Abstract
2-Arachidonoyl glycerol (2-AG) is the principal endogenously produced ligand for the cannabinoid CB1 and CB2 receptors (CBRs). The lack of potent and efficacious 2-AG ligands with resistance against metabolizing enzymes represents a significant void in the armamentarium of research tools available for studying eCB system molecular constituents and their function. Herein we report the first endocannabinoid glyceride templates with remarkably high potency and efficacy at CBRs. Two of our lead chiral 2-AG analogs, namely, (13S)- and (13R)-Me-2-AGs, potently inhibit excitatory neurotransmission via CB1 while they are endowed with excellent resistance to the oxidizing enzyme COX-2. Our SAR results are supported by docking studies of the key analog and 2-AG on the crystal structures of CB1.
Keywords: Endocannabinoids, 2-arachidonoyl glycerol, chirality, medicinal chemistry
The endogenous arachidonic acid (AA) derivatives N-arachidonoylethanolamine (1a, AEA, Figure 1a) and 2-arachidonoylglycerol (1b, 2-AG) are biosignaling molecules termed endocannabinoids (eCBs) because they bind to and modulate the two Gi/o-protein coupled cannabinoid receptors CB1 and CB2 (CBRs).1 Currently, CB1 and CB2 are well recognized drug targets for cardiometabolic, mood, and CNS disorders, as well as pain, inflammation and cancer. 2-AG is the major eCB in the CNS with concentrations approximately 170-fold higher than AEA. It selectively binds to CBRs, and it is a high-efficacy agonist at both CB1 and CB2.1,2 AEA is a low-efficacy agonist at CB1 with modest affinity for CB2 and also interacts with vanilloid receptors.3 Based upon these differences between 2-AG and AEA and on recent electrophysiology studies, it has been proposed that 2-AG is the principal eCB involved in rapid modulation of the CBRs’ function in the central nervous system (CNS).1,2,4,5 eCBs are synthesized enzymatically on demand from phospholipid precursors and are prone to enzymatic hydrolysis and oxidation. In the CNS the glyceride group of 2-AG is primarily hydrolyzed by monoacylglycerol lipase (MGL) and to a lesser extent by the α/β hydrolase domain containing 6 (ABHD6) whereas the ethanolamide group of AEA is cleaved by fatty acid amide hydrolase (FAAH).1,6−8
Figure 1.

(a) Structures and pharmacophoric regions (blue boxes) of AEA (1a) and 2-AG (1b). (b) Design of novel chiral 2-AG probes.
Moreover, the tetraolefinic chain (TC, Figure 1a) of the arachidonoyl structure is subject to oxidative metabolism by cyclooxygenases (COXs) lipoxygenases (LOXs) and cytochrome P450 enzymes leading to eicosanoid type ethanolamides and glycerides. Most of these metabolites exhibit distinct, non-CBR mediated biological actions with their biological role still to be fully elucidated.9−12
The diverse biological profile of eCB metabolites severely complicates the study of endocannabinoid signaling. For that reason oxidatively resistant endocannabinoid-like probes are essential for simplifying the interpretation of pharmacological data related to endocannabinoid physiology.
In the CNS the inducible form of COXs, COX-2, plays a predominant role in 2-AG biotransformations,1,11 resulting in metabolites that mask the pharmacology of the original lipid.12 Of note, the major COX-2 metabolite of 2-AG, prostaglandin E2 glyceryl ester, potentiates synaptic transmission and exhibits hyperalgesic and proinflammatory actions while it has been proposed as an endogenous agonist for the nucleotide P2Y6 receptor.1,11,13 In contrast, 2-AG suppresses synaptic transmission, and its signaling through CBRs is anti-inflammatory and antinociceptive.1 Therefore, stability against COX-2 is an important parameter to consider for the development of useful 2-AG probes.
In addition to being the soft spot for oxidative biotransformations the TC of the eCBs is the most critical pharmacophore for CBR recognition and activation. Its natural high flexibility complicates defining the pharmacophoric conformation(s) for CBRs and oxidative enzymes.14
As such, it is challenging to modify the TC in such a way that it maintains (or preferably enhances) activity for CBRs while at the same time increases stability for oxidative enzymes. So far, the few reported AEA analogs with structural changes at the TC pharmacophore exhibit much lower CB1 affinities when compared to AEA,14−17 Furthermore, despite multiple attempts over nearly 30 years, there has been no success toward developing 2-AG analogs with potent agonist properties for CBRs.18−22 At best, previous analogs exhibited 10-fold lower ability to activate CB1 receptors when compared to 2-AG,19 while there are no reports for 2-AG analogs with activity on CB2.
Recently, we introduced a novel design approach toward developing eCB analogs with conformationally constrained arachidonoyl chains, with higher potency for CBRs, and enhanced stabilities for oxidative and hydrolytic enzymes.14,23−25 The cornerstone of this approach is the incorporation of chiral methyl groups at the bisallylic carbons C7, C10, and C13 of the TC (Figure 1). Taking into consideration that the first step in the metabolism of the TC by oxidative enzymes is the abstraction of a hydrogen atom from these carbons, the rational behind our design is to block the sites where the oxidative metabolism starts.
In earlier work we demonstrated that this approach can be applied successfully on the AEA scaffold.23,25 Taking this approach, we discovered the first highly potent AEA-like CB1 agonist (AMG315) with resistance to both hydrolytic and oxidative enzymes. Currently AMG315 serves as a valuable tool for in vitro, in vivo, and structural studies.23,26
In the present study we explored applying our design approach to the 2-AG template, and we synthesized the C7- and C13-Me-2-AG ligands (Figure 1b).
Functional in vitro data as well as electrophysiology studies show that the lead (13S)- and (13R)-Me-2-AG probes are the first reported 2-AG analogs behaving as CB1 and CB2 agonists with potency and in vitro efficacy comparable to or higher than that of endogenous 2-AG. They also mimic the effects of 2-AG at CB1 in autaptic hippocampal neurons. Additionally, the compounds exhibit excellent stability against COX-2, thus validating our chiral lipid design approach. To improve our understanding regarding the binding motifs of our chiral glyceride templates with the CB1 receptor, we studied the interactions of the lead analog with CB1 through molecular docking. To the best of our knowledge such findings are reported here for first time regarding analogs of the principal endocannabinoid lipid 2-AG.
Synthesis of the chiral 2-AG ligands 2a–d, based on a chemoenzymatic approach developed in our laboratory, is summarized in Scheme 1.12,27 The requisite chiral Me-arachidonic acids 5a–d were synthesized in 12–15 steps from the respective commercially available methyl esters 3 and 4 by following enantioselective approaches previously developed in our laboratory.23,25
Scheme 1. Chemoenzymatic Synthesis of Chiral Me-2-AGs 2a–d.

Reagents and conditions: (a) 9, DMAP, EDCI, CH2Cl2, 0 °C to rt, overnight, 92–95%; (b) resin-immobilized lipase B from Candiada antarctica, anhydrous EtOH, rt, 2 h, 49–53%; (c) resin-immobilized lipase B from Candida antarctica, CH2Cl2, 0 °C, 5 h, 98%.
The 1,3-diacylglycerol 9 was coupled with Me-arachidonic acids 5a–d using 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI) in the presence of 4-dimethylaminopyridine (DMAP) to give the respective triglycerides 6a–d in excellent yields (92–95%).27 Treatment of 6a–d with resin-immobilized lipase B from Candiada Antarctica (Novozyme 435) in anhydrous EtOH afforded the required 2-AG ligands 2a–d in 49–53% yields along with the byproduct ethyl butyrate which was removed under reduced pressure. We also observed the formation of the transesterification side products Me-arachidonic acid ethyl esters (34–27% that were removed during column purification). In agreement with our earlier work,12,27 this approach provides the chiral 2-AG analogs essentially free of 1(3)-acylglycerol rearrangement byproducts as confirmed by 1H NMR data. Enzymatic acylation of glycerol 7 was carried out in anhydrous dichloromethane using vinylbutyrate 8 as an acyl transfer agent to furnish 1,3-butyroyl glycerol 9 in 98% yield.27
We first tested the four analogs for activity at cannabinoid receptors. Compounds capable of activating both CB1 and CB2, thus resembling the profile of the endogenous ligand, were then tested on COX-2 as it is the major metabolizing enzyme of the TC. Finally, we tested whether two key compounds mimicked the hallmark profile of 2-AG to suppress synaptic transmission in autaptic hippocampal neurons.
The standard CB1 and CB2 competitive ligand-binding assay using CP-55,940 as the radioligand has low reproducibility and accuracy for 2-AG and its analogs and was therefore excluded from our in vitro characterization.14,22 Instead, 2-AG and the four chiral analogs were tested for their ability to activate CB1 and CB2 receptors by measuring the decrease in forskolin-stimulated cAMP. Under physiological conditions CBRs couple predominantly to Gi proteins which inhibit adenylyl cyclase. This response is a downstream step in the signaling cascade initiated by agonist-liganded receptor and serves as a quantitative measure of cannabinoid receptor function. When adenylyl cyclase is stimulated by forskolin an agonist-induced decrease in cAMP levels can be measured.28 We used HEK 293 cells expressing rat CB1 (rCB1) or mouse CB2 (mCB2) as these cells are used routinely in our laboratories and provide robust results. Functional potencies (EC50) and efficacies (Emax) of the analogs are presented in Table 1 where data for 2-AG and the standard cannabinoid agonist CP-55,940 are also shown for comparison. The dose–response curves of the compounds for both the CB1 and CB2 are depicted in Figure 2.
Table 1. EC50 and Emax Values (and the Respective 95% Confidence Intervals) of 2-AG Ligands in Forskolin Stimulated cAMP Assay at Rat CB1 and Mouse CB2 Receptors.
Figure 2.
Concentration-dependent curves of inhibition of forskolin-stimulated cAMP accumulation at rat CB1 (A, B) and mCB2 (C, D) for 2-AG, 2-AG analogs, and standard CP55,940.
A cursory examination of the functional data for the CB1 receptor indicates that all four Me-2-AG isomers behave as CB1 agonists with potencies and efficacies comparable to or higher than that of the endogenous prototype. More specifically, the two C13 enantiomers AM8125 (13S) and AM8146 (13R) exhibit higher potency and efficacy than 2-AG with AM8146 being marginally more potent than AM8125. The two C7 enantiomers, AM11606 (7R) and AM11413 (7S), are equipotent to 2-AG.
Lastly, the efficacy of AM11606 is equal to 2-AG. However, AM11413 exhibits remarkably high efficacy on CB1, with an Emax exceeding that of the exogenous cannabinoid CB1/CB2 agonist standard CP55,940.
An evaluation of the c-AMP testing results of the four Me-2-AG isomers for the CB2 receptor shows that the (7S)-Me-2-AG isomer AM11413 is the least active of the four analogs as it exhibited very low efficacy at the highest concentration tested (1 μM). Thus, this 2-AG analog behaves as a potent and highly efficacious CB1 receptor agonist with no ability to activate CB2.
Its enantiomer, (7R)-Me-2-AG, AM11606, is 3 times more potent than 2-AG on CB2 but it exhibits lower efficacy when compared to the endogenous ligand and the standard CP55,940.
However, contrary to relatively low efficacy C7 enantiomers, the C13 enantiomers AM8125 (13S) and AM8146 (13R) behave as high efficacy agonists at CB2 with Emax values (50.1%, 42.8% respectively) exceeding that of 2-AG (Emax= 35.2%). The potency of the C13(S) and C13(R) analogs is comparable to the endogenous ligand while AM8125 exhibits the highest efficacy for CB2 which is comparable to the exogenous standard CP55,940.
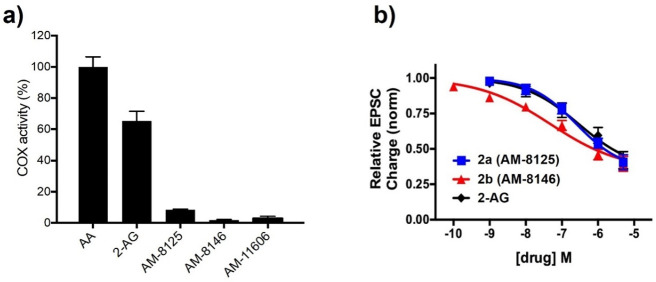
The three CB1 and CB2 receptor agonists (i.e., AM8125, AM8146, and AM11606) were tested as substrates on murine COX-2 along with the standards 2-AG and arachidonic acid. As we published earlier, we used a polarographic assay that measures oxygen consumption, and the results are presented in Figure 3a.23 In agreement with our design hypothesis our data show that incorporation of a chiral methyl group at the bisallylic carbons 7 and 13 enhances the stability of the TCs against COX-2.
Figure 3.
(a) Oxygenation of arachidonic acid (AA), 2-AG and key analogs by mCOX-2. The oxygen consumption was monitored using an oxygen electrode chamber as described in the experimental section in Supporting Information. (b) 2-AG, AM8125 (2a), and AM8146 (2b) all potently and efficaciously inhibit excitatory neurotransmission via CB1. EPSC: excitatory postsynaptic currents.
2-AG and the lead analogs AM8125 (2a) and AM8146 (2b) were further tested in autaptic hippocampal neurons, a well-characterized neuronal model of 2-AG-mediated endocannabinoid signaling.29 Autaptic neurons possess the eCB signaling machinery sufficient for depolarization induced suppression of excitation (DSE), a form of retrograde synaptic transmission, and are well-suited to second level pharmacological characterization of neuronal cannabinoid signaling.29 An effective 2-AG analog should yield similar potency and efficacy as the endogenous system. The response can be calibrated to the endogenous response in a given neuron. In agreement with the CB1 cyclase results, our data (Figure 2b) show that 2a closely mimics 2-AG while 2b is more potent, with the order of potency 2b > 2a ≈ 2-AG [EC50 2-AG (95% CI), 194 nM (73–513); 2a, 177 nM (88–354); 2b, 28 nM (8–98). Both compounds occlude DSE signaling at 5 μM (DSE in response to 3 s depolarization in the presence of 2a (5 μM), 0.94 ± 0.03; 2b (5 μM), 0.94 ± 0.04)].
In an effort to understand the interactions of our key analog AM8125 and 2-AG with the CB1 receptor, we performed molecular docking studies using the available crystal structures of CB1 receptor cocrystallized with the classical cannabinoid agonist AM841 (PDB code 5xr8)30 and the endocannabinoid agonist AMG315 (PDB code 8ghv).26 Docking results are shown in Table 2, while predicted docking poses of the ligands are depicted in Figures 4 and 5.
Table 2. Docking Results for 2-AG and AM8125 on the Crystal Structures of Exogenous AM841 (5xr8) and Endogenous AMG315 (8ghv) Agonists Bound hCB1.
| CB1 receptor cAMP data |
rigid docking score |
ligand energy after fully flexible ligand–receptor pocket refinement starting
from the active state |
||||
|---|---|---|---|---|---|---|
| compd | EC50, nM (95% CI) | Emax (%) (95% CI) | CB1 (5xr8) | CB1 (8ghv) | CB1 (5xr8) | CB1 (8ghv) |
| 2-AG | 25 (20.6–29.4) | 47.7 (40.3–55.1) | –31.1 | –26.7 | –55.9 | –66.2 |
| AM8125 | 17 (14.8–23.1 | 51.2 (48.6–56.3) | –27.0 | –31.1 | –54.5 | –69.3 |
Figure 4.
Predicted docking poses of 2-AG (A) and AM8125 (B) in the AM841-bound CB1 receptor binding pocket (PDB code 5xr8). 2-AG is shown as yellow sticks. AM8125 is shown as orange sticks with the (13S)-Me circled black. CB1 receptor helices and residues are shown in blue.
Figure 5.
Predicted docking poses of 2-AG (A) and AM8125 (B) in the AMG315-bound CB1 receptor binding pocket (PDB code 8ghv). 2-AG is shown as yellow sticks. AM8125 is shown as orange sticks with the (13S)-Me circled black. CB1 receptor helices and residues are shown in tan.
Initial docking calculations were performed with rigid positions of the residues in the active state binding pocket and flexible ligands. The polar headgroup of both 2-AG and AM8125 forms hydrogen bond interactions with residues in the top part of the orthosteric binding pocket. In the AM841-bound CB1 structure, H-bonds are observed between the polar headgroup of the ligands and the backbone of V110, F108 (N-term), and D266 (ECL2) (Figure 4). In the AMG315-bound CB1 structure, we instead observe H-bonds with H178 (TM2) and the backbone of K376 (TM7) while interactions with the backbone of D266 (ECL2) stay consistent (Figure 5). Observed variations in H-bond network are due to the minor flexibility (RMSD (heavy atoms) < 2 Å) of the binding pocket upon binding of different ligands. All mentioned H-bonds can be potentially present upon binding of AM8125 and 2-AG to CB1 receptor as H-bond forming atoms locate within 5 Å from the hydrophilic head of the ligands. In both cases, the hydrophobic tail of the molecules is buried deep into the orthosteric binding pocket. Lastly, the (13S)-methyl group of AM8125 interacts with the twin toggle residue F200.
Predicted docking scores are in the same range for both 2-AG and AM8125 and in agreement with the cAMP data. We additionaly, performed fully flexible optimization of the ligand and receptor pocket residues within 8 Å radius from the ligand starting from both active CB1 structures (PDB codes 5xr8 and 8ghv). The docking scores of this study are shown in Table 2, while the ligand positions are overlapping with the ones predicted by the rigid docking (data not shown).
In this work we extended our innovative chiral lipid design approach to include the principal endocannabinoid 2-AG. The SAR study led to the first 2-AG probes with in vitro functional potency and efficacy at CB1 and CB2 receptors comparable to or higher than the endogenous ligand.
Two of the analogs identified here, namely, (13S)- Me-2-AG (2a) and (13R)-Me-2-AG (2b), potently and efficaciously inhibit excitatory neurotransmission via CB1 while they are endowed with stability against COX-2. Our findings are highlighted with docking studies of 2-AG and the key analog on the crystal structures of exogenous and endogenous agonist bound hCB1. Further optimization of these excellent 2-AG templates is currently being pursued through the development of second generation chiral 2-AG analogs with remarkable potency and outstanding stability to all endocannabinoid hydrolyzing enzymes as well as COX-2.
Glossary
Abbreviations
- CB1
cannabinoid receptor 1
- CB2
cannabinoid receptor 2
- AEA
anandamide
- 2-AG
2-arachidonoylglycerol
- eCBs
endocannabinoids
- FAAH
fatty acid amide hydrolase
- MGL
monoacylglycerol lipase
- ABHD6
alpha/beta-Hydrolase domain containing 6
- COX-2
cyclooxygenases-2
- LOXs
lipooxygenases
- CNS
central nervous system
- SAR
structure–activity relationship
- HEK293
human embryonic kidney cell line
- NMR
nuclear magnetic resonance
- HPLC
high-performance liquid chromatography.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.4c00175.
Experimental procedures and spectroscopic, analytical, and physical data for all compounds; methods for cAMP assay, m-COX-2 stability, electrophysiology, and molecular modeling; reproductions of 1H and 13C NMR spectra for final compounds and representative triglyceride precursors; and additional references (PDF)
Author Contributions
§ S.P.N. and L.J. contributed equally. S.P.N.: project conception, design and supervision of experiments, synthesis and characterization of ligands, data analysis, manuscript writing. L.J., Y.L., M.-O.G.: design, synthesis, and characterization of ligands, manuscript writing. A.D.: cAMP studies. A.S.: design and supervision of electrophysiology studies, assistance in manuscript writing. S.K.: COX-2 stability studies. A.V.S.: molecular docking studies, assistance in manuscript writing. M.D.: electrophysiology studies. V.K.: design and supervision of molecular docking studies, assistance in manuscript writing. K.M.: design and supervision of cAMP and electrophysiology studies, assistance in manuscript writing. L.M.: design and supervision of COX-2 stability studies, assistance in manuscript writing. A.M.: project conception, design, and supervision of experiments, data analysis, manuscript writing.
NIDA is recognized for support [Grant DA009158 (A.M.) and Grant DA047858 (K.M.)].
The authors declare no competing financial interest.
Supplementary Material
References
- Lu H. C.; Mackie K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79 (7), 516–25. 10.1016/j.biopsych.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T.; Waku K. 2-Arachidonoylglycerol and the cannabinoid receptors. Chem. Phys. Lipids 2000, 108 (1–2), 89–106. 10.1016/S0009-3084(00)00189-4. [DOI] [PubMed] [Google Scholar]
- Smart D.; Gunthorpe M. J.; Jerman J. C.; Nasir S.; Gray J.; Muir A. I.; Chambers J. K.; Randall A. D.; Davis J. B. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1). Br. J. Pharmacol. 2000, 129 (2), 227–30. 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T.; Kishimoto S.; Oka S.; Gokoh M. Biochemistry, pharmacology and physiology of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Prog. Lipid Res. 2006, 45 (5), 405–46. 10.1016/j.plipres.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Katona I.; Freund T. F. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat. Med. 2008, 14 (9), 923–30. 10.1038/nm.f.1869. [DOI] [PubMed] [Google Scholar]
- Ahn K.; McKinney M. K.; Cravatt B. F. Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem. Rev. 2008, 108 (5), 1687–707. 10.1021/cr0782067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J.; Adamson C.; Butler D.; Janero D. R.; Makriyannis A.; Bahr B. A. Enhancement of endocannabinoid signaling by fatty acid amide hydrolase inhibition: a neuroprotective therapeutic modality. Life Sci. 2010, 86 (15–16), 615–23. 10.1016/j.lfs.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs W. R.; Blankman J. L.; Horne E. A.; Thomazeau A.; Lin Y. H.; Coy J.; Bodor A. L.; Muccioli G. G.; Hu S. S.; Woodruff G.; Fung S.; Lafourcade M.; Alexander J. P.; Long J. Z.; Li W.; Xu C.; Moller T.; Mackie K.; Manzoni O. J.; Cravatt B. F.; Stella N. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat. Neurosci. 2010, 13 (8), 951–7. 10.1038/nn.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzer C. A.; Marnett L. J. Endocannabinoid oxygenation by cyclooxygenases, lipoxygenases, and cytochromes P450: cross-talk between the eicosanoid and endocannabinoid signaling pathways. Chem. Rev. 2011, 111 (10), 5899–921. 10.1021/cr2002799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquhart P.; Nicolaou A.; Woodward D. F. Endocannabinoids and their oxygenation by cyclo-oxygenases, lipoxygenases and other oxygenases. Bba-Mol. Cell Biol. L 2015, 1851 (4), 366–376. 10.1016/j.bbalip.2014.12.015. [DOI] [PubMed] [Google Scholar]
- Alhouayek M.; Muccioli G. G. COX-2-derived endocannabinoid metabolites as novel inflammatory mediators. Trends Pharmacol. Sci. 2014, 35 (6), 284–92. 10.1016/j.tips.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Shelnut E. L.; Nikas S. P.; Finnegan D. F.; Chiang N.; Serhan C. N.; Makriyannis A. Design and synthesis of novel prostaglandin E2 ethanolamide and glycerol ester probes for the putative prostamide receptor(s). Tetrahedron Lett. 2015, 56 (11), 1411–1415. 10.1016/j.tetlet.2015.01.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruser A.; Zimmermann A.; Crews B. C.; Sliwoski G.; Meiler J.; Konig G. M.; Kostenis E.; Lede V.; Marnett L. J.; Schoneberg T. Prostaglandin E(2) glyceryl ester is an endogenous agonist of the nucleotide receptor P2Y(6). Sci. Rep 2017, 7 (1), 2380. 10.1038/s41598-017-02414-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliopoulos-Tsoutsouvas C.; Georgiadis M. O.; Ji L.; Nikas S. P.; Makriyannis A.. Natural compounds and synthetic drugs to target type-1 cannabinoid (CB1) receptor. In New Tools to Interrogate Endocannabinoid Signalling; Maccarrone M., Ed.; RSC, 2020; pp 48–88. [Google Scholar]
- Padgett L. W.; Howlett A. C.; Shim J. Y. Binding mode prediction of conformationally restricted anandamide analogs within the CB1 receptor. J. Mol. Signal. 2014, 3, 5. 10.1186/1750-2187-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Stelt M.; van Kuik J. A.; Bari M.; van Zadelhoff G.; Leeflang B. R.; Veldink G. A.; Finazzi-Agro A.; Vliegenthart J. F.; Maccarrone M. Oxygenated metabolites of anandamide and 2-arachidonoylglycerol: conformational analysis and interaction with cannabinoid receptors, membrane transporter, and fatty acid amide hydrolase. J. Med. Chem. 2002, 45 (17), 3709–20. 10.1021/jm020818q. [DOI] [PubMed] [Google Scholar]
- Finnegan D. F.; Shelnut E. L.; Nikas S. P.; Chiang N.; Serhan C. N.; Makriyannis A. Novel tail and head group prostamide probes. Bioorg. Med. Chem. Lett. 2015, 25 (6), 1228–31. 10.1016/j.bmcl.2015.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizzi A.; Cascio M. G.; Frosini M.; Ligresti A.; Aiello F.; Biotti I.; Brizzi V.; Pertwee R. G.; Corelli F.; Di Marzo V. Resorcinol-sn-Glycerol Derivatives: Novel 2-Arachidonoylglycerol Mimetics Endowed with High Affinity and Selectivity for Cannabinoid Type 1 Receptor. J. Med. Chem. 2011, 54 (24), 8278–8288. 10.1021/jm200529h. [DOI] [PubMed] [Google Scholar]
- Parkkari T.; Myllymaki M.; Savinainen J. R.; Saario S. M.; Castillo-Melendez J. A.; Laitinen J. T.; Nevalainen T.; Koskinen A. M.; Jarvinen T. Alpha-methylated derivatives of 2-arachidonoyl glycerol: synthesis, CB1 receptor activity, and enzymatic stability. Bioorg. Med. Chem. Lett. 2006, 16 (9), 2437–40. 10.1016/j.bmcl.2006.01.101. [DOI] [PubMed] [Google Scholar]
- Parkkari T.; Savinainen J. R.; Rauhala A. L.; Tolonen T. L.; Nevalainen T.; Laitinen J. T.; Gynther J.; Jarvinen T. Synthesis and CB1 receptor activities of novel arachidonyl alcohol derivatives. Bioorg. Med. Chem. Lett. 2004, 14 (12), 3231–4. 10.1016/j.bmcl.2004.03.093. [DOI] [PubMed] [Google Scholar]
- Suhara Y.; Oka S.; Kittaka A.; Takayama H.; Waku K.; Sugiura T. Synthesis and biological evaluation of several structural analogs of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Bioorg. Med. Chem. 2007, 15 (2), 854–67. 10.1016/j.bmc.2006.10.049. [DOI] [PubMed] [Google Scholar]
- Vadivel S. K.; Vardarajan S.; Duclos R. I. Jr; Wood J. T.; Guo J.; Makriyannis A. Conformationally constrained analogues of 2-arachidonoylglycerol. Bioorg. Med. Chem. Lett. 2007, 17 (21), 5959–63. 10.1016/j.bmcl.2007.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Ji L.; Eno M.; Kudalkar S.; Li A. L.; Schimpgen M.; Benchama O.; Morales P.; Xu S.; Hurst D.; Wu S.; Mohammad K. A.; Wood J. T.; Zvonok N.; Papahatjis D. P.; Zhou H.; Honrao C.; Mackie K.; Reggio P.; Hohmann A. G.; Marnett L. J.; Makriyannis A.; Nikas S. P. (R)-N-(1-Methyl-2-hydroxyethyl)-13-(S)-methyl-arachidonamide (AMG315): A Novel Chiral Potent Endocannabinoid Ligand with Stability to Metabolizing Enzymes. J. Med. Chem. 2018, 61 (19), 8639–8657. 10.1021/acs.jmedchem.8b00611. [DOI] [PubMed] [Google Scholar]
- Nikas S. P.; D’Souza M.; Makriyannis A. Enantioselective synthesis of (10S)- and (10R)-methyl-anandamides. Tetrahedron 2012, 68 (31), 6329–6337. 10.1016/j.tet.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papahatjis D. P.; Nahmias V. R.; Nikas S. P.; Schimpgen M.; Makriyannis A. Design and synthesis of (13S)-methyl-substituted arachidonic acid analogues: templates for novel endocannabinoids. Chem.—Eur. J. 2010, 16 (13), 4091–9. 10.1002/chem.200902880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna Kumar K.; Robertson M. J.; Thadhani E.; Wang H.; Suomivuori C. M.; Powers A. S.; Ji L.; Nikas S. P.; Dror R. O.; Inoue A.; Makriyannis A.; Skiniotis G.; Kobilka B. Structural basis for activation of CB1 by an endocannabinoid analog. Nat. Commun. 2023, 14 (1), 2672. 10.1038/s41467-023-37864-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadivel S. K.; Whitten K. M.; Makriyannis A. Chemoenzymatic synthesis of 2-arachidonoylglycerol, an endogenous ligand for cannabinoid receptors. Tetrahedron Lett. 2011, 52 (11), 1149–1150. 10.1016/j.tetlet.2011.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanastasiou I. P.; Georgiadis M. O.; Iliopoulos-Tsoutsouvas C.; Paronis C. A.; Brust C. A.; Tran N. K.; Ji L.; Ma X.; Wood J. T.; Zvonok N.; Tong F.; Bohn L. M.; Nikas S. P.; Makriyannis A. Improved cyclobutyl nabilone analogs as potent CB1 receptor agonists. Eur. J. Med. Chem. 2022, 230, 114027. 10.1016/j.ejmech.2021.114027. [DOI] [PubMed] [Google Scholar]
- Straiker A.; Mackie K. Depolarization-induced suppression of excitation in murine autaptic hippocampal neurones. J. Physiol-London 2005, 569 (2), 501–517. 10.1113/jphysiol.2005.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua T.; Vemuri K.; Nikas S. P.; Laprairie R. B.; Wu Y.; Qu L.; Pu M.; Korde A.; Jiang S.; Ho J. H.; Han G. W.; Ding K.; Li X.; Liu H.; Hanson M. A.; Zhao S.; Bohn L. M.; Makriyannis A.; Stevens R. C.; Liu Z. J. Crystal structures of agonist-bound human cannabinoid receptor CB1. Nature 2017, 547, 468. 10.1038/nature23272. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.