Abstract
INTRODUCTION
Plasma biomarkers of Alzheimer's disease and related dementias predict global cognitive performance and decline over time; it remains unclear how they associate with changes in different dementia syndromes affecting distinct cognitive domains.
METHODS
In a prospective study with repeated assessments of a randomly selected population‐based cohort (n = 787, median age 73), we evaluated performance and decline in different cognitive domains over up to 8 years in relation to plasma concentrations of amyloid beta 42/40 (Aβ42/40) ratio, phosphorylated tau181 (p‐tau181), neurofilament light chain (NfL), and glial fibrillary acidic protein (GFAP).
RESULTS
Cross‐sectionally, memory showed the strongest associations with p‐tau181, and attention, executive, and visuospatial functions with NfL. Longitudinally, memory decline was distinguishable with all biomarker profiles dichotomized according to data‐driven cutoffs, most efficiently with Aβ42/40. GFAP and Aβ42/40 were the best discriminators of decline patterns in language and visuospatial functions, respectively.
DISCUSSION
These relatively non‐invasive tests may be beneficial for clinical screening after replication in other populations and validation through neuroimaging or cerebrospinal fluid analysis.
Highlights
We performed a prospective study with up to 8 years of repeated domain‐specific cognitive assessments and baseline plasma Alzheimer's disease and related dementias biomarker measurements in a randomly selected population‐based cohort.
We considered distinct growth curves of trajectories of different cognitive domains and survival bias induced by missing data by adding quadratic time and applying joint modeling technique.
Cross‐sectionally, memory showed the strongest associations with plasma phosphorylated tau181, while attention, executive, and visuospatial functions were most strongly associated with neurofilament light chain.
Longitudinally, memory and visuospatial declines were most efficiently distinguished by dichotomized amyloid beta 42/40 profile among all plasma biomarkers, while language was by dichotomized glial fibrillary acidic protein.
These relatively non‐invasive tests may be beneficial for clinical screening; however, they will need replication in other populations and validation through neuroimaging and/or cerebrospinal fluid assessments.
Keywords: Alzheimer's disease and related dementias, amyloid beta 42/40 ratio, attention, cognitive decline, cognitive domains, glial fibrillary acidic protein, language, memory, neurofilament light chain, phosphorylated tau181, plasma biomarkers, visuospatial functions
1. INTRODUCTION
Positron emission tomography (PET) imaging and cerebrospinal fluid (CSF) biomarkers are now established modalities for detecting in vivo biological evidence of Alzheimer's disease (AD). 1 , 2 , 3 However, their limited accessibility, high cost, and low throughput hamper their widespread applications. Plasma biomarkers are relatively non‐invasive and low‐cost alternatives for monitoring the biology of AD and related disorders (ADRD). 3 , 4 , 5 Amyloid beta 42/40 (Aβ42/40) ratio, phosphorylated tau at threonine‐181 (p‐tau181), neurofilament light chain (NfL), and glial fibrillary acidic protein (GFAP) are among the most extensively studied blood biomarkers of ADRD. 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 Recent studies have shown strong correlations of plasma Aβ42/40 ratio and p‐tau181 with brain Aβ burden, as well as with concurrent and subsequent cognitive performance. 6 , 16 , 17 , 18 , 19 , 20 , 21 , 22 Plasma p‐tau181 shows good correlations with brain tau burden, while plasma NfL associates with magnetic resonance imaging (MRI)‐ and CSF‐assessed neurodegeneration in AD and ADRD. 11 , 12 , 23 Furthermore, plasma GFAP, measuring astrocyte reactivity, is closely related to abnormal Aβ accumulation as well as cognitive status in AD and, as with NfL, has been reported to be altered in non‐AD dementias. 24 , 25 , 26 , 27 , 28 Together, plasma biomarkers of ADRD are both cross‐sectionally and longitudinally associated with cognitive performance.
However, cognitive performance in earlier studies was most often aggregated as global cognition by averaging results from multiple cognitive domains. 22 , 25 , 29 , 30 , 31 , 32 Because different cognitive domains may become affected at various disease stages, and potentially reflect or predict distinct dementia presentations or syndromes, it is of interest to investigate cross‐sectional and longitudinal associations of plasma biomarkers with specific domains of cognition. These investigations are particularly needed in large population‐based cohorts, which are: (1) unselected for memory or other cognitive disorders, (2) less exclusive of general health factors and conditions, (3) more representative of broad communities than most clinic‐based studies, and (4) less well represented in the ADRD biomarker literature.
The novel aspects of the study include both population‐based recruitment and domain‐specific cognitive outcomes. By studying a population‐based unselected cohort, relatively unaffected by selection bias, we can better understand the natural trajectory of cognitive outcomes and their associations with plasma biomarkers in a broader context. This approach is valuable for uncovering early markers of cognitive changes and informing public health strategies. By examining domain‐specific cognitive outcomes, we gain a more nuanced understanding of how plasma biomarkers are associated with specific cognitive abilities, critical for tailoring interventions and treatments that target specific cognitive deficits.
We have recently reported cross‐sectional findings for plasma Aβ42/40 ratio, p‐tau181, NfL, and GFAP in a population‐based cohort of older adults, finding a bimodal distribution of Aβ42/40 that separated the population into two modes differentially associated with the other biomarkers, age, Clinical Dementia Rating (CDR), and a memory composite score. 33 Building on those cross‐sectional findings, we now examine associations between the same plasma biomarkers and both concurrent status and subsequent decline in performance over time in multiple cognitive domains, focusing on participants without dementia at study entry.
2. METHODS
2.1. Study setting and participants
The Monongahela–Youghiogheny Healthy Aging Team (MYHAT) is an ongoing population‐based study cohort drawn from a Rust Belt region of southwestern Pennsylvania, USA. MYHAT participants are assessed annually for the development of cognitive decline, mild cognitive impairment (MCI), and dementia. Study recruitment took place over two time periods (2006–2008 and 2016–2019) using age‐stratified random sampling from publicly available voter registration lists. Individuals were excluded if, at study entry, they were < 65 years old, not living in one of the designated towns, residing in long‐term care, had severe hearing or vision loss which precluded neuropsychological testing, or lacked decisional capacity to give informed consent. The full assessment was administered to participants with normal or mildly impaired cognition (age‐ and education‐corrected Mini‐Mental State Examination scores ≥ 21) 34 numbering 1982 in the original cohort and 703 in the second cohort. All study procedures were approved by the University of Pittsburgh Institutional Review Board, and all participants provided written informed consent. Further details have been reported previously. 35
2.2. Study assessments
Detailed assessment interviews included, but were not limited to, demographics, cognitive assessments, genotyping, and plasma biomarker measurements, all detailed in the following subsections.
2.2.1. Demographics
Age, sex, education (less than 8th grade or 8th to 11th grade [< high school, HS]; graduated from HS or General Educational Development [= HS]; some college, graduated from college program or graduate school [> HS]), and self‐identified race/ethnicity (White; Black or African American, more than one race [non‐White]).
RESEARCH IN CONTEXT
Systematic review: The authors searched and reviewed the extant literature using traditional (e.g., PubMed) sources. There were highly limited studies on associations between domain‐specific cognition composite as well as its longitudinal declines and Alzheimer's disease and related dementias and plasma biomarkers, but the relationships between global cognition and biomarkers were well studied. Those relevant citations are appropriately introduced and cited.
Interpretation: In this population‐based study (n = 787), memory showed the strongest cross‐sectional associations with plasma phosphorylated tau181, while attention, executive, and visuospatial functions had the strongest associations with neurofilament light chain. Longitudinally, after the application of data‐driven cutoffs, amyloid beta 42/40 profile most efficiently distinguished memory and visuospatial functions decline patterns, while glial fibrillary acidic protein distinguished declines in language.
Future directions: Future studies will focus on: (a) the potential confounding and effect modifications among the associations between cognitive domain and plasma biomarkers; (b) the validations and replications on the data‐driven cutoffs/thresholds; and (c) the biomarker method standardization, optimal panel selection, and thresholding.
2.2.2. Cognitive assessments
At baseline and each annual visit, a battery of neuropsychological tests was administered to evaluate cognitive functioning across five cognitive domains: attention/psychomotor speed (Digit Span, Trail‐Making Test A), executive functions (Trail‐Making Test B, clock drawing, verbal fluency for letters P&S), memory (Wechsler Memory Scale‐Revised, Logical Memory, 12‐item Face Name Associative Memory Exam, 36 and Fuld Object Memory Evaluation), language (Boston Naming Test, semantic verbal fluency, Indiana University Token Test), and visuospatial functions (Wechsler Adult Intelligence Scale‐3 Block Design and Benton Visual Form Discrimination). 37 , 38 To develop composite scores for each domain, we first created z scores initially standardizing each test score according to the sample baseline mean and standard deviation, and then averaging the standardized test scores within each domain for participants with at least one test score in that domain, 39 with higher composite scores indicating better cognitive performance.
2.2.3. Clinical dementia rating
CDR was grouped into 0 = cognitively normal (CN), 0.5 = MCI, ≥ 1 = dementia, as described previously. 33
2.2.4. Blood collection
Venous blood was collected after overnight fasting into purple‐top ethylenediaminetetraacetic acid tubes as described previously 33 for genotyping and plasma biomarkers.
2.2.5. APOE ε4
Apolipoprotein E (APOE) genotyping was carried out using blood or saliva samples as described previously, 33 and for this analysis was grouped into APOE ε4 carriers (with any ε4 allele) versus non‐carriers.
2.2.6. Plasma biomarker measurements
Plasma biomarker concentrations were assayed with single‐molecule array (SIMOA) technology using an HD‐X instrument from Quanterix at the Department of Psychiatry, University of Pittsburgh School of Medicine. Specimens were tested for p‐tau181 using the p‐tau181 V2 Advantage (#103714) assay. NfL, GFAP, Aβ42, and Aβ40 were analyzed with the Neurology 4‐Plex E (#103670) commercial assays, as detailed previously. 33 We performed two sets of analyses, the primary analysis including all samples that had measurable plasma biomarker signals, and a secondary analysis limiting the biomarker concentrations to those above the manufacturer's recommended detection limit, to test the hypothesis that the concentrations would be valid independent of the detection limits.
2.3. Statistical analysis
As previously reported, the log‐transformed Aβ40, Aβ42, and Aβ42/40 ratio were bimodally distributed in the MYHAT study cohort. 33 We applied the K‐medoids unsupervised clustering method 40 for the log‐transformed Aβ42/40 and identified two distinct clusters, which we labeled normal and abnormal groups based on the optimal cutoff/threshold values of −2.08 (i.e., 0.1249 in raw scale). We validated these groups by examining their associations with p‐tau181, NfL, and GFAP levels.
We designated as baseline the date of the annual MYHAT cognitive assessment closest to the blood draw date for plasma biomarkers, if these dates were no more than 6 months apart. We present baseline characteristics as medians (interquartile ranges [IQRs]) for continuous variables and frequencies (%) for categorical variables, both overall and by Aβ42/40 groups. To compare among groups, we used the Wilcoxon rank‐sum tests for continuous variables and Fisher exact test for categorical variables. We used all available outcome data from all cycles.
We used multiple linear regression models to examine cross‐sectional associations between each plasma biomarker and each of the five baseline cognitive domain composite scores (memory, attention/speed, language, executive functions, and visuospatial functions). Each model evaluated one biomarker at a time, while adjusting for demographics (age, sex, race, and education) and APOE ε4 genotype as they are known to affect cognitive performance.
To address the concerns about potential survival and attrition bias in the longitudinal study, we applied the joint modeling approach to analyze longitudinal relationships between plasma biomarkers and changes in domain‐specific cognitive composite scores over time. First, to verify whether joint modeling is necessary and determine the shape of the growth curve in each domain, we fitted a baseline joint model without including any plasma biomarker variable for each domain. Each baseline joint model consists of two submodels 41 adjusted for age, sex, race, education, and APOE ε4 genotype: (1) a growth curve model of domain‐specific cognitive decline, which uses a linear mixed‐effects regression model (LMM) with random intercepts for participants and random coefficients for time; and (2) an informative dropout model of time to death or becoming too ill to participate, which uses a Cox regression model, with the additional control for baseline CDR. Then, to examine the associations, we included each of the plasma biomarkers separately in the LMM submodel.
To visualize the trajectories at different biomarker levels and explore potential cutoff points, we categorized p‐tau181, NfL, and GFAP based on different thresholds: at the median (resulting in two groups), at the tertiles (resulting in three groups), and at the quartiles (resulting in four groups). Subsequently, we selected the cutoff method at tertiles as it most effectively distinguished the trajectory patterns between the corresponding groups. We further combined the first two tertile groups into one, therefore dichotomizing each biomarker into two groups (lower and higher groups) at the 66.66th percentile based on the findings from the tertile group analyses.
We also conducted several sensitivity analyses to evaluate the impact on our cross‐sectional and longitudinal findings of variations in (1) the cutoff for Aβ42/40 (dichotomizing it using a cutoff given by another clustering method), (2) the data type of plasma biomarkers (categorical or continuous values), (3) the quality control for the biomarker values (including or excluding those below the manufacturer's detection limits), and (4) the use of Aβ biomarkers (clustering based on Aβ42/40 ratio or on Aβ42 alone; see Method S1 in supporting information).
In addition, we conducted two secondary analyses to examine the potential effect modifications by sex and CDR on the associations between the rates of decline in cognitive composite scores and plasma biomarkers: (1) including three‐way interaction among sex, plasma biomarker, and time in each LMM submodel, and (2) additionally adjusting for CDR levels and three‐way interaction among CDR, plasma biomarker, and time in each submodel.
We applied the Benjamini–Hochberg procedure to control for the false discovery rate (FDR) in multiple hypothesis testing within the same domain.
We performed all analyses using R Statistical Software (v4.2.2; R Core Team 2022). We analyzed the joint models using the R JMBayes 42 package, incorporating regression‐spline‐approximation baseline hazard functions and 40,000 iterations.
3. RESULTS
3.1. Sample characteristics at baseline
Among all 2685 enrolled MYHAT participants, 920 had available plasma samples. 33 Of those, we excluded 65 participants with one or more plasma biomarkers for which measurement signals (called average enzyme per bead) were assigned by the instrument but without corresponding concentration values (41 in Aβ42/40, 41 in p‐tau181, 38 in NfL, and 38 in GFAP). Excluding another 30 whose “baseline” visits were beyond 6 months of blood draw, 9 whose CDR > = 1 at baseline and 29 whose APOE genotypes were missing, we included 787 individuals with CDR < 1 (dementia‐free) in the cross‐sectional analyses. Their median age was 73 years (IQR: 69 to 83), 63.5% were female, 95.4% were White, and 58.6% had more than high school (> HS) education (Table 1).
TABLE 1.
Baseline participant characteristics according to the Aβ42/40 ratio status.
| Aβ42/40 normal (N = 686) | Aβ42/40 abnormal (N = 101) | Total (N = 787) | ||
|---|---|---|---|---|
| Age, median (IQR), years | 73.0 (69.0, 80.0) | 83.0 (78.0, 87.0) | 73.0 (69.0, 80.0) | <0.001 |
| Sex Female, N(%) | 431(62.8%) | 69 (68.3%) | 500 (63.5%) | 0.320 |
| Race Non‐White, N(%) | 33 (4.8%) | 3 (3.0%) | 36 (4.6%) | 0.609 |
| Education, N(%) | 0.018 | |||
| < High School | 32 (4.7%) | 9 (8.9%) | 41 (5.2%) | |
| = High School | 240 (35.0%) | 45 (44.6%) | 285 (36.2%) | |
| > High School | 414 (60.3%) | 47 (46.5%) | 461 (58.6%) | |
| APOE ε4 carrier, N(%) | 152 (22.2%) | 20 (19.8%) | 172 (21.9%) | |
| MCI group, N(%) | 103 (15.0%) | 14 (13.9%) | 117 (14.9%) | 0.881 |
| Plasma biomarker b , median (IQR), pg/mL | ||||
| Aβ40 | 103.945 (83.647, 123.440) | 2.153 (1.377, 3.505) | 99.949 (60.702, 119.079) | <0.001 |
| Aβ42 | 6.825 (5.340, 8.161) | 0.625 (0.380, 0.841) | 6.398 (3.827, 7.918) | <0.001 |
| Aβ42/40 | 0.067 (0.059, 0.074) | 0.237 (0.169, 0.347) | 0.069 (0.060, 0.079) | <0.001 |
| p‐tau181 | 1.661 (1.185, 2.461) | 1.533 (0.806, 2.104) | 1.630 (1.151, 2.434) | 0.004 |
| NfL | 21.471 (16.218, 31.333) | 38.304 (28.590, 52.171) | 23.147 (16.955, 34.961) | <0.001 |
| GFAP | 122.001 (85.189, 175.047) | 221.199 (153.642, 341.984) | 129.964 (89.084, 194.295) | <0.001 |
| Comorbidity c , N(%) | ||||
| Diabetes | 168 (24.5%) | 30 (29.7%) | 198 (25.2%) | 0.270 |
| Hypertension | 462 (67.3%) | 77 (76.2%) | 539 (68.5%) | 0.085 |
| Kidney disease | 33 (4.8%) | 8 (7.9%) | 41 (5.2%) | 0.225 |
Note: This table presents the characteristics of the study population by Aβ42/40 status. Aβ42/40 normal is defined as Aβ42/40 0.1249, equivalent to exp(−2.08), while Aβ42/40 abnormal corresponds to Aβ42/40 > 0.1249. All continuous variables are presented as median and interquartile range: median, Q1, Q3, while categorical variables are shown as frequency and percentages: N (%).< High School: < 8th grade or 8th to 11th grade. = High School: graduated from high school or Generalized Educational Development. > High School: graduated from college, 4‐year college program, or graduate school. Non‐White: Black or African American, or more than one race. CN: CDR = 0, cognitive normal. MCI: CDR = 0.5, mild cognitively impaired.
Abbreviations: Aβ, amyloid beta; APOE, apolipoprotein E; CDR, Clinical Dementia Rating; GFAP, glial fibrillary acidic protein; IQR, interquartile range; MCI, mild cognitive impairment; NfL, neurofilament light chain; p‐tau, phosphorylated tau.
P values represent the comparison between Aβ42/40 normal and abnormal groups. For continuous variables, they were derived from the Wilcoxon rank‐sum tests, and for categorical variables, they were derived from Fisher exact tests. The significant level of 0.05 was used. Significant results are indicated in bold.
Plasma biomarkers were raw values before standardizations.
Participants self‐reported having been told by a health‐care professional that they had diabetes, hypertension until the collection of the plasma sample.
3.2. Characteristics of normal and abnormal Aβ groups
Of the 787 dementia‐free participants, 101 (12.83%) with abnormal plasma Aβ42/40 (Aβ42/40 > 0.1249 33 ) were older (median age 83 vs. 73 years) and less educated (46.5% vs. 60.3% had > HS education) than those with normal Aβ42/40 levels.
The abnormal group had lower Aβ42 levels which agrees with the literature for CSF Aβ42. 43 , 44 To the contrary, the Aβ42/40 ratio was higher in this group instead of the lower levels reported for CSF. 45 , 46 We attribute this disparity in Aβ42/40 to the bimodal distribution of Aβ40 in this population. Participants with abnormally low Aβ42 values also had extremely low Aβ40, resulting in a higher value of the Aβ42/40 ratio in the participants with higher Aβ42 (see Figure S1 in supporting information).
3.3. Characteristics of the CN and MCI groups at baseline
In this population, 117 (14.87%) MCI participants with CDR = 0.5 at baseline were older (median age 80 vs. 73 years), less educated (45.3% vs. 60.9% had > HS education), and had a higher proportion of APOE ε4 carriers (30.8% vs. 20.3%) than the CN group with CDR = 0 (Table S1 in supporting information).
3.4. Characteristics of the longitudinal sample
For the longitudinal analysis, we included 2820 records from 680 participants after excluding 107 individuals with no follow‐up visits. Those included were more educated and had higher baseline attention, language, executive, and visuospatial functions composite scores than those excluded (Table S2 in supporting information). The median follow‐up period was 3 years (range: 1–8 years; IQR: 2–4 years). The availability of participants, the number of informative dropout (i.e., death or too ill), and the missingness of the cognitive composite score per year are summarized in Table 2.
TABLE 2.
Missingness and availability of data in each year.
| Year | baseline | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|---|
| Number of participants a , N | 680 | 644 | 533 | 420 | 202 | 143 | 110 | 63 | 9 |
| Number of informative dropouts b , N | 24 | 52 | 72 | 93 | 91 | 113 | 115 | 37 | |
| Number of missing c , N | |||||||||
| Attention | 11 | 18 | 15 | 13 | 9 | 3 | 7 | 2 | 1 |
| Executive functions | 2 | 5 | 7 | 2 | 4 | 0 | 4 | 2 | 1 |
| Language | 13 | 18 | 11 | 12 | 10 | 4 | 6 | 2 | 1 |
| Memory | 18 | 26 | 22 | 18 | 12 | 6 | 14 | 8 | 1 |
| Visuospatial functions | 113 | 219 | 245 | 203 | 97 | 78 | 98 | 52 | 7 |
Number of participants with at least one cognitive score(s) available within the corresponding year.
Number of participants dropped out from the study due to death or too ill.
Number of missing cognitive composite scores at each year in each domain.
3.5. Cross‐sectional associations
In multivariable linear regression models evaluating the association between each plasma biomarker and domain‐specific cognitive composite score (Table S3 in supporting information and Figure 1), the following results were observed:
Baseline mean memory composite score was significantly higher in the abnormal Aβ42/40 group than in the normal Aβ42/40 group (β = 0.212, 95% confidence interval [CI] −0.02 to −0.40, adjusted P = 0.031).
Baseline mean memory composite score decreased as plasma p‐tau181, NfL and GFAP increased. Memory had the strongest inverse association with higher p‐tau181 (β = −0.111, 95% CI −0.17 to −0.05, adjusted P = 0.002) compared to other biomarkers.
Baseline mean attention and executive functions composite scores were inversely associated with plasma p‐tau181 and NfL. Both had stronger associations with NfL (β = −0.125, 95% CI −0.18 to −0.07, adjusted P < 0.001 and β = −0.107, 95% CI −0.16 to −0.06, adjusted P < 0.001) than with p‐tau181.
Baseline mean visuospatial functions composite score only associated with NfL (β = −0.134, 95% CI −0.22 to −0.04, adjusted P = 0.015).
FIGURE 1.
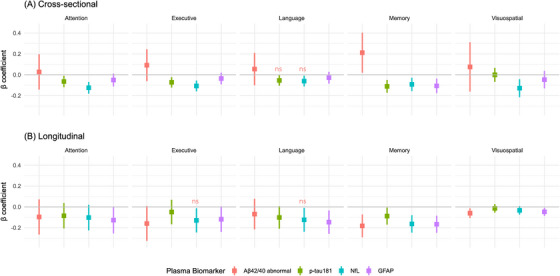
Cross‐sectional and longitudinal associations between plasma biomarkers and cognitive domain composite scores. Coefficient plot was generated to visualize the association between each baseline plasma biomarker and baseline domain‐specific cognitive composite score (A) and its rate of change (B). Each biomarker was individually added to a basic model adjusting for age, sex, race, education, and APOE allele. Aβ42/40 abnormal was defined as Aβ42/40 > 0.1249, equivalent to exp(−2.08). Plasma p‐tau181, NfL, and GFAP were standardized to their mean values and standard deviations in the cross‐sectional models (A), while were dichotomized based on their 66th percentiles in the longitudinal models (B). Estimated beta coefficients with the corresponding 95% confidence intervals were plotted for the associations of baseline plasma biomarkers with baseline or with the decline rate of neuropsychological test results. Estimates of beta coefficients in the cross‐sectional models (A) are presented in terms of “The difference in the mean baseline domain‐specific cognitive composite score in abnormal group compared to the mean in normal group” for Aβ42/40 or “The change in baseline domain‐specific cognitive composite score per standard deviation increase in baseline biomarker value” for p‐tau181, NfL, and GFAP. Estimates of beta coefficients in the longitudinal models (B) are presented in terms of “The difference between rates of change in the average domain‐specific cognitive composite scores in Aβ42/40 abnormal group compared with the normal group or p‐tau181, NfL, and GFAP higher groups compared to corresponding lower groups.” ns: The association became not significant after controlling for false discovery rate in multiple hypothesis testing on the same domain. Aβ, amyloid beta; APOE, apolipoprotein E; GFAP, glial fibrillary acidic protein; NfL, neurofilament light chain; p‐tau, phosphorylated tau.
3.6. Longitudinal associations
As shown in Table S4 in supporting information, the presence of a significant association parameter for either the random intercept or the random coefficient indicated that the risk of informative dropout was related to the random effect in the longitudinal model, which demonstrates that joint modeling is needed. We found joint modeling was necessary for modeling each of memory, attention, executive functions, and language, while fitting LMMs was sufficient for visuospatial functions. The memory, attention/speed, and language scores showed a quadratic trajectory over time, as indicated by significant coefficients of the time‐squared terms. In contrast, the executive functions and visuospatial functions scores demonstrated a linear trajectory.
3.6.1. Aβ42/40 groups
All average cognitive domain scores in the abnormal Aβ42/40 group declined faster than those in the normal group (Figure 2A and Figure S2 in supporting information); however, these differences in the rate of change were statistically significant only in the domains of memory (β = −0.182, standard deviation [SD] = 0.056, adjusted P = 0.001) and visuospatial functions (β = −0.060, SD = 0.024, adjusted P = 0.032; Table 3).
FIGURE 2.
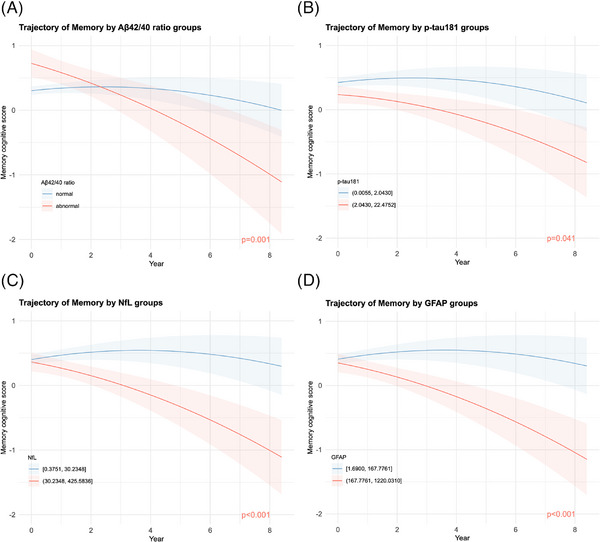
Trajectories of memory composite scores by plasma biomarker values. Trajectory plots were generated to visualize the estimated average memory composite score over the follow‐up period, based on (A) Aβ42/Aβ40, (B) p‐tau181, (C) NfL, and (D) GFAP groups. These plots were derived from linear mixed‐effects submodels of the joint models, accounting for longitudinal memory composite scores and informative dropout. Each plasma biomarker was individually added to a basic random coefficients model with age, sex, race, education, and APOE allele as fixed effects and time as random effects. The shaded areas represent Bayesian 95% credible intervals. The annotated raw P value indicates the significance of differences in decline rates between the abnormal group (red) and the normal group (blue) in (A), or between the higher group (red) and the lower group (blue) in (B)‐(D). The Aβ42/40 abnormal group was defined as plasma Aβ42/40 > 0.1249, equivalent to exp(−2.08). The cutoff values for other plasma biomarkers were their 66.66th percentile (upper tertile) and are shown in the figure legends. Aβ, amyloid beta; APOE, apolipoprotein E; GFAP, glial fibrillary acidic protein; NfL, neurofilament light chain; p‐tau181, phosphorylated tau.
TABLE 3.
Associations between baseline dichotomized plasma biomarkers and rate of change in domain‐specific cognitive composite score.
| Domain | Biomarker | β | SD | p value | Adjusted p value e |
|---|---|---|---|---|---|
| Memory | Aβ42/40 abnormal a | −0.182 | 0.056 | 0.001 | 0.001 |
| p‐tau181 T3 b | −0.088 | 0.042 | 0.041 | 0.041 | |
| NfL T3 c | −0.163 | 0.043 | <0.001 | <0.001 | |
| GFAP T3 d | −0.166 | 0.042 | <0.001 | <0.001 | |
| Attention | Aβ42/40 abnormal a | −0.096 | 0.086 | 0.257 | 0.257 |
| p‐tau181 T3 b | −0.085 | 0.062 | 0.155 | 0.207 | |
| NfL T3 c | −0.102 | 0.063 | 0.099 | 0.198 | |
| GFAP T3 d | −0.127 | 0.065 | 0.051 | 0.198 | |
| Language | Aβ42/40 abnormal a | −0.069 | 0.075 | 0.379 | 0.379 |
| p‐tau181 T3 b | −0.101 | 0.055 | 0.061 | 0.081 | |
| NfL T3 c | −0.124 | 0.058 | 0.036 | 0.072 | |
| GFAP T3 d | −0.146 | 0.057 | 0.005 | 0.020 | |
| Executive functions | Aβ42/40 abnormal a | −0.159 | 0.085 | 0.066 | 0.088 |
| p‐tau181 T3 b | −0.049 | 0.060 | 0.407 | 0.407 | |
| NfL T3 c | −0.129 | 0.059 | 0.030 | 0.088 | |
| GFAP T3 d | −0.118 | 0.062 | 0.054 | 0.088 | |
| Visuospatial functions | Aβ42/40 abnormal a | −0.060 | 0.024 | 0.012 | 0.032 |
| p‐tau181 T3 b | −0.014 | 0.021 | 0.493 | 0.493 | |
| NfL T3 c | −0.033 | 0.021 | 0.110 | 0.147 | |
| GFAP T3 d | −0.047 | 0.020 | 0.016 | 0.032 |
Note: This table shows the results of linear mixed effects submodels of the joint models to determine whether the decline rate of domain‐specific cognitive score varies between plasma biomarker levels. Each submodel used a domain‐specific cognitive composite score as the outcome variable and included a binarized baseline plasma biomarker in addition to a basic model containing age, sex, race, education, and APOE allele. The β coefficient represents the estimate of the effect of the interaction between each plasma biomarker and time. It is presented as “The difference in yearly change in domain‐specific cognitive composite scores between the abnormal group or highest tertile (Tertile‐3) and the normal group or the combination of the lowest and middle tertile groups (Tertile‐1 and Tertile‐2).” The comparison is made for each of Aβ42/40 ratio and other biomarkers respectively. The SD represents the standard deviation of the point estimate. P value is derived from an analysis of variance comparison to the basic model, with a significant level of 0.05. Significant results are indicated in bold.
Abbreviations: Aβ, amyloid beta; APOE, apolipoprotein E; GFAP, glial fibrillary acidic protein; NfL, neurofilament light chain; p‐tau, phosphorylated tau.
Aβ42/40 abnormal was defined as Aβ42/40 > 0.1249, equivalent to exp(‐2.08).
Highest tertile (Tertile‐3/T3) of p‐tau181 was (2.0430, 22.4752).
Highest tertile (Tertile‐3/T3) of NfL was (30.2348, 425.5836).
Highest tertile (Tertile‐3/T3) of GFAP was (167.7761, 1220.0310).
The adjusted p values were calculated by the Benjamini–Hochberg procedure to control for the false discovery rates in multiple testing on the same domain.
3.6.2. P‐tau181, NfL, and GFAP
The best separation of different trajectory patterns was provided by tertiles, which we designated from lowest to highest as Tertile‐1, Tertile‐2, and Tertile‐3. Rates of decline in the different cognitive domains were generally similar between Tertile‐1 and Tertile‐2 but both were significantly different from Tertile‐3 (Figure 3, Figure S3 and Table S5 in supporting information). Therefore, we dichotomized p‐tau181, NfL, and GFAP into lower (Tertile‐1 and Tertile‐2 combined) and higher (Tertile‐3) groups at the 66.66th percentile (Figure 2 and Table 3).
FIGURE 3.
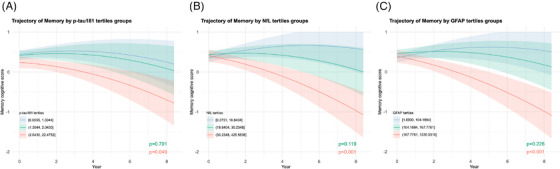
Trajectories of memory composite scores by plasma biomarker tertiles. Trajectory plots were generated to visualize the estimated average memory composite score over the follow‐up period, based on (A) p‐tau181, (B) NfL, and (C) GFAP tertile groups. The plots were derived from linear mixed‐effects submodels of the joint models, accounting for longitudinal domain‐specific composite scores and informative dropout. Each plasma biomarker was individually added to a basic random coefficients model with age, sex, race, education, and APOE carriership as the fixed effects and time as random effects. Shaded areas represent Bayesian 95% credible intervals. The annotated raw P value indicates the significance of differences in decline rates between the middle (green) or highest (red) tertile group and the lowest tertile group (blue). The cutoff values for these plasma biomarkers were based on their respective tertiles, as described in the figure legends. APOE, apolipoprotein E; GFAP, glial fibrillary acidic protein; NfL, neurofilament light chain; p‐tau, phosphorylated tau.
For p‐tau181, NfL, and GFAP, mean memory composite score showed 0.088 (SD = 0.042, adjusted P = 0.041), 0.163 (SD = 0.043, adjusted P < 0.001), and 0.166 (SD = 0.042, adjusted P < 0.001) faster declines in the higher groups, with the credible intervals well separated (Figure 2), than in the lower groups per year, respectively.
In the GFAP higher group, the mean language composite scores (β = −0.146, SD = 0.057, adjusted P = 0.020) and visuospatial functions (β = −0.047, SD = 0.020, adjusted P = 0.032) had significantly faster declines compared to those in the lower group.
No biomarkers were associated with the slopes of the attention and executive trajectories after controlling for the FDR.
3.6.3. Comparisons within memory domain
Comparing the lower groups of p‐tau181, NfL, and GFAP and the normal Aβ42/40 group (Figure 2), the shapes of the mean memory trajectories were similar. Over time, the average memory score in the normal/lower groups initially increased before declining, suggesting practice effects 47 over earlier cycles, whereas the average score in the corresponding lower and abnormal groups consistently decreased over time.
Comparing the higher and abnormal groups of the four biomarkers (Figure 2), at baseline, the estimated mean memory score in the Aβ42/40 abnormal group was much higher (with a credible interval even above 0.5) than the mean baseline scores in p‐tau181, NfL, and GFAP higher groups. However, over time, mean memory scores in the Aβ42/40 abnormal, NfL higher, and GFAP higher groups decreased to a value below −1.0 by year 8. This observation was consistent with the magnitude of effect (beta coefficient) of the interaction between Aβ42/40 abnormal and time being larger than the effects of any of the other interactions (Table 3). On the contrary, the trajectories grouped by p‐tau181 appeared more parallel than those grouped by other plasma biomarkers (Figure 2), and the beta coefficient of its interaction with time was the smallest one in magnitude (Table 3).
3.7. Secondary analyses
3.7.1. Sex effect modification on longitudinal associations
After refitting all longitudinal joint models further including three‐way interaction among sex, plasma biomarker, and time, we did not find any significant results (Table S6 in supporting information). Given the possibility of insufficient power to detect any significant effect modifications, we further looked at the estimates of those effect modifications; however, all magnitudes of the estimates were relatively small (< 0.100), suggesting the absence of modifying effect by sex.
3.7.2. CDR effect modification on longitudinal associations
Because most of our study cohort was CN, we did not have adequate power to detect any statistically significant modifying effects of CDR level on any longitudinal association between domain‐specific cognitive composite scores and plasma biomarkers (Table S7 in supporting information). Nevertheless, some relatively large (> 0.100) estimates of the effects of three‐way interactions in magnitude indicated that there tended to be an effect if the sample size was large enough. For example, the difference in memory composite score declines between two Aβ42/40 groups might be smaller in MCI group compared to CN; however, this difference was not statistically significant () based on the sample size in this study.
3.8. Sensitivity analyses
The results of the sensitivity analyses demonstrated that: (1) both cross‐sectional and longitudinal associations between domain‐specific cognition and plasma biomarkers were mostly robust with minor changes in the Aβ42/40 cutoff values (Result S1, Table S8, and Table S9 in supporting information); (2) several longitudinal associations lost statistical significance when treating plasma biomarkers as continuous variables (Result S1, Table S8, and Table S10 in supporting information); (3) the values that were below the manufacturer's detection limits did not substantially influence the clustering outcome despite small effects on the strength of some associations (Result S2, Table S11–S14, Figure S4, and S5 in supporting information); and (4) compared to Aβ42 alone, Aβ42/40 ratio gave a more narrowly defined abnormal group and more clearly separated trajectories in the memory and visuospatial functions domains (Result S3, Table S8, S9, S12, S14, S15, and Figure S6 in supporting information).
4. DISCUSSION
In individuals without dementia in the MYHAT longitudinal population‐based study of older adults, assessed annually up to 8 years after plasma collection, we found for the first time in a real‐world setting that plasma measures of Aβ42/40, p‐tau181, NfL, and GFAP provide comparable results to those obtained in more selective cohorts, providing support to their potential widespread applicability. Further, we examined relationships of these different plasma biomarkers with specific cognitive domains—memory, attention/psychomotor, language, visuospatial functions, and executive functions—not only cross‐sectionally but, more importantly, also their longitudinal trajectories, assessing which cognitive domain is the most strongly affected by a specific pathology. We show that, even in an overwhelmingly cognitively unimpaired population, these plasma biomarker profiles can distinguish the different patterns of domain‐specific cognitive decline. Our results suggest differential associations of plasma biomarkers with domain‐specific cognitive changes, implying that plasma biomarkers may have variable sensitivity to detecting cognitive changes arising from different domains.
We had expected Aβ42/40 and p‐tau181 to show the strongest associations with AD‐typical memory performance and faster memory decline relative to other cognitive domains, while expecting non–AD‐specific NfL and GFAP to show less differentiated associations across domains. These general patterns of results were only partially observed.
Regarding longitudinal trajectory patterns of biomarker‐cognitive domain associations, memory was the only cognitive domain in which decline was significantly differentiated with the groupings of all four plasma biomarkers. Abnormal Aβ42/40 and Tertile‐3 GFAP also showed faster declines in estimated mean visuospatial functions composite scores compared to normal Aβ42/40 and lower group of GFAP, respectively. GFAP was associated with the rates of change across more cognitive domains than were others, given it was the only one associated with language decline. Aβ42/40 was the most effective measure in distinguishing longitudinal cognitive decline in the memory and visuospatial domains between individuals with normal and abnormal biomarker profiles, while the rates of declines in memory between two p‐tau181 groups were least differentiable. These findings suggest that Aβ42/40 profile might be the best plasma biomarker for investigating the normal and abnormal memory decline patterns than the other biomarkers; however, plasma p‐tau181 may not be well suited for separating the clinical groups according to memory decline rates. Future work will include categorical outcomes such as incident dementia and etiologic subtypes to further investigate the predictive validity and utility of these biomarkers.
Although Aβ42/40 ratio is considered superior to Aβ42 alone to identify AD pathophysiology in CSF, 48 , 49 , 50 we examined both plasma Aβ42/40 and Aβ42 categorized as normal or abnormal. Note that participants with abnormally low Aβ42 values also had extremely low Aβ40 levels, resulting in higher Aβ42/40 ratios. Cross‐sectional analyses revealed robust associations between Aβ42, but not Aβ42/40, and the cognitive domains, as also reported by others. 20 , 31 , 51
A novel finding in our longitudinal models was that memory, attention, and language composite scores followed quadratic trajectories, in contrast to previous studies that only investigated linear decline. 22 , 29 , 31 , 52 This finding also accounted for selective survival bias by jointly modeling cognitive decline and informative dropout to estimate unbiased cognitive trajectories.
The abnormal Aβ42/40 group largely comprised individuals with lower Aβ42 and higher NfL and GFAP levels, meaning they had jointly altered biomarker profiles for Aβ pathology, neurodegeneration, and astroglial reactivity. This group showed monotonically faster declines and continued deterioration over time in memory and visuospatial functions. This finding confirms those of previous studies in which lower plasma Aβ42 was associated with faster cognitive decline, lower CSF Aβ42/40, and higher neocortical Aβ deposition. 7 , 51 , 52 , 53
We characterized plasma p‐tau181, NfL, and GFAP into three tertile groups based on their distributions and further dichotomized into two groups at the 66.66th percentiles, combining the two lower tertiles. All three highest tertile groups showed relatively faster declines in domain‐specific cognitive composite scores compared to lower tertile groups. A faster rate of change in memory composite score was significantly associated with each of the Tertile‐3 groups. 22 , 25 , 29 , 32 , 54 , 55 Of note, Tertile‐3 p‐tau181 was associated with faster memory decline, consistent with its role as an ADRD biomarker. However, faster declines in language and visuospatial domains were only observed in the GFAP Tertile‐3. Our results demonstrate the potential utility of upper tertile cutoffs of p‐tau181, NfL, and GFAP to predict domain‐specific cognitive changes.
In our main models, we included all individuals with measurable biomarker concentrations to avoid selection bias. However, our findings remained robust in the sensitivity analyses when we excluded values below the manufacturer's detection limits, demonstrating that their inclusion did not significantly skew the results. Furthermore, the results of the plasma Aβ42/40 clustering focusing on participants without dementia (CDR < 1) were comparable to those of our previous study that included all participants from the MYHAT cohort independent of cognitive status, 33 showing that the present results might be not significantly influenced by the exclusion of the participants with dementia.
Regarding strengths and limitations, we investigated the distribution and associations of plasma biomarkers in a population‐based cohort representative of older adults in its target geographic region. Our data thus have enhanced external validity (generalizability) compared to data from cohorts of self‐selected volunteers and referrals to clinical research settings. However, because the resulting cohort was largely of European descent, our findings should be investigated in more ethnoracially diverse study populations. The statistical models investigated both linear and quadratic trajectories of cognitive trajectories and considered survival bias using joint modeling. We examined multiple biomarkers individually, accounting for FDR and multiple comparisons. While beyond the scope of this study, we will further evaluate associations of cognitive decline with multiple biomarkers and their mutual interactions, to identify potential confounding and effect modification. 23 , 31 Our cutoffs/thresholds and clustering approaches were data‐driven; they will need to be replicated in other populations and examined in relation to PET and MRI imaging data. Furthermore, some previous studies have reported that the Quanterix/Simoa plasma Aβ42/40 assay used in this study may be outperformed by immunoprecipitation‐mass spectrometry (IP‐MS) assays. 56 Measurement of plasma Aβ42/40 by IP‐MS could not be performed in this study and there is currently no widely accessible IP‐MS platform for the other biomarkers. Additionally, the absence of plasma p‐tau217 data prevented us from examining its performance with plasma p‐tau181 in this cohort.
Key critiques of clinical trials that led to the recent US Food and Drug Administration approval of anti‐amyloid therapies some of which are now clinically available include enrichment for individuals with APOE ε4 genotype, lack of diversity of the study population in terms of recruitment source, and uncertainty if currently available plasma biomarkers will be useful for longitudinal monitoring of patients receiving the therapies. 57 , 58 The findings in this study—derived from those aged ≥ 65 years in the targeted area—are relevant to addressing some of these important points. The MYHAT study—by design—included mostly cognitively normal participants at baseline, allowing us to identify individuals at risk of cognitive decline, especially with the use of plasma surrogates for brain ADRD pathology. This is underscored by recent studies presented at the Clinical Trials in AD (CTAD conference 2023 59 ) that have shown much stronger beneficial effects of anti‐amyloid therapies when initiated among amyloid‐positive individuals with normal versus impaired cognition. Moreover, the population‐based design of this study reduced selection bias according to, for example, health status. This feature may potentially allow us in the future to examine the effects of disease‐relevant factors such as comorbid conditions, in the interest of personalizing treatment paradigms.
Plasma biomarkers are promising relatively non‐invasive and cost‐effective tools, compared to PET imaging and CSF assays, aiding in diagnosis and prognosis in various diseases that cause dementia. Population‐based studies are an essential step toward validating and calibrating them for eventual clinical use outside the specialty research and tertiary care setting. Future work will focus on biomarker method standardization, optimal panel selection, and thresholding. A major future contribution of these biomarkers may be to identify individuals for different intervention trials. Our current state of knowledge suggests that these biomarkers are not yet ready for clinical applications, but steady progress is being made toward that goal. Studies in real‐world populations are a step forward in that direction. Given the lack of widely available effective disease‐modifying therapeutic strategies, these measures are currently inappropriate for “screening” asymptomatic individuals outside research settings. This study adds to the rapidly expanding knowledge base about the potential of plasma biomarkers as research and clinical tools, by providing population‐based data from an under‐resourced US community. We also provide valuable insights into the relationships between individual biomarkers and domain‐specific cognitive declines.
While validating plasma ADRD biomarker performance in a population‐based sample is a step forward, several other issues remain to be addressed. One is the effect of co‐morbidities, both their direct effects on the different biomarkers and their independent, synergistic, or modulatory effects on cognitive trajectories over time. There is also an imperative need to assess how far the current results, mainly obtained in White individuals in the United States and Europe, can be generalized to diverse ethnic and geographic populations. Further, we need to extend the age range of these studies to examine their potential for early disease detection in the young‐old as well as their performance in the oldest old. Finally, it will be necessary to determine how these biomarkers perform in, for example, under‐resourced, rural, or remote populations, and how much they are affected by social determinants of health.
CONFLICT OF INTEREST STATEMENT
VLV received consulting fees from Eli Lilly and Life Molecular Imaging, and speaker honoraria from ACE Barcelona and BRI Japan—none related to this work. MG received honoraria from the University of Connecticut Health Center and from the Journal of the American Geriatrics Society, unrelated to this work. TKK has received honoraria from the University of Wisconsin Madison and the University of Pennsylvania and has an awarded patent (#WO2020193500A1), all unrelated to this work. All other authors declare that they have no conflicts of interest to disclose. Author disclosures are available in the supporting information.
CONSENT STATEMENT
All human subjects involved in this study provided informed consent prior to participation.
Supporting information
Supporting information
Supporting information
ACKNOWLEDGMENTS
The authors thank all MYHAT study personnel for their efforts and all MYHAT participants for their time, data, and specimens. PCLF is supported by Alzheimer's Association (AARFD‐22‐923814). VLV is supported by grant funding from the NIH (AG066468‐02, AG073267‐01), Aging Mind Foundation (DAF2255207), and the NHMRC IDEAS Grant G1005121. MG is supported by the NIH (R37 AG023651). TKK was funded by the NIH (1 R01 AG083874, U24 AG082930) and the Alzheimer's Association (AARF‐21‐850325). BB is supported by the Alzheimer's Association (AACSF‐20‐648075).
Zhang Y, Ferreira PCL, Jacobsen E, et al. Association of plasma biomarkers of Alzheimer's disease and related disorders with cognition and cognitive decline: The MYHAT population‐based study. Alzheimer's Dement. 2024;20:4199–4211. 10.1002/alz.13829
REFERENCES
- 1. Jack CR Jr, Bennett DA, Blennow K, et al. NIA‐AA Research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535‐562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Therriault J, Schindler SE, Salvad ó G, et al. Biomarker‐based staging of Alzheimer disease: rationale and clinical applications. Nat Rev Neurol. 2024;20(4):232‐244. [DOI] [PubMed] [Google Scholar]
- 3. Karikari TK, Ashton NJ, Brinkmalm G, et al. Blood phospho‐tau in Alzheimer disease: analysis, interpretation, and clinical utility. Nat Rev Neurol. 2022;18:400‐418. doi: 10.1186/s13195-020-00612-7 [DOI] [PubMed] [Google Scholar]
- 4. Balogun WG, Zetterberg H, Blennow K, Karikari TK. Plasma biomarkers for neurodegenerative disorders: ready for prime time? Curr Opin Psychiatry. 2023;36:112‐118. doi: 10.1097/YCO.0000000000000851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gonzalez‐Ortiz F, Kac PR, Brum WS, Zetterberg H, Blennow K, Karikari TK. Plasma phospho-tau in Alzheimer's disease: towards diagnostic and therapeutic trial applications. Mol Neurodegeneration. 2023;18:18. doi: 10.1186/s13024-023-00605-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schindler SE, Bollinger JG, Ovod V, et al. High‐precision plasma β‐amyloid 42/40 predicts current and future brain amyloidosis. Neurology. 2019;93:e1647‐e1659. doi: 10.1212/WNL.0000000000008081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fandos N, Pérez‐Grijalba V, Pesini P, et al. Plasma amyloid β 42/40 ratios as biomarkers for amyloid β cerebral deposition in cognitively normal individuals. Alzheimers Dement Diagn Assess Dis Monit. 2017;8:179‐187. doi: 10.1016/j.dadm.2017.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Janelidze S, Mattsson N, Palmqvist S, et al. Plasma P‐tau181 in Alzheimer's disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer's dementia. Nat Med. 2020;26:379‐386. doi: 10.1038/s41591-020-0755-1 [DOI] [PubMed] [Google Scholar]
- 9. Mielke MM, Hagen CE, Xu J, et al. Plasma phospho‐tau181 increases with Alzheimer's disease clinical severity and is associated with tau‐ and amyloid‐positron emission tomography. Alzheimers Dement. 2018;14:989‐997. doi: 10.1016/j.jalz.2018.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xiao Z, Wu W, Ma X, et al. Plasma Aβ42/Aβ40 and p‐tau181 predict long‐term clinical progression in a cohort with amnestic mild cognitive impairment. Clin Chem. 2022;68:1552‐1563. doi: 10.1093/clinchem/hvac149 [DOI] [PubMed] [Google Scholar]
- 11. Lewczuk P, Ermann N, Andreasson U, et al. Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer's disease. Alzheimers Res Ther. 2018;10:71. doi: 10.1186/s13195-018-0404-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mielke MM, Syrjanen JA, Blennow K, et al. Plasma and CSF neurofilament light: relation to longitudinal neuroimaging and cognitive measures. Neurology. 2019;93:e252‐e260. doi: 10.1212/WNL.0000000000007767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mattsson N, Andreasson U, Zetterberg H, Blennow K, for the Alzheimer's Disease Neuroimaging Initiative . Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2017;74:557‐566. doi: 10.1001/jamaneurol.2016.6117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cicognola C, Janelidze S, Hertze J, et al. Plasma glial fibrillary acidic protein detects Alzheimer pathology and predicts future conversion to Alzheimer dementia in patients with mild cognitive impairment. Alzheimers Res Ther. 2021;13:68. doi: 10.1186/s13195-021-00804-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mayer CA, Brunkhorst R, Niessner M, Pfeilschifter W, Steinmetz H, Foerch C. Blood Levels of Glial Fibrillary Acidic Protein (GFAP) in patients with neurological diseases. PLOS ONE. 2013;8:e62101. doi: 10.1371/journal.pone.0062101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nakamura A, Kaneko N, Villemagne VL, et al. High performance plasma amyloid‐β biomarkers for Alzheimer's disease. Nature. 2018;554:249‐254. doi: 10.1038/nature25456 [DOI] [PubMed] [Google Scholar]
- 17. Karikari TK, Benedet AL, Ashton NJ, et al. Diagnostic performance and prediction of clinical progression of plasma phospho‐tau181 in the Alzheimer's disease neuroimaging initiative. Mol Psychiatry. 2021;26:429‐442. doi: 10.1038/s41380-020-00923-z [DOI] [PubMed] [Google Scholar]
- 18. Karikari TK, Pascoal TA, Ashton NJ, et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer's disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020;19:422‐433. doi: 10.1016/S1474-4422(20)30071-5 [DOI] [PubMed] [Google Scholar]
- 19. Simrén J, Leuzy A, Karikari TK, et al. The diagnostic and prognostic capabilities of plasma biomarkers in Alzheimer's disease. Alzheimers Dement. 2021;17:1145‐1156. doi: 10.1002/alz.12283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Giudici KV, de Souto Barreto P, Guyonnet S, et al. Assessment of plasma amyloid‐β42/40 and cognitive decline among community‐dwelling older adults. JAMA Netw Open. 2020;3:e2028634. doi: 10.1001/jamanetworkopen.2020.28634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pascual‐Lucas M, Allué JA, Sarasa L, et al. Clinical performance of an antibody‐free assay for plasma Aβ42/Aβ40 to detect early alterations of Alzheimer's disease in individuals with subjective cognitive decline. Alzheimers Res Ther. 2023;15:2. doi: 10.1186/s13195-022-01143-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thomas KR, Bangen KJ, Edmonds EC, et al. Objective subtle cognitive decline and plasma phosphorylated tau181: early markers of Alzheimer's disease‐related declines. Alzheimers Dement Diagn Assess Dis Monit. 2021;13:e12238. doi: 10.1002/dad2.12238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marks JD, Syrjanen JA, Graff‐Radford J, et al. Comparison of plasma neurofilament light and total tau as neurodegeneration markers: associations with cognitive and neuroimaging outcomes. Alzheimers Res Ther. 2021;13:199. doi: 10.1186/s13195-021-00944-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Benedet AL, Milà‐Alomà M, Vrillon A, et al. Differences between plasma and cerebrospinal fluid glial fibrillary acidic protein levels across the Alzheimer disease continuum. JAMA Neurol. 2021;78:1471. doi: 10.1001/jamaneurol.2021.3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chatterjee P, Pedrini S, Doecke JD, et al. Plasma Aβ42/40 ratio, p‐tau181, GFAP, and NfL across the Alzheimer's disease continuum: a cross‐sectional and longitudinal study in the AIBL cohort. Alzheimers Dement. 2023;19:1117‐1134. doi: 10.1002/alz.12724 [DOI] [PubMed] [Google Scholar]
- 26. Bellaver B, Povala G, Ferreira PCL, et al. Astrocyte reactivity influences amyloid‐β effects on tau pathology in preclinical Alzheimer's disease. Nat Med. 2023;29:1775‐1781. doi: 10.1038/s41591-023-02380-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chatterjee P, Pedrini S, Stoops E, et al. Plasma glial fibrillary acidic protein is elevated in cognitively normal older adults at risk of Alzheimer's disease. Transl Psychiatry. 2021;11:27. doi: 10.1038/s41398-020-01137-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Montoliu‐Gaya L, Alcolea D, Ashton NJ, et al. Plasma and cerebrospinal fluid glial fibrillary acidic protein levels in adults with Down syndrome: a longitudinal cohort study. eBioMedicine. 2023;90:104547. doi: 10.1016/j.ebiom.2023.104547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rajan KB, Aggarwal NT, McAninch EA, et al. Remote blood biomarkers of longitudinal cognitive outcomes in a population study. Ann Neurol. 2020;88:1065‐1076. doi: 10.1002/ana.25874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cullen NC, Leuzy A, Janelidze S, et al. Plasma biomarkers of Alzheimer's disease improve prediction of cognitive decline in cognitively unimpaired elderly populations. Nat Commun. 2021;12:3555. doi: 10.1038/s41467-021-23746-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. He L, de Souto Barreto P, Aggarwal G, et al. Plasma Aβ and neurofilament light chain are associated with cognitive and physical function decline in non‐dementia older adults. Alzheimers Res Ther. 2020;12:128. doi: 10.1186/s13195-020-00697-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Arendonk J, Wolters FJ, Neitzel J, et al. Plasma neurofilament light chain (NfL) relates to preclinical changes in cognition, structural white matter integrity and markers of cerebral small‐vessel disease: a population‐based study. Alzheimers Dement. 2021;17:e053611. doi: 10.1002/alz.053611 [DOI] [Google Scholar]
- 33. Ferreira PCL, Zhang Y, Snitz B, et al. Plasma biomarkers identify older adults at risk of Alzheimer's disease and related dementias in a real‐world population‐based cohort. Alzheimers Dement. 2023;19:4507‐4519. doi: 10.1002/alz.12986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189‐198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 35. Ganguli M, Snitz B, Bilt JV, Chang C‐CH. How much do depressive symptoms affect cognition at the population level? The Monongahela‐Youghiogheny Healthy Aging Team (MYHAT) study. Int J Geriatr Psychiatry. 2009;24:1277‐1284. doi: 10.1002/gps.2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Papp KV, Amariglio RE, Dekhtyar M, et al. Development of a psychometrically equivalent short form of the Face‐Name Associative Memory Exam for use along the early Alzheimer's disease trajectory. Clin Neuropsychol. 2014;28:771‐785. doi: 10.1080/13854046.2014.911351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Benton AL. A visual retention test for clinical use. Arch Neurol Psychiatry. 1945;54:212. doi: 10.1001/archneurpsyc.1945.02300090051008 [DOI] [PubMed] [Google Scholar]
- 38. Ganguli M, Bilt JV, Lee C‐W, et al. Cognitive test performance predicts change in functional status at the population level: the MYHAT project. J Int Neuropsychol Soc. 2010;16:761‐770. doi: 10.1017/S1355617710000561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ganguli M, Chang C‐CH, Snitz BE, Saxton JA, Vanderbilt J, Lee C‐W. Prevalence of mild cognitive impairment by multiple classifications: the Monongahela‐Youghiogheny Healthy Aging Team (MYHAT) project. Am J Geriatr Psychiatry. 2010;18:674‐683. doi: 10.1097/JGP.0b013e3181cdee4f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kaufman L, Rousseeuw PJ, eds. Partitioning Around Medoids (Program PAM). John Wiley & Sons, Inc.; 1990:68‐125. doi: 10.1002/9780470316801.ch2 [DOI] [Google Scholar]
- 41. Rizopoulos D, Joint modeling of longitudinal and time‐to‐event data with applications in R 2021. [DOI] [PubMed]
- 42. Rizopoulos D. The R package JMbayes for fitting joint models for longitudinal and time‐to‐event data using MCMC. J Stat Softw. 2016;72:1‐46. doi: 10.18637/jss.v072.i07 [DOI] [Google Scholar]
- 43. Skoog I, Kern S, Zetterberg H, et al. Low cerebrospinal fluid Aβ42 and Aβ40 are related to white matter lesions in cognitively normal elderly. J Alzheimers Dis;n.d.;62:1877‐1886. doi: 10.3233/JAD-170950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sturchio A, Dwivedi AK, Young CB, et al. High cerebrospinal amyloid‐β 42 is associated with normal cognition in individuals with brain amyloidosis. e Clinical Medicine. 2021;38. doi: 10.1016/j.eclinm.2021.100988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lewczuk P, Matzen A, Blennow K, et al. Cerebrospinal fluid Aβ42/40 corresponds better than Aβ42 to amyloid PET in Alzheimer's disease. J Alzheimers Dis JAD. 2017;55:813‐822. doi: 10.3233/JAD-160722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pérez‐Grijalba V, Romero J, Pesini P, et al. Plasma Aβ42/40 ratio detects early stages of Alzheimer's disease and correlates with CSF and neuroimaging biomarkers in the AB255 study. J Prev Alzheimers Dis. 2019;6:34‐41. doi: 10.14283/jpad.2018.41 [DOI] [PubMed] [Google Scholar]
- 47. Dodge HH, Wang C‐N, Chang C‐CH, Ganguli M. Terminal decline and practice effects in older adults without dementia: the MoVIES project. Neurology. 2011;77:722‐730. doi: 10.1212/WNL.0b013e31822b0068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hansson O, Lehmann S, Otto M, Zetterberg H, Lewczuk P. Advantages and disadvantages of the use of the CSF amyloid β (Aβ) 42/40 ratio in the diagnosis of Alzheimer's disease. Alzheimers Res Ther. 2019;11:34. doi: 10.1186/s13195-019-0485-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shoji M, Matsubara E, Kanai M, et al. Combination assay of CSF tau, A beta 1‐40 and A beta 1‐42(43) as a biochemical marker of Alzheimer's disease. J Neurol Sci. 1998;158:134‐140. doi: 10.1016/s0022-510x(98)00122-1 [DOI] [PubMed] [Google Scholar]
- 50. Lewczuk P, Esselmann H, Otto M, et al. Neurochemical diagnosis of Alzheimer's dementia by CSF Abeta42, Abeta42/Abeta40 ratio and total tau. Neurobiol Aging. 2004;25:273‐281. doi: 10.1016/S0197-4580(03)00086-1 [DOI] [PubMed] [Google Scholar]
- 51. Seppala TT, Herukka S‐K, Hanninen T, et al. Plasma A 42 and A 40 as markers of cognitive change in follow‐up: a prospective, longitudinal, population‐based cohort study. J Neurol Neurosurg Psychiatry. 2010;81:1123‐1127. doi: 10.1136/jnnp.2010.205757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Verberk IMW, Hendriksen HMA, van Harten AC, et al. Plasma amyloid is associated with the rate of cognitive decline in cognitively normal elderly: the SCIENCe project. Neurobiol Aging. 2020;89:99‐107. doi: 10.1016/j.neurobiolaging.2020.01.007 [DOI] [PubMed] [Google Scholar]
- 53. Janelidze S, Stomrud E, Palmqvist S, et al. Plasma β‐amyloid in Alzheimer's disease and vascular disease. Sci Rep. 2016;6:26801. doi: 10.1038/srep26801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Therriault J, Benedet AL, Pascoal TA, et al. Association of plasma P‐tau181 with memory decline in non‐demented adults. Brain Commun. 2021;3:fcab136. doi: 10.1093/braincomms/fcab136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Chen S‐D, Huang Y‐Y, Shen X‐N, et al. Longitudinal plasma phosphorylated tau 181 tracks disease progression in Alzheimer's disease. Transl Psychiatry. 2021;11:1‐10. doi: 10.1038/s41398-021-01476-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Janelidze S, Teunissen CE, Zetterberg H, et al. Head‐to‐head comparison of 8 plasma amyloid‐β 42/40 assays in Alzheimer disease. JAMA Neurol. 2021;78:1375‐1382. doi: 10.1001/jamaneurol.2021.3180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Manly JJ, Deters KD. Donanemab for Alzheimer disease—who benefits and who is harmed? JAMA. 2023;330:510‐511. doi: 10.1001/jama.2023.11704 [DOI] [PubMed] [Google Scholar]
- 58. Manly JJ, Gilmore‐Bykovskyi A, Deters KD. Inclusion of underrepresented groups in preclinical Alzheimer disease trials—opportunities abound. JAMA Netw Open. 2021;4:e2114606. doi: 10.1001/jamanetworkopen.2021.14606 [DOI] [PubMed] [Google Scholar]
- 59. Sperling RA. Are we there yet (and how do we get there…)? J Prev Alzheimers Dis. 2023. doi: 10.14283/jpad.2023.128 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Supporting information


