Abstract
Purpose:
Adequate staging of early rectal neoplasms is essential for organ-preserving treatments, but MRI frequently overestimates the stage of those lesions. We aimed to compare the ability of magnifying chromoendoscopy and MRI to select patients with early rectal neoplasms for local excision.
Methods:
This retrospective study on a Tertiary Western cancer center included consecutive patients evaluated by magnifying chromoendoscopy and MRI who underwent en bloc resection of nonpedunculated sessile polyps larger than 20 mm, LSTs ≥ 20mm, or depressed-type lesions of any size (Paris 0-IIc). Sensitivity, specificity, accuracy, positive and negative predictive values of magnifying chromoendoscopy and MRI to determine which lesions were amenable to local excision (i.e., ≤T1sm1) were calculated.
Results:
Specificity of magnifying chromoendoscopy was 97.3% (95% CI 92.2–99.4), and accuracy was 92.7% (95%CI 86.7–96.6) for predicting invasion deeper than T1sm1 (not amenable to local excision). MRI had lower specificity (60.5%, 95% CI 43.4–76.0) and lower accuracy (58.3%, 95%CI 43.2–72.4). Magnifying chromoendoscopy incorrectly predicted invasion depth in 10.7% of the cases in which the MRI was correct, while magnifying chromoendoscopy provided a correct diagnosis in 90% of the cases in which the MRI was incorrect (p=0.001). Overstaging occurred in 33.3% of the cases in which magnifying chromoendoscopy was incorrect and 75% of the cases in which MRI was incorrect.
Conclusions:
magnifying chromoendoscopy is reliable for predicting invasion depth in early rectal neoplasms and selecting patients for local excision.
Keywords: magnifying chromoendoscopy, rectal cancer, rectal neoplasms, magnetic resonance imaging
INTRODUCTION
There are many organ preserving strategies for patients with early rectal neoplasms, such as transanal endoscopic microsurgery (TEM), transanal minimally invasive surgery (TAMIS), and endoscopic submucosal dissection (ESD). However, adequate selection of these patients is challenging.
Studies have suggested that endorectal ultrasound (ERUS) is the best exam to select patients for local excision (LE) (1–4). Nevertheless, ERUS has drawbacks: it is highly operator-dependent, has long learning curve, low ability to differentiate T1 substages (sm1, 2, and 3), and lower accuracy for larger lesions, especially those larger than 5 cm (1,5–8).
Precise staging of large lesions is crucial. Whereas understaging leads to suboptimal treatment and increased risk of recurrence; overstaging leads to overtreatment, which can negatively impact patients’ quality of life. Furthermore, adverse events following LE are more frequent in larger resections (9,10), underscoring the need for adequate preoperative staging.
Magnetic resonance imaging (MRI) is an established method for locoregional staging of rectal cancer. While a retrospective study by Balyasnikova et al. (2017) reported that MRI differentiated partial versus massive submucosal invasion with 89% of accuracy (11), other studies have suggested that it is not effective for benign rectal lesions (12), nor in differentiating T1 from T2 lesions (13–15). An ongoing multicentric clinical trial is evaluating the performance of MRI in staging rectal polyp planes (MINSTREL, NCT02532803). Meanwhile, MRI is still frequently used to define the treatment of large early rectal lesions when ERUS is not available or cannot be adequately performed.
Magnifying chromoendoscopy (MCE) is an alternative method to evaluate invasion depth of early colorectal neoplasms (16–19). This method is widespread in Japan, but not in Western countries, where MRI is still frequently used in those cases. Yet, studies in Western countries suggest that the sensitivity of MCE is reproducible (20,21) and its accuracy is not influenced by the lesion’s size (20,22). Thus, the aim of this study was to evaluate and compare the ability of MCE and MRI to select patients with early rectal neoplasms for LE in a Western cancer center.
MATERIALS AND METHODS
Subjects
The institutional Review Board approved the study and waived the requirement for informed consent. Medical records of consecutive patients with rectal lesions who were evaluated by MCE exclusively or by MCE and MRI from October/2011 to February/2020 were retrospectively reviewed, and only patients who underwent en bloc resection were included.
Inclusion criteria were rectal tumors with high risk of submucosal invasion, which included sessile polyps (nonpedunculated and >20 mm), LSTs ≥20mm, and depressed-type lesions of any size (Paris 0-IIc). Exclusion criteria were lesions with ulcerated surface, impairing adequate assessment of the pit pattern through MCE, and patients who had previously undergone chemoradiotherapy.
Our service is a reference cancer center and only accepts patients upon referral from a primary care provider. Therefore, all patients either had a biopsy confirming a malignant lesion, or another exam highly suspicious for neoplasia. As part of the staging workup, patients routinely undergo pelvic MRI and a complete colonoscopy. MCE is performed in cases of sessile polyps, laterally spreading tumors (LSTs), and depressed-type lesions. When MCE was performed before MRI and the lesion had non-invasive features, MRI was dismissed and the patient was referred to LE. If MCE and MRI results were discordant regarding the appropriateness of LE, the case was discussed in a tumor board meeting.
Diagnostic procedures
MCE was performed by a team of experienced endoscopists supervised by a senior endoscopist (F.S.K.). Conventional chromoendoscopy with 0.4% indigo carmine dye was performed with a standard white-light high-resolution endoscopic view, followed by magnification of up to x100, using the CF- Q160Z colonoscope (Olympus Medical Systems, Center Valley, PA). The lesions were classified according to the Kudo pit pattern classification. (23,24)
High-definition MRI was performed on GE HDXT 1.5T (GE Medical Systems Milwaukee, Wisconsin, USA), and GE Discovery MR7503.0T (GE Medical Systems Milwaukee, Wisconsin, USA), with a body-matrix coil centered over the pubic symphysis, without administration of contrast or bowel preparation. The images were interpreted by a team of radiologists with more than 5 years of experience in rectal MRI. Our institution’s radiologic report of rectal lesions is standardized and includes location, morphology, T and N categories, circumferential resection margin (CRM) status, and extramural vascular invasion (EMVI). The radiologists described the distinction of a preserved or compromised submucosal layer (hyperintense signal) when possible. When there was no hyperintense signal beneath the lesion but the muscular layer was preserved, the tumor was classified as T1sm3/T2. A second expert radiologist reassessed equivocal reports.
En bloc resection
After evaluation with MCE and MRI, patients were referred to LE or low anterior resection with total mesorectal excision (TME). In our institution, pathological reports of rectal neoplasia describe submucosal invasion according to the guidelines of the main societies of cancer of the colon and rectum (the submucosa is measured from the surface layer of the muscularis mucosae in μm, and lesions that invade less than 1000 μm are considered T1sm1). (25–27)
For patients treated by LE, the following criteria must be met for them to be considered cured: (i) well-differentiated adenocarcinoma, (ii) no lymphovascular invasion, (iii) budding grade 1, (iv) limited to the mucosal layer, or superficial submucosal invasion <1000 μm, and (v) free margins (26,27). Patients who do not meet all these criteria are referred to further treatment (TME).
Ability of MCE and MRI to select patients for local excision
MCE allows the identification of seven different pit patterns: I. round, II. asteroid, IIIs. small tubular, IIIL. large tubular, IV. dendritic or gyrus-like, Vi. irregular pattern, Vn. loss of pits with an amorphous structure. Lesions classified as Vn should not be referred to LE, as Vn pattern indicates massive submucosal invasion (T1sm>1), with a considerable risk of lymph node metastasis (23–25). Thus, we evaluated the ability of MCE to correctly differentiate lesions with extensive submucosal invasion from lesions without extensive submucosal invasion (T1sm1, intramucosal adenocarcinoma, or low-grade adenoma). Histopathological reports from the resected specimens were the gold standard for final staging.
As the ability of MRI to evaluate superficially invasive lesions is debatable (11,15), we evaluated three scenarios: (1) considering MRI T1/T2 as inconclusive and excluding from analysis, so patients with MRI ≥ T2 would be considered as having invasive lesions, and patients with MRI≤T1 would be considered as eligible for LE; (2) considering MRI≥ T1/T2 as indicative of invasive lesion, and MRI ≤T1 as eligible for LE; (3) considering MRI≥ T2 as indicative of invasive lesion, and MRI≤T1/T2 as eligible for LE.
We present the case of a patient evaluated by both MCE and MRI in Figures 1 and 2.
Figure 1.
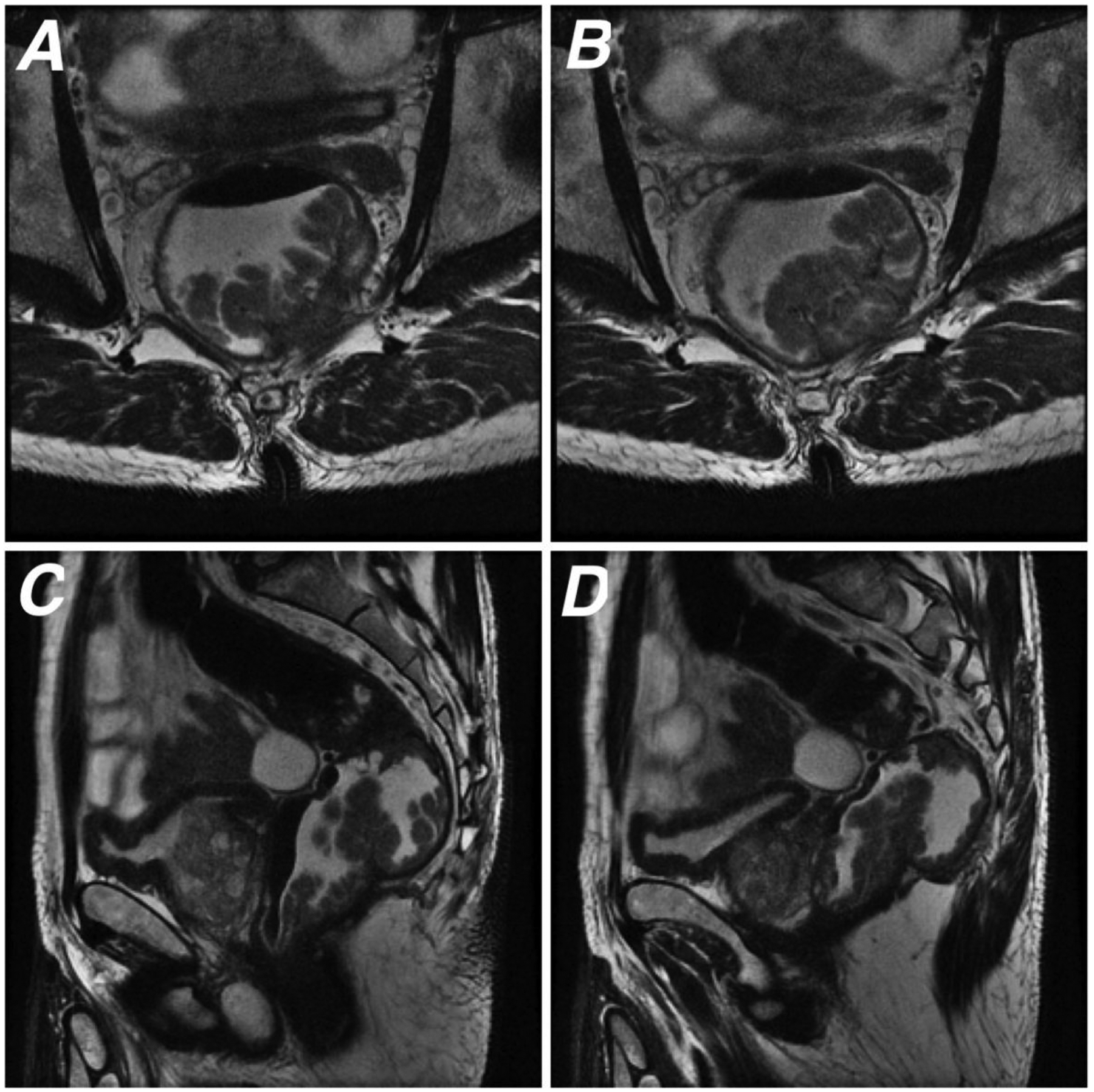
67 year-old male patient who underwent rectal MRI for baseline staging. (A and B) Oblique axial and (C and D) sagittal T2WI show a lower rectal polypoid tumor contained by the muscularis propria, consistent with T1/T2 lesion.
Figure 2.
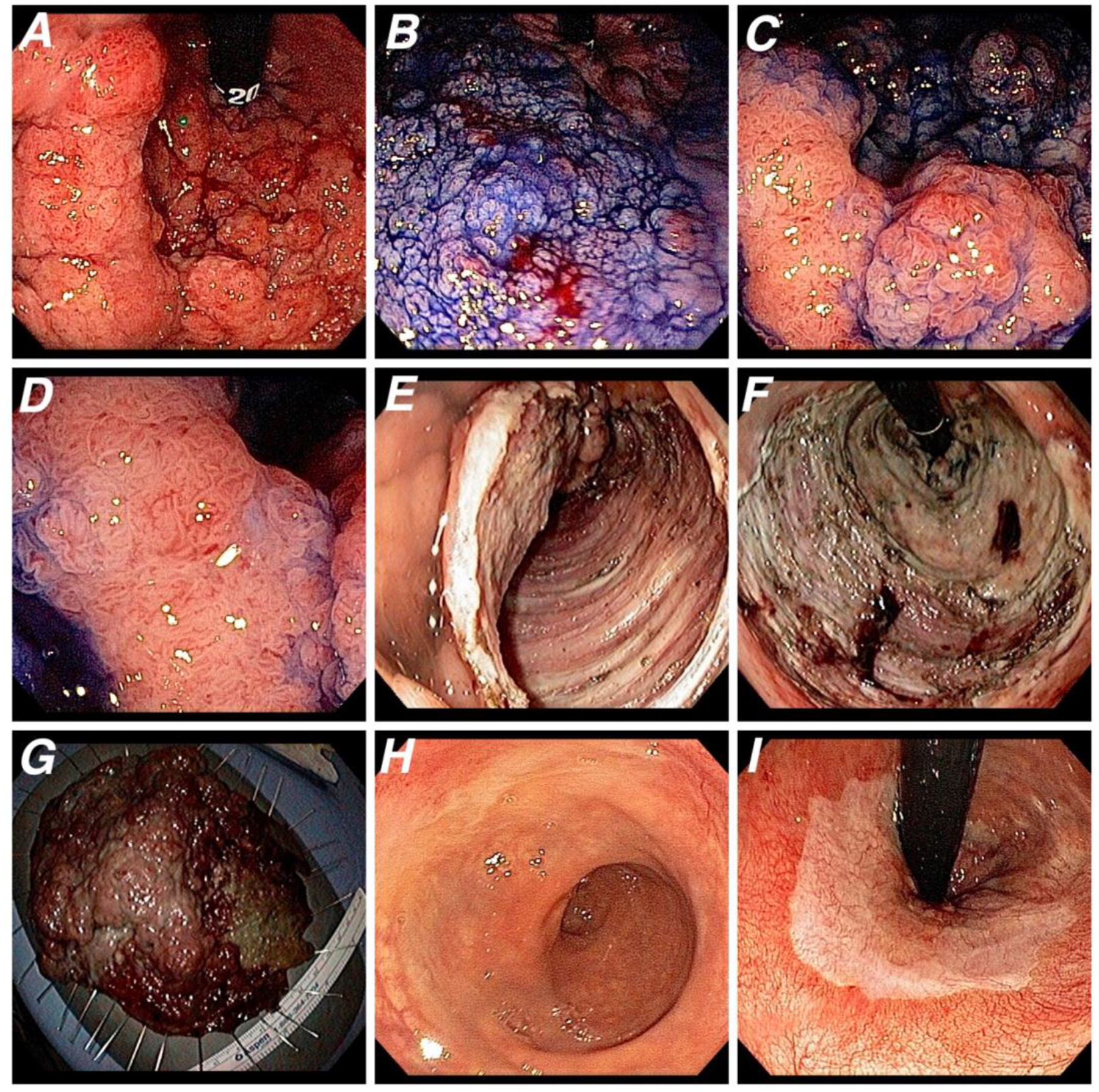
Same patient of Figure 1 was assessed and treated by colonoscopy. A. High-definition white light image (retroflexion view) shows a laterally spreading tumor, granular type, located in distal rectum, close to the dentine line. B. Lesion margins delineated with 0.4% índigo carmine dye spraying. C and D. Magnifying chromoendoscopy reveals Vi pit pattern, a noninvasive aspect that would correspond to either intramucosal adenocarcinoma or adenocarcinoma with superficial invasion of the submucosa (T1sm1). Endoscopic submucosal dissection (ESD) was indicated. E. Lesion partially dissected during the ESD procedure. F. The ulcer bed after the sucessfull en bloc resection. G. Resected specimen (115 × 110 mm). Histology revealed an intramucosal well diferentiated carcinoma without lymphovascular invasion and free margins. H and I. Four-year follow-up colonoscopy presenting a scar in the distal rectum with no signs of recurrence (H. Frontal view; I. Retroflexion view).
Statistical analysis
Chi-squared test or t-test were used to compare proportions and means, respectively. McNemar’s test was used to compare paired results of MCE and MRI, considering the scenario in which MRI had better accuracy as reference. Sensitivity, specificity, positive and negative predictive values (PPV and NPV, respectively) to predict deep submucosal invasion were calculated for each diagnostic modality with their respective 95% CI. Kappa statistic was calculated for the agreement between MCE or MRI and the histopathological report. Stata (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC) and Excel (Microsoft Corporation, Redmond, WA) were used for analyses. P values of 0.05 or less were considered significant.
RESULTS
In the period, 145 patients were evaluated by MCE, of whom 21 were excluded according to the exclusion criteria (Fig. 3). Thus, the final study cohort consisted of 124 patients, of whom 56 had also been evaluated with MRI. Eight patients had unsatisfactory MRI reports and were excluded from the analyses of diagnostic performance.
Figure 3.
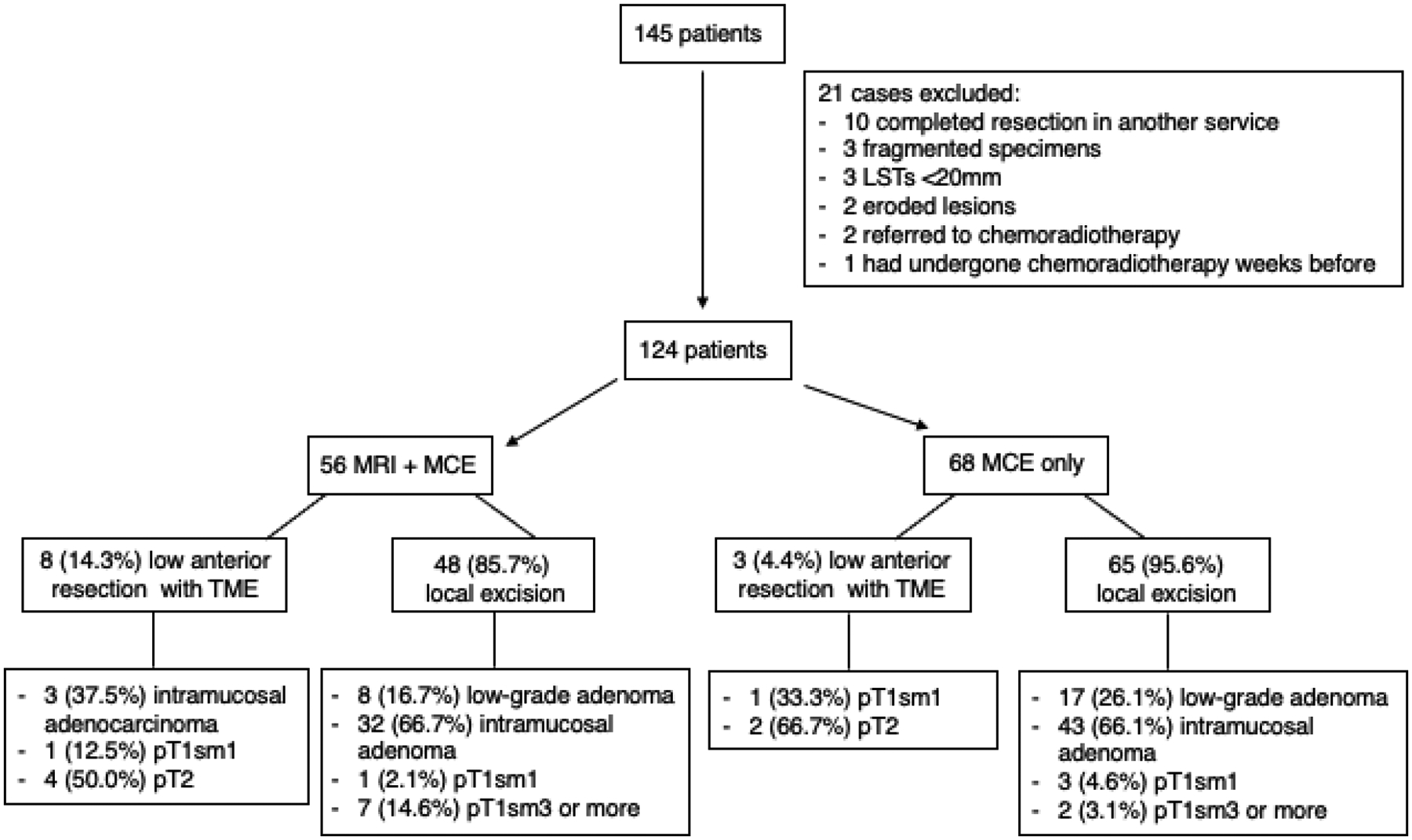
Included cases
The mean lesion size was 66.8 mm (SD 44.7). Table 1 describes the general characteristics of the patients. The most frequent histopathologic diagnosis was intramucosal adenocarcinoma (high-grade adenoma), representing 62.9% of the cases. The histopathological diagnosis according to MCE and MRI reports is shown in Table 2.
Table 1.
General characteristics of patients who underwent only MCE and patients who underwent MCE and MRI
| Only MCE (n=68) | MCE and MRI (n=56) | p value | Total (n=124) | |
|---|---|---|---|---|
| Age, y (mean) | 65.1 (SD 10.8) | 64.5 (SD 9.8) | 0.77 | 64.8 (SD 10.3) |
| Size, mm (mean) | 66.8 (SD 44.7) | 71.9 SD (36.2) | 0.49 | 69.1 (SD 40.2) |
| Paris classification | ||||
| LST-G homogeneous | 10 (14.7) | 7 (12.5) | 0.06 | 17 (13.7) |
| LST-G nodular mix | 33 (48.5) | 25 (44.6) | 58 (46.8) | |
| LST-NG flat elevated | 15 (22.1) | 6 (10.7) | 21 (16.9) | |
| LST-NG pseudodepressed | - | 1 (1.8) | 1 (0.8) | |
| ls | 4 (5.9) | 11 (19.6) | 15 (12.1) | |
| llc | - | 2 (3.6) | 2 (1.6) | |
| lp | - | 1 (1.8) | 1 (0.8) | |
| residual lesions | 6 (8.8) | 3 (5.4) | 9 (7.3) | |
| Pit Pattern | ||||
| III-L | 1 (1.5) | - | 0.19 | 1 (0.8) |
| IV | 16 (23.5) | 8 (14.3) | 24 (19.3) | |
| Vi | 47 (69.1) | 40 (71.4) | 87 (70.2) | |
| Vn | 4 (5.9) | 8 (14.3) | 12 (9.7) | |
| Histopathologic diagnosis | ||||
| Low-grade adenoma | 17 (25.0) | 8 (14.3) | 0.07 | 25 (20.2) |
| Intramucosal adenocarcinoma (high-grade adenoma) | 43 (63.2) | 35 (62.5) | 78 (62.9) | |
| pT1sm1 | 4 (5.9) | 2 (3.6) | 6 (4.8) | |
| ≥pT1sm2 | 4 (5.9) | 11 (19.6) | 15 (12.1) |
Table 2.
Histopathological diagnosis according to pit pattern or MRI classification.
| Low-grade adenoma | Intramucosal adenocarcinoma (high-grade adenoma) | pT1sm1 | ≥pT1sm2 | Total | ||
|---|---|---|---|---|---|---|
| Pit pattern | III-L | 1 (4.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.8) |
| IV | 11 (44.0) | 13 (16.7) | 0 (0.0) | 0 (0.0) | 24 (19.4) | |
| Vi | 13 (52.0) | 64 (82.1) | 4 (66.7) | 6 (40.0) | 87 (70.2) | |
| Vn | 0 (0.0) | 1 (1.3) | 2 (33.3) | 9 (60.0) | 12 (9.7) | |
| Total | 25 | 78 | 6 | 15 | 124 | |
| MRI | cT1 | 1 (12.5) | 3 (8.6) | 0 (0.0) | 2 (18.2) | 6 (10.7) |
| cT1/T2 | 3 (37.5) | 15 (42.9) | 1 (50.0) | 3 (27.3) | 22 (39.3) | |
| cT2 | 1 (12.5) | 9 (25.7) | 0 (0.0) | 4 (36.4) | 14 (25.0) | |
| cT2/T3a | 0 (0.0) | 1 (2.9) | 0 (0.0) | 0 (0.0) | 1 (1.8) | |
| cT3a | 1 (12.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.8) | |
| cT3b | 0 (0.0) | 1 (2.9) | 1 (50.0) | 1 (9.1) | 3 (5.4) | |
| cT3c | 0 (0.0) | 1 (2.9) | 0 (0.0) | 0 (0.0) | 1 (1.8) | |
| inconclusive | 2 (25.0) | 5 (14.3) | 0 (0.0) | 1 (9.1) | 8 (14.3) | |
| Total | 8 | 35 | 2 | 11 | 56 | |
Six patients had MRI reports showing cT2/T3 or cT3 lesions. The histopathologic report revealed low-grade adenoma in one, intramucosal adenocarcinoma in three, pT1sm1 in one, and pT2 in only one (Table 2).
The sensitivity, specificity, PPV and NPV of the MCE and MRI (in the three different scenarios) are presented in Table 3. Considering all 124 patients, the specificity of the MCE was 97.3% (95%CI 92.2–99.4), and it was not significantly different when only patients who had both MCE and MRI were included (97.4%, 95%CI 86.2–99.9). MRI had the highest specificity when considering MRI≥cT2 as positive for deep invasion (60.5%, 95%CI 43.4–76.0) (scenario 3), but still lower than the specificity of MCE.
Table 3.
Sensitivity, specificity, positive and negative predictive values (PPV and NPV, respectively), and kappa
| Sensitivity | 95% CI | Specificity | 95% CI | PPV | 95% CI | NPV | 95% CI | Accuracy | 95% CI | Kappa4 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MCE 1 | 60.0 | 32.3; 83.7 | 97.3 | 92.2; 99.4 | 75.0 | 42.8; 94.5 | 94.6 | 88.7; 98.0 | 92.7 | 86.7; 96.6 | 0.626 |
| MCE 2 | 60.0 | 26.2; 87.8 | 97.4 | 86.2; 99.9 | 85.7 | 42.1; 99.6 | 90.2 | 76.9; 97.3 | 89.6 | 77.3; 96.5 | 0.645 |
| MRI≥ cT1/T2 | 80.0 | 44.4; 97.5 | 10.5 | 2.9; 24.8 | 19.0 | 8.6; 34.1 | 66.7 | 22.3; 95.7 | 25.0 | 13.6; 39.6 | −0.043 |
| MRI≥ cT23 | 71.4 | 29.0; 96.3 | 21.0 | 6.0; 45.6 | 25.0 | 8.7; 49.1 | 66.7 | 22.3; 95.7 | 34.6 | 17.2; 55.7 | −0.117 |
| MRI≥ cT2 | 50.0 | 18.7; 81.3 | 60.5 | 43.4; 76.0 | 25.0 | 8.7; 49.1 | 82.1 | 63.1; 93.9 | 58.3 | 43.2; 72.4 | 0.077 |
including all patients
including only patients with both MCE and MRI
considering T1/T2 as inconclusive and excluding from analysis
kappa-statistic for the agreement between MCE or MRI and the histopathological report (<0.0: poor; 0.00 – 0.20: slight; 0.21 – 0.40: fair; 0.41 – 0.60: moderate; 0.61 – 0.80: substantial; 0.81 – 1.00: almost perfect)
MCE had higher positive and negative predictive values than MRI, regardless of the scenario. Accuracy was also higher for MCE. MRI had the highest accuracy when MRI≥cT2 was considered positive for deep invasion (scenario 3). Since MRI showed better accuracy in this scenario, we compared cases in which MCE was correct and MRI scenario 3 was incorrect (Table 4). MCE was incorrect in 3/28 (10.7%) cases in which the MRI was correct. In contrast, MCE provided a correct diagnosis in 18/20 (90%) cases in which the MRI was incorrect (p=0.001). In the 2/20 remaining cases where both were incorrect, both methods had understaged the lesion.
Table 4.
Comparison of MCE and MRI correct and incorrect results.
| MRI correct1 | MRI incorrect1 | Total | |
|---|---|---|---|
| MCE correct | 25 (89.3) | 18 (90.0) | 43 |
| MCE incorrect | 3 (10.7) | 2 (10.0) | 5 |
| Total | 28 | 20 | 48 |
MRI was considered correct when MRI≥cT2 was considered positive for deep invasion and MRI≤cT1/T2 was considered negative for deep invasion. Otherwise, MRI was considered incorrect. Patients with inconclusive MRI were excluded.
MCE incorrectly predicted invasion depth in 9/124 cases (4%) – 3 (33%) due to overstaging, and 6 (66%) due to understaging. Considering scenario 3, in which MRI had better accuracy, MRI incorrectly predicted invasion depth in 20/48 cases (41.7%), 15 (75%) due to overstaging, and 5 (25%) due to understaging.
Regarding nodal staging, MRI reported positive lymph nodes in 5/48 (10.4%) cases. In one case, it showed cT1N1; in two cases, it showed cT2N1; and in the other two cases, it showed cT3bN1. In one of the cT3N1 cases, MCE also showed a Vn (invasive) lesion. This patient underwent TME and the anatomopathological report showed a pT2N2a. In the other four cases, MCE showed Vi (non-invasive) lesions, and the treatment was determined in a tumor board meeting considering the patients’ clinical condition. Two patients (cT2N1 and cT1N1) underwent TME, and the histopathological report showed intramucosal adenocarcinoma. The other two patients (cT2N1 and cT3bN1) underwent LE (TEM and ESD), and the reports showed intramucosal adenocarcinoma and T1sm1, respectively. Those patients were followed for at least four years without recurrence.
DISCUSSION
This study highlights an important issue regarding the preoperative staging of rectal neoplasms. Although many studies have shown that MRI is unreliable in differentiating benign or superficially invasive rectal lesions from invasive lesions (12,14), MRI is still the most commonly employed method to stage early rectal cancer. In a recent Dutch population-based study published by Detering et al. in 2020, MRI was used as a single staging method in 5288/5539 (95.5%) patients undergoing upfront surgical treatment for primary cT1-T2 rectal cancer. The remaining 251 patients (5.5%) were evaluated by both ERUS and MRI, and in both situations MRI frequently overstaged pT1 tumors (55% of cases when it was used alone, and 31% of cases when it was used with ERUS) (15).
The present study was not designed to evaluate the diagnostic performance of the MRI, but we also observed a tendency of the MRI to overstage rectal lesions. This observation may be biased since we did not actively seek all operated patients with ≤pT1 tumors who might also have been evaluated by MRI. However, previous studies have shown that MRI can overstage early rectal lesions in more than 40% of the cases (12,15,28,29). In addition, four patients in the present study had an MRI showing cT3b or cT3c, but only one of them had a deeply invasive lesion. We likely overestimated the proportion of MRI false positives for deep invasion because we only included non-pedunculated sessile polyps, LSTs, and depressed-type lesions. Nevertheless, those findings highlight the importance of reporting the endoscopic aspect of the lesions, especially in studies on non-operative management of rectal cancer. None of the papers included by two systematic reviews on the “Watch and Wait” approach reported the pretreatment endoscopic aspect of the lesions. Up to 30% of the patients in those studies were classified as cT2, but only about half of the studies used ERUS besides MRI. (30,31)
Even though providers acknowledge that MRI is not an ideal method to evaluate early rectal cancer, results from a recent poll on Twitter®, posted by Dr. Sam Atallah, showed that many of them still rely on MRI to stage those lesions. (32) The survey asked about the next step in the case of a 68-year-old male with a 3 cm rectal polyp whose biopsy showed high-grade dysplasia. The conventional endoscopy could not tell if the lesion was invasive or not. Of the 849 respondents, 35% voted for LE, 25% for full staging workup, 33% for MRI, and 7% for re-biopsy. The fact that one-third indicated MRI for a 3 cm rectal polyp is noteworthy. Although the survey did not have scientific purposes, it provided valuable information regarding real-life practice.
Local excision, the most voted response, might be an option when there is doubt regarding the local stage of early lesions. It has been proposed that LE could be a diagnostic tool (or “excisional biopsy”) (15,33). This approach is supported by studies showing no difference in complications in salvage TME following TEM (34–37) or TAMIS (38). However, a meta-analysis by Chaouch et al. (2021) comparing the outcomes of primary TME with salvage TME following LE showed that the latter resulted in a significantly lower number of harvested lymph nodes (p= 0.006) (39). In addition, few studies reported data on long-term outcomes, so there is little evidence regarding recurrence rates in such cases.
Nevertheless, since salvage TME is an option when LE is not curative, MCE may offer an advantage over MRI. In the present study, we observed that MRI tends to overestimate the stage of early rectal lesions. In contrast, MCE only overestimated invasion depth in 3/124 cases (2.4%). Furthermore, MRI can also overestimate nodal staging. In Detering et al., MRI accused cN1 for tumors that were pN0 in 56% of the cases (15). In our cohort, MRI showed cN1 in 5/48 (10.4%) patients, four of whom actually had superficially invasive lesions (≤pT1sm1), which carry a very low risk of lymph node metastasis (<2%) and could be treated with LE. (25) In all those five cases, MCE had accurately predicted invasion depth. The high specificity (97.3%, 95% CI 92.2–99.4) and NPV of MCE in our study (94.6%, 95% CI 88.7–98.0) means that MCE showing a pit pattern not suggestive of deep invasion supports the referral to LE.
The present study is consistent with prior literature that reported a high accuracy of the MCE to differentiate T1sm1 from T1sm2/sm3 lesions (7,16–18). A strength of our study is that we included patients who had both MCE and MRI. The MCE was correct in 90% of the cases in which the MRI was incorrect. In contrast, MRI provided a correct diagnosis in only 11% of the cases in which the MCE was incorrect (p=0.001).
We must address the limitations of this study. Firstly, we did not include a comparison with ERUS, which is considered a better method than MRI for T staging of early lesions. (7) However, studies have shown that ERUS might have limited accuracy in real-life practice. (5) A systematic review and meta-analysis by O’Connell et al. (2019) showed that ERUS had modest to limited accuracy for benign and T1 lesions, respectively. (40) Moreover, in all 11 studies included, the mean lesion size was either not reported or lower than 43 mm, considerably lower than the mean size of the lesions in the present study, 67 mm. As previously mentioned, ERUS has lower accuracy for lesions larger than 50 mm (1,5–8), so it would be a suboptimal indication for the studied population.
Secondly, one can argue that the accuracy of MCE is operator dependent. However, MRI for rectal cancer staging is also observer-dependent and also requires specific training. Advances in image resolution, artificial intelligence, and computer-aided diagnosis in both colonoscopy and MRI may mitigate this dependence on observer experience and impact the performance of those exams in the future.
Lastly, this is a retrospective study and not all patients had MRI. Also, we did not have a blinded senior radiologist re-evaluate the MRI reports, not even the inconclusive reports. Thus, we cannot draw any definitive conclusion regarding the performance of MRI. Nevertheless, we aimed to demonstrate the real-life performance of both MCE and MRI. MCE images were not re-evaluated either, and all clinical decisions were made with the reports available. Yet, all MCE evaluations were supervised by a single senior endoscopist while a team of different radiologists interpreted the MRIs, which is a relevant limitation of this study.
Conclusion
MCE is a reliable method to predict depth of invasion in sessile polyps, LSTs ≥20mm, and depressed-type lesions. Therefore, if MCE is available, it should be used to assess patients with sessile polyps, LSTs ≥20mm, and depressed-type lesions before they are referred to TME or chemoradiotherapy.
Footnotes
Disclosure statement: the authors have no conflict of interest to declare.
REFERENCES
- 1.Pinto RA, Corrêa Neto IJF, Nahas SC, Rizkalah Nahas CS, Sparapan Marques CF, Ribeiro Junior U, et al. Efficacy of 3-Dimensional Endorectal Ultrasound for Staging Early Extraperitoneal Rectal Neoplasms. Dis Colon Rectum. 2017. May;60(5):488–96. [DOI] [PubMed] [Google Scholar]
- 2.Puli SR, Bechtold ML, Reddy JBK, Choudhary A, Antillon MR, Brugge WR. How Good is Endoscopic Ultrasound in Differentiating Various T Stages of Rectal Cancer? Meta-Analysis and Systematic Review. Ann Surg Oncol. 2009. Feb;16(2):254–65. [DOI] [PubMed] [Google Scholar]
- 3.Patel RK, Sayers AE, Kumar P, Khulusi S, Razack A, Hunter IA. The Role of Endorectal Ultrasound and Magnetic Resonance Imaging in the Management of Early Rectal Lesions in a Tertiary Center. Clinical Colorectal Cancer. 2014. Dec;13(4):245–50. [DOI] [PubMed] [Google Scholar]
- 4.Cote A, Graur F, Lebovici A, Mois E, Al Hajjar N, Mare C, et al. THE ACCURACY OF ENDORECTAL ULTRASONOGRAPHY IN RECTAL CANCER STAGING. Medicine and Pharmacy Reports. 2015. Jul 22;88(3):348–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashraf S, Hompes R, Slater A, Lindsey I, Bach S, Mortensen NJ, et al. A critical appraisal of endorectal ultrasound and transanal endoscopic microsurgery and decision-making in early rectal cancer. Colorectal Dis. 2012. Jul;14(7):821–6. [DOI] [PubMed] [Google Scholar]
- 6.Marusch F, Ptok H, Sahm M, Schmidt U, Ridwelski K, Gastinger I, et al. Endorectal ultrasound in rectal carcinoma – do the literature results really correspond to the realities of routine clinical care? Endoscopy. 2011. May;43(05):425–31. [DOI] [PubMed] [Google Scholar]
- 7.Morino M, Risio M, Bach S, Beets-Tan R, Bujko K, Panis Y, et al. Early rectal cancer: the European Association for Endoscopic Surgery (EAES) clinical consensus conference. Surg Endosc. 2015. Apr;29(4):755–73. [DOI] [PubMed] [Google Scholar]
- 8.Serra-Aracil X, Gálvez A, Mora-López L, Rebasa P, Serra-Pla S, Pallisera-Lloveras A, et al. Endorectal ultrasound in the identification of rectal tumors for transanal endoscopic surgery: factors influencing its accuracy. Surg Endosc. 2018;32(6):2831–8. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi T, Kudo S-E, Miyachi H, Sakurai T, Ishigaki T, Yagawa Y, et al. Management and risk factor of stenosis after endoscopic submucosal dissection for colorectal neoplasms. Gastrointest Endosc. 2017. Aug;86(2):358–69. [DOI] [PubMed] [Google Scholar]
- 10.Ohara Y, Toyonaga T, Tanaka S, Ishida T, Hoshi N, Yoshizaki T, et al. Risk of stricture after endoscopic submucosal dissection for large rectal neoplasms. Endoscopy. 2016. Jan;48(1):62–70. [DOI] [PubMed] [Google Scholar]
- 11.Balyasnikova S, Read J, Wotherspoon A, Rasheed S, Tekkis P, Tait D, et al. Diagnostic accuracy of high-resolution MRI as a method to predict potentially safe endoscopic and surgical planes in patients with early rectal cancer. BMJ Open Gastroenterol. 2017. Jul;4(1):e000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee L, Arbel L, Albert MR, Atallah SB, Hill J, Monson JRT. Radiologic Evaluation of Clinically Benign Rectal Neoplasms May Not Be Necessary Before Local Excision. Diseases of the Colon & Rectum. 2018. Oct;61(10):1163–9. [DOI] [PubMed] [Google Scholar]
- 13.Bipat S, Glas AS, Slors FJM, Zwinderman AH, Bossuyt PMM, Stoker J. Rectal Cancer: Local Staging and Assessment of Lymph Node Involvement with Endoluminal US, CT, and MR Imaging—A Meta-Analysis. Radiology. 2004. Sep;232(3):773–83. [DOI] [PubMed] [Google Scholar]
- 14.Oien K, Mjørud Forsmo H, Rösler C, Nylund K, Waage JE, Pfeffer F. Endorectal ultrasound and magnetic resonance imaging for staging of early rectal cancers: how well does it work in practice? Acta Oncologica. 2019. Apr 1;58(sup1):S49–54. [DOI] [PubMed] [Google Scholar]
- 15.Detering R, van Oostendorp SE, Meyer VM, van Dieren S, Bos ACRK, Dekker JWT, et al. MRI cT1–2 rectal cancer staging accuracy: a population-based study: Accuracy of staging MRI for early rectal cancer. Br J Surg [Internet]. 2020. Apr 16 [cited 2020 Oct 5]; Available from: http://doi.wiley.com/10.1002/bjs.11590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu K-I, Sano Y, Kato S, Fujii T, Nagashima F, Yoshino T, et al. Chromoendoscopy Using Indigo Carmine Dye Spraying with Magnifying Observation Is the Most Reliable Method for Differential Diagnosis between Non-Neoplastic and Neoplastic Colorectal Lesions: A Prospective Study. Endoscopy. 2004. Dec;36(12):1089–93. [DOI] [PubMed] [Google Scholar]
- 17.Kato S Magnifying colonoscopy as a non-biopsy technique for differential diagnosis of non-neoplastic and neoplastic lesions. WJG. 2006;12(9):1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emura F, Saito Y, Taniguchi M, Fujii T, Tagawa K, Yamakado M. Further validation of magnifying chromocolonoscopy for differentiating colorectal neoplastic polyps in a health screening center. J Gastroenterol Hepatol. 2007. Nov;22(11):1722–7. [DOI] [PubMed] [Google Scholar]
- 19.Konishi K, Kaneko K, Kurahashi T, Yamamoto T, Kushima M, Kanda A, et al. A comparison of magnifying and nonmagnifying colonoscopy for diagnosis of colorectal polyps: A prospective study. Gastrointestinal Endoscopy. 2003. Jan;57(1):48–53. [DOI] [PubMed] [Google Scholar]
- 20.Hurlstone DP. Efficacy of high magnification chromoscopic colonoscopy for the diagnosis of neoplasia in flat and depressed lesions of the colorectum: a prospective analysis. Gut. 2004. Feb 1;53(2):284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawaguti FS, Franco MC, Martins BC, Segateli V, Marques CFS, Nahas CSR, et al. Role of Magnification Chromoendoscopy in the Management of Colorectal Neoplastic Lesions Suspicious for Submucosal Invasion. Dis Colon Rectum. 2019;62(4):422–8. [DOI] [PubMed] [Google Scholar]
- 22.Tung S-Y, Wu C-S, Su M-Y. Magnifying colonoscopy in differentiating neoplastic from nonneoplastic colorectal lesions. Am J Gastroenterology. 2001. Sep;96(9):2628–32. [DOI] [PubMed] [Google Scholar]
- 23.Kudo S, Hirota S, Nakajima T, Hosobe S, Kusaka H, Kobayashi T, et al. Colorectal tumours and pit pattern. Journal of Clinical Pathology. 1994. Oct 1;47(10):880–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kudo S, Tamura S, Nakajima T, Yamano H, Kusaka H, Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointestinal Endoscopy. 1996. Jul;44(1):8–14. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018. Feb;23(1):1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Japanese Society for Cancer of the Colon and Rectum, Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020. Jan;25(1):1–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vogel JD, Felder SI, Bhama AR, Hawkins AT, Langenfeld SJ, Shaffer VO, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Colon Cancer. Diseases of the Colon & Rectum. 2022. Feb;65(2):148–77. [DOI] [PubMed] [Google Scholar]
- 28.Raynaud L, Mege D, Zappa M, Guedj N, Vilgrain V, Panis Y. Is magnetic resonance imaging useful for the management of patients with rectal villous adenoma? A study of 45 consecutive patients treated by transanal endoscopic microsurgery. Int J Colorectal Dis. 2018. Dec;33(12):1695–701. [DOI] [PubMed] [Google Scholar]
- 29.Park JS, Jang Y-J, Choi G-S, Park SY, Kim HJ, Kang H, et al. Accuracy of Preoperative MRI in Predicting Pathology Stage in Rectal Cancers: Node-for-Node Matched Histopathology Validation of MRI Features. Diseases of the Colon & Rectum. 2014. Jan;57(1):32–8. [DOI] [PubMed] [Google Scholar]
- 30.Dossa F, Chesney TR, Acuna SA, Baxter NN. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. The Lancet Gastroenterology & Hepatology. 2017. Jul;2(7):501–13. [DOI] [PubMed] [Google Scholar]
- 31.Dattani M, Heald RJ, Goussous G, Broadhurst J, São Julião GP, Habr-Gama A, et al. Oncological and Survival Outcomes in Watch and Wait Patients With a Clinical Complete Response After Neoadjuvant Chemoradiotherapy for Rectal Cancer: A Systematic Review and Pooled Analysis. Annals of Surgery. 2018. Dec;268(6):955–67. [DOI] [PubMed] [Google Scholar]
- 32.Sam Atallah MD. 68 yo healthy male has 3cm rectal polyp with high grade dysplasia. Endoscopically you can’t tell if it has invasive CA or not. What do you do next? #SoMe4Surgery @juliomayol @SWexner @ScottRSteeleMD @DCRjournal @SAGES_Updates @Neil_J_Smart @escp_tweets @RoelHompes @justinmaykel [Internet]. @SamAtallahMD. 2021. [cited 2021 Jul 15]. Available from: https://twitter.com/SamAtallahMD/status/1350508903793119232
- 33.Lee L, Burke JP, deBeche-Adams T, Nassif G, Martin-Perez B, Monson JRT, et al. Transanal Minimally Invasive Surgery for Local Excision of Benign and Malignant Rectal Neoplasia: Outcomes From 200 Consecutive Cases With Midterm Follow Up. Annals of Surgery. 2018. May;267(5):910–6. [DOI] [PubMed] [Google Scholar]
- 34.Levic K, Bulut O, Hesselfeldt P, Bülow S. The outcome of rectal cancer after early salvage TME following TEM compared with primary TME: a case-matched study. Tech Coloproctol. 2013. Aug;17(4):397–403. [DOI] [PubMed] [Google Scholar]
- 35.Dulskas A, Atkociunas A, Kilius A, Petrulis K, Samalavicius NE. Is Previous Transanal Endoscopic Microsurgery for Early Rectal Cancer a Risk Factor of Worse Outcome following Salvage Surgery A Case-Matched Analysis. Visc Med. 2019;35(3):151–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levic Souzani K, Bulut O, Kuhlmann TP, Gögenur I, Bisgaard T. Completion total mesorectal excision following transanal endoscopic microsurgery does not compromise outcomes in patients with rectal cancer. Surg Endosc [Internet]. 2021. Feb 24 [cited 2021 Jul 16]; Available from: http://link.springer.com/10.1007/s00464-021-08385-2 [DOI] [PubMed] [Google Scholar]
- 37.Hompes R, McDonald R, Buskens C, Lindsey I, Armitage N, Hill J, et al. Completion surgery following transanal endoscopic microsurgery: assessment of quality and short- and long-term outcome. Colorectal Dis. 2013. Oct;15(10):e576–81. [DOI] [PubMed] [Google Scholar]
- 38.Clermonts SHEM, Köeter T, Pottel H, Stassen LPS, Wasowicz DK, Zimmerman DDE. Outcomes of completion total mesorectal excision are not compromised by prior transanal minimally invasive surgery. Colorectal Dis. 2020. Jul;22(7):790–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaouch MA, Khan J, Gill TS, Mehrabi A, Reissfelder C, Rahberi N, et al. Early salvage total mesorectal excision (sTME) after organ preservation failure in rectal cancer does not worsen postoperative outcomes compared to primary TME: systematic review and meta-analysis. Int J Colorectal Dis [Internet]. 2021. Jul 9 [cited 2021 Jul 16]; Available from: https://link.springer.com/10.1007/s00384-021-03989-5 [DOI] [PubMed] [Google Scholar]
- 40.O’Connell E, Galvin R, McNamara DA, Burke JP. The utility of preoperative radiological evaluation of early rectal neoplasia: a systematic review and meta‐analysis. Colorectal Dis. 2020. Sep;22(9):1076–84. [DOI] [PubMed] [Google Scholar]


