Abstract
Objectives
Patients hospitalized for COVID‐19 frequently develop hypoxemia and acute respiratory distress syndrome (ARDS) after admission. In non‐COVID‐19 ARDS studies, admission to hospital wards with subsequent transfer to intensive care unit (ICU) is associated with worse outcomes. We hypothesized that initial admission to the ward may affect outcomes in patient with COVID‐19 ARDS.
Methods
This was a retrospective study of consecutive adults admitted for COVID‐19 ARDS between March 2020 and March 2021 at Stanford Health Care. Mortality scores at hospital admission (Coronavirus Clinical Characterization Consortium Mortality Score [4C score]) and ICU admission (Simplified Acute Physiology Score III [SAPS‐III]) were calculated, as well as ROX index for patients on high flow nasal oxygen. Patients were classified by emergency department (ED) disposition (ward‐first vs. ICU‐direct), and 28‐ and 60‐day mortality and highest level of respiratory support within 1 day of ICU admission were compared. A second cohort (April 2021‒July 2022, n = 129) was phenotyped to validate mortality outcome.
Results
A total of 157 patients were included, 48% of whom were first admitted to the ward (n = 75). Ward‐first patients had more comorbidities, including lung disease. Ward‐first patients had lower 4C and similar SAPS‐III score, yet increased mortality at 28 days (32% vs. 17%, hazard ratio [HR] 2.0, 95% confidence interval [95% CI] 1.0‒3.7, p = 0.039) and 60 days (39% vs. 23%, HR 1.83, 95% CI 1.04‒3.22, p = 0.037) compared to ICU‐direct patients. More ward‐first patients escalated to mechanical ventilation on day 1 of ICU admission (36% vs. 14%, p = 0.002) despite similar ROX index. Ward‐first patients who upgraded to ICU within 48 h of ED presentation had the highest mortality. Mortality findings were replicated in a sensitivity analysis.
Conclusion
Despite similar baseline risk scores, ward‐first patients with COVID‐19 ARDS had increased mortality and escalation to mechanical ventilation compared to ICU‐direct patients. Ward‐first patients requiring ICU upgrade within 48 h were at highest risk, highlighting a need for improved identification of this group at ED admission.
Keywords: acute respiratory distress syndrome, COVID‐19, disposition, emergency department
1. INTRODUCTION
1.1. Background
The COVID‐19 pandemic strained hospital capacity to unprecedented levels and highlighted the urgent need for early identification of patients at highest risk for poor outcomes. Multiple tools exist to identify patients at risk for clinical deterioration, such as the ROX index to predict failure of high flow nasal oxygen (HFNO) and progression to invasive mechanical ventilation, 1 , 2 the Coronavirus Clinical Characterization Consortium Mortality Score (4C score) 3 , 4 which is calculated at time of hospital admission to predict intensive care unit (ICU) admission and in‐hospital mortality, and the Simplified Acute Physiology Score III (SAPS‐III score, calculated at time of ICU admission) 5 , 6 to predict ICU mortality. While these tools may inform clinical care decisions after a patient is admitted, triage from the emergency department (ED) to the initial level of care is in practice often dictated by the patient's immediate respiratory support needs.
1.2. Importance
Patients with COVID‐19 infection present to the ED with variable disease severity. During the initial phase of the pandemic, approximately 10% of patients met acute respiratory distress syndrome (ARDS) criteria at time of ED presentation, while another ∼10% of patients developed ARDS after admission, 7 , 8 necessitating unplanned transfer to ICU. In non‐ARDS studies, patients transferred from hospital wards to ICU have increased mortality compared to patients directly admitted to ICU from the ED. 9 , 10 , 11 , 12 , 13 It is unknown whether outcomes among patients with COVID‐19 ARDS are associated with admission directly to ICU or initial admission to hospital wards with clinical deterioration necessitating subsequent ICU transfer.
1.3. Goals of this investigation
We hypothesized that among patients with COVID‐19 who meet ARDS criteria at some point during their hospital admission, admission to the ward (as opposed to directly to the ICU) is associated with worse patient outcomes.
2. METHODS
2.1. Design, setting, and selection of subjects
This retrospective cohort study was approved by the Stanford Institutional Review Board with a waiver of consent (IRB #56374). All patients more than 18 years of age who required ICU admission at either an academic or affiliated community hospital and met criteria for COVID‐19 ARDS at any time during hospitalization were included. COVID‐19 ARDS was defined by a positive SARS‐CoV‐2 polymerase chain reaction assay and Berlin criteria for ARDS. 14 Patients with a positive SARS‐CoV‐2 test who required >20 L/min HFNO and met all Berlin criteria except for the positive end‐expiratory criteria were also considered to have COVID‐19 ARDS, in anticipation of a revised consensus definition of ARDS. 15 For patients who did not have arterial blood gas measurements, a peripheral oxygen saturation to fraction of inhaled oxygen (SpO2/FiO2) ratio of <315 mmHg with an SpO2 ≤ 97% was used. 16
The primary cohort included patients admitted between March 2020 and March 2021 (n = 382). Patients were excluded if positive SARS‐CoV‐2 test was incidental (patient was asymptomatic, had a positive test >30 days before admission or previous hospitalization for COVID‐19 >30 days from index ICU admission), if they were transferred from an outside hospital with missing ED data, if they were admitted for comfort/hospice care, or if no ICU admission occurred (patient either died or transitioned to comfort/hospice care before ICU transfer, or admission order placed in error). Patients were excluded if they did not receive glucocorticoids, which reduce mortality and quickly became standard of care (Figure 1). All demographic, clinical variable, and outcome data were collected and analyzed on the primary cohort. A secondary cohort used for sensitivity analysis is described further below.
FIGURE 1.
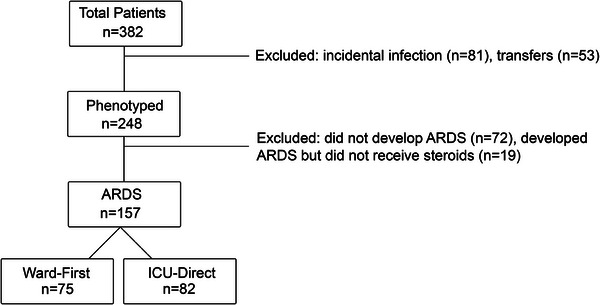
Study design and patient selection. All patients (n = 382) with a positive SARS‐CoV‐2 test who were admitted to medical intensive care unit (ICU) between March 2020 and March 2021 were screened. Patients (n = 134) with incidental infections or transferred from outside hospitals without emergency department (ED) admission data were excluded. Patients who did not meet criteria for acute respiratory distress (ARDS) (n = 72) or who did not receive steroids (n = 19) were excluded. Patients were classified either as ward‐first (n = 75) or ICU‐direct (n = 82) based on initial admission location.
2.2. Data collection
Patients in the primary cohort were clinically phenotyped by an MD reviewer in the REDCap database by recording baseline demographics and comorbidities, as well as clinical and laboratory values at time of ED triage, during ED stay, at time of hospital admission, and at time of ICU admission. The Stanford REDCap platform (http://redcap.stanford.edu) is maintained by the Stanford Medicine Research IT and subsidized by (1) the Stanford School of Medicine Research Office, and (2) through grant UL1TR001085.
2.3. Outcome measures
The primary outcomes were 28‐ and 60‐day mortality. The secondary outcomes were hospital and ICU length of stay, progression to invasive mechanical ventilation within 1 day of ICU admission, and days free of respiratory (both HFNO and ventilatory), cardiovascular (inotropes or vasopressors), or renal (continuous renal replacement therapy) support.
2.4. Mortality prediction scores
The 4C mortality prediction score was calculated using the worst recorded values in the ED before an admission order was placed. The 4C score includes peripheral oxygen saturation on room air; in patients who required home oxygen support before hospitalization, oxygen saturation on room air was replaced with oxygen saturation on home level of oxygen support. SAPS‐III mortality prediction scores were calculated at time of ICU admission using values within the first hour of arrival in the ICU for physiologic data and pH, and same day values for laboratory data. If data needed to calculate a component of the score were unavailable, that component was set to the least severe category. The ROX index was calculated at time of ICU admission.
The Bottom Line
Prior studies indicated that admission from the emergency department (ED) to the hospital ward with subsequent transfer to the intensive care unit (ICU) within 24 hours is associated with worse outcome when compared with direct admission to the ICU from the ED. It was previously unknown whether this concept holds true for patients with COVID‐19. In this retrospective cohort study of 157 patients, admission to the hospital ward first was associated with 15% higher mortality despite similar illness severity. Future studies should focus on identifying ED patients with occult critical illness at high risk of deterioration who may warrant initial ICU admission.
2.5. Data analysis
The data were graphed and analyzed in GraphPad Prism9. Differences between groups for 4C score, SAPS‐III score, ROX index, organ support‐free days, hospital‐free and ICU‐free days, laboratory values, patient age, and symptom duration were analyzed with Mann‒Whitney U‐test. Comorbid conditions, sex, race and ethnicity, chronic medication use, and proportion of patients who required invasive mechanical ventilation were analyzed with chi‐squared test. Comorbid conditions that were significantly different between ward‐first and ICU‐direct patients in the univariate analysis and their association with survival were analyzed with a Cox proportional hazard model. Co‐variants used for the proportional hazard model included disposition, history of connective tissue disease, malignancy, pulmonary disease, and chronic use of inhaled steroids. Kaplan‒Meier survival curves for 28‐ and 60‐day mortality were analyzed with Mantel‒Cox log rank test. Changes in respiratory support modality at time of ICU admission and on days 1 and 2 of ICU stay were visualized with a Sankey diagram.
2.6. Sensitivity analysis for primary outcomes
Given viral evolution over the course of the pandemic, 17 a sensitivity analysis was performed to determine whether associations between ED disposition and 28‐ and 60‐day mortality persisted in an expanded cohort. Patients admitted between April 2021 and July 2022 (n = 442) who met the same inclusion and exclusion criteria described above were classified as ward‐first or ICU‐direct (Figure S1). This second cohort was combined with the primary cohort and primary outcomes were analyzed using statistical methods described above.
3. RESULTS
3.1. Patient demographics and comorbidities
A total of 157 patients developed COVID‐19 ARDS between March 2020 and March 2021 (Figure 1). Patients were classified either as ward‐first (n = 75) or ICU‐direct (n = 82) based on initial admission location. All combinations of therapeutics received by patients are detailed in Table S1. Time from hospital admission to initiation of steroids was similar in ward‐first (median day 0, Q1‒Q3 range −1 to 1 day) and ICU‐direct (median day 0, Q1‒Q3 range −4 to 0 day, p = 0.132) patients.
Patient age, sex, race, and ethnicity were not different between ward‐first and ICU‐direct patients (Table 1). ICU‐direct and ward‐first patients had similar rates of cardiovascular, cerebrovascular, chronic liver, and renal disease, as well as diabetes. Use of systemic immunosuppressant medications and receipt of organ transplant was also not different between ward‐first and ICU‐direct patients. In contrast, ward‐first patients were more likely to have malignancy, connective tissue disease, and pulmonary comorbidities and to use chronic inhaled steroids compared to ICU‐direct patients (Table 1).
TABLE 1.
Patient demographics and comorbidities.
| Admission location | Ward‐first, n = 75 | ICU‐direct, n = 82 | p‐Value |
|---|---|---|---|
| Median age (Q1, Q3) (years) | 64 (50, 74) | 64 (50, 74) | 0.830 |
| Female | 29 (39) | 28 (34) | 0.556 |
| Pregnant, no.; mean gestation age (weeks) | 1 (33) | 2 (14.5) | |
| Race/ethnicity | 0.865 | ||
| White | 15 (20) | 15 (18) | |
| Black/African American | 3 (4) | 2 (2) | |
| Asian | 12 (16) | 13 (16) | |
| Hispanic/Latino/Spanish | 38 (51) | 47 (57) | |
| Hawaiian/Pacific Islander | 3 (4) | 1 (1) | |
| Other/combination/not specified | 4 (5) | 4 (5) | |
| Comorbidities | |||
| Cardiovascular disease a | 52 (69) | 56 (68) | 0.888 |
| Pulmonary disease b | 19 (25) | 9 (11) | 0.019 |
| Oxygen use at baseline, no. (mean L/min) c | 2 (2) | 3 (3) | 0.724 |
| Smoking status | 0.463 | ||
| Active | 2 (3) | 4 (5) | |
| Former | 34 (45) | 30 (37) | |
| Never | 39 (52) | 48 (59) | |
| Cerebrovascular disease d | 7 (9) | 12 (15) | 0.309 |
| Liver disease | 6 (8) | 3 (4) | 0.243 |
| Diabetes, with and without end organ damage | 40 (53) | 42 (51) | 0.791 |
| Renal disease e | 7 (9) | 7 (9) | 0.861 |
| Connective tissue disease | 6 (8) | 1 (1) | 0.040 |
| Malignancy f | 9 (12) | 2 (2) | 0.019 |
| Solid organ transplant recipient | 6 (8) | 5 (6) | 0.641 |
| Bone marrow transplant recipient | 2 (3) | 1 (1) | 0.508 |
| Psychiatric disease g | 19 (25) | 14 (17) | 0.205 |
| Chronic steroids, systemic | 8 (11) | 7 (9) | 0.650 |
| Chronic steroids, inhaled | 13 (17) | 3 (4) | 0.005 |
| Immunosuppressive medications h | 10 (13) | 6 (7) | 0.213 |
| DNR or DNR/DNI code status | 3 (4) | 7 (9) | 0.269 |
Note: Values rounded to nearest integer and, unless otherwise stated, expressed as no. (%). Data analyzed with chi‐squared or Mann‒Whitney U‐test as appropriate.
Bold value indicates the statistically significant.
Abbreviation: DNI, do not intubate; DNR, do not resuscitate; Q, quartile.
Cardiovascular disease includes previous myocardial infarction, congestive heart failure, arrythmia, peripheral vascular disease, or hypertension.
Pulmonary disease includes asthma, interstitial lung disease, chronic obstructive pulmonary disease, emphysema, chronic bronchitis, or any condition resulting in chronic oxygen use or chronic hypercapnia.
Oxygen used at baseline excludes isolated nocturnal continuous or bilevel positive airway pressure.
Cerebrovascular disease includes previous transient ischemic attack, stroke with residual deficits, or dementia.
Renal disease includes baseline creatinine >3 mg/dL, dialysis dependent, or previous kidney transplant.
Malignancy excludes any individuals where diagnosis occurred >5 years before index COVID‐19 admission.
Psychiatric disease includes physician‐diagnosed psychosis, anxiety, depression, post‐traumatic stress disorder, and substance use disorder (alcohol and drug, excludes marijuana).
Immunosuppressive medications includes active chemotherapy or antirejection medications (excludes steroids).
3.2. Admission from ED to the wards prior to ICU admission is associated with 28‐ and 60‐day mortality in COVID‐19 ARDS
Ward‐first patients had worse mortality at both 28 days (24/75 [32%] vs. 14/82 [17%], hazard ratio (HR) 1.97, 95% confidence interval [95% CI] 1.04‒3.72, p = 0.039) and 60 days (29/75 [39%] vs. 19/82 [23%], HR 1.83, 95% CI 1.04‒3.22, p = 0.037) compared to ICU‐direct patients (Figure 2), despite presenting to the ED after a similar duration of symptoms (median 7 vs. 8 days) and having lower ED‐based (4C, median 9 vs. 11, p = 0.023) and similar ICU‐based (SAPS‐III, median 60 vs. 59) mortality prediction scores (Table 2).
FIGURE 2.
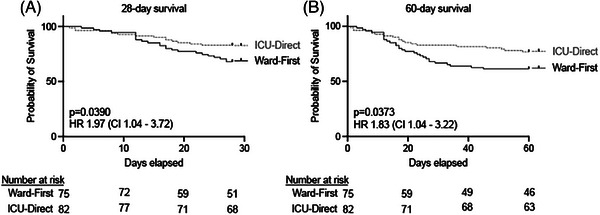
Admission to intensive care unit (ICU) was associated with reduced mortality in patients who developed COVID‐19‐associated acute respiratory distress syndrome (ARDS). The (A) 28‐day and (B) 60‐day survival rates were worse in ward‐first (n = 75) patients who developed COVID‐19 ARDS as compared to ICU‐direct patients (n = 82). CI, confidence interval; HR, hazards ratio.
TABLE 2.
Mortality prediction scores.
| Admission location | Time until ICU upgrade | |||||
|---|---|---|---|---|---|---|
| Ward‐first, n = 75 | ICU‐direct, n = 82 | p‐Value | <2 days, n = 25 | ≥2 days, n = 50 | p‐Value | |
| Symptom duration (days) a | 7 (4, 9) | 8 (6, 12) | 0.109 | 6 (5, 9) | 7 (4, 10) | 0.618 |
| ED mortality prediction | ||||||
| 4C score | 9 (6, 12) | 11 (7, 13) | 0.023 | 10 (7, 13) | 10 (7, 11) | 0.492 |
| ICU mortality prediction | ||||||
| SAPS‐III score | 60 (54, 67) | 59 (54, 69) | 0.928 | 62 (53, 67) | 58 (55, 68) | 0.860 |
Note: Values are expressed as median with interquartile range (Q1, Q3).
Bold value indicates the statistically significant.
Patients with unknown duration of symptoms were excluded from symptom duration analysis only (ward‐first: n = 1, and ICU‐direct: n = 2).
More ICU‐direct patients required mechanical ventilation at time of ICU admission (16/82 [20%] vs. 1/75 [1%, patient was intubated before transfer to ICU], p = 0.0003, Figure 3A,B) compared to ward‐first patients. Most ward‐first patients required HFNO at time of ICU admission (67/75 [89%] vs. 60/82 [73%], p = 0.0101, Figure 3B). A greater proportion of ward‐first patients then required escalation to mechanical ventilation within 1 day of ICU admission compared to ICU‐direct patients (27/74 [36%] vs. 9/66 [14%], p = 0.002, Figure 3A,B), despite having similar ROX index (ward‐first: median [interquartile range] 5.4 [4.1, 6.6]; ICU‐direct: median [interquartile range] 5.2 [4.2, 6.5]). In addition to worse mortality and rapid progression in respiratory support, ward‐first patients had fewer vasopressor‐free days compared to ICU‐direct patients in the first 28 days (median 24 vs. 27, p = 0.044, Table 3). Ward‐first patients also had fewer respiratory support‐free and hospital‐free days compared to ICU‐direct patients, but these differences were not statistically significant.
FIGURE 3.
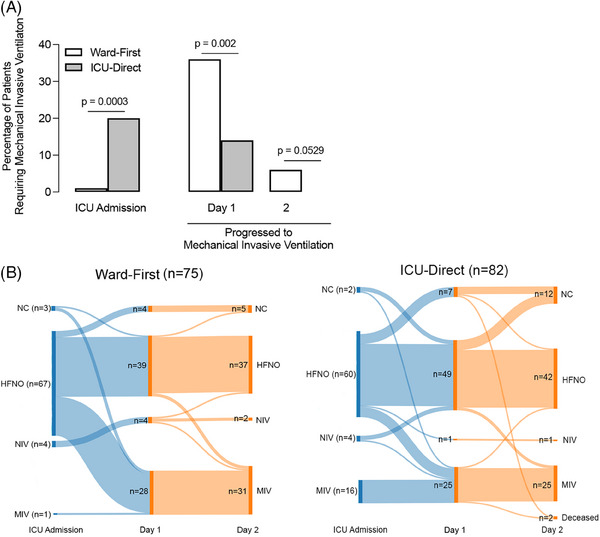
Ward‐first patients required more rapid escalation to mechanical ventilation within 1 day of intensive care unit (ICU) admission compared to ICU‐direct patients. (A) At time of ICU admission, a greater percentage of ICU‐direct patients (n = 16 out of 82, 20%) required mechanical invasive ventilation (MIV) as compared to ward‐first patients (n = 1 out of 75, 1%). However, more ward‐first patients (n = 27 out of 74, 36%) who were admitted to the ICU on high flow nasal oxygen (HFNO) or non‐invasive ventilation (NIV, inclusive of continuous positive airway pressure and bilevel positive airway pressure) progressed to requiring MIV within 1 day compared to ICU‐direct patients (n = 9 out of 66 patients, 14%). More ward‐first patients progressed to MIV on day 2 of ICU admission compared to ICU‐direct patients; however, this was not statistically significant. (B) Sankey diagrams illustrate progression of respiratory support needs for ward‐first (left) and ICU‐direct (right) patients at ICU admission and days 1 and 2 of ICU stay. NC, nasal cannula.
TABLE 3.
Secondary outcomes.
| Admission location | Time until ICU upgrade | |||||
|---|---|---|---|---|---|---|
| Ward‐first, n = 75 | ICU‐direct, n = 82 | p‐Value | <2 days, n = 25 | ≥2 days, n = 50 | p‐Value | |
| Respiratory‐free days a | 0 (0, 19) | 12 (0, 20) | 0.067 | 0 (0, 20) | 4 (0, 19) | 0.634 |
| Vasopressor‐free days b | 24 (0, 28) | 27 (18, 28) | 0.044 | 21 (0, 28) | 25 (0, 28) | 0.519 |
| CRRT‐free days | 28 (28, 28) | 28 (28, 28) | 0.280 | 28 (28, 28) | 28 (28, 28) | 0.642 |
| Hospital‐free days | 0 (0, 10) | 3 (0, 16) | 0.052 | 0 (0, 17) | 0 (0, 8) | 0.435 |
| ICU‐free days | 0 (0, 20) | 11 (0, 20) | 0.177 | 0 (0, 21) | 8 (0, 19) | 0.767 |
Note: Values are expressed as median with interquartile range (Q1, Q3). If patient died before or still required organ support, remained hospitalized or in the ICU at day 28, they were presumed to have zero organ‐free, hospital‐free, or ICU‐free days, respectively.
Bold value indicates the statistically significant.
Abbreviations: CRRT, continuous renal replacement therapy; ICU, intensive care unit.
Respiratory‐free days include total days out of 28 where patient did not require high flow nasal cannula and non‐invasive or invasive positive pressure ventilation.
Vasopressor‐free days include total days out of 28 where patient did not require vasoactive medications, including epinephrine, norepinephrine, vasopressin, phenylephrine, milrinone, dobutamine, or dopamine.
3.3. Association between COVID‐19 ARDS mortality, ED disposition, and comorbid conditions
Given the different rates of comorbid conditions between ward‐first and ICU‐direct patients, we next tested whether the association between 28‐ and 60‐day mortality and disposition location remained significant in a multivariable model. A history of malignancy, connective tissue disease or pulmonary disease was not associated with increased risk of 28‐ or 60‐day mortality (Table S2). Use of chronic inhaled steroids was associated with increased risk of 28‐day (HR 2.76, 95% CI 1.00‒6.89, p = 0.038) and 60‐day mortality (HR 2.78, 95% CI 1.13‒6.34, p = 0.020). Ward‐first disposition was associated with increased risk of 28‐day (HR 1.85, 95% CI 0.94‒3.76, p = 0.077) and 60‐day mortality (HR 1.68, 95% CI 0.92‒3.10, p = 0.093), but was no longer statistically significant when controlling for comorbid conditions.
3.4. Patients with rapid upgrade to ICU have worse mortality
Fifty‐seven percent of ward‐first patients required upgrade to ICU within 2 days of hospital admission (Figure S2). A shorter duration between hospital admission and upgrade to ICU was associated with worse 28‐day mortality (11/25 [44%] vs. 13/50 [26%], HR 2.5, 95% CI 1.0‒6.1, p = 0.0493, Figure 4), despite no difference in ED‐based or ICU‐based mortality prediction scores (Table 2). Secondary outcomes were similar in patients with rapid versus delayed ICU upgrade (Table 3). There were no differences in comorbid conditions between rapid versus delayed ICU upgrade groups (data not shown).
FIGURE 4.
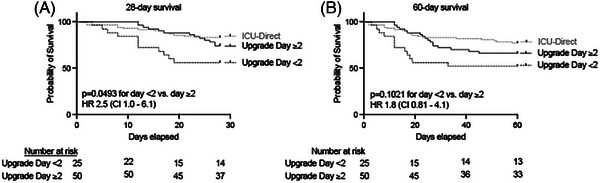
Shorter duration between hospital admission and upgrade to intensive care unit (ICU) was associated with worse 28‐day mortality in patients who developed acute respiratory distress syndrome (ARDS). The (A) 28‐day and (B) 60‐day survival curves in ward‐first patients (n = 75) who developed COVID‐19 ARDS and required upgrade to ICU in less than 2 days (n = 25) compared with patients who upgraded 2 days or later (n = 50) after admission. ICU‐direct survival curve was included for reference. CI, confidence interval; HR, hazards ratio.
3.5. Association between ED disposition and COVID‐19 ARDS mortality persisted in a sensitivity analysis of an independent cohort of COVID‐19 ARDS patients
A second cohort of patients (n = 65 ward‐first, n = 64 ICU‐first) admitted between April 2021 and July 2022 who developed COVID‐19 ARDS was combined with the primary cohort as a sensitivity analysis for the primary outcomes. Ward‐first patients had worse mortality at both 28 days (48/140 [34%] vs. 32/146 [22%], HR 1.57, 95% CI 1.02‒2.43, p = 0.0429) and 60 days (62/140 [44%] vs. 42/146 [29%], HR 1.72, 95% CI 1.17‒2.52, p = 0.0055) compared to ICU‐direct patients in this expanded cohort (Figure S3).
4. LIMITATIONS
This study has several limitations. First, criteria for ICU admission for COVID‐19 vary significantly by hospital, by region, and across time. Even within our own system, our community‐affiliated hospital supports patients with COVID‐19 who require HFNO on the ward or in the ICU depending on clinician judgment, while any patient with COVID‐19 on HFNO requires ICU admission at our academic hospital. To account for this variability in ICU admission criteria, we measured mortality risk and disease severity at time of ICU admission by calculating both the SAPS‐III score and ROX index, and found that these scores were not different between groups. Our sample likely excluded patients at our community site who were admitted to the ward on HFNO but never transferred to the ICU, and these patients may represent a distinct subset meeting ARDS criteria who improve and do not require the ICU; thus, we also analyzed 28‐ and 60‐day mortality in patients only admitted to our academic hospital and found that the HRs associated with ward‐first admission were similar (28‐day mortality: HR 2.0, 95% CI 0.92‒4.5; 60‐day mortality: HR 2.0, 95% CI 1.0‒3.9). This suggests that the association of ED disposition with mortality persists when including patients on HFNO who got better and may have been cared for on the ward at our community site. Overall mortality was also not different between our community and academic sites, suggesting that our findings are unlikely due to management differences between the hospitals. Nonetheless, interpretation of these data should consider practice setting. In addition, our findings should be validated in a larger multicenter cohort that includes all patients with COVID‐19 who met ARDS criteria.
The second limitation of our study is the demographic differences between ward‐first and ICU‐direct patients. Ward‐first patients had more comorbidities associated with worse outcomes in COVID‐19, including lung disease and malignancy, which may have contributed to their increased mortality despite lack of significance in our regression analysis. Notably, the 4C and SAPS‐III scores include comorbidities to risk stratify patients; however, ward‐first patients had worse mortality despite lower 4C scores and similar SAPS‐III scores as ICU‐direct patients. Patients who rapidly upgraded to the ICU also had worse mortality despite no differences in comorbid conditions compared to patients with delayed upgrade, suggesting that the trajectory of COVID‐19 ARDS illness more accurately reflects mortality risk than the snapshots provided by these scores at the time of hospital and ICU admission.
A final limitation of our study is that we stopped enrollment in July 2022 and thus included fewer patients infected with the omicron variant of COVID‐19, which remains the dominant circulating strain in the United States. Illness severity has declined in the omicron‐dominant era 17 ; thus, we hypothesized that inclusion of more patients with omicron would decrease the association between ED disposition and COVID‐19 ARDS mortality.
5. DISCUSSION
In non‐ARDS studies, patients transferred from hospital wards to ICU have increased mortality compared to patients directly admitted to ICU from the ED. 9 , 10 , 11 , 12 , 13 Most unplanned ICU transfers occur due to progression of disease, 18 with respiratory failure 18 or admission for a respiratory condition 19 having the highest predictive value for rapid upgrade to the ICU. In this cohort of patients with COVID‐19 ARDS, ED disposition to non‐ICU level of care was associated with more rapid escalation in respiratory support within 1 day of ICU admission and worse mortality compared to ICU‐direct patients. The association between ward‐first disposition and increased mortality persisted in our sensitivity analysis of patients admitted from 2021 to 2022, reflecting more recent viral variants.
Several scoring systems were developed to predict decompensation due to COVID‐19, yet none are widely adopted to aid in ED disposition decisions. A key finding in this work is that ward‐first patients experienced worse outcomes despite having lower ED‐based 4C scores and similar ICU‐based SAPS‐III scores compared to ICU‐direct patients. The median 4C score for both ward‐first and ICU‐direct patients corresponded to a “high‐risk” category, with estimated mortality of 31%‒35%, 4 which accurately predicted mortality in ward‐first patients (24/75, 32% at 28 days), but overestimated mortality in ICU‐direct patients (14/82, 17% at 28 days). Similarly, the median SAPS‐III score predicted mortality for ward‐first patients at 33.5% and ICU‐direct patients at 35.6%, demonstrating that this score also performed poorly for ICU‐direct patients. Because these scores were derived in large patient cohorts with a wider spectrum of disease severity, it is possible that the scores underperformed in ICU‐direct COVID‐19 ARDS because these patients represent the “extremes” of risk. Similarly, the ROX index at ICU admission was similar between groups, but ward‐first patients progressed to mechanical ventilation faster than ICU‐direct patients. Because the ROX index has better discriminatory function over time, 2 repeated assessments to capture an individual's trajectory rather than a single, static measurement may have more accurately predicted outcomes in ward‐first patients.
There are several plausible explanations for the association between initial ED disposition and patient outcomes. First, it is notable that ward‐first patients who upgraded to ICU often had rapid disease progression despite initiation of steroids and other therapies. Previous work has identified patients who have rapid evolution of disease as representing a distinct clinical phenotype with high mortality, 20 similar to our patient cohort. Inflammatory subphenotypes of COVID‐19 ARDS have also been identified where steroid treatment has a differential impact on mortality, 21 although it is currently unknown whether ARDS subphenotypes can be identified in patients before they progress to meet ARDS criteria. We hypothesized that ward‐first patients in our cohort who presented with initially less severe disease, but experienced rapid progression despite steroid therapy, may represent a distinct ARDS subphenotype compared to ICU‐direct patients. Identifying phenotypes before ARDS onset may improve triage in patients at risk for rapid progression.
System‐level factors also likely contributed to increased mortality in ward‐first patients. Respiratory decompensation is one of the most common indications for ICU admission; however, it is poorly recognized. Among patients admitted to hospital wards, delayed rapid response team activation commonly occurs due to unrecognized respiratory failure, and delay in recognition is associated with increased mortality. 22 , 23 Limited hospital resources during the COVID‐19 pandemic also required step‐down units to care for sicker patients and patients meeting ICU criteria who are cared for in non‐ICU settings have worse outcomes, 24 although this level of strain was not experienced at Stanford Health Care. Our methodology does not allow us to determine if different outcomes between ward‐first and ICU‐direct patients were due to phenotypic differences or due to differences in care provided in the ICU versus non‐ICU settings. HFNO was not allowed in non‐ICU patients at our academic hospital, so it is possible that a delay in receiving HFNO contributed to worse outcomes in ward‐first patients. However, HFNO was allowed outside the ICU at our community site and there was no difference in outcomes between ward‐first patients who received >20 L of HFNO prior to ICU transfer and ward‐first patients who first received HFNO in the ICU (28‐day mortality 2/6 [33%] vs. 6/14 [43%], p = 0.187). While there is limited power to rule out differences in outcomes, the lack of benefit with pre‐ICU administration of HFNO suggests that worse outcomes in ward‐first patients represent difference in host factors rather than differences in care between groups. Overall, the cause of increased mortality observed in ward‐first patients is likely multifactorial and future efforts should focus on both patient‐specific and system‐specific interventions to reduce mortality.
Ward‐first patients requiring intensive care within 48 h of admission were at highest risk for poor outcomes in our study, highlighting a need for improved identification of this group at time of hospital admission. This highlights the fact that existing risk stratification strategies largely focus on whether a patient will eventually require ICU admission, but not when, and most predictive tools perform similarly irrespective of timing of ICU upgrade. 25 Future work should focus on tools to assist ED disposition decisions that incorporate patient trajectory and timing of critical care interventions. While the global impact of COVID‐19 has evolved with less severe disease and declining mortality rates due to vaccines, therapeutics, and viral evolution, COVID‐19 infection continues to cause hospitalizations and ICU admissions similar to other endemic viruses, such as influenza. Tools to identify “rapid progressors” in patients infected with COVID‐19 are still needed and may translate to patients infected with other respiratory viral illnesses, which is a potential topic for future research.
In summary, we found that compared to direct ICU admission, admission from the ED to the ward is associated with increased mortality in patients who meet COVID‐19 ARDS criteria during their hospitalization, despite lower 4C and similar SAPS‐III risk scores at the time of hospital and ICU admission, respectively. Patients with rapid progression had the worst outcomes, and current risk stratification tools fail to identify these patients at the time of admission. Future work should focus on validating these findings in larger cohorts and developing tools to identify “rapid progressors” at the time of ED disposition.
AUTHOR CONTRIBUTIONS
Katie M. Lebold, Angela J. Rogers, and Jennifer G. Wilson contributed to the study design. All the authors contributed to the data acquisition, analysis and interpretation of data, and drafting and revision of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare they have no conflicts of interest.
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
ACKNOWLEDGMENTS
This study is supported by SAEM Resident Research Grant 2022–2023.
Biography
Katie Lebold, MD, PhD, is a resident in the Department of Emergency Medicine at Stanford University College of Medicine in Palo Alto, California, USA.

Lebold KM, Moore AR, Sanchez PA, et al. Association between emergency department disposition and mortality in patients with COVID‐19 acute respiratory distress syndrome. JACEP Open. 2024;5:e13192. 10.1002/emp2.13192
Prior presentation: Oral abstract presentation at the Society of Academic Emergency Medicine Annual Meeting, May 2023, Austin, TX, USA.
Supervising Editor: Nicholas Johnson, MD
REFERENCES
- 1. Prakash J, Bhattacharya PK, Yadav AK, Kumar A, Tudu LC, Prasad K. ROX index as a good predictor of high flow nasal cannula failure in COVID‐19 patients with acute hypoxemic respiratory failure: a systematic review and meta‐analysis. J Crit Care. 2021;66:102‐108. doi: 10.1016/j.jcrc.2021.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roca O, Caralt B, Messika J, et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high‐flow therapy. Am J Respir Crit Care Med. 2019;199(11):1368‐1376. doi: 10.1164/rccm.201803-0589OC [DOI] [PubMed] [Google Scholar]
- 3. Gordon AJ, Govindarajan P, Bennett CL, et al. External validation of the 4C mortality score for hospitalised patients with COVID‐19 in the RECOVER network. BMJ Open. 2022;12(4):e054700. doi: 10.1136/bmjopen-2021-054700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Knight SR, Ho A, Pius R, et al. Risk stratification of patients admitted to hospital with COVID‐19 using the ISARIC WHO Clinical Characterisation Protocol: development and validation of the 4C mortality score. BMJ. 2020;370:m3339. doi: 10.1136/bmj.m3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moreno RP, Metnitz PGH, Almeida E, et al. SAPS 3—from evaluation of the patient to evaluation of the intensive care unit. Part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31(10):1345‐1355. doi: 10.1007/s00134-005-2763-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Metnitz PGH, Moreno RP, Fellinger T, Posch M, Zajic P. Evaluation and calibration of SAPS 3 in patients with COVID‐19 admitted to intensive care units. Intensive Care Med. 2021;47(8):910‐912. doi: 10.1007/s00134-021-06436-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Singer AJ, Morley EJ, Meyers K, et al. Cohort of four thousand four hundred four persons under investigation for COVID‐19 in a New York Hospital and predictors of ICU care and ventilation. Ann Emerg Med. 2020;76(4):394‐404. doi: 10.1016/j.annemergmed.2020.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duca A, Memaj I, Zanardi F, et al. Severity of respiratory failure and outcome of patients needing a ventilatory support in the Emergency Department during Italian novel coronavirus SARS‐CoV2 outbreak: preliminary data on the role of helmet CPAP and non‐invasive positive pressure ventilation. eClinicalMedicine. 2020;24:100419. doi: 10.1016/j.eclinm.2020.100419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Valentini I, Pacilli AMG, Carbonara P, et al. Influence of the admission pattern on the outcome of patients admitted to a respiratory intensive care unit: does a step‐down admission differ from a step‐up one? Respir Care. 2013;58(12):2053‐2060. doi: 10.4187/respcare.02225 [DOI] [PubMed] [Google Scholar]
- 10. Renaud B, Brun‐Buisson C, Santin A, et al. Outcomes of early, late, and no admission to the intensive care unit for patients hospitalized with community‐acquired pneumonia. Acad Emerg Med. 2012;19(3):294‐303. doi: 10.1111/j.1553-2712.2012.01301.x [DOI] [PubMed] [Google Scholar]
- 11. Renaud B, Santin A, Coma E, et al. Association between timing of intensive care unit admission and outcomes for emergency department patients with community‐acquired pneumonia. Crit Care Med. 2009;37(11):2867‐2874. doi: 10.1097/CCM.0b013e3181b02dbb [DOI] [PubMed] [Google Scholar]
- 12. Molina JAD, Seow E, Heng BH, Chong WF, Ho B. Outcomes of direct and indirect medical intensive care unit admissions from the emergency department of an acute care hospital: a retrospective cohort study. BMJ Open. 2014;4(11):e005553. doi: 10.1136/bmjopen-2014-005553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fernando SM, Rochwerg B, Reardon PM, et al. Emergency department disposition decisions and associated mortality and costs in ICU patients with suspected infection. Crit Care. 2018;22(1):172. doi: 10.1186/s13054-018-2096-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526‐2533. doi: 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 15. Matthay MA, Arabi Y, Arroliga AC, et al. A new global definition of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2024;209(1):37‐47. doi: 10.1164/rccm.202303-0558WS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rice TW, Wheeler AP, Bernard GR, Hayden DL, Schoenfeld DA, Ware LB. Comparison of the SpO2/FiO2 ratio and the PaO2/FiO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132(2):410‐417. doi: 10.1378/chest.07-0617 [DOI] [PubMed] [Google Scholar]
- 17. Kojima N, Adams K, Self WH, et al. Changing severity and epidemiology of adults hospitalized with coronavirus disease 2019 (COVID‐19) in the United States after introduction of COVID‐19 vaccines, March 2021‒August 2022. Clin Infect Dis. 2023;77(4):547‐557. doi: 10.1093/cid/ciad276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dahn CM, Manasco AT, Breaud AH, et al. A critical analysis of unplanned ICU transfer within 48 hours from ED admission as a quality measure. Am J Emerg Med. 2016;34(8):1505‐1510. doi: 10.1016/j.ajem.2016.05.009 [DOI] [PubMed] [Google Scholar]
- 19. Delgado MK, Liu V, Pines JM, Kipnis P, Gardner MN, Escobar GJ. Risk factors for unplanned transfer to intensive care within 24 hours of admission from the emergency department in an integrated healthcare system. J Hosp Med. 2013;8(1):13‐19. doi: 10.1002/jhm.1979 [DOI] [PubMed] [Google Scholar]
- 20. Lascarrou JB, Gaultier A, Soumagne T, et al. Identifying clinical phenotypes in moderate to severe acute respiratory distress syndrome related to COVID‐19: the COVADIS study. Front Med. 2021;8:632933. doi: 10.3389/fmed.2021.632933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sinha P, Furfaro D, Cummings MJ, et al. Latent class analysis reveals COVID‐19‐related acute respiratory distress syndrome subgroups with differential responses to corticosteroids. Am J Respir Crit Care Med. 2021;204(11):1274‐1285. doi: 10.1164/rccm.202105-1302OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tirkkonen J, Skrifvars MB, Tamminen T, et al. Afferent limb failure revisited—a retrospective, international, multicentre, cohort study of delayed rapid response team calls. Resuscitation. 2020;156:6‐14. doi: 10.1016/j.resuscitation.2020.08.117 [DOI] [PubMed] [Google Scholar]
- 23. Barwise A, Thongprayoon C, Gajic O, Jensen J, Herasevich V, Pickering BW. Delayed rapid response team activation is associated with increased hospital mortality, morbidity, and length of stay in a tertiary care institution. Crit Care Med. 2016;44(1):54‐63. doi: 10.1097/CCM.0000000000001346 [DOI] [PubMed] [Google Scholar]
- 24. Simchen E, Sprung CL, Galai N, et al. Survival of critically ill patients hospitalized in and out of intensive care. Crit Care Med. 2007;35(2):449‐457. doi: 10.1097/01.CCM.0000253407.89594.15 [DOI] [PubMed] [Google Scholar]
- 25. Sutherland ME, Yarmis SJ, Lemkin DL, Winters ME, Dezman ZDW. National early warning score is modestly predictive of care escalation after emergency department‐to‐floor admission. J Emerg Med. 2020;58(6):882‐891. doi: 10.1016/j.jemermed.2020.03.032 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Supporting information
Supporting information
Supporting information


