Abstract
RNAs are increasingly considered valuable therapeutic targets, and the development of methods to identify and validate both RNA targets and ligands is more important than ever. Here, we utilized a bioinformatic approach to identify a hairpin-containing RNA G-quadruplex (rG4) in the 5′ untranslated region (5′ UTR) of DHX15 mRNA. By using a novel competitive small molecule microarray (SMM) approach, we identified a compound that specifically binds to the DHX15 rG4 (KD = 12.6 ± 1.0 μM). This rG4 directly impacts translation of a DHX15 reporter mRNA in vitro, and binding of our compound (F1) to the structure inhibits translation up to 57% (IC50 = 22.9 ± 3.8 μM). This methodology allowed us to identify and target the mRNA of a cancer-relevant helicase with no known inhibitors. Our target identification method and the novelty of our screening approach make our work informative for future development of novel small molecule cancer therapeutics for RNA targets.
Keywords: RNA targeting, DHX15, High-throughput screening, Small-molecule microarray, G-quadruplex, Translation inhibition
In recent years, RNAs have become attractive targets for small molecule medicinal chemistry campaigns.1 This is partly due to the sheer amount of RNA in cells—approximately 85% of the genome is transcribed into RNA, compared to the approximately 1–2% of the genome that encodes proteins.2,3 Further, just ∼15% of the proteome has been successfully targeted to date, leaving many disease-relevant proteins outside the realm of targetability with small molecules.4 Recent research suggests that several types of RNA (such as lncRNA, miRNA, and mRNA) can be effectively targeted with druglike molecules.5,6 Targeting mRNA presents a powerful opportunity to control the expression of pathogenic proteins that are challenging to target at the level of protein structure. Consequently, the development of novel screening methodologies that enable the rapid discovery of RNA-binding ligands with information about their binding modes and bias toward functional hits is critical.
Ideal RNA targets for small molecules should meet the requirements of both ligandability and functional relevance. Like proteins, many RNAs can fold into diverse secondary and three-dimensional structures with hydrophobic pockets in many structures throughout the protein databank (PDB).6−8 Moreover, these structures or conformations can be recognized by proteins or small molecules and regulate diverse functions such as translation, splicing, and RNA decay.9−13 In some cases, targeting ligandable structures with small molecules can result in functional changes caused by altering the thermodynamic stability or conformation of the RNA.14,15 For example, RNA G-quadruplexes (rG4s), which contain guanine-rich tracks that fold to form the high-order structure, have been regarded as attractive RNA targets.16 Several rG4s in 5′ untranslated regions (5′ UTR) have been targeted with small molecules (even with the FDA-approved drug Levofloxacin), resulting in translation inhibition.17−19 In particular, rG4s with duplex/hairpin structures embedded in loops have also attracted attention for their unique structures and protein binding (e.g., sc1 RNA-FMRP RGG, HIV-1 RNA U3).20,21 These intriguing RNA structures exhibit great potential for small molecule targeting.
Once an RNA target is identified, high-throughput screening (HTS) is an effective way to identify potential hit compounds. Multiple biochemical and biophysical techniques have been reported to screen compound libraries against RNA targets.22 Our lab has published extensively on the use of small molecule microarray (SMM) technology to identify RNA-binding compounds in an efficient manner.23,24 To discover hits, fluorescently labeled nucleic acids are incubated on glass slides that contain tens of thousands of small molecules spatially arrayed and covalently linked to the surface. Imaging and analysis of the slide reveals hit compounds bound to RNA. The development of SMM, new methodologies (e.g., multicolor imaging), and a large data set of orthogonal RNA-small molecule binding has resulted in a more comprehensive understanding of RNA targeting.24,25 Our lab seeks to continue to advance this understanding by further developing the SMM methodology. In this study, we describe a novel approach to SMM screening that allows us to identify small molecules that bind with a known binding mode to our target of interest.
To first identify a target, we employed a bioinformatic approach to identify noncanonical rG4s in the human transcriptome, revealing a hairpin-containing rG4 in the 5′ UTR of DEAH-box helicase 15 (DHX15) mRNA. We used multiple biochemical and biophysical techniques to establish the presence of this rG4 in the DHX15 mRNA and characterize its folding and impact on translation. To efficiently discover small molecule ligands for this rG4 with defined binding modes, we used a novel, biochemically competitive SMM screening approach. The most active identified compound (F1) bound reversibly to the rG4 (KD = 12.6 ± 1 μM) and inhibited translation of a DHX15 luciferase reporter in vitro. More broadly, our pipeline combining bioinformatics with biochemically competitive HTS efforts demonstrates a practical strategy for identifying targetable structured regions within complex mRNAs and ligands with defined binding modes.
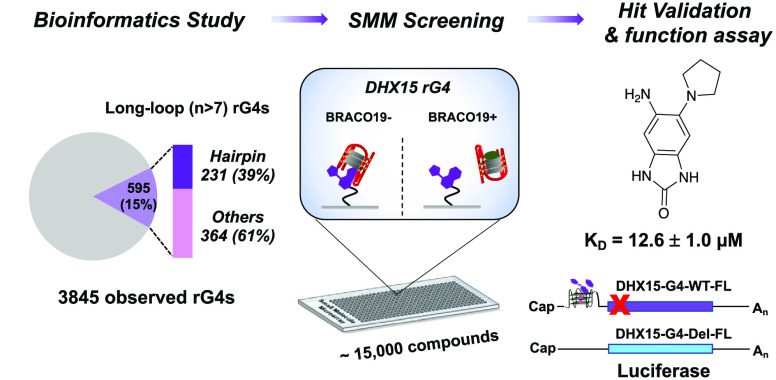
Many eukaryotic RNAs have been observed to contain rG4-forming sequences.26,27 To identify novel rG4s as targets, we assessed a public rG4-seq data set assembled to identify rG4s in human mRNAs.28 As shown in Figure 1A, a total of 3,845 rG4s were previously observed to form in K+ buffer by a transcriptional stalling method.29 While most rG4s were canonical quadruplex structures (G3–5N1–7G3–5N1–7G3–5N1–7), about 15% (595) of the rG4s were found to contain longer loops (n > 7). For those long-loop rG4s, 39% had the potential to form duplex (or hairpin) structures by base pairing within loops, making their 3D structures distinct from simpler G4s.30−32 Among the 230 putative hairpin-containing rG4 sequences corresponding to 72 genes (Supplementary File 1), a short RNA sequence from DHX15 mRNA was notable for three reasons. First, DHX15 protein is an RNA helicase involved in mRNA processing and immunity.33,34 It has been reported that DHX15 is overexpressed in and leads to the progression of several cancer types, including prostate cancer, Burkitt lymphoma, and acute myeloid leukemia.35−38 Second, as an RNA helicase, the DHX15 protein is difficult to target directly and selectively at the protein level with small molecules due to conserved nucleotide binding sites and complex ATP-dependent and -independent mechanisms.39,40 No chemical inhibitors targeting this helicase have been published, and targeting the structured mRNA of DHX15 presents a potential path forward to targeting this disease-relevant gene that is complementary to targeting the protein itself. Finally, the rG4 is embedded within the 5′ UTR of DHX15 mRNA (Figure 1B), which could potentially regulate its translation, as is the case with other rG4s.41 In addition, by evaluating 17 different cell lines, this quadruplex-forming sequence was found in 16 of them (Supplementary Figure 1). Together, these factors indicated that DHX15 rG4 could be a good target candidate for small molecules.
Figure 1.
Identification of an rG4 in DHX15 mRNA and in vitro study of G4 formation. (A) Bioinformatic study on the rG4s with long loops formed in human cells. The observed rG4s were obtained from reported rG4-seq data. (B) The hairpin-containing rG4 (secondary structure) discovered in the 5′ UTR region of DHX15 mRNA. (C) CD spectrum characterization of the folded/unfolded DHX15 rG4 RNA in KCl or LiCl buffer. (D) Cationic ion titration assay using K+ and Mg2+ to induce the RNA conformational change between stem-loop and hairpin-rG4 structures.
Characterization of DHX15 rG4 folding was first performed by using CD spectroscopy. To assess the folding conditions of the RNA, a short construct containing DHX15 rG4 was annealed and folded in buffer containing either KCl or LiCl prior to recording the CD spectrum. As shown in Figure 1C, RNA folded in KCl buffer exhibited a characteristic G4 structure, demonstrated by a positive peak at 263 nm and a negative peak at 240 nm. In contrast, a decreased and broadened peak was observed in the spectrum corresponding to the RNA folded in the LiCl buffer, implying the presence of a less folded RNA (Figure 1C). A conformational shift between quadruplex and duplex structures was also identified by utilizing a cationic ion titration assay, demonstrating that DHX15 rG4 formation is induced by the addition of K+ and inhibited by Mg2+ (Figure 1D). Additionally, a 1D imino proton NMR spectrum of unlabeled DHX15 confirmed the formation of a hairpin-containing G-quadruplex structure in the RNA (Supplementary Figure 2).31 Finally, by mixing well-studied pan-G4 molecules (i.e., TMPyP4 and BRACO19) with the RNA, dose-dependent quenching of the probe fluorescence was observed, again confirming the formation of the rG4 (Supplementary Figure 3).42,43
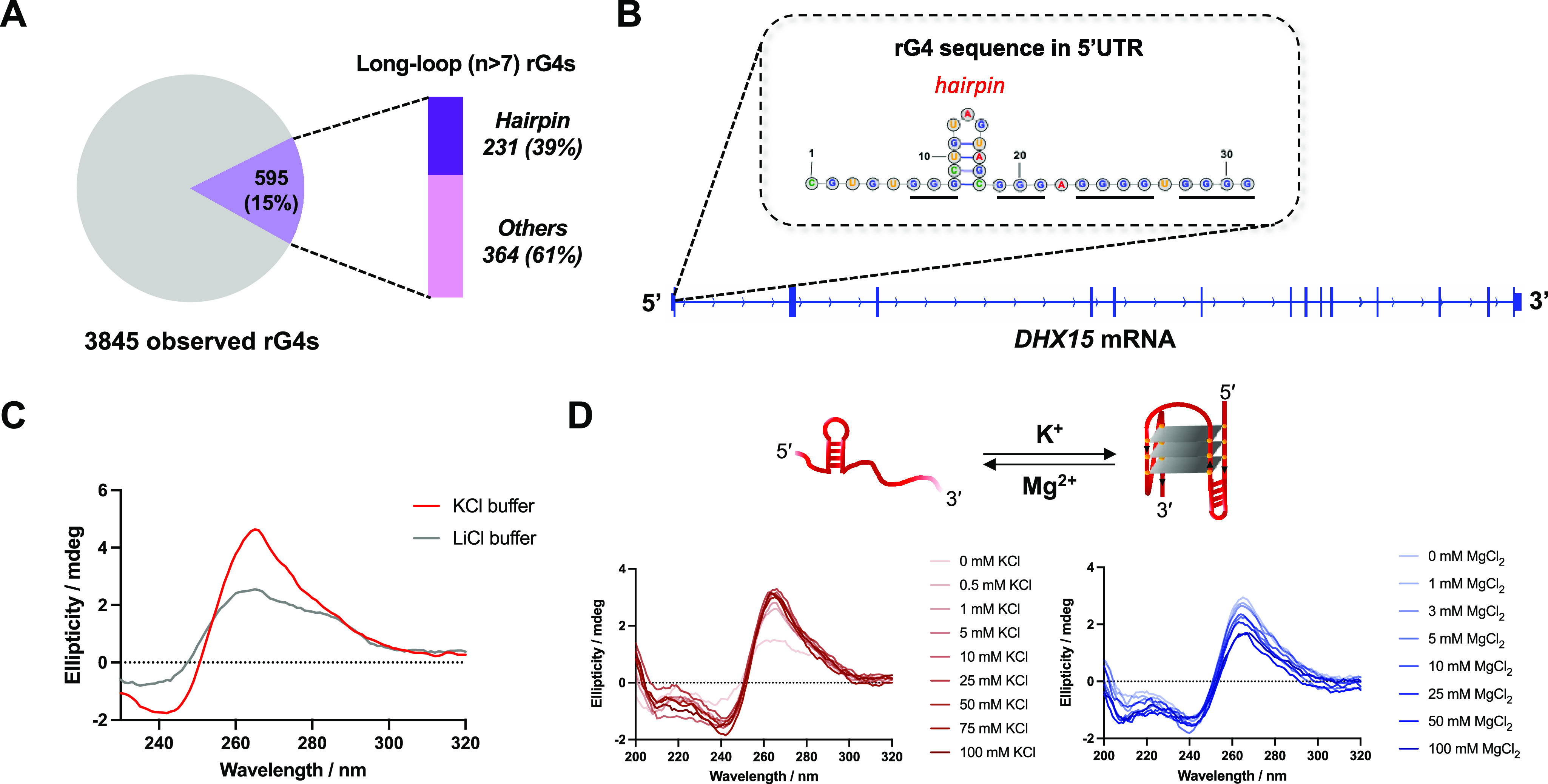
Having demonstrated folding of rG4 in vitro, a novel biochemically competitive high throughput SMM screen was performed to identify small molecule ligands that bind to rG4 (Figure 2A). SMM slides were fabricated as previously described,23 and a total of 14,802 compounds were screened. In one condition, slides were incubated with Cy5-DHX15 rG4. To identify compounds with a specific G4-binding mode, identical slides were incubated in parallel with both Cy5-DHX15 rG4 and BRACO19 (a well-known G4 binder selected as a control competitor). After incubation, scanning and data analysis revealed hits by quantification of fluorescence intensities of the spots on the slides. Z-scores were plotted on a 2D scatter plot, wherein the points in the highlighted area represent small molecules that were hits for DHX15 alone but not DHX15 incubated with the competing ligand, suggesting that these hits bind to the rG4 in the same manner as BRACO19 (Figure 2B). Among the screened compounds, 195 of them (hit rate: 1.3%) showed competitive behavior against BRACO19 (Supplementary File 2). In addition, hits were identified by comparing the Z-scores from the DHX15 rG4 screen with the Z-scores from a compiled data set of 23 other nucleic acid screens performed by our lab.25 By analyzing spot morphology and structural diversity, we recognized 17 compounds as competitive hits, yielding a final hit rate of 0.1%. Single-concentration screening by SPR and a fluorescence quenching assay were performed on commercial stocks of the hits to evaluate their binding ability toward the DHX15 rG4 (Supplementary Figure 4). Analysis of noncompetitive hits revealed that they were all promiscuous nucleic acid ligands in the SMM assay and were not studied further (Supplementary Figure 5). As a result, compound 11 was identified as the best binder following these assays, and it was chosen as our lead compound considering the observed competitive binding by BRACO19 (Z-scorerG4 = 3.9 vs Z-scorerG4+BRACO19 = 1.3) and Z-score profiling based on the compiled screening data set (Figure 1C,D).25,44 While unknown at the time of the screen, compound 11 was later found to be chemically unstable and prone to decomposition (Figure 3A and Supplementary Figure 6). We hypothesized that a decomposition product of compound 11 was responsible for the results of the screen, so we focused the rest of our study on fragments of compound 11.
Figure 2.
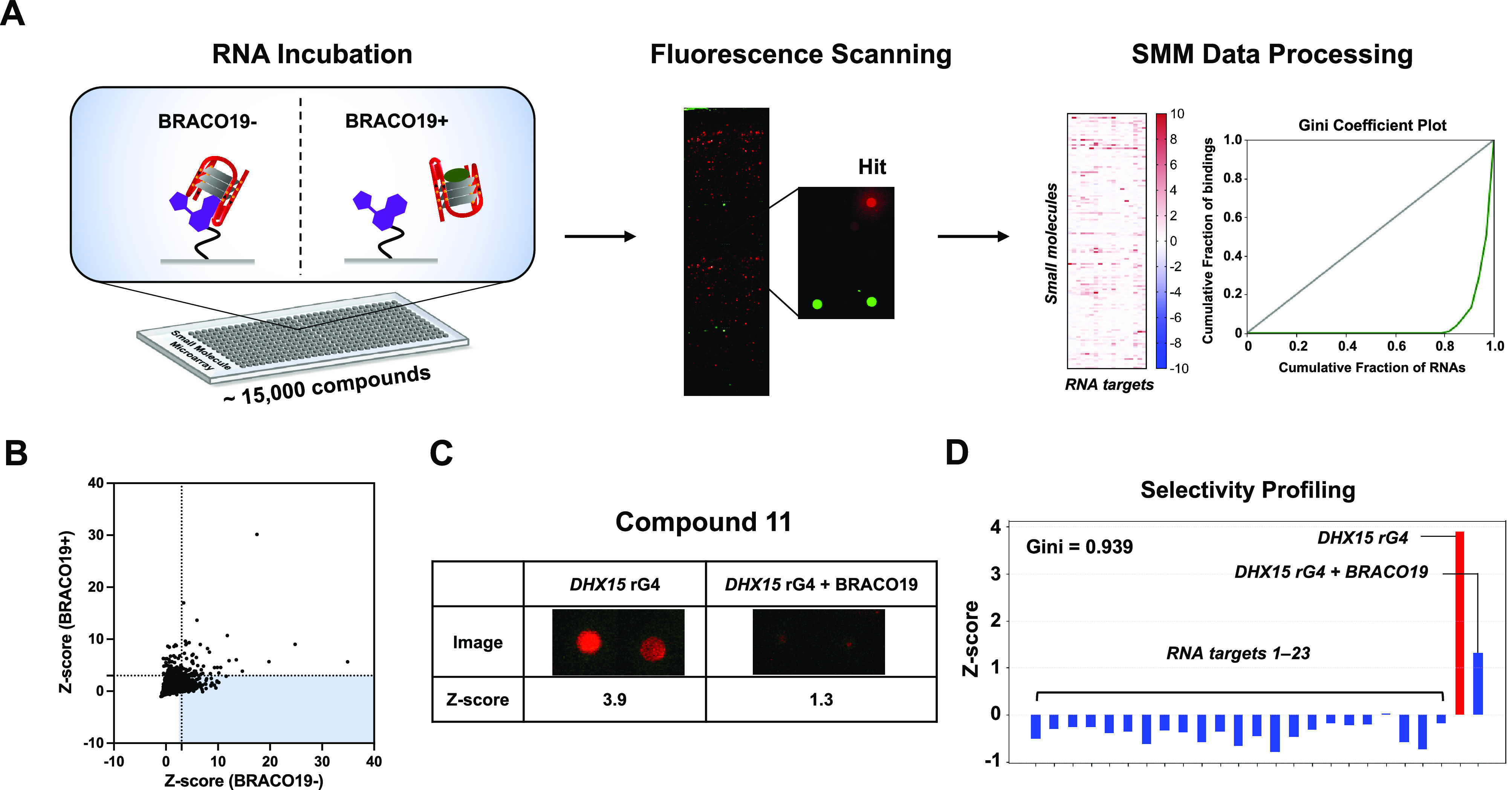
High-throughput screening using SMMs to identify DHX15 rG4 binders. (A) Schematic representation of the SMM workflow for an rG4 competitive microarray screening approach. The competitive screening strategy was used by incubating the RNA in the presence/absence of BRACO19. (B) Scatter plot of screening results showing the comparison of Z-scores in the presence/absence of BRACO19. (C) Identification of compound 11 as a BRACO19-competitive hit from SMM screening. (D) Z-score profiling of compound 11 based on the compiled screening data set.
Figure 3.
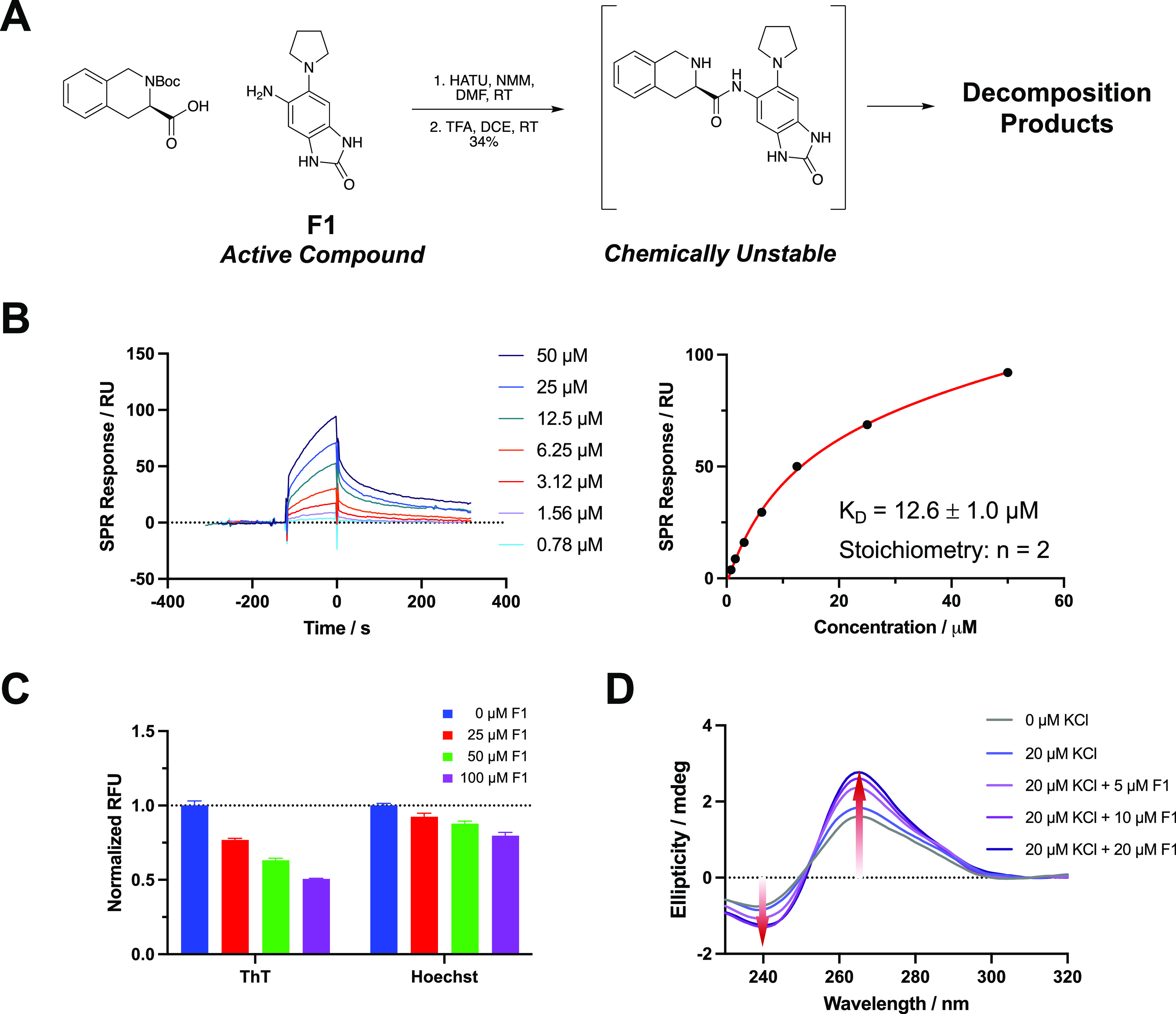
Identification of active compound via binding assays. (A) Chemical structure and synthetic preparation of compound 11 (observed to be chemically unstable). (B) Titration of F1 on SPR with dose-dependent binding curves and KD determination by fitting at steady state. (C) Dose-dependent FID assay using two fluorescent probes (ThT and Hoechst 33258). (D) CD spectra showing the DHX15 rG4 formation induced by addition of F1 at increasing concentrations.
To confirm the validity of the original hit, compound 11 was resynthesized to test in binding assays. Compound 11 was synthesized by first performing an amide coupling between a Boc-protected amino acid and the aniline F1 shown in Figure 3A. After deprotection and reverse phase purification, compound 11 was isolated with a 34% yield over two steps (Figure 3A). Upon preparation of stock solutions, it was observed that compound 11 readily decomposed in DMSO at room temperature upon standing. Liquid chromatography/mass spectrometry (LC/MS) revealed decomposition in solid and solution stocks of both the resynthesized and commercially available samples of compound 11 (Supplementary Figure 6). The rapid degradation of this compound, especially in solution, resulted in poor reaction yields (Figure 3A) and an inability of us to reliably test the compound in biophysical or biological assays. Although different samples provided different degradation mixtures, LC/MS analysis revealed that the masses for 11, F1, and F2 could be observed in all samples. Because the initial screen identified a competitive rG4 ligand with a good Z-score, we hypothesized that the original compound decomposed in such a way that a fragment of the compound remained immobilized to the slide and bound to the RNA during screening. Further, SPR assays were performed on pure compound 11 and a decomposed stock of compound 11, and a much higher binding signal was observed for the decomposed compound (Supplementary Figure 6).
As a starting point for the identification of the active fragment, we performed SPR on the compounds used to synthesize compound 11. The benzimidazole-2-one fragment (F1) and the R and S enantiomers of the deprotected tetrahydroisoquinoline (F2 and F3) were each evaluated for their ability to bind to the DHX15 rG4 (Supplementary Figure 7). Here, F1 displayed a strong binding signal and better binding kinetics than the other two fragments at 50 μM. By fitting the titration curves, F1 bound with a 2:1 stoichiometric ratio and had a KD of 12.6 ± 1.0 μM (Figure 3B), while F2 and F3 had much lower binding affinities (KD > 100 μM) (Supplementary Figure 8). Due to the observed binding activity of F1 to the rG4, we continued our study of this fragment alone.
Once we determined that F1 bound to the DHX15 rG4 by SPR, we focused on confirming its mode of binding. During screening, the original hit was biochemically competitive with by BRACO19, demonstrating that the hit was a G4 binder and likely stacked on the tetrads like BRACO19. To validate that our screen identified a lead compound that specifically stacks with the rG4, we performed an FID assay using ThT, a fluorescent G4-stacking compound. When F1 was titrated into solutions containing ThT and DHX15 rG4, ThT was displaced by up to 50%, indicating that F1 also stacks on the G4 tetrads (Figure 3C). In contrast, only ∼20% displacement was observed when 100 μM F1 was added to a solution of rG4 and Hoechst 33258, a fluorescent groove-binding ligand (Figure 3C). To further investigate the effect of F1 on rG4 folding, CD spectroscopy was performed. F1 was titrated into a solution of DHX15 RNA in a buffer containing 20 μM KCl, which meets the minimum requirement for rG4 folding. With increasing concentrations of F1, we observed the induction of rG4 formation, shown by the increased intensity of the characteristic spectrum (Figure 3D). This result suggests that F1 can shift the conformational equilibrium toward rG4 by binding and stabilizing its structure. Additionally, in a thermal melting assay by CD, stabilization of the DHX15 rG4 was observed in the presence of 50 μM F1 (ΔTm = 3.6 ± 0.3 °C) (Supplementary Figure 9). Taken together, these results suggest that our competitive HTS approach led to the identification of a ligand with the expected binding mode.
To briefly assess the target selectivity of our identified fragment, we compared the binding of F1 to DHX15 as well as five reported RNA/DNA constructs: KRAS s80 (three tandem G4s) and KRAS utr-z (single canonical G4), MYCN (hairpin-G4), U4/U6 snRNA (three-way junction and stem-loop), and dsDNA (duplex).45−47 Due to the hairpin-containing structural feature, we expected F1 to bind to DHX15 better than the nonhairpin-containing rG4s in KRAS transcript. We performed a direct binding assay on SPR using the three constructs and titrated in F1 at a starting concentration of 50 μM. As expected, we observed a much higher binding response for DHX15 rG4 compared to the other five RNA/DNA constructs. Notably, F1 showed a much higher binding affinity toward DHX15 than MYCN, a hairpin-G4 DNA, which was also reflected in SMM data. These results indicated a binding preference of F1 for DHX15 rG4 (Supplementary Figure 10).
During our bioinformatic search for a target, one reason we chose to pursue DHX15 was because rG4 is embedded in the 5′ UTR region, meaning it could potentially impact translation. To assess whether this rG4 has an impact on DHX15 translation, we designed two firefly luciferase reporter constructs, one containing the DHX15 rG4 (DHX15-G4-WT-FL) and one lacking the rG4 (DHX15-G4-Del-FL) (Figure 4A). Following plasmid construction and in vitro transcription, the resulting RNA constructs were tested in an in vitro translation assay. Each RNA was incubated with rabbit reticulocyte lysates, and then, firefly luciferase activity was measured for both constructs, with greater luciferase activity corresponding to greater translation. We found that, compared to the wild-type construct, the rG4 deletion construct showed double the luciferase activity (Figure 4B). This result indicates that the presence of the rG4 in the 5′ UTR of DHX15 inhibits translation, suggesting that small molecule ligands that stabilize rG4 could also act as inhibitors.
Figure 4.
Translation inhibition assay using an in vitro luciferase reporter system. (A) Schematic illustration of the DHX15 luciferase reporter system RNA constructs. (B) Relative luciferase activity of WT and G4 deletion RNA constructs. (C) Dose-dependent testing of F1 (μM) on translation inhibition of DHX15 mRNA. *P < 0.005, **P < 0.0005.
To ascertain the effect of our identified rG4-binding ligand on DHX15 mRNA translation, we evaluated F1 in the luciferase reporter system and performed a dose-dependent in vitro translation assay. As a result, treatment with F1 led to a dose-dependent response of translation inhibition (IC50 = 22.9 ± 3.8 μM), reaching a maximum inhibition value of 57% (Figure 4C and Supplementary Figure 11). In contrast, the translation efficiency of DHX15-G4-Del-FL was not significantly affected by the treatment with F1. BRACO19 was used as a positive control to compare its mode of inhibition to that of F1. At 10 μM, BRACO19 inhibited translation of the rG4-containing construct by ∼67% but did not inhibit translation of the deletion construct (Figure 4C). Additionally, a KRAS rG4 reporter construct representing a control rG4 without hairpin was treated with F1, and the translation was not affected (Supplementary Figure S12). Based on these data, we confirmed that F1 binds with a specific binding mode to the hairpin-containing rG4 in DHX15 and inhibits translation in a dose-dependent manner. Importantly, the compound also inhibits translation even in the presence of extensive rG4-forming sequences throughout the transcriptome during in vitro translation assays.
Here, we demonstrate a pipeline for the rapid discovery of rG4-targeting small molecules with defined binding modes by combining a bioinformatic approach, high-throughput SMM screening, and biophysical and functional assays. By utilizing a novel, biochemically competitive screening method, we identify compounds that bind to a newly characterized RNA target (DHX15 rG4) with a defined mode of interaction, streamlining the understanding of ligand binding and function properties.
In vitro translation assays demonstrate that this rG4 impacts DHX15 mRNA translation and can be modulated with small molecules. Still, it is important to note that considerable work is needed to evaluate the relevance of DHX15 to cancer growth and progression. While demonstrating potential, extensive work would be needed to evaluate the relevance of DHX15 to cancer and optimize compounds for improved binding affinity and efficacy. The unique structure of the DHX15 G4 (i.e., hairpin-quadruplex junction) could be leveraged in future ligand design efforts if informed by atomic resolution structural information about the mode of recognition of F1. These efforts could also potentially be informed by investigation of the noncompetitive hits from the screening. For example, it is already known that high-affinity, selective RNA binders can be developed via fragment-based strategies.48−50 While the current hit compound is likely not sufficient to be active in cellular assays, further optimization efforts as described above could yield a ligand suitable for evaluating activity in cells. Taken together, this work showcases a targeted RNA screening approach, offering valuable insights for future drug discovery efforts in RNA targeting beyond DHX15, exploring diverse RNA structures and shedding light on RNA as a potential therapeutic target for small molecules.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute (NCI), Center for Cancer Research, Project number Z01 BC011585 07 (PI, J. S. Schneekloth, Jr). We thank the members of the biophysics resource (Sergey G. Tarasov and Marzena Dyba) for helpful comments and suggestions on biophysical experiments. We thank Dongdong Wang from the NCI Molecular Targets Program for the help with the NMR experiment. This work also utilized the computational resources of the NIH HPC Biowulf cluster (http://hpc.nih.gov).
Glossary
Abbreviations
- DHX15
DEAH-box helicase 15
- SMM
small molecule microarray
- rG4
RNA G-quadruplex
- 5′-UTR
5′ untranslated region
- HTS
high-throughput screening
- BED
Browser Extendible Data
- SPR
surface plasmon resonance
- FID
fluorescent intercalator displacement
- CD
circular dichroism
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.3c00574.
Author Contributions
All authors have given approval to the final version of the manuscript. Conceived and designed experiments: J.S.S, P.R.P., M.Y., G.V.L., S.B., and K.Y. Designed compounds or contributed to synthetic route design: J.S.S. and P.R.P. Analyzed data: P.R.P., M.Y., G.V.L., S.B., and K.Y. Wrote/edited paper: J.S.S., P.R.P., M.Y., and S.B.
The authors declare no competing financial interest.
Supplementary Material
References
- Childs-Disney J. L.; Yang X.; Gibaut Q. M. R.; Tong Y.; Batey R. T.; Disney M. D. Targeting RNA structures with small molecules. Nat. Rev. Drug Discov 2022, 21 (10), 736–762. 10.1038/s41573-022-00521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An integrated encyclopedia of DNA elements in the human genome. Nature 2012, 489 (7414), 57–74. 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clamp M.; Fry B.; Kamal M.; Xie X.; Cuff J.; Lin M. F.; Kellis M.; Lindblad-Toh K.; Lander E. S. Distinguishing protein-coding and noncoding genes in the human genome. Proc. Natl. Acad. Sci. U. S. A. 2007, 104 (49), 19428–19433. 10.1073/pnas.0709013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins A. L.; Groom C. R. The druggable genome. Nat. Rev. Drug Discov 2002, 1 (9), 727–730. 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- Winkle M.; El-Daly S. M.; Fabbri M.; Calin G. A. Noncoding RNA therapeutics - challenges and potential solutions. Nat. Rev. Drug Discov 2021, 20 (8), 629–651. 10.1038/s41573-021-00219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner K. D.; Hajdin C. E.; Weeks K. M. Principles for targeting RNA with drug-like small molecules. Nat. Rev. Drug Discov 2018, 17 (8), 547–558. 10.1038/nrd.2018.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavita K.; Breaker R. R. Discovering riboswitches: the past and the future. Trends Biochem. Sci. 2023, 48 (2), 119–141. 10.1016/j.tibs.2022.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt W. M.; Calabrese D. R.; Schneekloth J. S. Jr Evidence for ligandable sites in structured RNA throughout the Protein Data Bank. Bioorg. Med. Chem. 2019, 27 (11), 2253–2260. 10.1016/j.bmc.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson S. W.; Turner A. W.; Arney J. W.; Saleem I.; Weidmann C. A.; Margolis D. M.; Weeks K. M.; Mustoe A. M. Discovery of a large-scale, cell-state-responsive allosteric switch in the 7SK RNA using DANCE-MaP. Mol. Cell 2022, 82 (9), 1708–1723.E10. 10.1016/j.molcel.2022.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon E. M.; Qiu Y.; Ghanbari Niaki A.; McLaughlin G. A.; Weidmann C. A.; Gerbich T. M.; Smith J. A.; Crutchley J. M.; Termini C. M.; Weeks K. M.; et al. mRNA structure determines specificity of a polyQ-driven phase separation. Science 2018, 360 (6391), 922–927. 10.1126/science.aar7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoetzel J.; Suess B. Structural Changes in Aptamers are Essential for Synthetic Riboswitch Engineering. J. Mol. Biol. 2022, 434 (18), 167631. 10.1016/j.jmb.2022.167631. [DOI] [PubMed] [Google Scholar]
- Chen J. L.; Zhang P.; Abe M.; Aikawa H.; Zhang L.; Frank A. J.; Zembryski T.; Hubbs C.; Park H.; Withka J.; et al. Design, Optimization, and Study of Small Molecules That Target Tau Pre-mRNA and Affect Splicing. J. Am. Chem. Soc. 2020, 142 (19), 8706–8727. 10.1021/jacs.0c00768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J. W.; Busa V. F.; Shao Y.; Leung A. K. L. Structure-Mediated RNA Decay by UPF1 and G3BP1. Mol. Cell 2020, 78 (1), 70–84.E6. 10.1016/j.molcel.2020.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disney M. D. Targeting RNA with Small Molecules To Capture Opportunities at the Intersection of Chemistry, Biology, and Medicine. J. Am. Chem. Soc. 2019, 141 (17), 6776–6790. 10.1021/jacs.8b13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly C. M.; Numata T.; Boer R. E.; Moon M. H.; Sinniah R. S.; Barchi J. J.; Ferre-D’Amare A. R.; Schneekloth J. S. Jr Synthetic ligands for PreQ(1) riboswitches provide structural and mechanistic insights into targeting RNA tertiary structure. Nat. Commun. 2019, 10 (1), 1501. 10.1038/s41467-019-09493-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte M. A.; Bolduc F.; Vannutelli A.; Mitteaux J.; Monchaud D.; Perreault J. P. Development of a highly optimized procedure for the discovery of RNA G-quadruplexes by combining several strategies. Biochimie 2023, 214 (Pt A), 24–32. 10.1016/j.biochi.2023.07.014. [DOI] [PubMed] [Google Scholar]
- Katsuda Y.; Sato S. I.; Inoue M.; Tsugawa H.; Kamura T.; Kida T.; Matsumoto R.; Asamitsu S.; Shioda N.; Shiroto S.; et al. Small molecule-based detection of non-canonical RNA G-quadruplex structures that modulate protein translation. Nucleic Acids Res. 2022, 50 (14), 8143–8153. 10.1093/nar/gkac580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaratnam S.; Torrey Z. R.; Calabrese D. R.; Banco M. T.; Yazdani K.; Liang X.; Fullenkamp C. R.; Seshadri S.; Holewinski R. J.; Andresson T.; et al. Investigating the NRAS 5′ UTR as a target for small molecules. Cell Chem. Biol. 2023, 30 (6), 643–657.E8. 10.1016/j.chembiol.2023.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L.; Velema W. A.; Lee Y.; Xiao L.; Mohsen M. G.; Kietrys A. M.; Kool E. T. Pervasive transcriptome interactions of protein-targeted drugs. Nat. Chem. 2023, 15, 1374. 10.1038/s41557-023-01309-8. [DOI] [PubMed] [Google Scholar]
- Vasilyev N.; Polonskaia A.; Darnell J. C.; Darnell R. B.; Patel D. J.; Serganov A. Crystal structure reveals specific recognition of a G-quadruplex RNA by a beta-turn in the RGG motif of FMRP. Proc. Natl. Acad. Sci. U. S. A. 2015, 112 (39), E5391–E5400. 10.1073/pnas.1515737112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpster C.; Boyle E.; Musier-Forsyth K.; Kankia B. HIV-1 genomic RNA U3 region forms a stable quadruplex-hairpin structure. Biophys Chem. 2021, 272, 106567. 10.1016/j.bpc.2021.106567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haniff H. S.; Knerr L.; Chen J. L.; Disney M. D.; Lightfoot H. L. Target-Directed Approaches for Screening Small Molecules against RNA Targets. SLAS Discov 2020, 25 (8), 869–894. 10.1177/2472555220922802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly C. M.; Abulwerdi F. A.; Schneekloth J. S. Jr Discovery of RNA Binding Small Molecules Using Small Molecule Microarrays. Methods Mol. Biol. 2017, 1518, 157–175. 10.1007/978-1-4939-6584-7_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan D.; Yang M.; Schneekloth J. S. Jr. Three-Color Imaging Enables Simultaneous Screening of Multiple RNA Targets on Small Molecule Microarrays. Curr. Protoc Chem. Biol. 2020, 12 (4), e87 10.1002/cpch.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdani K.; Jordan D.; Yang M.; Fullenkamp C. R.; Calabrese D. R.; Boer R.; Hilimire T.; Allen T. E. H.; Khan R. T.; Schneekloth J. S. Jr Machine Learning Informs RNA-Binding Chemical Space. Angew. Chem., Int. Ed. Engl. 2023, 62 (11), e202211358 10.1002/anie.202211358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.; Cheema J.; Zhang Y.; Deng H.; Duncan S.; Umar M. I.; Zhao J.; Liu Q.; Cao X.; Kwok C. K.; Ding Y. RNA G-quadruplex structures exist and function in vivo in plants. Genome Biol. 2020, 21 (1), 226. 10.1186/s13059-020-02142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney D.; Spiegel J.; Zyner K.; Tannahill D.; Balasubramanian S. The regulation and functions of DNA and RNA G-quadruplexes. Nat. Rev. Mol. Cell Biol. 2020, 21 (8), 459–474. 10.1038/s41580-020-0236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok C. K.; Marsico G.; Sahakyan A. B.; Chambers V. S.; Balasubramanian S. rG4-seq reveals widespread formation of G-quadruplex structures in the human transcriptome. Nat. Methods 2016, 13 (10), 841–844. 10.1038/nmeth.3965. [DOI] [PubMed] [Google Scholar]
- Kwok C. K.; Balasubramanian S. Targeted Detection of G-Quadruplexes in Cellular RNAs. Angew. Chem., Int. Ed. Engl. 2015, 54 (23), 6751–6754. 10.1002/anie.201500891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovskaya E.; Heddi B.; Bakalar B.; Richter S. N.; Phan A. T. Major G-Quadruplex Form of HIV-1 LTR Reveals a (3 + 1) Folding Topology Containing a Stem-Loop. J. Am. Chem. Soc. 2018, 140 (42), 13654–13662. 10.1021/jacs.8b05332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T. Q. N.; Lim K. W.; Phan A. T. A Dual-Specific Targeting Approach Based on the Simultaneous Recognition of Duplex and Quadruplex Motifs. Sci. Rep 2017, 7 (1), 11969. 10.1038/s41598-017-10583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H.; Kwok C. K. Spectroscopic analysis reveals the effect of hairpin loop formation on G-quadruplex structures. RSC Chem. Biol. 2022, 3 (4), 431–435. 10.1039/D2CB00045H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnsack K. E.; Kanwal N.; Bohnsack M. T. Prp43/DHX15 exemplify RNA helicase multifunctionality in the gene expression network. Nucleic Acids Res. 2022, 50 (16), 9012–9022. 10.1093/nar/gkac687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detanico T.; Virgen-Slane R.; Steen-Fuentes S.; Lin W. W.; Rhode-Kurnow A.; Chappell E.; Correa R. G.; DiCandido M. J.; Mbow M. L.; Li J.; Ware C. F. Co-expression Networks Identify DHX15 RNA Helicase as a B Cell Regulatory Factor. Front Immunol 2019, 10, 2903. 10.3389/fimmu.2019.02903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing Y.; Nguyen M. M.; Wang D.; Pascal L. E.; Guo W.; Xu Y.; Ai J.; Deng F. M.; Masoodi K. Z.; Yu X.; et al. DHX15 promotes prostate cancer progression by stimulating Siah2-mediated ubiquitination of androgen receptor. Oncogene 2018, 37 (5), 638–650. 10.1038/onc.2017.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.; Chen X.; Pan L.; Huang Y.; Cai Y.; Li J.; Li Y.; Wang S. RNA helicase DHX15 decreases cell apoptosis by NF-kappaB signaling pathway in Burkitt lymphoma. Cancer Cell Int. 2022, 22 (1), 92. 10.1186/s12935-021-02426-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L.; Li Y.; Zhang H. Y.; Zheng Y.; Liu X. L.; Hu Z.; Wang Y.; Wang J.; Cai Y. H.; Liu Q.; et al. DHX15 is associated with poor prognosis in acute myeloid leukemia (AML) and regulates cell apoptosis via the NF-kB signaling pathway. Oncotarget 2017, 8 (52), 89643–89654. 10.18632/oncotarget.20288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y.; Song Q.; Pascal L. E.; Zhong M.; Zhou Y.; Zhou J.; Deng F. M.; Huang J.; Wang Z. DHX15 is up-regulated in castration-resistant prostate cancer and required for androgen receptor sensitivity to low DHT concentrations. Prostate 2019, 79 (6), 657–666. 10.1002/pros.23773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadrick W. R.; Ndjomou J.; Kolli R.; Mukherjee S.; Hanson A. M.; Frick D. N. Discovering new medicines targeting helicases: challenges and recent progress. J. Biomol Screen 2013, 18 (7), 761–781. 10.1177/1087057113482586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K.; Nakano K.; Shimizu T.; Ohto U. The crystal structure of human DEAH-box RNA helicase 15 reveals a domain organization of the mammalian DEAH/RHA family. Acta Crystallogr. F Struct Biol. Commun. 2017, 73 (6), 347–355. 10.1107/S2053230X17007336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari S.; Bugaut A.; Huppert J. L.; Balasubramanian S. An RNA G-quadruplex in the 5′ UTR of the NRAS proto-oncogene modulates translation. Nat. Chem. Biol. 2007, 3 (4), 218–221. 10.1038/nchembio864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le V. H.; Nagesh N.; Lewis E. A. Bcl-2 promoter sequence G-quadruplex interactions with three planar and non-planar cationic porphyrins: TMPyP4, TMPyP3, and TMPyP2. PLoS One 2013, 8 (8), e72462 10.1371/journal.pone.0072462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar O. R.; Vegh A.; Somkuti J.; Smeller L. Characterization of a G-quadruplex from hepatitis B virus and its stabilization by binding TMPyP4, BRACO19 and PhenDC3. Sci. Rep 2021, 11 (1), 23243. 10.1038/s41598-021-02689-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursu A.; Childs-Disney J. L.; Angelbello A. J.; Costales M. G.; Meyer S. M.; Disney M. D. Gini Coefficients as a Single Value Metric to Define Chemical Probe Selectivity. ACS Chem. Biol. 2020, 15 (8), 2031–2040. 10.1021/acschembio.0c00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miglietta G.; Cogoi S.; Marinello J.; Capranico G.; Tikhomirov A. S.; Shchekotikhin A.; Xodo L. E. RNA G-Quadruplexes in Kirsten Ras (KRAS) Oncogene as Targets for Small Molecules Inhibiting Translation. J. Med. Chem. 2017, 60 (23), 9448–9461. 10.1021/acs.jmedchem.7b00622. [DOI] [PubMed] [Google Scholar]
- Yang M.; Carter S.; Parmar S.; Bume D. D.; Calabrese D. R.; Liang X.; Yazdani K.; Xu M.; Liu Z.; Thiele C. J.; Schneekloth J. S. Targeting a noncanonical, hairpin-containing G-quadruplex structure from the MYCN gene. Nucleic Acids Res. 2021, 49 (14), 7856–7869. 10.1093/nar/gkab594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornilescu G.; Didychuk A. L.; Rodgers M. L.; Michael L. A.; Burke J. E.; Montemayor E. J.; Hoskins A. A.; Butcher S. E. Structural Analysis of Multi-Helical RNAs by NMR-SAXS/WAXS: Application to the U4/U6 di-snRNA. J. Mol. Biol. 2016, 428 (5), 777–789. 10.1016/j.jmb.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller M. J.; Favorov O.; Li K.; Nuthanakanti A.; Hussein D.; Michaud A.; Lafontaine D. A.; Busan S.; Serganov A.; Aube J.; Weeks K. M. SHAPE-enabled fragment-based ligand discovery for RNA. Proc. Natl. Acad. Sci. U. S. A. 2022, 119 (20), e2122660119 10.1073/pnas.2122660119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist K. P.; Panchal V.; Gotfredsen C. H.; Brenk R.; Clausen M. H. Fragment-Based Drug Discovery for RNA Targets. ChemMedChem. 2021, 16 (17), 2588–2603. 10.1002/cmdc.202100324. [DOI] [PubMed] [Google Scholar]
- Suresh B. M.; Taghavi A.; Childs-Disney J. L.; Disney M. D. Fragment-Based Approaches to Identify RNA Binders. J. Med. Chem. 2023, 66 (10), 6523–6541. 10.1021/acs.jmedchem.3c00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.