Abstract
Ongoing assessment of patients with Alzheimer's disease (AD) in postapproval studies is important for mapping disease progression and evaluating real‐world treatment effectiveness and safety. However, interpreting outcomes in the real world is challenging owing to variation in data collected across centers and specialties and greater heterogeneity of patients compared with trial participants. Here, we share considerations for observational postapproval studies designed to collect harmonized longitudinal data from individuals with mild cognitive impairment or mild dementia stage of disease who receive therapies targeting the underlying pathological processes of AD in routine practice. This paper considers key study design parameters, including proposed aims and objectives, study populations, approaches to data collection, and measures of cognition, functional abilities, neuropsychiatric status, quality of life, health economics, safety, and drug utilization. Postapproval studies that capture these considerations will be important to provide standardized data on AD treatment effectiveness and safety in real‐world settings.
Keywords: Alzheimer's disease, core outcomes, data collection, population, real‐world evidence, study aims
1. BACKGROUND
Real‐world evidence (RWE) generation is becoming increasingly important to clinicians, regulatory agencies, and professional societies to inform patient access and understand the effectiveness, safety, and appropriate use of therapeutic agents. 1 , 2 RWE can also be used to support regulatory decision making, and the US Food and Drug Administration (FDA) has issued guidance on how RWE can help inform drug approval and support postapproval study requirements; 2 , 3 , 4 real‐world data collection as presented here is one type of Phase IV study in a clinical development program. Registries represent another approach to RWE generation and the prescribing information (PI) for ADUHELM (aducanumab‐avwa) and for LEQEMBI (lecanemab‐irmb) include reference to the Alzheimer's Network for Treatment and Diagnostics (ALZ‐NET) as a voluntary patient registry that collects postmarketing information on treatments for Alzheimer's disease (AD). 5 , 6 , 7 Registries, such as the ALZ‐NET registry, are data collection systems that capture a wide range of long‐term outcome data. 7 , 8 In contrast, both registry studies, such as the Centers for Medicare & Medicaid Services (CMS) National Patient Registry study, and Phase IV observational studies investigate a specific research question that determines the parameters for data collection. 8 , 9 , 10 Both data from registries and postapproval studies can support regulatory decisions, but data from registries are generally more representative of the real‐world population than postapproval studies and are preferable for assessing outcomes in subpopulations not included in Phase III trials; postapproval studies are preferred for assessing the clinical benefit and treatment strategies for novel drugs. 11 Since the US FDA accelerated approval of aducanumab and lecanemab (now traditionally approved) for the treatment of AD, 5 , 6 , 12 , 13 and with other anti‐amyloid beta (Aβ) monoclonal antibodies in clinical development, the focus on RWE generation in AD has intensified, specifically to understand the long‐term safety, effectiveness, and impact of anti‐Aβ monoclonal antibody treatment on patient‐related and healthcare resource utilization outcomes. In addition, Appropriate Use Recommendations for both aducanumab and lecanemab have been recently published to guide the introduction of these therapies into clinical practice. These recommendations highlight the need for careful patient selection, including the presence of amyloid pathology; the exclusion of patients at high risk of developing amyloid‐related imaging abnormalities (ARIA), such as those with cerebrovascular disease; recommendations for apolipoprotein E ε4 (APOE ε4) genotyping to assess ARIA risk; and monitoring of asymptomatic ARIA using serial magnetic resonance imaging. 14 , 15
Although randomized controlled trials (RCTs) are considered the gold‐standard study design for evidence‐based medicine, such studies have shortcomings in delivering generalizable information, as their methods can lack applicability in clinical practice. 1 , 16 , 17 RCTs are typically conducted in academic research settings or specialized trial sites by highly trained clinicians, where extrinsic factors are controlled, regimen adherence monitored, and interventions provided free of charge. 17 , 18 Participant selection can be narrow and homogeneous, which limits the generalizability of results; specific patient populations are not routinely included in RCTs, such as those of advanced age and/or with comorbidities. 1 , 17 , 19 African‐American, Hispanic, and Latino individuals have been historically underrepresented in AD clinical trials despite having a greater risk of developing AD than White individuals, and research has found that many commonly used exclusion criteria (eg, history of cardiovascular disease or diabetes) in AD clinical trials disproportionately affect African American, Hispanic, and Latino individuals. 20 , 21 In addition, treatment and follow‐up periods in RCTs are often short, potentially underestimating long‐term benefits and/or safety signals. 1 , 18
Real‐world studies aim to produce findings that translate research to routine practice, maximize generalizability, explore key treatment‐related factors driving clinical changes, and address considerations about the benefits, risks, and costs of an intervention. 17 RWE can be used to confirm the effectiveness and tolerability of treatments administered in routine conditions and support changes to labels (eg, new drug indications, new efficacy claims, addition of target populations, addition of administration information and patient‐preference data, safety revisions, and additional information on health‐related outcomes). 22 , 23 Owing to the longer duration, real‐world data collection has the potential to demonstrate treatment effects that may not be realized until later in the disease course, beyond the period of observation in a clinical trial. 1 , 18 Furthermore, observational studies may provide opportunities to evaluate larger, more diverse, and less restricted populations compared with clinical trials, with exclusion criteria generally based on safety rather than selecting a homogeneous sample. 1 , 17 , 22
Obtaining RWE requires efficient collection and robust analysis of data across disparate centers (eg, implementing common assessment tools, data quality systems, data exchange, data analysis, pooling of data, and strategies for interpretation across clinics). 8 Gathering data and comparing outcomes of patients with confirmed AD in routine clinical practice can be challenging because cognitive, functional, neuropsychiatric, and quality of life (QoL) outcomes may not be measured consistently. 22 There is also a lack of consensus on optimal clinical assessments for longitudinal observational studies across the disease continuum from mild cognitive impairment to dementia, with global and regional variations in the instruments used at each disease stage, as well as a lack of large historical longitudinal datasets in patients with confirmed anti‐Aβ pathology. Finally, a variety of different healthcare professionals manage patients with AD, including advanced practice providers, family medicine practitioners, internal medicine specialists, neurologists, medical directors of residential facilities, and geriatric psychiatrists, adding further complexity to the issue. As a result of these various factors, RWE in AD can be difficult to interpret across cohorts and has poor transferability across healthcare systems. 22
Given these challenges, a global International Collaboration for Real‐world Evidence in Alzheimer's Disease (ICARE AD) program was created to provide a structure for collecting standardized longitudinal data from eligible patients treated with aducanumab‐avwa in postapproval effectiveness studies. The program will no longer proceed owing to the national policy for coverage released by the CMS stating that anti‐Aβ monoclonal antibodies approved by the FDA through the accelerated approval pathway for AD will be covered by Medicare only for those enrolled in FDA‐ or National Institutes of Health‐approved trials conducted under an investigational new drug application, 24 thereby limiting the use in clinical practice. Despite this, and in light of the CMS confirming that broader coverage is now available for lecanemab following traditional FDA approval, 25 the authors of this paper believe the approach to and rationale behind the design of this program offers valuable learnings for the field as robust RWE in AD remains an unmet need. In addition, this proposed program would augment the current CMS National Patient Registry study as it has a longer follow‐up period (5 years vs 2 years) and collects additional data, such as QoL, lifestyle, and biomarker (for future analysis) data. 9 Data from this program would also complement data derived from the ALZ‐NET registry, which is a general database that collects data from patients receiving any FDA‐approved drug for AD, not specifically anti‐Aβ monoclonal antibodies, and does not collect QoL data. 26 Here we provide considerations for future studies assessing the use of anti‐Aβ monoclonal antibodies in a real‐world setting, with a structure to utilize predefined, consistent assessments that will provide harmonized effectiveness and safety data and patient‐ and caregiver‐reported outcomes.
2. STUDY AIMS
Long‐term clinical, QoL, and safety outcomes, including the incidence of ARIA, will be important to measure in future real‐world AD studies. In addition, tools that provide insights into healthcare resource utilization and AD‐related burden on informants/caregivers are valuable. Given an increasing emphasis placed on biomarkers, the collection of blood (plasma and serum) and optional cerebrospinal fluid for biobanking and potential genetic analysis is encouraged. Collectively, such resources and data could serve as a platform for nested studies aiming to support progress in specific areas of interest related to digital assessments, magnetic resonance imaging and ARIA monitoring, and use of new blood‐based biomarkers.
RESEARCH IN CONTEXT
Systematic review: A literature review highlighted that obtaining and comparing real‐world outcomes for patients with Alzheimer's disease (AD) is difficult owing to variation in data collected across centers.
Interpretation: There is a need to examine the long‐term, real‐world effectiveness and safety of therapies targeting known pathological processes of AD. We provide considerations for observational studies aiming to provide consistent longitudinal data from patients with AD, which will allow comparison of data across regions and transferability across healthcare systems to maximize data interpretability and inform best practices. Collecting data over a longer period and treating a more diverse population compared with registrational Phase III clinical trials will improve generalizability of findings.
Future directions: This paper aims to provide a framework for consistent data collection in real‐world AD registries and studies as additional treatments enter clinical practice.
3. STUDY POPULATIONS
The suggested eligibility criteria in Table 1 allow assessment of a larger and more heterogeneous patient population than is typically included in clinical trials, especially those who are historically underrepresented (eg, individuals who are ethnically/racially/geographically/socioeconomically diverse, have comorbid conditions or atypical disease, or are taking concomitant medications) to ensure that robust data are collected. 1 , 17 , 19 , 22 Expanded study eligibility can also enhance participant enrollment and retention, further driving patient diversity, 27 and ensure that study populations are representative of real‐world patients. 28 , 29
TABLE 1.
Suggested eligibility criteria.
| Standard inclusion criteria |
|---|
|
| Standard exclusion criteria |
|---|
|
Abbreviations: Aβ, amyloid beta; AD, Alzheimer's disease; QoL, quality of life.
4. STUDY OUTCOMES
Recommendations for primary, secondary, tertiary, and exploratory objectives and corresponding endpoints are described in Table 2.
TABLE 2.
Proposed study objectives and endpoints.
| Primary objective | Primary endpoints |
|---|---|
| Evaluate long‐term AD progression in treated patients with early‐stage AD (MCI due to AD or mild AD dementia) as determined by cognitive, functional, and neuropsychiatric measures and to describe the QoL, caregiver burden, and HCRU longitudinally |
Changes in cognition, functional abilities, and neuropsychiatric status as measured during routine visits from baseline through end of study by:
Changes in QoL, caregiver burden, and HCRU captured during routine visits from baseline through end of study by:
|
| Secondary objectives | Secondary endpoints |
|---|---|
| Evaluate incidence of AEs, including SAEs, in treated patients |
|
|
Assess incidence of treatment‐associated ARIA in real‐world practice per label recommendation for safety monitoring Estimate incidence of symptomatic ARIA in real‐world clinical practice Compare incidence of SAEs in patients with and without ARIA |
|
| Evaluate incidence of brain hemorrhage‐treated patients in real‐world clinical practice |
|
| Obtain descriptive statistics on characteristics of treatment population and drug utilization |
|
| Tertiary objective | Tertiary endpoint |
|---|---|
| Generate longitudinal biobank for future AD plasma or serum biomarker research |
|
| Exploratory objectives (where available) | Exploratory endpoints |
|---|---|
|
Evaluate long‐term treatment effects on biomarkers related to AD development or progression Collect samples for future genetic and biomarker research |
|
Abbreviations: AD, Alzheimer's disease; AE, adverse event; A‐IADL‐Q‐SV, Amsterdam Instrumental Activities of Daily Living Questionnaire Short Version; ARIA, amyloid‐related imaging abnormalities; ARIA‐E, amyloid‐related imaging abnormalities due to vasogenic edema; ARIA‐H, amyloid‐related imaging abnormalities due to micro‐hemorrhages, macro‐hemorrhages; CSF, cerebrospinal fluid; FAQ, Functional Activities Questionnaire; GDS‐SF, Geriatric Depression Scale Short Form; HCRU, healthcare resource utilization; MoCA, Montreal Cognitive Assessment; NPI‐Q, Neuropsychiatric Inventory Questionnaire; PGI‐C, Patient Global Impression of Change; PGI‐S, Patient Global Impression of Severity; QDRS‐IV, Quick Dementia Rating System Informant Version; QDRS‐PV, Quick Dementia Rating System Patient Version; QoL, quality of life; QoL‐AD, Quality of Life in Alzheimer's Disease; RUD, Resource Utilization in Dementia; SAE, serious adverse event; SF‐12, 12‐item Short Form Survey; ZBI, Zarit Burden Interview.
The recommended assessment tools were selected in collaboration with an external steering committee of international clinical experts and patient advisory groups, following a review and analysis of instruments utilized in cohorts and registries. The criteria for instrument selection considered sensitivity to assess changes in cognition, functional abilities, and neuropsychiatric status in early‐stage AD (mild cognitive impairment due to AD and mild AD dementia), which were identified through a comprehensive literature search of research analyzing test sensitivity. Further criteria included reliability, validity, ability to comprehensively assess AD clinical manifestations, and availability of existing data for untreated patients. Additionally, the measures need to be feasible for use in routine specialist practice, with appropriate formats for diverse populations, and easier and more rapid administration relative to clinical trial measures, facilitating efficient incorporation into the patient management workflow. Collection of descriptive information on the characteristics of patients with AD was recommended to gain a better understanding of AD, and the inclusion of QoL and health economic measures was intended to support measurement of the benefit/risk profile of future anti‐Aβ monoclonal antibodies entering clinical practice. Further details regarding the rationale for including specific assessment tools are provided in Table 3. An overview of the administration, validity, and limitations of the instruments is given in Data S1.
TABLE 3.
Rationale for suggested assessment tools.
| Assessment tool | Rationale for inclusion |
|---|---|
| Montreal Cognitive Assessment (MoCA) |
|
| Quick Dementia Rating System Informant Version/Patient Version (QDRS‐IV/PV) |
|
| Amsterdam Instrumental Activities of Daily Living Questionnaire Short Version (A‐IADL‐Q‐SV) |
|
| Neuropsychiatric Inventory Questionnaire (NPI‐Q) |
|
| Quality of Life in Alzheimer's Disease (QoL‐AD) |
|
| 12‐item Short‐Form Survey (SF‐12) |
|
| Patient Global Impression of Severity/Change (PGI‐S/C) | |
| Zarit Burden Interview (ZBI) | |
| Resource Utilization in Dementia (RUD) Lite Scale |
Abbreviations: AD, Alzheimer's disease; A‐IADL‐Q, Amsterdam Instrumental Activities of Daily Living Questionnaire; A‐IADL‐Q‐SV, Amsterdam Instrumental Activities of Daily Living Questionnaire Short Version; CDR‐G, Clinical Dementia Rating Global Score; CDR‐SB, Clinical Dementia Rating Scale‐Sum of Boxes; IADL, Instrumental Activities of Daily Living; MMSE, Mini‐Mental State Examination; MoCA, Montreal Cognitive Assessment; NPI, Neuropsychiatric Inventory; NPI‐Q, Neuropsychiatric Inventory Questionnaire; PGI‐C, Patient Global Impression of Change; PGI‐S, Patient Global Impression of Severity; QDRS‐IV, Quick Dementia Rating System Informant Version; QDRS‐PV, Quick Dementia Rating System Patient Version; QoL, quality of life; QOL‐AD, Quality of Life in Alzheimer's Disease; RUD, Resource Utilization in Dementia; SF‐12, 12‐item Short‐Form Survey; SF‐36, 36‐item Short Form Survey; ZBI, Zarit Burden Interview.
Data collected as part of a real‐world study may be compared with matched external control groups (ie, from existing AD cohorts that represent comparators for natural disease progression, historical health insurance claims, or future registries) to characterize real‐world, long‐term changes in cognition, functional abilities, and neuropsychiatric status and evaluate any differences between treated and untreated patients and differences among treatments. 30 RWE may also be compared with data from placebo arms of clinical trials while acknowledging the limitations of existing AD trial cohorts (such as lack of diversity) when interpreting comparisons with these groups. 19 , 21 In this proposed study, including patients eligible for treatment but who decline this could be considered, but patients who decline treatment may be difficult to follow up with, which could result in a misleading and partial dataset for such a comparator group.
In addition to measuring clinical outcomes, collecting blood and cerebrospinal fluid specimens is recommended for future biomarker and genetic studies in AD. Recent data have highlighted potential future applications of biomarkers, including as measures of disease progression, as prescreening tools for identifying patients with Aβ pathology and other AD‐related pathologies, and as screening tools for diagnosis and prognosis of patients to be included in clinical trials. 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 Biomarkers may also have a role in monitoring treatment response by demonstrating target engagement or as a surrogate of clinical benefit, as well as potentially predicting and monitoring side effects, such as ARIA due to vasogenic edema. 32 , 39 , 40 , 41 , 42 Biomarkers are now also being used to inform treatment decisions in clinical practice, with the aducanumab‐avwa and lecanemab‐irmb PIs stating that the presence of Aβ pathology must be confirmed prior to initiating treatment. 5 , 6 However, there remain a lack of consensus on the choice of biomarker assays and cutoffs, an absence of prospectively collected data, and missing validation of key assay performance characteristics, especially in real‐world populations. 31 , 39 , 43 Biobanking of longitudinal samples could enable biomarker substudies to be conducted and generate evidence for the validation, regulatory approval, and adoption of biomarkers as measures of brain AD pathology in patients with cognitive impairment, disease progression, and treatment response. Standardized procedures for sample collection and specimen storage need to be provided by the sponsors of the study.
5. DATA COLLECTION
Study durations of at least 5 years are recommended to ensure adequate observation of longitudinal changes, given that Aβ plaque levels have been found to continue to decline beyond 4 years of treatment with some anti‐Aβ monoclonal antibodies. 5
To improve the diversity of enrolled participants, the FDA recommends accounting for logistical and patient‐related factors that may limit participation in clinical studies, for example, reducing the burden of study participation by minimizing study visits and employing enrollment practices that enhance inclusiveness. 29 As such, study sites should be selected to represent a range of geographical locations and AD care settings (eg, academic centers, community memory clinics) with ethnically, racially, and socioeconomically diverse populations, as well as consideration of their ability to implement the core data elements. Data should typically be collected by trained site personnel as part of a structured interview (clinical assessment tools) or collected prospectively as part of an unstructured interview (medical history). For a real‐world study of this duration, it is recommended that data be gathered during routine visits conducted within the context of standard of care, with intervals for data collection of approximately 6 to 12 months. Self‐administered cognitive assessments, such as the Quick Dementia Rating System Patient Version (QDRS‐PV), which can be completed prior to the physician visit, may reduce study site burden, but they can be associated with some challenges, including reduced response validity (compliance, effort, motivation) because of a lack of monitoring, loss of qualitative data obtained from conventional evaluations, risk of interruptions, and lack of support for the patient if they are struggling to complete the task or experience technological problems. 48 , 68
An overview of the recommended schedule of data collection is shown in Figure 1. A detailed schedule of assessments is listed in Table S1; core data elements are noted to ensure consistency of clinical assessments and demographics collection, with data that may not be collected in routine practice considered optional to minimize study site burden and maximize real‐world relevance.
FIGURE 1.
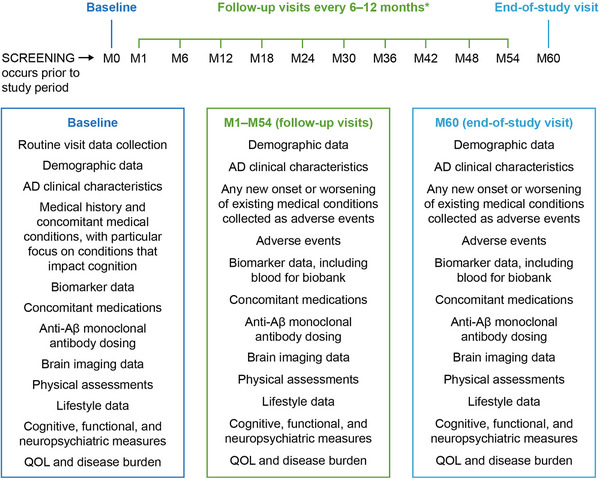
Schedule of data collection. *Study information may be collected at any routine clinical visits scheduled per local standard of care. Data from radiological assessments of MRI scans will be collected at each MRI visit. It is expected that after a patient reaches target dosing, information on each patient will be collected at the physician's discretion or as part of routine clinical practice, anticipated to be approximately every 6 to 12 months. AD, Alzheimer's disease; M, month; MRI, magnetic resonance imaging; QoL, quality of life.
TABLE 4.
Summary of proposed RWE benefits.
| Limitations of RCTs | Benefits of proposed RWE |
|---|---|
| Population diversity | Broader eligibility criteria; inclusion of community healthcare settings; minimal trial participant burden; inclusion of patients underrepresented in clinical trials |
| Study duration | Longer period of observation to evaluate longitudinal changes |
| Generalizability and transferability of data | Robust framework for collecting harmonized data, with core elements across all participating centers for consistent collection and interpretation; consensus on optimal clinical assessment tools |
| Selectivity and sensitivity of instruments | Range of well‐validated measures of cognition, functional abilities, neuropsychiatric status, quality of life, health economics, safety, and drug utilization |
| Healthcare‐related burden evaluation | Inclusion of patient‐ and caregiver‐reported outcomes, QoL, and HCRU measures in real‐world clinical context |
| Real‐world applicability | Clinical assessments tailored to standard‐of‐care clinical practice in varied healthcare settings |
| Data interpretation | Comparison with matched natural history cohorts, health insurance claims, or registries to evaluate differences between treated and untreated patients |
Abbreviations: HCRU, healthcare resource utilization; QoL, quality of life; RCT, randomized controlled trial; RWE, real‐world evidence.
6. LIMITATIONS OF PROPOSED REAL‐WORLD STUDIES
The potential limitations of a real‐world study are that some components may not be typical of clinical practice, namely, trained raters, extensive data and biomarker collection, and the requirement to enter results into a database, all of which may result in greater recruitment from research centers than other clinical settings. Moreover, the practicalities of implementing such a core dataset may be challenging, owing to regional variations in resource availability, differences in clinician specialty and training, the requirement for translation and validation of assessment tools, and reimbursement of AD biomarker investigations. The process of recruitment and consent in such a study may also introduce bias based on comorbidities, language, socioeconomic status, and ethnicity.
7. CONCLUSION
An important objective of the current AD treatment landscape is to monitor patient outcomes and evaluate disease progression. Real‐world studies can be compromised by inconsistent collection of data and use of nonstandardized endpoints, which can limit the generalizability of results. As summarized in Table 4, these considerations for collecting RWE in AD, adapted from the now closed ICARE AD program, may address some of the limitations of RCTs and help ensure consistent, longitudinal data collection across the AD continuum in routine practice to allow for transferability of data across healthcare systems.
AUTHOR CONTRIBUTIONS
All authors participated in the review of the literature and the drafting and review of the manuscript. All authors read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
J.E.G.: Provided consultation to Alpha Cognition, Biogen, Cognition Therapeutics, CND Life Sciences, EIP Pharma, Eisai, Eli Lilly, GE Healthcare, Genentech, Otsuka, and Roche. J.E.G. is the Chief Scientific Officer for Cognivue, the creator of the Quick Dementia Rating System, and holds the copyright with the New York University Grossman School of Medicine. J.E.G. is supported by National Institute on Aging (NIA) grants R01AG071514, R01AG701514S1, R56AG074889, R01AG071643, R01AG069765, R01AG057681, P01AG066584, and P30AG059295 and National Institute of Neurological Disorders and Stroke (NINDS) grants R01NS101483 and R01NS101483S1. J.L.C.: Provided consultation to Acadia, Actinogen, Acumen, AlphaCognition, Aprinoia, AriBio, Artery, Biogen, BioVie, Cassava, Cerecin, Diadem, EIP Pharma, Eisai, GemVax, Genentech, GAP Innovations, Janssen, Jocasta, Karuna, Lilly, Lundbeck, LSP, Merck, NervGen, Novo Nordisk, Oligomerix, Ono, Optoceutics, Otsuka, PRODEO, Prothena, ReMYND, Roche, Sage Therapeutics, Signant Health, Simcere, Sunbird Bio, Suven, SynapseBio, TrueBinding, Vaxxinity, and Wren Therapeutics pharmaceutical, assessment, and investment companies. J.L.C. is supported by National Institute of General Medical Sciences grant P20GM109025; NINDS grant U01NS093334; NIA grants R01AG053798, P20AG068053, P30AG072959, and R35AG71476; Alzheimer's Disease Drug Discovery Foundation (ADDF); Ted and Maria Quirk Endowment; and the Joy Chambers‐Grundy Endowment. M.L.B., C.deM., C.M.G., and M.R.S.: During the development of the study protocol and development of the manuscript, they were employees of Biogen and may hold stock, but have since left the company. I.R. is an employee of Biogen and may hold stock. R.F.A.: Supported by grants from Alzheimer's Association, CONICET (Consejo Nacional de Investigaciones Científicas y Tecnológicas de Argentina), Fleni Foundation, and subcontract funding from NIH (R01AGO53267). R.F.A. has served as a consultant or principal investigator for Bago, Biogen, Merck, Novo‐Nordisk, and Roche. A.A.: Received honoraria for consulting, participated in independent data safety monitoring boards, provided educational lectures, programs, and materials, and served on advisory boards for AbbVie, Acadia, Allergan, Alzheimer's Association, Alzheimer's Disease International (ADI), Axovant, AZ Therapies, Biogen, Eisai, Grifols, Harvard Medical School Graduate Continuing Education, JOMDD, Lundbeck, Merck, Prothena, Roche/Genentech, Novo Nordisk, Qynapse, Sunovion, Suven, and Synexus. A.A. receives royalties from Oxford University Press for a medical book on dementia and has received institutional grants and contract funding from NIA/NIH 1P30AG072980, AZ DHS CTR040636, Washington University St. Louis, Foundation for NIH (FNIH), and Gates Ventures. A. Atri's institution receives funding for clinical trials, biomarker and observational studies, contracts, and projects from AD consortia, foundations, and companies for which A.A. serves as the site principal investigator (past institution received funding for the Biogen EMERGE study; current institution receiving funding for the Eisai‐sponsored AHEAD 3‐45 study). H.C.: Supported by a foundation grant from Canadian Institutes for Health Research (CIHR), along with the Weston Foundation and the Baycrest Health Sciences Foundation. In the past 5 years, H.C. has participated as a site principal investigator in pharmaceutical trial activities sponsored by Alector LLC, Anavex Life Sciences, Hoffmann‐La Roche Limited, Lilly, TauRx, and Immunocal (site investigator for trials). H.C. participated as an unpaid advisor in 2020/21 for the establishment of an international database by Biogen (ICARE AD). C.P.: Member of the International Advisory Boards of Lilly and a consultant for Ads Neuroscience, AgenT, Alzhois, Euroimmune, Fujirebio, Roche, and Gilead. C.P. is an investigator in several clinical trials for AstraZeneca, Biogen, Esai, Lilly, Lundbeck, Neuroimmune, and Roche. V.R.P.: Provided consultation to Biogen and is supported by grants from the National Institutes of Health, Providence St. Joseph Health (Alzheimer's Translational Pillar [ATP]); Pacific Neuroscience Institute Foundation, including the generous support of the Singleton and McLoughlin families, and Saint John's Health Center Foundation. C.W.R.: Provided consultation to AbbVie, Actinogen, Alchemab Therapeutics, Biogen, Brain Health Scotland, Eisai, Lilly, Merck, Novo Nordisk, Roche, Roche Diagnostics, Signant Health, and Sygnature Discovery and is supported by grants from Biogen, AC Immune SA, and Roche. C.W.R. has intellectual property developed at the University of Edinburgh licensed to Linus Health and is Chief Executive Officer and Founder of Scottish Brain Sciences. S.A.M.S.: Supported by grants from Health∼Holland, Top sector Life Sciences & Health (PPP‐allowance; nos. LSHM20084 and LSHM19051), and ZonMW (nos. 7330502051 and 73305095008). Served as a consultant for Boehringer Ingelheim, Lundbeck, Takeda, and Toyama. S.A.M.S. is the developer and receives license fees for the use of the Amsterdam IADL Questionnaire from Alzheon, Axon Neuroscience, Genentech, Green Valley, Janssen, MedAvante, Roche, Vivoryon, and vTv Therapeutics. All funding, consultancy fees, and license fees are paid to S.A.M. Sikkes’ institution. Author disclosures are available in the supporting information.
Supporting information
Supporting Information
Table S1
ICMJE Disclosure Form
ACKNOWLEDGMENTS
The authors would like to thank the following individuals for their contributions to this publication: Sarah McEwen, PhD, of atai Life Sciences, for contributing to the early development of the manuscript while she was an employee of Pacific Brain Health Center, Pacific Neuroscience Institute, Santa Monica, CA, USA and Saint John's Cancer Institute, Santa Monica, CA, USA; Elizabeth Fisher, PhD, of Biogen, for contributing to the early development of the manuscript; Yuval Zabar, MD, of Biogen, for contributing to the later development of the manuscript; Jennifer Mitchell, PhD, and Catherine Sidaway, MBChB, both of Helios Medical Communications, Cheshire, UK, who provided medical writing and editorial support, funded by Biogen. The development of this manuscript was funded by Biogen. Role of the funding source: The sponsor developed the study design for ICARE AD.
Galvin JE, Cummings JL, Benea ML, et al. Generating real‐world evidence in Alzheimer's disease: Considerations for establishing a core dataset. Alzheimer's Dement. 2024;20:4331–4341. 10.1002/alz.13785
James E. Galvin and Jeffrey L. Cummings joint first authors.
REFERENCES
- 1. Nazha B, Yang JC‐H, Owonikoko TK. Benefits and limitations of real‐world evidence: lessons from EGFR mutation‐positive non‐small‐cell lung cancer. Future Oncol. 2021;17:965‐977. doi: 10.2217/fon-2020-0951 [DOI] [PubMed] [Google Scholar]
- 2. U.S. Food & Drug Administration (FDA) . Framework for FDA's real‐world evidence program 2018. (accessed March 8, 2022). https://www.fda.gov/media/120060/download [Google Scholar]
- 3. U.S. Food & Drug Administration (FDA) . Real‐world evidence 2022. (accessed January 27, 2023). https://www.fda.gov/science‐research/science‐and‐research‐special‐topics/real‐world‐evidence [Google Scholar]
- 4. Bonamici S. H.R.34—114th Congress (2015‐2016): 21st Century Cures Act 2016. (accessed March 22, 2023). https://www.congress.gov/bill/114th‐congress/house‐bill/34/
- 5. U.S. Food & Drug Administration (FDA) . ADUHELM: highlights of prescribing information (PI) 2023. (accessed March 19, 2023). https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761178s007lbl.pdf [Google Scholar]
- 6. U.S. Food & Drug Administration (FDA) . LEQEMBI: highlights of prescribing information (PI) 2023. (accessed October 5, 2023). https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761269Orig1s001lbl.pdf [Google Scholar]
- 7. Alzheimer's Network for Treatment and Diagnostics. ALZ‐NET 2023. (accessed March 19, 2023). https://www.alz‐net.org/
- 8. European Medicines Agency (EMA) . Guideline on registry‐based studies 2020. (accessed October 16, 2020). https://www.ema.europa.eu/en/documents/scientific‐guideline/guideline‐registry‐based‐studies_en.pdf [Google Scholar]
- 9. Centers for Medicare and Medicaid Services (CMS) . Prospective study on anti‐amyloid‐β monoclonal antibodies directed against amyloid for the treatment of Alzheimer's disease coverage of evidence development (the Anti‐aβ mAb CED study) 2023. (accessed October 5, 2023). https://www.cms.gov/files/document/ced‐study‐description.pdf [Google Scholar]
- 10. Yang W, Zilov A, Soewondo P, Bech OM, Sekkal F, Home PD. Observational studies: going beyond the boundaries of randomized controlled trials. Diabetes Res Clin Pract. 2010;88(1):S3‐S9. doi: 10.1016/S0168-8227(10)70002-4 [DOI] [PubMed] [Google Scholar]
- 11. Ismail RK, Sikkes NO, Wouters MW, et al. Postapproval trials versus patient registries: comparability of advanced melanoma patients with brain metastases. Melanoma Res. 2021;31:58‐66. doi: 10.1097/CMR.0000000000000707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. U.S. Food & Drug Administration (FDA) . FDA converts novel Alzheimer's disease treatment to traditional approval 2023. (accessed October 5, 2023). https://www.fda.gov/news‐events/press‐announcements/fda‐converts‐novel‐alzheimers‐disease‐treatment‐traditional‐approval [Google Scholar]
- 13. U.S. Food & Drug Administration (FDA) . FDA grants accelerated approval for Alzheimer's drug 2021. (accessed October 5, 2023). https://www.fda.gov/news‐events/press‐announcements/fda‐grants‐accelerated‐approval‐alzheimers‐drug [Google Scholar]
- 14. Cummings J, Rabinovici GD, Atri A, et al. Aducanumab: Appropriate Use Recommendations update. J Prev Alzheimers Dis. 2022;9:221‐230. doi: 10.14283/jpad.2022.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cummings J, Apostolova L, Rabinovici GD, et al. Lecanemab: Appropriate Use Recommendations. J Prev Alzheimers Dis. 2023;10:362‐377. doi: 10.14283/jpad.2023.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spieth PM, Kubasch AS, Penzlin AI, Illigens BM‐W, Barlinn K, Siepmann T. Randomized controlled trials—a matter of design. Neuropsychiatr Dis Treat. 2016;12:1341‐1349. doi: 10.2147/NDT.S101938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Depp C, Lebowitz BD. Clinical trials: bridging the gap between efficacy and effectiveness. Int Rev Psychiatry. 2007;19:531‐539. doi: 10.1080/09540260701563320 [DOI] [PubMed] [Google Scholar]
- 18. Atri A, Rountree SD, Lopez OL, Doody RS. Validity, significance, strengths, limitations, and evidentiary value of real‐world clinical data for combination therapy in Alzheimer's disease: comparison of efficacy and effectiveness studies. Neurodegener Dis. 2012;10:170‐174. doi: 10.1159/000335156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Banzi R, Camaioni P, Tettamanti M, Bertele’ V, Lucca U. Older patients are still under‐represented in clinical trials of Alzheimer's disease. Alzheimers Res Ther. 2016;8:32. doi: 10.1186/s13195-016-0201-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mitchell AK, Massett HA, Shakur M, Lockett J, Han SH. Analysis of exclusion criteria in NIA‐funded Alzheimer's disease and Alzheimer's disease‐related dementias clinical trials. Alzheimers Dement. 2021;17(10):e054416. doi: 10.1002/alz.054416 [DOI] [Google Scholar]
- 21. Franzen S, Smith JE, van den Berg E, et al. Diversity in Alzheimer's disease drug trials: the importance of eligibility criteria. Alzheimers Dement. 2022;18:810‐823. doi: 10.1002/alz.12433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gallacher J, de Reydet de Vulpillieres F, Amzal B, et al. Challenges for optimizing real‐world evidence in Alzheimer's disease: the ROADMAP project. J Alzheimers Dis. 2019;67:495‐501. doi: 10.3233/JAD-180370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tashkin DP, Amin AN, Kerwin EM. Comparing randomized controlled trials and real‐world studies in chronic obstructive pulmonary disease pharmacotherapy. Int J Chron Obstruct Pulmon Dis. 2020;15:1225‐1243. doi: 10.2147/COPD.S244942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoffman M. Biogen terminates Phase 4 ICARE AD trial of aducanumab in Alzheimer's disease 2022. (accessed July 28, 2022). https://www.neurologylive.com/view/biogen‐terminates‐phase‐4‐icare‐ad‐trial‐aducanumab‐alzheimer‐disease [Google Scholar]
- 25. Centers for Medicare and Medicaid Services (CMS) . Statement: broader Medicare coverage of LEQEMBI available following FDA traditional approval 2023. (accessed October 5, 2023). https://www.cms.gov/newsroom/press‐releases/statement‐broader‐medicare‐coverage‐leqembi‐available‐following‐fda‐traditional‐approval [Google Scholar]
- 26. Alzheimer's Association. ALZ‐NET protocol synopsis 2023. (accessed October 5, 2023). https://www.alznetproviders.org/‐/media/ALZNET/Resources/ALZ‐NET‐Protocol‐Synopsis.pdf
- 27. Benbow JH, Rivera DR, Lund JL, Feldman JE, Kim ES. Increasing inclusiveness of patient‐centric clinical evidence generation in oncology: real‐world data and clinical trials. Am Soc Clin Oncol Educ Book. 2022:116‐126. doi: 10.1200/EDBK_350574 [DOI] [PubMed] [Google Scholar]
- 28. U.S. Food & Drug Administration (FDA) . Diversity plans to improve enrollment of participants from underrepresented racial and ethnic populations in clinical trials; draft guidance for industry; availability 2022. (accessed February 6, 2023). https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/diversity‐plans‐improve‐enrollment‐participants‐underrepresented‐racial‐and‐ethnic‐populations [Google Scholar]
- 29. U.S. Food & Drug Administration (FDA) . Enhancing the diversity of clinical trial populations — eligibility criteria, enrollment practices, and trial designs guidance for industry 2020. (accessed February 6, 2023). https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/enhancing‐diversity‐clinical‐trial‐populations‐eligibility‐criteria‐enrollment‐practices‐and‐trial [Google Scholar]
- 30. Liu J, Barrett JS, Leonardi ET, et al. Natural history and real‐world data in rare diseases: applications, limitations, and future perspectives. J Clin Pharmacol. 2022;62(2):S38‐S55. doi: 10.1002/jcph.2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blennow K, Dage J, Bateman R, Hansson O. Recent advances in plasma biomarkers to improve preclinical and prodromal AD trials. Oral presentation at the 14th Clinical Trials on Alzheimer's Disease (CTAD) Annual Meeting, Boston, Massachusetts, USA, November 9‐12, 2021:(Abstract S1). [Google Scholar]
- 32. Sims JR, Lu M, Schade AE, Brooks DA, Mintun MA. TRAILBLAZER‐ALZ: three clinical trials of donanemab in early Alzheimer's disease: plasma P‐tau assays and the initial performance in clinical trials. Oral presentation at the 14th Clinical Trials on Alzheimer's Disease (CTAD) Annual Meeting, Boston, Massachusetts, USA, November 9‐12, 2021:(Abstract S2). [Google Scholar]
- 33. Verbel D, Gee M, Kaplow J, et al. Prediction of brain amyloid pathology using plasma Aβ42/40 ratio measured using the C2N PrecivityADTM test in the Mission AD study samples. Oral presentation at the 14th Clinical Trials on Alzheimer's Disease (CTAD) Annual Meeting, Boston, Massachusetts, USA, November 9‐12, 2021:(Abstract LBR08). [Google Scholar]
- 34. Pereira J, Janelidze S, Smith R, et al. Plasma GFAP is an early marker of Aβ but not tau pathology in Alzheimer's disease. Oral presentation at the 14th Clinical Trials on Alzheimer's Disease (CTAD) Annual Meeting, Boston, Massachusetts, USA, November 9‐12, 2021:(Abstract OC02). [Google Scholar]
- 35. Schindler S, Yarasheski K, West T, et al. Performance of the PrecivityAD™ blood test in detection of brain amyloidosis in cognitively normal and cognitively impaired individuals. Oral presentation at the 14th Clinical Trials on Alzheimer's Disease (CTAD) Annual Meeting, Boston, Massachusetts, USA, November 9‐12, 2021:(Abstract ROC09). [Google Scholar]
- 36. Ashton N, Milà‐Alomà M, Benedet A, et al. Plasma PTau231 as an early marker of amyloid‐β pathology for preclinical Alzheimer's disease trial selection. Oral presentation at the 14th Clinical Trials on Alzheimer's Disease (CTAD) Annual Meeting, Boston, Massachusetts, USA, November 9‐12, 2021:(Abstract LB01). [Google Scholar]
- 37. Piccirella S, Van Neste L, Fowler C, Doecke J, Uberti D, Kinnon P. AlzoSure® Predict, a simple, non‐invasive blood test to predict the early onset of Alzheimer's disease with the ability to identify MCI patients, before the clinical symptoms are identifiable (in the same test) 6 years in advance of clinical diagnosis. Oral presentation at the 14th Clinical Trials on Alzheimer's Disease (CTAD) Annual Meeting, Boston, Massachusetts, USA, November 9‐12, 2021:(Abstract LB03). [Google Scholar]
- 38. Sperling ER, Johnson K, Zhou J, et al. Introduction of plasma biomarker screening for the AHEAD 3‐45 study. Oral presentation at the 14th Clinical Trials on Alzheimer's Disease (CTAD) Annual Meeting, Boston, Massachusetts, USA, November 9‐12, 2021:(Abstract LB04). [Google Scholar]
- 39. Hansson O, Edelmayer RM, Boxer AL, et al. The Alzheimer's Association Appropriate Use Recommendations for blood biomarkers in Alzheimer's disease. Alzheimers Dement. 2022;18:2669‐2686. doi: 10.1002/alz.12756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hansson O, Nisenbaum L, Chen T, et al. Dose‐ and time‐dependent changes in plasma p‐tau181 in patients treated with aducanumab in the ENGAGE and EMERGE trials. Oral presentation at the 14th Clinical Trials on Alzheimer's Disease (CTAD) Annual Meeting, Boston, Massachusetts, USA, November 9‐12, 2021:(Abstract Late‐breaking Roundtable 8). [Google Scholar]
- 41. Swanson C, Dhadda S, Irizarry M, et al. Lecanemab: an assessment of the clinical effects, the correlation of plasma Aβ42/40 ratio with changes in brain amyloid PET SUVr, and safety from the core and open label extension of the Phase 2 proof‐of‐concept study, BAN2401‐G000‐201, in subjects with early Alzheimer's disease. Oral presentation at the 14th Clinical Trials on Alzheimer's Disease (CTAD) Annual Meeting, Boston, Massachusetts, USA, November 9‐12, 2021:(Abstract Late‐breaking Roundtable 5). [Google Scholar]
- 42. Piazza F, Caminiti SP, Zedde M, et al. Association of microglial activation with spontaneous ARIA‐E and CSF levels of anti‐aβ autoantibodies. Neurology. 2022;99:e1265‐e1277. doi: 10.1212/WNL.0000000000200892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rabe C, Bittner T, Jethwa A, et al. Utility of plasma Aβ1‐42/Aβ1‐40 as a screening tool is limited due to lack of robustness. Oral presentation at the 14th Clinical Trials on Alzheimer's Disease (CTAD) Annual Meeting, Boston, Massachusetts, USA, November 9‐12, 2021:(Abstract LBR11). [Google Scholar]
- 44. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695‐699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 45. O'Driscoll C, Shaikh M. Cross‐cultural applicability of the Montreal Cognitive Assessment (MoCA): a systematic review. J Alzheimers Dis. 2017;58:789‐801. doi: 10.3233/JAD-161042 [DOI] [PubMed] [Google Scholar]
- 46. Nasreddine ZS, MoCA test FAQ 2019. (accessed October 9, 2020). https://www.mocatest.org/faq/
- 47. Galvin JE. The Quick Dementia Rating System (QDRS): a rapid dementia staging tool. Alzheimers Dement. 2015;1:249‐259. doi: 10.1016/j.dadm.2015.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Galvin JE, Tolea MI, Chrisphonte S. Using a patient‐reported outcome to improve detection of cognitive impairment and dementia: the patient version of the Quick Dementia Rating System (QDRS). PLoS One. 2020;15:e0240422. doi: 10.1371/journal.pone.0240422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Duff K, Wan L, Levine DA, et al. The Quick Dementia Rating System and its relationship to biomarkers of Alzheimer's disease and neuropsychological performance. Dement Geriatr Cogn Disord. 2022;51:214‐220. doi: 10.1159/000524548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jutten RJ, Peeters CF, Leijdesdorff SM, et al. Detecting functional decline from normal aging to dementia: development and validation of a short version of the Amsterdam IADL Questionnaire. Alzheimers Dement (Amst). 2017;8:26‐35. doi: 10.1016/j.dadm.2017.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sikkes SAM, de Lange‐de Klerk ESM, Pijnenburg YAL, et al. A new informant‐based questionnaire for instrumental activities of daily living in dementia. Alzheimers Dement. 2012;8:536‐543. doi: 10.1016/j.jalz.2011.08.006 [DOI] [PubMed] [Google Scholar]
- 52. Sikkes SA, Knol DL, Pijnenburg YA, de Lange‐de Klerk ES, Uitdehaag BM, Scheltens P. Validation of the Amsterdam IADL Questionnaire©, a new tool to measure instrumental activities of daily living in dementia. Neuroepidemiology. 2013;41:35‐41. doi: 10.1159/000346277 [DOI] [PubMed] [Google Scholar]
- 53. Jutten RJ, Harrison JE, Brunner AJ, et al. The Cognitive‐Functional Composite is sensitive to clinical progression in early dementia: longitudinal findings from the Catch‐Cog study cohort. Alzheimers Dement (NY). 2020;6:e12020. doi: 10.1002/trc2.12020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jutten RJ, Dicks E, Vermaat L, et al. Impairment in complex activities of daily living is related to neurodegeneration in Alzheimer's disease–specific regions. Neurobiol Aging. 2019;75:109‐116. doi: 10.1016/j.neurobiolaging.2018.11.018 [DOI] [PubMed] [Google Scholar]
- 55. Verrijp M, Dubbelman MA, Visser LNC, et al. Everyday functioning in a community‐based volunteer population: differences between participant‐ and study partner‐report. Front Aging Neurosci. 2022;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dubbelman MA, Verrijp M, Facal D, et al. The influence of diversity on the measurement of functional impairment: an international validation of the Amsterdam IADL Questionnaire in eight countries. Alzheimers Dement (Amst). 2020;12:e12021. doi: 10.1002/dad2.12021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dubbelman MA, Verrijp M, Terwee CB, et al. Determining the minimal important change of everyday functioning in dementia: pursuing clinical meaningfulness. Neurology. 2022;99:e954‐e964. doi: 10.1212/WNL.0000000000200781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI‐Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233‐239. doi: 10.1176/jnp.12.2.233 [DOI] [PubMed] [Google Scholar]
- 59. Cummings J. The Neuropsychiatric Inventory Questionnaire: background and administration 1994. (accessed July 23, 2020). https://www.alz.org/careplanning/downloads/npiq‐questionnaire.pdf
- 60. Logsdon RG, Gibbons LE, McCurry SM, Teri L. Quality of life in Alzheimer's disease: patient and caregiver reports. J Ment Health Aging. 1999;5:21‐32. [Google Scholar]
- 61. Logsdon RG, Gibbons LE, McCurry SM, Teri L. Assessing quality of life in older adults with cognitive impairment. Psychosom Med. 2002;64:510‐519. doi: 10.1097/00006842-200205000-00016 [DOI] [PubMed] [Google Scholar]
- 62. Ware J, Kosinski M, Keller SD. A 12‐Item Short‐Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220‐233. doi: 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 63. Huo T, Guo Y, Shenkman E, Muller K. Assessing the reliability of the Short Form 12 (SF‐12) health survey in adults with mental health conditions: a report from the Wellness Incentive and Navigation (WIN) study. Health Qual Life Outcomes. 2018;16:34. doi: 10.1186/s12955-018-0858-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rampakakis E, Ste‐Marie PA, Sampalis JS, Karellis A, Shir Y, Fitzcharles M‐A. Real‐life assessment of the validity of Patient Global Impression of Change in fibromyalgia. RMD Open. 2015;1:e000146. doi: 10.1136/rmdopen-2015-000146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Viktrup L, Hayes RP, Wang P, Shen W. Construct validation of Patient Global Impression of Severity (PGI‐S) and Improvement (PGI‐I) questionnaires in the treatment of men with lower urinary tract symptoms secondary to benign prostatic hyperplasia. BMC Urol. 2012;12:30. doi: 10.1186/1471-2490-12-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Seng BK, Luo N, Ng WY, et al. Validity and reliability of the Zarit Burden Interview in assessing caregiving burden. Ann Acad Med Singap. 2010;39:758‐763. [PubMed] [Google Scholar]
- 67. Wimo A, Jonsson L, Zbrozek A. The Resource Utilization in Dementia (RUD) instrument is valid for assessing informal care time in community‐living patients with dementia. J Nutr Health Aging. 2010;14:685‐690. doi: 10.1007/s12603-010-0316-2 [DOI] [PubMed] [Google Scholar]
- 68. Tsoy E, Zygouris S, Possin KL. Current state of self‐administered brief computerized cognitive assessments for detection of cognitive disorders in older adults: a systematic review. J Prev Alzheimers Dis. 2021;8:267‐276. doi: 10.14283/jpad.2021.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Table S1
ICMJE Disclosure Form


