Index: 275 / 419 = 65%). Widespread-Heavy and CHOIR WPI/PF overlap on 83% of Fibro Index cases.
Table 3.
Contingency Tables and Overlap Across Fibromyalgia Criteria
| Fibro index |
Total fibro |
Total Counts | ||||||
|---|---|---|---|---|---|---|---|---|
| YES | NO | PPV OR NPV | YES | NO | PPV OR NPV | |||
| Widespread-Heavy (W-H) | yes | 415 | 1,372 | 23.2% | 637 | 1,150 | 35.6% | 1,787 |
| no | 458 | 16,275 | 97.3% | 1,044 | 15,689 | 93.8% | 16,733 | |
| sen or spc | 47.5% | 92.2% | 37.9% | 93.2% | ||||
| WPI/PF | yes | 419 | 1,262 | 24.9% | 623 | 1,058 | 37.1% | 1,681 |
| no | 454 | 16,385 | 97.3% | 1,058 | 15,781 | 93.7% | 16,839 | |
| sen or spc | 48.0% | 92.8% | 37.1% | 93.7% | ||||
| WPI/SS | yes | 275 | 777 | 26.1% | 419 | 633 | 39.8% | 1,052 |
| no | 598 | 16.870 | 96.6% | 1,262 | 16,206 | 92.8% | 17,468 | |
| sen or spc | 31.5% | 95.6% | 24.9% | 96.2% | ||||
| Overlap across criteria | ||||||||
| W-H and WPI/PF | yes | 348 | 833 | 500 | 681 | 1,181 | ||
| W-H and WPI/SS | yes | 225 | 514 | 332 | 407 | 139 | ||
| WPI/PF and WPI/SS | yes | 275 | 777 | 419 | 633 | 1,052 | ||
| W-H, WPI/PF, and WPI/SS | yes | 225 | 514 | 332 | 407 | 739 | ||
| Total Counts | 873 | 17,647 | 1,681 | 16,839 | 18,520 | |||
Abbreviation: Index Fibro, has ICD-10 diagnosis on index encounter; Total Fibro, has ICD-10 diagnosis associated with any encounter; W-H, Widespread-Heavy; WP1/SS, proxy combined CHOIR Widespread Pain Index and Symptom Severity Score derived from PROs; WPI/PF, proxy combined Widespread Pain Index and PROMIS Physical Function, sen, sensitivity; spc. specificity, PPV, positive predictive value, NPV, negative predictive value.
NOTE. Counts are shown except italicized percentages.
Informatic criteria show different sensitivity for detecting any diagnosis of fibromyalgia, i.e. Total Fibro. The sensitivity for Total Fibro is ~37% for both Widespread-Heavy and CHOIR WPI/PF, while it is 24.9% for CHOIR WPI/SS. Agresti-Coull 95% confidence intervals for CHOIR WPI/PF (34.7% - 39.4%) and CHOIR WPI/SS (22.9% - 27.1%) do not overlap, indicating that CHOIR WPI/PF identifies more Total Fibro cases than CHOIR WPI/SS. Examining overlap for Total Fibro indicates similar patterns as with Fibro Index. Consistent with these observed patterns, if one informatic criteria was not met, the other informatic criteria did not identify a large number of additional ICD-10 cases. For example, of the 1044 Total Fibro cases which were not clustered into Widespread-Heavy but did carry an ICD-10 Total Fibro diagnosis, 123 met CHOIR WPI/PF (11.7% Agresti-Coull 95% CI 9.9%−13.9%), and 87 met CHOIR WPI/SS (8.3% Agresti-Coull 95% CI 6.7%−10.2%).
On the other hand, participants classified as Widespread-Heavy who did not have an ICD-10 diagnosis did tend to meet CHOIR informatic criteria. Using the restricted dataset (N=18,520), of the 1372 Widespread-Heavy cases without Fibro Index diagnosis, 514 (37%) met CHOIR WPI/SS and 833 (61%) met CHOIR WPI/PF criteria. Similarly, of the 1150 Widespread-Heavy cases without Total Fibro diagnosis, 407 (35%) met CHOIR WPI/SS and 681 (59%) met CHOIR WPI/PF criteria.
Notably, the frequency of ICD-10 diagnosis occurring within given informatic criteria, i.e. the positive predictive value, is surprisingly low (Table 3). For Fibro Index, PPV values ranged from 23.2% – 26.1%. For Widespread-Heavy, this is consistent with data shown from the complete dataset in Fig 3 (476/2123 = 22.4%). For Total Fibro, PPV is slightly higher, ranging from 35.6% - 39.8%. Taken together, informatic criteria identify a substantial number of cases without ICD-10 diagnosis. Within the Widespread-Heavy subgroup, CHOIR criteria are met even if ICD-10 diagnoses are absent.
Male gender is less frequent in clinical fibromyalgia diagnosis than informatic proxies
In patients with a clinical diagnosis of fibromyalgia at the index encounter, 7% (N=69/987) are male (Table 1, Fig 4). Male gender is considerably more frequent in PRO diagnosis proxies (26–29% depending on PRO diagnosis, Table 1). Using multivariable logistic regression of ICD-10 Index Fibro and CHOIR WPI/SS PRO diagnosis on gender, we found that the odds of being male and having an Index Fibro diagnosis was significantly less compared to the CHOIR WPI/SS PRO diagnosis (n = 18520, LR chi2 (2) = 514.16, p<0.0001; odds ratio(Index Fibro) / odds ratio(CHOIR WPI/SS) = 0.164 95% CI 0.147–0.184). In a separate model meant to examine gender representation in ICD-10 Index Fibro compared with Widespread-Heavy classification, we observed a similar significantly lower odds of being male in patients with Index Fibro diagnosis compared with Widespread-Heavy assignment (n = 21423, LR chi2 (2) = 602.51, p<0.0001; odds ratio(Index Fibro) / odds ratio(Widespread-Heavy) = 0.157 95% CI 0.136–0.182). This suggests that PRO diagnosis criteria have greater male representation than Index Fibro.
Fig 4. More males meet approximated fibromyalgia criteria in comparison to those diagnosed clinically.
Counts of females and males are shown. A greater proportion of males meet approximated diagnostic criteria, 26% (278/1,052 CHOIR WPI/SS) and 27% (535/1999 CHOIR WPI/PF), or belong in the Widespread-Heavy cluster (29% 608/2123) than are diagnosed clinically with fibromyalgia 7% (69/987 Index Fibro).
Given potential age differences across diagnosis methods (Table 1), we sought to examine whether these were statistically significant with multivariable linear regression and post-estimation testing. Although there was no statistically significant difference in age between Index Fibro and CHOIR WPI/SS PRO diagnosis (n = 18520, F(2, 18517) = 249.59, p<0.0001; post-estimation Wald test (H0 Index Fibro = CHOIR WPI/SS) p=0.83), patients assigned to the Widespread Heavy cluster were older than those with Index Fibro (n = 21423, F(2, 21420) = 230.51, p<0.0001; linear combination(Index Fibro – Widespread Heavy = −3.2 years 95% CI −4.6 - −1.8). Age was statistically significantly different between Index Fibro and Widespread-Heavy (post-estimation Wald test, F(1, 21420) – 19.93, p < 0.0001).
As described above, a large group of patients in the Widespread-Heavy group did not have an ICD-10 diagnosis but did meet proxy diagnostic criteria. If underdiagnosis related to gender or age bias was contributing to the low prevalence of ICD-10 diagnosis cases in the Widespread-Heavy cluster, then we expected to find differences in gender and age across ICD-10 diagnosis group specifically in patients meeting proxy criteria, i.e. patients who we predict should have been diagnosed with fibromyalgia but were not. In the Widespread-Heavy cluster subgroup meeting CHOIR WPI/SS criteria (n=739, Widespread-Heavy n=1787, total N=18520), patients without an index ICD-10 diagnosis had statistically significantly different gender proportions (without Index Fibro: 153 male / 514 total; with Index Fibro: 15 male / 225 total; chi2(1) = 47.5, p < 0.001) and age (without Index Fibro: mean 53.6 years old (SD 0.6); with Index Fibro: mean 47.5 years old (SD 0.8), t-test p<0.0001) compared with those with an ICD-10 diagnosis. Similar results were obtained using CHOIR WPI/PF (n=1181, Widespread-Heavy n=1787, total N=18520) comparing across Index Fibro for gender (without Index Fibro: 250 male / 833 total; with Index Fibro: 27 male / 348 total; chi2(1) = 67.7, p < 0.001) and age (without Index Fibro: mean 54.5 years old (SD 13.7); with Index Fibro: mean 49.3 years old (SD 12.9), t-test p<0.0001). In both instances, Widespread-Heavy patients meeting proxy diagnostic criteria for fibromyalgia but lacking an ICD-10 diagnosis tended to be male and older.
Altogether, these results suggest that fibromyalgia is underdiagnosed at the initial encounter, with younger and more female-gendered patients receiving a clinical diagnosis than proxy diagnostic criteria would predict.
Discussion
We previously demonstrated that hierarchical clustering using only a pain body map could identify distinct subgroups differing in key pain characteristics. However, it was unknown if our hierarchical clustering approach could predict pain diagnoses, such as fibromyalgia. In this study, we find that patients in the Widespread – Heavy Cluster are more likely to have a fibromyalgia diagnosis. Importantly, we also observed that fibromyalgia is diagnosed in less than a quarter of the patients belonging to the Widespread-Heavy cluster. Although it is possible that fibromyalgia is appropriately diagnosed, we favor the interpretation that fibromyalgia is being underdiagnosed likely resulting in poor clinical outcomes. We propose that informatic measures could address this problem by prompting clinicians to perform formal evaluations.
We observed that more patients in our sample met informatic criteria for fibromyalgia diagnosis than were diagnosed clinically. We suspect this is due to failures in the application of ACR diagnostic criteria during the clinical encounter, i.e. underdiagnosis, for several reasons. First, ~75% of patients in the Widespread-Heavy group lacked an ICD-10 code at the index visit (Table 1, Fig 1, and Table 3). Of those, that lacked the ICD-10 code, 1/3 met CHOIR WPI/SS and 2/3 met CHOIR WPI/PF criteria (Table 3). Using a similar approach to the current study, an epidemiologic investigation using the 2012 US National Health Interview Survey found that only 27% of cases meeting a study-generated proxy diagnosis received a clinical diagnosis of fibromyalgia36. This is quite similar to our observed sensitivity estimates for Widespread-Heavy, CHOIR WPI/SS, and CHOIR WPI/PF (Table 3). Therefore, our study confirms prior work identifying discrepancies in clinical and proxy diagnoses with a unique dataset and approach. Both studies find fewer clinical diagnoses of fibromyalgia than predicted based on patient report.
Comparing our findings with the literature, ICD-10 prevalence in this study is lower than other reports, with greater female-to-male gender ratios and younger age than expected. In an early study of fibromyalgia prevalence41, a community sample of 391 people were clinically evaluated, and fibromyalgia was diagnosed in 3.4% women and 0.5% men, with an overall prevalence of 2% that increased with age28, 43. In another study using the ACR 2010 research criteria, age- and gender-adjusted prevalence was ~6%, with a 2-fold higher prevalence in women compared with men35. These prevalence estimates are consistent internationally and across multiple subsequent studies with female-to-male ratios of 8:1 with tender point exams and closer to 1–2:1 without tender point exams11, 18, 42. In our sample, ICD-10 diagnosis occurred in ~4% of patients (Table 1). Although similar to community-based estimates, fibromyalgia diagnosis rates are much greater in pain clinics, with up to 40% of patients meeting diagnostic criteria7, 22, 25. Additionally, we found a female-to-male ratio of 13:1 (Table 1), which is much larger than an expected 2:1 ratio. Taken together, the lower than expected prevalence estimate and greater than expected female-to-male gender ratio both support the interpretation that fibromyalgia is underdiagnosed.
Prior work supports the interpretation that our observed gender discrepancy arises from underdiagnosis. It is well documented that physicians are more likely to diagnose or even consider a diagnosis of fibromyalgia in women compared to men5. When strict diagnostic criteria that have no gender requirements are employed, only a slight majority of patients that received a diagnosis of fibromyalgia were women42. In contrast, 93% of index fibromyalgia diagnoses in our sample belonged to women. PRO diagnosis had fewer women (71–74%). Patients categorized into Widespread-Heavy at the index encounter but who did not have an ICD-10 fibromyalgia diagnosis had more men than those who had an ICD-10 diagnosis (Table 2). This suggests that strict diagnostic criteria were not applied leading to gender discrepancies42. Taken together, in our sample, clinical diagnosis of fibromyalgia is associated with younger, female-gendered patients compared with PRO diagnosis, likely representing underdiagnosis.
An argument against the interpretation of underdiagnosis is that, in patients without an ICD-10 diagnosis who satisfied informatic proxy diagnoses, clinicians had identified alternative causes of widespread pain and, therefore, appropriately did not diagnose fibromyalgia. However, the ACR 2016 criteria indicate that a clinical diagnosis of fibromyalgia should be made “irrespective of other diagnoses”38. In this scenario, a lack of an ICD-10 diagnosis would still be inappropriate. On the other hand, diagnostic coding may not fully reflect clinician evaluations. Factors like treatment for other conditions, concerns about the clinician-patient relationship, or economic considerations might contribute to the lack of an ICD-10 diagnosis. Although this is all possible, prevalence studies share these limitations35, 41 and still reported greater rates of diagnosis and lower female-to-male ratios. For these reasons, we favor the interpretation that there is underdiagnosis of fibromyalgia in current clinical practice above alternative interpretations.
Underdiagnosis of fibromyalgia is clinically important. It is likely that increasing clinical diagnosis rates of fibromyalgia would improve patient care, since undiagnosed fibromyalgia is associated with poor outcomes for patients and increased health care utilization2, 21, 37. In our prior study, we found that patients classified in the Widespread-Heavy group had poor 3-month outcomes, with minimal change in pain intensity and the second worst responder rate1. Given our current findings, we hypothesize that underdiagnosis of fibromyalgia, and likely undertreatment, accounts for these poor outcomes. Future work will focus on testing this hypothesis.
Improvement in diagnosis may be accelerated with the application of our PRO diagnosis methods as screening tools, especially since they are rapid, scalable, and specific. Patient reported information obtained electronically in a matter of minutes in the waiting room, as was done in our study, could trigger further evaluation by the clinician if certain criteria are reported, such as those used in this study for creating CHOIR WPI/SS. Moreover, machine learning applied to body map responses alone would accelerate screening, since the body map only takes seconds to complete. This sets our approach apart from prior work using highly complex datasets incorporating EMR data to predict chronic pain diagnosis14, 17. Future work will determine whether identification of possible fibromyalgia cases using Widespread-Heavy cluster membership could be used to prompt further formal application of ACR criteria to reduce unidentified cases. It is important to point out that a formal clinical evaluation remains critical for a fibromyalgia diagnosis3, 16, 19. However, our investigation demonstrates that an informatic proxy of ACR diagnostic criteria has utility in quickly identifying patients for the time-intensive, formal evaluation, potentially reducing bias and improving diagnosis.
It is also possible that making a clinical fibromyalgia diagnosis is less important than identifying characteristics of fibromyalgia which negatively influence patient outcomes and response to certain treatments. For example, “fibromyalgia-ness” reflected by high WPI and SSS scores in patients without a formal fibromyalgia diagnosis predicts worse post-operative pain following hip and knee arthroplasty8, 9. This literature combined with our observation that widespread pain is common without clinical fibromyalgia diagnosis, e.g., low rates of ICD-10 diagnosis in Widespread-Heavy, may argue for greater application of the transdiagnostic classification of nociplastic pain15, rather than fibromyalgia diagnosis per se. Nociplastic pain is thought to be centrally mediated and has been associated with worse outcomes for peripherally-targeted treatments commonly used for nociceptive pain, such as anti-inflammatory drugs, injections, or surgery15. It is also characterized by widespread pain and to some extent the nociplastic pain diagnosis overlaps or encompasses the disease of fibromyalgia. It is possible that patients without Index Fibro but classified in the Widespread-Heavy cluster would not meet ACR fibromyalgia criteria in a formal clinical evaluation but do have prominent nociplastic components to their pain syndrome. From this perspective, pain body map subgroups including the Widespread-Heavy cluster may provide a feasible way to operationalize nociplastic pain, allowing for future work examining the relationship between nociplastic pain, diagnosis, and treatment outcomes.
The current study benefits from a large sample of patients and a reporting of clinical diagnoses of fibromyalgia from 2016–2021 which allowed for direct comparison of clinical diagnoses with patients meeting informatic criteria. The use of informatic diagnosis proxies has face validity, since CHOIR WPI/SS and CHOIR WPI/PF incorporate measures that relate to the symptoms severity score in the ACR diagnostic criteria27. However, PRO diagnosis is ultimately a proxy of the ACR diagnostic criteria rather than a formal clinical evaluation to assess the diagnosis of fibromyalgia, which is a limitation of the current study design. Additionally, clinical diagnoses were only recorded if the ICD-10 code was present in the EMR, with the possibility that prior diagnoses or misreporting in the EMR could underestimate the prevalence of fibromyalgia.
In conclusion, the current study aimed to determine if hierarchical clustering of pain body map responses could predict the diagnosis of fibromyalgia. We found that patients belonging to the Widespread-Heavy cluster, a cluster with widespread pain distribution, worse symptoms, and poor outcomes, were significantly more likely to be diagnosed with fibromyalgia. Additionally, fibromyalgia may be clinically underdiagnosed, since more patients met proxy criteria than were clinically diagnosed with fibromyalgia at the same visit. More males met proxy criteria for a fibromyalgia diagnosis than were clinically diagnosed. Application of machine learning and rapid body map pain assessments could be used to limit bias and improve the diagnosis of fibromyalgia.
Supplementary Material
Highlights.
Pain patterns identified with hierarchical clustering of body map responses predict diagnosis.
In a large clinical sample, informatic criteria for fibromyalgia suggest underdiagnosis.
Algorithmic interpretation of body maps could reduce underdiagnosis by flagging clinicians.
Perspective:
This investigation applies hierarchical clustering to patient-reported, digital pain body maps, finding an association between body map responses and clinical fibromyalgia diagnosis. Rapid, computer-assisted interpretation of pain body maps would be clinically useful in prompting more detailed assessments for fibromyalgia, potentially reducing gender bias.
Footnotes
Disclosures:
The authors have no conflicts of interest to disclose. This study was supported by the University of Pittsburgh (ADW) and the NINDS (K23NS123429, BJA).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alter BJ, Anderson NP, Gillman AG, Yin Q, Jeong JH, Wasan AD. Hierarchical clustering by patient-reported pain distribution alone identifies distinct chronic pain subgroups differing by pain intensity, quality, and clinical outcomes. PLoS One. 16:e0254862, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Annemans L, Wessely S, Spaepen E, et al. Health economic consequences related to the diagnosis of fibromyalgia syndrome. Arthritis Rheum. 58:895–902, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Arnold LM, Clauw DJ, McCarberg BH. Improving the recognition and diagnosis of fibromyalgia. Mayo Clin Proc. 86:457–464, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold LM, Crofford LJ, Mease PJ, et al. Patient perspectives on the impact of fibromyalgia. Patient Educ Couns. 73:114–120, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barsky AJ, Peekna HM, Borus JF. Somatic symptom reporting in women and men. J Gen Intern Med. 16:266–275, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernik M, Sampaio TP, Gandarela L. Fibromyalgia comorbid with anxiety disorders and depression: combined medical and psychological treatment. Curr Pain Headache Rep. 17:358, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Brill S, Ablin JN, Goor-Aryeh I, Hyat K, Slefer A, Buskila D. Prevalence of fibromyalgia syndrome in patients referred to a tertiary pain clinic. J Investig Med. 60:685–688, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Brummett CM, Janda AM, Schueller CM, et al. Survey criteria for fibromyalgia independently predict increased postoperative opioid consumption after lower-extremity joint arthroplasty: a prospective, observational cohort study. Anesthesiology. 119:1434–1443, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brummett CM, Urquhart AG, Hassett AL, et al. Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol. 67:1386–1394, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clauw DJ. Fibromyalgia: an overview. Am J Med. 122:S3–s13, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Clauw DJ. Fibromyalgia: a clinical review. JAMA. 311:1547–1555, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Doebl S, Hollick RJ, Beasley M, Choy E, Macfarlane GJ. Comparing the Impact of Symptoms and Health Care Experiences of People Who Have and Have Not Received a Diagnosis of Fibromyalgia: A Cross-Sectional Survey Within the PACFiND Study. Arthritis Care Res (Hoboken). 2021 [DOI] [PubMed] [Google Scholar]
- 13.Dressler AM, Gillman AG, Wasan AD. A narrative review of data collection and analysis guidelines for comparative effectiveness research in chronic pain using patient-reported outcomes and electronic health records. J Pain Res. 12:491–500, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DuBrava S, Mardekian J, Sadosky A, et al. Using Random Forest Models to Identify Correlates of a Diabetic Peripheral Neuropathy Diagnosis from Electronic Health Record Data. Pain Med. 18:107–115, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Fitzcharles MA, Cohen SP, Clauw DJ, Littlejohn G, Usui C, Häuser W. Nociplastic pain: towards an understanding of prevalent pain conditions. Lancet. 397:2098–2110, 2021 [DOI] [PubMed] [Google Scholar]
- 16.Gittins R, Howard M, Ghodke A, Ives TJ, Chelminski P. The Accuracy of a Fibromyalgia Diagnosis in General Practice. Pain Med. 19:491–498, 2018 [DOI] [PubMed] [Google Scholar]
- 17.Gostine M, Davis F, Roberts BA, et al. Clinical Characteristics of Fibromyalgia in a Chronic Pain Population. Pain Pract. 18:67–78, 2018 [DOI] [PubMed] [Google Scholar]
- 18.Häuser W, Fitzcharles MA. Facts and myths pertaining to fibromyalgia. Dialogues Clin Neurosci. 20:53–62, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Häuser W, Sarzi-Puttini P, Fitzcharles MA. Fibromyalgia syndrome: under-, over- and misdiagnosis. Clin Exp Rheumatol. 37 Suppl 116:90–97, 2019 [PubMed] [Google Scholar]
- 20.Hood AM, Morais CA, Aroke EN, et al. Antiracism CoaliTION in Pain Research (ACTION-PR): Guiding Principles for Equity in Reporting. J Pain. 24:19–21, 2023 [DOI] [PubMed] [Google Scholar]
- 21.Hughes G, Martinez C, Myon E, Taïeb C, Wessely S. The impact of a diagnosis of fibromyalgia on health care resource use by primary care patients in the UK: an observational study based on clinical practice. Arthritis Rheum. 54:177–183, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Katz RS, Wolfe F, Michaud K. Fibromyalgia diagnosis: a comparison of clinical, survey, and American College of Rheumatology criteria. Arthritis Rheum. 54:169–176, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Kent ML, Tighe PJ, Belfer I, et al. The ACTTION-APS-AAPM Pain Taxonomy (AAAPT) Multidimensional Approach to Classifying Acute Pain Conditions. J Pain. 18:479–489, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleykamp BA, Ferguson MC, McNicol E, et al. The Prevalence of Psychiatric and Chronic Pain Comorbidities in Fibromyalgia: an ACTTION systematic review. Seminars in arthritis and rheumatism. 51:166–174, 2021 [DOI] [PubMed] [Google Scholar]
- 25.Marcus DA, Bernstein C, Albrecht KL. Brief, self-report fibromyalgia screener evaluated in a sample of chronic pain patients. Pain Med. 14:730–735, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathur VA, Trost Z, Ezenwa MO, Sturgeon JA, Hood AM. Mechanisms of injustice: what we (do not) know about racialized disparities in pain. Pain. 163:999–1005, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merriwether EN, Rakel BA, Zimmerman MB, et al. Reliability and Construct Validity of the Patient-Reported Outcomes Measurement Information System (PROMIS) Instruments in Women with Fibromyalgia. Pain Med. 18:1485–1495, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minerbi A, Fitzcharles MA. Fibromyalgia in Older Individuals. Drugs Aging. 38:735–749, 2021 [DOI] [PubMed] [Google Scholar]
- 29.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 41:582–592, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Palermo TM. Editorial: Introducing New Reporting Guidelines to Address Inclusion, Diversity, Equity, Antiracism, and Accessibility: Implementation at The Journal of Pain. J Pain. 24:22–23, 2023 [DOI] [PubMed] [Google Scholar]
- 31.Sarzi-Puttini P, Giorgi V, Marotto D, Atzeni F. Fibromyalgia: an update on clinical characteristics, aetiopathogenesis and treatment. Nature reviews. Rheumatology. 16:645–660, 2020 [DOI] [PubMed] [Google Scholar]
- 32.Scherrer KH, Ziadni MS, Kong JT, et al. Development and validation of the Collaborative Health Outcomes Information Registry body map. Pain reports. 6:e880, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sturgeon JA, Darnall BD, Kao MC, Mackey SC. Physical and psychological correlates of fatigue and physical function: a Collaborative Health Outcomes Information Registry (CHOIR) study. J Pain. 16:291–298.e291, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sturgeon JA, Dixon EA, Darnall BD, Mackey SC. Contributions of physical function and satisfaction with social roles to emotional distress in chronic pain: a Collaborative Health Outcomes Information Registry (CHOIR) study. Pain. 156:2627–2633, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vincent A, Lahr BD, Wolfe F, et al. Prevalence of fibromyalgia: a population-based study in Olmsted County, Minnesota, utilizing the Rochester Epidemiology Project. Arthritis care & research. 65:786–792, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walitt B, Nahin RL, Katz RS, Bergman MJ, Wolfe F. The Prevalence and Characteristics of Fibromyalgia in the 2012 National Health Interview Survey. PLoS One. 10:e0138024, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White KP, Nielson WR, Harth M, Ostbye T, Speechley M. Does the label “fibromyalgia” alter health status, function, and health service utilization? A prospective, within-group comparison in a community cohort of adults with chronic widespread pain. Arthritis Rheum. 47:260–265, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Wolfe F, Clauw DJ, Fitzcharles MA, et al. 2016. Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Seminars in arthritis and rheumatism. 46:319–329, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 38:1113–1122, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Wolfe F, Rasker JJ. The Evolution of Fibromyalgia, Its Concepts, and Criteria. Cureus.13:e20010, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 38:19–28, 1995 [DOI] [PubMed] [Google Scholar]
- 42.Wolfe F, Walitt B, Perrot S, Rasker JJ, Häuser W. Fibromyalgia diagnosis and biased assessment: Sex, prevalence and bias. PLoS One. 13:e0203755, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yunus MB, Holt GS, Masi AT, Aldag JC. Fibromyalgia syndrome among the elderly. Comparison with younger patients. J Am Geriatr Soc. 36:987–995, 1988 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.