Abstract
Objective:
Varenicline is a safe and effective aid to smoking cessation but most trials have involved frequent visits or intensive behavioral support unlike that typically provided in primary care. The current study examined if motivational text messages, sent via cellphone, would increase quit rates in smokers being treated with varenicline and 3 brief sessions in a family practice setting.
Methods:
This study was a randomized controlled, parallel-group smoking cessation trial. Intervention group participants (n = 74) received daily motivational text messages, additional texted tips in response to keywords, and weekly study questions while control group participants (n = 76) received only weekly study questions. Both groups received individualized counseling. Self-reported non-smoking and exhaled breath CO <10ppm were used to validate smoking abstinence at 3 weeks and 12 weeks.
Results:
Overall, 30.7% (46/150) of participants were abstinent at the 12 week follow-up and the abstinence rate did not differ between groups (INT 31.1% v. CON 30.3%, p = .91). The only predictor of abstinence at 12 weeks was use of varenicline during a previous quit attempt (p = .01). Intervention group participants were more likely to rate the text messaging program as good or excellent (p < .01), to recommend a similar program to family or friends (p < .01), and to complete positive smoking cessation activities (p = .04), when compared with the control group.
Conclusion:
Although there were no differences in quit rates between the intervention and control group, intervention group participants rated the text messaging system more favorably, were more likely to recommend the program to others, and were more likely to complete positive smoking cessation activities.
Cigarette smoking is the leading preventable cause of death and disease in many countries of the world (Eriksen, Mackay, Schulger, Gomeshtapeh, & Drope, 2015). Nearly one in five adults continue to smoke in countries such as the United States and United Kingdom (Eriksen et al., 2015). Although the smoking rate remains high, the majority of current smokers report wanting to quit smoking, with nicotine dependence being a major barrier to successful quitting (The Surgeon General, 1989; Tobacco Advisory Group of the Royal College of Physicians, 2000).
Varenicline has been shown to be safe and effective aid for smoking cessation and is the most efficacious single medication (Anthenelli et al., 2016; Cahill, Lindson-Hawley, Thomas, Fanshawe, & Lancaster, 2016). However, most of the randomized trials of varenicline provided relatively intensive behavioral support (e.g., more than 5 visits, often weekly) that is unlike the support typically provided in general medical care (Foulds et al., 2013; Stead, Koilpillai, Fanshawe, & Lancaster, 2016). Smoking cessation trials generally find a lower overall quit rate when less intensive in-person support is provided and one of the few placebo-controlled trials that did not find a benefit from varenicline provided a less frequent appointment schedule that is more typical of general medical practice (Steinberg et al., 2011). Thus, there is a need to identify low cost methods to provide systematic adjunct smoking cessation support to patients trying to quit in general medical settings such as primary care.
Cellphone ownership has been increasing in the United States, Europe, and many other countries. In 2015, 91% of US adults reported owning a cellphone capable of receiving text messages (Poushter, 2016), so sending motivational/informational text messages to the patient’s cellphone may be one potential way to provide additional support and contact during a quit attempt. Using motivational text messages to provide support to smokers trying to quit could provide many benefits such that the message can be received and read even if the recipient is unavailable at the time of receipt. Also, the messages can be interactive so that the smoker can get additional support when they need it. Recent studies have shown that smoking cessation text messages have been effective in helping smokers to quit (Whittaker, McRobbie, Bullen, Rodgers, & Gu, 2016).
The current study examined if motivational text messages, sent via cellphone, in addition to 3 brief counseling sessions and varenicline would increase quit rates. We hypothesized that smokers receiving regular motivational text messages to their cellphone would have higher abstinence rates at 12 weeks post target quit date compared to smokers who received control text messages. We also hypothesized that smokers who received regular motivational text messages (providing medication reminders and tips on positive quit behaviors) would have higher medication compliance rates and would engage in more positive smoking cessation activities. This study can provide important new data on the potential for text-based interventions to improve quit rates when added with brief counseling and medication in a general medical setting.
Methods
Subjects
All participants were recruited from the community via posters or by clinician referral for a study to help smokers quit using varenicline and motivational text messages to their cellphone. Recruitment occurred from December 2014 to October 2015. Participants were eligible if they were >20 years of age, smoked >4 cigarettes per day for at least 6 months, wanted to quit smoking in the next 30 days, had not used a drug or medicine as an aid to smoking cessation in the past month, had a cellphone capable of receiving text messages, and were willing to receive text messages. Participants were excluded if they were currently pregnant or trying to get pregnant or nursing, had a history of serious kidney disease, ever had a severe allergic reaction or other serious side effect while using varenicline, were not willing to use varenicline to make a quit attempt, reported uncontrolled mental illness, reported recent suicidal ideation (past 28 days), or had another household member participating in the study.
Baseline and Assessment Visit
Potential participants were screened for eligibility by phone and an assessment visit was scheduled. Visits were conducted at a local family medicine practice site that was part of the Penn State Ambulatory Research Network. At the assessment visit, participants provided a complete medical history including medications and they completed questionnaires about their tobacco use and health behaviors, including the Penn State Cigarette Dependence Index (Foulds et al., 2015), the Fagerstrom Test for Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fagerström, 1991), the Audit-C (Frank et al., 2008), a tobacco use history, importance and confidence to quit, INTERHEART (McGorrian et al., 2011), the Minnesota Withdrawal Scale (Hughes & Hatsukami, 1986), the PHQ-9, and the Perceived Stress Scale (Cohen, Kamarck, & Mermelstein, 1983). Participants were asked if they had used varenicline or any other methods (counseling or cold turkey) to quit in a previous quit attempt. Vitals (pulse, BP) and weight were collected and an exhaled carbon monoxide reading was taken using the PICO+ Smokerlyzer.
All participants received brief smoking cessation counseling lasting 10–20 minutes, chose a quit date within 30 days of the assessment visit, and were prescribed a 12 week supply of varenicline. Varenicline was supplied by Pfizer and was managed by the Penn State Hershey Investigational Drug Service. Participants were required to pick up the medication at the outpatient pharmacy and were instructed to begin taking it 7 days prior to their Target Quit Day (TQD) according to the standard dosing schedule (0.5mg once daily on Days 1–3, 0.5mg twice a day on Days 4–7, followed by 1mg twice a day for the remainder of a 12-week treatment period). The participant’s cellphone number was entered into the text messaging system and test welcome messages were sent to their phone. On the day they began taking varenicline they were instructed to text the word “Start” in response to this message.
Follow Up Contacts
Participants were followed up over the phone 7 days after their Target Quit Date (14 days after beginning varenicline). Participants were asked about current tobacco use, medication usage, and withdrawal symptoms. Medication usage was measured by asking the participants “Are you still taking Chantix?” If the participant responded yes, they were asked if they missed any doses, and if they did, how many doses they missed and if they missed any complete day of doses. If the participant was no longer taking Chantix, they were asked how many days ago they stopped taking the medication and why. During analysis, it was assumed that participants lost to follow-up were not taking their medication.
In-person follow-up visits were scheduled at 3 weeks and 12 weeks after the target quit day. At both follow-ups, participants were asked to complete questionnaires about their current tobacco use and health behaviors, medication usage, completion of brief questionnaires about positive smoking cessation activities and withdrawal symptoms (Hughes & Hatsukami, 1986) and all participants were provided with brief smoking cessation counseling (lasting <10 minutes). At the 3 week follow-up, participants were provided with an additional 8 week supply of varenicline. At all follow-ups, participants were asked about any changes in their physical or mental health and any reported adverse events were reviewed and coded by the study physician (JN).
Smoking Cessation
Point-prevalent abstinence from smoking was assessed at each in-person visit by asking the participant if they have used any tobacco products in the past 7 days and by measuring exhaled carbon monoxide levels. Participants were considered abstinent if they have not used any tobacco products in the past 7 days and they had an exhaled CO level less than 10ppm. Sustained abstinence was defined as no tobacco use since the initial target quit day and a CO level <10ppm at the 3 week and 12 week follow-up visits. Days to relapse were calculated from responses to the following questions at each follow-up visit, “Have you used any tobacco since your initial target quit day” and if yes, “How many days after your initial target quit date was it before you used a tobacco product.” The day of relapse was calculated from the response given at the first visit where the participant reported smoking. Participants were considered compliant with their medication if they took the medication for >28 days.
Smoking Cessation Activities and Text Messaging System Acceptability
An assessment of smoking cessation activities was measured at the 3 week follow-up by asking participants to rate the following statements on a scale from 0 (not at all) to 3 (Extremely true); 1. During my quit attempt I made a written list of all the reasons I wanted to quit smoking. 2. The day before my target quit day I made sure I got rid of all my cigarettes and tobacco. 3. When I quit smoking I told my friends, family, and colleagues that I am quitting. 4. When I quit smoking I asked my friends, family, and colleagues for their support in my quit attempt. 5. When I quit smoking I regularly tried to anticipate and avoid situations where I might be near cigarettes or people using tobacco. 6. When I quit smoking I made sure that I had a supply of snacks so that I did not feel hungry for both food and cigarettes. A total score (0–18) was calculated for each participant. In addition, a yes/no completion variable was created for each activity with a score of >0 considered completing the activity.
At the 12 week follow-up, participants were asked “Overall, how would you rate the helpfulness of the text messages you received over the past month for helping you stay off tobacco? (poor, satisfactory, good, or excellent)” They were also asked to provide comments about the text messaging system in the form of brief open-ended questions including “What aspects of the text messaging program did you find most helpful?” and “In what ways do you think the text messaging program could be more helpful?” Comments were read and coded by one researcher (SV) and unclear comments were discussed with two additional researchers (JY and EH) until consensus was met. Participants were also asked what proportion of the text messages they read (responses: just glanced at some of them, just glanced at most of them, read less than half of them, read most but not all, or read all of them). Finally, participants reported (yes/no) if they would recommend similar text messages to friends or family.
Study Design, Randomization, and Administration of Text Messages
This study was a randomized controlled, parallel-group smoking cessation trial conducted in a primary care setting. The study statistician (AB) generated a 1:1 block randomization sequence with block sizes of 10. The randomization IDs, group assignments (intervention or control) text messages and the timing of the messages were provided directly to a third party who specialized in mobile solutions and text messaging systems for research (Mosio). Researchers remained blind to the matching of the randomization IDs to the group assignments, which was done automatically when the participant was entered into the text messaging system.
Upon consenting to the study, each participant was sequentially assigned a randomization ID. The participant’s phone number and their sequential randomization ID were entered into the text messaging system, which was designed to automatically send the correct text message tips, according to intervention group or control group allocation, based on the randomization ID entered. All participants received administrative text messages welcoming to the study and reminding them of the follow-up visits (11 total messages). Intervention group participants received 2 text message motivational tips a day for 7 days prior to their target quit date (Pre-quit period), then following the Target Quit day, 5 tips a day for first 28 days (Month 1), 2 tips a day for the next 28 days (Month 2), then 1 tip a day for the final 28 days (Month 3). The tips were sent to participants on the following schedule; at 8am and 5pm during the Pre-quit period, at 8am, 11am, 2pm, 5pm, and 8pm during Month 1, at 8am and 5pm during Month 2, and at 5pm during Month 3. The timing of messages for this intervention closely resembled the message timing used in the Free and colleagues’ study (Free et al., 2011). In total, intervention participants received 239 messages over 91 days. Intervention participants were also able to text keywords Mood, Slip, and Crave to the system to instantly receive 3 additional motivational tips related to the keyword entered.
Content for the text message tips was taken from widely distributed smoking cessation educational campaigns and from internet sites and pamphlets. This included materials from the Center for Disease Control (CDC), National Cancer Institute (NCI) publications (such as Forever Free booklets (Brandon et al., 2004)), and various government health-related websites such as smokefree.gov (including their text messaging intervention tips database, smokefreeTXT, downloaded 1st September 2011). Additional tips were developed by the research team based on clinical experience with treating smokers. Once sample tips were identified from various sources, we conducted an online survey of members of the Society for Research on Nicotine and Tobacco (SRNT) and the Association for the Treatment of Tobacco Use and Dependence (ATTUD), inviting them to rate the helpfulness of over 200 widely used brief smoking cessation tips, organized by the stage of the quitting process (rating each tip on a scale from 0 = “not at all helpful” to 4 = “Extremely helpful”). Almost 300 experienced smoking cessation professionals rated the tips. The tips from this survey obtaining the highest scores were selected for inclusion in our current texting intervention. Examples of the text messaging content can be found in Table 1.
Table 1.
Example text message system content by time period
| Time period | Text message content examples |
|---|---|
| Before the quit day | Think about the things in your life that can trigger the urge to smoke. Make plans on how best to manage these situations. |
| The quit day | The first day will be easier if you can keep yourself active and busy, be around non-smokers, and keep your mind occupied. |
| The day after the quit day | Ask your family, friends, and coworkers for their support. Ask them not to smoke around you or leave cigarettes lying around. |
| Recently quit and beyond | Things to do to relieve stress besides smoking: reading, exercise, relaxation, deep breathing, prayer, meditation, or taking a walk. |
| Keyword: Mood | Having a cigarette never made a stressful situation go away. Tell yourself I can do it without a cigarette. Smoking is not an option. |
| Keyword: Craving | Where you are and what is going on can make you crave a cigarette. A change of scenery can really help. Go outside, or go to a different room. |
| Keyword: Slip | A slip is not unusual. Commit to quitting again right away. |
Control participants did not receive any motivational text messages and were not able to use the keyword (Mood, Slip, Crave) function. Both groups received one question per week (12 total), following the Target Quit Day asking “Have you smoked or used any tobacco product since your target quit day?” Participants were instructed to reply yes or no to each question. At any point during the study, participants could text the word “Stop” to the system to stop receiving study text messages.
Data Management and Statistical Analysis
This study was approved by the Penn State Hershey Institutional Review Board. Study data were collected and managed using REDCap (Research Electronic Data CAPture) electronic data capture tools (Harris et al., 2009) hosted at the Penn State Milton S. Hershey Medical Center and College of Medicine. REDCap is a secure, web-based application designed to support data capture for research studies. Study data was analyzed using SAS 9.4 software.
Power was estimated based on a two-sided Fisher’s Exact test. A sample size of 75 participants per group (150 total) would yield 80% power to detect a difference in quit rates in the two groups assuming a 50% 12-week quit rate in the intervention group and a 27% quit rate in the control group. 50% was the quit rate on varenicline at 12 weeks in a combined analysis of 8 randomized trials in which participants attended at least 10 visits over those 12 weeks (Foulds et al., 2013).
Means and frequencies were used to describe the quantitative data. Two-sided t-tests were utilized to identify differences in continuous variables between groups. Chi-square analysis was utilized to describe differences between categorical variables. To identify predictors of abstinence at 12 weeks, a multivariate logistic regression model was set up with all covariates in Table 1 with smoking status at 12 weeks as the dependent variable (abstinent = 1, smoking = 0) using the SAS stepwise procedure (iterative backwards and forwards stepwise) with selection entry and exit criteria set at p = .15. Based on the results of the first model, the final model was set up to include the intervention variable, any covariates from the first model that had a p-value ≤0.05, and the randomization variable. The “intent-to-treat” approach was utilized and data from all randomized participants (who collected their first dose of varenicline) included in the analysis, with those not attending follow-up classified as a continuing smoker (Gupta, 2011).
Results
A CONSORT diagram is included in Figure 1. Of the 259 participants assessed for eligibility, 48 were deemed ineligible, most commonly for a use of another smoking cessation medication to quit in the past month. Of the eligible participants, 9 participants were not interested in the study and 50 did not show to their scheduled assessment visit yielding 152 participants who consented to the study. Two consented participants were not included in analysis because they did not pick up their varenicline from the pharmacy or reply “Start” to the text messaging system as directed at the assessment visit. This left 150 participants for analysis (74 intervention and 76 control). Self-reported smoking status was collected from 134 (89.3%) participants at the phone call follow-up one week after the TQD. Self-reported smoking status and exhaled breath CO for biochemical verification was completed for 122 (81.3%) participants at the 3 week follow-up and for 113 (75.3%) participants at the 12 week follow-up. There were no differences in follow-up rates between groups at 3 and 12 weeks (p = .94 and p = .63, respectively).
Fig. 1.
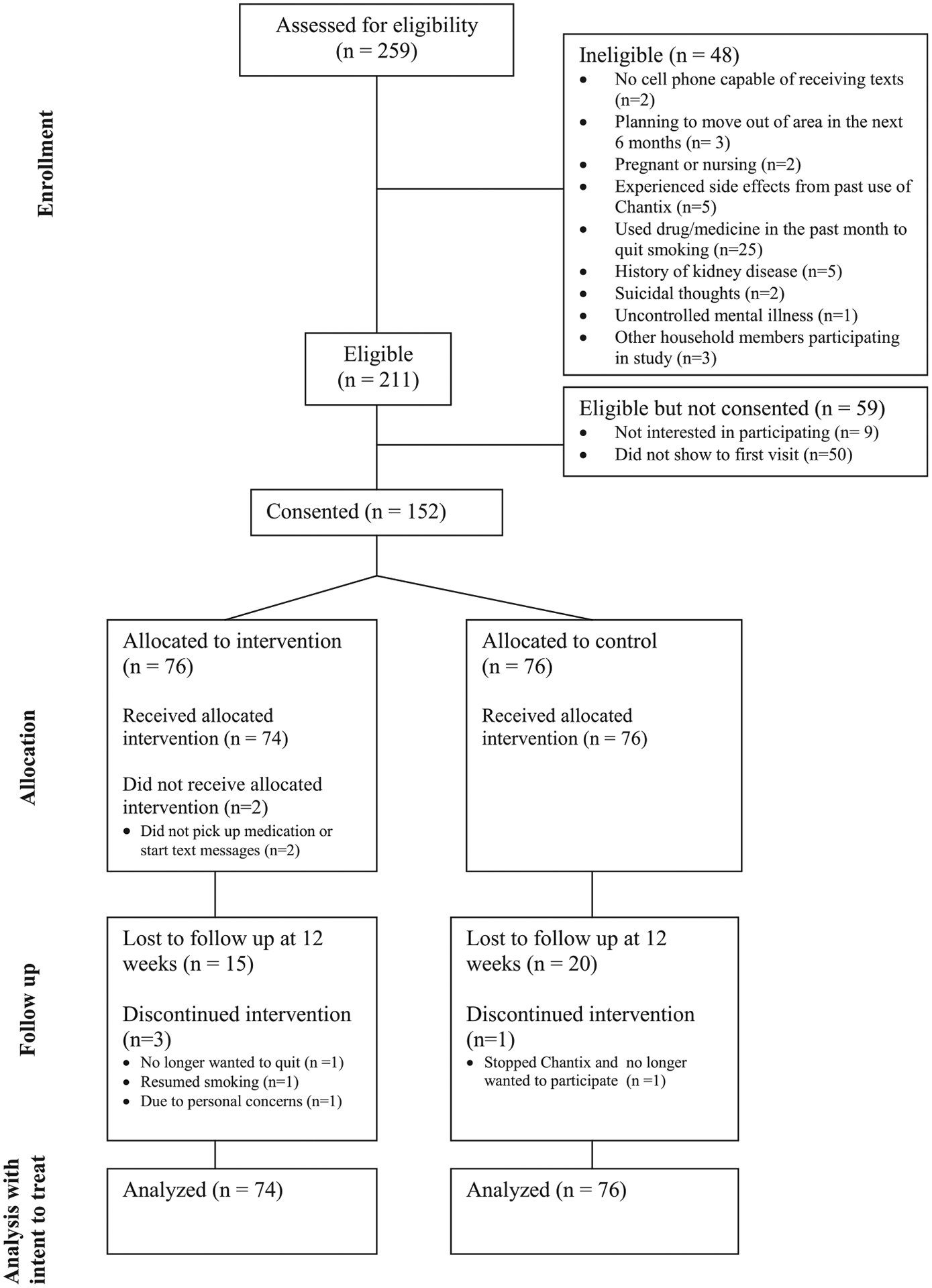
CONSORT diagram.
The baseline characteristics of the sample overall and by randomization group can be found in Table 2. At baseline, there were no significant differences between the intervention and control groups except that the control group had a higher exhaled CO (p = .02).
Table 2.
Baseline characteristics of the sample overall and by intervention and control group
| Characteristic | Overall (n = 150) | Intervention Group (n = 74) | Control Group (n = 76) | p-value |
|---|---|---|---|---|
| Mean age (SD) | 45.5 (11.9) | 46.6 (12.3) | 44.5 (11.6) | .29 |
| % (n) Male | 34.0 (51) | 39.2 (29) | 29.0 (22) | .19 |
| % (n) White | 87.3 (131) | 87.8 (65) | 86.8 (66) | .73 |
| Mean BMI | 30.9 (9.2) | 31.6 (8.0) | 30.3 (10.2) | .39 |
| % (n) Smoke menthol cigarettes | 54 (81) | 54.1 (40) | 54.0 (41) | .99 |
| Mean exhaled CO (SD) | 26.2 (14.1) | 23.4 (12.5) | 28.9 (15.0) | .02 |
| Mean years using tobacco (SD) | 25.9 (12.4) | 26.6 (12.7) | 25.3 (12.2) | .52 |
| % (n) used Chantix in prior quit attempt | 30.7 (46) | 24.3 (18) | 36.8 (28) | .10 |
| % (n) used other methods to quit (n = 149) | 69.3 (104) | 72.6 (53) | 67.1 (51) | .47 |
| Mean number of quit attempts (SD) | 4.8 (10.0) | 3.5 (4.0) | 6.2 (13.2) | .10 |
| Mean Importance to quit (SD) (n = 149) | 9.5 (1.1) | 9.4 (1.4) | 9.7 (.7) | .11 |
| Mean Confidence to quit (SD) (n = 149) | 8.7 (1.7) | 8.7 (1.7) | 8.7 (1.7) | .91 |
| Mean Cigarettes per day (SD) | 17.7 (6.8) | 17.4 (7.0) | 18.0 (6.7) | .57 |
| Mean Fagerstrom Score (SD) | 5.0 (1.8) | 4.8 (4.3) | 5.3 (4.9) | .09 |
| Mean PSCDI Score (SD) | 12.7 (3.2) | 12.2 (3.3) | 13.1 (3.1) | .09 |
| Mean PHQ9 Score (SD) | 3.4 (3.6) | 3.6 (4.1) | 3.2 (3.0) | .45 |
| Mean MNWS Score (SD) | 8.1 (5.9) | 8.2 (6.2) | 8.1 (5.7) | .92 |
| Mean Interheart Score (SD) | 18.5 (6.1) | 18.2 (5.9) | 18.8 (6.4) | .60 |
| Mean Audit C Score (SD) | 1.3 (1.2) | 1.2 (1.2) | 1.4 (1.1) | .56 |
| Mean Perceived Stress Score (SD) | 11.3 (5.8) | 11.4 (5.9) | 11.1 (5.8) | .80 |
Smoking Cessation Outcomes
At the 1 week phone call follow-up and 3 week and 12 week in person follow-up visits, there were no significant differences in point-prevalent abstinence between the intervention (INT) and control (CON) group participants (INT 27.0% abstinent v. CON 31.6%, p = .54, INT 37.8% v. CON 46.1%, p = .31 and INT 31.1% v. CON 30.3%, p = .91, respectively). There was also no difference in sustained abstinence at the 12 week follow-up visit between groups (INT 8.1% abstinent v. CON 11.8%, p = .45). The average time to relapse to smoking did not differ between groups (INT 16.0 days v. CON 16.5, p = .91), however intervention group participants were a little more likely to abstain from smoking on their TQD than control group participants (INT 60.8% abstinent v. CON 47.4%, p = .10).
In addition, medication compliance in the two groups was similar with both groups taking the medication approximately 12 days at the phone call follow-up (14 days after starting the medication) (p = .38) and 55 days at the 12 week follow-up (p = .96). Among those who were compliant with their medication (taking >28 days of medication) (n = 95/150, 63.3%), there was no difference in quit rates between groups (INT 44.7% abstinent v. CON 41.7%, p = .77). Overall, those who were compliant with their medication were more likely to be abstinent than those who were not compliant with their medication (Compliant 43.2% v. Non-compliant 9.1%, p < .01).
Finally, a stepwise logistic regression analysis using baseline variables (found in Table 2) to predict abstinence at the 12 week follow-up visit found that prior use of varenicline (β = 1.00, p = .01) was positively associated with quitting smoking. Randomization group was not a significant predictor of abstinence (β = .1781, p = .63).
Adverse Events
Overall, 64 expected adverse events related to quitting smoking or the use of varenicline were reported. 34.4% (22/64) of reported events were nausea, 26.6% (17/64) were vivid or unusual dreams, 32.8% (21/64) were nicotine withdrawal symptoms, and 3.1% (2/64) were rash. 3.1% (2/64) were related to having thoughts about death (1 reported at an unscheduled contact between the phone call and 3 week follow-up visit, 1 reported at the 12 week follow-up, both were taking the medication at the time of the report). There were 23 unexpected adverse events reported, 60.9% (14/23) which could be possibly related to quitting smoking or using varenicline and 39.1%(9/23) that were determined to be unrelated to the study treatment. Three of the unexpected events were considered serious adverse events because they were a serious medical event or required hospitalization (development of diabetes after stopping the medication, gallbladder surgery, and abdominal pain). No suicide attempts were reported.
Smoking Cessation Activities and Text Messaging System Acceptability
The intervention group was more likely to engage in positive smoking cessation activities than the control group (INT 10.5 total score, v. CON 9.1, p = .05). Specifically, intervention group participants were more likely to report carrying a supply of snacks so that they did not feel hungry for both food and cigarettes, compared to control group participants (p = .02). Responses to questions rating the text messaging system and information regarding the number of messages responded to can be found in Table 3. Overall, 79.6% of participants of attending the 12 week follow-up visit read all the text messages sent to their cell phone and responded on average to 9.3 of the 12 study questions. Control group participants responded to significantly more study questions than intervention group participants (p < .01).When examining the total number of text message questions responded to for all participants, not just for participants attending the 12 week follow-up, the results were similar (INT 7.0 questions v. CON 9.4, p < .01).
Table 3.
Text messaging system related outcomes among those attending 12 week follow-up
| Overall (N = 113) | Intervention (N = 57) | Control (N = 56) | P-value | |
|---|---|---|---|---|
| % (n) read all text messages (n = 112) | 79.5 (89) | 59.7 (34) | 100.0 (55) | <.01 |
| % (n) rate helpfulness of text messages as good or excellent (n = 112) | 41.1 (46) | 66.7 (38) | 14.6 (8) | <.01 |
| % (n) would recommend similar text messaging program to family or friends (n = 111) | 78.8 (89) | 96.4 (54) | 63.6 (35) | <.01 |
| Mean number of text study questions responded to (SD) (n = 113) | 9.3 (3.7) | 8.1 (4.0) | 10.5 (2.9) | <.01 |
| % (n) responding to all text study questions (n = 112) | 36.3 (41) | 21.1 (12) | 51.8 (29) | <.01 |
Within the intervention group, 40.5% (30/74) used keywords (stress, slip, or crave) to get additional text message support. On average these participants’ used keywords 3.3 times with a range of 1–13 times. 43.3% (13/30) of those who utilized keywords were abstinent at the 12 week follow-up, while only 22.7% (10/44) of those who did not use a keyword were abstinent (p = .06).
Coded responses to the open-ended questions about the most helpful aspects of the texting intervention and how it could be made more helpful are presented in Table 4. Importantly, almost 90% of respondents in the control group reported that the text messages they received (12 weekly questions plus 11 welcome texts/appointment reminders) were helpful as reminders of their intention to quit or they felt that the study questions were helpful as a way to hold them accountable during their quit attempt.
Table 4.
Responses from the open-ended questions related to the most helpful aspects of the text messaging program and how it could have been more helpful
| What aspects of the text messaging program did you find most helpful?a | ||
|---|---|---|
| Comment | Control (n = 35) | Intervention (n = 50) |
| Good reminder of quitting or felt held accountable, n (%)b | 31 (88.6) | 9 (18.0) |
| Not helpful or wanted more texts, n (%) | 4 (11.5) | 1 (2.0) |
| All of it was helpful, n (%) | 0 (0) | 6 (12.0) |
| Informative messages, n (%) | 0 (0) | 13 (26.0) |
| Encouraging, reinforcing or motivating messages, n (%) | 0 (0) | 11 (22.0) |
| Frequent contact, n (%) | 0 (0) | 3 (6.0) |
| Keywords/interactive aspect, n (%) | 0 (0) | 5 (10.0) |
| Other non-texting element, n (%) | 0 (0) | 2 (4.0) |
| In what ways do you think the text messaging program could be more helpful?a | ||
|---|---|---|
| Comment | Control (n = 35) | Intervention (n = 42) |
| More encouragement or more messages, n (%)b | 31(88.6) | 9 (21.4) |
| Fewer messages, n (%) | 0 (0) | 4 (9.5) |
| More interactive capabilities, n (%) | 3 (8.6) | 2 (4.8) |
| Change timing of messages, n (%) | 0 (0) | 5 (11.9) |
| Good as it is, n (%) | 1 (2.9) | 9 (21.4) |
| Other, n (%) | 0 (0) | 3 (9.5) |
received responses from 46.1% (35/76) of the control group and 67.6% (50/74) of the intervention group
% calculated based on the total number of responses in each group
received responses from 46.1% (35/76) of the control group and 56.8% (42/74) of the intervention group.
% calculated based on the total number of responses in each group
Nine participants pressed “STOP” at some point during the study to stop the messages (8 intervention and 1 control). The mean days participating in the program prior to pressing “STOP” was 46 days, with a range of 0–91 days. Two of the participants who pressed stop (1 intervention, 1 control) were lost to follow-up. Of the 7 participants not lost to follow-up, all reported relapsing prior to pressing stop. The mean number of days between relapsing and pressing stop was 41 days, ranging from 5 to 91 days. Due to a limitation of the texting system, it is believed that one person stopped the messages in error because they replied with a sentence that included the word stop, which triggered the system to terminate the texts to that individual.
Discussion
This study found no differences in abstinence rates between intervention and control group participants at the 1 week phone call follow-up or the 3 and 12 week in-person follow-ups, although those in the intervention group were slightly more likely to quit on their target quit day. In addition, significantly more participants randomized to the intervention group who completed the 12 week follow-up rated the helpfulness of the text message tips as good or excellent (67% v. 15%) and stated that they would recommend it to family or friends (96% v. 64%), compared with participants randomized to the control group. Prior use of varenicline was the only significant predictor of quitting in this study.
In contrast to our study, previous studies have reported that text messaging interventions are effective at promoting smoking cessation, compared to control interventions (Free et al., 2011; Scott-Sheldon et al., 2016; Spohr et al., 2015; Whittaker et al., 2016). Similar to our study, most of these studies used a decreasing message distribution schedule, included messages to be sent prior to the quit day, and included an option for on-demand support (i.e., keywords). Although the text messaging programs varied greatly between studies, there were no significant differences in quit rates between programs using decreasing, fixed, or variable message schedules or those which used a fixed or dynamic message track. In addition, a meta-analysis did not find any significant differences in quit rates between programs which included on-demand messaging and those that did not (Spohr et al., 2015).
There are several potential explanations for the lack of difference in quit rates between intervention and control group participants in this study. Perhaps most importantly, open-ended responses from control group participants suggest that they perceived the few text messages they received to be helpful. Control participants reported reading 100% of the messages sent to them, and although only a small proportion rated the messages as good or excellent, 63.6% of control participants said that they would recommend a similar program to family and friends. As many control participants stated simply knowing that they would be asked weekly about their smoking behaviors was perceived as support. This leads to the possibility that we did not observe a difference in the intervention and control groups because the control condition was more active than intended. Future studies to test the effectiveness of text messaging interventions should include a control group that does not receive any communications via text messaging that would be perceived as helpful.
This study also found that intervention participants were less likely to read the text messages they received compared to the control group. This could be due to the fact that intervention participants had more messages to read (246 incoming texts versus 23 total texts including welcome messages and reminders). In addition, during weeks 1–4 after the TQD, when intervention participants received 5 texts per day, these messages were always sent at the same times (8am, 11am, 2pm, 5pm, and 8pm). There were 2 comments from study participants specifically about the timing of the messages indicating that the consistent timing was “annoying” and that it “reminded them to smoke.” Therefore, it may be that some participants started ignoring texts arriving at those time points as they knew it was just another text from the smoking cessation program. Although studies have shown that a fixed or variable message schedule does not affect quit rates (Spohr et al., 2015), future studies should evaluate the most effective times of day to send messages.
We hypothesized that participants receiving medication reminders would have better medication compliance. However we found that intervention group participants did not take their medication for a greater number of days compared to participants in the control group during both the 2 week time period where they received daily reminders or throughout the entire 12 week period. Since packaging for Chantix is clearly labeled and each day of medication is clearly marked on the blister pack, the medication reminders provided by the study could be redundant, and additional reminders were not of benefit to intervention group participants.
Finally, only a minority of participants in the intervention group (40.5%) used keywords (slip, crave, or mood) to request additional support. This study found that participants within the intervention group who used keywords for additional support were more likely to quit smoking than those who did not text for additional support, highlighting the potential benefit of using the keyword feature. Few prior studies have not found on-demand messaging support like keywords to increase quit rates, however, these studies did not account for actual use of the on-demand option (Spohr et al., 2015). Future studies should incorporate an interactive feature and carefully measure use to determine if on-demand options can increase quit rates.
In summary, this study found no differences in abstinence rates between intervention and control group participants however, many important lessons on the feasibility and acceptability of using text messaging tips to aid a quit attempt were learned. First, it was determined that the few text messages received by the control group were perceived as supportive, suggesting that future studies should utilize a control group that does not receive any communication via text message. Second, intervention group participants suggested that receiving messages on a consistent schedule were perceived as annoying and predictable. Finally, less than half of participants in the intervention group utilized the keyword function for additional support. In this study, those who utilized keywords were more likely to quit, suggesting that encouraging use of keyword functionality in future studies may be important.
Acknowledgments
The authors would like to acknowledge the Penn State Clinical and Translational Research Institute, Penn State University CTSA, NIH/NCATS Grant number UL1 TR000127 for providing the REDCap data management tools used in this study. In addition, we are grateful to the Penn State Ambulatory Research Network (PSARN) for the use of their Primary Care sites.
This study was registered at clinicaltrials.gov (identifier: NCT02367391)
Declaration of interest
JF has done paid consulting work for pharmaceutical companies that manufacture or develop smoking cessation medicines (including Pfizer, GSK, J&J). The authors are primarily funded by grants from the National Institutes of Health (NIDA).
Funding
This study was funded by a grant from Pfizer Inc. under its Global Research Awards for Nicotine Dependence (GRAND #WS2391760) program (PI:JF).
References
- Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, … Evins AE (2016). Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): A double-blind, randomised, placebo-controlled clinical trial. Lancet, 387(10037), 2507–2520. doi: 10.1016/S0140-6736(16)30272-0 [DOI] [PubMed] [Google Scholar]
- Brandon TH, Meade CD, Herzog TA, Chirikos TN, Webb MS, & Cantor AB (2004). Efficacy and cost-effectiveness of a minimal intervention to prevent smoking relapse: Dismantling the effects of amount of content versus contact. Journal of Consulting and Clinical Psychology, 72(5), 797–808. doi: 10.1037/0022-006X.72.5.797 [DOI] [PubMed] [Google Scholar]
- Cahill K, Lindson-Hawley N, Thomas KH, Fanshawe TR, & Lancaster T (2016). Nicotine receptor partial agonists for smoking cessation. Cochrane Database System Review, (5), CD006103. doi: 10.1002/14651858.CD006103.pub7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, & Mermelstein R (1983). A global measure of perceived stress. Journal of Health and Social Behavior, 24(4), 385–396. doi: 10.2307/2136404 [DOI] [PubMed] [Google Scholar]
- Eriksen M, Mackay J, Schulger N, Gomeshtapeh F, & Drope J (2015). The tobacco atlas (5th ed.). Atlanta, Georgia: American Cancer Society, Inc. [Google Scholar]
- Foulds J, Russ C, Yu CR, Zou KH, Galaznik A, Franzon M, … Hughes JR (2013). Effect of varenicline on individual nicotine withdrawal symptoms: A combined analysis of eight randomized, placebo-controlled trials. Nicotine and Tobacco Research, 15(11), 1849–1857. doi: 10.1093/ntr/ntt066 [DOI] [PubMed] [Google Scholar]
- Foulds J, Veldheer S, Yingst J, Hrabovsky S, Wilson SJ, Nichols TT, & Eissenberg T (2015). Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking E-cigarette users. Nicotine & Tobacco Research : Official Journal of the Society for Research on Nicotine and Tobacco, 17(2), 186–192. doi: 10.1093/ntr/ntu204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D, DeBenedetti AF, Volk RJ, Williams EC, Kivlahan DR, & Bradley KA (2008). Effectiveness of the AUDIT-C as a screening test for alcohol misuse in three race/ethnic groups. Journal of General Internal Medicine, 23(6), 781–787. doi: 10.1007/s11606-008-0594-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Free C, Knight R, Robertson S, Whittaker R, Edwards P, Zhou W,… Roberts I (2011). Smoking cessation support delivered via mobile phone text messaging (txt2stop): A single-blind, randomised trial. Lancet, 378(9785), 49–55. doi: 10.1016/S0140-6736(11)60701-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SK (2011). Intention-to-treat concept: A review. Perspectives in Clinical Research, 2(3), 109–112. doi: 10.4103/2229-3485.83221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)–A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, & Fagerström KO (1991). The fagerström test for nicotine dependence: A revision of the fagerström tolerance questionnaire. British Journal of Addiction, 86(9), 1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x [DOI] [PubMed] [Google Scholar]
- Hughes JR, & Hatsukami D (1986). Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry, 43(3), 289–294. doi: 10.1001/archpsyc.1986.01800030107013 [DOI] [PubMed] [Google Scholar]
- McGorrian C, Yusuf S, Islam S, Jung H, Rangarajan S, Avezum A,… Investigators, I. (2011). Estimating modifiable coronary heart disease risk in multiple regions of the world: The INTERHEART modifiable risk score. European Heart Journal, 32(5), 581–589. doi: 10.1093/eurheartj/ehq448 [DOI] [PubMed] [Google Scholar]
- Poushter J (2016). Smartphone ownership and internet usage continues to climb in emerging economies. Retrieved from Pew Research Center; http://www.pewglobal.org/2016/02/22/smartphone-ownership-and-internet-usage-continues-to-climb-in-emerging-economies/ [Google Scholar]
- Scott-Sheldon L, Lantini R, Jennings E, Thind H, Rosen R, Salmoirago-Blotcher E, & Bock B (2016). Text messaging-based interventions for smoking cessation: A systematic review and meta-analysis. Journal of Medical Internet Research, 4(2). doi: 10.2196/mhealth.5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spohr SA, Nandy R, Gandhiraj D, Vemulapalli A, Anne S, & Walters ST (2015). Efficacy of SMS text message interventions for smoking cessation: A Meta-analysis. Journal of Substance Abuse Treatment, 56, 1–10. doi: 10.1016/j.jsat.2015.01.011 [DOI] [PubMed] [Google Scholar]
- Stead LF, Koilpillai P, Fanshawe TR, & Lancaster T (2016). Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database System Review, 3(CD008286). doi: 10.1002/14651858.CD008286.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg MB, Randall J, Greenhaus S, Schmelzer AC, Richardson DL, & Carson JL (2011). Tobacco dependence treatment for hospitalized smokers: A randomized, controlled, pilot trial using varenicline. Addictive Behaviors, 36(12), 1127–1132. doi: 10.1016/j.addbeh.2011.07.002 [DOI] [PubMed] [Google Scholar]
- The Surgeon General. (1989). Reducing the health consequences of smoking: 25 years of progress. Retrieved from Atlanta, Georgia. [PubMed] [Google Scholar]
- Tobacco Advisory Group of the Royal College of Physicians. (2000). Nicotine addiction in Britain: A report of the tobacco advisory group of the royal college of physicians. London, UK: Royal College of Physicians. [Google Scholar]
- Whittaker R, McRobbie H, Bullen C, Rodgers A, & Gu Y (2016). Mobile phone-based interventions for smoking cessation. Cochrane Database System Review, 4(CD006611). doi: 10.1002/14651858.CD006611.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]


