Abstract
INTRODUCTION
Evaluating whether genetic susceptibility modifies the impact of lifestyle‐related factors on dementia is critical for prevention.
METHODS
We studied 5170 participants from a French cohort of older persons free of dementia at baseline and followed for up to 17 years. The LIfestyle for BRAin health risk score (LIBRA) including 12 modifiable factors was constructed at baseline (higher score indicating greater risk) and was related to both subsequent cognitive decline and dementia incidence, according to genetic susceptibility to dementia (reflected by the apolipoprotein E [APOE] ε4 allele and a genetic risk score [GRS]).
RESULTS
The LIBRA was associated with higher dementia incidence, with no significant effect modification by genetics (hazard ratio for one point score = 1.09 [95% confidence interval, 1.05; 1.13]) in APOE ε4 non‐carriers and = 1.15 [1.08; 1.22] in carriers; P = 0.15 for interaction). Similar findings were obtained with the GRS and with cognitive decline.
DISCUSSION
Lifestyle‐based prevention may be effective whatever the genetic susceptibility to dementia.
Keywords: apolipoprotein E, cognitive decline, dementia, genetic risk score, modifiable risk factors
1. INTRODUCTION
As life expectancy increases and the population ages, cognitive aging and dementia, the most frequent form of pathological brain aging, have become a major concern worldwide. Dementia is a multifactorial syndrome, with sporadic Alzheimer's disease (AD) accounting for two thirds of cases, resulting from both genetic and environmental risk factors. While a large proportion of the heritable component of sporadic AD is attributed to epsilon alleles of apolipoprotein E (APOE), 75 additional common genetic risk loci were also mapped to sporadic AD in recent genome‐wide association studies (GWAS). 1 , 2 On the environmental side, modifiable lifestyle and related risk factors, which are actionable to primary prevention by individual behavioral changes, could represent ≥ 40% of the attributable risk of dementia and thus appear at the forefront of prevention. 3 The risk factors with the most compelling epidemiological evidence so far in relation to cognitive aging include psychosocial factors (low education level, depression, low cognitive stimulation), lifestyle (unhealthy diet, low physical activity, alcohol use, smoking), and related cardiometabolic health (diabetes, hypertension, obesity, high cholesterol). 3 , 4 In addition, individuals rarely encounter one risk factor alone and the combined study of the multiple risk factors for dementia could more accurately capture the additive effect of environmental exposure as a whole.
The LIfestyle for BRAin health risk score (LIBRA) uniquely combines and evaluates the additive effect of robust modifiable risk factors for dementia actionable by tailored interventions for primary prevention. 4 , 5 , 6 , 7 , 8 , 9 The LIBRA comprises a weighted score of modifiable components including: unhealthy lifestyle (unhealthy diet, physical inactivity, low engagement in cognitively stimulating activities, null or elevated alcohol consumption, and smoking), poor cardiometabolic health (history of heart disease, diabetes, high cholesterol, obesity, and hypertension), renal dysfunction, and depression, 5 with higher scores indicating a higher risk for dementia. 6 , 7 , 8 , 9 Initially developed upon a systematic literature review and Delphi expert study, 4 the LIBRA was highly associated with dementia when applied in observational cohorts. In the Maastricht Aging Study, each increase of one point of LIBRA was associated with a 25% increase in dementia risk, 5 and the LIBRA has been later externally validated in several other population‐based studies from mid‐life to older ages. 7 , 8 , 9
A crucial question for public health and prevention is whether the risk of dementia attributable to genetic susceptibility may be lowered by acting on modifiable risk factors through global prevention programs. This is a key concept of precision prevention, in which the right intervention should target the right population (in the right window of opportunity). However, epidemiological findings on gene‐by‐environment interactions in dementia have been inconsistent so far. In Europe, two studies reported lifestyle factors associated with dementia risk but only among older persons with low, 10 or conversely high, 11 genetic susceptibility, while UK and US studies reported no interaction effects. 12 , 13 , 14 None of these prior studies examined a comprehensive combination of modifiable factors as reflected by the LIBRA.
We leveraged a large cohort with a deep investigation of genomic and environmental risk factors and up to two decades of in‐person follow‐up for cognition and dementia, the Three‐City (3C) Study, to investigate the interaction between the LIBRA and genetic susceptibility in relation to dementia risk and cognitive trajectories.
RESEARCH IN CONTEXT
Systematic review: Some epidemiological studies found that the association of modifiable factors with risk of dementia is independent of the genetic susceptibility to the disease, while others reported null association among persons with a high genetic risk.
Interpretation: In a large cohort of older persons from western Europe (France), increasing number of unhealthy lifestyle‐related factors was associated with a higher risk of dementia and faster cognitive decline, independently of genetic risk factors for Alzheimer's disease.
Future directions: Implementation of the LIfestyle for BRAin health risk score (LIBRA) in preventive interventions should be considered in the future.
2. METHODS
2.1. Study population
The 3C Study is an ongoing prospective cohort initiated in 1999 to 2000, including 9294 non‐institutionalized community dwellers aged > 65 years from three French cities: Bordeaux (n = 2104), Dijon (n = 4931) and Montpellier (n = 2259). 15 The 3C protocol was approved by the consultative committee for the protection of persons participating in biomedical research of the Kremlin‐Bicêtre university hospital and Sud‐Méditerranée III and all participants provided written informed consent. At baseline, face‐to‐face interviews were conducted to collect sociodemographic data, lifestyle, and health parameters. Anthropometric and blood pressure measurements were performed, as well as a fasting blood sampling. APOE was genotyped and genome‐wide genotyping was ascertained among 6489 3C participants for the purpose of a GWAS on AD. 16
In‐person follow‐up visits were conducted every 2 to 3 years for 12 years in Dijon, 15 years in Montpellier, and 17 years in Bordeaux. The vital status of each participant was searched regularly via death certificates, participant's family, and/or physician and hospital records.
Of the 9294 participants from 3C who had completed the baseline clinical evaluation, we first excluded 1804 individuals with missing data for at least one of the LIBRA components at baseline, 1858 who did not participate in the GWAS, and 8 who had no APOE sequencing. Among the remaining 5624 participants, we further excluded 77 individuals who had dementia at baseline and 377 without at least one completed follow‐up visit, resulting in an analytical sample of 5170 participants (Figure S1 in supporting information). The excluded participants due to missing information for ≥ 1 LIBRA component, genomics or incident dementia, were slightly older than those included (74.9 vs. 73.9 years old), had slightly lower body mass index (BMI; 25.5 vs. 25.8 kg/m2), and were more often diabetics (12% vs. 9%; all P < 0.05; Table S1 in supporting information). However, they did not differ for other sociodemographic and health indicators including sex, educational level, income, smoking, or hypertension.
2.2. Ascertainment of dementia and cognitive change
Incident dementia cases were actively screened and dementia diagnosis was established based on a three‐step procedure. 15 First, trained psychologists administered a battery of neuropsychological tests at baseline and at each follow‐up visit. Second, the participants suspected to be demented by the neuropsychologist were secondarily examined by a neurologist to establish the diagnosis. Third, all potential cases were adjudicated by an independent committee of neurologists, who reviewed all existing information to establish the diagnosis and etiology based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition 17 and National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer's Disease and Related Disorders Association. 18
To analyze cognitive trajectories, we focused on global functioning, by combining the results in five neuropsychological tests assessed repeatedly from study baseline to the last follow‐up visit, which evaluate different cognitive domains: the Mini‐Mental State Examination (range of possible values, 0–30 points), 19 for global cognition; the Benton Visual Retention Test (range of possible values, 0–15 points), 20 for working memory attention; the Isaacs’ Set Test (range of sample values, 0–66 points), 21 for verbal fluency; and the Trail Making Tests, 22 assessed as the number of correct moves divided by time to perform the test in minutes; Part A (range of sample values, 0–84.6 moves/minute) and B (0–49.8 moves/minute), for processing speed and executive functions, respectively. More details on the cognitive tests have been described in Methods S1 in supporting information.
2.3. Ascertainment of the LIBRA
We calculated the LIBRA as a weighted sum of 12 lifestyle, cardiometabolic, and other health‐related components, 4 , 5 all defined at cohort baseline (Table 1). We were able to reconstruct all original components 5 in our study population. For certain components, we made slight adaptations in the definition criteria; we used study‐specific definitions for healthy diet, engagement in regular exercise and cognitively stimulating activities, as defined in our previous works; 23 , 24 and we included use of medications in addition to biological values or self‐report in the definition of diabetes, high cholesterol, and high depressive symptoms. The binary components (denoting high vs. low, or presence vs. absence of the risk factor) were then weighted based on the original scoring system definition (with positive weights assigned to risk factors and negative weights to protective factors) 5 and the LIBRA was computed as the weighted sum score of all the components for each individual.
TABLE 1.
Definition of the LIfestyle for BRAin health risk (LIBRA) score metrics.
| Metric | Definition | Weight to dementia risk a |
|---|---|---|
| Healthy diet | Intake of fruits and vegetables ≥ twice per day and intake of fish ≥ twice by week | −1.7 |
| Physical inactivity | 1.1 | |
| Dijon and Montpellier sites | Recreational walking <1 hour per day or no sport practice | |
| Bordeaux site | Recreational walking ≤ 1 hour per week or having <1 hour of sport or intensive leisure activity per week | |
| Engagement in cognitively stimulating activities b | Highest tertile of a cognitive stimulating activity score | −3.2 |
| Low to moderate alcohol consumption | Consumption of > 0 and < 14 units of alcohol per week | −1.0 |
| Smoking | Current smoker | 1.5 |
| Heart disease | History of: myocardial infarction, hospitalized stroke, coronary surgery/angioplasty, history of leg artery surgery if arteritis of the lower limbs | 1.0 |
| Diabetes | Fasting glycemia ≥ 7 mmol/L or taking diabetes medication | 1.3 |
| High cholesterol | Fasting blood cholesterol ≥ 6.2 mmol/L or taking lipid‐lowering medication | 1.4 |
| Obesity | Body mass index ≥ 30 kg/m2 | 1.6 |
| Hypertension | Blood pressure ≥ 140/90 mmHg or antihypertensive treatment | 1.6 |
| Renal dysfunction | Glomerular filtration rate <60 mL/min/1.73 m2 | 1.1 |
| Depressive symptomatology | Center for Epidemiologic Studies‐Depression scale ≥ 17 among men, ≥ 23 among women or taking an antidepressant treatment | 2.1 |
Weights were assigned as defined in the original score 4 (with positive weights for risk factors and negative weights for protective factors).
2.4. Genetics of dementia
The genetic susceptibility to dementia was studied using two complementary tools. First, APOE genotyping was performed using the fluorogenic 5′‐nuclease assay with TaqMan chemistry 25 and APOE ε4 carriers, defined as individuals having at least one ε4 risk allele (ε2/ε4, ε3/ε4, or ε4/ε4 genotype) were compared to non‐carriers (ε2/ε2, ε2/ε3, or ε3/ε3 genotype).
Second, we generated a genetic risk score (GRS) for AD by summing the number of independent risk alleles as identified in the latest published GWAS meta‐analysis, 2 each weighted by the corresponding regression coefficient. This GRS was built from genome‐wide genotyping data that were available in 3C participants and that were processed at the Centre National de Génotypage in Evry (France) using the Illumina Human610‐Quad BeadChip. Genotype data were imputed using the 1000 Genomes Phase 1 Version 3 reference panel following standard quality control, described in detail in previous publications. 26 To construct the GRS, we considered 68 AD‐associated common variants 2 showing high imputation quality in our study (minor allele frequency > 0.01 and imputation score ≥ 0.5; Table S2 in supporting information).
2.5. Statistical analyses
We primarily used the LIBRA and GRS as continuous variables in statistical models (i.e., for interaction tests) and scores were secondarily categorized to allow for stratified analyses when we needed to derive group‐level epidemiological indicators.
2.5.1. Incidence of dementia
To estimate hazard ratios (HR) of dementia with increasing LIBRA, we used cause‐specific Cox models taking into account potential competitive risk by death. The time to dementia onset was imputed at the mid‐point interval between the last visit without dementia and the visit at diagnosis. For participants who died without any dementia diagnosis, the time to dementia onset was censored at the time of death, if death occurred < 3 years after the last follow‐up visit, and at the last visit otherwise. To derive the crude cause‐specific absolute risk of dementia from the Cox model while accounting for competing risk by death, 27 we further specified a cause‐specific Cox model for death before dementia. For both dementia and death, Cox models considered the time since entry in the cohort as the timescale and used baseline hazard rates stratified by study center (to account for differences in the ability to meet proportional hazards assumption due to center‐specific follow‐up duration). Models were adjusted for age at baseline using a spline function, 28 sex, educational level, income, and genetic ancestry (represented by the four first principal components of population stratification, a common strategy to control for confounding due to subpopulation structure in genomics studies 29 ).
To investigate whether the associations between the LIBRA and dementia risk were modified by APOE ε4 or GRS, we examined two‐way interaction terms between the LIBRA (continuous) and each genetic tool (APOE ε4 or GRS [continuous]) in separate models. Absolute and relative risks of dementia for each increase of one point of LIBRA were estimated stratified on both APOE ε4 carrier status and increasing GRS levels (tertiles).
In all models, the log‐linearity and the proportional hazard assumptions were evaluated using penalized splines and the Schoenfeld residuals, respectively.
2.5.2. Cognitive decline
We estimated the evolution of global cognition, defined as the common factor underlying the five neuropsychological tests, using a latent process mixed model for multivariate continuous longitudinal outcomes. 30 The cognitive trajectory was modeled using a quadratic function of time, in years since cohort entry; within‐participant correlation was captured by a correlated random intercept and slopes on the time function. The models included an intercept that represented the cognitive scores at baseline, the quadratic functions of time, covariates (both as a simple effect and in interaction with time functions), and corresponding random effects to account for intra‐individual correlation. We examined the association of both LIBRA and genetic factors (and their potential interactions) with cognitive change (through time function parameters), adopting similar modeling strategy as with dementia risk analyses. As in the analysis of incident dementia, the assumption of a linear relationship between LIBRA and cognitive trajectory constituents was investigated using splines.
2.5.3. Supplementary analyses
As an additional analysis, we tested for potential differential associations of LIBRA to dementia risk across combined (APOE ε4‐by‐GRS) genetic risk groups, in a single model.
Moreover, in sensitivity analyses, we evaluated the robustness of our results to slight variations in the definition of our LIBRA and GRS scoring systems (Methods S1). We also secondarily excluded from the group of APOE ε4 carriers participants with the ε2 allele (i.e., ε2/ε4 genotype) as ε2 is known to compensate the increased risk conferred by the ε4 allele.
TABLE 2.
Baseline characteristics of participants according to increasing levels of LIfestyle for BRAin health risk (LIBRA) score (N = 5170).
| LIBRA score a | |||
|---|---|---|---|
| Low [−5.9; −1.0] | Intermediate [−1; 2.7] | High [2.7; 11.2] | |
| (n = 1383) | (n = 2503) | (n = 1284) | |
| Study center, N (%) | |||
| Bordeaux | 248 (17.9) | 578 (23.1) | 339 (26.4) |
| Dijon | 957 (69.2) | 1646 (65.8) | 836 (65.1) |
| Montpellier | 178 (12.9) | 279 (11.1) | 109 (8.5) |
| Sociodemographic characteristics | |||
| Age, mean (SD), years | 73.1 (5.0) | 73.7 (5.2) | 74.8 (5.4) |
| Women, N (%) | 936 (67.7) | 1485 (59.3) | 751 (58.5) |
| Education ≥ secondary school, N (%) | 724 (52.4) | 918 (36.7) | 354 (27.6) |
| Monthly income ≥ 1500€, N (%) | 966 (71.7) | 1464 (60.7) | 647 (52.1) |
| LIBRA score, mean (SD) | –2.43 (1.25) | 1.02 (0.97) | 4.29 (1.24) |
| Lifestyle and health characteristics (LIBRA components) | |||
| Healthy diet, N (%) | 520 (37.6) | 437 (17.5) | 74 (5.8) |
| Physical inactivity, N (%) | 98 (7.1) | 654 (26.1) | 562 (43.8) |
| Engagement in cognitively stimulating activities, N (%) | 1046 (75.6) | 525 (21.0) | 41 (3.2) |
| Alcohol consumption (> 0 and < 14 units of alcohol per week), N (%) | 973 (70.4) | 1171 (46.8) | 447 (34.8) |
| Current smoking, N (%) | 31 (2.2) | 113 (4.5) | 128 (9.9) |
| Heart disease, N (%) | 31 (2.2) | 213 (8.5) | 212 (16.5) |
| Diabetes, N (%) | 30 (2.2) | 179 (7.2) | 247 (19.2) |
| High cholesterol, N (%) | 399 (28.9) | 896 (35.8) | 694 (54.0) |
| Body mass index, mean (SD), kg/m2 | 24.7 (3.4) | 25.5 (3.8) | 27.5 (4.6) |
| Hypertension, N (%) | 950 (68.7) | 1913 (76.4) | 1181 (92.0) |
| Renal dysfunction, N (%) | 63 (4.6) | 248 (9.9) | 324 (25.2) |
| Depressive symptomatology, N (%) | 33 (2.4) | 209 (8.3) | 375 (29.2) |
| Genetic characteristics | |||
| APOE ε4 carrier, N (%) | 276 (20.0) | 495 (19.8) | 268 (20.9) |
| Genetic risk score b , N (%) | |||
| Low | 494 (35.7) | 817 (32.6) | 413 (32.2) |
| Medium | 443 (32.0) | 865 (34.6) | 415 (32.3) |
| High | 446 (32.2) | 821 (32.8) | 456 (35.5) |
Abbreviations: APOE, apolipoprotein E; SD, standard deviation.
Low, intermediate, and high LIBRA risk score categories were defined as the first quartile, second and third quartiles, and fourth quartile of the score, respectively. LIBRA ranges theoretically from −5.9 to +12.7.
Low, medium, and high genetic risk score categories were defined as the first, second, and third tertiles of the score, respectively.
Finally, we evaluated the robustness of our results to any bias due to the selection of healthier participants (those with complete information for LIBRA), by running a sensitivity analysis of LIBRA and incident dementia on the sample of n = 5900 participants with genomics data and followed for dementia (among whom n = 730 had missing information for at least one LIBRA component) and imputing the missing LIBRA information by multiple imputation. The Rubin rule was used for estimation of the within‐ and between‐imputation variances. 31
All statistical analyses were conducted using R software version 4.0.3 (R Foundation for Statistical Computing; RiskRegression 27 and lcmm 30 packages for cause‐specific Cox and multivariate latent process mixed models, respectively). Missing data for covariates (≤ 1% for education and income) were imputed using chained equations with fully conditional specification method, 32 whenever necessary.
3. RESULTS
At baseline, the participants were aged 73.9 years on average, 66% were female, and 39% had an education level higher than secondary school (Table 2). There was increasing proportion of participants from Bordeaux center and decreasing proportion from Dijon with increasing LIBRA scores (Table 2). Participants with higher LIBRA were slightly older and less likely to be female and to have higher levels of education and income (Table 2). LIBRA components were distributed widely across increasing score levels, with, by design, higher prevalence for unhealthy components (physical inactivity, smoking, heart disease, diabetes, high cholesterol and BMI, hypertension, renal dysfunction, and depression) and low prevalence for healthy components (healthy diet, engagement in cognitively stimulating activities, low to moderate alcohol consumption) with higher LIBRA values.
APOE ε4 carriers were quite evenly distributed across LIBRA levels (20.0% in lower vs. 20.9% in higher score levels). In contrast, participants with higher LIBRA were slightly more likely to have higher GRS score values. Both LIBRA (mean = 0.91 [standard deviation (SD) = 2.66], range −5.9 to 11.2) and GRS (mean = 4.51 [SD = 0.32], range 3.51–5.83) scores followed a Gaussian distribution (Figure S2 in supporting information).
In total, 652 (13%) participants developed dementia over a mean follow‐up time of 8.4 years (SD = 4.2; median = 8.5; range 1–19 years). The incidence rate of dementia increased with increasing LIBRA (1.1 [95% confidence interval (CI) 0.9; 1.2] per 100 person‐years [PY] in the lowest LIBRA quartile vs 2.3 [2.0; 2.7] per 100 PY in the highest quartile; Table 3). While dementia incidence was higher in participants with higher genetic risk (2.1 [1.8–2.5] vs. 1.4 [1.2–1.5] per 100 PY in APOE ε4 carriers vs. non‐carriers; 1.8 [1.6–2.0] vs. 1.3 [1.1–1.5] per 100 PY in participants with high vs. low GRS tertile, respectively), we found increasing incidence rates of dementia with increasing LIBRA in all categories of genetic risk. For example, in APOE ε4 carriers, incidence rates were 1.4 (95% CI 1.0; 1.9) per 100 PY in the low LIBRA versus 3.3 (2.5; 4.3) per 100 PY in the high LIBRA category.
TABLE 3.
Incidence rates of dementia according to increasing levels of LIfestyle for BRAin health risk (LIBRA) score and genetic risk (N = 5170).
| Overall population | LIBRA score a | |||
|---|---|---|---|---|
| Low | Intermediate | High | ||
| Overall population |
652/5170 1.5 (1.4–1.6) |
132/1383 1.1 (0.9–1.2) |
297/2503 1.4 (1.2–1.6) |
223/1284 2.3 (2.0–2.7) |
| APOE ε4 carrier status | ||||
| Non‐carrier | 479/4131 | 99/1107 | 215/2008 | 165/1016 |
| 1.4 (1.2–1.5) | 1.0 (0.8–1.2) | 1.2 (1.1–1.4) | 2.1 (1.8–2.5) | |
| Carrier | 173/1039 | 33/276 | 82/495 | 58/268 |
| 2.1 (1.8–2.5) | 1.4 (1.0–1.9) | 2.1 (1.6–2.5) | 3.3 (2.5–4.3) | |
| GRS b | ||||
| Low | 187/1724 | 44/494 | 85/817 | 58/413 |
| 1.3 (1.1–1.5) | 1.0 (0.7–1.3) | 1.2 (1–1.5) | 1.9 (1.4–2.4) | |
| Medium | 212/1723 | 41/443 | 96/865 | 75/415 |
| 1.5 (1.3–1.7) | 1.0 (0.7–1.4) | 1.4 (1.1–1.7) | 2.2 (1.7–2.7) | |
| High | 253/1723 | 47/446 | 116/821 | 90/456 |
| 1.8 (1.6–2.0) | 1.2 (0.9–1.6) | 1.6 (1.3–1.9) | 3.0 (2.4–3.7) | |
Abbreviations: APOE, apolipoprotein E; CI, confidence interval; GRS, genetic risk score.
Note: Data are number of incident cases/total and incidence rate per 100 person‐year (95% CI).
Low, intermediate, and high LIBRA risk score categories were defined as the first quartile, second and third quartiles, and fourth quartile of the score, respectively.
Low, medium, and high genetic risk score categories were defined as the first, second, and third tertiles of the score, respectively.
In multivariable‐adjusted analyses, a higher LIBRA was significantly associated with higher dementia risk, with no evidence of effect modification by genetic risk (Figure 1; P for interaction = 0.15 and = 0.57 for the interactions of LIBRA with APOE ε4 and the GRS, respectively). Each 1‐point increase in LIBRA was associated with a 9% increased risk of dementia (95% CI, 5%; 13%) in APOE ε4 non‐carriers and a 15% (95% CI, 8%; 22%) increased risk in carriers (Figure 1A). Similar results were found across GRS levels, adjusted on APOE ε4 status (HR = 1.10 [95% CI, 1.04; 1.16] in the low GRS vs. 1.12 [95% CI, 1.06; 1.18] in the high GRS tertile; Figure 1B). Associations of both LIBRA and GRS with dementia risk were log‐linear in all Cox models.
FIGURE 1.
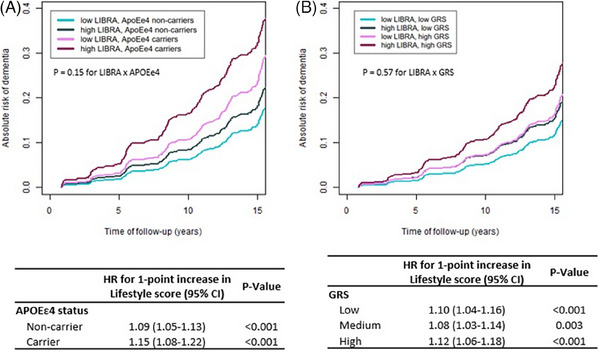
Multivariable‐adjusted absolute risk of dementia during the course of the study and corresponding relative risks (HR)a according to increasing levels of LIBRA score and genetic risk (N = 5170). a Relative risks (HR, as presented in tables) were estimated with cause‐specific Cox models censoring at death. The timescale was the delay between the date of inclusion and either the date of dementia, death, or the end of follow‐up, whichever came first. Absolute risks of dementia (as presented in plots) were estimated from the cause‐specific Cox model while accounting for competing risk by death by specifying a cause‐specific Cox model for death before dementia. All Cox models used baseline hazard rates stratified by study center and included: the LIBRA score (continuous), the genetic factor (APOE ε4 status in panel A, dementia GRS [tertiles] in panel B), the interactions of LIBRA score and the genetic factor, and covariates (age, sex, education level, income, genetic ancestry, and APOE ε4 status in panel B). The P value for LIBRA x GRS interaction test as embedded in (B) plot was estimated in a distinct model considering the GRS continuously. Curves represent the absolute risk of dementia at each time of follow‐up of an average study participant profile (a woman from the Bordeaux site, aged 74 years old at inclusion with educational level equal to middle school and monthly income between 1500 and 2250€). Note that the choice of profile is made to optimize the graphical representation and has no influence on the differences in HR estimated by the model (calculated for each increase of one point of LIBRA taken as a continuous variable). We chose two representative levels of LIBRA score (lower level = upper bound of the first quartile and higher level = lower bound of the fourth quartile), stratified in two levels of genetic factor (APOE ε4 carrier vs. no carrier in [A], and first tertile [low] vs. third tertile of GRS [high]). APOE, apolipoprotein E; CI, confidence interval; GRS, genetic risk score; HR, hazard ratio; LIBRA, LIfestyle for BRAin health risk score
In analyses of cognitive trajectories (Figure 2), results were consistent with those obtained with dementia risk. Each 1‐point increase in the LIBRA was associated with worse initial global cognition and with a steeper annual rate of cognitive decline, both in APOE ε4 carriers and non‐carriers (Figure 2A; P = 0.11 for LIBRA × APOE ε4 and = 0.41 for LIBRA × APOE ε4 × quadratic time function interaction terms), and both in low and high GRS levels (Figure 2B; P = 0.13 and = 0.23 for interaction terms). Among APOE ε4 non‐carriers, the estimated change in global cognition for each additional point of LIBRA was −0.11 (95% CI, −0.12; −0.10) standard units at inclusion, −0.14 (95% CI, −0.14; −0.12) at 7 years, and −0.17 (95% CI, −0.21; −0.13) at 15 years. In APOE ε4 carriers, the estimated change in global cognition for +1 point LIBRA was −0.09 (95% CI, −0.12; −0.06) standard units at inclusion, −0.13 (95% CI, −0.17; −0.09) at 7 years, and −0.16 (95% CI, −0.23; −0.09) at 15 years. Hence, for example, in APOE ε4 carriers, the average cognitive level attained by participants with a high LIBRA (> fourth quartile) after 5 years (69 standard units, see Figure 2A) would be reached approximately 2 years later if they had a low LIBRA (< first quartile) initially. That delay in cognitive aging among those with low (vs. high) LIBRA would extend to 3 years after 10 years of follow‐up. Findings were of similar magnitude in APOE ε4 non‐carriers or within levels of GRS.
FIGURE 2.
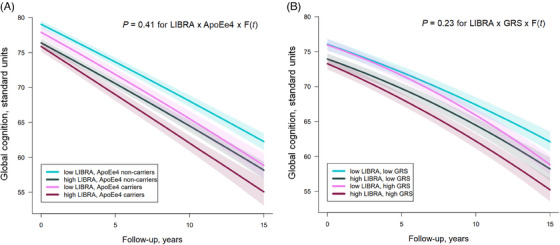
Multivariable‐adjusted mean trajectoriesa of change in global cognition for a specific profile by levels of LIfestyle for BRAin health risk (LIBRA) score at baseline and genetic risk (N = 5170). aEstimated using latent process mixed models for multivariate longitudinal outcomes that modeled repeated measures of five cognitive tests (MMSE, IST, BVRT, TMTA and TMTB) and considered a non‐linear trajectory with time approximated by a quadratic function of time (F[t] = 1, time, time2), with corresponding random effects. The models also included: an intercept representing the cognitive scores at baseline (with corresponding random effects); an indicator for the first cognitive visit; the LIBRA score (continuous), the genetic factor (APOE ε4 carrier status in [A], dementia genetic risk score [GRS, tertiles] in [B]), the interactions of LIBRA score and the genetic factor with the time function, and covariates (study center, age, sex, education level, income, genetic ancestry, and APOE ε4 status in [B]). A quadratic function of time was chosen based on the AIC, which indicated a better fit with quadratic compared to linear evolution ([A]: AIC = 500977.04 [linear]; AIC = 500695.46 [quadratic]; [B]: AIC = 515852.84 [linear]; AIC = 515494.54 [quadratic]). The P value for LIBRA x GRS x F(t) interaction test as embedded in (B) plot was estimated in a distinct model considering the GRS continuously. Curves represent the mean estimated trajectories (solid lines) with 95% CIs (indicated with shading) of an average study participant profile (a woman from the Bordeaux center, aged 74 years old at inclusion with educational level equal to middle school and monthly income between 1500 and 2250€). Note that the choice of profile is made to optimize the graphical representation and has no influence on the differences in trajectories estimated by the model (calculated for each increase of one point of LIBRA taken as a continuous variable). We chose two representative levels of LIBRA score (lower level = upper bound of the first quartile and higher level = lower bound of the fourth quartile), stratified in two levels of genetic factor (APOE ε4 carrier vs. no carrier in [A], and first tertile [low] vs. third tertile of GRS [high]). AIC, Aikaike information criterion; APOE, apolipoprotein E; BVRT, Benton Visual Retention Test; CI, confidence interval; GRS, genetic risk score; IST, Isaacs’ Set Test; LIBRA, LIfestyle for BRAin health risk score; MMSE, Mini‐Mental State Examination; TMT, Trail Making Tests
In supplementary analyses examining effect modification by combined APOE ε4 and GRS, we found no significant LIBRA‐by‐APOE ε4‐by‐GRS interaction on dementia risk (Figure S3 in supporting information). Furthermore, sensitivity analyses examining an alternative LIBRA using study‐specific weights or an alternative GRS from a GWAS based on clinically diagnosed cases, yielded similar results (Table S3, Figures S4 and S5 in supporting information). When limiting the definition of the cardiovascular disease component to heart disease (ignoring hospitalized stroke) to comply with the original LIBRA, or when excluding the n = 81 ε2/ε4 participants, findings were not modified (Pint = 0.17 and = 0.44 for the interaction term with APOE, and GRS, respectively, on the risk of dementia, for the updated LIBRA analysis; and Pint = 0.15 and = 0.90 for interactions when excluding the ε2/ε4 participants). Similarly, imputations of missing LIBRA yielded consistent results.
4. DISCUSSION
In this large cohort of French older persons, an increasing number of modifiable environmental/lifestyle factors for brain health, represented by the LIBRA, was associated with higher dementia risk and greater cognitive decline in any level of genetic susceptibility for dementia. Participants with both low and high genetic risk, based on carrying the APOE ε4 allele and/or scoring high on genetic risk beyond that conferred by the APOE ε4 (as captured by the GRS), had a greater risk of dementia and cognitive decline as the LIBRA increased.
The magnitude of association among the LIBRA, dementia, and cognitive function/decline was slightly stronger in older persons with higher genetic susceptibility compared to those with a low genetic risk, although differences were not statistically significant.
Our findings of independent additive genetic and environmental risks on dementia risk mean that even older persons with a genetic susceptibility for dementia, who are predicted to have worse cognitive trajectories and earlier age at dementia onset, 1 , 2 may be susceptible to the deleterious impact of environmental factors on brain aging. This is in line with the literature on cardiovascular diseases, suggesting that persons at greater genetic risk may be sensitive to prevention by lifestyle modifications to decrease their risk, 33 which is critically important for prevention in these highest risk groups. Although we found suggestion of stronger magnitude of association between the LIBRA and dementia endpoints in those at higher genetic risk, our findings suggest that universal prevention by promotion of a healthy lifestyle, regardless of the genetic status, would be efficient to lower dementia risk. In this perspective, some may consider that genetic risk screening is useless as a toolkit of prevention and should not be implemented as large‐scale public health initiatives. Conversely, one may argue that an apparent independence of environmental risk factors from genetic risk does not preclude from recommending genetic testing, as people at higher genetic risk may be more motivated and prone to adhere to prevention messages. For those at high genetic risk, knowing their genotype at an early disease stage could serve as a strong incentive to manage their risk by adopting preventive behaviors as soon as possible. Moreover, although no interaction emerged as we considered environment/lifestyle and GRSs globally, it remains plausible biologically that some of the diverse modifiable risk factors considered in LIBRA may interact in specific pathways encoded by the multiple genetic variants involved in AD. This could be explored agnostically throughout the entire genome in future research.
Additive effects between genetic and environmental/lifestyle risk on cognitive aging have been found in previous brain aging research, although studies, limited in number and heterogeneous in the way to account for environmental and genetic risk, did not yield consistent findings. Two northern European studies reported effect modification, with associations limited to persons with low genetic risk. Hence in a few hundred participants from the Finnish Cardiovascular Risk Factors, Aging and Dementia study, a higher LIBRA in late life was associated with increased dementia risk among APOE ε4 non‐carriers only. 7 Similarly, in the Rotterdam Study, optimal cardiovascular health level, reflected by a high Life's Simple 7 score (combining diet, body weight, and physical activity at recommended levels and the absence of smoking, hypertension, diabetes, and hypercholesterolemia) was associated with a decreased risk of dementia but only among APOE ε4 non‐carriers or participants with lower GRS. 10 As APOE ε4 is associated with higher mortality, 34 the absence of association among carriers in these studies could be explained by survival and selection bias at older ages, whereby ε4 carriers die prior to study entry or are less likely to be enrolled due to poor health. Conversely, in the Framingham Heart Study offspring and the Atherosclerosis Risk in Communities studies, no interaction was found between the Life's Simple 7 and APOE ε4 carrier status or a GRS on dementia risk. 14 , 35 Likewise, in both the Chicago Health and Aging Project and the China Cognition and Aging Study, associations between a healthy lifestyle score (encompassing diet, smoking, alcohol intake, exercise, and social and cognitive activity) and a slower cognitive decline were found independently of APOE ε4 carrier status. 36 , 37 This was also in accordance with post hoc analyses of two multi‐domain intervention trials, which showed that lifestyle changes were beneficial for cognition in older individuals at vascular risk, even among APOE ε4 carriers. 38 , 39 The largest analysis to date, from the UK Biobank, on a broad four‐component lifestyle score and a polygenic risk score, also found no evidence of interaction with dementia risk. 13 However, because the population was selected toward healthy lifestyles, with approximately two thirds of the sample classified in the favorable lifestyle category, those findings were not generalizable to populations with different cultural and health background.
The primary novelty of our study is its ability to encompass an extensive list of modifiable lifestyle‐related factors, in the form of the LIBRA, in the context of genetic background from a south‐western European population (France). It extends previous studies by investigating comprehensively environment/lifestyle and genetic factors, with a longer follow‐up for dementia and cognition, in‐person repeated cognitive testing, and systematic detection of dementia cases with validation by an independent committee of neurologists over up to two decades. Our study has also limitations. First, the LIBRA was estimated at baseline and data did not allow accounting for potential change during follow‐up. Second, as any long‐term observational study, loss to follow‐up occurred, and participants included were in better health than those excluded owing to missing data; selection of healthy volunteers primarily compromises the generalizability of findings; internal validity could also be compromised in the presence of collider bias, although the direction and magnitude of bias is difficult to predict. Third, gene‐by‐environment interaction studies require sufficient power and despite a fairly reasonable population size (several thousand participants appeared sufficient to detect gene‐by‐environment interactions in previous score‐based research 10 ), we cannot exclude that the null interaction we found is a false negative. However, this is unlikely, as the magnitude of associations of LIBRA to brain aging was relatively consistent in all strata of genetic risk, and associations were also consistent across two brain aging endpoints. Fourth, in spite of the prospective design and the exclusion of prevalent dementia cases at baseline, reverse causality cannot be excluded in this observational study.
In conclusion, our results provide evidence that increasing number of unhealthy environmental/lifestyle risk factors in older age is linearly associated with a higher risk of dementia and greater cognitive decline, in all groups of genetic risk for dementia. This suggests that preventive programs targeting lifestyle‐related factors may benefit all, whatever their genetic susceptibility to dementia. Yet the public health message may be especially important for those with high genetic predisposition, as encouraging these persons to modify some of their unhealthy behaviors and risk factors is likely to provide significant benefit in reducing cognitive aging and dementia.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare. Author disclosures are available in the supporting information.
CONSENT STATEMENT
All participants provided informed consent.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
The Three‐City Study is conducted under a partnership agreement between the Institut National de la Santé et de la Recherche Médicale (INSERM), the Institut de Santé Publique, Epidémiologie et Développement of the University of Bordeaux, and Sanofi‐Aventis. The Fondation pour la Recherche Médicale funded the preparation and initiation of the study. The Three‐City Study is also supported by the Caisse Nationale Maladie des Travailleurs Salariés, Direction Générale de la Santé, Mutuelle Générale de l'Education Nationale, Institut de la Longévité, Regional Governments of Aquitaine and Bourgogne, Fondation de France, Ministry of Research‐INSERM Programme “Cohortes et collections de données biologiques,” French National Research Agency COGINUT ANR‐06‐PNRA‐005 and ANR 2007LVIE 003, the Fondation Plan Alzheimer (FCS 2009‐2012), the Caisse Nationale pour la Solidarité et l'Autonomie (CNSA), and Roche Pharma. The sponsors were not involved in the design of the study or in the data analyses or manuscript elaboration. Jeanne Neuffer is supported by the grant “SilverBrainFood” within the framework of the “Future Investment Program” (Programme d'Investissements d'Avenir PIA3), “Competitiveness cluster structuring projects” (Projets structurants des pôles de compétitivité, PSPC) operated by BPI France. Dr. Maude Wagner is supported by a post‐doctoral fellowship from the French Foundation for Alzheimer's Research (alzheimerrecherche.org).
Neuffer J, Wagner M, Moreno E, et al. Association of LIfestyle for BRAin health risk score (LIBRA) and genetic susceptibility with incident dementia and cognitive decline. Alzheimer's Dement. 2024;20:4250–4259. 10.1002/alz.13801
REFERENCES
- 1. Winblad B, Amouyel P, Andrieu S, et al. Defeating Alzheimer's disease and other dementias: a priority for European science and society. Lancet Neurol. 2016;15:455‐532. doi: 10.1016/S1474-4422(16)00062-4 [DOI] [PubMed] [Google Scholar]
- 2. Bellenguez C, Küçükali F, Jansen IE, et al. New insights into the genetic etiology of Alzheimer's disease and related dementias. Nat Genet. 2022;54:412‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet North Am Ed. 2020;396:413‐446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Deckers K, van Boxtel MPJ, Schiepers OJG, et al. Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observational studies: major risk factors for dementia prevention. Int J Geriatr Psychiatry. 2015;30:234‐246. doi: 10.1002/gps.4245 [DOI] [PubMed] [Google Scholar]
- 5. Schiepers OJG, Köhler S, Deckers K, et al. Lifestyle for Brain Health (LIBRA): a new model for dementia prevention. Int J Geriatr Psychiatry. 2018;33:167‐175. doi: 10.1002/gps.4700 [DOI] [PubMed] [Google Scholar]
- 6. Deckers K, Köhler S, Ngandu T, et al. Quantifying dementia prevention potential in the FINGER randomized controlled trial using the LIBRA prevention index. Alzheimers Dement. 2021;17:1205‐1212. doi: 10.1002/alz.12281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deckers K, Barbera M, Köhler S, et al. Long‐term dementia risk prediction by the LIBRA score: a 30‐year follow‐up of the CAIDE study. Int J Geriatr Psychiatry. 2020;35:195‐203. doi: 10.1002/gps.5235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vos SJB, van Boxtel MPJ, Schiepers OJG, et al. Modifiable risk factors for prevention of dementia in midlife, late life and the oldest‐old: validation of the LIBRA index. JAD. 2017;58:537‐547. doi: 10.3233/JAD-161208 [DOI] [PubMed] [Google Scholar]
- 9. Pons A, LaMonica HM, Mowszowski L. Utility of the LIBRA index in relation to cognitive functioning in a clinical health seeking sample. JAD. 2018;62:373‐384. doi: 10.3233/JAD-170731 [DOI] [PubMed] [Google Scholar]
- 10. Licher S, Ahmad S, Karamujić‐Čomić H, et al. Genetic predisposition, modifiable‐risk‐factor profile and long‐term dementia risk in the general population. Nat Med. 2019;25:1364‐1369. doi: 10.1038/s41591-019-0547-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kivipelto M, Rovio S, Ngandu T, et al. Apolipoprotein E ɛ4 magnifies lifestyle risks for dementia: a population‐based study. J Cell Mol Med. 2008;12:2762‐2771. doi: 10.1111/j.1582-4934.2008.00296.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mulugeta A, Navale SS, Lumsden AL, Llewellyn DJ, Hyppönen E. Healthy lifestyle, genetic risk and brain health: a gene‐environment interaction study in the UK biobank. Nutrients. 2022;14:3907. doi: 10.3390/nu14193907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lourida I, Hannon E, Littlejohns TJ, et al. Association of lifestyle and genetic risk with incidence of dementia. JAMA. 2019;322:430‐437. doi: 10.1001/jama.2019.9879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tin A, Bressler J, Simino J, et al. Genetic risk, midlife life's simple 7, and incident dementia in the atherosclerosis risk in communities study. Neurology n.d.;99:e154‐e163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. the 3C study Group . Vascular factors and risk of dementia: design of the three‐city study and baseline characteristics of the study population. Neuroepidemiology. 2003;22:316‐325. doi: 10.1159/000072920 [DOI] [PubMed] [Google Scholar]
- 16. Kunkle BW, Grenier‐Boley B, Sims R, et al. Genetic meta‐analysis of diagnosed Alzheimer's disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat Genet. 2019;51:414‐430. doi: 10.1038/s41588-019-0358-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, 4th ed. 1994.
- 18. Blacker D, Albert MS, Bassett SS, Go RC, Harrell LE, Folstein MF. Reliability and validity of NINCDS‐ADRDA criteria for Alzheimer's disease. The National Institute of Mental Health Genetics Initiative. Arch Neurol. 1994;51:1198‐1204. doi: 10.1001/archneur.1994.00540240042014 [DOI] [PubMed] [Google Scholar]
- 19. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189‐198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 20. Benton AL. Manuel du test de rétention visuelle: applications cliniques et expérimentales. Editions du Centre de psychologie appliquée; 1953. [Google Scholar]
- 21. Isaacs B, Kennie AT. The set test as an aid to the detection of dementia in old people. Br J Psychiatry. 1973;123:467‐470. doi: 10.1192/bjp.123.4.467 [DOI] [PubMed] [Google Scholar]
- 22. Reitan RM. Validity of the trail making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271‐276. [Google Scholar]
- 23. Samieri C, Perier M‐C, Gaye B, et al. Association of cardiovascular health level in older age with cognitive decline and incident dementia. JAMA. 2018;320:657‐664. doi: 10.1001/jama.2018.11499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Naël V, Pérès K, Dartigues J‐F, et al. Vision loss and 12‐year risk of dementia in older adults: the 3C cohort study. Eur J Epidemiol. 2019;34:141‐152. doi: 10.1007/s10654-018-00478-y [DOI] [PubMed] [Google Scholar]
- 25. Dufouil C, Richard F, Fievet N, et al. APOE genotype, cholesterol level, lipid‐lowering treatment, and dementia: the three‐city study. Neurology. 2005;64:1531‐1538. doi: 10.1212/01.WNL.0000160114.42643.31 [DOI] [PubMed] [Google Scholar]
- 26. Chauhan G, Arnold CR, Chu AY, et al. Identification of additional risk loci for stroke and small vessel disease: a meta‐analysis of genome‐wide association studies. Lancet Neurol. 2016;15:695‐707. doi: 10.1016/S1474-4422(16)00102-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ozenne B, Lyngholm SA, Scheike T, Torp‐Pedersen C, Alexander GT. riskRegression: predicting the risk of an event using cox regression models. R J. 2017;9:440. doi: 10.32614/RJ-2017-062 [DOI] [Google Scholar]
- 28. Eilers PHC, Marx BD. Flexible smoothing with B ‐splines and penalties. Statist Sci. 1996;11:89‐121. doi: 10.1214/ss/1038425655 [DOI] [Google Scholar]
- 29. Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome‐wide association studies. Nat Genet. 2006;38:904‐909. doi: 10.1038/ng1847 [DOI] [PubMed] [Google Scholar]
- 30. Proust‐Lima C, Philipps V, Liquet B. Estimation of extended mixed models using latent classes and latent processes: the R package lcmm. J Stat Soft. 2017;78(2):1‐56. doi: 10.18637/jss.v078.i02 [DOI] [Google Scholar]
- 31. Rubin DB. Multiple imputation for nonresponse in surveys. John Wiley & Sons, Ltd; 1987. doi: 10.1002/9780470316696.fmatter [DOI] [Google Scholar]
- 32. Van Buuren S, Brand JPL, Groothuis‐Oudshoorn CGM, DB Rubin. Fully conditional specification in multivariate imputation. J Stat Comput Simul. 2006;76:1049‐1064. doi: 10.1080/10629360600810434 [DOI] [Google Scholar]
- 33. Khera AV, Emdin CA, Drake I, et al. Genetic risk, adherence to a healthy lifestyle, and risk of coronary artery disease. J Am Coll Cardiol. 2017;69(11):2560. doi: 10.1016/S0735-1097(17)35949-1 [DOI] [Google Scholar]
- 34. Wolters FJ, Yang Q, Biggs ML, et al. The impact of APOE genotype on survival: results of 38,537 participants from six population‐based cohorts (E2‐CHARGE). PLoS One. 2019;14:e0219668. doi: 10.1371/journal.pone.0219668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Peloso GM, Beiser AS, Satizabal CL, et al. Cardiovascular health, genetic risk, and risk of dementia in the Framingham Heart Study. Neurology. 2020;95:e1341‐e1350. doi: 10.1212/WNL.0000000000010306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jia J, Zhao T, Liu Z, et al. Association between healthy lifestyle and memory decline in older adults: 10 year, population based, prospective cohort study. BMJ. 2023;380:e072691. doi: 10.1136/bmj-2022-072691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dhana K, Aggarwal NT, Rajan KB, Barnes LL, Evans DA, Morris MC. Impact of the apolipoprotein E ε4 allele on the relationship between healthy lifestyle and cognitive decline: a population‐based study. AJE. 2021;190:1225‐1233. doi: 10.1093/aje/kwab033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Solomon A, Turunen H, Ngandu T, et al. Effect of the Apolipoprotein E genotype on cognitive change during a multidomain lifestyle intervention: a subgroup analysis of a randomized clinical trial. JAMA Neurol. 2018;75:462. doi: 10.1001/jamaneurol.2017.4365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Andrieu S, Guyonnet S, Coley N, et al. Effect of long‐term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo‐controlled trial. Lancet Neurol. 2017;16:377‐389. doi: 10.1016/S1474-4422(17)30040-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


