Abstract
INTRODUCTION
We examined the efficacy of a multidomain intervention in preventing cognitive decline among Japanese older adults with mild cognitive impairment (MCI).
METHODS
Participants aged 65‐85 years with MCI were randomized into intervention (management of vascular risk factors, exercise, nutritional counseling, and cognitive training) and control groups. The primary outcome was changes in the cognitive composite score over a period of 18 months.
RESULTS
Of 531 participants, 406 completed the trial. The between‐group difference in composite score changes was 0.047 (95% CI: −0.029 to 0.124). Secondary analyses indicated positive impacts of interventions on several secondary health outcomes. The interventions appeared to be particularly effective for individuals with high attendance during exercise sessions and those with the apolipoprotein E ε4 allele and elevated plasma glial fibrillary acidic protein levels.
DISCUSSION
The multidomain intervention showed no efficacy in preventing cognitive decline. Further research on more efficient strategies and suitable target populations is required.
Highlights
This trial evaluated the efficacy of multidomain intervention in individuals with MCI.
The trial did not show a significant difference in preplanned cognitive outcomes.
Interventions had positive effects on a wide range of secondary health outcomes.
Those with adequate adherence or high risk of dementia benefited from interventions.
Keywords: cognitive decline, cognitive training, dementia, diet, mild cognitive impairment, multidomain intervention, nutrition, physical exercise, randomized control trial
1. BACKGROUND
Global estimates show that around 46.8 million people were living with dementia in 2015, a figure expected to triple by 2050. 1 Dementia is becoming a serious public health problem that not only impacts the quality of life of individuals with dementia but also affects their caregivers and society given the rising costs of health and social care services. 2 Although disease‐modifying drugs for Alzheimer's disease (AD) have recently obtained accelerated approval, 3 dementia mechanisms involve multiple factors, including AD pathology. Moreover, several modifiable risk factors contributing to approximately 40% of global dementia cases have been identified. 4 Consequently, targeting multiple risk factors and mechanisms simultaneously may be essential to achieve an optimal preventive effect.
To date, several large randomized controlled trials, 5 including the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) trial, 6 have shown the efficacy of multidomain interventions targeting multiple modifiable risk factors simultaneously in the general population, especially in those at high risk for dementia, although the FINGER trial demonstrated a small effect size (Cohen's d = 0.127). 6 Moreover, a recent systematic review and meta‐analysis 7 concluded that multidomain interventions exerted limited beneficial effects on cognitive function, with such effects being stronger among populations at increased genetic risk for dementia, for instance, people with the apolipoprotein E (APOE) ε4 allele. However, further studies are still needed to develop more effective preventive strategies and identify effective target populations more likely to respond to multidomain interventions. The World‐Wide FINGERS Network was established in 2017. 5 This network facilitates the harmonization of research methodologies across randomized controlled trials, while emphasizing the importance of customizing interventions to suit different populations and environments. 5
The Japan‐Multimodal Intervention Trial for the Prevention of Dementia (J‐MINT) aimed to verify whether a multidomain intervention that involved the management of vascular risk factors, group‐based physical exercise and physical activity self‐monitoring, nutritional counseling, and cognitive training could prevent the progression of cognitive decline among Japanese older adults with mild cognitive impairment (MCI), which was operationally defined based on age, education, and neuropsychological test results. 8
2. METHODS
2.1. Study design
J‐MINT is an 18‐month, multicenter, randomized controlled trial conducted at five Japanese institutions. All study procedures were reviewed and approved by the Institutional Review Boards of all institutions. The study protocol has been published elsewhere. 8 This trial followed the Consolidated Standards of Reporting Trials guideline. 9
2.2. Participants
The J‐MINT recruited participants aged 65‐85 years who had age‐ and education‐adjusted cognitive decline with a standard deviation (SD) of 1.0 or more from the reference threshold for one or more of the four cognitive domains, namely, memory, attention, executive function, and processing speed, from hospitals, memory clinics, and/or community‐based cohorts. Participants who needed to preclude and/or restrict physical exercise were excluded. All inclusion and exclusion criteria are shown in the supplemental methods (Appendix pages 4 and 5).
All participants received a full explanation regarding the purpose, nature, and potential risks of this trial and provided written informed consent before participation.
2.3. Interventions
Participants in both groups were treated for diabetes, hypertension, and dyslipidemia according to relevant clinical practice guidelines in Japan. 8 Moreover, participants in the control group received general health‐related information in writing every 2 months. 8
The intervention group further received intervention programs, which included group‐based physical exercises, nutritional counseling, and cognitive training.
Participants were encouraged to engage in group‐based physical exercise sessions lasting 90 min once a week for 78 sessions. Exercise sessions included muscle stretching, muscle strength training, aerobic exercise, dual‐task training, and group meetings. 8 During the group meetings, health‐related information was provided to promote healthy behaviors, and participants were encouraged to discuss their physical, social, and cognitive activities among themselves. Participants were advised to monitor their daily steps, heart rate (HR), and sleep using an iPad Air tablet computer synchronized to their Fitbit Inspire HR activity monitor. During the coronavirus disease‐2019 (COVID‐19) pandemic, group‐based physical exercise sessions were restricted in accordance with the declaration of a state of emergency by the government. Under such circumstances, online intervention programs were provided for physical exercise. 10
Individual nutritional counseling was offered by qualified health consultants. Participants were scheduled to receive face‐to‐face counseling (60 min per session) once and telephone counseling four times over 6 months, for a total of three and 12 face‐to‐face and telephone counseling sessions over the 18‐month intervention period, respectively. For the first 6 months, nutritional counseling focused on improving lifestyle and dietary behavior. From the next 7‐18 months, nutritional counseling included guidance on dietary intake required to improve cognitive and physical condition and guidance on chewing and swallowing function and oral care. Moreover, participants were instructed to maintain a well‐balanced diet, increase dietary diversity, and increase intakes of fish and seafood, milk and dairy products, fruits, vegetables, and green tea. 8
Participants were encouraged to engage in cognitive training individually using the Brain HQ, 8 which was customized for the J‐MINT trial. Over the 18‐month intervention period, participants were instructed to engage in intensive training lasting at least 30 min per day for at least 4 days per week during the 4th to 6th, 10th to 12th, and 16th to 18th months (intensive training periods).
RESEARCH IN CONTEXT
Systematic review: We conducted a search on PubMed for randomized controlled trials investigating multidomain interventions for preventing cognitive decline. The efficacy of multidomain interventions is inconclusive. Further studies are needed to develop effective preventive strategies and verify target populations likely to respond well to interventions.
Interpretation: While the multidomain intervention did not demonstrate overall efficacy in preventing cognitive decline, subanalyses indicated that consistent adherence to interventions yielded beneficial effects on cognitive function and secondary health outcomes. Moreover, subgroup analyses indicated that multidomain interventions might be particularly advantageous for older adults with the apolipoprotein E ε4 allele and elevated plasma glial fibrillary acidic protein levels, although these findings require validation in other studies.
Future directions: Our trial underscores the potential of adequate adherence to multidomain intervention in preventing cognitive decline. Further research is necessary to identify more effective preventive strategies and target populations that are more responsive to multidomain interventions.
2.4. Outcomes
The primary outcome was the change from baseline in a global composite score combining several neuropsychological tests at the 18‐month follow‐up. Neuropsychological tests in this trial included the Mini–Mental State Examination (MMSE) 11 for global cognitive function; the Logical Memory I and II subset of the Wechsler Memory Scale‐Revised (WMS‐R) 12 ; the Free and Cued Selective Reminding Test (FCSRT) 13 for memory function; Digit Span of the Wechsler Adult Intelligence Scale (WAIS)‐III 14 ; Trail Making Test (TMT) 14 ; Digit Symbol Substitution Test (DSST) subset of the WAIS‐III 14 ; and letter word fluency test 15 for executive function/processing speed. The composite score was generated by averaging the Z‐scores for each neuropsychological test standardized by the baseline mean and SD for each test in the full‐analysis set (FAS). When calculating the composite score, inverse Z‐scores were used for the TMT given that a lower score for this test indicates better function.
Secondary outcomes associated with cognitive changes included changes in the global composite score from baseline at the 6‐ and 12‐month follow‐ups, changes in the scores for each neuropsychological test from baseline at the 6‐, 12‐, and 18‐month follow‐ups, and incident dementia. Other secondary outcomes included changes in each component of the comprehensive geriatric assessment (basic and instrumental activities of daily living, frailty status, dietary diversity, nutritional status, appetite, depressive symptoms, fall risk, social network, health‐related quality of life, sleep quality, social participation, handicap from hearing loss, anthropometric measurements, and physical performance) from baseline at the 6‐ and 18‐month follow‐ups. Details regarding secondary outcome measurements are provided in the supplemental methods (Appendix pages 5 to 7). All outcome measurements were centrally collected using the electronic data capture (EDC) system.
2.5. Sample size
Given the lack of multidomain intervention trials using our global composite score consisting of several neuropsychological tests, our sample size calculation was based on a previous randomized controlled trial that evaluated the effects of an exercise program combined with physical and cognitive tasks in 308 older adults with MCI confirmed to have age‐adjusted cognitive decline. 16 In this previous trial, the intervention group showed a significantly greater change in the MMSE score than the control group (intervention group vs control group, 0.00 ± 2.48 vs −0.80 ± 2.48) after the 40‐week trial. Based on that previous trial, we hypothesized that the present study would also be likely to detect a difference in the change in cognitive function between the intervention and control groups. Using a two‐sided significance level of 5% and a statistical power of 80%, the total sample size required by the t‐test was calculated to be 302. In addition, after accounting for a dropout rate of 40% at the final follow‐up in each trial site, the total required sample size was estimated to be 500 participants.
2.6. Randomization and masking
Participants were centrally randomized at a 1:1 allocation ratio into a multidomain intervention and control group using the dynamic allocation method via stratification according to the following variables: (1) age at enrollment (65 to 74 years vs 75 to 85 years); (2) sex (female vs male); (3) MMSE score (24 to 27 vs 28 to 30); and (4) presence of memory impairment (amnestic vs non‐amnestic). Participants were randomized electronically via a web‐based system constructed by a trial statistician (FK) and a co‐investigator (AH). Research staff members assessing the primary outcome data were blinded to the group assignment and were not involved in the intervention programs. However, research staff members providing the intervention programs and participants were not blinded to the group allocation.
2.7. Statistical methods
2.7.1. Analysis set
The following four analysis sets were used in this trial: (1) the intention‐to‐treat (ITT) analysis set, which included all subjects randomized regardless of whether they received the intervention programs or general health instruction; (2) the FAS, which included subjects who received the intervention program or general health instruction at least once and had at least one postbaseline neuropsychological assessment; (3) the per‐protocol set (PPS), which included subjects who received the intervention program or general health instruction for 18 months; and (4) the safety analysis set (SAS), which included subjects who received the intervention program or general health instruction at least once.
2.7.2. Analysis of primary and secondary outcomes
The primary and secondary efficacy analyses were performed using the FAS. To evaluate differences in cognitive changes from baseline at the 18‐month follow‐up between the intervention and control groups, the mixed‐effects model for repeated measures (MMRM) with an unstructured covariance structure was used with group, time of visit, group‐by‐time interaction, age at randomization (65 to 74 years, 75 to 85 years), sex (male, female), presence of memory impairment at randomization (amnestic, non‐amnestic), and baseline composite cognitive score as covariates. Cohen's d was computed to estimate effect size. For the primary outcome, analyses using the ITT and PPS were also performed. In the ITT analysis, missing values were imputed using fully conditional specification methods with 500 imputations. Imputation was performed using baseline characteristics, including age at enrollment, sex, presence of memory impairment, group, and composite cognitive score at baseline and each follow‐up. In the secondary continuous variables, the same analyses were performed using the MMRM; however, the MMSE was added at randomization as a covariate for endpoints other than MMSE. For the secondary categorical variables, generalized estimating equation models with a logistic link function were used, in which we used an unstructured correlation. Covariates were the same as that used when analyzing continuous variables. Frequencies of adverse events in the intervention and control groups were summarized.
2.7.3. Prespecified subanalyses
The subanalysis on the FAS according to the adherence rate to group‐based physical exercise sessions (≥70%, <70%, and control group) was performed using the MMRM with the same covariates mentioned earlier.
Prespecified subgroup analyses on the FAS were also performed using the MMRM with the same covariates mentioned earlier, years of education (≤9 vs >9 years), threshold of age‐ and education‐adjusted cognitive decline (1.0 to <1.5 SD vs ≥1.5 SD), presence of memory impairment (amnestic vs non‐amnestic), APOE phenotype (APOE ε4 carrier vs APOE ε4 non‐carrier), and dementia‐related plasma biomarkers (amyloid beta [Aβ] composite biomarker, tau phosphorylated at threonine 181 [p‐tau181], neurofilament light chain [NfL], and glial fibrillary acidic protein [GFAP]). Aβ composite biomarker was assessed using the immunoprecipitation‐mass spectrometry assay at Shimadzu Techno Research. Aβ composite values were computed by combining normalized scores of Aβ precursor protein 669‐711/Aβ 1‐42 and Aβ 1‐40/Aβ 1‐42 using a reported cutoff value of 0.376, as suggested previously. 17 Other biomarkers, such as p‐tau181, NfL, and GFAP, were measured using the single‐molecule array (SimoaTM) platform. Cutoff values for these three biomarkers were determined by referring to “normal datasets,” which included as subjects those who satisfied the following inclusion criteria from another cohort study (named SD‐BATON) conducted at the National Center for Geriatrics and Gerontology independently of the J‐MINT: (1) aged 65 to 85 years, (2) cognitively unimpaired (Clinical Dementia Rating = 0), and (3) negative visual interpretation on Aβ‐PET imaging. Cutoff values for these three biomarkers were as follows: p‐tau181, 2.222 pg/mL; NfL, 23.594 pg/mL; and GFAP, 278.105 pg/mL. Details regarding the calculations are presented in the supplemental methods (Appendix pages 7 and 8).
2.7.4. Post hoc subanalyses
A post hoc subgroup analysis according to age at enrollment (65 to 74 vs 75 to 85 years) was performed. To investigate variations in domain‐specific cognitive changes – specifically in the memory, executive functioning, and processing speed domains – between the intervention and control groups in FAS, we conducted the same analyses using MMRM. The memory domain comprised the FCSRT and logical memory tests, while the executive function domain encompassed TMT (shifting part B–part A), digit span, and letter word fluency test. Additionally, the processing speed domain included DSST and TMT part A. Furthermore, post hoc analysis based on cognitive training adherence was also performed. During the intensive training period for a total of 9 months, participants were instructed to perform cognitive training lasting at least 30 min per day for ≥4 days per week. Therefore, those who engaged in cognitive training lasting ≥30 min per day for >156 days during the 18‐month intervention period were included in the adherent intervention group (≥156 days, <156 days, and control group).
All statistical analyses were performed by a study statistician (FK) SAS (SAS Institute, Inc., Cary, NC, USA), with p values of <0.05 indicating statistical significance. This trial was registered with the University Hospital Medical Information Network Clinical Trials Registry as No. UMIN000038671 (https://upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno = R000044075).
2.8. Monitoring
On‐site monitoring was conducted at each institution to ensure that patient rights were protected, the reported data were accurate, and the execution of the trial was compliant with the approved protocol. The monitor ensured that (1) written informed consent was obtained from all participants before their participation in this trial, (2) primary outcome data reported in the EDC were complete and accurate, and (3) all serious adverse events were reported appropriately.
3. RESULTS
3.1. Participant characteristics
Between November 26, 2019 and December 28, 2020, 1677 participants were screened, among whom 531 were randomly assigned to the multidomain intervention (n = 265) or control group (n = 266; Figure 1). Eventually, 406 (76%) participants completed the neuropsychological tests at the 18‐month follow‐up. Dropout rates were similar between the intervention (59 participants, 22%) and control (66 participants, 25%) groups (p = 0.147). The FAS included 433 participants (82% of all randomized participants) after excluding 81 participants who withdrew immediately after randomization and 17 participants without any postbaseline assessment of neuropsychological tests. The baseline characteristics of the 433 participants included in the FAS were well balanced (Table 1).
FIGURE 1.
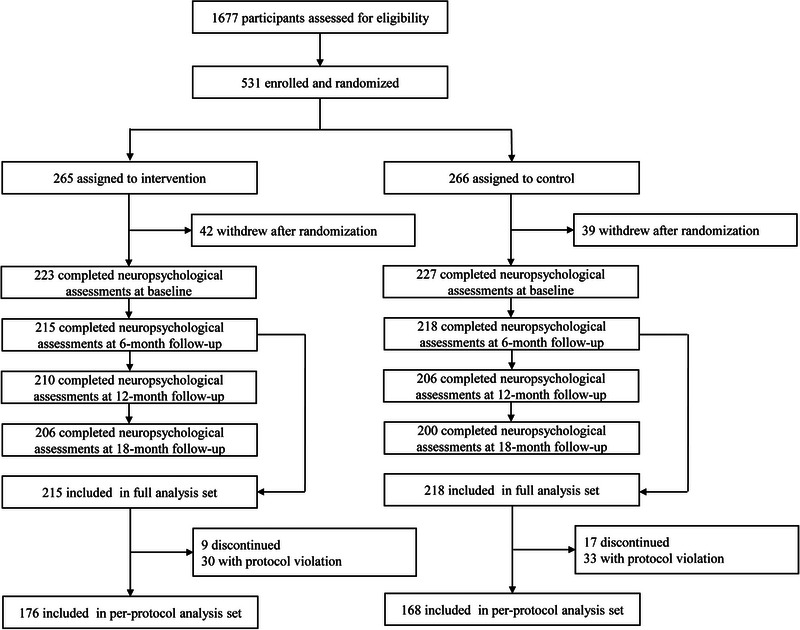
Flowchart for study inclusion and exclusion.
TABLE 1.
Baseline characteristics of participants included in full‐analysis set.
| Intervention group (n = 215) | Control group (n = 218) | |
|---|---|---|
| Sex | ||
| Male | 103 (48%) | 104 (48%) |
| Female | 112 (52%) | 114 (52%) |
| Age, years | 74.3 (5.0) | 74.4 (4.8) |
| Education, years | 12.6 (2.5) | 12.5 (2.4) |
| Barthel Index | 99.4 (2.7) | 99.4 (3.3) |
| Lawton Index | ||
| Male (/5) | 4.8 (0.4) | 4.9 (0.4) |
| Female (/8) | 7.9 (0.3) | 7.9 (0.3) |
| Age‐ and education‐adjusted cognitive decline | ||
| 1.0 to < 1.5 SD | 76 (35%) | 86 (39%) |
| ≥1.5 SD | 139 (65%) | 132 (61%) |
| Presence of memory impairment | 85 (40%) | 85 (39%) |
| APOE ε4 carrier a | 70/212 (33%) | 54/214 (25%) |
| Comorbidities | ||
| Diabetes | 32 (15%) | 36 (17%) |
| Hypertension | 100 (47%) | 100 (46%) |
| Dyslipidemia | 80 (37%) | 77 (35%) |
| Neuropsychological test | ||
| Composite score (mean Z‐score) | −0.02 (0.56) | 0.02 (0.60) |
| MMSE | 27.8 (1.9) | 27.6 (1.8) |
| FCSRT | 44.6 (6.5) | 44.8 (5.3) |
| Logical memory | ||
| Immediate recall | 16.8 (7.5) | 17.4 (7.6) |
| Delayed recall | 11.5 (7.7) | 11.8 (7.8) |
| Digit symbol substitution test | 53.1 (14.3) | 54.7 (16.0) |
| Trail Making Test | ||
| Part A | 60.0 (25.7) | 60.8 (27.1) |
| Part B | 121.4 (62.1) | 116.3 (63.4) |
| Digit span | ||
| Forward | 8.1 (1.9) | 8.3 (2.0) |
| Backward | 5.2 (1.6) | 5.3 (1.8) |
| Letter word fluency test | 9.4 (3.4) | 9.3 (3.3) |
| Dementia‐related plasma biomarkers a | ||
| Aβ (composite biomarkers) | 0.05 (0.72) | −0.09 (0.77) |
| p‐tau181 (pg/mL) | 1.94 (0.89) | 1.97 (1.09) |
| NfL (pg/mL) | 24.44 (11.48) | 23.57 (13.40) |
| GFAP (pg/mL) | 235.01 (171.31) | 220.11 (110.68) |
Note: Data are presented as n (%), n/N (%), or mean (SD).
Abbreviations: Aβ, amyloid beta; APOE, apolipoprotein E; FCSRT, Free and Cued Selective Reminding Test; GFAP, glial fibrillary acidic protein; MMSE, Mini–Mental State Examination; NfL, neurofilament light chain; p‐tau181, tau phosphorylated at threonine 181; SD, standard deviation.
Data are not available for all randomized participants.
3.2. Interventional effects on cognitive outcomes
Intervention effects on cognitive outcome measurements in the FAS are summarized in Table S1. The primary efficacy analysis showed no significant mean difference in the change in composite cognitive score from baseline at the 18‐month follow‐up between the intervention and control groups (mean difference: 0.047; 95% CI −0.029 to 0.124; p = 0.226; Cohen's d = 0.087) (Table S1 and Figure 2A). Analyses of each neuropsychological test revealed that the letter word fluency test scores in the intervention group improved by a mean of 0.827 (95% CI 0.356 to 1.298) points; however, no significant mean difference was observed between the intervention and control groups (mean difference: 0.625; 95% CI −0.039 to 1.289; p = 0.065). Notably, analyses using the PPS and ITT showed similar results (Tables S2 and S3). During the intervention period, 5/207 (2%) and 6/196 (3%) participants in the intervention and control groups progressed to dementia (p = 0.766).
FIGURE 2.

Primary outcomes. (A) Changes in composite score from baseline to 18‐month follow‐up in full‐analysis set. (B) Changes in composite score from baseline to 18‐month follow‐up according to group‐based physical exercise session attendance rates in full‐analysis set. *Mean difference in changes in composite score between adherent intervention and control groups was significant (p < 0.05). †Mean difference in changes in composite score between adherent and non‐adherent intervention groups was significant (p < 0.05).
3.3. Interventional effects on secondary outcomes
Analyses of secondary outcomes showed that the intervention had significant beneficial effects on dietary diversity, number of participating groups, handicap from hearing loss, body mass index, fat‐free mass, and systolic blood pressure values at the 18‐month follow‐up (Tables S4 and S5).
3.4. Subanalyses
3.4.1. Analysis according to the adherence rate to group‐based physical exercise sessions
The individual mean number of exercise session attendance was 64.9 ± 15.8 out of 78 sessions (83%). Subanalysis based on attendance rate of group‐based physical exercise sessions (≥70%, <70%, and control group) showed that the adherent intervention group (≥70%) exhibited a significant interventional effect compared to the non‐adherent intervention group (mean difference: 0.233; 95% CI 0.079 to 0.388; p = 0.003; Cohen's d = 0.540) and control group (mean difference: 0.080; 95% CI 0.001 to 0.159; p = 0.047; Cohen's d = 0.160) (Table S6 and Figure 2B). Analyses of secondary outcomes showed significant differences in changes in dietary diversity, body mass index, body composition (fat mass and fat‐free mass), physical performance (gait speed and five times sit‐to‐stand test), and incident physical frailty between the adherent intervention and control groups (Tables S7 and S8).
3.4.2. Subgroup analyses
Subgroup analyses according to the threshold of age‐ and education‐adjusted cognitive decline indicated that participants with cognitive deficits of ≥1.5 SD below the reference threshold might likely benefit from multidomain interventions; however, mean differences between the intervention and control groups were not significant (mean difference: 0.088; 95% CI −0.012 to 0.187; p = 0.084) (Table S9).
Subgroup analyses showed that a significant interventional effect on the cognitive composite score among older adults with the APOE ε4 allele (mean difference: 0.164; 95% CI 0.011 to 0.317; p = 0.035; Cohen's d = 0.377) (Table S9 and Figure 3).
FIGURE 3.
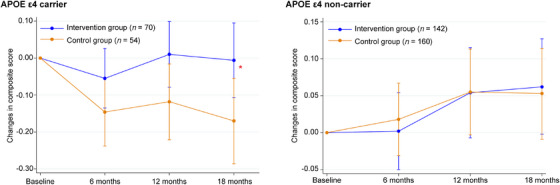
Changes in composite score from baseline at 18‐month follow‐up according to apolipoprotein E status in full‐analysis set. *Mean difference in changes in composite score between intervention and control groups was significant (p < 0.05). APOE, apolipoprotein E.
The results of subgroup analyses according to dementia‐related plasma biomarkers are depicted in Figure 4. Participants with lower values for the four biomarkers generally showed an increase in cognitive composite scores after 18 months, while those with higher values showed stable or decreased cognitive composite scores. The intervention and control groups showed no significant intergroup differences in the analyses according to Aβ, p‐tau181, or NfL (Table S9 and Figure 4). However, a significant intervention effect was observed among older adults with GFAP levels of ≥278.105 pg/mL (mean difference: 0.231; 95% CI 0.009 to 0.453; p = 0.042; Cohen's d = 0.428).
FIGURE 4.
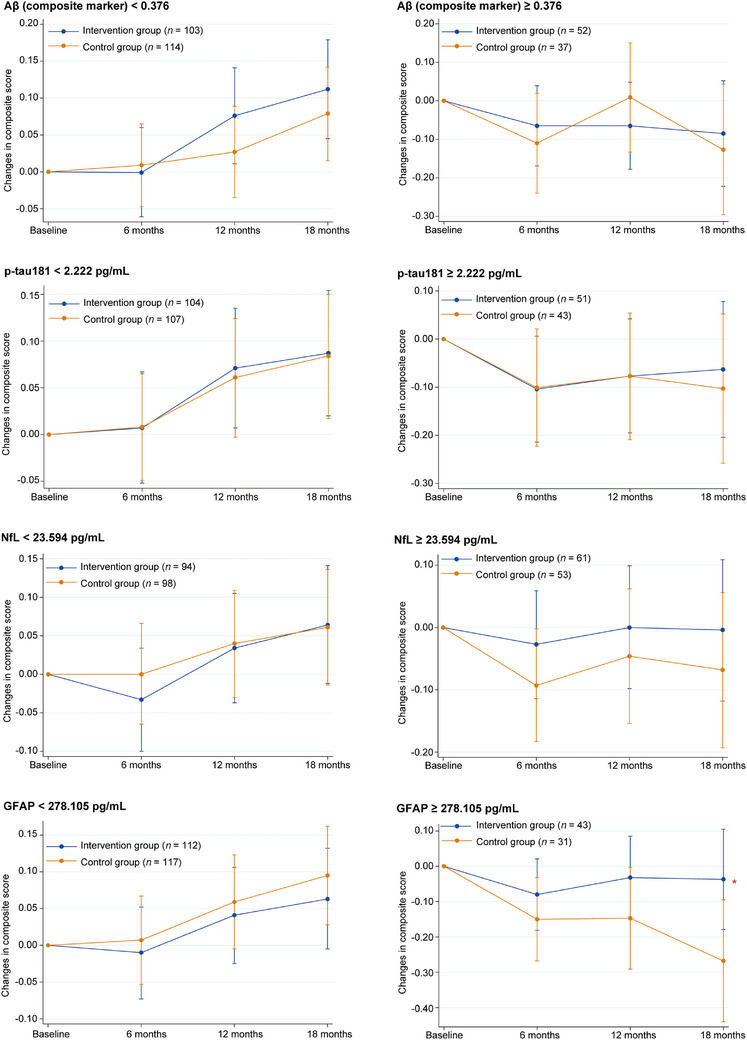
Changes in composite score from baseline at 18‐month follow‐up according to dementia‐related plasma biomarkers in full‐analysis set. *Mean difference in changes in composite score between intervention and control groups was significant (p < 0.05). Aβ, amyloid beta; GFAP, glial fibrillary acidic protein; NfL, neurofilament light chain; p‐tau181, tau phosphorylated at threonine 181.
3.5. Post hoc analyses
3.5.1. Subgroup analyses based on age at enrollment
Subgroup analyses according to age at enrollment showed that the young older adults (age 65 to 74 years) in the intervention group showed improvements in cognition (Table S9 and Figure S1). This was despite the fact that the mean differences between the intervention and control groups were not significant (mean difference: 0.096; 95% CI −0.006 to 0.197; p = 0.066).
3.5.2. Intervention effects on domain‐specific cognitive function
Table S10 displays the impact of the intervention on domain‐specific cognitive function. No significant effects were detected on any domain‐specific cognitive outcomes; nonetheless, the executive function domain showed potential susceptibility to intervention (mean difference: 0.088; 95% CI −0.007 to 0.183; p = 0.068).
3.5.3. Analysis according to the adherence of cognitive training
The intervention group engaged in ≥30 min of cognitive training per day for a mean of 69.2 ± 96.9 days. Only 38 participants (18%) in the intervention group engaged in ≥30 min of cognitive training per day for ≥156 days. The adherent intervention group (≥156 days) showed a significant interventional effect compared with the non‐adherent intervention group (mean difference: 0.245; 95% CI: 0.105 to 0.384; p = 0.001; Cohen's d = 0.628) and control group (mean difference: 0.248; 95% CI: 0.111 to 0.385; p < 0.001; Cohen's d = 0.611) at the 18‐month follow‐up (Table S11 and Figure S2).
3.6. Adverse events
Table 2 summarizes all adverse events. In the SAS (n = 447), 83 (37%) and 65 participants (29%) in the intervention and control groups reported at least one adverse event, respectively.
TABLE 2.
Adverse events in safety analysis set during trial.
| Intervention group (n = 223) | Control group (n = 224) | |
|---|---|---|
| All adverse events | 128 | 90 |
| At least one serious adverse event | 83 (37%) | 65 (29%) |
| Blood and lymphatic system disorders | 0 (0%) | 1 (<1%) |
| Cardiac disorders | 7 (3%) | 4 (2%) |
| Ear and labyrinth disorders | 3 (1%) | 0 (0%) |
| Endocrine disorders | 1 (<1%) | 0 (0%) |
| Eye disorders | 15 (7%) | 17 (8%) |
| Gastrointestinal disorders | 7 (3%) | 7 (3%) |
| General disorders and administration site conditions | 0 (0%) | 1 (<1%) |
| Infections and infestations | 8 (4%) | 3 (1%) |
| Injury, poisoning, and procedural complications | 18 (8%) | 15 (7%) |
| Investigations | 4 (2%) | 1 (<1%) |
| Metabolism and nutrition disorders | 3 (1%) | 5 (2%) |
| Musculoskeletal and connective tissue disorders | 13 (6%) | 5 (2%) |
| Neoplasms: benign, malignant, and unspecified | 15 (7%) | 15 (7%) |
| Nervous system disorders | 9 (4%) | 6 (3%) |
| Renal and urinary disorders | 1 (<1%) | 2 (1%) |
| Reproductive system and breast disorders | 0 (0%) | 1 (<1%) |
| Respiratory, thoracic, and mediastinal disorders | 2 (1%) | 2 (1%) |
| Skin and subcutaneous tissue disorders | 2 (1%) | 0 (0%) |
| Vascular disorders | 3 (1%) | 0 (0%) |
| Unspecified | 2 (1%) | 0 (0%) |
4. DISCUSSION
The J‐MINT is the first trial to examine the efficacy of a multidomain intervention for over 18 months in preventing cognitive decline among Japanese older adults with MCI. Despite facing challenges posed by the COVID‐19 pandemic, J‐MINT successfully completed the trial. Nevertheless, the multidomain intervention did not demonstrate significant efficacy in preventing cognitive decline in individuals with MCI.
Several trials have been conducted on multidomain interventions in the past decade, but their results have varied. 18 In the current study, multidomain interventions did not show significant efficacy in preventing cognitive decline, whereas positive effects on a wide range of secondary health outcomes were observed. Additionally, maintaining adequate adherence to physical exercise sessions (≥70%) and cognitive training led to significant improvements in cognition. Our findings align with the results of the recent FINGER trial, indicating that high adherence to a multidomain intervention program can improve cognition across all domains. 19 However, it is important to interpret our results cautiously because the non‐adherent intervention group (physical exercise session) exhibited tendencies toward poorer physical performance, including reduced gait speed and muscle strength, lower health‐related quality of life, and a higher risk of falls (Table S12), which could be associated with cognitive decline. Moreover, participants with comparable conditions placing them at high risk of cognitive decline might have been included in the control group. These considerations, alongside unmeasured variables in the trial, could have confounded the effect of multidomain interventions in the subanalysis.
Furthermore, we observed that the intervention group with high attendance in cognitive training sessions demonstrated cognitive improvements at the 6‐month follow‐up. However, the adherent intervention group (cognitive training) displayed higher cognitive function at baseline, particularly in TMT part B and DSST (Table S13), which could also have confounded the intervention effect, while no difference was noted in physical function. In this study, participants were directed to engage in cognitive training for a minimum of 30 min per day, at least 4 days a week, during the intensive training periods. However, the study's low adherence to cognitive training, which stood at only 18%, presents a significant challenge. One potential explanation for the lower adherence among participants with MCI is the difficulty in using the technology associated with tablet computers. To tackle this issue, it is essential to not only develop age‐friendly digital devices and applications but also provide technological support both before and during the intervention. Furthermore, there is a clear need to develop cognitive training programs that older adults find enjoyable or that can be undertaken collaboratively with peer participants in group settings.
Subgroup analyses showed that multidomain intervention benefits participants with the APOE ε4 allele, which is a genetic risk factor for AD. Among APOE ε4 carriers, those in the control group showed a decrease in their cognition at the 18‐month follow‐up, whereas those in the intervention group were able to maintain their levels of cognition. This result was consistent with the subgroup analysis of the FINGER trial and the MAPT study. They showed that APOE ε4 carriers, but not APOE ε4 non‐carriers, significantly benefited from the interventions. 20 , 21 Therefore, it is believed that multidomain interventions have the potential to delay the progression of cognitive decline among older adults at a greater genetic risk for dementia. Nevertheless, insufficient evidence has been available from intervention trials to discuss and conclude on the efficacy for preventing cognitive decline among older adults at greater genetic risk for dementia.
Notably, investigating the relationship between the interventional effects and brain pathological status related to dementia is of paramount importance. To address this, we analyzed four plasma biomarkers – Aβ composite, p‐tau181, NfL, and GFAP – which are considered to reflect brain pathology for amyloid (A), tau (T), neurodegeneration (N), and neuroinflammation (I), respectively. 22 To the best of our knowledge, no previous studies addressed the efficacy of multidomain interventions according to the levels of plasma “ATNI” biomarkers. The results appeared to be reasonable that subjects with lower values of the four biomarkers (less probable having corresponding brain pathology) generally showed increased cognitive composite score after 18 months, which might reflect a learning effect. However, those with higher biomarker values showed decreased scores (Figure 4).
Because APOE ε4 carriers showed significant interventional effects, participants with higher values of AD‐specific biomarkers – such as Aβ composite 17 and p‐tau181 23 – were expected to also show significant effects. However, the results did not demonstrate such relationships, and only subjects with higher GFAP levels showed a significant effect of the intervention (Figure 4). Reports have shown that plasma GFAP, a biomarker of astroglial activation and astrocytosis, which indicates brain inflammation, was associated with AD pathology and could predict incident dementia. 24 Stocker et al. reported that an elevated plasma GFAP level was associated with clinical AD incidence 9 to 17 years before diagnosis, and it occurred earlier than those for p‐tau181 and NfL (within 9 years). 25 Therefore, we consider that neuroinflammation, especially at earlier stages of the AD continuum, is more susceptible to the effects of the multidomain interventions compared with other pathological status such as amyloid, tau, and neurodegeneration. Further studies are needed to deepen our understanding of the relationship between interventional effects and brain pathological status.
Post hoc subgroup analyses indicated that the intervention might be expected to be beneficial in a relatively young older population at high risk of dementia. Among the multidomain prevention trials designed to prevent cognitive decline or incident dementia with a large sample size, 6 , 21 , 26 , 27 the FINGER trial – which included relatively young older adults aged 60 to 77 years – demonstrated that a multidomain intervention program had significant effects on cognition. 6 However, other trials focusing on older adults aged ≥70 years failed to show a significant intervention effect on primary cognitive outcomes. 21 , 26 , 27 In our stratified analysis according to age, young older adults (65 to 74 years) in the intervention group showed an improvement in their cognition, whereas old older adults (75 to 85 years) showed a decrease in their cognition regardless of allocation (Table S9 and Figure S1). Although the reason remains unclear, our trial suggests that young older adults (65 to 74 years) at high risk of dementia would be an effective target population for preventing cognitive decline. Nonetheless, a recent study involving 172 older adults aged 70 to 89 years, each with at least two dementia risk factors targeted by the intervention, revealed that a 2‐year, personalized, multidomain intervention resulted in enhanced cognitive function. 28 This result implies that initiating interventions is not too late and highlights the potential importance of personalized or tailored interventions among older adults in the late stages of life.
In this trial, the intervention group reported adverse events relatively more frequently than did the control group. This is because adverse events in the intervention group were recorded during the intervention period, whereas those in the control group were recorded only at the 6‐, 12‐, and 18‐month follow‐up periods.
Findings from J‐MINT could be used as a reference for the social implementation of dementia risk reduction in the future. J‐MINT recruited MCI participants from community‐based cohorts and hospitals including memory clinics. 8 Therefore, multidomain interventions can be implemented in community and memory clinic settings. We are currently launching a feasibility trial in some municipalities. While the effect sizes of multidomain interventions may have been characterized as small on both individual and group levels within a short‐term study period, 7 their societal impact could be substantial over the long term. Further long‐term studies examining the societal implications of multidomain interventions would be warranted. In the memory clinic setting, Lecanemab, an anti‐beta‐amyloid monoclonal antibody, was recently approved in Japan. Multidomain intervention can widely cover older adults at high risk of dementia, including those who do not meet eligibility criteria for Lecanemab.
This trial had a few potential limitations. The COVID‐19 pandemic impacted the delivery of interventions, especially group‐based exercise. Some participants canceled their attendance at the group‐based exercise sessions owing to the fear of infection. It is possible that we might have overlooked or underestimated the efficacy of multidomain interventions. In the current study, the control group also received interventions, albeit less intensive, including the management of vascular risk factors and general health‐related information every 2 months. This study design may have posed challenges in detecting a significant effect of multidomain interventions. Finally, as our trial focused on Japanese older adults with MCI, the generalizability of our findings to other populations may be limited.
In conclusion, the J‐MINT did not reveal significant efficacy of multidomain intervention in preventing cognitive decline. Nevertheless, the multidomain intervention exhibited effectiveness across a broad spectrum of secondary health outcomes. Subanalyses indicated that consistent adherence to multidomain interventions could potentially prevent cognitive decline. Moreover, findings from the J‐MINT suggest that multidomain interventions for 18 months may hold particular value for older adults with elevated GFAP levels and those carrying the APOE ε4 allele, although confirmation of these findings is warranted through additional studies. Further research is necessary to identify more effective preventive strategies and target populations that exhibit greater responsiveness to multidomain interventions.
AUTHOR CONTRIBUTIONS
Hidenori Arai and Takashi Sakurai designed the study. Fumie Kinoshita performed statistical analysis. Hiroyasu Akatsu, Yoshinori Fujiwara, Masafumi Kuzuya, Makoto Michikawa, Susumu Ogawa, Hiroyuki Suzuki, Hajime Takechi, and Hiroyuki Umegaki collected the data. Taiki Sugimoto wrote and prepared the manuscript. All authors were involved in the reading and editing of the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit the manuscript for publication. Takashi Sakurai, Taiki Sugimoto, Nanae Matsumoto, and Kazuaki Uchida accessed and verified the data. Hidenori Arai and Takashi Sakurai are guarantors of this work.
CONFLICT OF INTEREST STATEMENT
National Center for Geriatrics and Gerontology (NCGG), the employer of Akinori Nakamura, has filed and registered the following patents: AU 2016322342, AU 2019205010, CN 201680053338.0, EP 16846400.6, EP 19192797.9, IN 201847013746, JP 6467512, and US 15/752498. Akinori Nakamura is the inventor of these patents, but all patent rights have been transferred to NCGG. All other authors declare no competing interests. Author disclosures are available in the Supporting information.
CONSENT STATEMENT
All participants provided written informed consent before participating in the trial.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
We would like to thank all participants, all members of the J‐MINT study group, and on‐site study staff for their efforts in conducting assessments and interventions. We thank the BioBank of the National Center for Geriatrics and Gerontology for providing quality control of the laboratory. We also thank Dr. Naoyuki Sato for performing measurements of the plasma biomarker (p‐tau 181 and NfL). We further thank Dr. Takahiko Tokuda for measurement of the plasma biomarker (GFAP). This work was financially supported by the Japan Agency for Medical Research and Development under Grant JP22de0107002, JP23ae0101077, and JP23dk0207052. The funders had no role in the study design, methods, data collection, analysis, or preparation of the paper.
Collaborators of the J‐MINT
Hiroyasu Akatsu, Takafumi Ando, Yutaka Arahata, Hidenori Arai, Masahiko Bundo, Katsuya Dezaki, Takehiko Doi, Yoshinori Fujiwara, Toshiki Fukuzaki, Ryoichi Hanazawa, Keigo Hinakura, Akihiro Hirakawa, Akihiro Hirashiki, Kentaro Horibe, Ai Iizuka, Tsutomu Inatomi, Takashi Kato, Shuji Kawashima, Ai Kimura, Fumie Kinoshita, Kaori Kinoshita, Nobuyuki Kishino, Izumi Kondo, Manabu Kokubo, Masafumi Kuzuya, Sangyoon Lee, Yasutomo Matsui, Nanae Matsumoto, Makoto Michikawa, Hiroyuki Minami, Hisayuki Miura, Akinori Nakamura, Shumpei Niida, Keiji Nishihara, Kenichiro Nomoto, Susumu Ogawa, Yusuke Okada, Aiko Osawa, Rei Otsuka, Naoki Saji, Takashi Sakurai, Hiroyuki Sato, Kenji Sato, Naoyuki Sato, Akiko Satoh, Daichi Shigemizu, Hiroyuki Shimada, Atsuya Shimizu, Taiki Sugimoto, Hirokazu Suzuki, Hiroko Suzuki, Hiroyuki Suzuki, Keisuke Suzuki, Yusuke Suzuki, Hajime Takechi, Akinori Takeda, Shinya Takeda, Haruhiko Tokuda, Masashi Tsujimoto, Hiroyuki Umegaki, Satomu Wakayama, Yukihiko Washimi, Akiko Yamaoka, Fumihiko Yasuno, and Hiroshi Yoshino.
Sakurai T, Sugimoto T, Akatsu H, et al. Japan‐Multimodal Intervention Trial for the Prevention of Dementia: A randomized controlled trial. Alzheimer's Dement. 2024;20:3918–3930. 10.1002/alz.13838
The clinical trial registration number: This trial was registered with the UMIN‐CTR (UMIN000038671).
REFERENCES
- 1. Prince M, Wimo A, Guerchet M, Ali GC, Wu YT, Prina M. World Alzheimer Report 2015 – The Global Impact of Dementia: Alzheimer's Disease International; 2015.
- 2. 2022 Alzheimer's disease facts and figures. Alzheimers Dement. 2022;18:700‐789. doi: 10.1002/alz.12638 [DOI] [PubMed] [Google Scholar]
- 3. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer's disease. N Engl J Med. 2023;388:9‐21. doi: 10.1056/NEJMoa2212948 [DOI] [PubMed] [Google Scholar]
- 4. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413‐446. doi: 10.1016/S0140-6736(20)30367-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kivipelto M, Mangialasche F, Snyder HM, et al. World‐wide FINGERS network: a global approach to risk reduction and prevention of dementia. Alzheimers Dement. 2020;16:1078‐1094. doi: 10.1002/alz.12123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at‐risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255‐2263. doi: 10.1016/S0140-6736(15)60461-5 [DOI] [PubMed] [Google Scholar]
- 7. Hafdi M, Hoevenaar‐Blom MP, Richard E. Multi‐domain interventions for the prevention of dementia and cognitive decline. Cochrane Database Syst Rev. 2021;11:CD013572. doi: 10.1002/14651858.CD013572.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sugimoto T, Sakurai T, Akatsu H, et al. The Japan‐multimodal intervention trial for prevention of dementia (J‐MINT): the study protocol for an 18‐month, multicenter, randomized, controlled trial. J Prev Alzheimers Dis. 2021;8:465‐476. doi: 10.14283/jpad.2021.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schulz KF, Altman DG, Moher D, CONSORT Group . CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. doi: 10.1186/1741-7015-8-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Röhr S, Arai H, Mangialasche F, et al. Impact of the COVID‐19 pandemic on statistical design and analysis plans for multidomain intervention clinical trials: experience from world‐wide FINGERS. Alzheimers Dement (N Y). 2021;7:e12143. doi: 10.1002/trc2.12143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189‐198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 12. Wechsler D. Wechsler Memory Scale‐Revised. Psychological Corporation; 1981. [Google Scholar]
- 13. Grober E, Buschke H. Genuine memory deficits in dementia. Dev Neuropsychol. 1987;3:13‐36. doi: 10.1080/87565648709540361 [DOI] [Google Scholar]
- 14. Wechsler D. Manual for the Wechsler Adult Intelligence Scale. Psychological Corp; 1955. [Google Scholar]
- 15. Lezak MD, Lezak MD. Neuropsychological Assessment. Oxford University Press; 2004. [Google Scholar]
- 16. Shimada H, Makizako H, Doi T, et al. Effects of combined physical and cognitive exercises on cognition and mobility in patients with mild cognitive impairment: a randomized clinical trial. J Am Med Dir Assoc. 2018;19:584‐591. doi: 10.1016/j.jamda.2017.09.019 [DOI] [PubMed] [Google Scholar]
- 17. Nakamura A, Kaneko N, Villemagne VL, et al. High performance plasma amyloid‐β biomarkers for Alzheimer's disease. Nature. 2018;554:249‐254. doi: 10.1038/nature25456 [DOI] [PubMed] [Google Scholar]
- 18. Solomon A, Stephen R, Altomare D, et al. Multidomain interventions: state‐of‐the‐art and future directions for protocols to implement precision dementia risk reduction. A user manual for Brain Health Services‐part 4 of 6. Alzheimers Res Ther. 2021;13:171. doi: 10.1186/s13195-021-00875-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ngandu T, Lehtisalo J, Korkki S, et al. The effect of adherence on cognition in a multidomain lifestyle intervention (FINGER). Alzheimers Dement. 2022;18:1325‐1334. doi: 10.1002/alz.12492 [DOI] [PubMed] [Google Scholar]
- 20. Solomon A, Turunen H, Ngandu T, et al. Effect of the apolipoprotein E genotype on cognitive change during a multidomain lifestyle intervention: a subgroup analysis of a randomized clinical trial. JAMA Neurol. 2018;75:462‐470. doi: 10.1001/jamaneurol.2017.4365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Andrieu S, Guyonnet S, Coley N, et al. Effect of long‐term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo‐controlled trial. Lancet Neurol. 2017;16:377‐389. doi: 10.1016/S1474-4422(17)30040-6 [DOI] [PubMed] [Google Scholar]
- 22. Hampel H, Hu Y, Cummings J, et al. Blood‐based biomarkers for Alzheimer's disease: current state and future use in a transformed global healthcare landscape. Neuron. 2023;111:2781‐2799. doi: 10.1016/j.neuron.2023.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Janelidze S, Mattsson N, Palmqvist S, et al. Plasma P‐tau181 in Alzheimer's disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer's dementia. Nat Med. 2020;26:379‐386. doi: 10.1038/s41591-020-0755-1 [DOI] [PubMed] [Google Scholar]
- 24. Cicognola C, Janelidze S, Hertze J, et al. Plasma glial fibrillary acidic protein detects Alzheimer pathology and predicts future conversion to Alzheimer dementia in patients with mild cognitive impairment. Alzheimers Res Ther. 2021;13:68. doi: 10.1186/s13195-021-00804-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stocker H, Beyer L, Perna L, et al. Association of plasma biomarkers, p‐tau181, glial fibrillary acidic protein, and neurofilament light, with intermediate and long‐term clinical Alzheimer's disease risk: results from a prospective cohort followed over 17 years. Alzheimers Dement. 2023;19:25‐35. doi: 10.1002/alz.12614 [DOI] [PubMed] [Google Scholar]
- 26. Bischoff‐Ferrari HA, Vellas B, Rizzoli R, et al. Effect of vitamin D supplementation, omega‐3 fatty acid supplementation, or a strength‐training exercise program on clinical outcomes in older adults: the DO‐HEALTH randomized clinical trial. JAMA. 2020;324:1855‐1868. doi: 10.1001/jama.2020.16909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moll van Charante EP, Richard E, Eurelings LS, et al. Effectiveness of a 6‐year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster‐randomised controlled trial. Lancet. 2016;388:797‐805. doi: 10.1016/S0140-6736(16)30950-3 [DOI] [PubMed] [Google Scholar]
- 28. Yaffe K, Vittinghoff E, Dublin S, et al. Effect of personalized risk‐reduction strategies on cognition and dementia risk profile among older adults: the SMARRT randomized clinical trial. JAMA Intern Med. 2024;184:54‐62. doi: 10.1001/jamainternmed.2023.6279 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


