Abstract
In cancer therapy, the modulation of tumor suppressor proteins represents a critical frontier in developing innovative treatments. A promising direction in this field is the strategic upregulation of tumor suppressor proteins, a paradigm illustrated by the development of compounds designed to enhance the activity of the p53 protein. This protein, often called the “guardian of the genome”, is crucial in preventing cancer development by inducing cell cycle arrest, apoptosis, and senescence in response to DNA damage and oncogenic stress. However, p53 function is compromised in many cancers, leading to unchecked cell proliferation and tumor progression. Addressing this challenge, a novel approach focuses on manipulating the p53/MDM2 signaling pathway to restore p53’s tumor-suppressive functions.
Important Compound Classes
Title
Compounds and Method for Upregulation of p53 through Induction of MDM2 Degradation
Patent Publication Number
WO 2023/250318 A1
URL: https://patents.google.com/patent/WO2023250318A1/en?oq=WO+2023%2f250318+A1)
Publication Date
December 28, 2023
Priority Application
US 63/353783
Priority Date
June 20, 2022
Inventors
Wang, B.; Yang, X.; Tripathi, R.
Assignee Company
Georgia State University Research Foundation, Inc. [US/US]; 30 Courtland Street, NE, Alumni Hall, Suite 326, Atlanta, Georgia 30303 (US)
Disease Area
Cancer
Biological Target
MDM2/p53
Summary
Enhancing p53 Activity through MDM2 Degradation: Central to this innovative strategy is targeting MDM2, a negative regulator of p53. Under normal conditions, MDM2 binds to p53, leading to its ubiquitination and subsequent degradation, effectively limiting p53’s tumor-suppressive activities. However, developing compounds that induce MDM2 degradation can prevent the downregulation of p53, thereby enhancing its tumor suppressor function. This approach offers a direct method to combat p53-inactive tumors and serves as a model for leveraging the body’s tumor-suppressing mechanisms to fight cancer.
The specific application of this strategy in leukemia serves as a beacon, illuminating its potential to tackle cancers with a known disruption in the p53 pathway. Leukemia cells, characterized by their rapid proliferation and resistance to apoptosis, often exhibit dysregulated p53/MDM2 interactions. By reinstating p53 activity through targeted MDM2 degradation, these compounds offer a promising path for the development of more effective leukemia therapies. Moreover, this strategy holds promise for broader applications across various types of cancers where the p53 pathway is compromised.
Integrating this approach with other therapeutic strategies, such as targeted protein degradation (TPD) and lysosomal degradation of extracellular targets, underscores the multifaceted nature of modern cancer therapy. This integration highlights the versatility of targeting the intracellular environment—either by removing oncogenic proteins through TPD or enhancing the activity of tumor suppressor proteins. Such comprehensive strategies can offer a more robust arsenal against cancer, tailoring treatments to the specific molecular underpinnings of the disease.
The development of compounds for upregulating tumor suppressor proteins unfurls new vistas in cancer therapy, kindling hope for treatments that more effectively harness the body’s natural defenses against cancer. As research progresses, the integration of these novel approaches with existing and emerging therapeutic strategies holds the promise of enhancing the precision and efficacy of cancer treatments, propelling us toward a future where cancer can be more effectively managed or even cured.
Key Structures
Biological Assay
Western blot and cytotoxicity assay
Biological Data
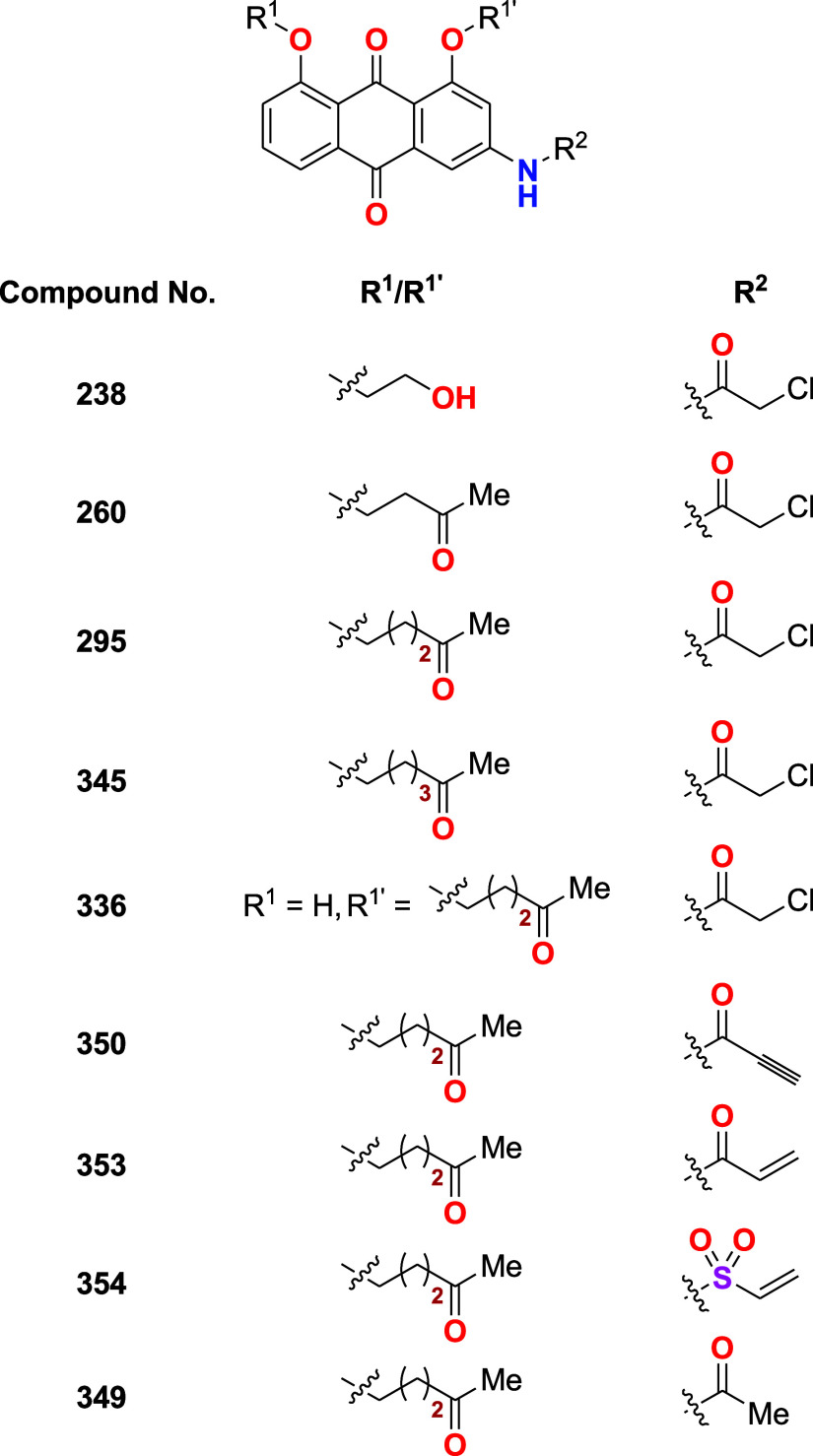
The table presents the IC50 values of select compounds, indicative of their efficacy in EU-1
cells using compound 238 as positive control. Western
blot studies show compound 295 decreases MDM2 level and
increases p53 level in a dose- and time-dependent manner.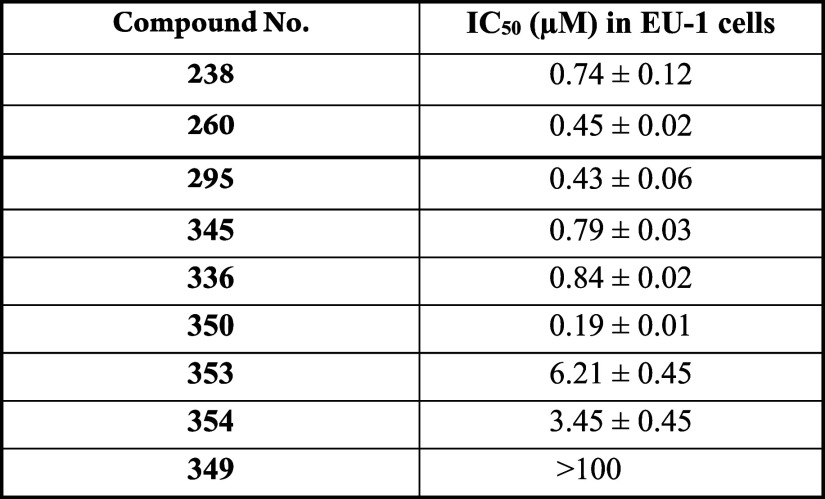
Recent Review Articles
The author declares no competing financial interest.
References
- Wang S.; He F.; Tian C.; Sun A. From PROTAC to TPD: Advances and Opportunities in Targeted Protein Degradation. Pharmaceuticals 2024, 17, 100. 10.3390/ph17010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.; Xue H.; Jin J. Applications of Protein Ubiquitylation and Deubiquitylation in Drug Discovery. J. Biol. Chem. 2024, 107264. 10.1016/j.jbc.2024.107264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaseem A. M. Advancements in MDM2 Inhibition: Clinical and Pre-Clinical Investigations of Combination Therapeutic Regimens. Saudi Pharm. J. 2023, 31, 101790. 10.1016/j.jsps.2023.101790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvalim C.; Datta A.; Lee S. C. Role of p53 in Breast Cancer Progression: An Insight into p53 Targeted Therapy. Theranostics 2023, 13, 1421–1442. 10.7150/thno.81847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar A.; Wang S. Therapeutic Strategies to Activate p53. Pharmaceuticals 2023, 16, 24. 10.3390/ph16010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente A. T. S.; Salvador J. A. R. MDM2-Based Proteolysis-Targeting Chimeras (PROTACs): An Innovative Drug Strategy for Cancer Treatment. Int. J. Mol. Sci. 2022, 23, 11068. 10.3390/ijms231911068. [DOI] [PMC free article] [PubMed] [Google Scholar]