Abstract
Background
PTEN loss and aberrations in PI3K/AKT signaling kinases associate with poorer response to abiraterone acetate (AA) in metastatic castration-resistant prostate cancer (mCRPC). In this study, we assessed antitumor activity of the AKT inhibitor capivasertib combined with enzalutamide in mCRPC with prior progression on AA and docetaxel.
Methods
This double-blind, placebo-controlled, randomized phase 2 trial, recruited men ≥ 18 years with progressing mCRPC and performance status 0–2 from 15 UK centers. Randomized participants (1:1) received enzalutamide (160 mg orally, once daily) with capivasertib (400 mg)/ placebo orally, twice daily on an intermittent (4 days on, 3 days off) schedule. Primary endpoint was composite response rate (RR): RECIST 1.1 objective response, ≥ 50 % PSA decrease from baseline, or circulating tumor cell count conversion (from ≥ 5 at baseline to < 5 cells/7.5 mL). Subgroup analyses by PTENIHC status were pre-planned.
Results
Overall, 100 participants were randomized (50:50); 95 were evaluable for primary endpoint (47:48); median follow-up was 43 months. RR were 9/47 (19.1 %) enzalutamide/capivasertib and 9/48 (18.8 %) enzalutamide/placebo (absolute difference 0.4 % 90 %CI −12.8 to 13.6, p = 0.58), with similar results in the PTENIHC loss subgroup. Irrespective of treatment, OS was significantly worse for PTENIHC loss (10.1 months [95 %CI: 4.6–13.9] vs 14.8 months [95 %CI: 10.8–18]; p = 0.02). Most common treatment-emergent grade ≥ 3 adverse events for the combination were diarrhea (13 % vs 2 %) and fatigue (10 % vs 6 %).
Conclusions
Combined capivasertib/enzalutamide was well tolerated but didn’t significantly improve outcomes from abiraterone pre-treated mCRPC.
Keywords: Enzalutamide, AKT-inhibitor, PTEN, Prostate cancer, Phase II randomized trial
Highlights
-
•
RE-AKT is a multicentre, randomized, double-blinded phase II trial in mCRPC.
-
•
Combined capivasertib and enzalutamide did not increase enzalutamide’s antitumour activity in mCRPC.
-
•
Albeit well tolerated, capivasertib exposure is reduced when enzalutamide is given.
1. Introduction
Prostate cancer (PC) is one of the commonest causes of cancer-related death [1], with a mainstay of its systemic therapy remaining androgen deprivation therapy (ADT) [2]. Metastatic disease invariably becomes castration-resistant (mCRPC), although androgen receptor (AR) dependency continues [3]. This led to development of AR pathway inhibitors (ARPI) including abiraterone acetate (AA), enzalutamide, apalutamide and darolutamide [4], [5], [6], [7]. Mechanisms of resistance to ARPI include but are not limited to AR amplification, mutations [8] and constitutively active splice variants [9].
PI3K/AKT pathway activation, mainly due to PTEN loss, is a mechanism of resistance to AR blockade [10]. PTEN loss and aberrations in PI3K/AKT signaling kinases (PIK3CA/PIK3CB/AKT1/AKT2) associate with worse outcomes and poorer response to AA in mCRPC [11], [12]. This led to studies combining ARPIs with AKT blockade [13], [14]. Here, we present the RE-AKT trial that explored antitumor activity of capivasertib, and enzalutamide compared to enzalutamide alone in men with mCRPC previously treated with AA and docetaxel.
2. Materials and methods
2.1. Study design and participants
RE-AKT (ISRCTN17168679, NCT02525068) was a multicenter, double-blind, placebo-controlled, randomized phase II trial (1:1) conducted in 15 UK centers (Appendix A). Eligibility criteria included: age ≥ 18 years; 1–2 prior lines of taxane therapy; ≥ 12 weeks of prior AA; histologic PC diagnosis; Eastern Cooperative Oncology Group (ECOG) performance status 0–2; PSA ≥ 10 ng/mL; castrate serum testosterone (full list in Appendix B). Patients provided written informed consent before enrollment. The study was co-sponsored by The Royal Marsden Hospital and The Institute of Cancer Research (ICR) UK; approved by a Research Ethics Committee (14/LO/0259); coordinated centrally by The Clinical Trials and Statistics Unit at ICR (ICR-CTSU); conducted to the principles of good clinical practice and overseen by independent data monitoring and steering committees.
2.2. Randomization and masking
Eligible patients were randomly allocated (1:1) centrally using an interactive web response system (IWRS, Cenduit Solutions) to receive enzalutamide/capivasertib or enzalutamide/placebo. Allocation used a minimization algorithm with a random element incorporated, with center, number of prior chemotherapy lines and prior response to abiraterone as balancing factors. Participants and clinicians were masked to treatment allocation.
2.3. Procedures
Participants received capivasertib 400 mg or matching placebo, orally, twice daily on an intermittent (4 days on and 3 days off) dosing schedule. Enzalutamide 160 mg was administered orally, once daily. Patients continued on study treatment until disease progression (clinical or radiological), unacceptable toxicity or patient decision to discontinue. Clinical assessments took place 1 and 2 weeks after the start of treatment, then at the start of every new 4-week cycle, including monitoring of adverse events (graded according to Common Terminology Criteria for Adverse Events (CTCAE version 4.0), performance status, physical examination, routine bloods, and symptom review. Pain was assessed using the Brief Pain Inventory Short Form (BPI-SF) [15], [16].
Radiological assessments (CT and bone scans) were every 12 weeks. Circulating tumor cell (CTC) counts were measured every 4 weeks for the first 12 weeks, and thereafter every 12 weeks. CTC counts were not made available to the treating physician. PSA serum measurements were collected every cycle if available, and every 12 weeks at a minimum. Blood samples for correlative biomarker studies were taken every 4 weeks.
PTEN protein immunohistochemical (PTENIHC) expression was determined as previously described [11]. In short, nuclear and cytoplasmic staining intensity were assessed by a pathologist blinded to clinical outcome data using H-scores [(% of weak staining cells) × 1] + [(% of moderate staining cells) × 2] + [(% of strong staining cells) × 3] ranging from 0 (minimum) to 300 (maximum). A binary classification scheme was used with PTEN loss defined as H-score ≤ 10.
2.4. Outcomes
The primary endpoint was response rate, defined as a composite of: radiological objective response (by RECIST 1.1 [17]), a decrease in PSA of ≥ 50 % from baseline, and conversion of CTC count (from ≥ 5 at baseline to < 5 cells per 7·5 mL blood). PSA and CTC responses required confirmation in a second consecutive assessment at least 4 weeks later and absence of radiological progression. In assessing response, only PSA and CTC assessments from 12 weeks onwards (to coincide with the first RECIST assessment) were considered, unless a PSA or CTC response was maintained after 12 weeks of treatment (without radiological response at 12 weeks).
Secondary endpoints included: radiographic progression free survival (rPFS), defined as the time from randomization to first RECIST 1.1 progression, bone scan progression defined by Prostate Cancer Working Group 2 [18], or death; overall survival (OS), defined as time from randomization to death; best percentage change in PSA from baseline while on treatment, as well as at 12 weeks (or earlier if therapy was discontinued); CTC count falls by 30 %; maximal CTC percentage decline; CTC percentage decline at 12 weeks; skeletal-related events (including palliative external-beam radiotherapy, new symptomatic fractures, spinal cord compression, or tumor–related orthopedical surgery); pain palliation using the BPI-SF worst pain intensity score; and tolerability.
2.5. Statistical analyses
Assuming a response rate of 17 % with enzalutamide alone in the post‐abiraterone and docetaxel setting [19], 50 patients per group allowed the detection of at least 40 % response rate in the enzalutamide and capivasertib combination group (one‐sided α = 0.05, 82 % power). For secondary time‐to‐event outcomes, we targeted a hazard ratio of 0.60 (one‐sided α 0.10; 80 % power), requiring 70 events, and equating to an increase in median PFS from 5 to 8.3 months when adding capivasertib, and in OS from 10 to 16.7 months.
Populations of analysis included the intention to treat (ITT, all randomly assigned participants), the safety population (SP, all who received at least one dose of either study drug) and evaluable population for the analysis of the primary endpoint. The latter was defined as all participants in the SP meeting all eligibility criteria; patients were excluded from this population only if they discontinued treatment prior to 12 weeks for reasons considered unrelated to trial treatment or disease. Sensitivity analyses of the primary endpoint on the ITT population were performed, with patients discontinuing prior to 12-week assessments considered non-responders. Analysis of all other efficacy endpoints were performed on the ITT population. Toxicity is reported on the SP.
Response rates (RR) are reported with 95 % confidence intervals (CIs). Treatment effect is estimated by the absolute difference in RR, presented with 90 % CI and one-sided p-values from Fisher’s exact test as per the trial design. Other estimates are presented with 95 % CIs. Percentage changes from baseline PSA, sum of target lesions (RECIST 1.1), and CTC counts are presented as waterfall plots, and treatment groups compared with Mann-Whitney tests. For rPFS, patients alive and without progression are censored at last scheduled disease assessment. Patients alive at the end of follow-up are censored for analysis of OS. Both endpoints are summarized by Kaplan-Meier estimates, with median times reported and groups compared with the log-rank test. BPI-SF was assessed using standard scoring algorithms [16] with a focus on worst pain intensity score and analgesic score.
Pre-planned subgroup analyses to assess interaction of PTENIHC and treatment were performed using logistic (for binary endpoints) or Cox proportional hazards (for time to event endpoints) models with interaction terms and considered exploratory in nature. Analyses are based on a database snapshot taken April 7, 2022, and performed with Stata software (version 17).
3. Results
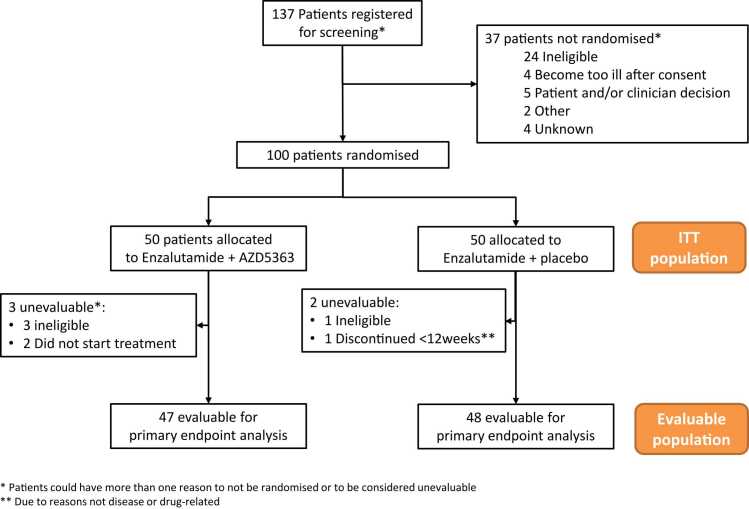
Between 06/07/2016, and 06/09/2019, 137 patients were registered for screening; 100 were subsequently randomized (Fig. 1). Median follow-up was 43 months. Baseline characteristics are presented in Table 1. Although well balanced in terms of patient demographics, diagnostic features, and previous treatments (Table C.1), in the enzalutamide/placebo group fewer patients presented with RECIST-measurable disease.
Fig. 1.
Consort diagram.
Table 1.
Baseline characteristics.
| Enzalutamide/capivasertib (N = 50) |
Enzalutamide/placebo (N = 50) |
|||
|---|---|---|---|---|
| n | % | n | % | |
| Age (years)a | 72.3(67.5, 77.9) | 71.5 (67.7, 76.2) | ||
| Ethnicity | ||||
| White | 47 | 94 | 50 | 100 |
| Other | 3 | 6 | 0 | 0 |
| Primary tumor staging at diagnosis (T) | ||||
| T0/T1 | 1 | 2 | 2 | 4 |
| T2 | 11 | 22 | 3 | 6 |
| T3 | 16 | 32 | 24 | 48 |
| T4 | 9 | 18 | 6 | 12 |
| Unknown | 13 | 26 | 15 | 30 |
| Lymphadenopathy at diagnosis (N) | ||||
| N0 | 16 | 32 | 19 | 38 |
| N1/N2 | 19 | 38 | 13 | 26 |
| Unknown | 15 | 30 | 18 | 36 |
| Metastatic disease at diagnosis | ||||
| M1 | 30 | 60 | 29 | 58 |
| Total Gleason score at diagnosis | ||||
| ≤ 7 | 22 | 44 | 23 | 46 |
| ≥ 8 | 20 | 40 | 17 | 34 |
| Unknown | 8 | 16 | 10 | 20 |
| Time since histological confirmation of prostate cancer (years)a | 6.7 (4.2, 11.1) n = 48 | 5.9 (2.9, 8.7) n = 49 | ||
| Time since confirmation of castrate resistant disease (years)a | 3.7 (2.4, 5.4) n = 47 | 3.7 (2.6, 5.4) n = 50 | ||
| Disease presentation at trial entry | ||||
| Measurable soft-tissue disease (± bone lesions) | 31 | 62 | 25 | 50 |
| Non-measurable soft-tissue disease (± bone lesions) | 10 | 20 | 8 | 16 |
| Bone lesions only | 9 | 18 | 17 | 34 |
| Site of metastatic disease at trial entryb | ||||
| Lung | 8 | 16 | 8 | 16 |
| Lymph node | 33 | 66 | 27 | 54 |
| Liver | 7 | 14 | 8 | 16 |
| Bone | 46 | 92 | 47 | 94 |
| CTC count at trial entry | ||||
| CTC < 5 | 17 | 34 | 12 | 24 |
| CTC ≥ 5 | 33 | 66 | 38 | 76 |
| PSA at trial entry (ng/mL)a | 144.2 (60, 240.3) | 245 (79.3, 591) | ||
| Prior lines of chemotherapyc | ||||
| One | 29 | 58 | 29 | 58 |
| Two | 21 | 42 | 21 | 42 |
| Prior response to abiraterone?c | ||||
| No | 17 | 34 | 16 | 32 |
| Yes | 33 | 66 | 34 | 68 |
| PTEN Status | ||||
| PTEN Normal | 29 | 58 | 33 | 66 |
| PTEN Loss | 16 | 32 | 14 | 28 |
| Unknown | 5 | 10 | 3 | 6 |
Presented as median (first Q1-third Q3 quartiles).
Patients may have reported more than 1 lesion site.
Balancing factors at randomization; Response to abiraterone pre-defined in the protocol as ≥ 50 % PSA decline or RECIST 1.1 ORR.
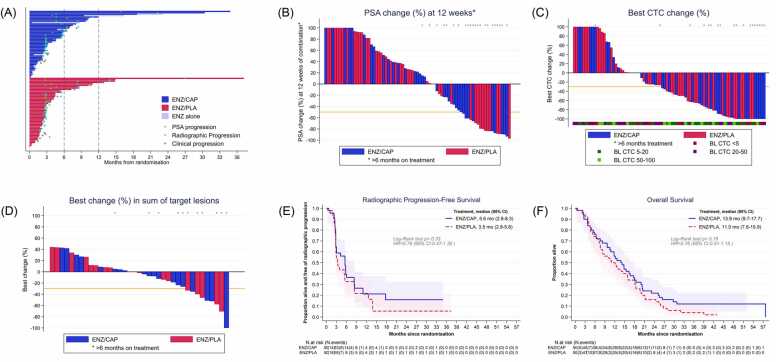
Ninety-eight patients (48 enzalutamide/capivasertib, 50 enzalutamide/placebo) were included in the SP; two patients were found ineligible after randomization, before starting study treatment. Median time on combination treatment was 2.9 months (interquartile range (IQR): 0.9–6.3) for enzalutamide/capivasertib, 2.7 months (1.8–4.5) for enzalutamide/placebo. Patients who discontinued capivasertib were permitted to continue enzalutamide alone: 12 patients (25 %) continued on enzalutamide alone for a median of 2.4 months (1.5–7.6). Fifteen patients (31 %) allocated to enzalutamide/capivasertib and nine patients (18 %) in the enzalutamide/placebo group remained on study treatment for more than 6 months (Fig. 2A).
Fig. 2.
Antitumor activity by allocated treatment group.
a) Swimmer plot of time on treatment for each patient according to treatment group, indicating periods where enzalutamide/capivasertib, patients received enzalutamide alone. Treatment periods of ≥ 6 months and ≥ 12 months are highlighted. PSA=prostate-specific antigen. b) Percentage change from baseline in PSA at 12 weeks. c) Best percentage change from baseline in CTC at any time during allocated treatment. d) Best percentage change from baseline in sum of target lesions at any time during allocated treatment e)) Kaplan Meier curve for radiographic progression-free survival by treatment group. f) Kaplan Meier curve for overall survival by treatment group. ENZ: enzalutamide, CAP: capivasertib, PLA: placebo.
3.1. Antitumor activity
Ninety-five patients (47 enzalutamide/capivasertib, 48 enzalutamide/placebo) were evaluable for at least one component of the composite RR, with 72 (76 %) evaluable for RECIST 1.1 response, 82 (86 %) for PSA response, and 80 (84 %) for CTC conversion. No differences in composite RR were observed between the groups: 9/47 (19.1 %) enzalutamide/capivasertib vs 9/48 (18.8 %) enzalutamide/placebo (absolute difference 0.4 % 90 %CI − 12.8 to 13.6, p = 0.58; Table 2). Radiological response was observed in 4/35 (11.4 %) evaluable enzalutamide/capivasertib patients vs 5/37 (13.5 %) with enzalutamide/placebo; PSA response was observed in 7/38 (18.4 %) and 8/42 (19.0 %), respectively; and CTC conversion in 2/29 (6.9 %) and 5/34 (14.7 %), respectively.
Table 2.
Antitumor activity (measured by composite response) by treatment group (evaluable population).
| Enzalutamide/capivasertib |
Enzalutamide/placebo |
Difference % |
p-value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | R | %R | 95 %CI | N | R | %R | 95 %CI | Diff | 90 %CI | |||||
| Composite response | 47 | 9 | 19.1 | (9.1 | 33.3) | 48 | 9 | 18.8 | (8.9 | 32.6) | 0.4 | (− 12.8 | 13.6) | 0.58 |
| RECIST 1.1 response | 35 | 4 | 11.4 | (3.2 | 26.7) | 37 | 5 | 13.5 | (4.5 | 28.8) | -2.1 | (− 14.9 | 10.7) | |
| Confirmed PSA fall > = 50 % | 38 | 7 | 18.4 | (7.7 | 34.3) | 42 | 8 | 19.0 | (8.6 | 34.1) | -0.6 | (− 15.0 | 13.7) | |
| Confirmed CTC conversion | 29 | 2 | 6.9 | (0.8 | 22.8) | 34 | 5 | 14.7 | (5.0 | 31.1) | -7.8 | (− 20.4 | 4.8) | |
| RECIST 1.1 or PSA response | 39 | 8 | 20.5 | (9.3 | 36.5) | 43 | 8 | 18.6 | (8.4 | 33.4) | 1.9 | (− 12.5 | 16.3) | 0.52 |
| Composite response by PTENIHCstatus | ||||||||||||||
| PTENIHC Normal (N = 62) | 29 | 9 | 31.0 | (15.3 | 50.8) | 33 | 8 | 24.2 | (11.1 | 42.3) | 6.8 | (− 11.9 | 25.5) | 0.38 |
| PTENIHC Loss (N = 26) | 13 | 0 | 0.0 | (0.0 | 24.7) | 13 | 1 | 7.7 | (0.2 | 36.0) | -7.7 | (− 19.8 | 4.5) | 0.50 |
N: number of patients.
R: Number of responses.
%R: Response Rate, 95 %CI: 95 % exact confidence interval for proportions; 90 % CI: normal approximation for difference of proportions.
P-value: 1-sided exact Fisher’s test.
There were no differences between treatment groups in percentage change from baseline in PSA at 12 weeks (p = 0.28, Fig. 2B), best percentage change in CTC counts while on treatment (p = 0.24, Fig. 2C), or in the sum of target lesions (p = 0.70, Fig. 2D). Median rPFS in the enzalutamide/capivasertib group was 5.6 months (95 %CI: 2.8–8.3) and 3.5 months (95 %CI: 2.8–5.6) in the enzalutamide/placebo group, with a hazard ratio (HR) of 0.78 (95 % CI: 0.47–1.30, p = 0.33, Fig. 2E). Median OS for enzalutamide/capivasertib was 13.9 months (95 %CI: 9.7–17.7), and 11.0 months (95 %CI: 7.6–15.9) for enzalutamide/placebo, with HR 0.76 (95 % CI: 0.51–1.15, p = 0.19, Fig. 2F).
Six (12 %) enzalutamide/capivasertib patients experienced at least one skeletal event compared to 14 (28 %) enzalutamide/placebo patients (absolute difference 16 % 95 %CI 0.6–31.4, p = 0.04). No statistically significant differences were found between groups in changes in worst pain nor analgesic BPI-SF scores (Table C.2).
3.2. Antitumor activity by PTENIHC status
PTENIHC status was available in 92/100 patients (71/92 from diagnostic biopsies,21/92 from fresh biopsies): 63/92 (68.5 %) were PTENIHC normal and 29/92 (31.5 %) PTENIHC loss; PTENIHC was available in 88/95 (92.6 %) evaluable patients, with 62/88 (70.5 %) PTENIHC normal, and 26/88 (29.5 %) PTENIHC loss. Composite RR by PTENIHC status is presented in Table 2 and Table C.3. A breakdown of composite RR by PTENIHC status and biopsy type is presented in Table C.4. No significant differences were found between treatment groups within each PTENIHC subgroup. Given the small number of responses in the PTENIHC loss group, a formal test for interaction of PTENIHC and treatment was not done.
Only one response was observed in 26 PTENIHC loss patients (3.8 %) across both treatment groups, while 17/62 responses (27.4 %) were observed in PTENIHC normal patients (p = 0.009). This difference was demonstrated not only in PSA falls, but also in RECIST responses and CTC counts (Table C.3). For PTEN IHC normal patients, similar composite response rates across treatment groups were seen: enzalutamide/capivasertib 9/29 (31 %) vs enzalutamide/placebo 8/33 (24.2 %).
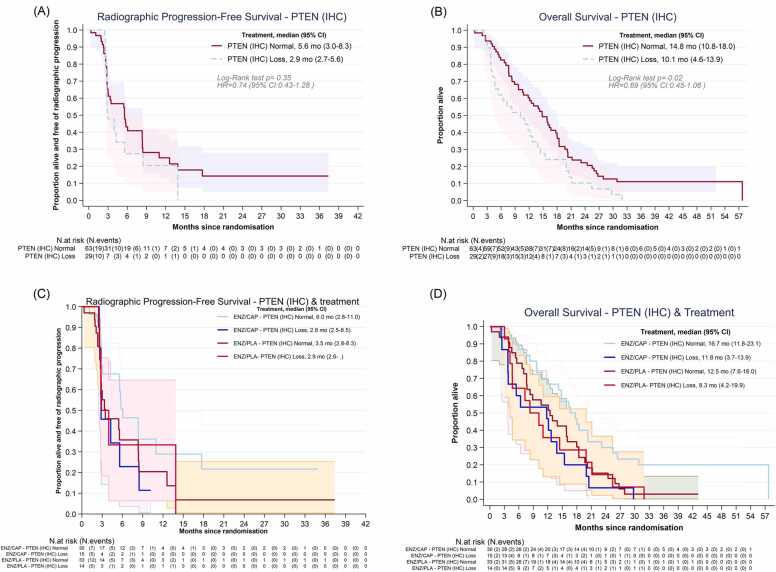
For all other efficacy endpoints, the role of PTENIHC loss as a biomarker for poor outcome was confirmed (Figs. 3A and 3B, Fig. D.1, Table C.5). Median rPFS for PTENIHC loss was 2.9 months (95 %CI: 2.7–5.6) compared to 5.6 months (95 %CI: 3.0–8.3) in PTENIHC normal patients (p = 0.35). OS was significantly worse for PTENIHC loss compared to PTENIHC normal patients, regardless of their treatment (10.1 months [95 %CI: 4.6–13.9] vs 14.8 months [95 %CI: 10.8–18]; log-rank p = 0.02). We did not, however, find signals of differential effect of AKT inhibitor treatment and PTENIHC status across all these endpoints (Figs. 3C and 3D).
Fig. 3.
Exploring role of PTENIHCas prognostic or predictive marker. a) Radiographic Progression-Free Survival by PTENIHC status. b) Overall Survival by PTENIHC status. c) Radiographic Progression-Free Survival by PTENIHC status and treatment group. d) Overall Survival by PTENIHC status and treatment group.ENZ: enzalutamide, CAP: capivasertib, PLA: placebo.
3.3. Safety and tolerability
Of the 98 patients starting treatment, 34 (72 %) in the enzalutamide/capivasertib group and 20 (40 %) in the enzalutamide/placebo group had at least one dose reduction or interruption during blinded treatment mainly due to adverse events (AE). Main reasons for discontinuation of the combination were disease progression (65 % enzalutamide/capivasertib vs 74 % enzalutamide/placebo), AE (25 % vs 10 %) and patient or clinician’s decision (10 % vs 16 %). Five patients (11 %) in the enzalutamide/capivasertib group continued to receive open label enzalutamide after discontinuing the combination due to AE for a median of 4 further cycles (IQR: 3–8 cycles).
The most common treatment-emergent AE while on blinded treatment on the enzalutamide/capivasertib vs enzalutamide/placebo group were fatigue (60 % vs 52 %), diarrhea (75 % vs 30 %), decreased appetite (38 % vs 34 %) and nausea (42 % vs 30 %) (Fig. D.2). Most grade 3 or higher AE were diarrhea (13 % vs 2 %), fatigue (10 % vs 6 %), anemia (10 % vs 14 %) and back pain (8 % vs 4 %). In the combination group, one grade 4 toxicity (diarrhea) was considered related to capivasertib; one further grade 5 event (intracranial hemorrhage resulting in death) was considered unrelated to either treatment. All other non-prostate cancer deaths occurred > 30 days after discontinuing treatment and were considered unrelated to treatment. Ten serious adverse reactions (SAR) were reported in five enzalutamide/capivasertib patients vs three SAR in two enzalutamide/placebo patients (Table C.6).
4. Discussion
Alterations in the PI3K/AKT/PTEN pathway occurs in up to 50 % of mCRPC cancers and are associated with cell proliferation and ARPI resistance [20]. Preclinical studies showed crosstalk between AR and PI3K/AKT signaling and support dual inhibition having superior antitumor activity, especially in PTEN-deficient tumors [21], [22]. Capivasertib is a potent and selective inhibitor of the three AKT isoforms that has been investigated in multiple tumors including breast and PC [23]. A phase III trial recently described that combining capivasertib with fulvestrant improved PFS compared to fulvestrant alone in estrogen-receptor positive metastatic breast cancer previously treated with aromatase inhibition [24]. In mCRPC, capivasertib in combination with enzalutamide [14], or AA [25], has an acceptable toxicity profile with antitumor activity reported. The phase I/II ProCAID trial evaluated capivasertib with docetaxel and reported a significant improvement in OS for the combination [26]. Based on these data, two phase III trials, CAPItello-280; (NCT05348577, docetaxel and capivasertib in mCRPC) and CAPItello-281 (NCT04493853i, capivasertib and AA in PTEN-deficient, de novo, metastatic, hormone-sensitive PC) are being pursued.
In our phase II trial, in a heavily pre-treated population after AA and docetaxel, we did not find evidence of a difference in RR between enzalutamide/capivasertib and enzalutamide/placebo. Nevertheless, a higher proportion of patients receiving capivasertib stayed on study treatment for more than 6 months, and, albeit non-statistically significant, larger rPFS and OS were also observed in the combination group. Moreover, patients on enzalutamide/capivasertib had significantly fewer skeletal events. The 4 days on/3 days off capivasertib schedule was well tolerated in keeping with phase I data [14], [25]. It is noteworthy that in our Phase I we observed that enzalutamide nearly halved capivasertib PK exposure [14], resulting in a lower exposure than what had been observed in earlier studies of the compound, possibly impacting on capivasertib efficacy in our study.
In our pre-planned biomarker analysis, the proportion of PTENIHC loss was slightly lower than previously reported [11], [12]. However, we have confirmed in a prospective trial the prognostic role of PTENIHC loss in late stage mCRPC. When stratifying by PTENIHC status there was, however, no differential RR or rPFS between treatment groups. Although PTENIHC loss is an established biomarker of sensitivity to the combination of the AKT inhibitor ipatasertib and AA in mCRPC patients ARPI naïve [13], other clinical experiences, as well as our data, would suggest this is not the case in more advanced stages probably due to the expression of AR splice variants in these tumors which are not blocked by enzalutamide[27].
5. Conclusions
The combination of capivasertib and enzalutamide is well tolerated but, at the levels of PK exposure that can be attained in the presence of enzalutamide, does not increase antitumor activity compared to enzalutamide alone in post abiraterone and taxane mCRPC.
Funding
This research was conducted with support from an Investigator-Sponsored Study Programme of AstraZeneca and endorsed by Cancer Research UK (Grant no. CTUQQR-Dec22/100004CRUKE/12/050). Astellas Pharma Europe Ltd provided enzalutamide free of charge to participating study centers through an investigator sponsored research grant from Astellas.
The de Bono translational team acknowledge research funding for this work from Prostate Cancer UK, the Movember Foundation through the London Movember Centre of Excellence (CEO13_2-002), the Prostate Cancer Foundation, Cancer Research UK (Centre Programme grant), Experimental Cancer Medicine Centre grant funding from Cancer Research UK and the Department of Health, and Biomedical Research Centre funding to the Royal Marsden.
CRediT authorship contribution statement
Christy Ralph: Writing – review & editing, Resources, Investigation. Ursula McGovern: Writing – review & editing, Resources, Investigation. Peter Hoskin: Writing – review & editing, Resources, Investigation. Bora Gurel: Writing – review & editing, Resources, Investigation, Data curation. Jenny Nobes: Writing – review & editing, Resources, Investigation. Simon Crabb: Writing – review & editing, Resources, Investigation. Zafar Malik: Writing – review & editing, Resources, Investigation. Robert J Jones: Writing – review & editing, Resources, Investigation. Alison Birtle: Writing – review & editing, Resources, Investigation. Joanna Gale: Writing – review & editing, Resources, Investigation. Peter Sankey: Writing – review & editing, Resources, Investigation. Pasquale Rescigno: Writing – review & editing, Writing – original draft, Visualization, Resources, Methodology, Investigation. Emma Hall: Writing – review & editing, Supervision, Methodology, Funding acquisition, Conceptualization. Suzanne Carreira: Writing – review & editing, Resources, Investigation, Data curation. Johann S de Bono: Writing – review & editing, Supervision, Resources, Methodology, Investigation, Funding acquisition, Conceptualization. Penny Flohr: Writing – review & editing, Resources, Investigation, Data curation. Susana Miranda: Writing – review & editing, Resources, Investigation, Data curation. Claudia Bertan: Writing – review & editing, Resources, Investigation, Data curation. Suneil Jain: Writing – review & editing, Resources, Investigation. Nuria Porta: Writing – review & editing, Writing – original draft, Visualization, Methodology, Formal analysis. Duncan McLaren: Writing – review & editing, Resources, Investigation. Laura Finneran: Writing – review & editing, Writing – original draft, Visualization, Formal analysis, Data curation. Eliot Chadwick: Writing – review & editing, Resources, Investigation. Ruth Riisnaes: Writing – review & editing, Resources, Investigation, Data curation. Aude Espinasse: Writing – review & editing, Project administration. Ines Figueiredo: Writing – review & editing, Resources, Investigation, Data curation. Ana Ferreira: Writing – review & editing, Resources, Investigation, Data curation. Mateus Crespo: Writing – review & editing, Resources, Investigation, Data curation. Daniel Nava Rodrigues: Writing – review & editing, Resources, Investigation, Data curation.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: JdB reports advisory board fees from many companies including Acai Therapeutics, Amgen, Astra Zeneca, Astellas, Bayer, Bioxcel Therapeutics, Boehringer Ingelheim, Cellcentric, Crescendo, Daiichi, Dark Blue Therapeutics, Eisai, Genentech/Roche, Genmab, GSK, Harpoon, ImCheck Therapeutics, Janssen, Merck Serono, Merck Sharp & Dohme, Menarini/Silicon Biosystems, MetaCurUm, Myricx, Novartis, Nurix Therapeutics, Oncternal, Orion, Pfizer, Qiagen, Sanofi Aventis, Sierra Oncology, Taiho, Takeda, Tango Therapeutics, Terumo, Vertex Pharmaceuticals. He is an employee of The ICR, which have received funding or other support for his research work from Acai Therapeutics, Amgen, AstraZeneca, Astellas, Bayer, Cellcentric, Crescendo, Daiichi, Genentech, Genmab, GSK, Harpoon, Immunic Therapeutics, Janssen, Merck Serono, Merck Sharp & Dohme, Menarini/Silicon Biosystems, MetaCurUm, Myricx, Nurix Therapeutics, Oncternal, Orion, Pfizer, Qiagen, Sanofi Aventis, Sierra Oncology, Taiho, Vertex Pharmaceuticals. The ICR have a commercial interest in abiraterone, PARP inhibition in DNA repair defective cancers and PI3K/AKT pathway inhibitors (no personal income). JDB was named as an inventor, with no financial interest for patent 8,822,438, submitted by Janssen that covers the use of abiraterone acetate with corticosteroids. EH reports that their institution has received an Investigator Initiated Research grant (IIR) from AstraZeneca for the central coordination of the trial. EH reports grants received by their institution as contribution to support central trial costs for non-commercial trials from Accuray, Varian Medical Systems, AstraZeneca, Janssen-Cilag, Bayer, Roche Products, and Merck Sharp and Dohm. SJ reports advisory boards and speaker fees received from AAA/Novartis, Accord, Astellas, Astra Zeneca, Bayer, Boston Scientific, Janssen and Pfizer, as well as consultancy fees received from Boston Scientific and BXT Nanotherapy. SJ also reports conferences travel received from Bayer and Janssen. AJB reports honoraria from Janssen-Cilag, consulting or advisory roles from Roche, Astellas Medivation, Janssen Oncology, AstraZeneca, Sanofi, Bayer Schering Pharma, Bristol-Myers-Squib, Merck Serono and Pfizer. AJB also reports speakers’ fees received from Bayer, Janssen Oncology and Pfizer. RJ reports honoraria from Astellas Pharma, Janssen, AstraZeneca, MSD Oncology, Bristol Myers Squibb, Pfizer, Novartis, Ipsen, Bayer, Roche/Genentech, Merck Serono, Eisai, WebMD, Advanced Accelerator Applications/Novartis and Elsevier. RJ also reports speakers’ fees received from Merck Serono, Pfizer, Janssen, Astellas Pharma, MSD Oncology, AstraZeneca, Ipsen, Bristol Myers Squibb/Celgene and Bayer. RJ also reports research Funding received from Roche (Inst), Astellas Pharma (Inst), AstraZeneca (Inst), Exelixis (Inst), Clovis Oncology (Inst) and Bayer (Inst), as well as travel, accommodations and expenses received from Ipsen, Bayer, Janssen, Astellas Pharma, MSD, Merck Serono and Pfizer.
PR, NP, LF, AE, SM, PF, JG, JN, RR, BG, DR, IF, SC, CB, AF, MC, SC, ZM, CR, UMC, PH, PS, EC and DML have no conflicts to declare.
Acknowledgement
Capivasertib was discovered by AstraZeneca subsequent to a collaboration with Astex Therapeutics (and its collaboration with the Institute of Cancer Research and Cancer Research Technology Limited). This study represents independent research supported by the National Institute of Health Research (NIHR) Biomedical Research Centre at the Royal Marsden NHS Foundation Trust and the Institute of Cancer Research, London. Professor Johann de Bono is a National Institute for Health Research (NIHR) Senior Investigator. PR is supported by the Prostate Cancer Foundation Young Investigator Awards. The Institute of Cancer Research (ICR) Clinical Trials and Statistics Unit (ICR-CTSU), London, UK, also receives programme grant funding from Cancer Research UK (C1491/A25351, CTUQQR-Dec22/100004).
The views expressed in this article are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
This work uses data provided by patients and collected by the NHS as part of their care and support. We wish to thank all of our collaborators, the RE-AKT Trial Management Group members past and present, the RE-AKT Independent Data Monitoring Committee and the ICR-CTSU mCRPC Steering committee. And we want to thank especially all of the patients and their families for making this research possible.
Data sharing
The ICR-CTSU supports the wider dissemination of information from its research and increased cooperation between investigators. Trial data are collected, managed, stored, shared, and archived according to ICR-CTSU Standard Operating Procedures to ensure the enduring quality, integrity, and utility of the data. Formal requests for data sharing are considered in line with ICR-CTSU procedures with due regard given to funder and sponsor guidelines. Requests are via a standard proforma describing the nature of the proposed research and extent of data requirements. Data recipients are required to enter a formal data sharing agreement that describes the conditions for release and requirements for data transfer, storage, archiving, publication, and intellectual property. Requests are reviewed by the Trial Management Group (TMG) in terms of scientific merit and ethical considerations including patient consent. Data sharing is undertaken if proposed projects have a sound scientific or patient benefit rationale as agreed by the TMG and approved by the Independent Data Monitoring and Steering Committee as required. Restrictions relating to patient confidentiality and consent will be limited by aggregating and anonymising identifiable patient data. Additionally, all indirect identifiers that could lead to deductive disclosures will be removed in line with Cancer Research UK Data Sharing Guidelines.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.ejca.2024.114103.
Appendix A. Supplementary material
Supplementary material
References
- 1.Bray F., et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Huggins C., Hodges C.V. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J Clin. 1972;22(4):232–240. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 3.Chen C.D., et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10(1):33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 4.de Bono J.S., et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scher H.I., et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 6.Fizazi K., et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2019;380(13):1235–1246. doi: 10.1056/NEJMoa1815671. [DOI] [PubMed] [Google Scholar]
- 7.Chi K.N., et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381(1):13–24. doi: 10.1056/NEJMoa1903307. [DOI] [PubMed] [Google Scholar]
- 8.Romanel A., et al. Plasma AR and abiraterone-resistant prostate cancer. Sci Transl Med. 2015;7(312) doi: 10.1126/scitranslmed.aac9511. 312re10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharp A., et al. Androgen receptor splice variant-7 expression emerges with castration resistance in prostate cancer. J Clin Invest. 2019;129(1):192–208. doi: 10.1172/JCI122819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz S., et al. Feedback suppression of PI3Kalpha signaling in PTEN-mutated tumors is relieved by selective inhibition of PI3Kbeta. Cancer Cell. 2015;27(1):109–122. doi: 10.1016/j.ccell.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferraldeschi R., et al. PTEN protein loss and clinical outcome from castration-resistant prostate cancer treated with abiraterone acetate. Eur Urol. 2015;67(4):795–802. doi: 10.1016/j.eururo.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rescigno P., et al. PI3K/AKT pathway deleterious mutations in lethal prostate cancer. Ann Oncol. 2018;29:viii293. [Google Scholar]
- 13.Sweeney C., et al. Ipatasertib plus abiraterone and prednisolone in metastatic castration-resistant prostate cancer (IPATential150): a multicentre, randomised, double-blind, phase 3 trial. Lancet. 2021;398(10295):131–142. doi: 10.1016/S0140-6736(21)00580-8. [DOI] [PubMed] [Google Scholar]
- 14.Kolinsky M.P., et al. A phase I dose-escalation study of enzalutamide in combination with the AKT inhibitor AZD5363 (capivasertib) in patients with metastatic castration-resistant prostate cancer. Ann Oncol. 2020;31(5):619–625. doi: 10.1016/j.annonc.2020.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cleeland C.S., et al. Dimensions of the impact of cancer pain in a four country sample: new information from multidimensional scaling. Pain. 1996;67(2-3):267–273. doi: 10.1016/0304-3959(96)03131-4. [DOI] [PubMed] [Google Scholar]
- 16.Cleeland CS . The brief pain inventory user guide; 2009. Available from: 〈http://www.mdanderson.org/education-and-research/departments-programs-and-labs/departments-and-divisions/symptom-research/symptom-assessment-tools/BPI_UserGuide.pdf〉.
- 17.Eisenhauer E.A., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 18.Scher H.I., et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26(7):1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brasso K., et al. Enzalutamide antitumour activity against metastatic castration-resistant prostate cancer previously treated with docetaxel and abiraterone: a multicentre analysis. Eur Urol. 2015;68(2):317–324. doi: 10.1016/j.eururo.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 20.Jamaspishvili T., et al. Clinical implications of PTEN loss in prostate cancer. Nat Rev Urol. 2018;15(4):222–234. doi: 10.1038/nrurol.2018.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carver B.S., et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell. 2011;19(5):575–586. doi: 10.1016/j.ccr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulholland D.J., et al. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell. 2011;19(6):792–804. doi: 10.1016/j.ccr.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coleman N., et al. Clinical development of AKT inhibitors and associated predictive biomarkers to guide patient treatment in cancer medicine. Pharmgenom Pers Med. 2021;14:1517–1535. doi: 10.2147/PGPM.S305068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner N.C., et al. Capivasertib in hormone receptor–positive advanced breast cancer. N Engl J Med. 2023;388(22):2058–2070. doi: 10.1056/NEJMoa2214131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shore N., et al. A Phase I study of capivasertib in combination with abiraterone acetate in patients with metastatic castration-resistant prostate cancer. Clin Genitourin Cancer. 2023;21(2):278–285. doi: 10.1016/j.clgc.2022.11.017. [DOI] [PubMed] [Google Scholar]
- 26.Crabb S.J., et al. Pan-AKT inhibitor capivasertib with docetaxel and prednisolone in metastatic castration-resistant prostate cancer: a randomized, Placebo-controlled phase II trial (ProCAID) J Clin Oncol. 2021;39(3):190–201. doi: 10.1200/JCO.20.01576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sweeney C.J., et al. Phase Ib/II study of enzalutamide with samotolisib (LY3023414) or Placebo in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res. 2022;28(11):2237–2247. doi: 10.1158/1078-0432.CCR-21-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material