Abstract
Background
This study explored the integration of conductive threads into a microfluidic compact disc (CD), developed using the xurographic method, for a potential sweat biosensing platform.
Material/Methods
The microfluidic CD platform, fabricated using the xurographic method with PVC films, included venting channels and conductive threads linked to copper electrodes. With distinct microfluidic sets for load and metering, flow control, and measurement, the CD’s operation involved spinning for sequential liquid movement. Impedance analysis using HIOKI IM3590 was conducted for saline and artificial sweat solutions on 4 identical CDs, ensuring reliable conductivity and measurements over a 1 kHz to 200 kHz frequency range.
Results
Significant differences in |Z| values were observed between saline and artificial sweat treatments. 27.5 μL of saline differed significantly from 27.5 μL of artificial sweat, 72.5 μL of saline from 72.5 μL of artificial sweat, and 192.5 μL of saline from 192.5 μL of sweat. Significant disparities in |Z| values were observed between dry fibers and Groups 2, 3, and 4 (varying saline amounts). No significant differences emerged between dry fibers and Groups 6, 7, and 8 (distinct artificial sweat amounts). These findings underscore variations in fiber characteristics between equivalent exposures, emphasizing the nuanced response of the microfluidic CD platform to different liquid compositions.
Conclusions
This study shows the potential of integrating conductive threads in a microfluidic CD platform for sweat sensing. Challenges in volume control and thread coating degradation must be addressed for transformative biosensing devices in personalized healthcare.
Keywords: Electrochemical Techniques, Microfluidics, Sweat, Textiles
Introduction
In recent years, there has been significant progress in development of microfluidic platforms for biosensing applications. However, the field of sweat biosensing still faces challenges that limit its precision, sensitivity, and real-world applicability. Existing technologies often grapple with issues related to on-body collection, precision in volume control, and degradation of sensing components. This study aimed to address these limitations and introduce a transformative approach to sweat biosensing through the integration of conductive threads into a microfluidic compact disc (CD) platform.
Since the 1990s, researchers have been investing their efforts in refining microfluidic technologies [1]. To date, microfluidic platforms have been classified into several categories, including lab-on-a-chip (LOC), lab-on-paper (LOP), droplet-based microfluidic, and lab-on-a-disc (LOD) systems [2–5]. Among these, the microfluidic compact disc (CD) platform, also known as a centrifugal microfluidic CD, stands out for its inherent simplicity and efficiency. Al-Faqheri et al [6] introduced an active valving on the microfluidic CD platform using paraffin wax to seal venting chambers/holes. The new valving system was more resilient against bursting at high frequencies and significantly reduced the direct heating of samples and reagents in the microfluidic CD system. Sayad et al [5] demonstrated the integration of loop-mediated isothermal amplification (LAMP) in microfluidic CD for rapid foodborne pathogen detection. Those achievements promise further microfluidic CD development, improvement, and expanding the field of application.
The main materials of e-textiles are conductive threads capable of conducting electricity from parts of units. In this case, conductive fibers or fibers coated with various conductive coating materials can be used to create conductive threads. The ability to incorporate an array of multiple textile sensors capable of selectively detect different analytes absorbed into the fabric would enable the creation of a powerful textile multisensory platform akin to a lab-on-fabric device. Although conductive threads can be produced using various methods, the wrapping and coating methods are the most common in the industry [7,8]. Interestingly, textile-based sensors have the same targets as wearable sensors, which are biomarkers in human sweat, an epidermally available biofluid containing various analytes [9]. The ability to detect biosensing perspiration (sweating) levels may open a new avenue for the early detection of pathological conditions [10]. The importance of sweat in situ quantitative analysis for disease diagnosis and physiological health monitoring has led to significant advancements in wearable electrochemical sweat sensors. These sensors now offer complex and fully multiplexed systems, enabling simultaneous detection of multiple analytes. The choice of sensing technique depends on the chemical characteristics and concentration range of the target biomarkers in sweat. Additionally, the promising subfield of photoelectrochemical detection has garnered attention in analytical chemistry [11–15]. Furthermore, real-time sweat detection and analysis can be used as a physical reference for fitness assessment and to indicate hyperhidrosis in non-medical situations [16,17].
Electrochemical sweat sensors have emerged as a critical technology for real-time sweat detection and analysis, offering high sensitivity, selectivity, quick response times, and simplicity in design. Advancements in this field, such as amperometry, potentiometry, voltammetry, and photoelectrochemical detection, have expanded the potential applications of electrochemical sensors, including protein-specific immunosensors [11,12,15]. Promising studies have already demonstrated the effectiveness of thread-based electrochemical sweat sensors in assessing fitness levels and predicting fatigue [18]. These textile sensors, with their three-dimensional structures, can accurately measure sweat quantity and perspiration levels in real-time [19].
Existing sweat biosensing technologies, while promising, often fall short of providing precise and real-time analysis due to inherent challenges. On-body sweat collection remains a challenge, leading to potential inaccuracies in analyte detection. Moreover, conventional platforms may struggle with volume control, compromising the reliability of measurements. The degradation of sensing components over time further hinders the long-term effectiveness of current technologies.
The present study sought to bridge these gaps by leveraging the unique properties of conductive threads within the microfluidic CD platform. This integration introduces a novel dimension to sweat biosensing, addressing challenges related to volume control, on-body collection, and long-term reliability. By combining the efficiency of microfluidic CDs with the electrical properties of conductive threads, we aim to pave the way for a new era in personalized healthcare and industrial applications.
The objectives of this study encompass the fabrication and characterization of a microfluidic CD platform integrated with conductive threads, followed by an in-depth analysis of its performance in sweat biosensing. Our focus was on overcoming the limitations of existing technologies and demonstrating the potential of this integrated approach for transformative applications. The xurographic method was employed for CD fabrication, providing a scalable and efficient means to integrate conductive threads.
Material and Methods
Design and Fabrication of the Microfluidic CD Platform
The microfluidic CD platform was fabricated with 6 layers of polyvinyl chloride (PVC) films using the xurographic method. Each layer of microfluidic CD was designed in computer-aided design (CAD) software and cut using a cutter plotter, as illustrated in Figure 1. Each layer was stacked and bonded using a heat lamination process at 180°C. It consisted of 2 layers of 80-μm PVC film for the top and bottom (with alignment holes), 4 layers of 250-μm PVC film for the microfluidic components, and 1 layer of 250-μm PVC film for the microfluidic component and venting channels. The 80-μm foils were used as the top and bottom cover with the inlet and ventilation holes. The copper (Cu) electrodes were used as a conductive bridge between the conductive thread (inside the measurement chamber) and the connection to the impedance analyzer crocodile clips. The electrodes were also fabricated using xurographic technique. The total length of the thread was 40 mm, so the total distance between electrodes was 35 mm. This thread length corresponds to the size of the chamber optimized for a 4-set CD. The thread is placed in the chamber between 2 layers of self-adhesive copper tape. The lower layer of copper tape is positioned on the bottom layer of the microfluidic CD, while the upper layer secures the thread at a distance of 35 mm. Then, the chamber is closed during the hot lamination process.
Figure 1.
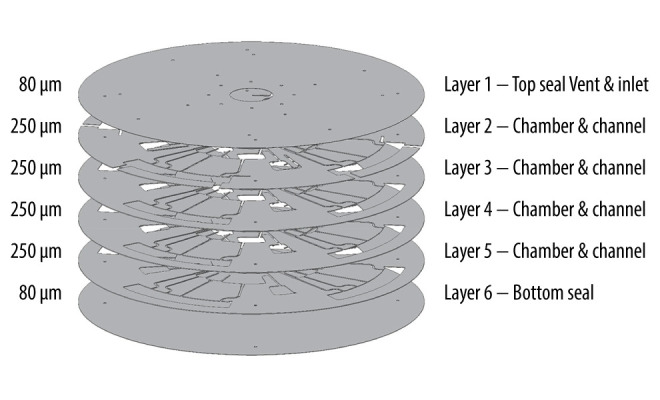
Schematic illustration showing the layers and assembly of microfluidic CD.
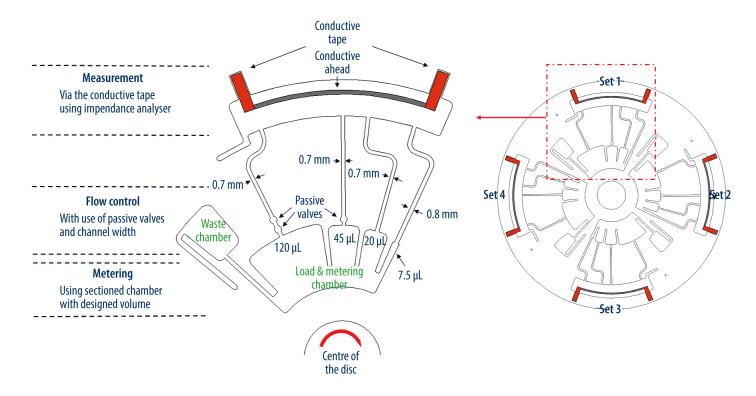
The microfluidic CD consisted of 4 identical microfluidic sets, as shown in Figure 2. Each microfluidic set was designed to perform 3 processes: 1) load and metering, 2) flow control, and 3) measurement.
Figure 2.
The design of microfluidic CD section – load and metering, flow control, measurement.
The load and metering part was designed to store the initially loaded liquid (during rest) and then the meter loaded liquid into the designated section based on the volume while the balance/excess flowed to the waste chamber. In each set of this microfluidic CD, there were 4 metered sections categorized as Section 1 – 7.5 μL, Section 2 – 20 μL, Section 3 – 45 μL and Section 4 – 120 μL. The metering process started when the microfluidic CD was spun at a lower speed (below the burst frequency).
Flow control occurred right after the loaded liquid was metered. The flow control sequenced the flow from the smallest volume to the highest volume section into the analysis/measurement chamber. To control the flow from the smallest volume to the biggest, the hydraulic diameter (DH) of the channels and the passive valves were introduced according to the theoretical consideration reported by Thio et al [20]. The channel for the first section had the biggest channel width (0.8 mm), followed by the rest of the section channels with a width of 0.7 mm. These adjustments were not sufficient for used liquids, so the first and second sections burst at the same time. To ensure sequential liquid delivery, the third section was designed with an additional passive valve, whereas the fourth section was designed with 2 additional passive valves. These adjustments were sufficient for used liquids, and section 3 and section 4 burst at different frequencies.
The measurement chamber was the final part of the microfluidic network designed in this disc, where the impedance measurements were taken via 2 conductive tapes connected at the end of each conductive thread.
Dimensions of the Microfluidic Channels
The specific dimensions of the microfluidic channels have been provided. Details include the channel widths for each section, the hydraulic diameter (DH) adjustments, and the introduction of passive valves for flow control. These adjustments are based on theoretical considerations, as reported by Thio et al [20]. The CAD file for the designs with exact dimensions is included in the Supplementary Figure 1.
Specification of the Conductive Thread
Raw Material
The Silver-Tech 120 thread is composed of a blend of polyamide and polyester that has been coated with silver. This unique combination results in a sewing and embroidery thread that possesses conductive properties.
Construction Type
The Silver-Tech 120 thread can be classified as a hybrid thread that has been skillfully engineered to provide enhanced functionality for conductive seams and surfaces.
Product Properties
The Silver-Tech 120 thread is a specialized sewing and embroidery thread that distinguishes itself by the presence of a silver coating. Importantly, it has been demonstrated to have no detrimental effects on cells in the Cytotoxicity Test, as per the DIN EN ISO 10993-5 standard. This ensures its compatibility for various applications, such as textile electrodes that function as sensors and actuators.
Technical Specifications
Ticket Number: 120
Embroidery Thread
40 Tex Number: 28 Make-up: 2500 m FS Number of Colors: 1 Minimum Needle Size in Nm: 75 Minimum Needle Size in No: 11 Resistance: Less than 530 Ω/m
Available Color
The Silver-Tech 120 thread is offered in color number 1000, which gives it a raw, silver-grey appearance due to its silver content. It is important to note that, over time, the grey color may gradually darken as a result of the presence of silver and the natural oxidation processes (AMANN Group, 2017).
Solutions and Chemicals Used
The Sodium Chloride Infundibile HF 9g/L Solution for Infusion, produced by HEMOFARM AD VRŠAC was used. It is a sterile infusion solution that contains 9 g/L of sodium chloride. This versatile solution allows for expression of doses in different units, such as mEq or mmol of sodium, as well as the mass of sodium or the mass of sodium salt. It is important to take into account the conversion factors: 1 g NaCl is equal to 394 mg of sodium, 17.1 mEq or 17.1 mmol of both sodium and chlorine. In the field of medicine, an intravenous infusion of this solution with a concentration of 0.9% sodium chloride results in an approximate tonicity of 308 mOsm/L. This particular characteristic makes it suitable for intravenous use, as it provides a well-balanced concentration of sodium chloride for medical and therapeutic purposes. This Sodium Chloride Infundibile HF solution adheres to established standards, offering flexibility in the expression of dosages and contributing to its applicability in a variety of medical contexts.
Artificial Sweat pH 6.8, Nanochemazone, Chemazone, Inc. Canada. Synthetic Perspiration/Artificial Perspiration Product Number: NCZ-APS-0011-20. The pH of the product can be customized according to specific requirements within the pH range of 2.0 to 9.0, and in this investigation pH of 6.8 has been used. The ready-to-use Synthetic Eccrine Perspiration accurately replicates the composition of genuine human eccrine sweat. It consists of 19 amino acids, the 7 most abundant minerals, and the 4 most prevalent metabolites, all at pH 4.5. The concentrations in this product closely align with those experimentally determined for adult human eccrine sweat. The stabilized version of the Synthetic Perspiration solution undergoes preservation with a fungicide and bactericide, ensuring a shelf life of 2 years when stored at room temperature.
Theory of Microfluidic Compact Disc Operation
The developed microfluidic platform was tested using 2 different liquids: (a) saline (Hemofarm, AD, Vršac, Serbia) and (b) standard artificial sweat solution. The standard artificial sweat of pH 6.8 was used (Artificial Sweat pH 6.8, Nanochemazone, Chemazone, Inc., Canada). Up until the testing phase, the prepared fluids were kept at 4°C in the dark. Two discs of the same design were fabricated; the first was tested with saline whereas the second was tested with artificial sweat. Each electrode’s electrical continuity was measured to ensure the threads were electrically connected. The thread (Silver-Tech 120) was supplied by AMANN Group (BÖnnigheim, Germany) and it belongs to their Silver-Tech series (AMANN Group, 2017).
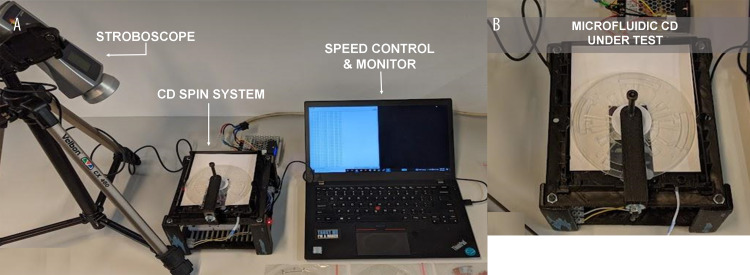
Before the target liquids were loaded into the LOAD chamber, impedance measurements were taken on the thread of each set. This was considered as the reference/dry thread measurement. Then, a total of 220 μL of liquid was carefully loaded into the LOAD chamber. When the liquid was loaded, the disc was then placed on the spin system and spun with a gradual increase of RPM (revolutions per minute) ranging from 0 to 1000 RPM. A microcontroller (ATmega328) was used for controlling the stepper motor rotation, consistent with our previous reports [21–23]. Testo 477 LED stroboscope lighting was synced with the RPM, to provide a still image-like video for observation of the liquid’s movement in the disc, as shown in Figure 3. As the disc was spun, the liquid in the disc moved towards the sectioned/metered portion of the LOAD chamber. The excess solution was drained into the waste chamber.
Figure 3.
(A) Microfluidic CD spin system set up consist of stroboscope, CD spin system and computer (speed control and monitor). (B) The microfluidic CD under test installed on the spin system.
The RPM was increased until the first and the second section in the LOAD chamber (7.5 μL and 20 μL) burst out of the LOAD chamber into the MEASUREMENT chamber. Then, the disc slowly and gradually slowed until it stopped. The impedance analyzer probe was then fixated to the conductive tape of the thread for the measurement to be taken and recorded. Once completed, the disc was spun again gradually until the subsequent metered section in the LOAD chamber bursts out into the MEASUREMENT chamber, where the disc was stopped again for the measurement to be taken. The sequence of the solution bursting out of the LOAD chamber was designed using the passive valving technique and channel dimension manipulation. It was designed to sequence the burst-out order from 7.5 μL+20 μL, 45 μL, and 120 μL respectively. This step was repeated for all of the sets. The recorded measurement took the total addition of solution in the MEASUREMENT chamber as the resulting volume. The first measurement on the thread were taken using 27.5 μL of liquid sample, 72.5 μL for the second measurement, and 192.5 μL for the third measurement.
Finally, 4 CDs were fabricated. The tolerances achieved were less than ±0.4 mm in total. Moreover, each fabricated CD contained 4 identical chambers with manually assembled electrodes. These electrodes were thoroughly checked with a multimeter before utilization for Hioki measurements. To ensure reliable conductivity, each electrode was tested multiple times during the fabrication process. If a significant resistance increment was detected, we replaced the affected parts of the electrode before CD lamination.
Impedance Analysis
Electrical continuity testing using a multimeter was performed on dry electrodes. Once the electrical continuity test was performed to confirm the electrical connectivity of each electrode, impedance analysis was conducted using HIOKI IM3590, an impedance analyzer for frequency sweep from 1 kHz to 200 kHz (500 data points). Once the reference (short circuit) measurement was completed, the dry electrode was measured, and then the target liquid was added (saline or artificial sweat) on the electrodes to perform a measurement. The impedance (modulus and angle) was measured over the frequency range.
Statistical Analysis
One-way ANOVA with post hoc Tukey HSD test was performed to analyze differences between the obtained values for all 4 measurements in both discs, with the level of significance set at P<0.05 Post hoc analyses for specific comparisons were performed for |Z| and theta values between different groups to identify nuanced differences.
Results
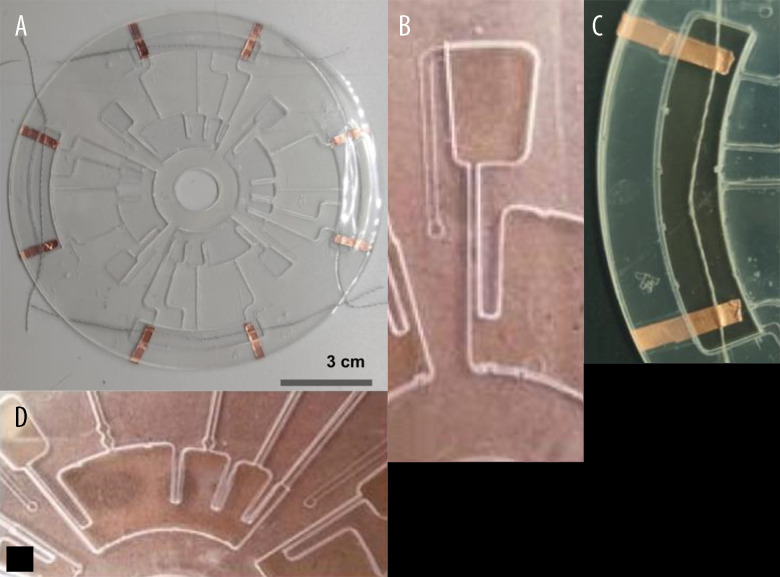
The results of the microfluidic CD fabrication are shown in Figure 4. The microfluidic CD consisted of 4 different layers of chambers and channels with additional layers as the top (vent and inlet) and bottom seals. The total weight of the fabricated microfluidic CD was 5 g. Figure 4A shows an actual image of the fabricated microfluidic CD platform integrated with conductive threads of Silver-Tech 120 for potential sweat biosensing.
Figure 4.
(A) Actual image of the fabricated microfluidic CD, (B) waste chamber, (C) MEASUREMENT section, (D) LOAD section.
As illustrated in Figure 4C, the integrated conductive thread microfluidic CD using commercially available Silver-Tech 120 threads consisted of 4 major sections with Cu electrodes, as shown in Table 1. Table 1 summarizes the testing of target liquid (ie, saline or artificial sweat) on Cu electrodes to obtain impedance over frequency range, then we calculated conductance (G) and the difference in conductance relative to dry measurement (ΔG) as an output of this study. The observed burst frequency (RPM) was experimentally determined to be in the range of 320 to 520 RPM, as shown in Table 2. The values of observed burst frequency were in accordance with theoretical expectations.
Table 1.
Preparation of analyzed liquid samples (saline and artificial sweat).
| Sets | Liquids | Volume (μL) | Output | Thread | Electrode |
|---|---|---|---|---|---|
| 1 | Saline/artificial sweat | 192.5 | G, ΔG, |Z|, Θ | Silver-tech 120* | Cu |
| 2 | Saline/artificial sweat | 192.5 | G, ΔG, |Z|, Θ | Cu | |
| 3 | Saline/artificial sweat | 27.5 72.5 192.5 |
G, ΔG, |Z|, Θ | Cu | |
| 4 | Saline/artificial sweat | 27.5 72.5 192.5 |
G, ΔG, |Z|, Θ | Cu |
Technical data: (NEN ISO 2062). Linear density (dtex) (DIN EN ISO 2060): 93 * 3; Linear density (Nm) (DIN EN ISO 2060) 108/3; Optical Diameter 0.23 mm; Breaking force (cN) (DIN EN ISO 2062) 1260; Elongation at break (%) (DIN EN ISO 2062)-16; Resistance <530 Ω/m.
Table 2.
Speed of disc (burst frequency).
| Sectioned chamber (μL) | Theoretical burst frequency (RPM) | Observed burst frequency (RPM) | |||
|---|---|---|---|---|---|
| SET 1 Cu – saline/artificial sweat |
SET 2 Cu – saline/artificial sweat |
SET 3 Cu – saline/artificial sweat |
SET 4 Cu – saline/artificial sweat |
||
| 7.5 | 385 | 320 | 320 | 350 | 370 |
| 20 | 400 | 320 | 320 | 350 | 370 |
| 45 | >400* | 480 | 470 | 470 | 490 |
| 120 | >400* | 510 | 520 | 520 | 500 |
The theoretical speed was calculated using the burst frequency formula reported in Madou et al [24] without the account for additional passive valves.
One-way ANOVA with post hoc Tukey HSD test was used to analyze the differences between obtained values for all 4 measurements in both discs. For |Z| values, there were significant differences between Group 1 (dry fibers) and Groups 2, 3, and 4 (different amounts of saline) with P values of 0.0001, 0.0000, and 0.0000, respectively. However, the trend was present, as can be seen in Figure 5, but no significant differences were found between Group 5 (dry fibers) and Groups 6, 7, and 8 (different amounts of artificial sweat), as their P values were 0.4147, 0.1546, and 0.1374, respectively. For impedance angle values, there were no significant differences between Group 1 (dry fibers) and Groups 2, 3, and 4 (different amounts of saline), as their P values were 0.9916, 0.8836, and 0.8575, respectively. Similarly, no significant differences were observed between Group 5 (dry fibers) and Groups 6, 7, and 8 (different amounts of artificial sweat), with P values of 0.9999, 0.9996, and 0.9990, respectively. In addition, for |Z| values, Group 2 (27.5 μL of saline) showed a significant difference compared to Group 6 (27.5 μL of artificial sweat) with a P value of 0.0111; Group 3 (72.5 μL of saline) showed a significant difference compared to Group 7 (72.5 μL of artificial sweat) with a P value of 0.0000; Group 4 (192.5 μL of saline) showed a significant difference compared to Group 8 (192.5 μL of sweat) with a P value of 0.0000. For theta values, post hoc analysis revealed Group 2 (27.5 μL of saline) and Group 6 (27.5 μL of artificial sweat) did not show a significant difference in impedance angle values, with a P value of 1.0000; Group 3 (72.5 μL of saline) and Group 7 (72.5 μL of artificial sweat) did not show a significant difference in theta values, with a P value of 0.9944; and Group 4 (192.5 μL of saline) and Group 8 (192.5 μL of sweat) did not show a significant difference in theta values, with a P value of 0.9949. These results indicate that the equivalence between the corresponding saline and sweat treatment groups (Groups 2 and 6, Groups 3 and 7, and Groups 4 and 8) showed no significant differences in impedance angle values, suggesting similar properties between the exposure to equivalent amounts of liquids. However, there were significant differences observed in |Z| values, indicating variations in fiber characteristics between the equivalent exposures. Finally, comparisons were performed between various amounts of liquids and the data suggested the following: The |Z| value comparisons within saline groups (Groups 2 (27.5 μL), 3 (72.5 μL), and 4 (192.5 μL) showed significant differences, with Group 3 having the highest |Z| value, followed by Group 4, and Group 2 having the lowest |Z| value. For artificial sweat groups, Groups 6 (27.5 μL), 7 (72.5 μL), and 8 (192.5 μL) had significantly different |Z| values, with Group 7 having the highest |Z| value, followed by Group 8, and Group 6 having the lowest |Z| value. No significant differences were found in impedance angle values among all the compared groups.
Figure 5.
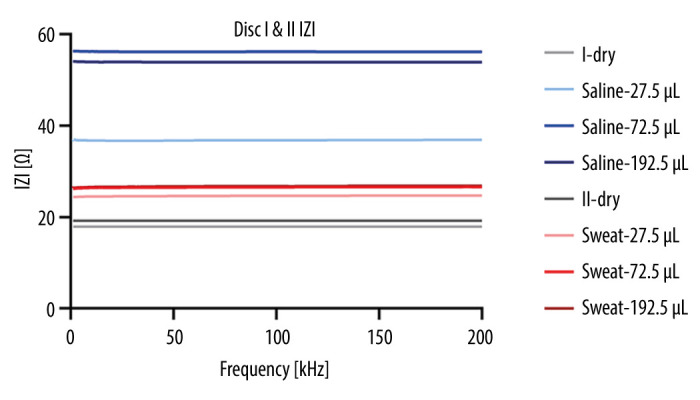
The mean values of all types of measurement.
Discussion
This study presents (1) for the first time, an application of a precise, cost-effective, and rapid prototyping xurography technique to fabricate the lightweight CD-like platform for potential biomedical applications; (2) a comprehensive electrochemical analysis of a system consisting of thread-biological fluids electrodes; and (3) a microfluidic CD as a scaffold for the fabrication of an electrochemical analysis system as biosensors for future biomedical and industrial applications [25–27]. The present study represents an advancement in the field of microfluidic biosensing, introducing a novel approach that integrates conductive threads into a microfluidic compact disc (CD) platform. This approach, facilitated by the precise, cost-effective, and flexible rapid prototyping xurography technique, allows for the fabrication of a lightweight CD-like platform, offering potential applications in both biomedical and industrial settings. This study marks the first application of conductive fibers in a microfluidic CD system, presenting an innovative combination of technologies for potential sweat biosensing. The flexibility of the middle layer material, combined with the integration of conductive threads, represents a novel direction for microfluidic CD development. To the best of our knowledge, the integration and characterization of conductive fibers within microfluidic CD systems have not been previously explored. In this study, the microfluidic CD platform was carefully designed and fabricated with 6 layers, including chambers and channels, with additional top and bottom seals. The successful fabrication of the microfluidic CD, as depicted in Figure 4, showcases the potential of the xurography technique for rapid and precise prototyping of complex microfluidic systems. The resulting lightweight CD-like platform, weighing only 5 grams, highlights its suitability for portable and point-of-care applications.
For electroanalytical applications, thread-based microfluidic devices have shown promising performance. Agustini et al [26] demonstrated the use of thread microchannels for electrochemical micro-flow injection analysis without the need for any treatment before use. A study by Carneiro et al [27] combined 3D-printed microfluidic structures with cotton threads for amperometric detection of antioxidants in wine samples, achieving low limits of detection and excellent sensitivity compared to other sensors.
In this study, the fabrication method chosen was determined by its ability to produce a part with desired properties. In a short amount of time, this method allows for the lamination of multilayered structures carved from pressure-sensitive and thermally activated adhesive foils (less than 15 minutes). While the advantage of flexibility should be considered in the context of the fabrication technique and platform versatility, we acknowledge that the physical deformability of the microfluidic CD poses complexities in precision during analysis. Future research will be dedicated to exploring and addressing these complexities, focusing on optimizing device design, materials, and mechanical properties to ensure the precision and accuracy of analyte detection in various applications.
Various fabrication techniques for microfluidic devices are used to achieve maximum performance while lowering fabrication costs. The main directions of development include silicon-based microfabrication, which is essentially based on microelectromechanical systems (MEMS) fabrication techniques, or, as an alternative material, glass, rapid prototyping in polymers (including elastomers: polydimethylsiloxane, poly (methyl methacrylate), and thermoplastics like 3D printing, and paper-based techniques. Our research in detection development combines a microfluidic platform that aims to detect the electrical parameters of the conductive fiber integrated into the cells of the microfluidic system. To the best of our knowledge, conductive fibers and their characterization under the influence of physiological liquids have not been previously implemented in microfluidic CD systems.
Sweat is becoming an extremely valuable analyte in many biomedical applications, as well as for various types of industrial applications. Sweat, as a rich source of electroconductive electrolytes such as sodium and potassium, holds great significance in numerous biomedical and clinical settings. It has been explored for a variety of applications, including early diagnosis of cystic fibrosis, assessment of hydration levels, and as an indicator of various physiological conditions [8]. Additionally, sweat conductivity has been investigated in industrial applications, offering insights into the effects of moisture on electrical properties in textiles and e-textile applications. With the integration of conductive fibers into the microfluidic CD platform, this study harnessed the potential of sweat as a valuable analyte for biosensing applications. The comprehensive electrochemical analysis conducted in this study offers valuable insights into the behavior of the integrated system comprising conductive threads, copper electrodes, and 2 types of conductive solutions (saline and artificial sweat). The choice of electrodes plays a crucial role in the performance of the electrochemical system, as their conductivity significantly affects the measurement outcomes. Copper (Cu) was selected as electrode material due to its favorable conductivity properties. Concerning electrodes, copper-based electrodes are the best choice for various electronic applications. The main criterion for electrode material selection is conductivity. The conductivity of annealed copper is 59×106 siemens/meter. In this study, the impedance pattern of saline and artificial sweat was tested on Cu electrodes, as shown in Figures 6 and 7. Data from the National Institute of Standards and Technology indicate a conductivity of κ=14.5 mS/cm (and resistivity ρ=68.9 Ω/cm) for a 0.9% saline solution at 22°C [28]. The mean value for the sweat conductivity is 16 150 mΩ/cm [29]. The electric resistance (nominal) of silver-coated polyamide/polyester hybrid thread Silver-Tech 120 in a published work was reported to be <530 Ω/m [30]. The artificial sweat conductivity ranges from 2.5 to 40 mS/cm [31,32]. Silver-based conductive threads for creating sewn circuits in biomedical applications due to broad-spectrum antimicrobial, antibacterial, and antistatic properties. These threads have excellent thermal conductivity and stability and are gentle on the skin. A recent study by Qureshi et al [33] compared the impedance change of 2 types of sensors as a function of moisture content to assess the sensor’s utility. Embroidered electrodes exhibit significant impedance activity due to a somewhat polarizable electric characteristic of the moist substrate. It behaves like a lossy capacitor, with a dielectric constant that changes proportionally with moisture and conductivity that increases as leakage occurs.
Figure 6.
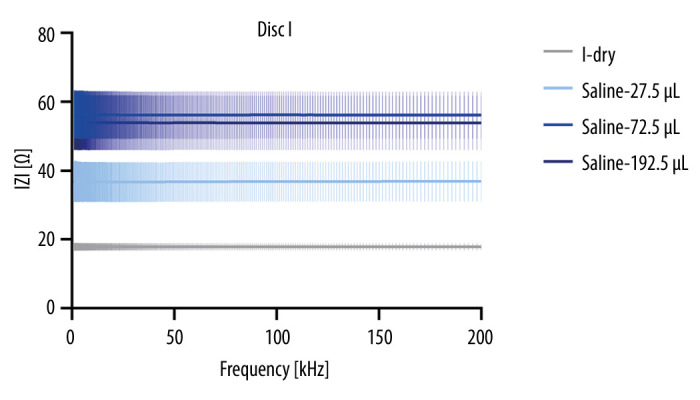
Impedance modulus of dry disc and measurements with saline – mean and standard deviation.
Figure 7.
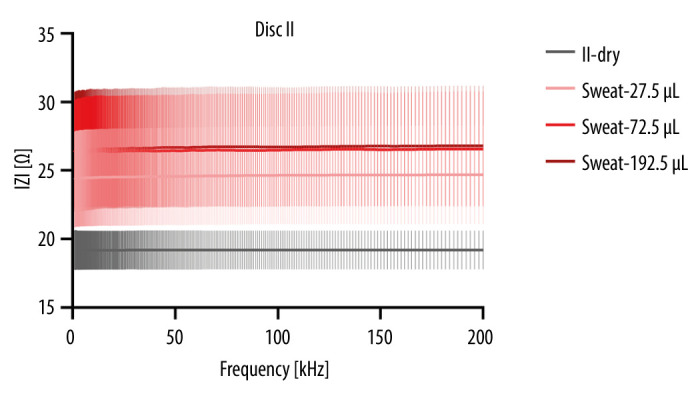
Impedance modulus of dry disc and measurements with artificial sweat – mean and standard deviation.
Saline and artificial sweat share certain similarities and differences in their composition and properties. Both solutions aim to replicate the electrolyte balance and physiological characteristics, making them valuable tools in various scientific and industrial applications, as described in the report similarly investigating the effect of various physiological solutions on fibers [34]. However, a notable difference between them lies in their burst frequency, as observed in our study, a divergence attributed to variations in viscosity and manufacturing tolerance. While both solutions strive to mimic the ionic composition of physiological bodily fluids, the dissimilarities in these physical aspects can lead to divergent burst frequencies.
In a study by Zhao et al [35], a flexible and wearable microfluidic device known as the microfluidic thread/fabric-based analytical device was developed for sweat sensing and monitoring. This skin-mounted band integrated hydrophilic dot patterns with a hydrophobic surface, enabling precise analysis of local sweat loss, pH, chloride, and glucose concentrations in sweat [35]. Li et al [36] summarized recent advancements in wearable microfluidic sensors for healthcare monitoring. These sensors, attached to the skin, provided high precision assessment of physiological information such as sweat loss, metabolites, and electrolyte balance. Curto et al [10] introduced an autonomous and wearable microfluidic platform capable of real-time pH analysis in sweat. The platform incorporated an optical detection system with a surface mount light-emitting diode and a light photosensor, enabling successful real-time monitoring of sweat pH during physical activities. Ahlawat, Bhatia, and Kumar highlighted the growing significance of thread-based microfluidic devices as inexpensive and disposable alternatives for detecting biomarkers in urine or blood [37]. The study emphasized the advantages of multifilament threads, including low cost, light weight, easy availability, and hydrophilic nature, making them suitable for various microfluidics-based technologies. Capillary channels within threads facilitated easy fluid flow, eliminating the need for external pumping systems. Thread-based microfluidic sensors were considered advantageous over paper-based sensors due to their broader material choices and cost-effectiveness [37].
Based on previous research, the conductivity of the base fabrics has a notable influence on the mechanical strain experienced during laundry conditions. Moreover, wet chemical processes applied to textiles significantly affect the electrical resistance of conductive textile materials. These materials are utilized as conductive tracks and transmission lines in electronic textile (e-textile) applications, as indicated in studies by Simegnaw et al [38], Jia et al [19], Zhao et al [35], Li et al [36], Curto et al [10], and Ahlawat et al [37]. Introducing rotation in the microfluidic compact disc (CD) induces centripetal force, which facilitates fluid movement within the disc. This innovative technique enables precise manipulation of small fluid volumes and controlled delivery of analytes to specific chambers, thereby enhancing the accuracy and sensitivity of biosensing. By examining the conductance values and impedance patterns of electrodes in response to varying volumes of saline and artificial sweat, valuable insights are gained into the dynamic behavior of the system. A comprehensive understanding of the electrochemical characteristics exhibited by the integrated system under different physiological fluid volumes is pivotal in optimizing the platform’s overall performance and sensitivity. Our findings align with the detection of liquid quantities and notably distinguish between saline solutions, exhibit a discernible trend with artificial sweat, and offer a reasonable basis for comparing results with the aforementioned studies.
The integration of conductive fibers within the microfluidic CD platform opens up exciting possibilities for various biomedical and industrial applications. In the field of biomedicine, the ability to biosense physiological fluids such as sweat offers numerous advantages, including non-invasiveness, real-time monitoring, and potential early detection of diseases. For instance, the detection of specific biomarkers in sweat could provide valuable insights into an individual’s health status and help diagnose conditions such as cystic fibrosis, diabetes, and dehydration. Moreover, the integration of conductive threads into wearable biosensing devices could revolutionize fitness monitoring and personalized healthcare by providing continuous, unobtrusive monitoring of vital signs.
While this study presents a novel in vitro biosensing platform, several limitations warrant consideration. The characterization of the fiber’s structural aspects, although recognized as essential, remains an aspect that could provide deeper insights into the system. Building upon the current study, there are several avenues for future research that could address its limitations and expand its scope. Further investigations should encompass diverse fiber types, a wider range of parameters, and the inclusion of frequencies below 1 kHz in the electrochemical impedance spectroscopy (EIS). By embarking on these enhancements, the study seeks to refine its contributions to the understanding of the biosensing platform’s capabilities and potential applications.
Further investigations into the electrochemical behavior of different electrode materials and conductive solutions are warranted to optimize the platform’s performance for specific applications. Exploring the influence of various physiological factors, such as temperature and pH, on the conductive thread’s behavior will also be important for ensuring the platform’s robustness and applicability under diverse conditions. Moreover, the integration of specific sensing elements within the microfluidic CD platform, such as antibodies or molecular probes, will enable targeted detection of biomolecules in sweat, enhancing the platform’s specificity and sensitivity. Miniaturization and wearable integration of the biosensing system will be crucial for translating the technology into real-world applications.
In conclusion, this study demonstrates the potential of integrating conductive threads within a microfluidic CD platform for future sweat sensing applications. The combination of rapid prototyping xurography and conductive fibers opens up new opportunities for the development of innovative biosensing technologies with diverse applications in biomedical and industrial fields. Further advancements in materials, fabrication techniques, and sensor integration will pave the way for transformative biosensing devices, ushering in a new era of personalized and non-invasive healthcare monitoring. Despite the promising results obtained in this study, several challenges and future directions must be addressed to fully realize the potential of the integrated microfluidic CD platform. The observed behavior of the system with very small volumes of tested solutions highlights the need for precise control and standardization of liquid volumes to ensure precise and reliable measurements. It can be observed in Figure 8 and particularly in Figure 9 that the measurements with the smallest amount of liquid did not have reliable outputs, since the amount was apparently not enough to completely wet the electrode and capillary forces moved the liquid during measurements. It was also observed that the conductance decreased with an increased volume of saline or artificial sweat. The Ag coating of the thread is covered with cracks and damage (Stavrakis et al, 2021). By wetting it, the cracks in the coating become wider and small coating flakes are disengaged or partially detached from the coating layer. This results in partial reductance of a conductive path; therefore, conductance will be reduced. Probably, the threads will become damaged from the artificial sweat or saline and wetted, which can cause the silver coating of the thread to develop cracks and degrade the conductive coating separated and hence, reducing conductivity and increasing capacitance. This is evident in Figure 9, where the ΔG increases as the total volume in the chamber increases.
Figure 8.
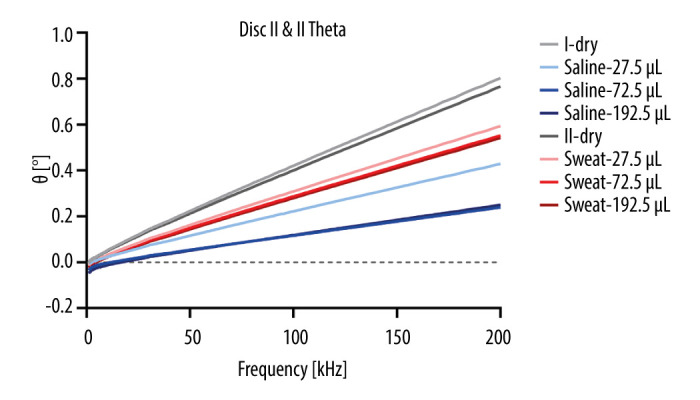
Mean values of theta for all measurements (standard deviation not shown due to small values).
Figure 9.
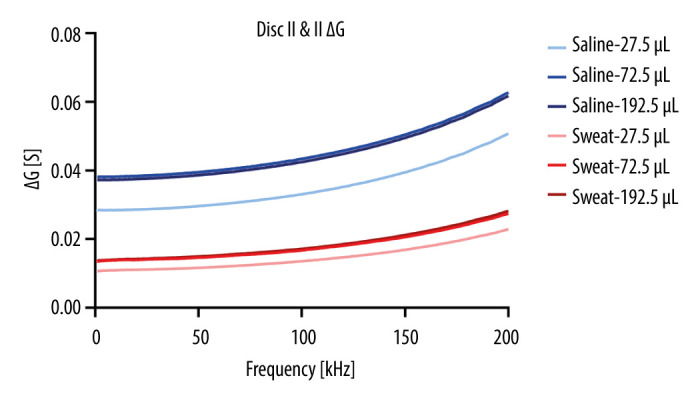
ΔG over frequency for all samples.
The innovative approach employed in the study involves the integration of conductive threads into a microfluidic CD platform for the purpose of sweat biosensing. This approach introduces a novel dimension to the field, combining rapid prototyping xurography with conductive fibers to facilitate the development of a lightweight and cost-effective biosensing device. Additionally, it opens up new avenues for personalized healthcare monitoring. The effective fabrication of the microfluidic CD, as depicted in Figure 4, exemplifies the potential of the xurography technique in creating intricate microfluidic systems with precise control. This method allows for the lamination of multilayered structures within a remarkably short timeframe, highlighting its efficiency in rapid prototyping. The resulting CD-like platform, weighing only 5 grams, showcases its portability, making it suitable for point-of-care applications and continuous health monitoring. In future applications, the integration of specific sensing elements, such as antibodies or molecular probes, can be explored to enhance the platform’s specificity and enable targeted detection of biomolecules in sweat. This study sets the stage for further investigations into optimizing the platform’s performance, exploring diverse electrode materials, and expanding the scope of physiological factors considered.
The behavior observed in the system when tested with very small volumes of solutions, as illustrated in Figure 8 and particularly in Figure 9, emphasizes the importance of precise control and standardization of liquid volumes to ensure accurate measurements. The challenges associated with the reliability of outputs, especially with minimal liquid volumes, can be attributed to incomplete wetting of the electrode caused by capillary forces. Figure 6 shows that the conductance decreases as the volume of saline or artificial sweat increases, indicating potential damage to the conductive thread coating. The cracks and damages in the silver coating of the thread, exacerbated by wetting, contribute to the reduction in conductivity and an increase in capacitance. This phenomenon underscores the significance of material durability, particularly when exposed to physiological fluids, and suggests the need for further exploration in material engineering to enhance the robustness of the conductive fibers. Future research endeavors will aim to refine the capabilities of the platform, address these challenges, and explore advanced materials and fabrication techniques to pave the way for transformative developments in the field of microfluidic biosensing.
Conclusions
This study has explored the optimal configuration of multiplex and cost-effective techniques for fabricating microfluidic devices on compact discs (CDs) specifically designed for biomedical applications and general health monitoring purposes. By integrating low-cost microfluidics on CDs with foils produced by the xurography method, we have developed chambers that enable the analysis of the intricate interaction between conductive thread and physiological liquids. To scrutinize the effects of different quantities of saline and artificial sweat, we employed comprehensive methods based on electrical impedance analysis. The obtained results reveal that our microfluidic CD system has exceptional sensitivity in detecting changes in impedance patterns within a small volume range, thereby eliminating the need for extensive calibration.
Supplementary Data
Design dimensions
Footnotes
Conflict of interest: None declared
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Declaration of Figures’ Authenticity: All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: This research received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 872370, and European Union’s Horizon 2020 research and innovation programme under the grant agreement No. 854194, and University Malaya Partnership Grant No. RK006-2021
References
- 1.Aeinehvand MM, Ibrahim F, Al-Faqheri W, et al. Recent advances in the development of micropumps, microvalves and micromixers and the integration of carbon electrodes on centrifugal microfluidic platforms. International Journal of Nanotechnology. 2018;15(1–3):53–68. [Google Scholar]
- 2.Francis J, Stamper I, Heikenfeld J, Gomez EF. Digital nanoliter to milliliter flow rate sensor with in vivo demonstration for continuous sweat rate measurement. Lab Chip. 2018;19(1):178–85. doi: 10.1039/c8lc00968f. [DOI] [PubMed] [Google Scholar]
- 3.Seok Y, Batule BS, Kim MG. Lab-on-paper for all-in-one molecular diagnostics (LAMDA) of zika, dengue, and chikungunya virus from human serum. Biosens Bioelectron. 2020;165:112400. doi: 10.1016/j.bios.2020.112400. [DOI] [PubMed] [Google Scholar]
- 4.Amirifar L, Besanjideh M, Nasiri R, et al. Droplet-based microfluidics in biomedical applications. Biofabrication. 2022;14(2):022001. doi: 10.1088/1758-5090/ac39a9. [DOI] [PubMed] [Google Scholar]
- 5.Sayad A, Ibrahim F, Mukim Uddin S, et al. A microdevice for rapid, monoplex and colorimetric detection of foodborne pathogens using a centrifugal microfluidic platform. Biosens Bioelectron. 2018;100:96–104. doi: 10.1016/j.bios.2017.08.060. [DOI] [PubMed] [Google Scholar]
- 6.Al-Faqheri W, Ibrahim F, Thio THG, et al. Vacuum/Compression Valving (VCV) using parrafin-wax on a centrifugal microfluidic CD platform. PLoS One. 2013;8(3):e58523. doi: 10.1371/journal.pone.0058523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatterjee K, Tabor J, Ghosh TK. Electrically conductive coatings for fiber-based E-textiles. Fibers. 2019;7(6):51. [Google Scholar]
- 8.Bilir MZ, Gök MO. The effect of perspiring on conductivity in electronic textile design. Marmara Fen Bilimleri Dergisi. 2017;29(2):48–53. [Google Scholar]
- 9.Possanzini L, Decataldo F, Mariani F, et al. Textile sensors platform for the selective and simultaneous detection of chloride ion and pH in sweat. Sci Rep. 2020;10(1):17180. doi: 10.1038/s41598-020-74337-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curto VF, Coyle S, Byrne R, et al. Concept and development of an autonomous wearable micro-fluidic platform for real time pH sweat analysis. Sensors and Actuators B: Chemical. 2012;175:263–70. [Google Scholar]
- 11.Biosensors. Free Full-Text. Epidermal wearable biosensors for monitoring biomarkers of chronic disease in sweat. [Accessed November 24, 2023]. https://www.mdpi.com/2079-6374/13/3/313 . [DOI] [PMC free article] [PubMed]
- 12.Gao F, Liu C, Zhang L, et al. Wearable and flexible electrochemical sensors for sweat analysis: A review. Microsyst Nanoeng. 2023;9(1):1–21. doi: 10.1038/s41378-022-00443-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao W, Emaminejad S, Nyein HYY, et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature. 2016;529(7587):509–14. doi: 10.1038/nature16521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammond KB, Turcios NL, Gibson LE. Clinical evaluation of the macroduct sweat collection system and conductivity analyzer in the diagnosis of cystic fibrosis. J Pediatr. 1994;124(2):255–60. doi: 10.1016/s0022-3476(94)70314-0. [DOI] [PubMed] [Google Scholar]
- 15.Heikenfeld J. Technological leap for sweat sensing. Nature. 2016;529(7587):475–76. doi: 10.1038/529475a. [DOI] [PubMed] [Google Scholar]
- 16.Curto VF, Fay C, Coyle S, et al. Real-time sweat pH monitoring based on a wearable chemical barcode micro-fluidic platform incorporating ionic liquids. Sensors and Actuators B: Chemical. 2012;171–172:1327–34. [Google Scholar]
- 17.Stojanović GM, Radetić MM, Šaponjić ZV, et al. A textile-based microfluidic platform for the detection of cytostatic drug concentration in sweat samples. Applied Sciences. 2020;10(12):4392. [Google Scholar]
- 18.Terse-Thakoor T, Punjiya M, Matharu Z, et al. Thread-based multiplexed sensor patch for real-time sweat monitoring. npj Flex Electron. 2020;4(1):1–10. [Google Scholar]
- 19.Jia J, Xu C, Pan S, et al. Conductive thread-based textile sensor for continuous perspiration level monitoring. Sensors. 2018;18(11):3775. doi: 10.3390/s18113775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thio THG, Soroori S, Ibrahim F, et al. Theoretical development and critical analysis of burst frequency equations for passive valves on centrifugal microfluidic platforms. Med Biol Eng Comput. 2013;51(5):525–35. doi: 10.1007/s11517-012-1020-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thiha A, Ibrahim F, Joseph K, et al. A novel microfluidic compact disc to investigate electrochemical property changes between artificial and real salivary samples mixed with mouthwashes using electrical impedance analysis. PLoS One. 2023;18(2):e0280381. doi: 10.1371/journal.pone.0280381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thiha A, Ibrahim F. A Colorimetric Enzyme-Linked Immunosorbent Assay (ELISA) detection platform for a point-of-care dengue detection system on a lab-on-compact-disc. Sensors. 2015;15(5):11431–41. doi: 10.3390/s150511431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uddin SM, Ibrahim F, Sayad AA, et al. A portable automatic endpoint detection system for amplicons of loop mediated isothermal amplification on microfluidic compact disk platform. Sensors. 2015;15(3):5376–89. doi: 10.3390/s150305376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madou MJ, Lee LJ, Daunert S, et al. Design and fabrication of CD-like microfluidic platforms for diagnostics: Microfluidic functions. Biomedical Microdevices. 2001;3(3):245–54. [Google Scholar]
- 25.Weng X, Kang Y, Guo Q, et al. Recent advances in thread-based microfluidics for diagnostic applications. Biosens Bioelectron. 2019;132:171–85. doi: 10.1016/j.bios.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agustini D, Fedalto L, Bergamini MF, Marcolino-Junior LH. Microfluidic thread based electroanalytical system for green chromatographic separations. Lab Chip. 2018;18(4):670–78. doi: 10.1039/c7lc01267e. [DOI] [PubMed] [Google Scholar]
- 27.Carneiro MCCG, Moreira FTC, Dutra RAF, et al. Homemade 3-carbon electrode system for electrochemical sensing: Application to microRNA detection. Microchemical Journal. 2018;138:35–44. [Google Scholar]
- 28.Sauerheber R, Heinz B. Temperature effects on conductivity of seawater and physiologic saline, mechanism and significance. Chem Sci J. 2015;6:4. [Google Scholar]
- 29.Licht TS, Stern M, Shwachman H. Measurement of the electrical conductivity of sweat: Its application to the study of cystic fibrosis of the pancreas. Clin Chem. 1957;3(1):37–48. [PubMed] [Google Scholar]
- 30.Stavrakis AK, Simić M, Stojanović GM. Electrical characterization of conductive threads for textile electronics. Electronics. 2021;10(8):967. [Google Scholar]
- 31.Hourlier-Fargette A, Schon S, Xue Y, et al. Skin-interfaced soft microfluidic systems with modular and reusable electronics for in situ capacitive sensing of sweat loss, rate and conductivity. Lab Chip. 2020;20(23):4391–403. doi: 10.1039/d0lc00705f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ortega L, Llorella A, Esquivel JP, Sabaté N. Self-powered smart patch for sweat conductivity monitoring. Microsyst Nanoeng. 2019;5(1):1–10. doi: 10.1038/s41378-018-0043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qureshi S, Stojanović GM, Simić M, et al. Silver conductive threads-based embroidered electrodes on textiles as moisture sensors for fluid detection in biomedical applications. Materials. 2021;14(24):7813. doi: 10.3390/ma14247813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz D, Magen YK, Levy A, Gefen A. Effects of humidity on skin friction against medical textiles as related to prevention of pressure injuries. Int Wound J. 2018;15(6):866–74. doi: 10.1111/iwj.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Z, Li Q, Chen L, et al. A thread/fabric-based band as a flexible and wearable microfluidic device for sweat sensing and monitoring. Lab Chip. 2021;21(5):916–32. doi: 10.1039/d0lc01075h. [DOI] [PubMed] [Google Scholar]
- 36.Li S, Ma Z, Cao Z, et al. Advanced wearable microfluidic sensors for healthcare monitoring. Small. 2020;16(9):1903822. doi: 10.1002/smll.201903822. [DOI] [PubMed] [Google Scholar]
- 37.Ahlawat S, Bhatia R, Kumar B. Impact of thread-based microfluidic devices in modern analysis: An update on recent trends and applications. Current Analytical Chemistry. 2023;19(4):281–97. [Google Scholar]
- 38.Simegnaw AA, Malengier B, Tadesse MG, et al. Study the electrical properties of surface mount device integrated silver coated vectran yarn. Materials. 2022;15(1):272. doi: 10.3390/ma15010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Design dimensions