Abstract
Determining egg quality is the foremost challenge in assisted reproductive technology (ART). Although extensive advances have been made in multiple areas of ART over the last 40 years, oocyte quality assessment tools have not much evolved beyond standard morphological observation. The oocyte not only delivers half of the nuclear genetic material and all of the mitochondrial DNA to an embryo but also provides complete developmental support during embryonic growth. Oocyte mitochondrial numbers far exceed those of any somatic cell, yet little work has been done to evaluate the mitochondrial bioenergetics of an oocyte. Current standard oocyte assessment in in vitro fertilization (IVF) centers include the observation of oocytes and their surrounding cell complex (cumulus cells) via stereomicroscope or inverted microscope, which is largely primitive. Additional oocyte assessments include polar body grading and polarized light meiotic spindle imaging. However, the evidence regarding the aforementioned methods of oocyte quality assessment and IVF outcomes is contradictory and non-reproducible. High-resolution microscopy techniques have also been implemented in animal and human models with promising outcomes. The current era of oocyte imaging continues to evolve with discoveries in artificial intelligence models of oocyte morphology selection albeit at a slow rate. In this review, the past, current, and future oocyte imaging techniques will be examined with the goal of drawing attention to the gap which limits our ability to assess oocytes in real time. The implications of improved oocyte imaging techniques on patients undergoing IVF will be discussed as well as the need to develop point of care oocyte assessment testing in IVF labs.
Keywords: oocyte imaging, non-invasive oocyte screening, light microscopy, polarized light microscopy, Raman spectroscopy, artificial intelligence, machine learning, microfluidics, oocyte mitochondria, fertility, live and fixed cell imaging
Non-invasive oocyte imaging techniques in assisted reproductive technologies are scarce and underdeveloped. The future of oocyte imaging methods should be low cost and safe with high-resolution technology.
Graphical Abstract
Graphical Abstract.
Introduction
If oocyte quality imaging techniques are improved, modern reproductive medicine will have an unparalleled opportunity to improve fertility treatment outcomes. Concerning data from the World Health Organization show one in six people experience infertility [1] and approximately 50% of all cases are attributed to female etiologies. Even with modern in vitro fertilization (IVF) technologies, only 44.5% of women under 35 years of age have an IVF cycle that results in a live birth [2]. This number steadily decreases as women age. Since women are born with all the oocytes they will ever have, each oocyte retrieved during a costly cycle of IVF is vital. According to the American Society for Reproductive Medicine (ASRM), the average cost of one IVF cycle in the US is $12 400 [3], and Katz et al. estimated the total average cost to have one live birth is approximately $60,000 [4]. Assisted reproductive technology (ART) is not covered by the majority of private insurances and less than half of states mandate fertility insurance coverage laws [5]. Furthermore, coverage is only given to individuals who meet various imposed criteria such as marital status, heterosexuality, and age. Given the extreme financial burden of IVF both in the US and globally, optimizing all aspects of IVF cycles is necessary for patients to achieve their dreams of a healthy live birth. The national live birth rates (LBR) for women using autologous oocytes reported by the Society for Assisted Reproductive Technology (SART), in less than 35-year olds is 44.5%, 32.4% for 35- to 37-year olds, 20.2% for 38- to 40-year olds, 9.6% for 41- to 42-year olds, and 2.9% in greater than 42-year olds [2]. The most common group requiring ART and IVF are the fastest growing group, women 40 and above, who generally have lesser quality oocytes. Patients who cannot achieve a live birth with autologous oocytes may need to turn to donor eggs, which is a financially and emotionally challenging decision. Yet, there is no good selection method for screening oocytes due to a lack of imaging methods. Currently, the quality of an egg is unknown until after fertilization and implantation. This is reflected in the fact that the average LBR of women utilizing donor oocytes is only 41.4% in the US in 2021 [6]. Advancements in oocyte quality imaging could hopefully help improve these outcomes. Oocyte quality can be negatively affected by age [7, 8], obesity [9, 10], lifestyle factors [11, 12], and genetics [13]. Any one of these factors could cause infertility; therefore, determining the quality of oocytes will lead to improved chances of conception and help patients make informed decisions about their care. A high-quality, chromosomally normal (euploid) oocyte is not known as such until after fertilization, embryogenesis, implantation, and a healthy live birth. Determining oocyte quality prior to fertilization and implantation could reduce the timeline to a live birth. However, none of the commonly used imaging techniques provide definitive information about the quality of oocytes in an embryology lab. Despite improvements in culture media, the introduction of intracytoplasmic sperm injection (ICSI) [14] and embryo and oocyte vitrification [15], limited progress has been made in oocyte quality assessments. There is no intra or interlaboratory standardization of oocyte grading amongst embryologists therefore leading to high subjectivity [16, 17]. Effective technology designed to screen live oocytes for the highest quality could significantly improve live birth rates and would enable patients and practitioners alike to make better informed decisions regarding their care plan. An additional benefit of choosing the most robust oocytes from an IVF cycle may include mitigating the challenges that couples face in countries and societies that prohibit the cryopreservation of supernumerary embryos [18, 19]. Furthermore, patients undergoing oocyte cryopreservation for elective or medical reasons could have more confidence that only the healthiest oocytes with the best chance for a live birth are cryopreserved for the future. Lastly, the goal of an IVF cycle is to achieve a single healthy live birth and elective single embryo transfer is the only effective means to achieve this [20]. ASRM and SART recommend only transferring one embryo in patients less than 35 years old, two or less in 38- to 40-year olds, and three or less in >40-year olds to minimize the risks of multiple gestations [21]. If the quality of the oocyte and resultant embryo were known prior to implantation, physicians and patients would have more confidence to consent to the transfer of one healthy embryo.
Non-invasive oocyte screening methods are essential to our understanding of how to select the highest quality oocytes used for insemination and transfer. Currently, the standard of care oocyte assessments consists of morphological assessments via light microscopy. This method of visualization is subjective, non-standardized, and does not provide positive predictive value of oocyte fertilization or implantation. With the advancement of microscopy, additional screening techniques have been implemented in IVF laboratories over the years. These include meiotic spindle imaging via polarized light microscopy, confocal Raman spectroscopy, and fluorescence life-time imaging to name a few. The future of non-invasive oocyte screening incorporates microfluidic devices and artificial intelligence (AI) methods that leverage machine learning, deep learning, and neural networks through sophisticated algorithms to detect oocyte morphology and competence. These AI platforms could be the future of non-invasive oocyte screening. This review will describe the past, current, and future oocyte imaging methods, the advantages, and limitations of each technology and where the future of imaging will take us.
Current oocyte assessment techniques in ART
Current assessments of oocyte quality in IVF labs include simplistic, subjective, and minimal visualization methodologies [22]. Removal of the cumulus oocyte complex (COC) from oocytes of patients undergoing IVF with ICSI is necessary to allow for visualization of the nuclear maturity [22–24]. Typically, in an embryology laboratory, light microscopy is utilized to check for oocyte maturity, which depends on the stage of meiosis [25]. The visualization of a large nucleus also known as the germinal vesicle (GV) within the oocyte indicates; it is arrested at prophase meiosis I and is unable to be fertilized. A metaphase I oocyte (MI) occurs after GV breakdown and is arrested at metaphase meiosis I. It is characterized by having no nucleus or polar body (PB). Once oocytes complete meiosis I, a PB is extruded rendering a haploid oocyte (MII) that is arrested in the metaphase of meiosis II until fertilization takes place [24].
Polar Body assessments
Extrusion of the first polar body (PB) from an oocyte indicates its maturity, and the appearance of the PB has been used to determine the quality of an oocyte [24, 26–28]. The PB should be smooth and not fragmented. However, the impact of PB quality on oocytes and resulting IVF outcomes is debatable. DeSantis et al. analyzed 873 oocytes derived from 382 patients and divided them into four groups based upon their PB morphology. Group I was of normal size and had a smooth surface, group II was fragmented, group III displayed a rough surface, and group IV was large in size. Between the four PB classification groups, the fertilization rates were not statistically significant (59, 57, 64, and 60%; p ≥ 0.05). Additionally, the number of high-quality day 2 embryos was not statistically significant amongst groups I, II, and III (14, 12, and 17%; p ≥ 0.05), while the low number of embryos in group IV did not allow for comparative statistical analysis to the other groups [26]. The morphological characteristics of 470 MII oocyte polar bodies from 80 ICSI cycles were observed by Halvaei et al. and they equally found no significant difference in fertilization (61.5 versus 59.8%; p ≥ 0.05) or good embryo formation (66.5 versus 55.6%; p ≥ 0.05) when fragmented polar bodies were compared with normal polar bodies [29]. Subjectivity and variability amongst embryologists performing this grading is a potential reason that PB morphology has no predictive value. On the contrary, other studies reported that first PB morphology significantly correlated with embryo quality, clinical implantation, and pregnancy rates [30]. Younis et al. divided 553 MII oocytes according to their PB morphology (group I: normal intact round or ovoid polar bodies, group II: normal and abnormal, group III: abnormal, fragmented polar bodies). Of the 176 embryos transferred, they observed significantly higher implantation (31, 9, and 2%; p ≤ 0.001) and pregnancy rates (61, 24, and 5%; p ≤ 0.001) when the PB had a normal morphology [30]. Ebner et al. reported an increase in embryo fragmentation and a decrease in blastocyst development resulting from oocytes with abnormal polar bodies [27]. Ebner et al. divided 644 MII oocytes from 60 women and grouped them according to intact (group 1; 279) or fragmented (group 2; 365) polar bodies. A significant increase in fragmentation on day 2 of development (13.2% versus 10.9%; p ≤ 0.05) and decrease in blastocyst formation (42.2% versus 54.9%; p ≤ 0.025) was seen in the group with a fragmented compared to intact PB [27]. Light microscopic observations of PB’s are limited and there is insufficient evidence to deem this a reliable oocyte assessment tool.
Oocyte morphological assessments and IVF outcomes
Embryologists utilize light microscopy to notate extracytoplasmic and cytoplasmic abnormalities when visualizing oocytes for intracytoplasmic sperm injection [17, 25, 31, 32]. Oocyte abnormalities include unusual zona pellucida (ZP) or the extracellular matrix surrounding an oocyte. Abnormal coloring and size of the ZP may be observed [33]. Perivitelline space, which is the space between the ZP and oocyte, can be abnormally large (Figure 1) [34]. Dark cytoplasm and the presence of vacuoles are also observed abnormalities [35, 36]. However, these observations are subjective, variable and data on the outcomes of these abnormalities are disparate. Ten et al. assessed oocytes from 160 ICSI cycles and found oocytes containing dark cytoplasm had an 83% decrease of having good quality embryos compared to the control. Additionally, good-quality embryos were 1.8 times more likely to have been derived from oocytes with a larger than normal perivitelline space [36]. In contrast, Esfandiari et al. found no differences in fertilization (63% vs. 58% after IVF, 69.5% vs. 75.1% after ICSI; p ≥ 0.05), embryo development (43.6% vs. 42.6% for grade I embryos and 35.9% vs. 36.4% for grade II embryos; p ≥ 0.05), implantation and clinical pregnancy rates (7.6% vs. 8% and 25% vs. 24.4%; p ≥ 0.05), respectively, of transfers of embryos derived from oocytes of the “dark granular” appearance [37]. A systematic review of 52 articles concluded some aspects of oocyte abnormalities have a negative effect whereas others do not. Dark cytoplasm, homogeneous granularity of cytoplasm, and abnormal oocyte shape had no influence on treatment outcome. On the contrary, they found fragmented PBs, dark ZP, and a large PV space with debris, which is likely to affect some treatment outcomes [35]. These inconsistent outcomes of light microscopy observations of oocyte abnormalities demonstrate that this method is not reliable, adequate, or sufficient to choose the best-quality oocytes.
Figure 1.
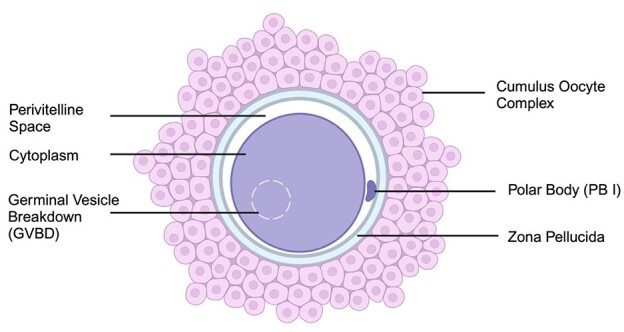
Graphical abstract of a mature, metaphase II oocyte. Created with BioRender.com.
Oocyte imaging techniques
Microscopy has evolved over the years allowing for higher resolution of biological structures up to the nanometer level. Many forms of optical imaging have been utilized to image oocytes and their surrounding COC such as polarized light microscopy, standard fluorescence microscopy, Raman spectroscopy, laser scanning confocal microscopy, and hyperspectral microscopy [38–42]. However, none of these techniques are used in real-time embryology laboratories as they can be damaging to the oocytes, expensive, and cumbersome. This section will review several studies performed utilizing these various optical imaging techniques and their discoveries of oocyte structure and function.
Polarized light microscopy for meiotic spindle visualization
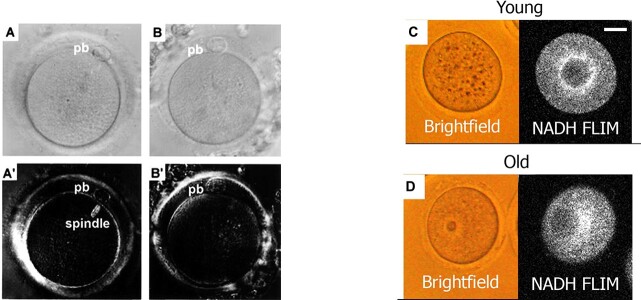
Polarized light microscopy has been utilized to observe meiotic spindles in oocytes. This differs from traditional light microscopy as the light waves oscillate in only one plane for increased contrast and improvement of image quality when examining birefringent objects (objects that have different refractive indices depending on the polarization and direction of light and thus double refract light in two orthogonal directions) [43, 44]. The optimal IVF cycle has approximately 70% maturity of oocytes; however, further imaging techniques utilizing polarized light microscopy could determine critical genetic characteristics of these oocytes. During the metaphase of meiosis II, microtubules of the meiotic spindle align chromosomes along the equator. The meiotic spindle is critical for proper segregation of chromosome alignment, genetic diversity, and reduction of chromosomes in the oocyte by half to prepare it for fertilization [45]. Aneuploidy, or the incorrect number of chromosomes, is responsible for causing poor reproductive outcomes, as people with ovaries undergo ovarian aging [46]. It is predicted that more than 50% of oocytes from women over 40 are aneuploid leading to miscarriages or failed implantation, largely caused by defects in the meiotic spindle [47–49]. Imaging the meiotic spindle has been utilized as a non-invasive way to determine oocyte quality in IVF labs and has the potential to be used in the selection of euploid oocytes prior to fertilization. With conventional light microscopy, it is not possible to visualize the meiotic spindle due to the translucent appearance and lack of contrast [43]. Although fluorescent labels and colored dyes could be used to visualize the meiotic spindle, they are invasive and damaging to the oocytes, rendering them unusable [50, 51]. A non-invasive technique to visualize the meiotic spindle of oocytes is using polarized light microscopy (PolScope) that can visualize birefringent structures such as microtubules of the spindle [52]. PolScope observation studies (Figure 2) have confirmed the safety of use on human oocytes and showed no difference in fertilization or viable embryo rates when exposed to the PolScope [51]. Utilizing PolScope imaging, comparisons have been made between the presence of a spindle and its size to oocyte quality and IVF outcomes, however results remain contradictory. Wang et al. examined 1544 oocytes from 136 ICSI cycles for the presence or absence of a meiotic spindle. In less than 35-year olds (74 cycles), 84.3% displayed a birefringent spindle, 75.1% in 35- to 37-year olds (26 cycles), 82.3% in 38- to 40-year olds (32 cycles), and 75% in greater than 40-year-olds (4 cycles). A significant increase in fertilization (69.4% versus 62.9%; p ≤ 0.05), good-quality day 3 embryos (66.3% versus 55.4%; p ≤ 0.01), and blastocyst development (51.1% versus 30.3%; p ≤ 0.001) rates were seen in oocytes when a spindle was observed [41]. Tomari et al. showed a relationship between meiotic spindle size and embryo developmental potential. 1302 oocytes were divided into groups based on the spindle size: group A (<90 μm2), group B (90–120 μm2), and group C (>120 μm2). Oocytes with a spindle size of 90-120 μm2 (group B) had a statistically significant higher fertilization rate compared to groups A and C (83.8% vs. 75.6% and 74.5%; p ≤ 0.01), respectively. The blastocyst formation rate was significantly higher in group B compared to group A (53.1% v.s 40.6%; p ≤ 0.05) and the clinical pregnancy rate in group B was significantly higher than groups A and C (29.5% vs. 9.4% and 12.0%; p ≤ 0.05), respectively [42]. On the contrary, De Santis et al. and Chamayou et al. have not shown significant differences in pregnancy and implantation rates or embryo quality when observing the presence and quality of the meiotic spindle [53, 26]. Chamayou et al. did not find a significant relationship between meiotic spindle presence or absence and clinical pregnancy (20.8% vs. 17.5%; p ≥ 0.05) or implantation rates (11.9% vs. 9.7%; p ≥ 0.05) when observing 967 embryos transferred [53]. De Santis et al. analyzed the retardance or density of microtubules of the meiotic spindle in oocytes of women with a mean age of 35.0 ± 4.9. They revealed no significant differences between spindle length and oocyte quality (<34 years old: 2.41 nm, 35–29: 2.36 nm, and ≥ 40: 1.94 nm) or the resulting embryo quality (grade A embryos: 2.74 nm, grade B + C embryos: 2.36 nm) when comparing the average maximum spindle retardance [26]. As demonstrated with these conflicting results, PolScope imaging of oocytes is not uniform in its ability to predict IVF outcomes. Although PolScope is non-invasive and usable in real time, it is not standard of care in IVF labs due to inconsistent results and inefficiency.
Figure 2.
Human oocytes imaged on differential interference contrast (DIC) and PolScope to determine the presence or absence of a meiotic spindle (left). (A) DIC image of MII oocyte without visualization of the meiotic spindle. (A’) Visualization of the image of oocyte “A” using the PolScope. (B) DIC image of MII oocyte, cannot visualize the meiotic spindle. (B′) Image with PolScope; however, no meiotic spindle is visualized [41]. Bright field and FILM images of young and old mice (right). (C) NADH FLIM bright areas show uniform distribution of mitochondria. (D) NADH FLIM shows a strong mitochondria signal around the periphery instead of a uniform distribution [65].
High-resolution optical imaging techniques
Confocal Raman spectroscopy
Confocal Raman spectroscopy (CRS) employs inelastic scattering to produce interactions of light and matter. Photon scattering allows for identification of molecules and the molecular bonds of cells [17]. Although CRS is non-invasive, it does require long imaging times of live cells which could be detrimental to oocytes retrieved during IVF. However, CRS has been utilized to observe changes in molecular structures of mouse oocytes. Wood et al. used CRS to scan for differences between GV and MII oocytes and discovered that GV oocytes contained a small, central lipid deposit and a large polar lipid deposit, whereas MII oocytes have one large central lipid deposit [54]. Line scans were also employed across the diameter of the oocyte and compared via principal component analysis (PCA). The line scans were used as maturation markers to determine differences between GV and MII’s [54]. This technique has potential to predict oocyte development competency via non-invasive imaging. An additional use for Raman microscopy is the ability to image morphological aspects of aging oocytes. Oocyte age is an important factor in fertility and numerous studies have shown the decline in oocyte quality as women age [55]. Oocyte imaging of older oocytes can provide us with insight into their function, structure, and ability to choose the healthiest oocytes from a group of developing follicles. An experiment by Bogliolo et al. employed Raman spectroscopy to detect chemical modifications induced by age-related oxidative stress in mouse oocytes [56]. They superovulated 4- to 8-week-old mice (young) and 48- to 52-week-old mice (old) and their MII oocytes were retrieved from the oviducts. Their experimental design divided the young mice into three groups: (A) oocytes were processed immediately after collection, (B) cultured in vitro for 10 hours before processing, and (C) exposed to 10 mM hydrogen peroxide to produce oxidative stress. Their fourth group D consisted of MIIs from the old mice. Spectra from CRS were analyzed statistically via PCA and showed young oocytes had significant differences in lipid and protein components compared to all other groups (Figure 3). Raman spectroscopy is also used to visualize damage either internally or externally post vitrification and warming of oocytes. Vitrification is an ultra-rapid method of cryopreservation which prevents inter and intracellular ice crystal formation [57]. Successful vitrification of oocytes is achieved by exposing them to permeable and non-permeable cryoprotectant agents (CPA’s) such as sucrose, ethylene glycol and dimethyl sulfoxide. Although CPAs are protective during vitrification, at high volumes and exposure times, they become toxic to oocytes [58–61]. Utilizing Raman spectroscopy to image oocytes post vitrification and warming could have huge implications for donor egg banks to screen their donors for any structural damage sustained during the vitrification process that is missed by light microscopy. Rusciano et al. investigated biochemical modifications of the ZP and cytoplasm of vitrified bovine oocytes. They had three experimental groups: (1) vitrified and warmed oocytes, (2) exposure to cryoprotectants only, and (3) control group. The results of PCA showed vitrification induced a protein transformation whereas lipids were tightly packed and maintained in the zona. The findings found vitrification and subsequent warming process causes zona hardening [63]. Zona hardening occurs after natural fertilization; however, when exposed to CPAs, a large influx in intracellular calcium concentration occurs, yielding a hardened ZP [62]. This is clinically relevant because although ICSI is the method of fertilization, a hardened zona could still interfere with endometrial implantation. Therefore, assisted hatching is performed on the ZP of a blastocyst to circumvent zona hardening. An additional observation from this study was protein structural loss which alludes to protein denaturation from the extreme cold exposure [63]. Protein denaturation could have sequelae that are unknown and unseen without improvement of oocyte imaging to identify changes caused by vitrification.
Figure 3.
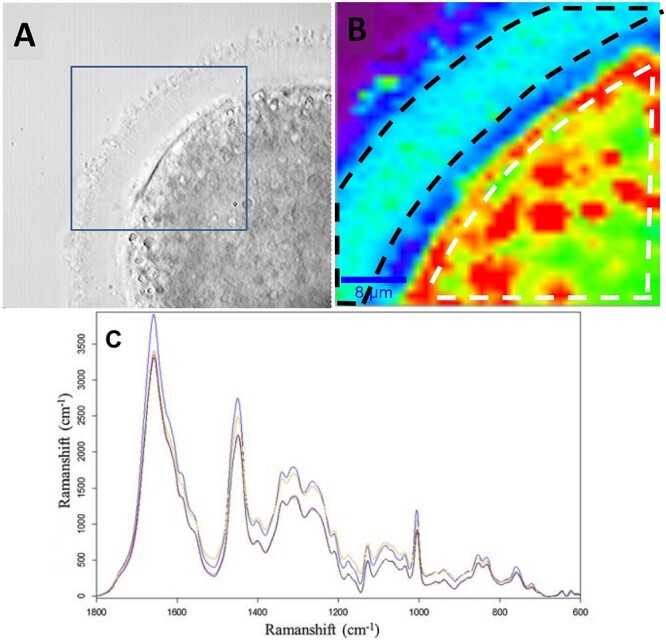
Images from a control oocyte obtained from brightfield and Raman imaging (top). (A) Brightfield image of an oocyte. The square represents the portion imaged via Raman. (B) Raman image of oocyte from (A). The white dashed line measures the area used for the average spectrum of the cytoplasmic region of the oocyte. The black dashed line represents the area used to measure spectra of the ZP [63]. (C) Average Raman spectra of young oocytes (blue), in-vitro aged (black), oxidative damaged (pink), and old (yellow). The spectra demonstrate a molecular fingerprint of macromolecular components [56].
Fluorescence lifetime imaging microscopy
Fluorescence lifetime imaging microscopy (FLIM) is a technique to measure metabolic profiles and competency of oocytes and embryos. FLIM detects molecular variations of fluorophores by measuring differences in how long they remain in an excited state before they decay and emit a photon [64]. FLIM detects electron transporters in oocytes, flavin adenine dinucleotide (FAD) and nicotinamide adenine dinucleotide dehydrogenase (NADH), which play a pivotal role in oxidative phosphorylation. The visualization of these electron transporters therefore paints the metabolic picture of an oocyte. This technique can be useful as it is non-invasive and can detect mitochondrial differences between young and old oocytes, metabolic dysfunctions, and other mutations which affect mitochondrial metabolic status [65]. Mice with knockout of Clpp, a mutation that affects metabolism and fertility, were compared to controls for metabolic oocyte dysfunction via FLIM. Old and young mouse oocytes were also compared to determine metabolic oocyte dysfunction and eight metabolic parameters were observed; fluorescence intensity (I), short lifetime (T1), long lifetime (T2), and fraction engaged with enzyme (F) for NADH and FAD [66]. The Clpp−/− mice displayed more mitochondrial abnormalities compared to the Clpp+/+. When young and old mouse oocytes were visualized via FLIM, highly significant differences in the metabolic parameters were seen (Figure 2). FLIM could also be used to detect different metabolic states in cumulus cell (CC) samples. Venturas et al. measured the metabolic states of CCs to determine if there was an association with patient age, body mass index (BMI), anti-Müllerian hormone (AMH), a marker of ovarian reserve secreted by antral follicles and oocyte maturity [67]. FLIM detected significant NADH and FAD changes that correlate with patient age and AMH, however, not BMI. FLIM also demonstrated NADH and FAD levels are associated with oocyte maturity [68]. FLIM is a novel non-invasive technique that could potentially be used in the IVF lab to detect mitochondrial defects that adversely affect oocyte metabolism and integrity [65]. A better understanding of oocyte physiology could help patients and their physicians identify the best oocytes to optimize pregnancy outcomes.
Live and fixed cell imaging of oocyte mitochondria
Oocyte mitochondria are dynamic organelles, and their structure, quantity, and distribution are important for many cellular processes. Therefore, oocyte mitochondrial bioenergetics are an important field of study to exploit for cellular diagnostics of egg quality. Mitochondria structure and distribution in mouse oocytes has been observed using live-cell images stained with fluorescent dyes, confocal imaging and FLIM. AL-Zubaidi et al. used live-cell imaging and ratiometric assessment in mouse oocytes to determine the spatial and temporal aspects of its mitochondrial membrane potential [69]. After live-cell imaging on a confocal microscope with targeted mitochondrial probes, they discovered membrane potential increases throughout oocyte maturation. They also reported an increase in mitochondrial membrane potential near the first meiotic spindle compared to the rest of the cytoplasm. Lounas et al. analyzed the mitochondrial morphology of porcine CCs by staining them with Tetramethylrhodamine, Methyl Ester, Perchlorate (TMRM), and 2D confocal microscopy. Lounas et al. discovered different mitochondrial morphologies in the CCs. Mitochondrial heterogeneity is hypothesized to regulate communication and the transport of different molecules between the cumulus and the oocyte [86]. The studies demonstrated the dynamic changes of mitochondria throughout oocyte maturation. Clark et al. studied the presence of multidrug resistance transporter-1 (MDR-1) in oocyte mitochondrial membrane and discovered its importance in maintaining oocyte homeostasis and protection from oxidative stress [70]. This same group examined fixed-cell images of mouse oocytes on a confocal microscope to determine the effects of the Mdr1a mutation on oocyte quality. The results showed more than a 3-fold increase in aneuploidies, more abnormal meiotic spindles in the mutant compared to the wildtype, and twice the amount of mitochondrial single nucleotide polymorphisms in mutant compared to wild type mice oocytes (Figure 4). The mutant mouse also displayed lower adenosine triphosphate levels compared to wild type, demonstrating the importance of MDR-1 in oocyte mitochondrial physiology [71].
Figure 4.
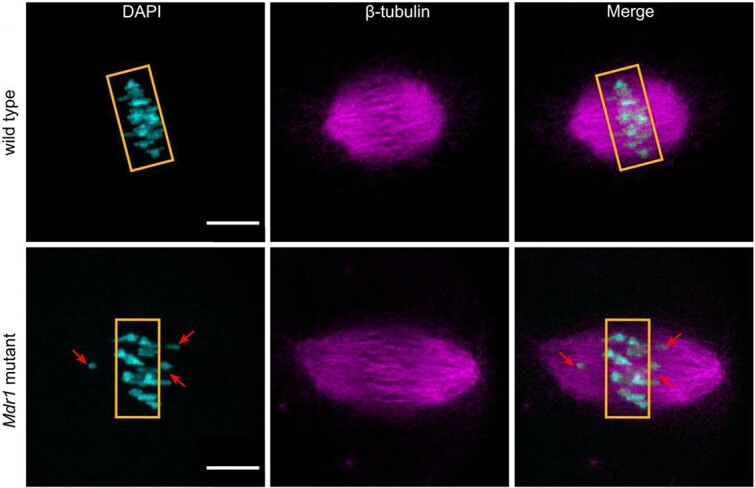
Whole mounted mutant oocytes stained with anti-tubulin antibody (magenta) and DAPI (cyan) to reveal chromosomes and spindle alignment. The bottom left image shows misaligned chromosomes in mutants (34%) compared to wt (15%) [71].
Microfluidic devices for oocyte evaluation
Simple perfusion apparatus
A simple perfusion apparatus (SPA) was developed in 2015 by Angione et al. to analyze oocytes at the single cell level while maintaining temperature, perfusion of drugs, media, labeling reagents, and high-resolution imagining [72]. The SPA uses a hydrodynamic trap array of eight chambers that allows for the flow of oocytes into individual channels for experimentation purposes (Figure 5) and prevention of cell damage. SPA allows for non-invasive longitudinal studies of oocyte imaging and all the while measuring any changes due to manipulation via sperm, fluorescent dyes, media, etc. Having the capability to analyze the distinct molecular characteristics of individual oocytes with the use of SPA, non-invasive techniques could provide insight into the developmental potential of a woman’s oocytes [73]. Understanding the physiology of an oocyte that could lead to more efficient oocyte cryopreservation outcomes is critical. Microfluidic devices have helped investigate oocyte membrane transport properties with the use of CPAs to better understand their osmotic fluctuations during cryopreservation. These investigations could be greatly beneficial to optimize donor egg bank freezing protocols or if specific donors have different membrane transport properties than others. Guo et al. utilized a microfluidic device to trap single mouse oocytes and analyze its osmotic responses to CPAs and cryopreservation [74]. Guo et al. evaluated whether the microfluidic device was safe by staining the oocytes with acridine orange/ethidium bromide after perfusion into the device to assess for toxicity. All oocytes survived. They also took photomicrographs of the change in oocyte volume when exposed to varying types and concentrations of CPAs. They observed that the permeability changes with alterations in solute concentrations and the types of CPAs. Also, the oocytes membrane permeability increases as the solute concentration increases, which helps to determine cryobiological properties of an oocyte during freezing.
Figure 5.
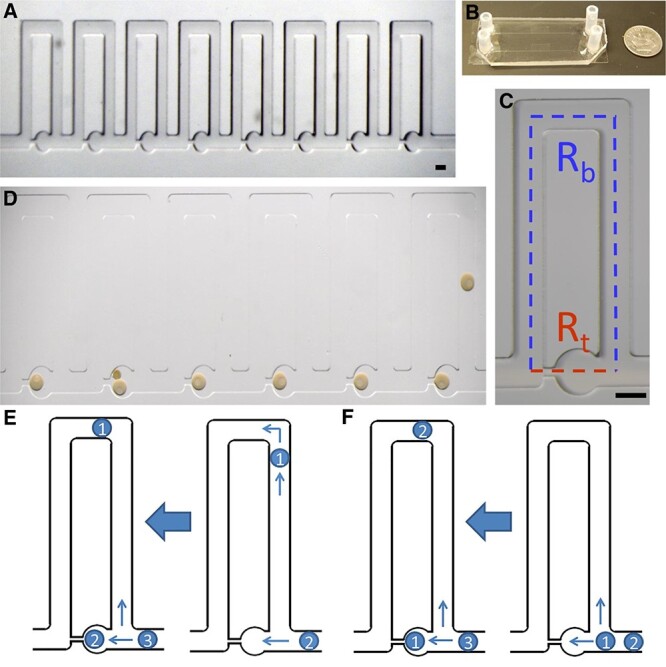
Simple perfusion apparatus. (A) 8-traps of the hydrodynamic trap array. (B) Macroscale view of the SPA. (C) Individual trap. Red represents trapping channel and blue represents the bypass channel. (D) Individual, immature sea star oocytes within the traps. Oocytes are loaded from the right to left in the device. (E) Indirect trapping: occurs when trap resistance is greater than the bypass channel resistance and oocyte goes in bypass channel. (F) Direct trapping: occurs when trap resistance is lower than bypass channel resistance and oocyte goes in trap [72].
AI oocyte assessments
AI, machine learning, deep learning, and neural networks have enormous potential for oocyte and embryo assessments because it removes the issue of subjectivity between embryologists, requires less manipulation, and is non-toxic to gametes as well as efficient for use in the lab [75].
Neural networks
Neural network is a methodology of AI that teaches computers to process data similar to the human brain. Targosz et al. used neural networks to classify oocyte maturity status. DeepLabV3Plus was used for analysis of the oocyte images to extract specific regions of them. The extracted areas of oocytes were transferred to SqueezeNet architecture for their classification. This paper demonstrated a completed neural network program that automatically classifies human oocytes into metaphase II (MII), metaphase I (MI), and prophase I (P1/GV), and degenerate categories [76]. Although oocyte classification via AI provides efficiency in the IVF laboratory, screening of nuclear and cytoplasmic maturity and abnormalities will provide a more in-depth, non-invasive observation of high-quality oocytes. Semantic image segmentation is a technique for deep learning that analyzes images by assigning parts of the image in semantic terms [77]. Targosz et al. used 71 deep neural network models to complete semantic oocyte segmentation (Figure 6). They trained their algorithm to classify oocytes based on their morphological appearance. This included cytoplasmic coloring, granularity, vacuoles, first and fragmented PB, perivitelline space, CCs, and GVs. Their training accuracy reached an impressive 79% [78]. This non-invasive advanced learning methodology could provide methods to evaluate oocyte competency and prediction tools to avoid waste of oocytes that are non-viable. AI has the potential to increase ART success rates due to the safe and effective methodology.
Figure 6.
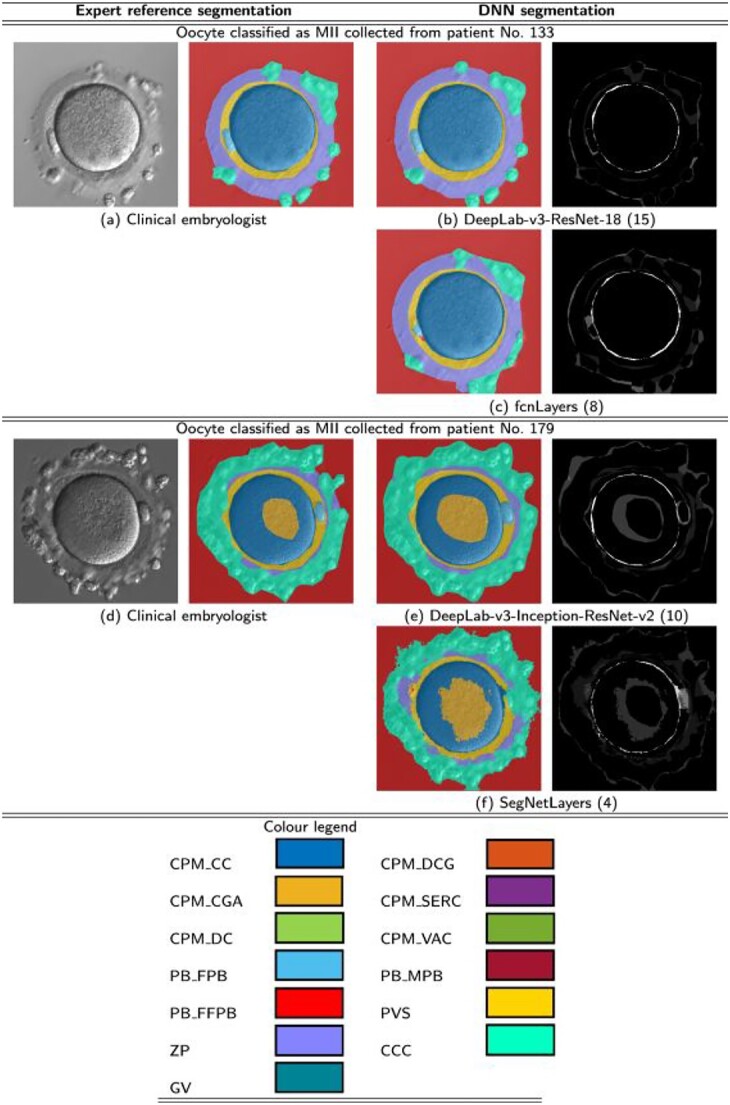
Semantic segmentation results obtained from deep neural networks. (A and D) MII oocytes segmented by an embryologist. (B, C, E, F) MII oocytes segmented from deep neural networks. These graphical visualizations display minimal differences in segmentation from an embryologist and a network [76].
Machine learning algorithms
Numerous companies in fertility are now producing AI concepts to revolutionize non-invasive, machine learning tools to assess and predict oocyte and embryo viability [79]. Other AI companies have developed machine learning assessments of oocyte quality [80, 81]. One system was created through the production of millions of images of embryos and oocytes, and an algorithm was built by clinically testing it in greater than 50 000 embryos. It utilizes computer vision technology to analyze embryos and oocytes in 3D and performs 10% better at predicting implantation compared to a clinical embryologist [82]. A different AI software was created using a neural network trained on 16 373 images of oocytes and their outcomes [83]. It uses a 2D image analysis of mature oocytes to create a predictive score of oocyte quality to usable blastocyst development [83]. Utilizing this technology, 16 261 oocytes were scored according to the algorithm and a correlation to blastocyst quality was made. The results showed a positive correlation with blastocyst quality and oocyte scoring [84]. It is important to acknowledge there are many confounders that may affect AI to correctly predict blastulation and implantation outcomes. These include sperm quality, oocyte morphological abnormalities, ICSI procedure and age. It is important to validate an AI program based upon the many factors involved in IVF. A study by Lim et al. assessed how the ICSI procedure, sperm quality, and oocyte dysmorphisms affect the prediction of blastulation from oocyte images through this technology [85]. They found that post-ICSI images had higher predictive algorithm scores compared to pre-ICSI images (p ≤ 0.001) meaning oocyte images through AI have higher prediction of blastulation when post-ICSI images are used. They also found the efficacy of prediction was not affected by sperm quality. Although AI is promising and innovative, even more advancements need to be made to detect further details of oocyte characteristics such as mitochondria quantity and density.
Discussion
The goal of reproductive medicine is to increase the pregnancy rates both in autologous and donor oocyte cycles, but our greatest hurdle is inadequate assessments of the oocyte. Many invasive oocyte assessment methods have been introduced and used for research purposes in non-human and human studies, however, the development of non-invasive, real-time point-of-care oocyte assessments via microscopy has not progressed to a place where oocyte imaging improves clinical outcomes. There are subjective and superficial light microscopy techniques and PolScope visualization of meiotic spindles but both with contradictory outcomes on their ability to improve live birth rates. There are emerging data on high-resolution microscopy imaging such as confocal, Raman spectroscopy, and FLIM; however, real-time assessments with these types of microscopies are not practical in a clinical setting during an oocyte retrieval. Advanced microscopy modalities are prohibitive due to the high costs of the microscopes, the large footprint of the equipment, extended time of oocyte imaging prior to insemination, and exposure to toxic labeling reagents. The future of IVF should rely on the ability to have low cost, compact instruments that are gamete, and embryo safe with high-resolution imaging methods. Examples of technologies that could potentially improve the oocyte imaging techniques include enhanced microfluidic devices, machine learning, and computer vision assessments to identify the structural variations including cytoplasmic granularities and uniformity, ZP glycoproteins, and PB symmetry. Improved resolution and safe labeling protocols will help to evaluate the quantity and patterning of critical organelles such as oocyte mitochondria and endoplasmic reticulum. However, the challenge of poor imaging will continue to plague reproductive medicine until point of care, non-invasive imaging is optimized.
Contributor Information
Caitlin F Boylan, University of North Carolina, Chapel Hill, NC, USA; Eastern Virginia Medical School, Norfolk, VA, USA.
Keshia M Sambo, Institute for Biochemistry and Biology, University of Potsdam, Potsdam, Germany.
Genevieve Neal-Perry, University of North Carolina, Chapel Hill, NC, USA.
Lynae M Brayboy, Department of Neuropediatrics Charité-Universitätsmedizin Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany; Klinik für Pädiatrie m. S. Neurologie, Charité Campus Virchow Klinikum, Berlin, Germany; Department of Reproductive Biology, Bedford Research Foundation, Bedford, MA, USA.
References
- 1. World Health Organization . Infertility Prevalence Estimates, 1990–2021. Geneva: World Health Organization; 2023. [Google Scholar]
- 2. Society for Assisted Reproductive Technology . Preliminary National Summary Report for 2021. Birmingham, AL. Society for Assisted Reproductive Technology (SART); 2023. [Google Scholar]
- 3. American Society for Reproductive Medicine (ASRM) . In vitro fertilization treatment. Vitro Fertil Treat Reprod., 2015, Washington, D.C. ASRM 2015. [Google Scholar]
- 4. Katz P, Showstack J, Smith JF, Nachtigall RD, Millstein SG, Wing H, Eisenberg ML, Pasch LA, Croughan MS, Adler N. Costs of infertility treatment: results from an 18-month prospective cohort study. Fertil Steril 2011; 95:915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. RESOLVE . The National Infertility Association. Insurance coverage by state: RESOLVE: the National Infertility Association. McLean, VA. RESOLVE 2023. [Google Scholar]
- 6.Society For Assisted Reproductive Technology. Preliminary National Summary Report for 2021. Birgmingham, AL. Society for Assisted Reproductive Technology (SART); 2023. [Google Scholar]
- 7. Capalbo A, Hoffmann ER, Cimadomo D, Maria Ubaldi F, Rienzi L. Human female meiosis revised: new insights into the mechanisms of chromosome segregation and aneuploidies from advanced genomics and time-lapse imaging. Hum Reprod Update 2017; 23:706–722. [DOI] [PubMed] [Google Scholar]
- 8. Cimadomo D, Fabozzi G, Vaiarelli A, Ubaldi N, Ubaldi FM, Rienzi L. Impact of maternal age on oocyte and embryo competence. Front Endocrinol 2018; 9:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sermondade N, Huberlant S, Bourhis-Lefebvre V, Arbo E, Gallot V, Colombani M, Fréour T. Female obesity is negatively associated with live birth rate following IVF: a systematic review and meta-analysis. Hum Reprod Update 2019; 25:439–451. [DOI] [PubMed] [Google Scholar]
- 10. Snider AP, Wood JR. Obesity induces ovarian inflammation and reduces oocyte quality. Reproduction 2019; 158:R79–R90. [DOI] [PubMed] [Google Scholar]
- 11. Goswami D, Conway GS. Premature ovarian failure. Hum Reprod Update 2005; 11:391–410. [DOI] [PubMed] [Google Scholar]
- 12. Setti AS, Halpern G, Braga DPDAF, Iaconelli A, Borges E. Maternal lifestyle and nutritional habits are associated with oocyte quality and ICSI clinical outcomes. Reprod Biomed Online 2022; 44:370–379. [DOI] [PubMed] [Google Scholar]
- 13. Jankowska K. Premature ovarian failure. Menopausal Rev 2017; 2:51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O’Neill CL, Chow S, Rosenwaks Z, Palermo GD. Development of ICSI. Reproduction 2018; 156:F51–F58. [DOI] [PubMed] [Google Scholar]
- 15. Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, Ubaldi FM, Vanderpoel S, Racowsky C. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod 2017; 2:139–155, dmw038v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ebner T. Selection based on morphological assessment of oocytes and embryos at different stages of preimplantation development: a review. Hum Reprod Update 2003; 9:251–262. [DOI] [PubMed] [Google Scholar]
- 17. Sciorio R, Miranian D, Smith GD. Non-invasive oocyte quality assessment. Biol Reprod 2022; 106:274–290. [DOI] [PubMed] [Google Scholar]
- 18. Kliebisch T, Bielfeld A, Krüssel J, Baston-Büst D. The German middleway as precursor for single embryo transfer. A retrospective data-analysis of the Düsseldorf University Hospitalʼs Interdisciplinary Fertility Centre – UniKiD. Geburtshilfe Frauenheilkd 2016; 76:690–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Turone F. Italy to pass new law on assisted reproduction. BMJ 2004; 328:9-a-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coetsier T, Dhont M. Avoiding multiple pregnancies in in-vitro fertilization: who’s afraid of single embryo transfer? Hum Reprod 1998; 13:2663–2664. [DOI] [PubMed] [Google Scholar]
- 21. Practice Committee of the American Society for Reproductive Medicine . Practice Committee for the Society for Assisted Reproductive Technologies. Guidance on the limits to the number of embryos to transfer: a committee opinion. Fertil Steril 2021; 116:651–654. [DOI] [PubMed] [Google Scholar]
- 22. Balaban B, Barut T, Urman B. Assessment of oocyte quality. In: Nagy ZP, Varghese AC, Agarwal A (eds.), Practical Manual of In Vitro Fertilization. New York, NY: Springer New York; 2012: 105–119. [Google Scholar]
- 23. Eppig J. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod Fertil Dev 1996; 8:485. [DOI] [PubMed] [Google Scholar]
- 24. He M, Zhang T, Yang Y, Wang C. Mechanisms of oocyte maturation and related epigenetic regulation. Front Cell Dev Biol 2021; 9:654028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rienzi L, Balaban B, Ebner T, Mandelbaum J. The oocyte. Hum Reprod 2012; 27:i2–i21. [DOI] [PubMed] [Google Scholar]
- 26. De Santis L, Cino I, Rabellotti E, Calzi F, Persico P, Borini A, Coticchio G. Polar body morphology and spindle imaging as predictors of oocyte quality. Reprod Biomed Online 2005; 11:36–42. [DOI] [PubMed] [Google Scholar]
- 27. Ebner T, Moser M, Sommergruber M, Yaman C, Pfleger U, Tews G. First polar body morphology and blastocyst formation rate in ICSI patients. Hum Reprod 2002; 17:2415–2418. [DOI] [PubMed] [Google Scholar]
- 28. Wei Y, Zhang T, Wang Y-P, Schatten H, Sun Q-Y. Polar bodies in assisted reproductive technology: current progress and future Perspectives1. Biol Reprod 2015; 92:19. [DOI] [PubMed] [Google Scholar]
- 29. Halvaei I, Khalili MA, Soleimani M, Razi MH. Evaluating the role of first polar body morphology on rates of fertilization and embryo development in ICSI cycles. Int J Fertil Steril 2011; 5:110–115. [PMC free article] [PubMed] [Google Scholar]
- 30. Younis JS, Radin O, Izhaki I, Ben-Ami M. Does first polar body morphology predict oocyte performance during ICSI treatment? J Assist Reprod Genet 2009; 26:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bartolacci A, Intra G, Coticchio G, dell’Aquila M, Patria G, Borini A. Does morphological assessment predict oocyte developmental competence? A systematic review and proposed score. J Assist Reprod Genet 2022; 39:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yu EJ, Ahn H, Lee JM, Jee BC, Kim SH. Fertilization and embryo quality of mature oocytes with specific morphological abnormalities. Clin Exp Reprod Med 2015; 42:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sauerbrun-Cutler M-T, Vega M, Breborowicz A, Gonzales E, Stein D, Lederman M, Keltz M. Oocyte zona pellucida dysmorphology is associated with diminished in-vitro fertilization success. J Ovarian Res 2015; 8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ferrarini Zanetti B, De Almeida P, Ferreira Braga D, Souza Setti A, De Cássia Sávio Figueira R, LaconelliA Jr, BorgesE Jr. Is perivitelline space morphology of the oocyte associated with pregnancy outcome in intracytoplasmic sperm injection cycles? Eur J Obstet Gynecol Reprod Biol 2018; 231:225–229. [DOI] [PubMed] [Google Scholar]
- 35. Nikiforov D, Grøndahl ML, Hreinsson J, Andersen CY. Human oocyte morphology and outcomes of infertility treatment: a systematic review. Reprod Sci 2022; 29:2768–2785. [DOI] [PubMed] [Google Scholar]
- 36. Ten J, Mendiola J, Vioque J, De Juan J, Bernabeu R. Donor oocyte dysmorphisms and their influence on fertilization and embryo quality. Reprod Biomed Online 2007; 14:40–48. [DOI] [PubMed] [Google Scholar]
- 37. Esfandiari N, Burjaq H, Gotlieb L, Casper RF. Brown oocytes: implications for assisted reproductive technology. Fertil Steril 2006; 86:1522–1525. [DOI] [PubMed] [Google Scholar]
- 38. Tan TCY, Dunning KR. Non-invasive assessment of oocyte developmental competence. Reprod Fertil Dev 2022; 35:39–50. [DOI] [PubMed] [Google Scholar]
- 39. Genicot G, Leroy JLMR, Soom AV, Donnay I. The use of a fluorescent dye, Nile red, to evaluate the lipid content of single mammalian oocytes. Theriogenology 2005; 63:1181–1194. [DOI] [PubMed] [Google Scholar]
- 40. Vanroose G, Nauwynck H, Soom AV, Ysebaert M-T, Charlier G, Oostveldt PV, De Kruif A. Structural aspects of the zona pellucida of in vitro-produced bovine embryos: a scanning electron and confocal laser scanning microscopic study. Biol Reprod 2000; 62:463–469. [DOI] [PubMed] [Google Scholar]
- 41. Wang W-H, Meng L, Hackett RJ, Keefe DL. Developmental ability of human oocytes with or without birefringent spindles imaged by PolScope before insemination. Hum Reprod 2001; 16:1464–1468. [DOI] [PubMed] [Google Scholar]
- 42. Tomari H, Honjo K, Kunitake K, Aramaki N, Kuhara S, Hidaka N, Nishimura K, Nagata Y, Horiuchi T. Meiotic spindle size is a strong indicator of human oocyte quality. Reprod Med Biol 2018; 17:268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Caamaño J, Muñoz M, Diez C, Gómez E. Polarized light microscopy in mammalian oocytes. Reprod Domest Anim 2010; 45:49–56. [DOI] [PubMed] [Google Scholar]
- 44. Polarized RD, Microscopy L. Application of Molecular Methods and Raman Microscopy/Spectroscopy in Agricultural Sciences and Food Technology. London: Ubiquity Press; 2019: 193–198. [Google Scholar]
- 45. Albertini DF. The mammalian oocyte. In: Plant, T.M., Zeleznik, A.J. (eds) Knobil and Neill’s Physiology of Reproduction, 4th ed. Cambridge, MA: Academic Press; 2015: 59–97. [Google Scholar]
- 46. Charalambous C, Webster A, Schuh M. Aneuploidy in mammalian oocytes and the impact of maternal ageing. Nat Rev Mol Cell Biol 2023; 24:27–44. [DOI] [PubMed] [Google Scholar]
- 47. Thomas C, Cavazza T, Schuh M. Aneuploidy in human eggs: contributions of the meiotic spindle. Biochem Soc Trans 2021; 49:107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Blyth U, Craciunas L, Hudson G, Choudhary M. Maternal germline factors associated with aneuploid pregnancy loss: a systematic review. Hum Reprod Update 2021; 27:866–884. [DOI] [PubMed] [Google Scholar]
- 49. Eichenlaub-Ritter U, Vogt E, Yin H, Gosden R. Spindles, mitochondria and redox potential in ageing oocytes. Reprod Biomed Online 2004; 8:45–58. [DOI] [PubMed] [Google Scholar]
- 50. Zaninovic N, Gosden R, Veeck GL. Visualization of meiotic spindles and chromosomes in living human oocytes using conventional Hoffman modulation microscopy. Fertil Steril 2005; 84:S368–S369. [Google Scholar]
- 51. Keefe D, Liu L, Wang W, Silva C. Imaging meiotic spindles by polarization light microscopy: principles and applications to IVF. Reprod Biomed Online 2003; 7:24–29. [DOI] [PubMed] [Google Scholar]
- 52. Oldenbourg R. Polarized light microscopy of spindles. Methods Cell Biol 1998; 61:Elsevier:175–208. [DOI] [PubMed] [Google Scholar]
- 53. Chamayou S, Ragolia C, Alecci C, Storaci G, Maglia E, Russo E, Guglielmino A. Meiotic spindle presence and oocyte morphology do not predict clinical ICSI outcomes: a study of 967 transferred embryos. Reprod Biomed Online 2006; 13:661–667. [DOI] [PubMed] [Google Scholar]
- 54. Wood BR, Chernenko T, Matthäus C, Diem M, Chong C, Bernhard U, Jene C, Brandli AA, McNaughton D, Tobin MJ, Trounson A, Lacham-Kaplan O. Shedding new light on the molecular architecture of oocytes using a combination of synchrotron Fourier transform-infrared and Raman spectroscopic mapping. Anal Chem 2008; 80:9065–9072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gleicher N, Kushnir VA, Albertini DF, Barad DH. Improvements in IVF in women of advanced age. J Endocrinol 2016; 230:F1–F6. [DOI] [PubMed] [Google Scholar]
- 56. Bogliolo L, Murrone O, Di Emidio G, Piccinini M, Ariu F, Ledda S, Tatone C. Raman spectroscopy-based approach to detect aging-related oxidative damage in the mouse oocyte. J Assist Reprod Genet 2013; 30:877–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Clark NA, Swain JE. Oocyte cryopreservation: searching for novel improvement strategies. J Assist Reprod Genet 2013; 30:865–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Best BP. Cryoprotectant toxicity: facts, issues, and questions. Rejuvenation Res 2015; 18:422–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chang C-C, Shapiro DB, Nagy ZP. The effects of vitrification on oocyte quality. Biol Reprod 2022; 106:316–327. [DOI] [PubMed] [Google Scholar]
- 60. Mukaida T, Oka C. Vitrification of oocytes, embryos and blastocysts. Best Pract Res Clin Obstet Gynaecol 2012; 26:789–803. [DOI] [PubMed] [Google Scholar]
- 61. Katayama KP, Stehlik J, Kuwayama M, Kato O, Stehlik E. High survival rate of vitrified human oocytes results in clinical pregnancy. Fertil Steril 2003; 80:223–224. [DOI] [PubMed] [Google Scholar]
- 62. Larman MG, Sheehan CB, Gardner DK. Calcium-free vitrification reduces cryoprotectant-induced zona pellucida hardening and increases fertilization rates in mouse oocytes. Reproduction 2006; 131:53–61. [DOI] [PubMed] [Google Scholar]
- 63. Rusciano G, De Canditiis C, Zito G, Rubessa M, Roca MS, Carotenuto R, Sasso A, Gasparrini B. Raman-microscopy investigation of vitrification-induced structural damages in mature bovine oocytes. PloS One 2017; 12:e0177677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Datta R, Heaster TM, Sharick JT, Gillette AA, Skala MC. Fluorescence lifetime imaging microscopy: fundamentals and advances in instrumentation, analysis, and applications. J Biomed Opt 2020; 25:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sanchez T, Zhang M, Needleman D, Seli E. Metabolic imaging via fluorescence lifetime imaging microscopy for egg and embryo assessment. Fertil Steril 2019; 111:212–218. [DOI] [PubMed] [Google Scholar]
- 66. Sanchez T, Wang T, Pedro MV, Zhang M, Esencan E, Sakkas D, Needleman D, Seli E. Metabolic imaging with the use of fluorescence lifetime imaging microscopy (FLIM) accurately detects mitochondrial dysfunction in mouse oocytes. Fertil Steril 2018; 110:1387–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Venturas M, Yang X, Kumar K, Wells D, Racowsky C, Needleman DJ. Metabolic imaging of human cumulus cells reveals associations among metabolic profiles of cumulus cells, patient clinical factors, and oocyte maturity. Fertil Steril 2021; 116:1651–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Venturas M, Yang X, Kumar K, Wells D, Racowsky C, Needleman DJ. Metabolic imaging of human cumulus cells reveals associations among metabolic profiles of cumulus cells, patient clinical factors, and oocyte maturity. Fertil Steril 2021; 116:1651–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. AL-Zubaidi U, Liu J, Cinar O, Robker RL, Adhikari D, Carroll J. The spatio-temporal dynamics of mitochondrial membrane potential during oocyte maturation. Mol Hum Reprod 2019; 25:695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Clark H, Knapik LO, Zhang Z, Wu X, Naik MT, Oulhen N, Wessel GM, Brayboy LM. Dysfunctional MDR-1 disrupts mitochondrial homeostasis in the oocyte and ovary. Sci Rep 2019; 9:9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nabi D, Bosi D, Gupta N, Thaker N, Fissore R, Brayboy LM. Multidrug resistance transporter-1 dysfunction perturbs meiosis and Ca2+ homeostasis in oocytes. Reproduction 2023; 165:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Angione SL, Oulhen N, Brayboy LM, Tripathi A, Wessel GM. Simple perfusion apparatus for manipulation, tracking, and study of oocytes and embryos. Fertil Steril 2015; 103:281–290.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Brayboy LM, Wessel GM. The double-edged sword of the mammalian oocyte – advantages, drawbacks and approaches for basic and clinical analysis at the single cell level. Mol Hum Reprod 2016; 22:200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Guo X, Chen Z, Memon K, Chen X, Zhao G. An integrated microfluidic device for single cell trapping and osmotic behavior investigation of mouse oocytes. Cryobiology 2020; 92:267–271. [DOI] [PubMed] [Google Scholar]
- 75. Swain J, VerMilyea MT, Meseguer M, Ezcurra D, Fertility AI Forum Group, Ezcurra D, Letterie G, Sánchez P, Trew G, Swain J, Meseguer M, Nayot D, et al. AI in the treatment of fertility: key considerations. J Assist Reprod Genet 2020; 37:2817–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Targosz A, Myszor D, Mrugacz G. Human oocytes image classification method based on deep neural networks. Biomed Eng Online 2023; 22:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Liu X, Deng Z, Yang Y. Recent progress in semantic image segmentation. Artif Intell Rev 2019; 52:1089–1106. [Google Scholar]
- 78. Targosz A, Przystałka P, Wiaderkiewicz R, Mrugacz G. Semantic segmentation of human oocyte images using deep neural networks. Biomed Eng Online 2021; 20:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chow DJX, Wijesinghe P, Dholakia K, Dunning KR. Does artificial intelligence have a role in the IVF clinic? Reprod Fertil 2021; 2:C29–C34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fairtility . Why Chloe. Tel Aviv-Yafo, Israel. Fairtility; 2023. [Google Scholar]
- 81. Yelke HK, Ozkara G, Yuksel B, Kumtepe Colakoglu Y, Aygun M, Brualla A, Erlich I, Hickman C, Selimoglu S, Okten B, Kahraman S. O-007 Simplifying the complexity of time-lapse decisions with AI: CHLOE (Fairtility) can automatically annotate morphokinetics and predict blastulation (at 30hpi), pregnancy and ongoing clinical pregnancy. Hum Reprod 2022; 37:deac104.007. [Google Scholar]
- 82. Erlich I, Ben-Meir A, Har-Vardi I, Grifo J, Wang F, Mccaffrey C, McCulloh D, Or Y, Wolf L. Pseudo contrastive labeling for predicting IVF embryo developmental potential. Sci Rep 2022; 12:2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fjeldstad J, Mercuri N, Meriano J, Krivoi A, Campbell A, Smith R, Berrisford K, Drezet C, Casper R, Nayot D. O-204 Non-invasive AI image analysis unlocks the secrets of oocyte quality and reproductive potential by assigning ‘Magenta’ scores from 2-dimensional (2-D) microscope images. Hum Reprod 2022; 37:deac104.119. [Google Scholar]
- 84. Mercuri N, Fjeldstad J, Krivoi A, Meriano J, Nayot D. A non-invasive, 2-dimensional (2D) image analysis artificial intelligence (AI) tool scores mature oocytes and correlates with the quality of subsequent blastocyst development. Fertil Steril 2022; 118:e78–e79. [Google Scholar]
- 85. Lim AYX, Zepeda A, Hickman C, Kantor B. P-310 Bringing transparency to oocyte assessment: the importance of including confounders when building artificial intelligence (AI) based support tools to quantify oocyte viability. Hum Reprod 2023; 38:dead093.668. [Google Scholar]
- 86. Lounas A, Lebrun A, Laflamme I, Vernoux N, Savage J, Tremblay ME, Germain M, Richard FJ. A 3D analysis revealed complex mitochondria morphologies in porcine cumulus cells. Sci Rep 2022; 12:15403. [DOI] [PMC free article] [PubMed] [Google Scholar]