Posterior reversible encephalopathy syndrome (PRES) is a distinctive neuroradiological disorder characterized by the abrupt onset of neurological symptoms, including severe headache, altered levels of consciousness ranging from drowsiness to coma, seizures, and visual impairments.1 The underlying cause of PRES typically arises from endothelial dysfunction triggered by factors such as fluctuating blood pressure, acute renal failure, drug toxicity, immunosuppressive agents, electrolyte imbalances, infections, and autoimmune disorders.1,2 Electroencephalography (EEG) plays a crucial role in evaluating encephalopathy and identifying nonconvulsive status epilepticus in patients with PRES.3 However, definitive EEG findings specific to PRES have not been established. A high prevalence of certain EEG abnormalities, such as generalized or posterior focal background slowing, nonconvulsive electrographic status epilepticus, and periodic discharges, has been observed in individuals with PRES.3-5 Despite this, there is a limited number of reported cases, particularly in the pediatric population, focusing on PRES-associated periodic lateralized epileptiform discharges (PLEDs).3-16 Therefore, our objective is to present a rare and noteworthy pediatric case involving PRES-associated PLEDs, along with comprehensive clinical information, and to review the existing literature.
A 10-year-old girl, previously monitored in the pediatric intensive care unit for acute extremity compartment syndrome following an earthquake, presented with symptoms including blurred vision, sudden-onset agitation, confusion, gaze deviation, and focal motor and sensory seizures. She was born following an uneventful pregnancy and delivery, with the nonconsanguineous marriage of her parents. She achieved normal developmental milestones.
On physical and neurological examination, she appeared lethargic with the following vital signs: temperature 37.5°C, blood pressure 145/90 mm Hg, heart rate 102 beats/min, and respiratory rate 22 breaths/min. Mid-dilated pupils, lack of eye contact, and an inability to follow light sources were noted. In addition, intermittent bilateral gaze deviation and a positive Babinski sign were observed. Spontaneous and symmetrical movement of both the upper and lower extremities was evident.
Laboratory tests revealed the followings: leukocytosis (23.2 × 109/L), anemia (hemoglobin: 7.8 g/dL), thrombocytosis (platelet: 645 × 109L), hyponatremia (sodium: 127 mEq/L), hypocalcemia (calcium: 7.4 mg/dL), and hypomagnesemia (magnesium: 1.2 mg/dL). Kidney function tests, including blood urea nitrogen (BUN; 2 mg/dL) and creatinine (0.21 mg/dL), were unremarkable. In addition, the erythrocyte sedimentation rate was normal (20 mm/h), whereas the level of C-reactive protein was elevated (25 mg/dL). Serological tests for viral infections and polymerase chain reaction tests on both nasopharyngeal swabs and cerebrospinal fluid (CSF) were negative. Cerebrospinal fluid examination showed no evidence of central nervous system (CNS) infection, with normal cell count, color, and sugar and protein values. Moreover, cultures from blood, urine, and CSF samples demonstrated no signs of microbial growth.
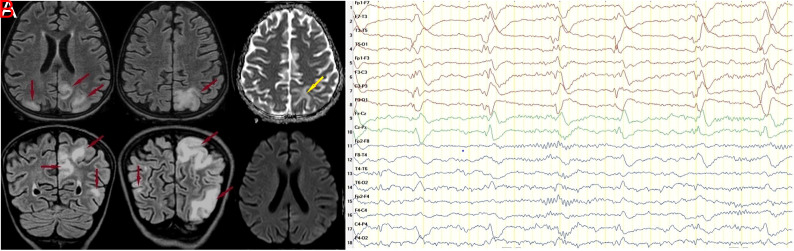
Brain MRI demonstrated cortical and subcortical hyperintensities in the left dominant bilateral temporal, parietal, and occipital lobes on T2-weighted and fluid attenuation inversion recovery (FLAIR) sequences, suggestive of potential vasogenic cerebral edema. Additional investigations using apparent diffusion coefficient (ADC) mapping indicated increased signal intensity, whereas diffusion-weighted imaging (DWI) revealed isointensity in these specific regions (Figure 1A). Electroencephalography showed a predominant slowing of posterior background activity on the left side and left-dominant bilateral PLEDs plus fast activity (Figure 1B).
Figure 1.
(A) Sixteen hours after the onset of typical symptoms, a fluid-attenuated inversion recovery (FLAIR) MRI sequence revealed abnormal subcortical and cortical hyperintensities consistent with vasogenic edema in the bilateral temporal, parietal, and occipital lobes, with more prominent in the left hemisphere (red arrows). Apparent diffusion coefficient (ADC) mapping demonstrates cortical/subcortical vasogenic edema with hyperintensity (yellow arrow). Diffusion-weighted imaging (DWI) showed isointensity in these areas. (B) Twenty-two hours after the onset of typical symptoms, the electroencephalography demonstrated mild slowing of the background rhythm, left dominant slowing of the posterior background activity and posteriorly left dominant bilateral PLEDs plus fast activity.
Based on these findings, the patient was diagnosed with PRES-associated PLEDs due to hypertension and electrolyte imbalances. We postulated that these conditions could be associated with limb ischemia and sepsis resulting from extremity compartment syndrome, rather than renal dysfunction. The patient presented with focal sensory seizures, such as visual hallucinations and focal tonic seizures. Antiseizure medication (30 mg/kg/day, levetiracetam) and antihypertensive agents were started. Concurrently, replacement therapies were initiated to correct hyponatremia, hypocalcemia, and hypomagnesemia. Within a day, notable improvement in the patient’s neurological deficit was observed. While epileptic discharges persisted on EEG, clobazam (0.5 mg/kg/day) effectively prevented seizures recurrence. Follow-up EEG on day 6 demonstrated mild unilateral slowing of the posterior background activity. After 1 month, a control brain MRI showed complete resolution of the abnormalities initially identified. The dose of clobazam was gradually tapered, and seizures did not recur for the subsequent 3 months. She exhibited remarkable improvement in motor and cognitive skills, including attention and perception abilities.
The underlying pathophysiology of PRES remains controversial, with multiple theories including impaired cerebrovascular autoregulation (disrupted cerebral perfusion regulation) and endothelial dysfunction.1,3 The diagnosis of PRES involves a combination of clinical evaluation, imaging, and electrophysiological studies, with the exclusion of other potential causes. These causes include ischemic or hemorrhagic stroke, meningitis, infectious or autoimmune encephalitis, uremic or hepatic encephalopathy, primary angiitis of the CNS, malignancies such as lymphoma and gliomatosis cerebri, and epileptic disorders.1,17 Ischemic stroke typically manifests with focal neurological deficits such as hemiparesis/hemiplegia and facial asymmetry. In contrast, PRES often presents with reversible symptoms, including headaches, altered mental status, and focal seizures. Acute ischemia reveals restricted diffusion on DWI and ADC, appearing as hyperintensity on DWI with corresponding hypointensity on ADC. Conversely, typical findings for PRES involve hyperintensity on FLAIR images and ADC mapping, with isointensity on DWI in the parieto-occipital or posterior frontal cortical/subcortical regions.17,18 CNS infections can lead to symptoms similar to PRES, and CSF analysis can help rule out infectious causes. Moreover, metabolic encephalopathies such as uremic or hepatic encephalopathy and primary angiitis of the CNS can induce similar neurological symptoms. Differentiating these conditions from PRES requires additional blood tests and neuroimaging studies.17,18
Periodic lateralized epileptiform discharges are frequently observed in acute neurological conditions, but its association with PRES is rare. To date, PLEDs on EEG have been documented in a limited number of PRES cases: 13 in adults and 6 in children (Supplementary Table 1).3-16 Posterior reversible encephalopathy syndrome is predominantly identified in young to middle-aged adults, with a higher prevalence in females.1 In the existing literature, gender information was provided for 11 of the 19 patients with PRES-associated PLEDs, of which 9 (82%) were female. Similarly, our patient was also female.
Supplementary Table 1.
Clinical summary and characteristics of our patient and previously reported patients with PRES-associated PLEDs
| Patient | Age (years) | Sex | Brain MRI involvement | EEG | Accompanying disorder/Etiology | Seizure type(s) | Treatment (and ASMs) | Follow-up EEG (time) | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Fitzpatrick et al. 6 | ND | ND | Bilateral involvement (details ND) | PLEDs (details ND) | Hypertension | ND | ND | ND | ND |
| Fitzpatrick et al. 6 | ND | ND | Bilateral involvement (details ND) | PLEDs (details ND) | Hypertension | ND | ND | ND | ND |
| Bhatt et al. 7 | 47 | F | Bilateral temporo-occipital lobes | PLEDs (details ND) | Non-small cell carcinoma of the lung on etoposide treatment | No seizure | Discontinuation of etoposide | Normal (10th day) | Good (seizure free) |
| Skiba et al. 8 | 28 | M | Bilateral parieto-occipital lobes | Posteriorly dominant bilateral PLEDs, slowing of the posterior background rhythm | Thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, hypertension, chronic renal failure | Generalized tonic-clonic, focal-nonmotor-sensory | Intravenous antihypertensives, valproic acid, phenytoin and levetiracetam | Normal (time ND) | Recurring several generalized tonic-clonic seizures |
| Kastrup et al. 4 | 60 | F | Bilateral frontal, temporal and parieto-occipital lobes | Left dominant bilateral PLEDs, slowing of the posterior background rhythm | Facioscapulohumeral dystrophy, electrolyte imbalance | Generalized and focal motor | Correction of electrolyte imbalance, clonazepam, valproic acid | Normal (21th day) | Good (seizure free) |
| Choi et al. 9 | 54 | F | Unilateral medial temporal, parieto-occipital lobes | Posteriorly dominant unilateral PLEDs, slowing of the posterior background rhythm | Subacute encephalopathy with seizures in alcoholics syndrome | Focal to bilateral tonic-clonic | Levetiracetam | ND | Poor (persisting aphasia and motor weakness) |
| Cherian et al. 10 | 32 | F | Bilateral parieto-occipital (left dominant) and frontal lobes | Unilateral (right) occipital PLEDs, slowing of the posterior background rhythm | Crohn’s disease on mesalamine treatment (immunomodulator), pulmonary tuberculosis | Convulsive status epilepticus | Valproic acid | Slowing of the posterior background rhythm (time ND) | Good (seizure free) |
| Kandemir et al. 11 | 60 | F | Bilateral parieto-occipital lobes | Left dominant bilateral temporo-parieto-occipital PLEDs, slowing of the posterior background rhythm | Metastatic lung cancer, usage of carboplatin and paclitaxel therapy | Generalized tonic-clonic, focal-nonmotor-sensory | Dexamethasone, phenytoin infusion, levetiracetam | Slowing of the background and PLEDs on both temporo-parieto-occipital regions (17th and 50th days) | Recurring seizures, death secondary to cardiopulmonary arrest due to cancer progression |
| Silveira et al. 12 | 20 | F | Bilateral frontal, posterior temporal, and parietooccipital lobes | Unilateral (right) temporo-parieto-occipital PLEDs plus fast activity | Acute intermittent porphyria, hypertension | Generalized tonic-clonic | Antihypertensives, lorazepam, levetirasetam, high dose steroids | ND | Good (seizure free) |
| Kamiya-Matsuoka et al. 3 | Adult (detail ND) | ND | Bilateral posterior parieto-occipital lobes, and thalamus | Left dominant bilateral occipital PLEDs | Malignancy (details ND) | ND | ND | Symmetric generalized rhythmic discharges (time ND) | Poor (details ND) |
| Subramaniam et al. 13 | 77 | M | Left posterior regions | Unilateral (left) posterior PLEDs | ND | ND | Four ASMs (details ND) | ND | ND |
| Matsumoto et al. 14 | 79 | F | Unilateral (left) rectal gyrus, temporo-parietal lobes, insular cortex, and thalamus | Unilateral (left) PLEDs | Epilepsy, chronic phase of subarachnoid hemorrhage | Focal motor | Levetiracetam, general anesthesia, valproic acid, perampanel | Normal (time ND) | Poor, (motor and sensory aphasia and right hemispatial neglect, recurring seizures) |
| Fisher et al. 15 | 40 | F | Bilateral parieto-occipital and left frontal (with punctate hemorrhages) | Unilateral (right) occipital and posterior temporal PLEDs, focal occipital electrographic seizures | Liver transplant for alcoholic cirrhosis on prednisone, tacrolimus, and mycophenolate for acute rejection, hypertension | Focal-nonmotor-sensory, focal motor, focal to bilateral tonic-clonic | Antihypertensive, levetiracetam, pregabalin, lacosamide, midazolam, propofol, ketamine, topiramate, clobazam, transcranial direct current stimulation | Interictal temporo-occipital spikes (time ND) | Good (seizure free) |
| Cordelli et al. 16 (Total six children) | ND | ND | ND | PLEDs (details ND) | ND | ND | ND | ND | ND |
| Present case | 10 | F | Left dominant bilateral temporo-parieto-occipital lobes | Left dominant bilateral PLEDs plus fast activity, slowing of the posterior background rhythm | Acute extremity compartment syndrome, hypertension, hyponatremia, hypocalcemia, hypomagnesemia | Focal-nonmotor-sensory, focal motor | Antihypertensive, correction of electrolyte imbalance, levetiracetam, clobazam | Mild unilateral (left) slowing of the posterior background rhythm (6th day) | Good (seizure free) |
ASM, antiseizure medication; EEG, electroencephalography; F, female; M, male; MRI, magnetic resonance imaging; ND, not documented; PLEDs, periodic lateralized epileptiform discharges; PRES, posterior reversible encephalopathy syndrome.
A comprehensive analysis of PLEDs on EEG was meticulously detailed for 10 of the 19 patients. Among these cases, unilateral PLEDs were evident in 60% of patients, whereas bilateral PLEDs were observed in 40%. In addition, a combination of PLEDs plus fast activity was noted in just 1 patient.12 Notably, our patient exhibited a similar pattern with PLEDs plus fast activity. Follow-up EEG was documented for 8 of the 19 patients, revealing EEG abnormalities, including background slowing (n = 2), interictal spikes (n = 1), generalized rhythmic discharges (n = 1), and PLEDs (n = 1) in 50% of this subgroup. However, the time intervals for these follow-up EEGs varied considerably, ranging from 10 to 50 days, and were often not reported for most patients.
Electroencephalography patterns associated with PRES encompass diffuse or focal slowing of the background rhythm, generalized and/or focal epileptiform discharges, electrographic status epilepticus, and PLEDs.4 Reiher et al19 proposed a classification for PLEDs, dividing them into 2 primary categories: “PLEDs proper” (involving PLEDs without rhythmic discharges) and “PLEDs plus” (encompassing PLEDs with rhythmic discharges). The “PLEDs proper” category was further subdivided into 3 subcategories labeled as classes 1, 2, and 3. In addition, “PLEDs plus” was classified into 2 distinct classes: class 4 (encompassing PLEDs with brief rhythmic discharges lasting 1 second or less) and class 5 (characterized by more prolonged rhythmic discharges). Notably, the “PLEDs plus” category carried a higher risk of seizure recurrence when compared to “PLEDs proper.”19 Despite our patient being classified as class 5 “PLEDs plus,” an absence of seizure recurrence was observed during follow-up, leading to the decision for early discontinuation of antiseizure medication.
Outcomes were documented in 9 of the 19 patients with PRES-associated PLEDs. Among this group, seizures persisted beyond the acute phase of PRES in 44% of patients, whereas no recurrence was observed in the remaining patients. Moreover, 2 patients (22%) experienced persistent neurological deficits such as aphasia and motor weakness.9,14 Unfortunately, 1 patient died because of progression of the primary disease.11 In contrast, in our patient, seizures did not recur over the subsequent 3 months, and no neurological deficits were observed.
In a retrospective analysis of 46 adult patients who experienced PRES, Kamiya-Matsuoka et al3 demonstrated a correlation between the location of radiological lesions and the specific region displaying focal abnormalities on routine EEG. However, our patient did not exhibit an obvious correlation. Interestingly, despite 3 patients with PRES-associated PLEDs in the literature showing bilateral hemispheric involvement on MRI, their EEG revealed unilateral PLEDs. A similar observation was noted in a study by Kastrup et al,4 involving the assessment of EEGs from 49 adult patients with PRES, where no distinct correlation was established between MRI involvement and EEG findings. The occurrence of PRES-associated PLEDs in children was initially documented by Cordelli et al.16 In this retrospective analysis of 111 children diagnosed with PRES, 61.9% of children showed slowing of background activity in the posterior regions, while 5.7% exhibited PLEDs.
In conclusion, to the best of our knowledge, this report represents the first documented pediatric case of PRES-associated PLEDs, including information on prognosis in the current literature. Further studies are needed to determine the prevalence and eventual outcomes of PLEDs in children diagnosed with PRES.
Footnotes
Informed Consent: Written informed consent for publication of this case report were obtained from the patient’s parents in compliance with the national ethics regulation.
Peer-review: Externally peer-reviewed.
Author Contributions: Conception – M.Y., S.Ç.; Design – M.Y., M.H.; Supervision – T.K., S.T.; Materials – M.Y., M.H., T.K.; Data Collection and/or Processing – M.Y., S.Ç.; Analysis and/or Interpretation – M.Y., S.Ç., S.T.; Literature Review – M.Y., S.Ç., M.H.; Writing Manuscript – M.Y., M.H.; Critical Review – T.K., S.T.
Declaration of Interests: The authors have no conflicts of interest to declare.
References
- 1. Fischer M, Schmutzhard E. Posterior reversible encephalopathy syndrome. J Neurol. 2017;264(8):1608 1616. ( 10.1007/s00415-016-8377-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Canpolat M, Kaya Özçora GD, Poyrazoğlu H, et al. Long-term follow-up of patients with a diagnosis of posterior reversible encephalopathy syndrome. Turk Arch Pediatr. 2021;56(6):569 575. ( 10.5152/TurkArchPediatr.2021.21072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kamiya-Matsuoka C, Tummala S. Electrographic patterns in patients with posterior reversible encephalopathy syndrome and seizures. J Neurol Sci. 2017;375:294 298. ( 10.1016/j.jns.2017.02.017) [DOI] [PubMed] [Google Scholar]
- 4. Kastrup O, Gerwig M, Frings M, Diener HC. Posterior reversible encephalopathy syndrome (PRES): electroencephalographic findings and seizure patterns. J Neurol. 2012;259(7):1383 1389. ( 10.1007/s00415-011-6362-9) [DOI] [PubMed] [Google Scholar]
- 5. Lin L, Drislane FW. Lateralized periodic discharges: a literature review. J Clin Neurophysiol. 2018;35(3):189 198. ( 10.1097/WNP.0000000000000448) [DOI] [PubMed] [Google Scholar]
- 6. Fitzpatrick W, Lowry N. PLEDs: clinical correlates. Can J Neurol Sci. 2007;34(4):443 450. ( 10.1017/S0317167100007332)18062453 [DOI] [Google Scholar]
- 7. Bhatt A, Farooq MU, Bhatt S, Majid A, Kassab MY. Periodic lateralized epileptiform discharges: an initial electrographic pattern in reversible posterior leukoencephalopathy syndrome. Neurol Neurochir Pol. 2008;42(1):55 59. [PubMed] [Google Scholar]
- 8. Skiba V, Etienne M, Miller JA. Development of chronic epilepsy after recurrent episodes of posterior reversible encephalopathy syndrome associated with periodic lateralized epileptiform discharges. Seizure. 2011;20(1):93 95. ( 10.1016/j.seizure.2010.10.005) [DOI] [PubMed] [Google Scholar]
- 9. Choi JY, Kwon J, Bae EK. A pathophysiologic approach for subacute encephalopathy with seizures in alcoholics (SESA) syndrome. J Clin Neurosci. 2014;21(9):1649 1652. ( 10.1016/j.jocn.2013.11.045) [DOI] [PubMed] [Google Scholar]
- 10. Cherian A, Soumya CV, Iype T, et al. Posterior reversible encephalopathy syndrome with PLEDs-plus due to mesalamine. J Neurosci Rural Pract. 2014;5(1):72 75. ( 10.4103/0976-3147.127882) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kandemir M, Küçükkaya B, Tepe MS, Yalçıner ZB, Salepçi NT. Reversible posterior leukoencephalopathy syndrome due to carboplatin and paclitaxel therapy. Balk Med J. 2015;32(4):421 425. ( 10.5152/balkanmedj.2015.15487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Silveira DC, Bashir M, Daniel J, Lucena MH, Bonpietro F. Acute intermittent porphyria presenting with posterior reversible encephalopathy syndrome and lateralized periodic discharges plus fast activity on EEG. Epilepsy Behav Case Rep. 2016;6:58 60. ( 10.1016/j.ebcr.2016.08.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Subramaniam T, Jain A, Hall LT, et al. Lateralized periodic discharges frequency correlates with glucose metabolism. Neurology. 2019;92(7):e670 e674. ( 10.1212/WNL.0000000000006903) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matsumoto A, Hanayama H, Matsumoto H, et al. Asymmetric posterior reversible encephalopathy syndrome secondary to epilepsy occurring in the chronic phase of subarachnoid hemorrhage. Surg Neurol Int. 2022;13:129. ( 10.25259/SNI_244_2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fisher RS, McGinn RJ, Von Stein EL, et al. Transcranial direct current stimulation for focal status epilepticus or lateralized periodic discharges in four patients in a critical care setting. Epilepsia. 2023;64(4):875 887. ( 10.1111/epi.17514) [DOI] [PubMed] [Google Scholar]
- 16. Cordelli DM, Marra C, Ciampoli L, et al. Posterior Reversible encephalopathy Syndrome in infants and young children. Eur J Paediatr Neurol. 2021;30:128 133. ( 10.1016/j.ejpn.2020.10.009) [DOI] [PubMed] [Google Scholar]
- 17. Tetsuka S, Ogawa T. Posterior reversible encephalopathy syndrome: a review with emphasis on neuroimaging characteristics. J Neurol Sci. 2019;404:72 79. ( 10.1016/j.jns.2019.07.018) [DOI] [PubMed] [Google Scholar]
- 18. Anderson RC, Patel V, Sheikh-Bahaei N, et al. Posterior reversible encephalopathy syndrome (PRES): pathophysiology and neuro-imaging. Front Neurol. 2020;11:463. ( 10.3389/fneur.2020.00463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reiher J, Rivest J, Grand’Maison F, Leduc CP. Periodic lateralized epileptiform discharges with transitional rhythmic discharges: association with seizures. Electroencephalogr Clin Neurophysiol. 1991;78(1):12 17. ( 10.1016/0013-4694(91)90013-t) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a