Abstract
Quantitative analysis of electroencephalography (qEEG) is a potential source of biomarkers for neonatal encephalopathy (NE). However, prior studies using qEEG in NE were limited in their generalizability due to individualized techniques for calculating qEEG features or labor-intensive pre-selection of EEG data. We piloted a fully automated method using commercially available software to calculate the suppression ratio (SR), absolute delta power, and relative delta, theta, alpha, and beta power from EEG of neonates undergoing 72 h of therapeutic hypothermia (TH) for NE between April 20, 2018, and November 4, 2019. We investigated the association of qEEG with degree of encephalopathy (modified Sarnat score), severity of neuroimaging abnormalities following TH (National Institutes of Child Health and Development Neonatal Research Network [NICHD-NRN] score), and presence of seizures. Thirty out of 38 patients met inclusion criteria. A more severe modified Sarnat score was associated with higher SR during all phases of TH, lower absolute delta power during all phases except rewarming, and lower relative delta power during the last 24 h of TH. In 21 patients with neuroimaging data, a worse NICHD-NRN score was associated with higher SR, lower absolute delta power, and higher relative beta power during all phases. QEEG features were not significantly associated with the presence of seizures after correction for multiple comparisons. Our results are consistent with those of prior studies using qEEG in NE and support automated qEEG analysis as an accessible, generalizable method for generating biomarkers of NE and response to TH. Additionally, we found evidence of an immature relative frequency composition in neonates with more severe brain injury, suggesting that automated qEEG analysis may have a use in the assessment of brain maturity.
Keywords: Quantitative electroencephalography, Neonatal encephalopathy, Hypoxic-ischemic encephalopathy
Introduction
Neonatal encephalopathy (NE) secondary to hypoxia-ischemia is caused by interruption in cerebral blood flow and oxygen delivery around time of birth and can lead to significant lifelong neurologic morbidity [1]. Therapeutic hypothermia (TH), the only currently available therapy, decreases metabolic demands and attenuates secondary energy failure that leads to ongoing injury. TH reduces major disability and death by 1/3rd in neonates with moderate to severe NE [2]. Current methods to identify which neonates are at risk for worse neurodevelopmental outcomes despite TH are imprecise. Ideally, biomarkers would be available before or during TH, while the secondary phase of injury is ongoing, to identify additional therapeutic windows, shorten time to accurate prognostication, and identify patients who may require additional monitoring and novel treatments.
Neonates undergoing TH are commonly monitored with continuous video electroencephalography (cEEG) [3]. CEEG provides excellent temporal resolution and is ideal for monitoring dynamic brain electrical activity in real time, both to detect seizures as well as characterize background brain activity, which can provide prognostic information distinct from the presence or absence of seizures. In a recent meta-analysis, background patterns such as suppression-burst or low-voltage/isoelectric patterns were shown to be highly specific and sensitive to predict worse NE outcomes [4]. While conventional automated seizure detectors based on rhythmicity analysis are limited in their use in neonates due to the slow rhythms seen in neonatal seizures, quantitative analysis (quantitative analysis of electroencephalography [qEEG]) can be used to characterize features of the neonatal background activity. QEEG analyses that characterize these background features have demonstrated prognostic utility, including calculation of duration and amplitude of interburst intervals [5], and the use of spectral analysis to measure power across frequency bands [6–9]. Amplitude-integrated EEG (aEEG) is a simple form of qEEG analysis that has been used extensively for seizure detection and prognostication in NE. AEEG patterns associated with a poor prognosis in NE include discontinuity, burst suppression, and persistently low-voltage tracings [10].
However, many of the previous studies using qEEG in neonates with NE were limited in their generalizability due to the use of individualized techniques for calculating qEEG features or labor-intensive pre-selection of EEG data. Studies using machine learning algorithms, often difficult to integrate for widespread clinical use and susceptible to inaccuracies when used on datasets that differ from the training dataset, have tended to focus on improving seizure detection in neonates [11], though a few have focused on early grading of NE severity [12]. In this study, we piloted a fully automated method for calculating qEEG features from clinical cEEG monitoring data using a commercially available software without pre-selection of EEG data. We evaluated the association of qEEG features with established markers of clinical severity in NE to evaluate whether our methodology would generate comparable results to prior studies using bespoke techniques for qEEG analysis.
Methods
Clinical data were obtained from the Johns Hopkins Hospital (JHH) Neuroscience Intensive Care Nursery (NICN) Program Administrative Database (IRB NA 00034540), the Identification of Diagnostic and Prognostic Biomarkers for Perinatal Hypoxic-Ischemic Brain Injury Study (BIN study, IRB NA 00026068), and Clinical Practice of Continuous Video-EEG Outside of the Epilepsy Monitoring Unit Study (IRB NA 00044076) in compliance with the Health Insurance Portability and Accountability Act of 1996 (HIPAA). The BIN study, a prospective cohort of neonates undergoing TH for NE at a single center, received approval from the Johns Hopkins University (JHU) Institutional Review Board (IRB) and was exempt of informed consent until 2017, when informed consent became required to access medical records. The Neuroscience Intensive Care Nursery (NICN) program coordinator (CP) identified patients with NE and after agreement by the treating clinical team, a study team member discussed inclusion of the patient with their parents and obtained informed consent.
Participants
Patients were drawn from the BIN study between April 20, 2018, and November 4, 2019. Patients were excluded for incomplete EEG data, off-label use of TH (<35 weeks gestational age), partial TH course (<72 h), nonperipartum events, causes of NE or death not compatible with potential hypoxic-ischemic injury, or the need for extracorporeal membrane oxygenation (Fig. 1).
Fig. 1.
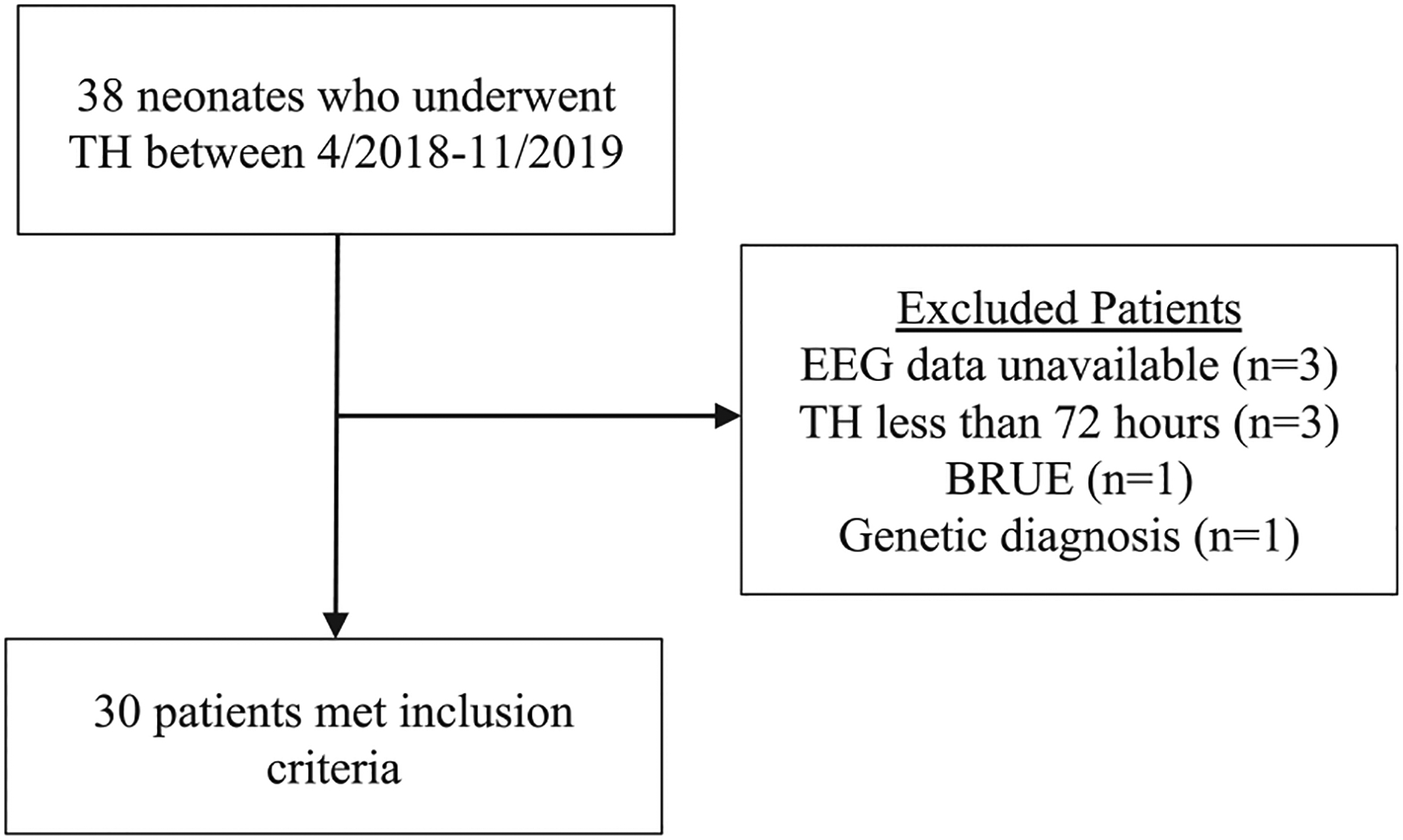
Patient inclusion and exclusion. EEG, electroencephalography; BRUE, brief resolved unexplained event; TH, therapeutic hypothermia.
Quantitative EEG Analysis
Longitudinal quantitative data were computed from the raw EEG tracings using Persyst version 14.E (Prescott, AZ: Persyst Development Co.), a commercially available software that provides automated (1) spectral power analysis calculated using fast Fourier transformations for the following frequency bands: delta, 0.5–4 Hz; theta, 4–8 Hz; alpha, 8–13 Hz; and beta, 13–30 Hz and (2) calculation of the suppression ratio (SR; the fraction of the EEG in suppression, defined as ≥0.5 s at ≤3 μV, averaged over a 60 s epoch). While other qEEG features may be calculated, these features are included as part of the standard qEEG display with the software package. Moreover, EEG power and the interburst interval/presence of burst suppression (approximated through SR as no direct interburst interval calculation is available through this software) have been well established to have prognostic value in NE. QEEG features were computed using Persyst’s automated artifact reduction which suppresses muscle (electromyography), eye movement, and electrode artifact. All qEEG measures were averaged across both hemispheres. The relative power in each frequency band was calculated by dividing the absolute power in each frequency band by the absolute power across all frequency bands. QEEG data were then averaged by phase of TH, defined as the first 24 h of TH; 24–48 h of TH; 48–72 h of TH; rewarming (6 h); and post-rewarming (typically 6 h).
Clinical Variables
Clinical data were obtained from electronic medical records. Race was assigned based on maternal race. Sex and gestational age were assigned by the NICU team. The most severe modified Sarnat score, which grades the degree of encephalopathy as mild, moderate, or severe, during the first 6 h of admission to the NICU was determined by members of the study team (RCV, CP, FJN) [13].
The presence and timing of seizures was determined based on medical records (for patients who had suspected seizures prior to initiation of EEG monitoring) or by the presence of seizures confirmed by cEEG as described in the EEG report and as marked on the raw EEG record. Total seizure burden was not calculated as part of this pilot study and epochs with seizures were not excluded from the qEEG analysis. Presence and timing of phenobarbital administration was determined based on clinical notes (for patients who received phenobarbital prior to arrival at our center) or by documentation in the medication administration record. Given the long half-life of phenobarbital, once a patient received a single dose of phenobarbital they were treated as exposed during all subsequent phases of TH.
Brain MRI scans obtained after TH were scored by a pediatric neuroradiologist (AT) using the NICHD-NRN scoring system, which grades severity of injury based on lesion distribution and extent with a higher score indicating more severe injury [14]. Patients with MRIs obtained after day of life 10 or patients who only underwent a subset of our comprehensive imaging protocol were not scored.
Statistical Analysis
All statistical analyses were carried out in STATA version 17 (College Station, TX: StataCorp LLC). The relationship between clinical variables, including degree of encephalopathy based on modified Sarnat score at presentation (mild, moderate, or severe), MRI NICHD-NRN score, and the presence of seizures were investigated using Fischer’s exact tests. For the statistical analysis, the following qEEG features were used: SR, absolute delta power, and relative power across each of the delta, theta, alpha, and beta frequency bands. Given the small sample size, the distributions of the qEEG variables were assessed for normality with Shapiro-Wilk tests. The results of the Shapiro-Wilk tests showed that the distributions of many of the qEEG variables deviated significantly from normality, so nonparametric tests were used for subsequent analysis. Nonparametric tests for trends across ordered groups were conducted to determine if qEEG features at each phase of TH differed by modified Sarnat score (mild, moderate, or severe) or by NICHD-NRN score (0–3) [15]. Kruskal-Wallis tests were performed to determine if qEEG features at each phase of TH differed between neonates with or without seizures.
The role of phenobarbital exposure as a potential confounder was explored. Nonparametric tests for trends across ordered groups were performed to evaluate the relationship between phenobarbital exposure and the modified Sarnat score and the MRI NICHD-NRN score. Kruskal-Wallis tests were performed to determine whether there was an association between prior or concurrent phenobarbital exposure at each phase of TH and qEEG features. Each analysis was corrected for multiple comparisons using the Benjamini-Hochberg procedure for false discovery rate correction [16].
Results
Demographics
Thirty out of 38 patients screened met study inclusion criteria including complete EEG data (Fig. 1). Patient demographic and clinical characteristics are summarized in Table 1. The cohort of included patients had an average gestational age of 39.7 weeks and was 53% male. Sixty percent of patients had moderate to severe encephalopathy by modified Sarnat scoring and 47% of the 21 patients who had neuroimaging appropriate for MRI NICHD-NRN scoring had abnormal scores following TH. MRIs for these patients were obtained at a median of day of life 5 (IQR 2 days). Twenty-seven percent of patients developed clinical and/or EEG-only seizures in the immediate post-natal period with a median time of seizure onset at 31 h post-birth (IQR 64.7 h) with 1 patient having somewhat delayed onset of seizures at day of life five. In neonates, status epilepticus has been defined as a continuous seizure lasting 30 min or a series of seizures whose total duration exceeds 50% of a given 1 h period or both [17] – by these criteria, one of the patients in our cohort met criteria for status epilepticus. Four patients (13%) were exposed to phenobarbital during TH for treatment of seizures. Of these, all four received bolus phenobarbital doses, but only two were started on maintenance. One patient was treated for clinical seizures prior to EEG confirmation. Two additional patients received phenobarbital after TH was completed for seizures and thus were not counted as exposed for the purposes of the qEEG analysis. Patients who had seizures during TH but were not treated with phenobarbital received other medications including levetiracetam, fosphenytoin, and midazolam.
Table 1.
Patient demographic and clinical characteristics
| Characteristic - number (%) | |
|---|---|
| Male sex | 16 (53) |
| Race | |
| White | 12 (40) |
| Black | 7 (23) |
| Hispanic | 6 (20) |
| Asian | 4 (13) |
| Other/not reported | 1 (3) |
| Modified Sarnat score | |
| Mild | 12 (40) |
| Moderate | 10 (33) |
| Severe | 8 (27) |
| Abnormal MRI | 8 (27) |
| NICHD-NRN score | |
| 0 | 16 (53) |
| 1A | 2 (7) |
| 1B | 0 (0) |
| 2A | 1 (3) |
| 2B | 0 (0) |
| 3 | 2 (7) |
| Not available | 9 (30) |
| Seizures* | 8 (27) |
| EEG-confirmed seizures** | 6 (20) |
| Status epilepticus | 1 (3) |
| Exposure to phenobarbital during TH | 4 (13) |
| Characteristic - median (range) | |
| GA (weeks) | 39.7 (2) |
| Birth weight (grams) | 3,449 (640) |
| Time to initiation of active cooling from birth (hours) | 4.1 (2.6) |
| Time to start of seizures from birth (hours) | 31.3 (64.7) |
TH, therapeutic hypothermia; GA, gestational age; MRI, magnetic resonance imaging; NICHD-NRN, National Institutes of Child Health and Development Neonatal Research Network.
Two patients developed seizures after completion of TH.
Two patients developed clinical seizures prior to being placed on cEEG monitoring.
The degree of encephalopathy based on modified Sarnat score at presentation was not significantly associated with the MRI NICHD-NRN score following TH (Fischer’s exact test = 0.807) or clinical and/or EEG-confirmed seizures (Fischer’s exact test = 0.126). A higher MRI NICHD-NRN score was not associated with clinical and/or EEG-confirmed seizures (Fischer’s exact test = 0.140).
Relationship of QEEG to Sarnat Score
Throughout all phases of TH, a more severe modified Sarnat score at admission was associated with a higher SR (nonparametric tests for trend: z-scores 2.6–3.1, p values <0.009; Table 2; Fig. 2). A more severe modified Sarnat score was also associated with lower absolute delta power (nonparametric tests for trend: z-scores – 2.9 to −2.7, p values <0.007) during all phases, except rewarming. Additionally, a more severe modified Sarnat score was associated with lower relative delta power during the last 24 h of TH (nonparametric tests for trend: z-score = −2.7, p value = 0.008). Relative alpha, theta, or beta power was not associated with the modified Sarnat score. We performed secondary pairwise analyses using Kruskal-Wallis tests to compare qEEG differences between the mild and moderate and moderate and severe groups by Sarnat score to see if the more severe patients were driving any significant results, but none of these results remained significant after correction for multiple comparisons.
Table 2.
Association of quantitative EEG features by phase of TH with markers of clinical severity in neonates with encephalopathy using nonparametric tests for trend (modified Sarnat score, NICHD-NRN score) or the Kruskal-Wallis test (presence of clinical and/or electrographic seizures)
| QEEG feature | Modified Sarnat score (z-score [p value]) | NICHD-NRN score (z-score [p value]) | Seizures (H[1] [p value]) |
|---|---|---|---|
| 0–24 h of TH | |||
| Suppression ratio | 3.1 (0.002)* | 2.5 (0.011)* | 6.2 (0.013) |
| Absolute delta power | −2.8 (0.005)* | −2.5 (0.014)* | 3.3 (0.067) |
| Relative delta power | −1.8 (0.072) | −1.5 (0.134) | 7.1 (0.008) |
| Relative alpha power | 2.0 (0.042) | 1.7 (0.097) | 6.2 (0.013) |
| Relative beta power | 1.3 (0.181) | 2.4 (0.017)* | 0.8 (0.373) |
| 24–48 h of TH | |||
| Suppression ratio | 3.0 (0.003)* | 2.4 (0.015)* | 4.1 (0.044) |
| Absolute delta power | −2.7 (0.007)* | −2.5 (0.013)* | 1.6 (0.205) |
| Relative delta power | −2.0 (0.042) | −2.6 (0.008)* | 5.5 (0.019) |
| Relative alpha power | 1.7 (0.085) | 2.5 (0.012)* | 4.9 (0.028) |
| Relative beta power | 1.8 (0.072) | 2.5 (0.013)* | 1.3 (0.260) |
| 48–72 h of TH | |||
| Suppression ratio | 3.0 (0.002)* | 2.3 (0.022)* | 3.9 (0.049) |
| Absolute delta power | −2.9 (0.003)* | −2.5 (0.012)* | 0.1 (0.708) |
| Relative delta power | −2.7 (0.008)* | −2.5 (0.012)* | 3.9 (0.049) |
| Relative alpha power | 2.2 (0.025) | 2.6 (0.009)* | 3.2 (0.075) |
| Relative beta power | 2.1 (0.037) | 2.7 (0.008)* | 1.3 (0.260) |
| Rewarming | |||
| Suppression ratio | 2.6 (0.009)* | 2.5 (0.012)* | 2.4 (0.122) |
| Absolute delta power | −2.3 (0.022) | −2.4 (0.017)* | 0.0 (0.963) |
| Relative delta power | −2.3 (0.022) | −1.0 (0.340) | 1.2 (0.281) |
| Relative alpha power | 2.0 (0.042) | 1.4 (0.152) | 1.3 (0.260) |
| Relative beta power | 2.0 (0.045) | 2.7 (0.007)* | 2.1 (0.146) |
| Post-rewarming | |||
| Suppression ratio | 3.1 (0.002)* | 2.7 (0.007)* | 3.0 (0.083) |
| Absolute delta power | −2.8 (0.005)* | −2.7 (0.007)* | 0.6 (0.425) |
| Relative delta power | −2.2 (0.029) | −1.0 (0.328) | 2.4 (0.122) |
| Relative alpha power | 2.2 (0.025) | 1.5 (0.128) | 2.9 (0.091) |
| Relative beta power | 2.5 (0.011) | 2.5 (0.011)* | 4.1 (0.044) |
An asterisk indicates where p value remained significant after adjustment for multiple comparisons using the Benjamini-Hochberg procedure.
NICHD-NRN, National Institutes of Child Health and Development Neonatal Research Network.
Fig. 2.
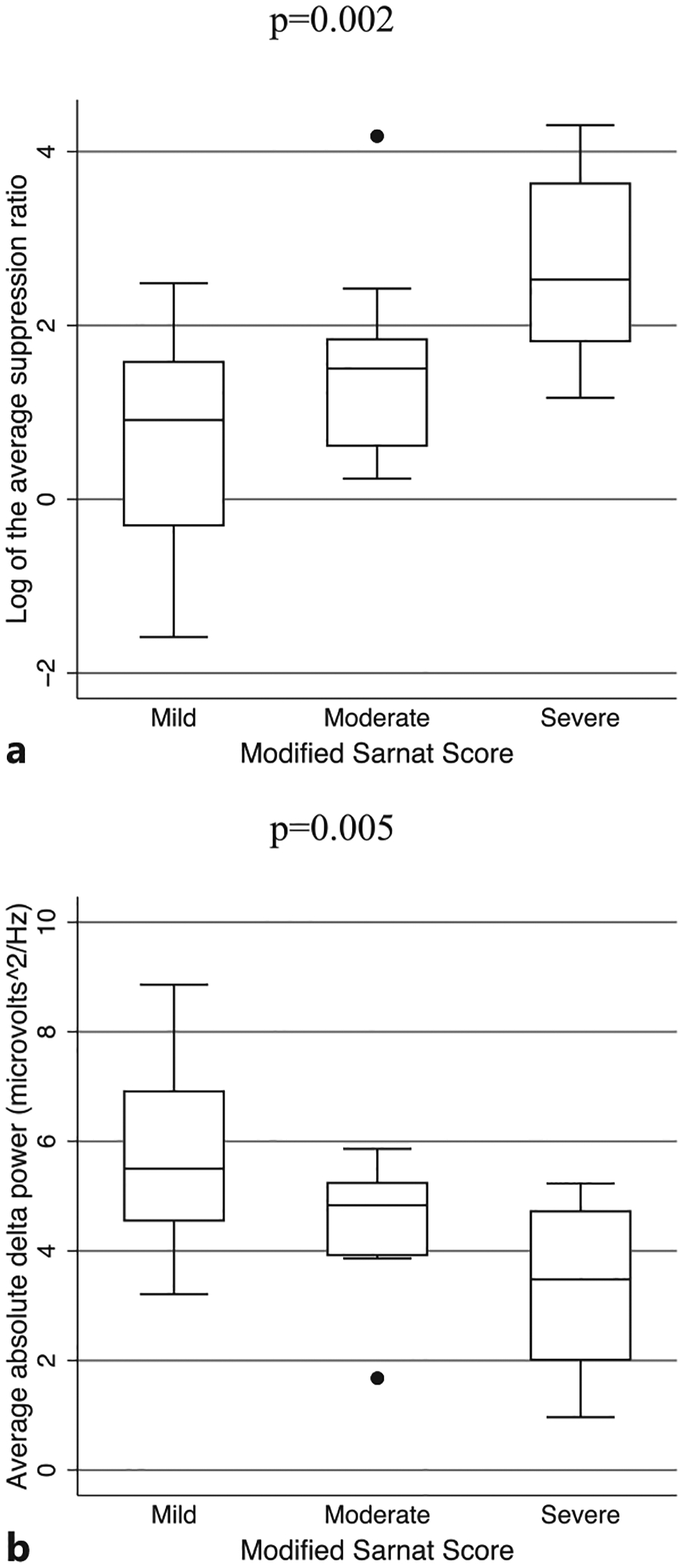
Boxplots showing the median and interquartile range of the SR (a) and absolute delta power (b) by modified Sarnat score in the first 24 h of TH in 30 neonates with NE. All results shown remained significant after correction for multiple comparisons.
Relationship of QEEG to Severity of Injury on MRI
In the 21 patients who had MRIs that were appropriate for NICHD-NRN scoring, a higher NICHD-NRN score was associated with a higher SR (nonparametric tests for trend: z-scores 2.3–2.7, p values <0.02) and lower absolute delta power (nonparametric tests for trend: z-scores −2.4 to −2.7, p values <0.02) during all phases of TH, rewarming, and post-rewarming (Fig. 3; Table 2). Of note, this relationship was most significant during the post-rewarming period. Additionally, a higher NICHD-NRN score was associated with higher relative beta power throughout all phases of TH, rewarming, and post-rewarming (nonparametric tests for trend: z-scores 2.4–2.7, p values <0.02; Fig. 3, Table 2). During the latter 48 h of TH, higher relative alpha power was associated with a higher NICHD-NRN score (nonparametric tests for trend: z-scores 2.5–2.6, p values = 0.012). Relative theta power was not associated with the NICHD-NRN score.
Fig. 3.
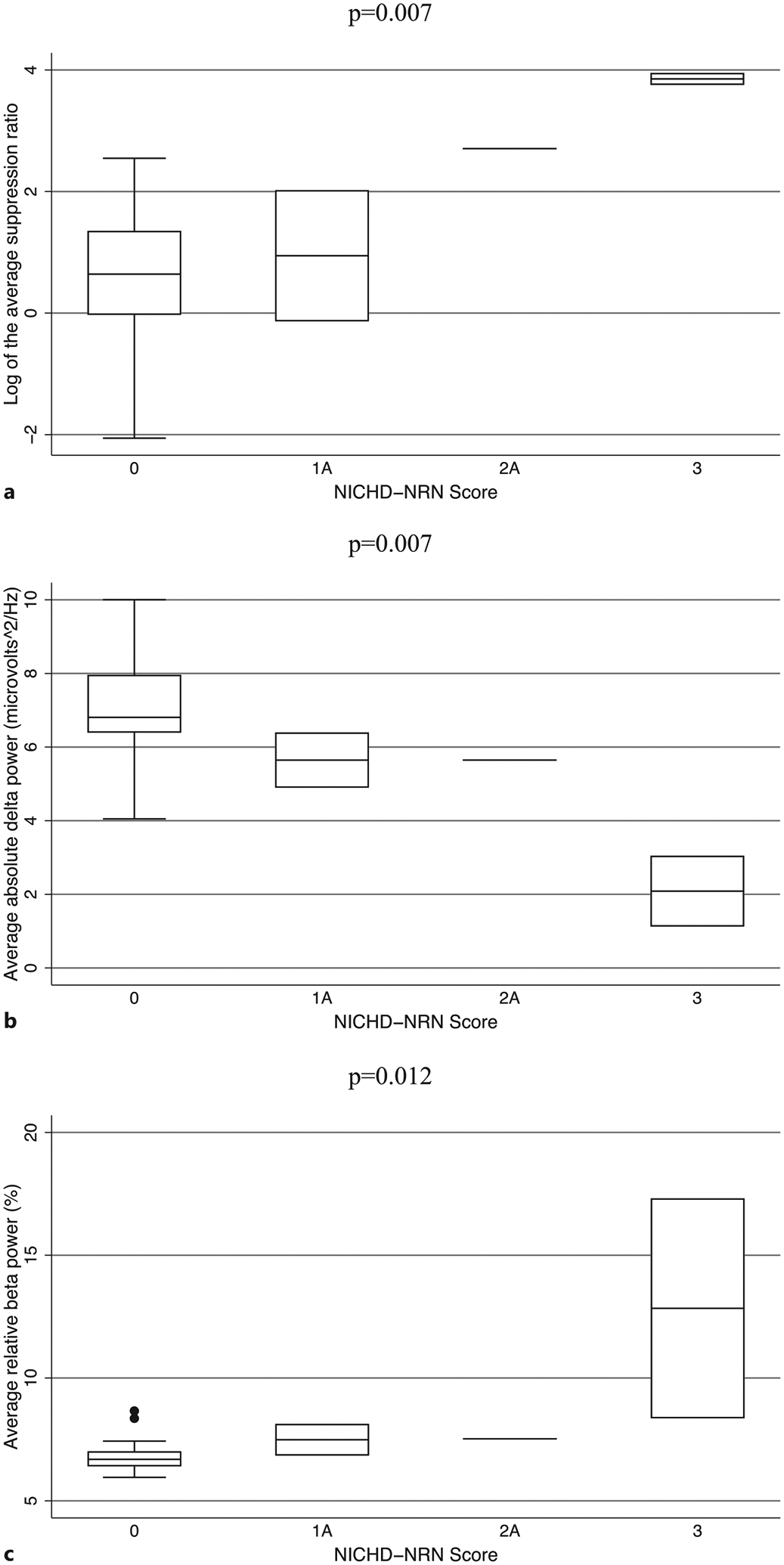
Boxplots showing the median and interquartile range of the SR (a), absolute delta power (b), and relative beta power (c) by NICHD-NRN imaging score in the post-rewarming phase of TH in 21 neonates with NE. All results shown remained significant after correction for multiple comparisons.
We performed secondary pairwise analyses using Kruskal-Wallis tests to compare differences in qEEG measures between adjacent severity groups (e.g., NICHD-NRN score of 0 vs. 1A, 1A vs. 1B), but none of these results were significant. None of the associations between qEEG and clinical and/or EEG-confirmed seizures survived correction for multiple comparisons (Table 2).
Effect of Phenobarbital Exposure on QEEG
There was no significant association of phenobarbital exposure with the Sarnat score (nonparametric test for trend: z-score 1.6, p value 0.09) or MRI NICHD-NRN score (nonparametric test for trend: z-score −0.68, p value 0.49). Prior or concurrent phenobarbital exposure was not associated with SR, absolute delta power, or relative delta, theta, alpha, or beta power by phase of TH (H[1] 0.01–5.03, p values 0.02–0.93 after correction for multiple comparisons).
Discussion
In this study, we piloted a fully automated approach to EEG background analysis in neonates undergoing TH for NE using a commercially available software. Prior studies have focused on identifying shorter epochs free from artifact [6, 8, 9]. Instead, we chose to analyze EEG data from all 72 h of TH and up to an additional 12 h of rewarming and post-rewarming monitoring and relied on automated artifact reduction. By avoiding the need to pre-select EEG data, our method can be implemented in large cohorts with prolonged monitoring duration and is entirely applicable to clinical practice. With this approach, we found that multiple qEEG features were associated with worse severity of NE, as evidenced by Sarnat score at presentation and MRI NICHD-NRN score following TH. Both a higher SR and lower absolute power in the delta frequency band were associated with more severe injury. These findings are consistent with prior studies relying on bespoke methods that found that EEG suppression and lower overall power were associated with more severe injury and worse imaging outcomes [6, 8, 9]. Additionally, our findings are consistent with those of the literature on aEEG in NE which similarly suggest low EEG power and discontinuity/burst suppression are poor prognostic indicators [10]. Importantly, the fact that we were able to replicate the findings of other authors supports the validity of our automated method.
Additionally, we found that relative delta power was lower while relative alpha and beta power were higher in more severely affected neonates. The normal EEG background in healthy neonates is composed predominantly of delta activity, divided into 50–60% active sleep, 30–40% quiet sleep, and brief periods of wakefulness [18]. In healthy neonates, sleep is associated with higher relative delta power due to the “tracé-alternant” pattern and high-voltage slow-wave activity [19]. The trend toward faster frequencies seen in neonates with more severe NE suggests that NE may disrupt normal sleep-wake cycling [20]. Moreover, as normal brain maturation progresses throughout gestation, premature fast features such as delta brushes slowly disappear [21]. Our finding that the relative composition of the background frequency mix continued to include faster frequencies throughout the monitoring period suggests that the brain injury in NE may also disrupt the normal maturation of postnatal brain activity. Thus, analysis of frequency composition via separation into qEEG power bands may be useful to estimate the degree of developmental arrest or dysmaturity in NE patients. This may be especially true in patients with ongoing brain injury despite TH [22]. We found that the strongest relationship between qEEG features and MRI findings suggestive of persistent injury was in the post-rewarming period. While some patients may reverse early signs of injury on conventional EEG [23], potentially due to TH, a subset of patients will have persistent abnormalities. Our results support that persistent functional abnormalities, as evidence by neurophysiologic abnormalities late in TH, correspond to structural injury, evidenced by MRI findings.
Due to the small number of patients, particularly those with EEG-confirmed seizures, our study was not powered to find associations between qEEG features and the presence of seizures. An abnormal early EEG background, including features such as excessive discontinuity, burst suppression, or extremely low voltage, has previously been associated with development of seizures in neonates with NE [24]. A prior study looking for qEEG features associated with seizures similarly found that lower total power across all frequency bands in the first hour of recording was associated with the later development of seizures [25]. Although our study was not powered to conclusively support a relationship between qEEG features and seizures, future studies should investigate whether qEEG analysis during the early portions of TH may be helpful in identifying neonates who are at high risk for developing seizures and may require more frequent cEEG review and/or longer cEEG monitoring.
Our study has limitations. As a pilot study, our sample size was limited, though overall the distribution of patients across disease severity was robust. As such, we were not powered to use multivariate models to investigate potential confounders such as phenobarbital exposure or to stratify our analyses by sex. We could not perform meaningful analyses to compare qEEG differences between individual levels of injury severity. We did not account for seizure burden in our analysis as this study was focused primarily on addressing background features, but the lack of significant associations between qEEG features and the presence of seizures, which typically show increased power, suggests the seizures themselves did not skew the overall qEEG analysis. We did not find that prior or concurrent phenobarbital exposure was significantly associated with qEEG features, making this exposure less concerning as a possible confounder, though our sample size for patients exposed to phenobarbital was quite small. Additionally, automated EEG analysis is vulnerable to artifacts, and we did not pre-select artifact-free periods for analysis in this study but instead relied on automated artifact reduction in order to improve ease of use and generalizability of the analysis. Further validation of this automated method in a large group of neonates and against other markers of clinical severity will be necessary particularly with regard to the impact of seizures on the qEEG analysis. Ultimately, future studies using this automated qEEG analysis should be carried out to establish any association between qEEG features and long-term neurodevelopmental outcomes.
Our results support the role for qEEG analysis as a potential source of biomarkers of neonatal NE and response to TH. Biomarkers that identify neonates at highest risk for adverse outcomes or neonates with ongoing injury during TH are needed to improve therapeutic strategies and assist with prognostication for clinicians and families. Automated qEEG analysis presents an opportunity for an early and ongoing evaluation of brain activity accessible to both the trained neurophysiologist as well as neonatologists and is applicable to both research and clinical settings.
Acknowledgments
The author would like to acknowledge Michael J. Guess for his technical support in the analysis.
Funding Sources
This research was supported by the following grants: NIH/NICHD R01HD086058 (A.E., F.N.J., E.G., D.V., C.D.); NIH KO8NS096115 and 3K08NS096115-03S1 (RC-V); the JHU-SOM Clinician Scientist Award (RC-V); the Thomas Wilson Foundation (RC-V); and the American Academy of Pediatrics Resident Research Grant (EC).
Footnotes
Statement of Ethics
This study protocol was reviewed and approved by the Institutional Review Board at Johns Hopkins Hospital through the Neuroscience Intensive Care Nursery (NICN) Program Administrative Database (IRB NA 00034540), the Identification of Diagnostic and Prognostic Biomarkers for Perinatal Hypoxic-Ischemic Brain Injury Study (BIN study, IRB NA 00026068), and Clinical Practice of Continuous Video-EEG Outside of the Epilepsy Monitoring Unit Study (IRB NA 00044076) in compliance with the Health Insurance Portability and Accountability Act of 1996 (HIPAA). Written informed consent was required for study participation and was obtained from the patient’s parent or guardian prior to study inclusion.
Conflict of Interest Statement
A research version of the quantitative EEG software program Persyst version 14.E (Prescott, AZ: Persyst Development Co.) used in the data analysis was provided by the manufacturer.
Data Availability Statement
The raw datasets generated during and/or analyzed during the current study are not publicly available as patients did not consent to data sharing as part of the study. Further inquiries can be directed to the corresponding author.
References
- 1.Douglas-Escobar M, Weiss MD. Hypoxic-ischemic encephalopathy: a review for the clinician. JAMA Pediatr. 2015;169(4):397–403. [DOI] [PubMed] [Google Scholar]
- 2.Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: an updated systematic review and meta-analysis. Arch Pediatr Adolesc Med. 2012;166(6):558–66. [DOI] [PubMed] [Google Scholar]
- 3.Shellhaas RA, Chang T, Tsuchida T, Scher MS, Riviello JJ, Abend NS, et al. The American Clinical Neurophysiology Society’s guideline on continuous electroencephalography monitoring in neonates. J Clin Neurophysiol. 2011;28(6):611–7. [DOI] [PubMed] [Google Scholar]
- 4.Awal MA, Lai MM, Azemi G, Boashash B, Colditz PB. EEG background features that predict outcome in term neonates with hypoxic ischaemic encephalopathy: a structured review. Clin Neurophysiol. 2016; 127(1):285–96. [DOI] [PubMed] [Google Scholar]
- 5.Dereymaeker A, Matic V, Vervisch J, Cherian PJ, Ansari AH, De Wel O, et al. Automated EEG background analysis to identify neonates with hypoxic-ischemic encephalopathy treated with hypothermia at risk for adverse outcome: a pilot study. Pediatr Neonatol. 2019;60(1):50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Govindan RB, Massaro A, Vezina G, Tsuchida T, Cristante C, du Plessis A. Does relative or absolute EEG power have prognostic value in HIE setting? Clin Neurophysiol. 2017;128(1):14–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed R, Temko A, Marnane W, Lightbody G, Boylan G. Grading hypoxic-ischemic encephalopathy severity in neonatal EEG using GMM supervectors and the support vector machine. Clin Neurophysiol. 2016;127(1):297–309. [DOI] [PubMed] [Google Scholar]
- 8.Jain SV, Zempel JM, Srinivasakumar P, Wallendorf M, Mathur A. Early EEG power predicts MRI injury in infants with hypoxic-ischemic encephalopathy. J Perinatol. 2017; 37(5):541–6. [DOI] [PubMed] [Google Scholar]
- 9.Kota S, Massaro AN, Chang T, Al-Shargabi T, Cristante C, Vezina G, et al. Prognostic value of continuous electroencephalogram delta power in neonates with hypoxic-ischemic encephalopathy. J Child Neurol. 2020;35(8):517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandrasekaran M, Chaban B, Montaldo P, Thayyil S. Predictive value of amplitude-integrated EEG (aEEG) after rescue hypothermic neuroprotection for hypoxic ischemic encephalopathy: a meta-analysis. J Perinatol. 2017;37(6):684–9. [DOI] [PubMed] [Google Scholar]
- 11.Abbasi H, Unsworth CP. Applications of advanced signal processing and machine learning in the neonatal hypoxic-ischemic electroencephalogram. Neural Regen Res. 2020;15(2):222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raurale SA, Boylan GB, Mathieson SR, Marnane WP, Lightbody G, O’Toole JM. Grading hypoxic-ischemic encephalopathy in neonatal EEG with convolutional neural networks and quadratic time-frequency distributions. J Neural Eng. 2021;18(4):046007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 1976;33(10):696–705. [DOI] [PubMed] [Google Scholar]
- 14.Shankaran S, McDonald SA, Laptook AR, Hintz SR, Barnes PD, Das A, et al. Neonatal magnetic resonance imaging pattern of brain injury as a biomarker of childhood outcomes following a trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. J Pediatr. 2015;167(5):987–93.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuzick J A wilcoxon-type test for trend. Stat Med. 1985;4(1):87–90. [DOI] [PubMed] [Google Scholar]
- 16.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57(1):289–300. [Google Scholar]
- 17.Abend NS, Wusthoff CJ, Goldberg EM, Dlugos DJ. Electrographic seizures and status epilepticus in critically ill children and neonates with encephalopathy. Lancet Neurol. 2013;12(12):1170–9. [DOI] [PubMed] [Google Scholar]
- 18.Dereymaeker A, Pillay K, Vervisch J, De Vos M, Van Huffel S, Jansen K, et al. Review of sleep-EEG in preterm and term neonates. Early Hum Dev. 2017;113:87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korotchikova I, Connolly S, Ryan CA, Murray DM, Temko A, Greene BR, et al. EEG in the healthy term newborn within 12 hours of birth. Clin Neurophysiol. 2009;120(6):1046–53. [DOI] [PubMed] [Google Scholar]
- 20.Korotchikova I, Stevenson NJ, Walsh BH, Murray DM, Boylan GB. Quantitative EEG analysis in neonatal hypoxic ischaemic encephalopathy. Clin Neurophysiol. 2011;122(8):1671–8. [DOI] [PubMed] [Google Scholar]
- 21.Niedermeyer E, Schomer DL, Lopes da Silva FH. Niedermeyer’s electroencephalography: basic principles, clinical applications, and related fields. 6th ed., Philadelphia, Pa, London: Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 22.Castro Conde JR, Gonzalez Barrios D, Gonzalez Campo C, Gonzalez Gonzalez NL, Reyes Millan B, Sosa AJ. Visual and quantitative electroencephalographic analysis in healthy term neonates within the first six hours and the third day of life. Pediatr Neurol. 2017;77:54–60 e1. [DOI] [PubMed] [Google Scholar]
- 23.Murray DM, Boylan GB, Ryan CA, Connolly S. Early EEG findings in hypoxic-ischemic encephalopathy predict outcomes at 2 years. Pediatrics. 2009;124(3):e459–67. [DOI] [PubMed] [Google Scholar]
- 24.Glass HC, Wusthoff CJ, Shellhaas RA, Tsuchida TN, Bonifacio SL, Cordeiro M, et al. Risk factors for EEG seizures in neonates treated with hypothermia: a multicenter cohort study. Neurology. 2014;82(14):1239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain SV, Mathur A, Srinivasakumar P, Wallendorf M, Culver JP, Zempel JM. Prediction of neonatal seizures in hypoxic-ischemic encephalopathy using electroencephalograph power analyses. Pediatr Neurol. 2017;67:64–70 e2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw datasets generated during and/or analyzed during the current study are not publicly available as patients did not consent to data sharing as part of the study. Further inquiries can be directed to the corresponding author.


