Abstract
Renal pathologists identify the protein component of renal amyloid deposits by immunohistochemistry using antibodies against known amyloidogenic proteins. The majority of amyloid cases can be categorized by a simple antibody panel that includes immunoglobulin light chains lambda and kappa, and serum amyloid A. In some instances, however, these reagents do not recognize materials that stain with Congo red or yield ambiguous staining results, thus creating a diagnostic dilemma. Chemical analysis of fibrils extracted from such a nonreactive renal biopsy led to the discovery of a previously unknown amyloid formed from leukocyte chemotactic factor 2 (LECT2). Over the past 8 years, we received 285 renal amyloid samples, of which 31 remained unclassified. In an effort to determine whether any of the latter samples were LECT2 related, tandem mass spectrometry was performed. In all, 7 of the 31 cases were identified as an amyloid LECT2 (ALECT2), a finding confirmed immunohistochemically using a LECT2-specific antibody. The deposits strongly stained for Congo red and, in most cases, had distinctive morphological features with diffuse involvement of the interstitium, arteries, and glomeruli. Hence, we believe that ALECT2 represents the third common form of renal amyloidosis.
Keywords: amyloid, LECT2, leukocyte chemotactic factor 2, renal biopsy
The diagnosis of amyloidosis is based on the finding of characteristic extracellular amorphous material, which is Congo red positive and apple green birefringent with polarization. By electron microscopy, amyloid is composed of characteristic randomly oriented, 7–10 nm nonbranching fibrils. Despite its unifying morphology, all amyloid is not the same. There are currently more than 25 proteins known to be capable of forming amyloid,1 and new amyloid proteins are still being described. This complexity is important to recognize because the clinical management of this disease varies widely depending on the responsible protein.
Systemic amyloidosis often involves the kidneys and is diagnosed on renal biopsy. The most prevalent type, designated AL, is derived from immunoglobulin light chains, although many other kinds of amyloid deposit in this organ include those formed from serum amyloid A protein (AA), and, less commonly, fibrinogen (AFib), transthyretin (ATTR), lysozyme (ALys), and apolipoproteins AI or AII (AApoAI, AApoAII).1 Recently, yet another example of renal amyloidosis—namely that composed of leukocyte chemotactic factor 2 (LECT2)—was described and exhibited extensive congophilic deposits in the glomeruli, interstitium, and vessels.2 In an effort to identify additional cases of ALECT (LECT2-associated amyloidosis), we conducted a retrospective study that involved 31 previously unclassified samples (out of 285 amyloid-containing specimens) received in our laboratory over the past 8½ years in which the nature of the deposits could not be established immunohistochemically.
In this report, we describe seven new cases of LECT2 amyloidosis and provide information on the morphological pattern of this form of renal amyloidosis.
RESULTS
Among the 21,598 kidney biopsy specimens sent to our laboratory over the past 8½ years, 285 (1.3%) were found histochemically to contain apple green birefringent congophilic deposits that, by electron microscopy, were observed to have typical ultrastructural features of amyloid. The two most common forms of amyloidosis identified were AL (86.3%) or AA (7.0%) (Table 1). The majority of cases were identified immunologically; however, 31 instances could not be typed when immunostained by anti-κ, anti-λ, anti-AA, or anti-fibrinogen antisera either due to the absence of staining or ambiguous staining. To determine the nature of the amyloid in these cases, the material was extracted and chemically analyzed by tandem mass spectrometry. In seven instances, the periodic acid-Schiff-negative deposits were identified as LECT2 and this finding was confirmed immunohistochemically (Figure 1). Among the remaining 24, the amyloid in 4 was composed of transthyretin (ATTR); in 1, it was derived from immunoglobulin-γ heavy chain (AH); in 6, it was composed of AL; 6 others were derived from AA; and in 7, there was insufficient sample for analysis. It was noteworthy that the LECT2-associated amyloid was intensely congophilic, as compared with that formed from light chain, serum amyloid A, or transthyretin.
Table 1 |.
Frequency of amyloid type in 285 renal biopsy specimens
| Amyloid type | Number | Percentage (%) |
|---|---|---|
| AL | 246 | 86.3 |
| AA | 20 | 7.0 |
| ALECT2 | 7 | 2.5 |
| ATTR | 4 | 1.4 |
| AH | 1 | 0.3 |
| Unknown | 7 | 2.5 |
Abbreviations: AA, serum amyloid A-associated amyloid; AH, heavy chain-associated amyloid; AL, light chain-associated amyloid; ALECT2, leukocyte chemotactic factor 2-associated amyloid; ATTR, transthyretin-associated amyloid.
Figure 1 |. LECT2-associated renal amyloidosis (light microscopy).
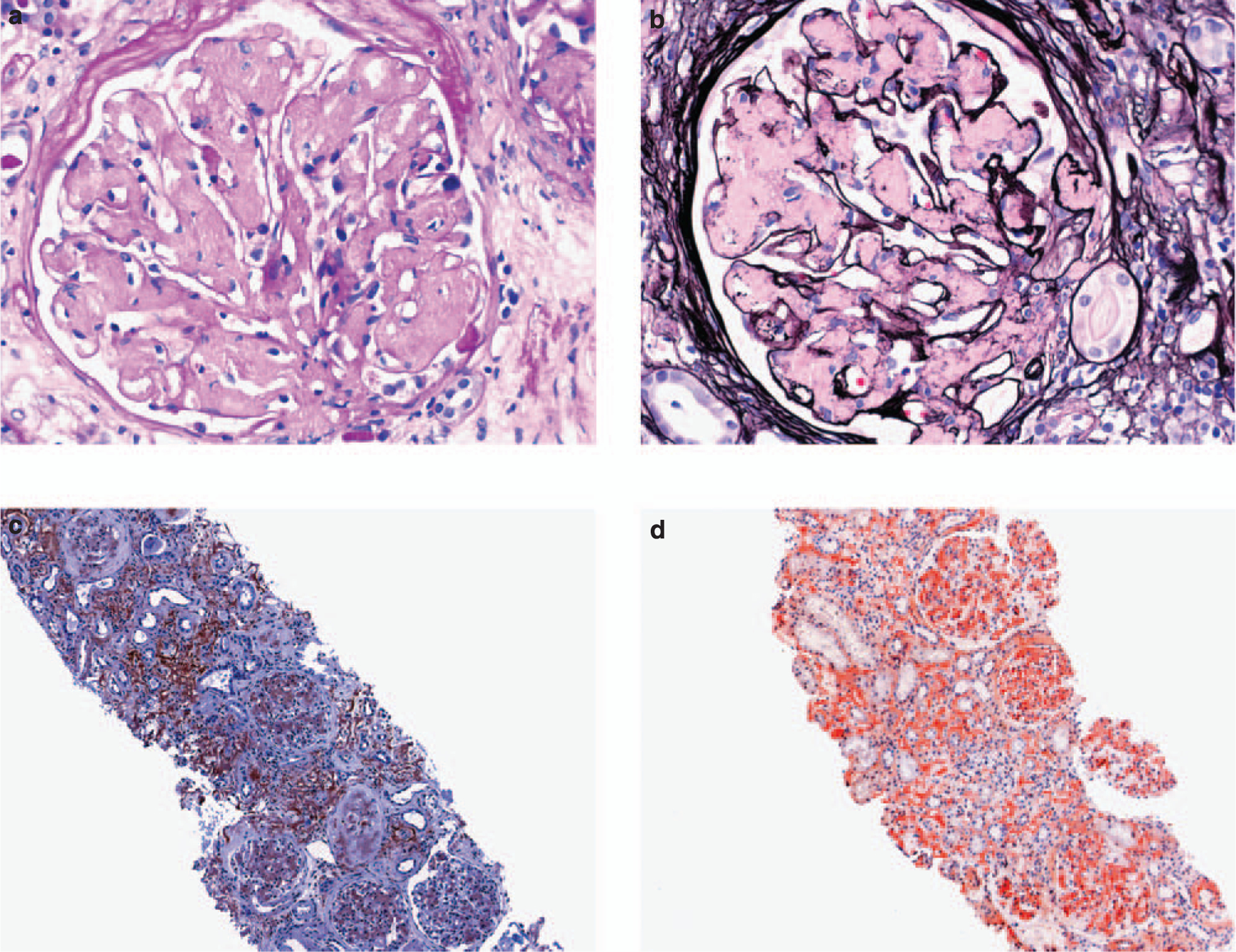
(a) Extensive mesangial replacement by periodic acid-Schiff and (b) Jones methenamine silver-negative amorphous material (× 400). (c) Reactivity of glomerular and interstitial amyloid deposits with an anti-LECT2 antibody (immunoperoxidase technique, × 100). (d) Uptake of Congo red stain by interstitial and glomerular amyloid deposits (× 100). LECT2, leukocyte chemotactic factor 2.
The mean age of patients with LECT2 amyloidosis was 67.8 years (range: 58–84 years). There were a total of four women and three men. Five of seven patients were Hispanic. There was one Caucasian (1/7) and one Native American (1/7).
There was extensive amyloid deposition in all cases of LECT2 amyloidosis. The interstitial compartment was involved in all seven cases, with all but one showing striking and diffuse interstitial deposition. The mesangial regions showed marked, diffuse, global expansion, and displacement by congophilic deposits, in all cases with one exception (Figure 1). In addition, many of the arteries and arterioles were affected (Table 2).
Table 2 |.
Distribution of LECT2-associated amyloid within the kidney
| Case no. | Mesangium | GBM | Interstitium | Arterioles | Arteries |
|---|---|---|---|---|---|
| 1 | ++++a | + | ++++ | ++ | +++ |
| 2 | ++++ | +++ | + | ++ | N/A |
| 3 | ++ | − | +++ | + | ++ |
| 4 | ++++ | + | ++++ | ++ | ++ |
| 5 | − | − | ++++ | + | + |
| 6 | ++++ | + | ++++ | +++ | + |
| 7 | ++++ | − | ++++ | +++ | − |
Abbreviations: GBM, glomerular basement membrane; LECT2, leukocyte chemotactic factor 2; N/A, arteries not present in specimen.
LECT2 deposits: −, absent; +, <25%; ++, 25–50%; +++, 50–75%; ++++, 75–100%.
DISCUSSION
The morphological pattern of amyloid distribution in all LECT2 specimens was similar to that reported originally,2 that is, with extensive involvement of all compartments of the kidney. In this respect, LECT2 deposition differed somewhat from other types of renal amyloid. For example, the amyloid formed from fibrinogen A α-chain is strikingly selective for the glomeruli;3 apolipoprotein AI is reported to be restricted to the medullary interstitium, with conspicuous absence from the cortex in both the interstitium and the glomeruli;4 AA predominately targets the glomeruli, but occasionally is seen in the vasculature with little or no deposition within the glomeruli or interstitium.5
Immunohistochemistry is the usual method used for typing renal amyloid and, in one report, successfully showed the amyloid type in 96.6% of cases.1 However, it comes with diagnostic pitfalls and occasionally cases are not able to be definitively typed using this method. Many of these unknown cases represent AL amyloid, which is occasionally nonreactive with commercial light-chain antibodies,6 but it is also possible that these unknown cases represent a rare form of amyloid, which was not tested for, or even an as yet uncharacterized amyloid. Chemical analysis of formalin-fixed paraffin-embedded tissue by tandem mass spectrometry was used in this study to determine the nature of the amyloid protein in cases that could not be typed by our routine immunohistochemical panel. Depending on the size of the biopsy specimen and the amount of amyloid present, as few as four to six 4-mm-thick sections may be sufficient to make a diagnosis of amyloid type using this method. In some cases, in which no tissue remained in the paraffin block, results were obtained from previously stained sections, such as hematoxylin and eosin, Jones methenamine silver, Masson trichrome, and periodic acid-Schiff reagent.
Correct identification of the amyloid subtype prevents confusion and additional testing often performed in the setting of an unknown type of amyloidosis. Even more importantly, it prevents potentially dangerous therapy, which can occur if it is wrongly concluded that the amyloid, although not staining for κ- or λ-light chains, is due to abnormal paraprotein production caused by a subtle plasma cell dyscrasia. This may lead to the incorrect use of chemotherapy and even bone marrow transplantation.
Little is known about the pathogenesis or prognosis of LECT2 amyloidosis. The process seemingly is confined to the kidney; however, there are only limited chemical and no postmortem data on patients with this disorder. In contrast to other types of renal amyloid, LECT2, which is produced by the liver,7 is neither mutated,2 an acute-phase reactant, nor a product of a clonal population.8
In summary, ALECT2 was the third most common form of renal amyloidosis in our series. Therefore, we recommend that nephropathologists consider this diagnosis in cases that are ‘non-reactive’ with conventionally used immunohistochemical reagents and especially in those with strong congophilia, extensive mesangial expansion, and diffuse interstitial and vascular involvement.
MATERIALS AND METHODS
Light microscopy
Briefly, kidney biopsies were fixed in buffered formalin, dehydrated in graded alcohols, and embedded in paraffin using standard techniques. Serial 3-μm-thick sections were cut and treated with hematoxylin and eosin, Jones methenamine silver, Masson trichrome, and periodic acid-Schiff reagent. In addition, the tissue was stained with Congo red wherein the diagnosis of amyloid was confirmed by Congo red positivity with apple green birefringence when viewed by polarizing microscopy.
Immunohistochemistry
Samples were placed in Michel’s media, washed in buffer, and frozen in a cryostat. Sections, cut at 5 μm, were rinsed in buffer and reacted with fluorescein-tagged polyclonal rabbit anti-human antibodies to IgG, IgA, IgM, C3, C4, C1q, fibrinogen, and κ-, and λ-light chains (all from Dako, Carpenteria, CA, USA) for 1 h, rinsed, and a coverslip applied using aqueous mounting media. For AA and LECT2 detection, 4-μm-thick paraffin-embedded sections were cut, deparaffinized, rehydrated, and blocked with normal horse serum for 5 min, followed by reaction for 1 h with a 1:200 dilution of mouse anti-human amyloid A protein mAb (DakoCytomation, Glostrup, Denmark) or a 1:40 dilution of a goat anti-human LECT2 mAb (R&D Systems, Minneapolis, MN, USA). Immunoreactions were visualized using 3,3’-diaminobenzidine as the substrate (Vector Laboratories, Burlingame, CA, USA).
Electron microscopy
Pieces of 1-mm thickness were removed from each end of the renal biopsy specimen and dehydrated using graded alcohols, followed by embedding in epon/araldite embedding resin. Sections of 1-μm thickness sections were cut using an ultramicrotome, stained with toluidine blue, and examined in a Jeol JEM-1011 electron microscope (Jeol, Tokyo, Japan). Photomicrographs were routinely taken at × 5000, × 12,000, and × 20,000 magnifications. To confirm the fibrillar nature of the deposits, appropriate areas were examined at × 50,000.
Chemical analysis
Amyloid fibrils were extracted from 8 to 30 4-μm-thick formalin-fixed, paraffin-embedded sections using 6 mol/l guanidine HCl and, after reduction and alkylation, followed by trypsin digestion, were analyzed by tandem mass spectrometry, as described previously.9,10
ACKNOWLEDGMENTS
This study was supported, in part, by USPHS Research Grant CA 10056 from the National Cancer Institute. AS is an American Cancer Society Clinical Research Professor.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
REFERENCES
- 1.von Hutten H, Mihatsch M, Lobeck H et al. Prevalence and origin of amyloid in kidney biopsies. Am J Surg Pathol 2009; 33: 1198–1205. [DOI] [PubMed] [Google Scholar]
- 2.Benson MD, James S, Scott K et al. Leukocyte chemotactic factor 2: a novel renal amyloid protein. Kidney Int 2008; 74: 218–222. [DOI] [PubMed] [Google Scholar]
- 3.Lachmann HJ, Chir B, Booth DR et al. Misdiagnosis of hereditary amyloidosis as AL (primary) amyloidosis. N Engl J Med 2002; 346: 1786–1791. [DOI] [PubMed] [Google Scholar]
- 4.Gregorini G, Izzi C, Obici L et al. Renal apolipoprotein A-I amyloidosis: a rare and usually ignored cause of hereditary tubulointerstitial nephritis. J Am Soc Nephrol 2005; 16: 3680–3686. [DOI] [PubMed] [Google Scholar]
- 5.Verine J, Mourad N, Desseaux K et al. Clinical and histological characteristics of renal AA amyloidosis: a retrospective study of 68 cases with a special interest to amyloid-associated inflammatory response. Hum Pathol 2007; 38: 1798–1809. [DOI] [PubMed] [Google Scholar]
- 6.Picken MM, Herrera GA. The burden of ‘sticky’ amyloid. Typing challenges. Arch Pathol Lab Med 2007; 131: 850–851. [DOI] [PubMed] [Google Scholar]
- 7.Yamagoe S, Akasaka T, Uchida T et al. Expression of a neutrophil chemotactic protein LECT2 in human hepatocytes revealed by immunochemical studies using polyclonal and monoclonal antibodies to a recombinant LECT2. Biochem Biophys Res Commun 1997; 237: 116–120. [DOI] [PubMed] [Google Scholar]
- 8.Nagai H, Hamada T, Uchida T et al. Systemic expression of a newly recognized protein, LECT2, in the human body. Pathol Int 1998; 48: 882–886. [DOI] [PubMed] [Google Scholar]
- 9.Murphy CL, Wang S, Williams T et al. Characterization of systemic amyloid deposits by mass spectrometry. Methods Enzymol 2006; 412: 48–62. [DOI] [PubMed] [Google Scholar]
- 10.Murphy CL, Eulitz M, Hrncic R et al. Chemical typing of amyloid protein contained in formalin-fixed paraffin-embedded biopsy specimens. Am J Clin Pathol 2001; 116: 135–142. [DOI] [PubMed] [Google Scholar]


