Abstract
BACKGROUND:
The World Health Organization Classification (WHO) of Urinary and Male Genital Tumors has recently been updated to its 5th edition. The new edition presents a comprehensive approach to the classification of urinary and male genital tumors with an incorporation of morphologic, clinical, and genomic data.
OBJECTIVE:
This review aims to update the new classification of bladder cancer in the 5th edition and to highlight important changes in nomenclatures, diagnostic criteria, and molecular characterization, as compared to the 4th edition.
METHODS:
The pathologic classification of bladder cancer in the 5th edition of WHO Classification of Urinary and Male Genital Tumours was compared to that in the 4th edition. PubMed was searched using key words, including bladder cancer, WHO 1973, WHO 1998, WHO 2004, WHO 2016, histology, pathology, genomics, and molecular classification in the time frame from 1973 to August of 2022. Other relevant papers were also consulted, resulting in the selection of 81 papers as references.
RESULTS:
The binary grading of papillary urothelial carcinoma (UC) is practical, but it may be oversimplified and contribute to “grade migration” in recent years. An arbitrary cutoff (5%) has been proposed for bladder cancers with mixed grades. The diagnosis of papillary urothelial neoplasm with low malignant potential has been dramatically reduced in recent years because of overlapping morphology and treatment with low-grade papillary UC. An inverted growth pattern should be distinguished from true (or destructive) stromal invasion in papillary UC. Several methods have been proposed for pT1 tumor substaging, but it is often challenging to substage pT1 tumors in small biopsy specimens. Bladder UC shows a high tendency for divergent differentiation, leading to several distinct histologic subtypes associated with an aggressive clinical behavior. Molecular classification based on the genomic analysis may be a useful tool in the stratification of patients for optimal treatment.
CONCLUSIONS:
The 5th edition of WHO Classification of Urinary and Male Genital Tumours has made several significant changes in the classification of bladder cancer. It is important to be aware of these changes and to incorporate them into routine clinical practice.
Keywords: Bladder cancer, WHO, urothelial carcinoma, grading, staging, heterogeneity, molecular classification, histologic subtypes
INTRODUCTION
Bladder cancer is a common malignancy with a global incidence of 573, 278 new cases in 2020, representing 3% of all human cancers [1]. The incidence of bladder cancer is four times higher in men than in women, making it the 6th most common cancer in men [2, 3]. Bladder cancer is generally more prevalent in developed countries compared to developing nations [4]. In the United States, bladder cancer is the 4th most common cancer in men affecting 81,180 new patients per year and causing 17,100 deaths in 2021 [3]. The most common type of bladder cancers is urothelial carcinoma (UC), which represents more than 90% of all bladder cancers in the Western countries. Bladder UCs originate from precursor lesions in the urothelium and progress along dual-track, referred to as papillary and non-papillary, which leads to clinically and morphologically different forms of the disease [5, 6]. The classification of bladder cancer has undergone several modifications in recent years [7–11], incorporating new molecular and genomic data into the classification scheme which holds the promise to improve the diagnosis, treatment, and prognosis of patients affected by this disease [12–14].
The 5th edition of World Health Organization (WHO) Classification of the Urinary and Male Genital Tumours provides a timely update on the pathology and genomics of neoplastic diseases in the bladder [15]. The time interval between the 5th edition and the 4th edition is 6 years, only half of that between the 4th edition and 3rd edition [7, 10, 16]. Nonetheless, there have been significant new advancements in the histology and genomics of bladder cancer which have been included in this edition. In this review, we will emphasize new approaches to the diagnosis, nomenclature, cancer grading, and molecular features of urothelial tumors. Non-urothelial tumors, neuroendocrine, mesenchymal, and other neoplastic diseases are beyond the scope of this summary.
METHODS
The classification of bladder cancer in the 5th edition of WHO Classification of Urinary and Male Genital Tumours was compared to that in the 4th edition, which revealed several significant changes, including cancer grading, histologic subtypes, and molecular classification based on genomic analysis. Literature search performed in Pubmed using the key words, including bladder cancer, WHO 1973, WHO 1998, WHO 2004, WHO 2016, histology, pathology, grading, staging, T1 substaging, histologic variants or subtypes, genomic analysis, and molecular classification in the time frame from 1973 to August of 2022. A total of 81 related papers and publications were selected as references.
RESULTS
Grading of papillary urothelial carcinoma
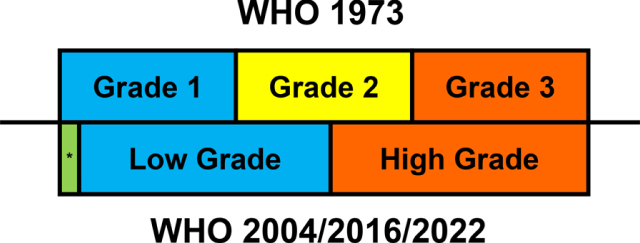
Papillary urothelial carcinoma (UC) exhibits a continuous spectrum of cytological atypia and architectural disorder on a scale from low grade tumors resembling normal urothelium to high grade tumors with pronounced cytoarchitectural atypia. Grading is the most important factor in the treatment decision for patients with noninvasive papillary UC [16, 17], which is largely based on the degree of cytological atypia of the urothelium lining fibrovascular cores, such as nuclear enlargement, pleomorphism, hyperchromasia, coarse chromatin, prominent nucleoli, irregular nuclear contours, and frequent mitoses. Architectural disorders, including complex papillae showing frequent fusion and branching as well as disorderly orientation of tumor cells along the papillae (or loss of polarity), are also incorporated into the grading criteria. Several grading systems have been proposed by various organizations since the introduction of the WHO 1973 three-tiered numeric grading system (Fig. 1) [7, 8, 10, 16]. These grading systems can effectively assess the risk of cancer progression and recurrence in papillary UC, but they have considerable interobserver variability due to the presence of overlapping morphologic features in different grades [18–20].
Fig. 1.
Correlation among different WHO grading systems of papillary urothelial carcinoma. The 1973 system uses a 3-tier numeric grading, and the 2004/2016/2022 system uses a binary grading. While low grade tumors include most of grade 1 tumors and grade 2 tumors with relatively less atypia, high grade tumors include all grade 3 tumors and grade 2 tumors with more atypia. * Papillary urothelial neoplasm of low malignant potential is a separate entity from papillary UC and corresponds to the very low end of grade 1 tumors.
The binary grading of papillary UC (low grade vs high grade) continues to be used in the 5th edition of WHO classification. Since it was first proposed in 1998, the binary grading system has been adopted by the WHO in the 3rd and 4th editions as well [7, 8, 10, 16]. Low-grade papillary UC shows mild cytoarchitectural atypia, while high-grade papillary UC exhibits severe cytoarchitectural atypia. An advantage of the binary grading system is that the diagnosis of high-grade papillary UC correlates well with positive urine cytology [21]. In addition, it correlates well with the concept of bladder cancer development along dual papillary/non-papillary track [5, 22]. However, the distinction between low- and high-grade tumors remains somewhat subjective, as similar to previous editions, with the cutoff between mild and severe atypia not clearly defined. Therefore, it is difficult to determine whether papillary UC with moderate or borderline atypia belongs to the low-grade or high-grade group. Although this binary grading system is less prone to interobserver variability than a three- or four-tier grading systems, it oversimplifies the complexity of the grading, because papillary UC shows a continuous spectrum of cytoarchitectural atypia. As the spectrum of changes within one grade is wide in the binary grading system, tumors at different ends of the same grade may have different biologic behaviors and inconsistent clinical outcomes.
Since the introduction of this binary grading system, there has been a significant “grade migration” from low-grade to high-grade in the diagnosis of papillary UC [23, 24]. Pathologists diagnose papillary UC as high-grade at a significantly increased frequency, while the diagnosis of low-grade papillary UC is correspondingly decreasing. However, the “grading migration” does not seem to correlate with disease progression and outcomes in clinical analysis [24]. This grade migration has a significant impact on clinical management, since low-grade and high-grade papillary UC are managed differently. As the cancer grading system is based on a subjective visual analysis of morphology, it needs to be revised based on scientific evidence and validated by independent studies on large patient cohorts. In addition to histologic grades, the risk for cancer recurrence and progression is also related to several other factors, such as tumor size, multifocality, history of prior recurrence, and intravesical therapy [17]. Ancillary studies, such as immunohistochemical (IHC) and molecular tests, may improve the grading reproducibility and lead to a better correlation with clinical outcomes [25, 26]. Mutations in TP53 gene and allelic loss of chromosome 9, particularly in the CDKN2A locus, are common findings in high-grade papillary UC [27]. By IHC, high-grade tumors are often associated with loss of CD44 and increased proliferation activity (e.g. Ki-67 index >5%) [26, 28]. Overall, cancer grading approach is based on microscopic morphology, and the incorporation of ancillary markers is not generally advocated in routine pathology practice.
Papillary urothelial carcinoma with mixed grades
Heterogeneity of cancer grade is a common feature in papillary UC, which occurs in as many as one third of papillary tumors (Fig. 2) [29–31]. Most papillary UCs with mixed grades have a considerable high-grade component (>10%), and these tumors show similar clinical outcomes to those with pure high-grade. However, several studies have demonstrated that papillary tumors with only a minor high-grade component are associated with clinical outcome similar to that of low-grade papillary UC [30–32]. Different thresholds are used to define a minor high-grade component in papillary UC with mixed grades, which may lead to a poor interobserver reproducibility and contribute to “grade migration” [30–32]. In the 5th edition, 5% of the high-grade component is recommended as the cutoff to define the overall grade in papillary UC with mixed grades. The papillary tumors with <5% of high-grade component are classified as “predominantly low-grade with a minor high-grade component”, while those with ≥5% of high-grade component are classified as high-grade tumors. This approach may aid the risk stratification and help optimize the management of patients with tumors exhibiting different grades. Interestingly, the Genitourinary Pathology Society (GUPS) recently recommended a different cutoff for papillary UC with mixed grades [33]. When the high-grade component accounts for <10% in papillary UC with mixed grades, a diagnosis of “noninvasive low-grade papillary UC with a focal (<10%) noninvasive higher grade component” should be rendered. In addition, it is recommended to add a comment that “There is limited data on the prognostic significance of a minor component of high-grade tumor in an otherwise lower grade carcinoma, and the studies suggest that they generally behave more like low-grade tumors.” Further large prospective studies are needed to determine the significance of the extent of a high-grade component in a predominantly low-grade tumor to predict its clinical behavior.
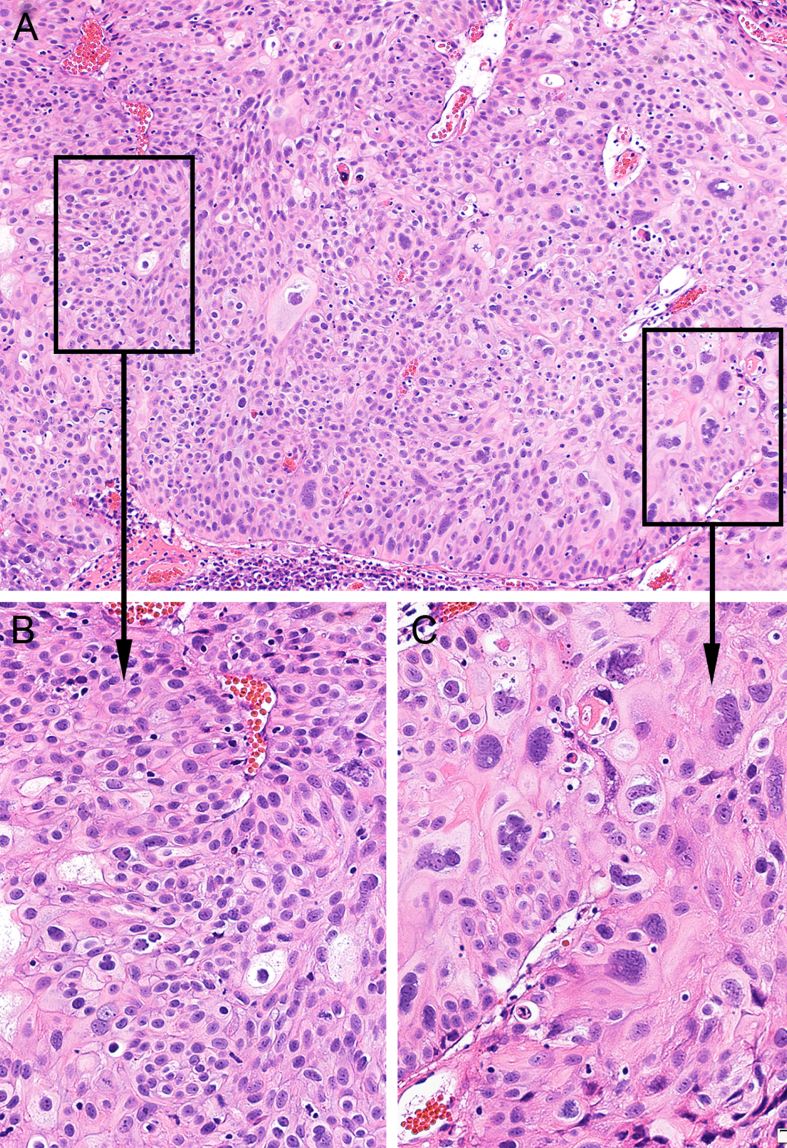
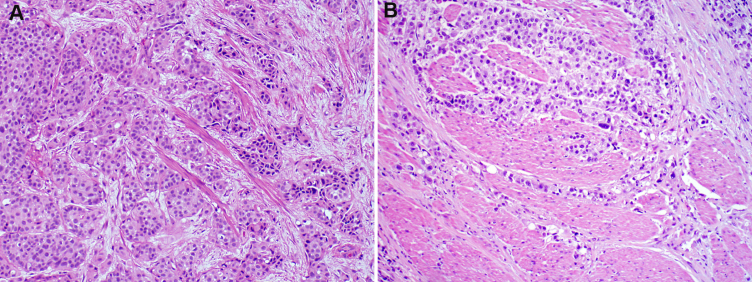
Fig. 2.
Papillary urothelial carcinoma mixed grades. A. The tumor shows predominantly low-grade features with focal high-grade (×100). B. Low-grade component shows mild to moderate cytologic atypia (×200). C. High-grade component shows severe cytologic atypia (×400).
Papillary urothelial neoplasm with low malignant potential
Papillary urothelial neoplasm of low malignant potential (PUNLMP) is retained as a distinct diagnostic category in the 5th edition. PUNLMP is characterized by papillary fibrovascular structures lined by thickened urothelium that lacks discernible cytological atypia (Fig. 3) [34]. Although the urothelial lining appears thicker or more cellular than normal urothelium, it has no loss of cellular polarity. Occasionally, PUNLMP may demonstrate an inverted growth pattern [35]. Several studies have shown that PUNLMP has a lower risk of cancer recurrence and progression than low-grade papillary UC [34–36]. The nomenclature of PUNLM can avoid the “carcinoma” label on patients with such an indolent tumor, but PUNLMP should be followed in the same manner as low-grade papillary UC, as it still carries a low risk for cancer recurrence. However, it may be difficult to differentiate PUNLMP from low-grade papillary UC even among experienced urologic pathologists [37, 38]. One recent study has shown that the pathologic diagnosis of PUNLMP has been significantly decreased in recent years from 31.3% in 1990–2000 to 3.2% in 2000–2010 to 1.1% in 2010–2018 [39]. The treatment and follow-up guidelines for PUNLMP and low-grade papillary UC are not dissimilar in major urological societies [40, 41], suggesting that PUNLMP may be incorporated into low-grade papillary UC as one category [42].
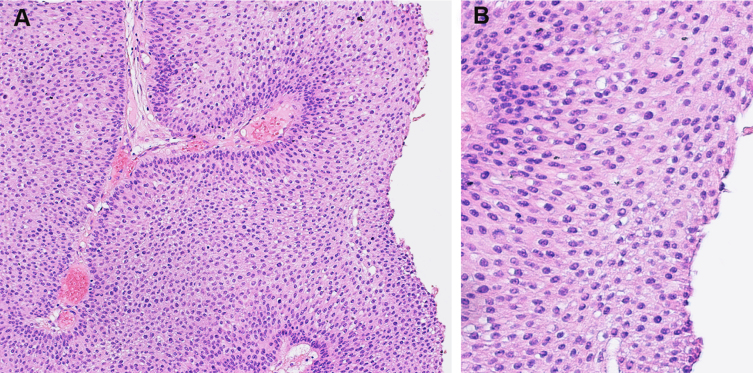
Fig. 3.
Papillary urothelial neoplasm of low malignant potential. A. The overlying urothelium is thickened (×100). B. The urothelium shows minimal cytologic atypia (×200).
Papillary urothelial neoplasms with an inverted growth pattern
Papillary UC sometimes show an inverted growth pattern, which is characterized by invagination of tumor cells into the lamina propria forming large nests with broad pushing borders (Fig. 4). The stromal involvement does not reach the muscularis propria (MP), unlike nested subtype UC which is usually deeply invasive into the MP. Sometimes it may be difficult to distinguish papillary UC with the inverted growth pattern from invasive UC. The inverted growth pattern shows large nests with broad, smooth, pushing borders and retains the basement membrane around them. Invasive UC is characterized by small and irregularly shaped nests. Furthermore, invasive UC often induces stromal reactive changes, such as retraction artefact, paradoxical differentiation, and desmoplasia. Papillary urothelial tumors with an inverted growth pattern exhibit a wide spectrum of morphologic and cytologic features [43]. In the 5th edition, the diagnosis of inverted urothelial papilloma is generally reserved for those with almost exclusively inverted morphology. In papillary UC, the inverted growth pattern is typically coexistent with the exophytic papillary pattern. When the inverted pattern is prominent (>80%) or exclusive, the designation of “noninvasive papillary UC with an inverted growth pattern” may be used, distinguishing such tumors from invasive UC [16, 33].
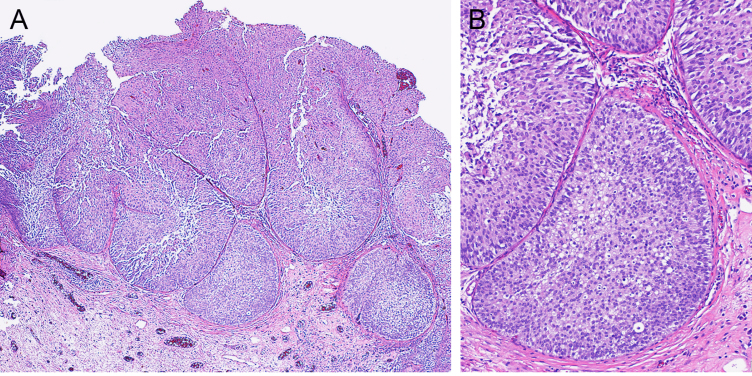
Fig. 4.
Papillary urothelial carcinoma shows an inverted growth pattern. A. The tumor shows large nests with broad pushing borders in the lamina propria (×40). B. The tumor shows low-grade features (×100).
Flat urothelial lesions
Urothelial carcinoma in situ (UCIS) is the only flat neoplastic entity that is recognized in the 5th edition. UCIS shows severe cytoarchitectural atypia like that in high-grade papillary UC except for papillary formation. These atypical features are usually easily identified at a low to intermediate magnification. UCIS shows several morphologic patterns, such as large cell, small cell, plasmacytoid, pagetoid, and clinging. The presence of these patterns does not have significant clinical implications, except for the plasmacytoid which is associated with discontinuous involvement of the urothelium (Fig. 5) [44, 45]. Rare cases of UCIS with in situ glandular differentiation (adenocarcinoma in situ) have been reported [46]. On IHC, UCIS often shows abnormal full-thickness immunoreactivity for CK20, increased expression of p53, and decreased expression of CD44 [26]. Other markers, such as CK5/6 and Ki-67, may also have some utility in the distinction between CIS and reactive urothelial atypia [26, 45]. However, none of these IHC markers is highly sensitive or specific, especially in equivocal lesions. Overall, histology remains the diagnostic gold standard for UCIS and routine use of IHC is not recommended.
Fig. 5.
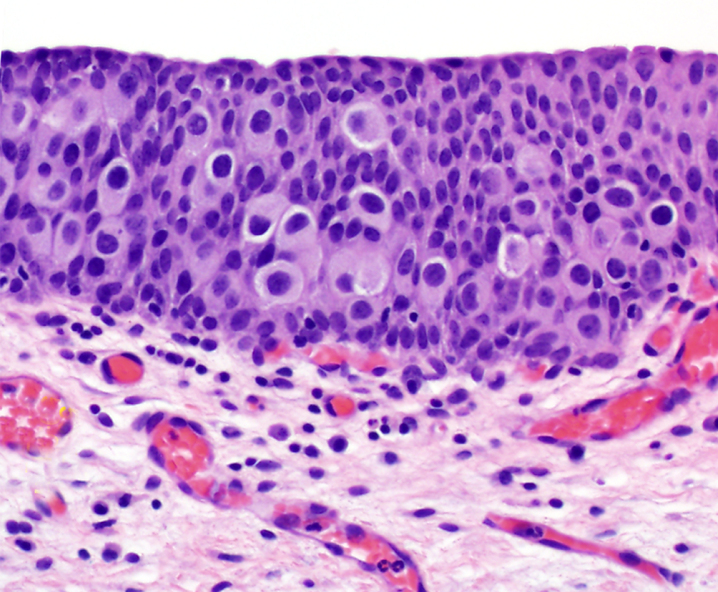
Urothelial carcinoma in situ shows a pagetoid growth pattern (×200).
Several other flat lesions were described in the 4th edition, but they are not recognized as distinct neoplastic lesions in the 5th edition. “Urothelial dysplasia” is a controversial diagnostic term for a flat lesion that encompasses various changes thought to be preneoplastic in nature but fall short of the diagnosis of UCIS. Nonetheless, “Urothelial dysplasia” is not a synonym of “intraepithelial neoplasia” in the urinary tract. The lack of well-defined objective criteria has led to poor reproducibility in the diagnosis of “urothelial dysplasia” and its clinical significance remains unclear [47, 48]. “Urothelial proliferation of uncertain malignant potential” (UPUMP) is another lesion that is no longer recognized as a distinct entity in the 5th edition. UPUMP includes papillary and flat urothelial hyperplasia with no or minimal cytologic atypical. The GUPS recommends the term “atypical urothelial proliferation (AUP)” with a comment suggesting that this lesion may represent a precursor to an early noninvasive low-grade papillary urothelial carcinoma, as it often harbors chromosome 9 alterations and FGFR3 gene mutations [49]. If flat urothelial hyperplasia exhibits considerable cytologic atypia that is worrisome for UCIS, the diagnostic designation of urothelial dysplasia may be considered.
pT1 cancer substaging
Bladder cancer is staged using the TNM system in the 5th edition, but pT1 bladder cancer invading the lamina propria (LP) exhibits considerable heterogeneity in clinical outcome [50, 51]. Upstaging of pT1 cancer in subsequent radical cystectomy specimens is common and has been reported in nearly 40% of cases [52]. The depth or extent of LP invasion is a strong predictor of outcome in patients with pT1 tumor, although several other factors, such as tumor size, multifocality, recurrence, lymphovascular invasion, patient age, and prior treatment, are also important in the risk stratification [17, 50]. It is generally believed that pT1 substaging in transurethral resection specimens has prognostic value [7, 16, 33, 51]. The extent of LP invasion may be evaluated by micrometric measurement or based on the distinct histoanatomical landmarks in the LP, such as muscularis mucosae (MM) and vascular plexus [53–55]. The most common method is to use the MM as an anatomic landmark - pT1a tumor invades above the MM, and pT1b tumor invades into the MM or beyond. This method is relatively simple and can be performed on small tumors, but it is highly dependent on specimen’s orientation to the surface urothelium. Furthermore, MM is not always visible in TUBRT specimens because of its discontinuous distribution or displacement by tumor. It is important to differentiate MM from MP in invasive bladder cancer because of the significant difference in cancer staging and treatment (Fig. 6) [56]. Sometimes, vascular plexus in the LP may be used as a substitute for the MM [57]. Others have used percentage of specimen with invasive tumor, diameter of invasive tumor, number of invasive tumor foci, and depth of invasion in millimeters from the basement membrane, but these methods are time-consuming and not always accurate [58–61]. Some pathologists use focal or extensive invasion to substage pT1 disease. Focal invasion or microinvasion has been defined by the presence of an invasive tumor involving <1 high power field, greatest diameter of invasive tumor <1 mm and in depth of <2 mm, or invasive tumor present above the muscularis mucosae [51]. It remains unclear which criterion is the most effective in the pT1 substaging, and comparisons of various methods are needed in well-designed prospective studies to assess their accuracy. The 5th edition recommends that an attempt to substage pT1 disease may be made by the pathologist using any of the above criteria [16].
Fig. 6.
Urothelial carcinoma invades different types of smooth muscle tissue. A. muscularis mucosae (pT1b) (×100). B muscularis propria (pT2) (×100).
Lymphovascular invasion (LVI) is another risk factor associated with a high propensity for cancer recurrence and progression in pT1 bladder cancer [62]. However, it may be difficult to assess LVI, particularly in TURBT specimens, as there are frequent retraction, distortion, and carryover artifacts, which may mimic LVI. Strict morphologic criteria such as the presence of endothelial lining, should be applied in the diagnosis of LVI. The use of IHC with endothelial markers (CD31, CD34, and D2-40) can aid the diagnosis of LVI by confirming the presence of endothelial cells. Endothelial markers are not, however, recommended as a screening test for LVI in TURBT or cystectomy specimens [16, 26].
Divergent differentiation and histologic subtype
Bladder UC, particularly invasive UC, has a high propensity for divergent differentiation along other nonurothelial lineages leading to the emergence of squamous, glandular, trophoblastic, and Mullerian differentiation [63, 64]. Squamous differentiation characterized by intercellular bridges or various keratinization production is the most common form of divergent differentiation and reported in 30–40% of cases [65, 66]. Glandular differentiation is the second most common divergent differentiation with up to 18% of bladder UC showing glandular features [65]. True glandular differentiation consists of malignant intestinal glands resembling colorectal adenocarcinoma and should be distinguished from the pseudo-glandular luminal spaces in otherwise conventional UC. Although squamous and glandular differentiation are more frequently observed in locally advanced diseases, they are not significantly associated with worse cancer-specific survival in stage-by-stage comparison [67]. Rarely, invasive UC may show trophoblastic differentiation with an elevation of β-hCG in serum [68]. Interestingly, a considerable proportion of patients with metastatic UC without apparent trophoblastic histology also have β-hCG elevation, which has been used as a marker for monitoring response to therapy [69]. Müllerian differentiation in bladder UC is usually composed of clear cell adenocarcinoma [70].
Bladder UC may also progress to a variety of distinct histologic subtypes or variants (Table 1) [63, 64]. In the 5th edition, “subtype” is a preferred term, as “variant” may cause confusion with genetics and other fields. Although these subtypes show different microscopic features from those in the conventional UC, they are still intrinsically of urothelial origin (Fig. 7). Some UC subtypes, such as nested, tubular and microcystic subtypes, mimic benign lesions, which may pose a diagnostic challenge. Some subtypes, such as micropapillary, plasmacytoid, sarcomatoid, and small cell carcinoma subtypes, show highly aggressive clinical behaviors. These aggressive subtypes are considered to represent a high-risk factor in the treatment and prognosis, which may warrant a more aggressive treatment than those used for conventional UC. Several UC subtypes demonstrate distinct genomic changes that may underlie their aggressive behaviors [63, 64].
Table 1.
Divergent differentiation and histologic subtype in urothelial carcinoma
| •Conventional or usual urothelial carcinoma (UC) - Pure UC with no divergent differentiation or subtype morphology |
| •UC with Divergent differentiation |
| ∘ Squamous |
| ∘ Glandular |
| ∘ Trophoblastic |
| ∘ Mullerian |
| •UC Subtype |
| ∘ Micropapillary |
| ∘ Nested |
| ∘ Tubular and microcystic |
| ∘ Large nested |
| ∘ Lymphoepithelioma-like |
| ∘ Small cell carcinoma |
| ∘ Plasmacytoid |
| ∘ Sarcomatoid |
| ∘ Lipid-rich |
| ∘ Lymphoepithelioma-like |
| ∘ Clear cell |
| ∘ Giant cell |
| ∘ Poorly differentiated |
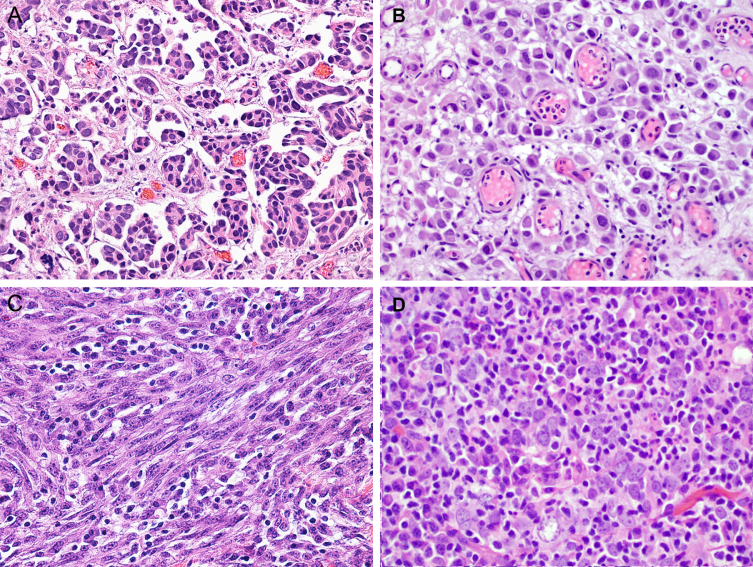
Fig. 7.
Different urothelial carcinoma subtypes. A. Micropapillary (×200). B. Plasmacytoid (×200). C. Sarcomatoid (×200). D. Lymphoepithelioma-like subtype (×200).
Micropapillary subtype is characterized by small morula-like tumor nests without fibrovascular cores surrounded by empty spaces or lacunae. The presence of multiple small nests within the same lacuna is typical. It has a high propensity for metastasis and is associated with aggressive behavior, which may necessitate an early cystectomy treatment in some patients with non-muscle-invasive disease. Overexpression and amplification of ERBB2 is more frequent in micropapillary UC and may represent a potential target for therapy [71].
Plasmacytoid UC subtype shows discohesive individual tumor cells with eccentric nuclei and abundant eosinophilic cytoplasm which resemble plasma cells. The tumor cells diffusely infiltrate the bladder wall with minimal stromal reaction and have a high tendency for peritoneal spread, leading to a high rate of positive resection margin in cystectomy specimens. The presence of somatic mutations of CDH1 (leading to frequent loss of E-cadherin expression) is a hallmark molecular feature of these tumors, which has been documented in approximately 80% of plasmacytoid subtypes [72].
Sarcomatoid UC comprises of mesenchymal neoplastic cells with loss of epithelial phenotype admixed with those showing partial retention of epithelial features. The mesenchymal component may show features of heterologous differentiation, such as osteosarcoma, chondrosarcoma, rhabdomyosarcoma, and angiosarcoma. The survival of patients with sarcomatoid UC is generally poor, and the presence of heterologous components may be associated with even a more adverse behavior [71]. Sarcomatoid UC is characterized by frequent mutations of TP53 genes in nearly all cases and inactivating RB1 mutations in approximately half of them combined with downregulation of homotypic adherence genes and dysregulation of the EMT network [15].
In the 5th edition, small cell carcinoma is discussed in a separate chapter dedicated to neuroendocrine tumors involving the urinary tract and male genital organs. Like its counterparts in the lungs and other organs, bladder small cell carcinoma is composed of poorly differentiated malignant cells with scant cytoplasm, high nuclear/cytoplasmic ratio, and salt and pepper granular chromatin. Similar to sarcomatoid UC, small cell carcinoma subtype shows frequent mutations of the TP53/RB1 genes and displays lineage plasticity driven by a urothelial-neural phenotypic switch [73, 74]. It is characterized by an immune-null phenotype that is depleted of immune cell infiltration. Furthermore, small cell carcinoma expresses a high level of adenosine receptor A2A (ADORA2A), an immune checkpoint receptor, which may represent a potential therapeutic target for this highly lethal subtype of bladder cancer [73].
Molecular classification of muscle-invasive bladder cancer
A number of contemporary studies have analyzed the genomic profile of muscle-invasive bladder cancer (MIBC) on multiple molecular platforms, including somatic DNA mutations, copy number variations, DNA methylation, mRNA expressions, microRNA expressions, microbe analysis, and proteomic analysis [11–14, 75]. These comprehensive analyses demonstrated a remarkable molecular diversity in MIBC, which may underlie a wide spectrum of clinical behaviors as well as varied responses to conventional and targeted therapies. Several different molecular classification systems based on genomic profiling have been proposed [11–14, 75–78]. The original mRNA classification was proposed by the Lund Group and identified five subcategories. The TCGA group identified five molecular subtypes of bladder cancer, while a recent meta-analysis based on 1750 cases of muscle invasive bladder cancer identified six consensus molecular classes: luminal papillary, luminal nonspecified, luminal unstable, stroma-rich, basal/squamous, and neuroendocrine-like. TP53 mutations are frequent in the neuroendocrine-like, basal-squamous, and luminal-unstable subtypes, while FGFR3 mutations are enriched in the luminal-papillary subtype. Overall, the luminal-unstable subtype shows the most genomic alterations. The MD Anderson and The University of North Carolina groups proposed a classification of bladder cancer with two major categories referred to as luminal and basal subtypes. Although the names and numbers of subtypes are somewhat different in these classification systems, there are strong evidences to support that top-level separation occurs at the basal and luminal differentiation checkpoint (Fig. 8). The luminal UC appears to evolve through the papillary track, while the basal UC develops via the nonpapillary track [5]. Although papillary UC are almost exclusively luminal subtype, invasive bladder UC can be luminal or basal subtype. The invasive UC with a luminal expression signature likely evolve from the preexisting papillary tumor and represent a progression of superficial papillary tumors. Further studies revealed that various UC histologic subtypes are associated with characteristic molecular subtypes [15, 73, 79]. For example, micropapillary and plasmacytoid subtypes are almost exclusively of the luminal subtype [79], while sarcomatoid and small cell subtypes show basal molecular signatures [15, 73, 79].
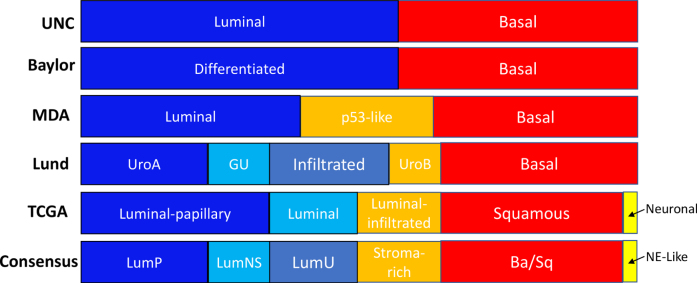
Fig. 8.
Different molecular classification systems of muscle-invasive bladder cancers. International consensus classification proposes 6 distinct molecular subtypes, which is based on a meta-analysis of 1750 cases from 18 datasets. Ba/Sq×basal/squamous; LumNS×luminal nonspecified; LumP×luminal papillary; LumU×luminal unstable; MDA×MD Anderson Cancer Center; NE-like×neuroendocrine-like; TCGA×the Cancer Genome Atlas; UNC×University of North Carolina. Modified with permission from Kamoun et al. Eur Urol. 2020;77(4):420-433.
Although the molecular classification of bladder cancer based on the genomic mRNA expression profiling provides valuable insights into its biological behavior, it cannot be easily applied to the routine clinical practice because the analytical method is technologically complex and costly. Recent studies have found that IHC may be used to aid the molecular classification of bladder UC [80]. A small set of luminal (GATA3, CK20, and uroplakin II) and basal (CK5/6 and CK14) markers can be effectively used to classify bladder cancers into luminal and basal categories, although the performance of this classifier remains to be validated in large independent cohorts [81].
Novel molecular markers have great promise in improving the prognostic power and reproducibility of the current histology-based grading, particularly in the metastatic setting, allowing the identification of patients who may benefit from targeted therapy. It becomes evident that high-quality immunohistochemistry and molecular testing are essential in the molecular classification of bladder cancer with significant diagnostic, prognostic and predictive implications. However, the availability of immunohistochemistry and molecular testing may be limited in low- and middle-income countries. As the WHO classification is proposed for worldwide use, major emphasis has been placed on histopathological criteria in the 5th edition. In summary, microscopic features represent the gold standard of pathological classification of bladder cancer, but molecular features represent an emerging auxiliary information aiding the clinical decision process.
CONCLUSIONS
Bladder cancer is a heterogeneous disease which exhibits a wide spectrum of clinical and pathologic features. The classification of bladder cancer has been traditionally based on morphologic assessment with the aid of IHC. However, recent genomic studies have revealed that distinct alterations of DNA and RNA in bladder cancer may underlie its diverse clinicopathologic features, leading to the molecular classification of bladder cancer. These advances fundamentally change our understanding of the disease and expand the diagnostic and therapeutic options for patients affected by bladder cancer. The 5th edition of the WHO Classification of Urinary and Male Genital tumors provides significant revisions of bladder cancer classification with an incorporation of new morphologic and genomic data. Although the application of molecular profiling has provided insightful information on the diverse behavior of bladder cancer, morphology remains the gold standard in the taxonomy of bladder cancer. This practical approach with combination of morphologic, immunohistochemical, genomic, and clinical data may represent the optimal paradigm of bladder cancer classification, expanding the diagnostic and therapeutic options for patients affected by bladder cancer.
ACKNOWLEDGMENTS
We would like to thank Mrs. Stephanie Garza for editorial assistance.
FUNDING
This study was supported by Cancer Prevention and Research Institute of Texas (CPRIT) Grant RP220021 to BC.
AUTHOR CONTRIBUTIONS
CCG, BC: Conception, performance of work, interpretation of results, writing and review of the article. SSS: Conception, interpretation of the results, and review of the article.
ETHICAL CONSIDERATIONS
This study, as a literature review is exempt from any requirement for Institutional Review Board approval. No human or animal research was involved in the elaboration of this manuscript.
CONFLICTS OF INTEREST
BC is an Editorial Board member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer-review. CCG and SSS do not have a conflict of interest associated with this publication.
REFERENCES
- [1]. Bladder cancer fact sheet. International Agency for Research on Cancer; 2020. https://gco.iarc.fr/today/fact-sheets-cancers. https://gco.iarc.fr/today/fact-sheets-cancers
- [2]. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- [3]. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022 CA Cancer J Clin. 2022;72(1):7–33. [DOI] [PubMed] [Google Scholar]
- [4]. Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: A global overview and recent trends. Eur Urol. 2017;71(1):96–108. [DOI] [PubMed] [Google Scholar]
- [5]. Guo CC, Czerniak B. Bladder cancer in the genomic era. Arch Pathol Lab Med. 2019;143(6):695–704. [DOI] [PubMed] [Google Scholar]
- [6]. Czerniak B, Dinney C, McConkey D. Origins of bladder cancer. Annu Rev Pathol. 2016;11:149–74. [DOI] [PubMed] [Google Scholar]
- [7]. Moch H HP, Ulbright TM, Retuer VE. WHO Classification of Tumours of the Urinary System and Male Genital Organs. 4 ed. Lyon, France: IARC Press; 2016. [Google Scholar]
- [8]. Epstein JI, Amin MB, Reuter VR, Mostofi FK. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol. 1998;22(12):1435–48. [DOI] [PubMed] [Google Scholar]
- [9]. Mostofi FKSL, Torloni HHistological typing of urinary bladder tumours Geneva, Switzerland: World Health Organization; 1973. [Google Scholar]
- [10]. Eble JSG, Epstein J, Sesterhenn I Pathology and genetics of tumours of the urinary system and male genital organs, 3 ed. Lyon, France: IARC Press; 2004. [Google Scholar]
- [11]. Kamoun A, de Reyniès A, Allory Y, et al. A consensus molecular classification of muscle-invasive bladder cancer. Eur Urol. 2020;77(4):420–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Choi W, Porten S, Kim S, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25(2):152–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell. 2017;171(3):540–56 e525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Damrauer JS, Hoadley KA, Chism DD, et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc Natl Acad Sci U S A. 2014;111(8):3110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Guo CC, Majewski T, Zhang L, et al. Dysregulation of EMT drives the progression to clinically aggressive sarcomatoid bladder cancer. Cell Reports. 2019;27(6):1781–93.e1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Board WCoTE Urinary and male genital tumours, 5 ed. Lyon (France): International Agency for Research on Cancer. 2022 [Google Scholar]
- [17]. Kamat AM, Hahn NM, Efstathiou JA, et al. Bladder cancer. Lancet. 2016;388(10061):2796–810. [DOI] [PubMed] [Google Scholar]
- [18]. van Rhijn BW, van Leenders GJ, Ooms BC, et al. The pathologist’s mean grade is constant and individualizes the prognostic value of bladder cancer grading. Eur Urol. 2010;57(6):1052–7. [DOI] [PubMed] [Google Scholar]
- [19]. Soukup V, Čapoun O, Cohen D, et al. Prognostic performance and reproducibility of the 1973 and 2004/2016 world health organization grading classification systems in non-muscle-invasive bladder cancer: A european association of urology non-muscle invasive bladder cancer guidelines panel systematic review. Eur Urol. 2017;72(5):801–13. [DOI] [PubMed] [Google Scholar]
- [20]. Compérat EM, Burger M, Gontero P, et al. Grading of urothelial carcinoma and the new “World Health Organisation Classification of Tumours of the Urinary System and Male Genital Organs 2016”. Eur Urol Focus. 2019;5(3):457–66. [DOI] [PubMed] [Google Scholar]
- [21]. Rosenthal DLWE, Kurtycz DFI. The Paris System for reporting urinary cytology Cham (Switzerland): Springer International Publishing; 2016. [Google Scholar]
- [22]. Sylvester RJ, Rodríguez O, Hernández V, et al. European association of urology (EAU) prognostic factor risk groups for Non-muscle-invasive Bladder Cancer (NMIBC) incorporating the WHO 2004/2016 and WHO 1973 classification systems for grade: An update from the EAU NMIBC guidelines panel. Eur Urol. 2021;79(4):480–8. [DOI] [PubMed] [Google Scholar]
- [23]. Lokeshwar SD, Ruiz-Cordero R, Hupe MC, Jorda M, Soloway MS. Impact of 2004 ISUP/WHO classification on bladder cancer grading. World J Urol. 2015;33(12):1929–36. [DOI] [PubMed] [Google Scholar]
- [24]. Klaassen Z, Soloway MS. European Association of Urology and American Urological Association/Society of Urologic Oncology Guidelines on risk categories for non-muscle-invasive bladder cancer may lead to overtreatment for low-grade Ta bladder tumors. Urology. 2017;105:14–7. [DOI] [PubMed] [Google Scholar]
- [25]. Akgul M, MacLennan GT, Cheng L. The applicability and utility of immunohistochemical biomarkers in bladder pathology. Hum Pathol. 2020;98:32–55. [DOI] [PubMed] [Google Scholar]
- [26]. Amin MB, Trpkov K, Lopez-Beltran A, Grignon D. Best practices recommendations in the application of immunohistochemistry in the bladder lesions: Report from the International Society of Urologic Pathology consensus conference. Am J Surg Pathol. 2014;38(8):e20–34. [DOI] [PubMed] [Google Scholar]
- [27]. Cheng L, Zhang S, MacLennan GT, Williamson SR, Lopez-Beltran A, Montironi R. Bladder cancer: Translating molecular genetic insights into clinical practice. Hum Pathol. 2011;42(4):455–81. [DOI] [PubMed] [Google Scholar]
- [28]. Quintero A, Alvarez-Kindelan J, Luque RJ, et al. Ki-67 MIB1 labelling index and the prognosis of primary TaT1 urothelial cell carcinoma of the bladder. Journal of Clinical Pathology. 2006;59(1):83–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Cheng L, Neumann RM, Nehra A, Spotts BE, Weaver AL, Bostwick DG. Cancer heterogeneity and its biologic implications in the grading of urothelial carcinoma. Cancer. 2000;88(7):1663–70. [DOI] [PubMed] [Google Scholar]
- [30]. Gofrit ON, Pizov G, Shapiro A, et al. Mixed high and low grade bladder tumors–are they clinically high or low grade? J Urol. 2014;191(6):1693–6. [DOI] [PubMed] [Google Scholar]
- [31]. Reis LO, Taheri D, Chaux A, et al. Significance of a minor high-grade component in a low-grade noninvasive papillary urothelial carcinoma of bladder. Human pathology. 2016;47(1):20–5. [DOI] [PubMed] [Google Scholar]
- [32]. May M, Brookman-Amissah S, Roigas J, et al. Prognostic accuracy of individual uropathologists in noninvasive urinary bladder carcinoma: A multicentre study comparing the 1973 and 2004 World Health Organisation classifications. Eur Urol. 2010;57(5):850–8. [DOI] [PubMed] [Google Scholar]
- [33]. Amin MB, Comperat E, Epstein JI, et al. The genitourinary pathology society update on classification and grading of flat and papillary urothelial neoplasia with new reporting recommendations and approach to lesions with mixed and early patterns of neoplasia. Adv Anat Pathol. 2021;28(4):179–95. [DOI] [PubMed] [Google Scholar]
- [34]. Holmäng S, Hedelin H, Anderström C, Holmberg E, Busch C, Johansson SL. Recurrence and progression in low grade papillary urothelial tumors. J Urol. 1999;162(3 Pt 1):702–7. [DOI] [PubMed] [Google Scholar]
- [35]. Maxwell JP, Wang C, Wiebe N, Yilmaz A, Trpkov K. Long-term outcome of primary Papillary Urothelial Neoplasm of Low Malignant Potential (PUNLMP) including PUNLMP with inverted growth. Diagn Pathol. 2015;10:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Pan CC, Chang YH, Chen KK, Yu HJ, Sun CH, Ho DM. Prognostic significance of the 2004 WHO/ISUP classification for prediction of recurrence, progression, and cancer-specific mortality of non-muscle-invasive urothelial tumors of the urinary bladder: A clinicopathologic study of 1,515 cases. Am J Clin Pathol. 2010;133(5):788–95. [DOI] [PubMed] [Google Scholar]
- [37]. Tuna B, Yörükoglu K, Düzcan E, et al. Histologic grading of urothelial papillary neoplasms: Impact of combined grading (two-numbered grading system) on reproducibility. Virchows Archiv. 2011;458(6):659. [DOI] [PubMed] [Google Scholar]
- [38]. Yorukoglu K, Tuna B, Dikicioglu E, et al. Reproducibility of the 1998 World Health Organization/International Society of Urologic Pathology classification of papillary urothelial neoplasms of the urinary bladder. Virchows Archiv. 2003;443(6):734–40. [DOI] [PubMed] [Google Scholar]
- [39]. Hentschel AE, van Rhijn BWG, Bründl J, et al. Papillary urothelial neoplasm of low malignant potential (PUN-LMP): Still a meaningful histo-pathological grade category for Ta, noninvasive bladder tumors in 2019? Urologic Oncology: Seminars and Original Investigations. 2020;38(5):440–8. [DOI] [PubMed] [Google Scholar]
- [40]. Babjuk M, Burger M, Compérat EM, et al. European association of urology guidelines on non-muscle-invasive bladder cancer (TaT1 and Carcinoma In Situ) - 2019 update. European Urology. 2019;76(5):639–57. [DOI] [PubMed] [Google Scholar]
- [41]. Chang SS, Boorjian SA, Chou R, et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. The Journal of Urology. 2016;196(4):1021–9. [DOI] [PubMed] [Google Scholar]
- [42]. Jones TD, Cheng L. Noninvasive papillary urothelial neoplasia (NIPUN): Renaming cancer. Urologic Oncology: Seminars and Original Investigations. 2021;39(5):286–90. [DOI] [PubMed] [Google Scholar]
- [43]. Hodges KB, Lopez-Beltran A, Maclennan GT, Montironi R, Cheng L. Urothelial lesions with inverted growth patterns: Histogenesis, molecular genetic findings, differential diagnosis and clinical management. BJU Int. 2011;107(4):532–7. [DOI] [PubMed] [Google Scholar]
- [44]. Compérat E, Jacquet SF, Varinot J, et al. Different subtypes of carcinoma in situ of the bladder do not have a different prognosis. Virchows Arch. 2013;462(3):343–8. [DOI] [PubMed] [Google Scholar]
- [45]. McKenney JK. Urothelial carcinoma in situ: Diagnostic update. Pathology. 2021;53(1):86–95. [DOI] [PubMed] [Google Scholar]
- [46]. Chan TY, Epstein JI. In situ adenocarcinoma of the bladder. Am J Surg Pathol. 2001;25(7):892–9. [DOI] [PubMed] [Google Scholar]
- [47]. McKenney JK. Precursor lesions of the urinary bladder. Histopathology. 2019;74(1):68–76. [DOI] [PubMed] [Google Scholar]
- [48]. Cheng L, Cheville JC, Neumann RM, Bostwick DG. Natural history of urothelial dysplasia of the bladder. Am J Surg Pathol. 1999;23(4):443–7. [DOI] [PubMed] [Google Scholar]
- [49]. van Oers JM, Adam C, Denzinger S, et al. Chromosome 9 deletions are more frequent than FGFR3 mutations in flat urothelial hyperplasias of the bladder. Int J Cancer. 2006;119(5):1212–5. [DOI] [PubMed] [Google Scholar]
- [50]. Kardoust Parizi M, Enikeev D, Glybochko PV, et al. Prognostic value of T1 substaging on oncological outcomes in patients with non-muscle-invasive bladder urothelial carcinoma: A systematic literature review and meta-analysis. World Journal of Urology. 2020;38(6):1437–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Amin SEMB, Greene FL, et al. , AJCC Cancer Staging Manual. 8th ed. New York: Springer. [Google Scholar]
- [52]. Shariat SF, Palapattu GS, Karakiewicz PI, et al. Discrepancy between clinical and pathologic stage: Impact on prognosis after radical cystectomy. Eur Urol. 2007;51(1):137–49 discussion 149-51. [DOI] [PubMed] [Google Scholar]
- [53]. Sanfrancesco J, McKenney JK, Leivo MZ, Gupta S, Elson P, Hansel DE. Sarcomatoid urothelial carcinoma of the bladder: Analysis of 28 cases with emphasis on clinicopathologic features and markers of epithelial-to-mesenchymal transition. Archives of Pathology & Laboratory Medicine. 2016;140(6):543–51. [DOI] [PubMed] [Google Scholar]
- [54]. Fransen van de Putte EE, Otto W, Hartmann A, et al. Metric substage according to micro and extensive lamina propria invasion improves prognostics in T1 bladder cancer. Urol Oncol. 2018;36(8):361.e367-1.e313. [DOI] [PubMed] [Google Scholar]
- [55]. Leivo MZ, Sahoo D, Hamilton Z, et al. Analysis of T1 bladder cancer on biopsy and transurethral resection specimens: Comparison and ranking of T1 quantification approaches to predict progression to muscularis propria invasion. Am J Surg Pathol. 2018;42(1):e1–10. [DOI] [PubMed] [Google Scholar]
- [56]. Hwang MJ, Kamat AM, Dinney CP, Czerniak B, Guo CC. Bladder cancer involving smooth muscle of indeterminate type or muscularis mucosae in transurethral biopsy specimens. Am J Clin Pathol. 2020;154(2):208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57]. Paner GP, Ro JY, Wojcik EM, Venkataraman G, Datta MW, Amin MB. Further characterization of the muscle layers and lamina propria of the urinary bladder by systematic histologic mapping: Implications for pathologic staging of invasive urothelial carcinoma. Am J Surg Pathol. 2007;31(9):1420–9. [DOI] [PubMed] [Google Scholar]
- [58]. Cheng L, Weaver AL, Neumann RM, Scherer BG, Bostwick DG. Substaging of T1 bladder carcinoma based on the depth of invasion as measured by micrometer: A new proposal. Cancer. 1999;86(6):1035–43. [DOI] [PubMed] [Google Scholar]
- [59]. Lopez-Beltran A, Cheng L. Stage T1 bladder cancer: Diagnostic criteria and pitfalls. Pathology. 2021;53(1):67–85. [DOI] [PubMed] [Google Scholar]
- [60]. Brimo F, Wu C, Zeizafoun N, et al. Prognostic factors in T1 bladder urothelial carcinoma: The value of recording millimetric depth of invasion, diameter of invasive carcinoma, and muscularis mucosa invasion. Hum Pathol. 2013;44(1):95–102. [DOI] [PubMed] [Google Scholar]
- [61]. van Rhijn BW, van der Kwast TH, Alkhateeb SS, et al. A new and highly prognostic system to discern T1 bladder cancer substage. Eur Urol. 2012;61(2):378–84. [DOI] [PubMed] [Google Scholar]
- [62]. Mathieu R, Lucca I, Rouprêt M, Briganti A, Shariat SF. The prognostic role of lymphovascular invasion in urothelial carcinoma of the bladder. Nat Rev Urol. 2016;13(8):471–9. [DOI] [PubMed] [Google Scholar]
- [63]. Lopez-Beltran A, Henriques V, Montironi R, Cimadamore A, Raspollini MR, Cheng L. Variants and new entities of bladder cancer. Histopathology. 2019;74(1):77–96. [DOI] [PubMed] [Google Scholar]
- [64]. Amin MB. Histological variants of urothelial carcinoma: Diagnostic, therapeutic and prognostic implications. Mod Pathol. 2009;22(Suppl 2):S96–118. [DOI] [PubMed] [Google Scholar]
- [65]. Wasco MJ, Daignault S, Zhang Y, et al. Urothelial carcinoma with divergent histologic differentiation (mixed histologic features) predicts the presence of locally advanced bladder cancer when detected at transurethral resection. Urology. 2007;70(1):69–74. [DOI] [PubMed] [Google Scholar]
- [66]. Linder BJ, Boorjian SA, Cheville JC, et al. The impact of histological reclassification during pathology re-review–evidence of a Will Rogers effect in bladder cancer? J Urol. 2013;190(5):1692–6. [DOI] [PubMed] [Google Scholar]
- [67]. Kim SP, Frank I, Cheville JC, et al. The impact of squamous and glandular differentiation on survival after radical cystectomy for urothelial carcinoma. J Urol. 2012;188(2):405–9. [DOI] [PubMed] [Google Scholar]
- [68]. Przybycin CG, McKenney JK, Nguyen JK, et al. Urothelial carcinomas with trophoblastic differentiation, including choriocarcinoma: Clinicopathologic series of 16 cases. Am J Surg Pathol. 2020;44(10):1322–30. [DOI] [PubMed] [Google Scholar]
- [69]. Douglas J, Sharp A, Chau C, et al. Serum total hCGβ level is an independent prognostic factor in transitional cell carcinoma of the urothelial tract. Br J Cancer. 2014;110(7):1759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70]. Grosser D, Matoso A, Epstein JI. Clear cell adenocarcinoma in men: A series of 15 cases. Am J Surg Pathol. 2021;45(2):270–6. [DOI] [PubMed] [Google Scholar]
- [71]. Ching CB, Amin MB, Tubbs RR, et al. HER2 gene amplification occurs frequently in the micropapillary variant of urothelial carcinoma: Analysis by dual-color in situ hybridization. Mod Pathol. 2011;24(8):1111–9. [DOI] [PubMed] [Google Scholar]
- [72]. Al-Ahmadie HA, Iyer G, Lee BH, et al. Frequent somatic CDH1 loss-of-function mutations in plasmacytoid variant bladder cancer. Nature Genetics. 2016;48(4):356–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73]. Yang G, Bondaruk J, Cogdell D, et al. Urothelial-to-neural plasticity drives progression to small cell bladder cancer. iScience. 2020;23(6):101201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74]. Chang MT, Penson A, Desai NB, et al. Small-cell carcinomas of the bladder and lung are characterized by a convergent but distinct pathogenesis. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 2018;24(8):1965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75]. Cancer Genome Atlas Research N. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507(7492):315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76]. Seiler R, Ashab HAD, Erho N, et al. Impact of molecular subtypes in muscle-invasive bladder cancer on predicting response and survival after neoadjuvant chemotherapy. European Urology. 2017;72(4):544–54. [DOI] [PubMed] [Google Scholar]
- [77]. Sjödahl G, Lauss M, Lövgren K, et al. A molecular taxonomy for urothelial carcinoma. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 2012;18(12):3377–86. [DOI] [PubMed] [Google Scholar]
- [78]. Mo Q, Nikolos F, Chen F, et al. Prognostic power of a tumor differentiation gene signature for bladder urothelial carcinomas. JNCI: Journal of the National Cancer Institute. 2018;110(5):448–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79]. Guo CC, Dadhania V, Zhang L, et al. Gene expression profile of the clinically aggressive micropapillary variant of bladder cancer. Eur Urol. 2016;70(4):611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80]. Dadhania V, Zhang M, Zhang L, et al. Meta-analysis of the luminal and basal subtypes of bladder cancer and the identification of signature immunohistochemical markers for clinical use. EBioMedicine. 2016;12:105–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81]. Guo CC, Bondaruk J, Yao H, et al. Assessment of luminal and basal phenotypes in bladder cancer. Sci Rep. 2020;10(1):9743. [DOI] [PMC free article] [PubMed] [Google Scholar]