Abstract
INTRODUCTION
Accurate epidemiologic estimates for dementia are lacking for American Indians, despite substantive social and health disparities.
METHODS
The Strong Heart Study, a population‐based cohort of 11 American Indian tribes, conducted detailed cognitive testing and examinations over two visits approximately 7 years apart. An expert panel reviewed case materials for consensus adjudication of cognitive status (intact; mild cognitive impairment [MCI]; dementia; other impaired/not MCI) and probable etiology (Alzheimer's disease [AD], vascular bain injury [VBI], traumatic brain injury [TBI], other).
RESULTS
American Indians aged 70–95 years had 54% cognitive impairment including 10% dementia. VBI and AD were primary etiology approximately equal proportions (>40%). Apolipoprotein (APO) Eε4 carriers were more common among those with dementia (p = 0.040). Plasma pTau, glial fibrillary acidic protein (GFAP), and neurofilament light chain (NfL) were higher among those with cognitive impairment, but not amyloid beta (Aβ). Cognitive intact had mean 3MSE 92.2 (SD 6.4) and mean Montreal Cognitive Assessment (MoCA) score of 21.3 (SD 3.2).
DISCUSSION
This is the first population‐based study to estimate the prevalence of vascular and Alzheimer's dementias in a population‐based study of American Indians.
Highlights
The Strong Heart Study is a population‐based cohort of American Indian tribes, conducted over 30+ years and three US geographic regions (Northern Plains, Southern Plains, Southwest).
Our teams conducted detailed cognitive testing, neurological examination, and brain imaging over two visits approximately 7 years apart. An expert panel reviewed collected materials for consensus‐based adjudication of cognitive status (intact; MCI; dementia; other impaired/not MCI) and probable underlying etiology (AD; VBI; TBI; other).
In this cohort of American Indians aged 70–95, 54% were adjudicated with cognitive impairment, including approximately 35% MCI and 10% dementia. These data expand on prior reports from studies using electronic health records, which had suggested prevalence, and incidence of dementia in American Indians to be more comparable to the majority population or non‐Hispanic White individuals, perhaps due to latent case undercounts in clinical settings.
Vascular and neurodegenerative injuries were approximately equally responsible for cognitive impairment, suggesting that reduction of cardiovascular disease is needed for primary prevention.
Traumatic injury was more prevalent than in other populations, and common among those in the “other/not MCI” cognitive impairment category.
Mean scores for common dementia screening instruments—even among those adjudicated as unimpaired—were relatively low compared to other populations (mean unimpaired 3MSE 92.2, SD 6.4; mean unimpaired MoCA 21.3, SD 3.2), suggesting the need for cultural and environmental adaptation of common screening and evaluation instruments.
Keywords: American Indians, cognition, dementia, epidemiology, health disparities
1. BACKGROUND
Prevalence of Alzheimer's disease and related dementias (ADRD) is high, affecting more than 10% of those aged 65 years or more, 1 with risk doubling every 5 years. 2 The Lancet Commission has identified 12 modifiable “life‐course” risk factors responsible for an estimated 40% of the population attributable risk for dementia, 3 with age, genetics, and other factors accounting for the other 60%. However, disparities in socioeconomics, historical, and sociological factors, with accompanying higher burden of risk factors and comorbidities, are likely to result in disparate prevalence, incidence, and risk models for some groups, especially Indigenous peoples. 4 , 5 American Indians, for example, endure a high prevalence of hypertension, diabetes, and depression; low access to resources and education; but also have high social support and resilience. 6 , 7 However, despite such high risk, little is yet established about the epidemiology of ADRD in American Indians and other Indigenous peoples.
From electronic healthcare records data, dementia prevalence in older American Indians and Alaska Natives has been estimated at 9%, similar to non‐Hispanic White individuals (10%), 8 with 30%–35% cumulative lifetime risk of impairment after age 65. 9 However, population‐based research has suggested, based on application of conventional thresholds for standardized cognitive tests, that approximately half of American Indians over age 65 may have detectable cognitive impairment and dementia. 7 , 10 , 11 The discrepancies in these findings have yet to be reconciled.
Health records data are likely subject to substantive data bias. Up to 50% of those with dementia may be missing notations in their medical records, a disparity that is especially problematic for populations experiencing barriers to care. 12 , 13 Data biases resulting from inadequate care access, poor post‐diagnosis care, and systematic case undercounting may differentially influence lead time and post‐diagnosis survival, thereby resulting in misestimation even of prevalence and incidence. 14 Compounding such problems for investigations of racial‐ethnic disparities is poor accuracy of racial‐ethnic data in electronic health records, 15 , 16 with as much as 40% missingness and broad inconsistency in selection categories provided across systems, a data inadequacy driven, perhaps in part, by low priority placed on collection of such data by healthcare providers and administrators. 17 However, epidemiologic studies using passively collected health or administrative data rely on key assumptions, including equal access to care, equal quality in care standards, and equivalent data quality across comparison groups. When such assumptions cannot be upheld, 18 , 19 , 20 such as for American Indians and other Indigenous US populations in the context of dementia research, interpretation of studies using administrative data must be done with care.
In contrast, population‐based studies can improve on such data biases. More time‐consuming and resource‐demanding to conduct, cohort studies that define participation prospectively and collect data using consistent, objective, comprehensive methodology specific to the condition of interest can resolve many limitations of interpretation that may prove problematic for convenience sampling from administrative records. Defining dementia using such gold standard methodology utilizes neurologist and neuropsychologist evaluations to define the presence and type of cognitive impairment as well as likely underlying etiology, a multi‐hour process. Although small studies have reported on dementia and cognitive impairment using clinical data, no study has yet to define these conditions using case adjudication by expert panel consensus for defined case status, including mild cognitive impairment (MCI), dementia, cognitive impairment that is not MCI (IN‐MCI), or for probable underlying etiology such as Alzheimer's disease (AD), vascular brain injury (VBI), or traumatic brain injury (TBI) in any population‐based study of American Indians. This is the first report to detail epidemiologic estimates of the prevalence of these conditions in this unique, underserved, understudied population.
2. METHODS
2.1. Design, setting, participants
The Strong Heart Study (SHS) and its ancillary Cerebrovascular Disease and Consequences in American Indians (CDCAI) comprise a >30‐year longitudinal study of persons claiming American Indian ancestry, from tribes and communities currently located in the US Northern Plains, Southern Plains, and Southwest. The SHS initial visit in 1989–1991 recruited 67% of eligible residents; CDCAI follow‐up visits in 2010–2013 (Visit 1, N = 818, then aged 65–95 years) and again in 2017–2019 (Visit 2, N = 403) recruited 87% and 76%, respectively, of survivors from the population‐based, baseline SHS cohort. Details of participation in CDCAI—including numbers for recruitment, retention, and loss to follow‐up over the multiple stages of the cohort study—have been previously described in detail, including CONSORT diagrams. 21 , 22 All participating institutional, Indian Health Service (IHS), and tribal review boards approved study protocols; all participants provided written informed consent. Of note: whereas terminology preferences differ across individuals, tribes, and communities, this study uses the contemporary legal term “American Indian,” signifying those who trace heritage or ancestry to the original peoples of the North American continent, in particular from regions now considered part of the contiguous United States.
RESEARCH IN CONTEXT
Systematic review: Prior research using electronic healthcare records data suggests that dementia prevalence and incidence in older American Indians is similar to that in non‐Hispanic White individuals. However, those findings likely represent significant undercounts of true population risk, due to disparities in healthcare access, presentation to care, and post‐diagnosis care standards; differential standards in dementia diagnosis; and inaccuracy in race reporting. Prospective, population‐based epidemiologic estimates for Alzheimer's disease and related dementias have yet to be elucidated for American Indians or other Indigenous United States (US) populations.
Interpretation: This is the first community‐facing, population‐based study to prospectively collect data on cognition and comorbidities, with the explicit purpose of estimating the prevalence and incidence of dementia and probable underlying etiologies in American Indians across three heterogeneous US regions. These data represent a gold standard epidemiologic assessment of these complex conditions in an understudied population.
Future directions: Future research should validate underlying pathology, especially with gold standard positron emission tomography imaging, to evaluate the stage and extent of disease in this population. Other Indigenous US groups also warrant similar epidemiologic examination, including American Indians from other regions, Alaska Natives, Native Hawaiians, and Pacific Islanders/Native Guamanians.
2.2. Data collection, variables
Detailed methods covering collection of neuropsychological data, structural magnetic resonance imaging (MRI), and clinical examinations including plasma assays, are provided in the Supplement . In short, cognitive data included the Modified Mini Mental Status Examination (3MSE), Wechsler Adult Intelligence Scale digit symbol substitution test (WAIS‐IV DSST), Controlled Oral Word Association F,A,S Test (COWA), California Verbal Learning Test 2nd edition short form (CVLT); Montreal Cognitive Assessment (MoCA), Number Span forward and backward tasks, Benson Complex Figure copy and recall, animal and vegetable naming tests, Trail Making Test (TMT) A and B, Craft Story immediate and delayed recall, and Multilingual Naming Test (MINT). Functional examinations included the Wide Range Achievement Test reading (WRAT), and Functional Activities Questionnaire (FAQ) of instrumental activities of daily living (ADL). MRI data at both visits included infarcts, hemorrhages, white matter hyperintensity (WMH) lesions (leukoaraiosis), sulcal enlargement, and ventricular dilation, and volumetric estimation for entorhinal, hippocampal, and parahippocampal atrophy. Short Physical Performance Battery (SPPB) measured lower body function. Medical history, self‐reported age (years), sex (male or female), years of formal education, smoking (yes or no), and recent alcohol use (yes or no) were self‐reported. Bilingual status (yes or no) was assessed based on self‐reported ability to speak tribal or Native language moderately or very well, given the context of all participants speaking English fluently and conducting testing in English. Hypertension (systolic blood pressure ≥ 140 mmHg, diastolic ≥ 90 mmHg, and/or use of medications); diabetes mellitus (fasting blood glucose ≥ 126 mg/dL and/or medications); chronic kidney disease (estimated glomerular filtration rate < 60 mL/min); and body mass index were measured. Plasma was collected and assayed using Quanterix platform for phosphorylated tau (pTau181), amyloid beta (Aβ40, Aβ42), glial fibrillary protein (GFAP), neurofilament light chain (NfL). Apolipoprotein E ε4 (APOEε4) carrier status was measured by genotyping.
2.3. Case review and consensus adjudication
The protocol for panel based case review and consensus adjudication utilized accumulated neuropsychological, imaging, and clinical data and was based on NIA‐AA (Albert–McKhann) criteria for MCI, dementia, and (other) impairment not MCI. 23 , 24 This or similar protocol is also used by other large research programs on dementia, such as the Alzheimer's Disease Research Centers programs overseen by the National Alzheimer's Coordinating Center; 25 is harmonizable with DSM‐5 clinical criteria; and is comparable to other (cardiovascular) event adjudication methods conducted by the parent SHS study. 26 A panel of physicians and content experts met regularly, with attendance at a minimum including a neuroepidemiologist, a neuropsychologist, and a neurologist, to review participant data packets.
First, assignment of cognitive status (cognitive intact, MCI, dementia, IN‐MCI, unable to determine) was by (a) ascertaining lower‐than expected performance on cognitive tests (for age, education), or change in performance (for repeated tests), and (b) evaluating specific cognitive domains of especially poor performance, relative to other domains. MCI was defined as mild to moderate impairment or change in one or more domains, but retention of the ability to live independently (maintain ADL). Dementia was characterized as moderate to severe loss affecting at least two domains, with a loss of ability to maintain ADL. Impaired not MCI (IN‐MCI) was assigned to those with one or more domains with abnormal functioning, but a pattern of impairment that did not meet the clinical definition of MCI or dementia. 25 Unable to determine was assigned to those with extensive missing data, such as those with full domains of missing test information, or whose data were too inconsistent for determination. The data used for these determinations included cognitive test scores and FAQ/iADLs; sociodemographic, cultural, and environmental considerations such as age, sex, bilingual ability, educational history, and performance on WRAT were also considered when assigning cognitive status.
Probable underlying etiology (primary and secondary: AD, VBI, TBI, other) were then assigned to those with cognitive impairment, by evaluating specifics of performance across test domains, in the context of sociodemographic, clinical, neurological, and imaging data. Primary etiology, and when relevant secondary etiology, were assigned on the basis of the likely contributing pathology underlying any observed cognitive change; those with only a primary (“sole”) etiology assignment were distinguished from those with 2+ etiologies (“mixed”). Modifying status such as mobility, sight, or hearing limitations as well as clinical factors, such as the history of brain injury, that could explain specific patterns of cognitive test performance were considered. The data included in these reviews included behaviors (smoking, alcohol use); clinical factors (diabetes, hypertension, dyslipidemia, kidney disease, obesity, migraines, seizures, traumatic injury, stroke); physical function (SPPB); imaged features (infarct, hemorrhage, white matter hyperintensities, sulcal and ventricle atrophy, volumetrics including hippocampus and entorhinal cortex). For quality control purposes, 3%–5% of cases were randomly selected for duplicate review, adjudication, and comparison.
2.4. Statistical analyses
Tabulation across categories of cognitive status, primary and mixed etiology, and by sex included calculation of mean and standard deviation (SD) or count and percent (%) for characteristics of interest. Testing for differences across adjudicated categories used one‐way analysis of variance (ANOVA) (continuous explanatory variable) or chi2 (dichotomous). Testing for differences in continuous cognitive test scores, comparing dichotomous sociodemographic features (e.g., male/female) used T‐tests. Receiver operating characteristic (ROC) analysis calculated the area under the curve (AUC), sensitivity, and specificity for cognitive impairment (MCI, dementia, IN‐MCI combined), based on continuous features such as cognitive test scores. The empirically selected optimal cut‐point was established using the Liu method, which maximizes sensitivity and specificity. 27 LASSO (logistic) regression with bootstrap standard errors was used to select the best set of (visit 2) explanatory cognitive test scores for discriminating cognitive impairment, along with age, sex, education, and field center. Ridgeline plots provided a graphical summary of distribution (kernel density) for continuous characteristics, over categorical features. All analyses were conducted using Stata v17 or R v4.
3. RESULTS
3.1. Quality control
We conducted 12 blinded duplicate review assessments, or 3% of the total. Of these, 10 had consistent cognitive and etiology assignments (83%), one had a differential in status assignment (MCI, dementia), and one had a differential in etiology assignment (VBI, AD). Based on these duplicated assessments, intra‐system variability in consensus review was low. For the two with differing adjudications, we retained the later assignment. Unable to determine status due to missing or inconsistent data was the determination for n = 6 (1.5%), leaving N = 397 with either intact (n = 181), MCI (n = 140), dementia (n = 41), or impaired not MCI (n = 35).
3.2. Prevalence of cognitive syndromes and etiologies
In this large, heterogeneous, population‐based study of American Indian elders (Visit 2: age range 72–95 years; mean 78.1, SD 4.7), MCI was adjudicated in 35% and dementia in 10%; impaired not MCI status was present in nearly 9% (Table 1 ). Primary etiology assessments were fairly consistent between MCI and dementia: AD was ascertained as responsible for 41%–44% of MCI and dementia cases, VBI for 44%–51%, TBI for 1%–2%, and other etiology for 6%–10%. The I‐MCI group was determined to have TBI as primary etiology in 34% and other or unknown etiology in 66%. With respect to single versus mixed etiologies, AD as sole etiology was more common in MCI than dementia (31%, 24%, respectively), as was VBI etiology (38%, 22%, respectively); however, the reverse is true for AD‐VBI mixed etiology, which was more common in dementia (37%) than in MCI (14%).
TABLE 1.
Number and prevalence of adjudicated cognitive syndrome by assigned etiology among American Indian participants of the Strong Heart Study (2017–2019).
| MCI | Dementia | IN‐MCI | |
|---|---|---|---|
| N = 140 | N = 41 | N = 35 | |
| 35.3% | 10.3% | 8.8% | |
| Primary consensus etiology | |||
| AD | 57 (41%) | 18 (44%) | 0 |
| VBI | 71 (51%) | 18 (44%) | 0 |
| TBI | 2 (1%) | 1 (2%) | 12 (34%) |
| Other (e.g., LBD) | 10 (6%) | 4 (10%) | 21 (60%) |
| Unable to determine | 0 | 0 | 2 (6%) |
| Mixed consensus etiology | |||
| AD primary (sole) | 44 (31%) | 10 (24%) | 0 |
| VBI primary (sole) | 53 (38%) | 9 (22%) | 0 |
| TBI primary (sole) | 1 (0.7%) | 1 (2%) | 9 (26%) |
| AD & VBI (mixed) | 19 (14%) | 15 (37%) | 0 |
| TBI & any other etiology (mixed) | 12 (9%) | 1 (2%) | 4 (11%) |
| Other etiologies (mixed) | 1 (0.7%) | 1 (2%) | 0 |
Note: Values provided as n (column %) unless otherwise indicated. Cognitive intact N = 181 (45.7%).
Abbreviations: AD, Alzheimer's disease; IN‐MCI, impaired cognition not MCI; LBD Lewy body disease; MCI, mild cognitive impairment; TBI, traumatic brain injury; VBI, vascular brain injury.
3.3. Sociodemographics of cognitive syndromes
Comparing the cognitive intact category (46% of cohort) with the three categories of cognitive impaired (MCI, dementia, IN‐MCI), those determined to have dementia were oldest (mean age nearly 80 years), those with MCI of intermediate age (mean age nearly 79 years), and those with intact cognition and IN‐MCI youngest (mean ages 77 years; Table 2 ). Education was highest among the cognitive intact group (mean 13.6 years), intermediate among MCI and IN‐MCI (mean 12.5‐12.7), and lowest for dementia (mean 12.0). Differences in education were statistically significant (p < 0.001) but differences in age could not exclude the role of chance (p = 0.051). Those with MCI and dementia were more likely to be male than those determined as cognitive intact (32%–33% vs. 24%); IN‐MCI were even more likely male (46%), although this difference was not significant (p = 0.057).
TABLE 2.
Selected sociodemographics, risk, and AT(N) plasma biomarker features by adjudicated cognitive status among American Indian participants of the Strong Heart Study (2017–019).
| Overall | Cognitive intact | MCI | Dementia | IN‐MCI | ||
|---|---|---|---|---|---|---|
| Parameter | N = 397 |
N = 181 45.6% |
N = 140 35.3% |
N = 41 10.3% |
N = 35 8.8% |
p‐Value |
| Age, years | 78.0 (4.7) | 77.4 (4.8) | 78.5 (4.7) | 79.8 (4.9) | 77.2 (3.2) | 0.051 |
| Sex, male; % | 30% | 24% | 33% | 32% | 46% | 0.057 |
| Education, years | 13.0 (2.5) | 13.6 (2.4) | 12.7 (2.4) | 12.0 (2.5) | 12.5 (3.0) | 0.001 |
| APOE ε4 carriers; % | 21% | 18% | 19% | 35% | 31% | 0.040 |
| pTau181 pg/mL | 7.5 (13.5) | 7.6 (17.3) | 6.8 (4.7) | 10.2 (18.4) | 6.6 (4.3) | <0.001 |
| Aβ40 pg/mL | 143.4 (44.6) | 140.1 (43.6) | 143.9 (45.3) | 155.8 (41.7) | 143.7 (49.0) | 0.762 |
| Aβ42 pg/mL | 8.4 (2.8) | 8.2 (2.9) | 8.5 (2.9) | 8.8 (2.3) | 8.3 (2.5) | 0.210 |
| Aβ42/40 ratio | 0.059 (0.014) | 0.059 (0.014) | 0.059 (0.014) | 0.057 (0.009) | 0.060 (0.013) | 0.052 |
| GFAP pg/mL | 174.7 (90.0) | 162.2 (80.9) | 175.9 (88.0) | 216.2 (88.6) | 186.5 (125.3) | 0.005 |
| NfL pg/mL | 40.2 (26.6) | 34.9 (21.3) | 42.4 (28.7) | 53.7 (31.1) | 43.7 (30.9) | <0.001 |
Note: Values provided as mean (SD) unless otherwise indicated. p‐values based on one‐way ANOVA (continuous) or chi2 (dichotomous) tests.
Abbreviations: Aβ, amyloid beta; APOE, apolipoprotein E; GFAP, glial fibrillary protein; IN‐MCI, impaired cognition not MCI; MCI, mild cognitive impairment; NfL, neurofilament light chain; pTau, phosphorylated tau.
3.4. APOE ε4 carrier status and plasma ATN marker values across cognitive categories
Apolipoprotein E (APOE) ε4 carrier status was statistically different across adjudicated cognitive status categories (p = 0.040), with cognitive intact and MCI similar to each other (18%–19% carriers) and dementia and IN‐MCI also similar to each other, but with a higher proportion of APOE ε4 carriers (31%–35%). Some plasma ATN and related markers (pTau, GFAP, NfL) were also associated with cognitive status, with worse plasma marker values among those adjudicated with dementia and intermediate values among those with MCI (Table 2 ). However, Aβ (40, 42, ratio) plasma marker values were not statistically different across cognitive categories. Those adjudicated with IN‐MCI were most similar to MCI, in plasma marker features.
3.5. Normative neuropsychological functioning (cognitive intact)
The cognitive intact category had overall high cognitive functioning, with mean 3MSE score 92.2 (SD 6.4) and mean MoCA score 21.3 (SD 3.2), both multidomain tests of general functioning (Table 3 ). Sex differences were detected with females significantly performing better than males on WAIS digit symbol coding test (processing speed), COWA F,A,S test (phonemic fluency), CVLT‐II SF long delay free recall (delayed verbal memory) and vegetable naming test (semantic fluency); males performed significantly better than females on MINT (pictographic memory, confrontation naming).
TABLE 3.
Neuropsychological test scores, overall and by sex, among cognitively intact American Indian participants of the Strong Heart Study (2017–2019).
| Cognitive intact | ||||
|---|---|---|---|---|
| Parameter |
All intact N = 181 |
Female N = 137 |
Male N = 44 |
p‐Value |
| 3MSE | 92.2 (6.4), 61–100 | 92.7 (6.4), 61–100 | 90.7 (6.1), 74–100 | 0.888 |
| WAIS DSST | 47.7 (13.5), 13–76 | 49.2 (13.8), 13–76 | 43.2 (11.4), 20–70 | 0.032 |
| COWA | 29.4 (10.9), 2–60 | 29.8 (10.5), 2–60 | 28.2 (11.8), 9–55 | 0.008 |
| CVLT LF | 6.0 (1.7), 0–9 | 6.2 (1.6), 0–9 | 5.5 (2.0), 0–9 | <0.001 |
| MoCA | 21.3 (3.2), 12–28 | 21.4 (3.4), 12–28 | 21.0 (2.7), 16–27 | 0.538 |
| MINT | 28.3 (1.9), 23–32 | 28.1 (2.0), 23–32 | 28.8 (1.6), 25–32 | <0.001 |
| Number forward | 7.0 (2.2), 3–13 | 7.0 (2.3), 3–13 | 7.0 (2.0), 4–12 | 0.717 |
| Number backward | 5.0 (1.8), 1–9 | 4.9 (1.7), 1–9 | 5.2 (2.1), 2–9 | 0.302 |
| Benson copy | 16.0 (1.3), 11–17 | 16.0 (1.3), 11–17 | 15.9 (1.4), 13–17 | 0.958 |
| Benson recall | 10.3 (3.1), 0–17 | 10.4 (3.2), 0–17 | 10.0 (2.8), 2–17 | 0.553 |
| Craft story repeat (paraphrase) | 11.5 (3.9), 0–21 | 11.8 (3.8), 2–19 | 10.7 (4.1), 0–21 | 0.986 |
| Craft story recall (paraphrase) | 10.3 (3.7), 0–18 | 10.5 (3.7), 2–17 | 9.7 (3.9), 0–18 | 0.802 |
| Animal naming | 15.5 (4.2), 6–28 | 15.6 (4.4), 6–28 | 15.0 (3.7), 7–22 | 0.204 |
| Vegetable naming | 11.0 (3.1), 3–19 | 11.7 (2.8), 5–19 | 9.1 (3.1), 3–18 | <0.001 |
| TMT A (seconds) | 55.8 (25.6), 20–132 | 54.6 (24.8), 20–126 | 59.7 (27.9), 23–132 | 0.502 |
| TMT B (seconds) | 148.2 (60.4), 58–300 | 147.4 (59.9), 58–300 | 150.5 (62.7), 59–300 | 0.241 |
Notes: Values provided as mean (SD), Range unless otherwise indicated. p‐values based on T‐tests. Smaller values on all tests represent better performance, except for TMT A/B wherein longer time needed to complete a task represents poorer performance.
Abbreviations: 3MSE, Mini‐Mental Status Examination; COWA, Controlled Oral Word Association test; CVLT LF, California Verbal Learning Test (short form) long delay free recall; MINT, Multilingual Naming Test; MoCA, Montreal Cognitive Assessment; TMT A/B, Trails Making Test A and B; WAIS DSST, Weschler Adult Intelligence Scale digit symbol substitution task.
Empirically defined classification thresholds suggested the optimal threshold to classify any impairment (MCI, dementia, IN‐MCI) with 3MSE was <90.5 and for MoCA < 19.5; to classify dementia alone with 3MSE was <92.5 and MoCA < 19.5 (Table 4 ). However, there was moderate to low performance for any given test to individually classify impairment; Trail Making Test A and B performed best, with AUC = 0.6–0.7 for any impairment and AUC 0.7–0.8 for dementia. Variable selection using LASSO regression (in combination with four core features: age, sex, education, center) empirically identified 10 neuropsychological tests that best classify cognitive impairment (3MSE, MoCA, WAIS coding, COWA, CVLT LF, MINT, Benson delay, Craft delay, animal naming, Trails B; model AUC = 0.89) and to discriminate dementia (WAIS, CVLT LF, MoCA, MINT, Number forward, Number backward, Benson copy, Benson recall, Craft story recall, Vegetable naming; AUC = 0.99).
TABLE 4.
Performance metrics for individual neuropsychological tests in discriminating between cognitive intact and impaired cognition (MCI, dementia, In‐MCI) among American Indian participants of the Strong Heart Study (2017–2019).
| Any cognitive impairment (MCI, dementia, IN‐MCI), vs. intact | Dementia, vs. intact | |||
|---|---|---|---|---|
| Parameter | Empirical, optimal cut point | ROC (AUC, sensitivity, specificity) at cut point | Empirical, optimal cut point | ROC (AUC, sensitivity, specificity) at cut point |
| 3MSE a | 90.5 | 0.31 (0.36, 0.26) | 92.5 | 0.28 (0.20, 0.35) |
| WAIS DSST a , b | 42.5 | 0.39 (0.49, 0.28) | 42.5 | 0.28 (0.27, 0.28) |
| COWA a | 26.5 | 0.36 (0.31, 0.41) | 22.5 | 0.33 (0.39, 0.26) |
| CVLT LF a , b | 6.5 | 0.39 (0.24, 0.53) | 6.5 | 0.33 (0.13, 0.53) |
| MoCA a , b | 19.5 | 0.29 (0.26, 0.31) | 19.5 | 0.21 (0.11, 0.31) |
| MINT a , b | 28.5 | 0.40 (0.29, 0.51) | 27.5 | 0.25 (0.14, 0.35) |
| Number forward b | 6.5 | 0.43 (0.38, 0.49) | 5.5 | 0.41 (0.56, 0.25) |
| Number backward b | 4.5 | 0.39 (0.33, 0.44) | 3.5 | 0.26 (0.35, 0.17) |
| Benson copy b | 16.5 | 0.41 (0.39, 0.42) | 15.5 | 0.33 (0.26, 0.39) |
| Benson recall a , b | 9.5 | 0.35 (0.35, 0.34) | 7.5 | 0.27 (0.35, 0.19) |
| Craft story repeat (paraphrase) | 9.5 | 0.38 (0.47, 0.28) | 8.5 | 0.27 (0.31, 0.22) |
| Craft story recall (paraphrase) a , b | 8.5 | 0.36 (0.39, 0.33) | 8.5 | 0.27 (0.21, 0.33) |
| Animal naming a | 14.5 | 0.36 (0.32, 0.39) | 12.5 | 0.28 (0.31, 0.25) |
| Vegetable naming a , b | 9.5 | 0.35 (0.39, 0.32) | 8.5 | 0.22 (0.23, 0.22) |
| TMT A (seconds) | 53.5 | 0.66 (0.74, 0.59) | 81.5 | 0.81 (0.79, 0.83) |
| TMT B (seconds) a | 162.5 | 0.64 (0.58, 0.69) | 172.5 | 0.65 (0.58, 0.72) |
Abbreviations: 3MSE, mini mental status examination; APOE, apolipoprotein E; COWA, Controlled Oral Word Association test; CVLT LF, California Verbal Learning Test (short form) long delay free recall; IN‐MCI, impaired cognition not MCI; MCI, mild cognitive impairment; MINT, Multilingual Naming Test; MoCA, Montreal Cognitive Assessment; ROC(AUC), receiver operating characteristic area under the curve; TMT A/B, Trails Making Test A and B; WAIS DSST, Weschler Adult Intelligence Scale digit symbol substitution task.
Items empirically identified by LASSO regression to discriminate any cognitive impairment (MCI, dementia, In‐MCI), in addition to age, sex, education, and center, with 100 repetitions bootstraps; 14‐variable model AUC: 0.898.
Items empirically identified by similar model to discriminate dementia (14‐variable model), AUC: 0.99.
3.6. Distribution of neuropsychological performance, by adjudicated cognitive case status
Graphical examination of test score distributions by adjudicated case status (Figure 1 ), suggests that tests related to multidomain or general cognition (3MSE, MoCA), memory (Craft story, Benson figure recall), language (MINT, fluency tasks), working memory and executive function (number span backward), and processing speed (WAIS digit) decline linearly between intact, MCI, and dementia; whereas other tests split into a bimodal distribution with presence of dementia (3MSE, MoCA, Craft story, Benson figure, MINT, number span). The IN‐MCI category was a distinct group with inconsistent patterns across cognitive domains.
FIGURE 1.
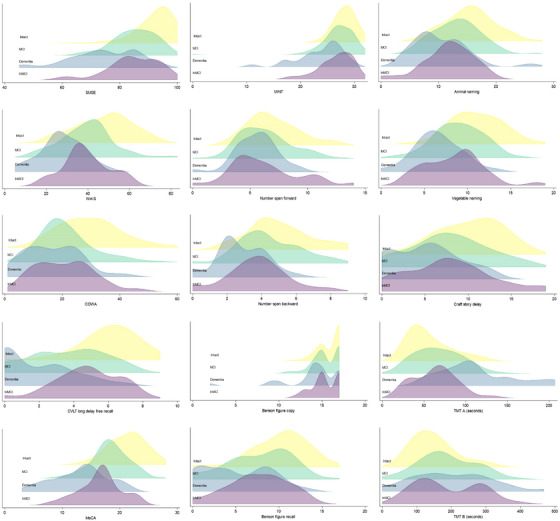
Ridgeline plot (overlaid density plots) of distribution of neuropsychological test scores, by cognitive status category, among American Indian participants of the Strong Heart Study (2017–2019). Categories include cognitive intact (yellow), mild cognitive impairment (MCI, green), dementia (blue), and intact not MCI (purple). Cognitive tests include Modified Mini Mental Status Examination (3MSE), Wechsler Adult Intelligence Scale 4th edition digit symbol substitution coding test (WAIS DSST), Controlled Oral Word Association F,A,S Test (COWA), California Verbal Learning Test 2nd edition short form long delay free recall (CVLT), Montreal Cognitive Assessment (MoCA), Multilingual Naming Test (MINT), Number Span forward and backward tasks, Benson complex figure copy and recall, animal and vegetable naming tests, Craft Story delayed recall, and Trail Making Test (TMT) A and B
4. DISCUSSION
This study reports the prevalence of cognitive impairment at approximately 54% in American Indians over age 65, and 10% in dementia. Furthermore, the underlying etiologies of AD and VBI were roughly equivalently responsible, with VBI etiology slightly heavier in weight. These are the first formally defined, population‐based epidemiologic estimates of MCI, dementia, and IN‐MCI, and of underlying etiologies of AD, VBI, and TBI, defined by expert consensus for this population, providing novel information for clinicians and researchers interested in AD and related dementias, especially in the context of racial‐ethnic health disparities.
Of note, these prevalence estimates differed markedly from previous estimates generated from studies using electronic healthcare records data. However, these data are consistent with expectations that healthcare or administrative records data are subject to underreporting of cases, perhaps due in part to differences in healthcare access as well as differences in race‐reporting in such systems. Our data (impairment > 50%) are also consistent with expectations based on the use of conventional diagnostic thresholds for cognitive impairment in standardized cognitive testing, with common cutoffs falling below the study population mean. Furthermore, psychometrics of standard cognitive tests has found minimal differences in test performance in this population, 28 further emphasizing that conventional methods of dementia assessment in the clinic may be adequate for case identification. Thus, it is likely that prior estimates were true undercounts, and that achieving more equitable case recognition may require improvements to causal disparities, such as in access to healthcare and in quality of healthcare delivery—although more research is needed to test these hypotheses.
Our population prevalence estimates are also higher than those estimated for other racial/ethnic groups, even when using comparable methods for data collection and inference. The Cardiovascular Health Study, 29 the Northern Manhattan Study, 30 and the Harmonized Cognitive Assessment Protocol 31 have reported similar population‐based expert consensus adjudication methodologies among non‐Hispanic White individuals (NHW), African Americans (AA), and Hispanic/Latino (H/L) aged > 65 years. Combining findings from these studies, estimated ranges for adjudicated MCI and dementia, respectively, are NHW 12‐21% and 2‐9%; AA 22‐25% and 2‐15%; H/L 20‐28% and 5‐10%.
Given our findings of 35% MCI, 10% dementia (45% combined) in American Indians, a prevalence ratio comparing American Indians with NHW (14%–30%) may be estimated to be 1.5–3.2; a corresponding ratio for AA (24%–40%) to be 1.1–1.9 and for H/L (25%–38%) to be 1.2–1.8. Despite such substantial disparities, American Indians are not included in most population and clinic‐based research on dementia and Alzheimer's disease. Indirect calculated like these across unharmonized studies should be interpreted with caution; thus, additional research that more systematically included American Indians and other Indigenous or underrepresented US populations, such as Alaska Natives, Native Hawaiians, and Pacific Islanders, is critically needed. Direct cross‐comparisons with prospective, systematically collected data are needed to fully and equitably establish high‐quality epidemiologic estimates for these important sources of morbidity and mortality.
Other key findings from this report include the relative impact of vascular and Alzheimer's diseases in etiologic assessments for MCI and dementia. Conventional findings in majority populations have identified AD as responsible for 60%–80% of dementias, 32 although the predominance of mixed, rather than primary, pathologies in dementia incidence is increasingly recognized. 33 , 34 However, our findings placed VBI as primary cause, and AD as secondary. These findings echo prior research of much higher cardiovascular risk observed for this population, 35 , 36 , 37 , 38 as well as the very high prevalence of related VBI risk factors, such as hypertension and diabetes (>80%, >50%, respectively). 39 Public health programs aimed to reduce or prevent dementia prevalence in this population may need to prioritize cardiovascular and VBI risk reduction; mirroring successful reductions in the majority population may provide insights into public health strategies and opportunities. 40
Despite these substantive disparities, the interpretation of race‐specific differences should be done with caution. Differences in education, socioeconomics, language, healthcare access, social history, and related socioeconomic factors can confound cognitive test performance as well as cognitive functioning in a manner that is not inherent to racial/ethnic identity, but for which race/ethnicity often serves as a convenient proxy. 41 Such differential population contexts can bias test score interpretability, resulting in many of the observed differences across race, ethnicity, and culture. 42 , 43 As an example, tests commonly used to screen for dementia have poor discriminant properties for populations with low literacy, 44 , 45 suggesting that education, not racial identity, is a key factor in score variance across populations in those studies. Ongoing work to validate these standardized neuropsychological tests among older American Indians is consistent with these hypotheses–that education and bilingual status, in particular, are key contextual criteria when evaluating performance and scoring. 28 , 46 However, this work is ongoing: formal psychometrics are needed for population‐specific interpretability in score performance, reliability, and generalizability for all cognitive tests, and clinical diagnostics should be evaluated empirically for accuracy and utility in all populations.
Prior studies have not consistently reported on the IN‐MCI (Impaired not MCI) group as an independent category, attributed in part to inconsistent data collection of this category across Alzheimer's Disease Research Centers, resulting in some authors collapsing this category with <MCI (e.g., intact) category, 47 , 48 or dropping them from the analysis, 49 due to uncertainty in the data interpretation and consensus procedures. In our study, the IN‐MCI group was predominantly adjudicated as having traumatic etiology, a risk factor that is more common in this population than in the general population. 50 , 51 Future research is likely warranted with greater attention to this impairment category, especially among populations with high rates of head injury or other non‐conventional sources of cognitive risk.
These data are not without limitations. Those with severe impairment may have not survived or been able to participate or may have participated but had incomplete data and thus adjudication was indeterminate, which would result in partial case underestimates. Prior reports on selective survival between SHS baseline (1989–91) and CDCAI visit 1 (2010–13) suggest that participants who were lost to follow‐up were more likely older, male, diabetic, and hypertensive but not more likely to have heart events, compared with those who were successfully recruited. 52 Similarly, a comparison of participant characteristics among those who did and did not participate in CDCAI visit 1 and visit 2 (2017–19) suggests that those lost to follow‐up were also more likely to have chronic kidney disease, depressive symptoms, and prior stroke. 22 These patterns are consistent with the potential for selective survival, with acceleration over time, and greater relevance to vascular brain injury between CDCAI visits 1 and 2. If present, bias due to selective survival could contribute to case underestimation. Second, our collection of instrumental activities of daily living was self‐reported and did not include family or friend co‐respondents for additional perspective on functional status. Third, this cohort was representative of American Indian elders from tribes and communities in the Northern and Southern Plains and the Southwest and may not be generalizable to other groups. Fourth, our ROC analyses, intended as exploratory, may involve some circularity in logic, since the same cognitive tests used as a set to define cognitive impairment were then evaluated for association with that categorization; therefore, the specific findings from these analyses should be interpreted with caution, and instead serve as hypothesis generation for future investigations.
Despite these limitations, the many strengths of this study include a deep and rich, standardized protocol with extensive quality control and community‐representation, eliciting high participation in a rigorous set of procedures. Our case review and consensus protocols capitalized on interdisciplinary dementia specialist expertise, and a consistent adjudication procedure—an advantage over prior works.
5. CONCLUSION
Overall, these data reliably represent the first population‐based epidemiologic estimates of prevalence for MCI and dementia, as well as for AD and VBI etiology, in a large population‐based study of American Indians. Clinicians and researchers may find these data useful, as well as the relative contributions of key cognitive, imaging, and clinical features, as they evaluate risk in patients, participants, and communities with underserved or minoritized racial‐ethnic identities.
AUTHOR CONTRIBUTIONS
Astrid M. Suchy‐Dicey, Lonnie Nelson, Dedra S. Buchwald, and Thomas J. Grabowski collected data from participant interactions. Astrid M. Suchy‐Dicey, Kimiko Domoto‐Reilly, Lonnie Nelson, Suman Jayadev, and Kristoffer Rhoads conducted case reviews and consensus adjudication. Astrid M. Suchy‐Dicey composed the hypotheses, analyzed the data, and wrote the manuscript. All authors (Astrid M. Suchy‐Dicey, Kimiko Domoto‐Reilly, Lonnie Nelson, Suman Jayadev, Dedra S. Buchwald, Thomas J. Grabowski, and Kristoffer Rhoads) contributed substantive edits and comments on the scientific concept and written materials.
CONFLICT OF INTEREST STATEMENT
The authors have no financial interests to declare. Author disclosures are available in the supporting information.
CONSENT STATEMENT
All participating institutional, Indian Health Service (IHS), and tribal review boards approved study protocols; all participants provided written informed consent.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
The authors thank all Strong Heart Study staff, participants, and communities. The authors acknowledge Kyra Oziel who provided research support. The opinions expressed in this paper are solely the responsibility of the author(s) and do not necessarily reflect the official views of the Indian Health Service or the National Institutes of Health (NIH). This study has been funded in whole or in part with federal funds from the National Institutes of Health, including R01HL093086 (Buchwald), P50AG005136 (Grabowski), K01AG057821 (Suchy‐Dicey). The Strong Heart Study has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, National Institute of Health, Department of Health and Human Services, under contract numbers 75N92019D00027, 75N92019D00028, 75N92019D00029, & 75N92019D00030. The study was previously supported by research grants: R01HL109315, R01HL109301, R01HL109284, R01HL109282, and R01HL109319 and by cooperative agreements: U01HL41642, U01HL41652, U01HL41654, U01HL65520, and U01HL65521.
Suchy‐Dicey AM, Domoto‐Reilly K, Nelson L, et al. Epidemiology and prevalence of dementia and Alzheimer's disease in American Indians: Data from the Strong Heart Study. Alzheimer's Dement. 2024;20:4174–4184. 10.1002/alz.13849
DATA AVAILABILITY STATEMENT
These data are the property of the sovereign tribal nations from which they were gathered. Scientific use of these data can be requested from the Strong Heart Study (https://strongheartstudy.org).
REFERENCES
- 1. 2023 Alzheimer's disease facts and figures. Alzheimers Dement. 2023;19:1598‐1695. [DOI] [PubMed] [Google Scholar]
- 2. Cao Q, Tan CC, Xu W, et al. The prevalence of dementia: a systematic review and meta‐analysis. J Alzheimers Dis. 2020;73:1157‐1166. [DOI] [PubMed] [Google Scholar]
- 3. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet. 2020;396:413‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walker JD, Spiro G, Loewen K, Jacklin K. Alzheimer's disease and related dementia in indigenous populations: a systematic review of risk factors. J Alzheimers Dis. 2020;78:1439‐1451. [DOI] [PubMed] [Google Scholar]
- 5. Nguyen HXT, Bradley K, McNamara BJ, Watson R, Malay R, LoGiudice D. Risk, protective, and biomarkers of dementia in indigenous peoples: a systematic review. Alzheimers Dement. 2024;20:563‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Suchy‐Dicey A, Eyituoyo H, O'Leary M, et al. Psychological and social support associations with mortality and cardiovascular disease in middle‐aged American Indians: the Strong Heart Study. Soc Psychiatry Psychiatr Epidemiol. 2022;57:1421‐1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Suchy‐Dicey AM, Oziel K, Sawyer C, et al. Educational and clinical associations with longitudinal cognitive function and brain imaging in American Indians: the Strong Heart Study. Neurology. 2022;99:e2637‐e2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matthews KA, Xu W, Gaglioti AH, et al. Racial and ethnic estimates of Alzheimer's disease and related dementias in the United States (2015‐2060) in adults aged ≥65 years. Alzheimers Dement. 2019;15:17‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12:216‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suchy‐Dicey A, Shibata D, Cholerton B, et al. Cognitive correlates of MRI‐defined cerebral vascular injury and atrophy in elderly American Indians: the Strong Heart Study. J Int Neuropsychol Soc. 2020;26:263‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Verney SP, Suchy‐Dicey AM, Cholerton B, et al. The associations among sociocultural factors and neuropsychological functioning in older American Indians: the Strong Heart Study. Neuropsychology. 2019;33:1078‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duwe EA, Petterson S, Gibbons C, Bazemore A. Ecology of health care: the need to address low utilization in American Indians/Alaska Natives. Am Fam Physician. 2014;89:217‐218. [PubMed] [Google Scholar]
- 13. Kim G, Bryant AN, Goins RT, Worley CB, Chiriboga DA. Disparities in health status and health care access and use among older American Indians and Alaska Natives and non‐Hispanic Whites in California. J Aging Health. 2012;24:799‐811. [DOI] [PubMed] [Google Scholar]
- 14. Warren LA, Shi Q, Young K, Borenstein A, Martiniuk A. Prevalence and incidence of dementia among indigenous populations: a systematic review. Int Psychogeriatr. 2015;27:1959‐1970. [DOI] [PubMed] [Google Scholar]
- 15. Klinger EV, Carlini SV, Gonzalez I, et al. Accuracy of race, ethnicity, and language preference in an electronic health record. J Gen Intern Med. 2015;30:719‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cusick MM, Sholle ET, Davila MA, Kabariti J, Cole CL, Campion TR Jr. A method to improve availability and quality of patient race data in an electronic health record system. Appl Clin Inform. 2020;11:785‐791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yan C, Zhang X, Yang Y, et al. Differences in health professionals' engagement with electronic health records based on inpatient race and ethnicity. JAMA Netw Open. 2023;6:e2336383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Griffin‐Pierce T, Silverberg N, Connor D, et al. Challenges to the recognition and assessment of Alzheimer's disease in American Indians of the southwestern United States. Alzheimers Dement. 2008;4:291‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hendrie HC. Lessons learned from international comparative crosscultural studies on dementia. Am J Geriatr Psychiatry. 2006;14:480‐488. [DOI] [PubMed] [Google Scholar]
- 20. Henderson JN, Traphagan JW. Cultural factors in dementia: perspectives from the anthropology of aging. Alzheimer Dis Assoc Disord. 2005;19:272‐274. [DOI] [PubMed] [Google Scholar]
- 21. Suchy‐Dicey AM, Shibata D, Best LG, et al. Cranial magnetic resonance imaging in elderly American Indians: design, methods, and implementation of the cerebrovascular disease and its consequences in American Indians study. Neuroepidemiology. 2016;47:67‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suchy‐Dicey AM, Oziel K, Sawyer C, et al. Educational and Clinical Associations With Longitudinal Cognitive Function and Brain Imaging in American Indians: The Strong Heart Study. Neurology. 2022;99(24):e2637‐e2647. doi: 10.1212/WNL.0000000000201261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beekly DL, Ramos EM, van Belle G, et al. The National Alzheimer's Coordinating Center (NACC) database: an Alzheimer disease database. Alzheimer Dis Assoc Disord. 2004;18:270‐277. [PubMed] [Google Scholar]
- 26. Zhang Y, Galloway JM, Welty TK, et al. Incidence and risk factors for stroke in American Indians: the Strong Heart Study. Circulation. 2008;118:1577‐1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu X. Classification accuracy and cut point selection. Stat Med. 2012;31:2676‐2686. [DOI] [PubMed] [Google Scholar]
- 28. Suchy‐Dicey AM, Vo TT, Oziel K, et al. Psychometric Properties of Controlled Oral Word Association (COWA) Test and Associations With Education and Bilingualism in American Indian Adults: The Strong Heart Stud. Assessment. 2024;31(3):745‐757. doi: 10.1177/10731911231180127. Epub 2023 Jun 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lopez OL, Kuller LH, Fitzpatrick A, Ives D, Becker JT, Beauchamp N. Evaluation of dementia in the cardiovascular health cognition study. Neuroepidemiology. 2003;22:1‐12. [DOI] [PubMed] [Google Scholar]
- 30. Wright CB, DeRosa JT, Moon MP, et al. Race/ethnic disparities in mild cognitive impairment and dementia: the Northern Manhattan Study. J Alzheimers Dis. 2021;80:1129‐1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Manly JJ, Jones RN, Langa KM, et al. Estimating the prevalence of dementia and mild cognitive impairment in the US: the 2016 Health and Retirement Study harmonized cognitive assessment protocol project. JAMA Neurol. 2022;79:1242‐1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Association As . What is Dementia? Types of Dementia. Alzheimer's Association; 2023. [Google Scholar]
- 33. Attems J, Jellinger KA. The overlap between vascular disease and Alzheimer's disease–lessons from pathology. BMC Med. 2014;12:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community‐dwelling older persons. Neurology. 2007;69:2197‐2204. [DOI] [PubMed] [Google Scholar]
- 35. Jillella DV, Crawford S, Lopez R, Zafar A, Tang AS, Uchino K. Vascular risk factor prevalence and trends in Native Americans with ischemic stroke. J Stroke Cerebrovasc Dis. 2022;31:106467. [DOI] [PubMed] [Google Scholar]
- 36. Kirkpatrick AC, Stoner JA, Donna‐Ferreira F, et al. High rates of undiagnosed vascular cognitive impairment among American Indian veterans. Geroscience. 2019;41:69‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Howard BV, Lee ET, Cowan LD, et al. Rising tide of cardiovascular disease in American Indians. The Strong Heart Study. Circulation. 1999;99:2389‐2395. [DOI] [PubMed] [Google Scholar]
- 38. Muller CJ, Noonan CJ, MacLehose RF, et al. Trends in cardiovascular disease morbidity and mortality in American Indians over 25 years: the Strong Heart Study. J Am Heart Assoc. 2019;8:e012289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shibata D, Suchy‐Dicey A, Carty CL, et al. Vascular risk factors and findings on brain MRI of elderly adult American Indians: the Strong Heart Study. Neuroepidemiology. 2019;52:173‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mensah GA, Wei GS, Sorlie PD, et al. Decline in cardiovascular mortality: possible causes and implications. Circ Res. 2017;120:366‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Manly JJ. Advantages and disadvantages of separate norms for African Americans. Clin Neuropsychol. 2005;19:270‐275. [DOI] [PubMed] [Google Scholar]
- 42. Berry CM, Clark MA, McClure TK. Racial/ethnic differences in the criterion‐related validity of cognitive ability tests: a qualitative and quantitative review. J Appl Psychol. 2011;96:881‐906. [DOI] [PubMed] [Google Scholar]
- 43. Pedraza O, Mungas D. Measurement in cross‐cultural neuropsychology. Neuropsychol Rev. 2008;18:184‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Paddick SM, Gray WK, McGuire J, Richardson J, Dotchin C, Walker RW. Cognitive screening tools for identification of dementia in illiterate and low‐educated older adults, a systematic review and meta‐analysis. Int Psychogeriatr. 2017;29:897‐929. [DOI] [PubMed] [Google Scholar]
- 45. Wu Y, Zhang Y, Yuan X, Guo J, Gao X. Influence of education level on MMSE and MoCA scores of elderly inpatients. Appl Neuropsychol Adult. 2023;30(4):414‐418. doi: 10.1080/23279095.2021.1952588. Epub 2021 Jul 15. [DOI] [PubMed] [Google Scholar]
- 46. Suchy‐Dicey AM, Vo TT, Oziel K, et al. Psychometric reliability, validity, and generalizability of 3MSE scores among American Indian adults: the Strong Heart Study. J Int Neuropsychol Soc. 2024:1‐10. doi: 10.1017/S1355617723011438. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 47. Koepsell TD, Monsell SE. Reversion from mild cognitive impairment to normal or near‐normal cognition: risk factors and prognosis. Neurology. 2012;79:1591‐1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Graves LV, Edmonds EC, Thomas KR, Weigand AJ, Cooper S, Bondi MW. Evidence for the utility of actuarial neuropsychological criteria across the continuum of normal aging, mild cognitive impairment, and dementia. J Alzheimers Dis. 2020;78:371‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Milani SA, Marsiske M, Cottler LB, Chen X, Striley CW. Optimal cutoffs for the Montreal Cognitive Assessment vary by race and ethnicity. Alzheimers Dement (Amst). 2018;10:773‐781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Daugherty J, Waltzman D, Sarmiento K, Xu L. Traumatic brain injury‐related deaths by race/ethnicity, sex, intent, and mechanism of injury—United States, 2000‐2017. MMWR Morb Mortal Wkly Rep. 2019;68:1050‐1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Peterson AB, Sarmiento K, Xu L, Haileyesus T. Traumatic brain injury‐related hospitalizations and deaths among American Indians and Alaska natives—United States, 2008‐2014. J Safety Res. 2019;71:315‐318. [DOI] [PubMed] [Google Scholar]
- 52. Suchy‐Dicey AM, Muller CJ, Madhyastha TM, et al. Telomere length and magnetic resonance imaging findings of vascular brain injury and central brain atrophy: the Strong Heart Study. Am J Epidemiol. 2018;187:1231‐1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Data Availability Statement
These data are the property of the sovereign tribal nations from which they were gathered. Scientific use of these data can be requested from the Strong Heart Study (https://strongheartstudy.org).


