Abstract
Black men are disproportionately affected by type 2 diabetes (T2D) and experience higher diabetes-related complications than non-Hispanic White men. To address the complex barriers in diabetes self-management for Black men, we implemented a 3-month peer-led and empowerment-based Diabetes Self-Management Education (DSME) and Support (DSMS) intervention in Metro Detroit. Twenty-five Black men ≥55 years of age with self-reported T2D were randomized to the intervention group (n=12)—10 hr of DSME and 9 hr of DSMS—or enhanced usual care (EUC) group (n=13)—10 hr of DSME. Peer leaders (n = 3) were trained by certified diabetes care and education specialists (CDCESs) to cofacilitate the support sessions. Outcomes (hemoglobin A1c [HbA1c], diabetes self-care activities, and diabetes distress) were assessed preintervention and postintervention. In the intervention and EUC groups, mean HbA1c decreased by 0.20% (p = .52, SD = 0.99) and 0.13% (p = .68), respectively. General diet (p = .03, M change: 1.32, SD = 1.71) and blood glucose monitoring (p < .05, M change: 0.50, SD = 0.74) scores improved among those in the intervention group. General diet scores also improved in the EUC group: mean change: 1.77, p = .08, although changes were not statistically significant. Changes in diabetes distress scores differed based on the number of sessions attended, with a significant decrease in those attending 7 to 12 sessions (n = 7), >50%, (p = .003, M change: −5.71, SD = 3.20). Implementing a peer-led DSMS program for Black men was feasible, adopted, and led to positive changes in outcomes. Scaling up the intervention and assessing sustainability is warranted.
Keywords: type 2 diabetes, Black men, self-management education/support, peer leaders
Introduction
In the United States, 11.6% of adults aged 18 years or older are living with type 2 diabetes (T2D) (Centers for Disease Control and Prevention [CDC], 2023). T2D risk is associated with the built environment, particularly in urban settings such as Metro Detroit, Michigan (Amuda & Berkowitz, 2019). T2D ranks as the eighth leading cause of death in the Metro Detroit area (Tian, 2023). Among individuals 45 to 64 years of age and 65 years and older residing in Metro Detroit, 16.1% and 23.3%, respectively, are living with a diabetes diagnosis (CDC, 2024). The prevalence of diabetes is significantly higher in Black men, who are at an increased risk of developing the condition and are more likely to die from its complications than non-Hispanic White men (CDC, 2023; Michigan Department of Community Health [MDCH], 2019). Black men are more likely to experience blood glucose levels above target ranges relative to non-Hispanic White men (CDC, 2023). In 2021, age-adjusted diabetes death rates per 100,000 people in Michigan were 32.5 for White males, 18.4 for White females, 50.0 for Black males, and 35.0 for Black females (Michigan Department of Health & Human Services [MDHHS] & Division for Vital Records & Health Statistics, 2022).
The need to exhibit strength, confidence, emotional restraint, and authority serves as an obstacle for Black males in adopting guidance from health care professionals and accessing support from family and community members (Hawkins et al., 2015, 2017). Black men may encounter unique challenges when trying to adopt health-promoting behaviors. Challenges include a lack of social support, poor relationships with health care providers as a result of mistrust, financial constraints due to high medical fees, the burden of long working hours, and low health literacy due in part to systemic barriers (Harvey & Alston, 2011; Hawkins et al., 2015, 2017; Liburd et al., 2007; Muvuka et al., 2020).
Gender-related norms among Black men also include the “superman syndrome,” which is the belief that Black men can manage their health without the help of health care professionals (Harvey & Alston, 2011; Hawkins et al., 2015, 2017; Liburd et al., 2007). The literature emphasizes the role of gender in health behavior management and highlights the conflict between male gender norms and adapting self-management behaviors and engagement with health care (Courtenay, 2000). Findings underscore the importance of incorporating health messaging in diabetes self-management that aligns with the values and goals specific to Black men. To promote gender-specific programming, male interventionists such as peer leaders, should be integrated to reduce the stigma associated with seeking health care, to enhance communication with health care providers, and to address specific beliefs that undermine health (Fleming et al., 2014).
Peer leaders, who are trained lay individuals, play an integral role in improving T2D self-management within minority communities by providing diabetes education, assisting with goal setting, problem-solving, and offering social and emotional support (Lee et al., 2018; Spencer et al., 2013). In racially marginalized populations, the involvement of peer leaders who share cultural identity and have strong connections in the community is vital for improving diabetes management (Heisler, 2007, 2010; Peers for Progress, 2010). Although studies have identified the effectiveness of interventions that utilize peer leader models to enhance blood glucose monitoring and clinical outcomes (such as hemoglobin A1c [HbA1c] levels) among racially marginalized populations, these interventions are not tailored to meet the needs of Black men (Balls-Berry et al., 2015). Particularly, the lack of content customization and messaging adjustment is coupled with the predominant use of female peer leaders in these studies, with women generally comprising most participants in diabetes intervention studies (Sherman et al., 2017). Matching peer leaders with participants based on race and gender has the potential to enhance the appropriateness of the intervention content and messaging (Hurt et al., 2015; Maulsby et al., 2013; Sherman et al., 2017).
While there is limited research on the use of male peer leaders with Black men living with T2D, male peer leaders have been used to address other health concerns, such as HIV, to provide health education and support to Black men (Maulsby et al., 2013). There is a gap however, in that this research primarily consists of nonrandomized feasibility studies with small sample sizes. It is important to assess whether the use of male peer leaders as interventionists can improve diabetes self-management outcomes through empirically tested intervention methods.
The most effective approaches to diabetes management incorporate Diabetes Self-Management Education (DSME) and Support (DSMS). These two components encompass the necessary knowledge, skills, and abilities for individuals to care for their diabetes on an ongoing basis. DSME takes into account the individual’s needs, goals, and life experiences, guided by evidence-based standards (Powers et al., 2016). Research findings demonstrate the effectiveness of DSME in improving clinical outcomes, quality of life, and cost-effectiveness in the short term (Lee et al., 2018; Spencer et al., 2013). However, it is acknowledged that DSME alone is insufficient to sustain the substantial self-management efforts required for T2D. To maintain these improvements, ongoing DSMS is needed, which involves activities to support the implementation and continuation of behaviors essential for managing T2D (Davis et al., 2022). DSMS can be behavioral, educational, psychosocial, and/or clinical in nature, and both health professionals and trained peer leaders can effectively deliver DSMS (Beck et al., 2017; Piatt et al., 2010, 2012; Piatt & Zgibor, 2010; Powers et al., 2016; Tang et al., 2011; Tang, Funnell, Noorulla, et al., 2012; Tang, Funnell, & Oh, 2012).
The empowerment philosophy provided the foundation for both the DSME and DSMS intervention strategies and the peer leader training program (Funnell et al., 2005, 2007). When using this philosophy, participants reflect on their self-management practices, discuss the emotional aspects of living with diabetes, and play an active role in structured, person-centered processes for establishing goals and resolving issues (Funnell, 2023; Funnell et al., 2005). Educational strategies include self-discovery and problem-based learning, self-determined goal setting, shared decision-making, creating equal partnerships, and using person-centered communication and nonstigmatizing language (Funnell, 2023; Kloss et al., 2022).
Empowerment-based DSME and DSMS have been effective in other studies that have used peer leaders in partnership with certified diabetes care and education specialists (CDCESs) (Funnell et al., 2005; Piatt et al., 2018; Tang, Funnell, & Oh, 2012). A study by Tang, Funnell, and Oh (2012) used a participant-driven model, where individuals had control over how often support was provided. This model advanced self-sufficiency and suggested that training peer leaders to provide empowerment-based DSMS had sustained positive effects on self-care behaviors (e.g., diet and insulin use) and metabolic/cardiovascular health outcomes (e.g., glycemia and serum cholesterol) (Tang, Funnell, & Oh, 2012). In DSMS sessions with peer leaders, emphasis was placed on supporting participants with self-management skills and emotional coping, which positively affected diabetes-related health behaviors and health outcomes. In the Funnell et al. (2005) study, the combination of the culturally sensitive and patient-centered approach resulted in positive outcomes and participant empowerment. Group support, efficiency, and sustained positive changes were reported by participants during the 1-year follow-up period. The program, guided by empowerment principles, stressed informed decision-making and behavioral goal setting, and integrated clinical, psychosocial, and behavioral components. As evidenced by feedback from participants and educators, the program placed value in trusting participants’ abilities to manage their diabetes and in the importance of the empowerment-focused approach in DSME (Funnell et al., 2005). See Tang and Funnell for a more detailed explanation of the empowerment-based training approach (Funnell et al., 2005; Tang, Funnell, & Oh, 2012).
Project Support, Education, and Evaluation in Diabetes (SEED) used an empowerment-based approach to both DSME and DSMS, focusing on selecting and training peer leaders who, as individuals with diabetes themselves, brought shared experiences and empathy to the program (Piatt et al., 2018). The training content was rooted in the empowerment philosophy and focused on person-centered goal setting using the Specific, Measurable, Actionable, Relevant, and Timely (SMART) criteria. Through group-based problem-solving sessions, self-discovery, and ongoing support, participants engaged in collaborative learning, and shared insights and strategies for effective diabetes self-management. These studies and the work of others (Spencer et al., 2013) highlight the importance of peer leaders who adopt an empowerment-based approach in DSMS for adults living with T2D.
Integrating DSMS into communities, and leveraging available resources and community infrastructures are critical for lifelong diabetes management. There is limited research on how to best organize and implement these interventions in real-world community settings, particularly for Black men. This study aimed to examine the feasibility and acceptability of an innovative model of DSMS using peer leaders within a community-based setting. Findings may provide valuable insights into adapting and implementing effective and sustained peer-led DSMS programming for Black men with T2D, subsequently advancing our understanding in the field.
Method
Pilot Study Overview
The Michigan Men’s Diabetes Project (MenD) was first piloted as a 12-week randomized clinical control trial. The aim of the pilot was to understand the feasibility and acceptability of the Peer-Led Diabetes Self-Management Support (PLDSMS) intervention and to estimate the intervention effect size on the primary outcome of diabetes self-management behaviors and changes in HbA1c, and on secondary outcomes that include diabetes self-efficacy and distress.
Setting
Participants were men who self-identified as Black and were older than 55 years. The program was conducted virtually using Zoom for Health at the University of Michigan, which is a Health Insurance Portability and Accountability Act (HIPAA) compliant platform (Zoom for U-M, n.d.).
Recruitment
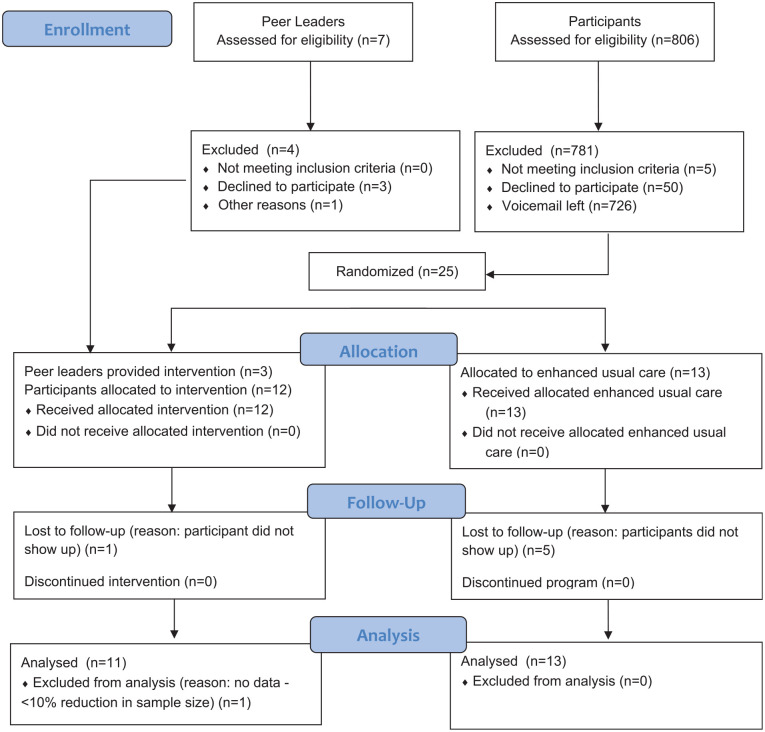
A total of three peer leaders and 25 participants were recruited—13 participants were randomized to the enhanced usual care (EUC) group and the remaining 12 to the intervention group (Figure 1). Participant inclusion criteria included a T2D diagnosis for at least 6 months prior to program start date and full-time residency in the Metro Detroit area. Diagnosis was determined via self-report or through verification of medical records, and determination was dependent on the method of recruitment. Eligible participants must have also been under the current care of a physician for diabetes. To confirm current care, at the baseline assessment, individuals were asked whether they currently had a doctor, and if so, were asked to provide their doctor’s contact information. The University of Michigan Office of Research DataDirect system was used as the primary tool for participant recruitment. DataDirect is a self-serve data tool available to Michigan Medicine employees and includes more than 4 million patients from the University of Michigan (University of Michigan Medical School, n.d.). All but two participants were recruited through DataDirect. The Michigan Center for Urban African American Aging Research Participant Resource Pool (MCUAAAR PRP) was also utilized. The MCUAAAR PRP is a volunteer registry that can be accessed by scholars conducting research focused on African Americans who meet their study criteria (60.1% of male PRP members have a clinical diagnosis of T2D) (“Participant Resource Pool,” n.d.). Those with any of the following conditions were not eligible to participate: a status of nonambulatory; serious health conditions; psychiatric illness (severity requiring hospitalization); or serious diabetes complications (e.g., blindness that would impede meaningful participation). Individuals from DataDirect and the MCUAAAR PRP were identified based on the specified eligibility criteria. Contact details were then obtained, and the study staff reached out via phone, inviting those eligible to participate in the study.
Figure 1.
Michigan Men’s Diabetes Project Flow Diagram
Two of the three peer leaders who led the intervention were recruited from the MCUAAAR PRP. The third peer leader was a participant in a prior peer leader study (Piatt et al., 2021). To be eligible, peer leaders were required to have had a clinical diagnosis of T2D for at least 1 year prior to the start date of the study; be a current resident of Metro Detroit; have at least an eighth-grade education; be willing to commit to 24 hr of training; be actively working on his or her own diabetes self-management goals; and be willing to serve as a peer leader. Exclusion criteria mirrored that of the participants.
Intervention Design
Peer leaders received 24 hr of group training over a 3-month period by CDCESs. The CDCESs used training materials relevant to Black men with T2D. Knowledge and skills that included measurement of empowerment-based facilitation, communication, and goal setting, were assessed. A complete outline of the content covered, and skills assessed, is described elsewhere (Hawkins et al., 2021; Tang, Nwankwo, et al., 2012).
Participants were randomly assigned to either the EUC group or intervention group. Those in the EUC received 10 hr of virtual DSME over 6 weeks facilitated by one of the project CDCESs. Those in the intervention group received 10 hr of virtual DSME cofacilitated by a CDCES and two peer leaders. Although the DSME was not part of a recognized or certified program, all the recommended content from the Diabetes Self-Management Education and Support (DSMES) standards was addressed for both groups (Powers et al., 2016). In addition, the intervention group received 9 hr of PLDSMS over 6 weeks with the trained peer leaders. Those in the EUC group received six sessions of DSME, while the intervention group received ongoing support (i.e., 12 sessions). The CDCES was not present for the PLDSMS sessions, but was available via telephone if needed to answer content questions. To continue the partnership, the CDCESs led monthly leadership development sessions with the peer leaders via Zoom to discuss concerns and questions, and to practice empowerment-based communication skills and behavioral strategies.
A rolling recruitment was used for this study so start dates were staggered for participants. Sessions were held from July 2021 to October 2021 for seven of the 12 participants in the intervention group, and August 2021 to November 2021 for the remaining five participants. For those in the EUC group, sessions were held from July 2021 to August 2021 for seven of the 13 participants, and August 2021 to September 2021 for the remaining six participants. All participants received a reminder phone call and text the day before the first session. Peer leaders received US$10/hr for training, leader review sessions, DSME sessions, and DSMS sessions. Additional detail on intervention design is presented elsewhere (Hawkins et al., 2021).
Primary Outcome Measures
Primary (HbA1c and self-care activities) and secondary outcomes were collected at baseline and at 3 months (intervention end). Participants received US$20 after each completed assessment. HbA1c data were collected using a point-of-care Siemens DCA Vantage Analyzer, which is accurate and precise (Whitley et al., 2015). Participants were asked to provide nonfasting finger stick capillary blood samples. Self-care activities were measured using the Summary of Diabetes Self-Care Activities (SDSCA) measure (Toobert et al., 2000). The scale measures self-care activities, with subscales assessed independently due to low interitem correlations. The brief self-report questionnaire measures management behaviors that include blood glucose monitoring, foot care, physical activity, and dietary management (general and specific diet). Items are measured on a scale of 0 to 7, representing days per week, and subsequently averaged to compute subscale scores. A higher score suggests better self-care. Participation (session attendance) was measured to assess intervention feasibility (Pearson et al., 2020).
It is important to highlight that there is a distinction between timepoint data collection and session attendance. Participants who missed sessions were still asked to complete timepoint assessments. All participants were contacted and scheduled for each assessment.
Secondary Outcome Measures
Secondary outcomes included body mass index (BMI; kg/m2); blood pressure (mmHg); depressive symptom severity; diabetes self-efficacy; and diabetes distress. BMI was calculated using height, measured in centimeters using a stadiometer, and weight, measured in pounds on a calibrated digital scale. Height and weight measurements were converted to meters and kilograms, respectively, to properly calculate BMI. Blood pressure was measured using an automated machine that obtained three readings with 1 min between each reading. The readings were averaged and recorded.
Depressive symptom severity was measured using the 2-item Patient Health Questionnaire (PHQ-2) (Kroenke et al., 2003). The PHQ-2 was reported to have construct and criterion validity. Scores range from 0 to 6, with a score of ≥3 indicating probable major depressive disorder (Kroenke et al., 2003).
To measure diabetes self-efficacy, the 8-item Diabetes Self-Efficacy Scale (DSES) was used (α = .85; test retest validity = 0.80) (Lorig et al., 2009). Items range from 1 (not confident at all) to 10 (totally confident); the higher the score, the higher the self-efficacy. Diabetes-related distress was assessed using the 17-item Diabetes Distress Scale (α = .93) (Polonsky et al., 2005). The scale ranges from 1 (no problem) to 6 (serious problem).
Statistical Analyses
We performed a paired sample t test using Rubin’s method to test for differences in HbA1c, diabetes self-care activities, BMI, blood pressure, depressive symptoms, diabetes self-efficacy, and diabetes distress from preintervention (baseline) to 3 months postintervention among those who participated in peer-led DSMS and those who did not participate. Multiple imputation was used to handle missing data among the control group at follow-up, with the assumption that data were missing at random. Five imputed data sets were created, and the Markov chain Monte Carlo (MCMC) method was used. Descriptive statistics were used to report program participant demographic characteristics at baseline.
To assess the effect of session attendance on the outcomes, the paired sample t test was performed. This tested for differences in the primary and secondary outcome measures among the proportion who attended >50% and ≤50% of the PLDSMS sessions. Statistical significance across all models was defined as having a value of p < .05.
SAS software version 9.4 (SAS Institute, Inc.) was used for all statistical analyses. Study data were collected and managed using REDCap electronic data capture tools, CTSA: UL1TR002240 (Harris et al., 2009).
We used the CONsolidated Standards Of Reporting Trials (CONSORT) reporting guidelines (Schulz et al., 2010) (Supplemental Table S1). Individuals were asked to provide written informed consent prior to participating. The study protocol was approved by the University of Michigan Medical School Institutional Review Board (IRBMED) HUM00190932.
Results
Among the intervention group, 11 of the 12 (92%) participants completed both the preassessment and postassessment, and among the EUC group, 8 of the 13 (62%) participants completed both the preassessment and postassessment (Table 1 and Figure 2).
Table 1.
Program Participant Characteristics at Baseline a
| All participants | Treatment | Control | Peer leaders | |
|---|---|---|---|---|
| (N=28) | (n=12) | (n=13) | (n=3) | |
| Age (years) | ||||
| Mean (SD) | 62.8 (7.1) | 64.4 (5.6) | 62.1 (5.8) | 59.7 (16.6) |
| Range | 42 - 75 | 57 - 74 | 56 - 74 | 42 – 75 |
| Hemoglobin A1c | ||||
| Mean (SD) | 7.5 (1.7) | 7.7 (2.2) | 7.4 (1.3) | 7.2 (0.9) |
| Range | 5.7 – 12.8 | 6.0 – 12.8 | 5.7 – 10.5 | 6.6 – 8.2 |
| Highest degree or level of school completed | ||||
| Some college | 11 (39.3) | 5 (41.7) | 5 (38.5) | 1 (33.3) |
| College graduate | 4 (14.3) | 1 (8.3) | 1 (7.7) | 2 (66.7) |
| Graduate degree | 12 (42.9) | 5 (41.7) | 7 (53.8) | 0 (0) |
| Unknown | 1 (3.5) | 1 (8.3) | 0 (0) | 0 (0) |
| Employment Status | ||||
| Full-time | 13 (46.4) | 5 (41.7) | 7 (53.8) | 1 (33.3) |
| Retired | 11 (39.3) | 5 (41.7) | 4 (30.8) | 2 (66.7) |
| Unknown | 4 (14.3) | 2 (16.6) | 2 (15.4) | 0 (0) |
| Marital Status | ||||
| Single | 6 (21.4) | 2 (16.7) | 3 (23.1) | 1 (33.3) |
| Married | 20 (71.4) | 10 (83.3) | 8 (61.5) | 2 (66.7) |
| Unknown | ||||
| Annual Household Income | ||||
| <$50,000 | 7 (25.0) | 5 (41.7) | 1 (7.7) | 1 (33.3) |
| ≥$50,000 | 21 (75.0) | 7 (58.3) | 12 (92.3) | 2 (66.7) |
| Insurance Status (select all that apply) | ||||
| Group plan through employer | 18 (64.3) | 6 (50.0) | 10 (76.9) | 2 (66.7) |
| Medicare | 13 (46.4) | 6 (50.0) | 6 (46.2) | 1 (33.3) |
| Received DSME b | ||||
| Yes | 25 (89.3) | 11 (91.7) | 11 (84.6) | 3 (100) |
| No | 3 (10.7) | 1 (8.3) | 2 (15.4) | 0 (0) |
Reported as “n” and prevalence (%).
Diabetes Self-Management Education.
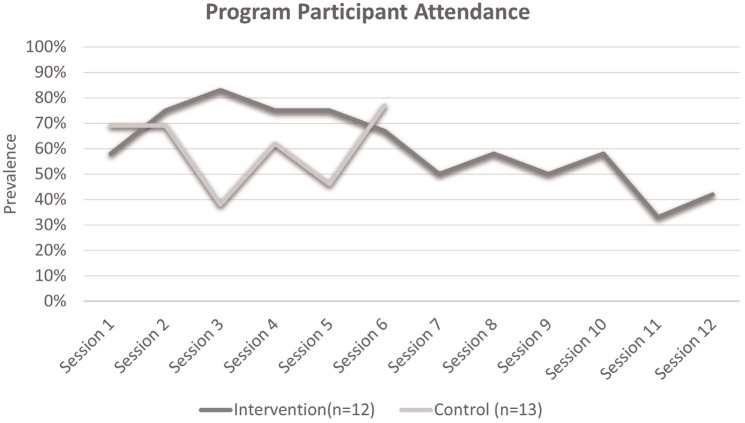
Figure 2.
Program participant attendance over time. The intervention group attended 12 sessions, while those in the control group were expected to attend six sessions
Note. Intervention and control group sessions were independently held.
A nonlinear relationship between time and participation rate was present among the control group. Session 6 marked the highest rate of participation (77%, 10 of 13).
HbA1c levels decreased by 0.20% (SD = 0.99, p = .52) from baseline to 3 months in the intervention group (Table 2), and by 0.13% (p = .68) in the control group (Table 3). Among those in the intervention group, there was a statistically significant difference in general diet (M change: 1.32, SD = 1.71, p = .03) and blood glucose monitoring scores from baseline to 3 months (M change: 0.50, SD = 0.74, p < .05). Among those who attended more than half of the total number of sessions held, a difference in diabetes distress scores from baseline to 3 months (M change: −5.71, SD = 3.20) was statistically significant (p = .003), representing improvement.
Table 2.
Changes in Primary and Secondary Outcome Measures From Baseline, Intervention Group (n=11)
| Outcome measures (primary and secondary) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Self-care activities a | Blood Pressure | |||||||||||
| Hemoglobin A1c |
General diet | Specific diet | Exercise | Blood glucose testing | Foot-care | BMI | Systolic | Diastolic | DepressiveSymptom severity (PHQ-2) | Diabetes self-efficacy b | Diabetes distress c |
|
| Program session participation | ≤ 50% (1-6 sessions) (n=4) |
|||||||||||
| Mean Change d | 0.10 (0.42) | 2.00 (0.82) | −0.38 (2.95) | 0.50 (1.08) | 0.38 (0.75) | 0.75 (1.85) | −0.36 (1.63) | −9.50 (7.94) | −2.25 (7.80) | −1.50 (2.38) | 0.09 (2.70) | −0.75 (14.06) |
| Standard error | 0.21 | 0.41 | 1.48 | 0.54 | 0.38 | 0.92 | 0.81 | 3.97 | 3.90 | 1.19 | 1.35 | 7.03 |
| t-value e | 0.47 | 4.90 | −0.25 | 0.93 | 1.00 | 0.81 | −0.45 | −2.39 | −0.58 | −1.26 | 0.07 | −0.11 |
| P-value | 0.67 | 0.02 f | 0.82 | 0.42 | 0.39 | 0.48 | 0.68 | 0.10 | 0.60 | 0.30 | 0.95 | 0.92 |
| Program Session Participation | > 50% (7-12 sessions) ( n =7) | |||||||||||
| Mean Change d | −0.37 (1.21) | 0.93 (2.01) | 0.64 (1.41) | 1.21 (2.21) | 0.57 (0.79) | 0.21 (1.80) | −0.28 (0.47) | −2.38 (7.57) | −0.60 (8.20) | 0.14 (1.22) | 0.38 (0.77) | −5.71 (3.20) |
| Standard error | 0.46 | 0.76 | 0.53 | 0.84 | 0.30 | 0.68 | 0.18 | 3.09 | 3.67 | 0.46 | 0.29 | 1.21 |
| t-value e | −0.81 | 1.21 | 1.45 | 1.92 | 0.32 | −1.54 | −0.77 | −0.16 | 0.31 | 1.29 | −4.72 | |
| P-value | 0.45 | 0.27 | 0.27 | 0.20 | 0.10 | 0.76 | 0.17 | 0.48 | 0.88 | 0.77 | 0.25 | 0.003 f |
|
Total
( n =11) | ||||||||||||
| Mean Change d | −0.20 (0.99) | 1.32 (1.71) | 0.27 (2.02) | 0.95 (1.85) | 0.50 (0.74) | 0.41 (1.74) | −0.31 (0.96) | −5.23 (8.15) | −1.33 (7.57) | −0.45 (1.81) | 0.27 (1.60) | −3.91 (8.47) |
| Standard error | 0.30 | 0.51 | 0.61 | 0.56 | 0.22 | 0.53 | 0.29 | 2.58 | 2.52 | 0.55 | 0.48 | 2.55 |
| t-value e | −0.67 | 2.56 | 0.45 | 1.71 | 2.24 | 0.78 | −1.06 | −2.03 | −0.53 | −0.83 | 0.56 | −1.53 |
| p value | .52 | .03 f | .66 | .12 | .05 f | .45 | .31 | .07 | .61 | .42 | .58 | .16 |
BMI = Body Mass Index. Missing: n=1.
Measured using the Summary of Diabetes Self-Care Activities (SDSCA). b Measured using the 8-item Diabetes Self-Efficacy Scale (DSES). c Assessed using the 17-item Diabetes Distress Scale. d Reported as mean change (standard deviation). e Calculated using the paired sample t test for evaluating the changes in primary and secondary outcomes from baseline. f Significant at the 0.05 level (2-tailed).
Table 3.
Changes in Primary and Secondary Outcome Measures From Baseline, Control Group (n=13)
| Outcome measures (Primary and secondary) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Self-Care activities a | Blood pressure | |||||||||||
| Hemoglobin A1c |
general diet | Specific diet | Exercise | Blood glucose testing | Foot-Care | BMI | Systolic | Diastolic | Depressive Symptom severity(PHQ-2) |
Diabetes self- efficacy b |
Diabetes distress c |
|
| Mean Change d | −0.13 | 1.77 | 0.74 | 2.02 | 2.44 | 0.04 | −0.41 | 10.34 | 0.15 | 0.09 | 0.33 | −0.68 |
| Standard error | 0.31 | 0.98 | 0.49 | 1.44 | 1.89 | 0.96 | 0.50 | 8.17 | 7.3 | 0.52 | 0.78 | 3.28 |
| t-value e | −0.43 | 1.81 | 1.51 | 1.40 | 1.29 | 0.04 | −0.82 | 1.27 | 0.02 | 0.18 | 0.43 | −0.21 |
| p value | .68 | .08 | .14 | .20 | .24 | .97 | .43 | .24 | .98 | .87 | .67 | .84 |
BMI = Body Mass Index.
Measured using the Summary of Diabetes Self-Care Activities (SDSCA). b Measured using the 8-item Diabetes Self-Efficacy Scale (DSES). c Assessed using the 17-item Diabetes Distress Scale. d Reported as mean change. e Calculated using the paired sample t test for evaluating the changes in primary and secondary outcomes from baseline.
General diet scores increased from baseline in both the intervention (M change: 1.32, SD = 1.71, p = .03) and control groups (M change: 1.77, p = .08). However, changes in the control group were not statistically significant. Relatedly, blood glucose monitoring scores increased from baseline in both the intervention (M change: 0.50, SD = 0.74, p = .05) and control groups (M change: 2.44, p = .24). Changes in the control group were not statistically significant. Systolic (M change: –5.23, SD = 8.15, p = .07) and diastolic (M change: –1.33, SD = 7.57, p = .61) blood pressure decreased in the intervention group, whereas systolic (M change: 10.34, p = .24) and diastolic (M change: 0.15, p = .98) blood pressure increased in the control group.
Discussion
This 3-month pilot randomized clinical control trial sought to examine the feasibility and acceptability of a novel self-management education and support program for Black men with T2D, while also measuring changes in primary and secondary outcomes. Our study contributes to addressing health disparities among Black men, a population often underserved in research and health care. The partnership between the CDCES and peer leaders, and the empowerment-based approach underscored the uniqueness and importance of this intervention program. Participants in the intervention group exhibited positive changes in HbA1c levels, general diet and blood glucose monitoring scores, and blood pressure. The control group also experienced some positive improvements in health outcomes, but changes were not significant. Participant engagement varied over the sessions, with participation rates peaking at 83% in the intervention group. On average, though, rates were slightly lower compared with other peer-led diabetes prevention interventions (Tang et al., 2014). Although all sessions were held on Zoom, suggesting increased accessibility, preliminary feedback indicated that there were challenges around using Zoom software, mainly for older men. Reasons beyond difficulties with the software may have also contributed to fluctuations in engagement over time.
As demonstrated in our study, peer-led support interventions are effective in lowering HbA1c levels and improving self-management behaviors, particularly among those with low medication taking and self-management support levels (Moskowitz et al., 2013). Our results align with existing literature on the positive impact of peer-led programs on HbA1c and self-management behaviors. Culturally tailored interventions, as evidenced by a diabetes self-management program for Chinese Americans, have proven effective in improving diabetes self-management skills (Sun et al., 2012). Peer-led programs, such as one implemented in a low-income population in Mali, have led to improvements in glycemic stability and anthropometric measures (Debussche et al., 2018). Additional studies support the current findings, although mixed. Extended peer support slightly improved blood pressure, as reported in one study (Simmons et al., 2015), and potentially improved cardiovascular health, as reported in another study (Tang et al., 2015). Other studies reported no significant changes in glycemic stability with community-based DSME and peer support, but significant reductions in diabetes distress (Ju et al., 2018; Presley et al., 2020). Importantly, none of the aforementioned studies used empowerment in any aspect of the training or in any of the intervention components, aside from Debussche et al. (2018). Here, the authors described the intervention as being an empowerment-based program, grounded in theory. The approach shaped the design of the educational sessions, sessions that included tailored handouts and new curricula. These studies collectively emphasize the need for a complete understanding of the specific health outcomes targeted by peer-led interventions, while considering duration, participant characteristics, and level of support provided.
Black men experience worse diabetes-related health outcomes that include higher rates of lower limb amputations, compared with other racial and ethnic groups. The convergence of race and gender places Black men in a compounded situation, where they face distinct challenges that hinder meaningful participation in peer-led diabetes self-management programs. Understanding the specific challenges faced by Black men is necessary for designing interventions that resonate with their experiences. Tailoring diabetes self-management and support programs to the unique needs and experiences of Black men can enhance the effectiveness of these programs. This may include addressing social and systemic factors that contribute to health disparities.
Designing and implementing interventions requires active community engagement and a great degree of cultural sensitivity that are often inherent in peer-led programs (Okoro et al., 2018). Black male peer leaders living in the same area, with the same health condition, and of similar age and socioeconomic status, by virtue, share similarities with the participants. Programs that are culturally relevant and cognizant of the challenges faced by Black men can foster trust and increase participation rates. Collaborating with community leaders and stakeholders can support recruitment efforts and lead to greater acceptance of program objectives and strategies among the target population.
Our findings also underscore the importance of empowerment-based approaches, especially for Black men. Empowering individuals to take ownership of their health and well-being can serve as a means for positive behavior change. The integration of empowerment strategies into peer-led interventions may contribute to overcoming systemic challenges and deepening a sense of autonomy and self-advocacy among Black men living with T2D. The intervention integrated targeted health education tailored to address the health literacy needs of Black men. Increasing support and education around diabetes management that emphasizes the importance of self-care within the context of culture and gender can empower individuals to make informed decisions about their health.
Limitations
Black men living with T2D are more likely to have functional limitations that include lower limb amputation and limited mobility due to diabetes-related complications. Although virtual programs address barriers to in person attendance, there are limitations. Future studies may find value in measuring the impact of a virtual versus in-person intervention. While efforts were made to reduce loss to follow-up, some participants did not complete the follow-up assessment, affecting generalizability. Of note, there were no statistically significant differences in baseline characteristics between controls with preintervention and postintervention data and those with no follow-up data. The lack of significant differences in baseline characteristics between the two groups indicates that minimal bias was introduced as a result of attrition. In this study, we evaluated differences in psychosocial factors; however, the study was not inclusive of all factors that may have affected participant engagement. Individuals were not randomly selected to participate, which may have introduced self-selection bias. Participants who chose to enroll may differ systematically from those who did not, further affecting the generalizability of our findings. Many of the participants were generally healthy prior to enrollment and their enthusiasm may have influenced the observed positive outcomes. Finally, the sample size was small and the follow-up period in our study was limited to 3 months. Although this time period was adequate to assess short-term outcomes, an extended follow-up period is needed to evaluate whether the observed positive changes were sustained.
Recommendations
Future research should address these limitations through the refinement of recruitment strategies to improve generalizability and through the exploration of a wider array of relevant psychosocial factors. Extending the follow-up period to assess the long-term impact on health outcomes, specifically as it relates to HbA1c, is also warranted. Psychosocial factors, including social support, mental health, and stress, can significantly affect engagement and outcomes in diabetes self-management programs. Examining these additional factors could provide further insight into participant experiences. Including age of diabetes onset as a predictor of peer-led diabetes intervention efficacy was not the objective of this study; however, investigators may find usefulness in evaluating diabetes duration as a predictor of acceptability, which may influence future program development. Utilizing a combination of objective measures and qualitative data could also enhance the robustness of future studies.
Initial and continued engagement from participants is important for the success of peer-led programs. Assessing the sustainability of HbA1c reductions postintervention and utilizing a difference-in-differences technique with an increased sample size will provide a more comprehensive understanding of intervention effect. These recommendations will help close existing gaps and contribute to the literature on effective peer-led interventions for Black men.
Supplemental Material
Supplemental material, sj-docx-1-jmh-10.1177_15579883241258318 for The Michigan Men’s Diabetes Project Randomized Clinical Control Trial: A Pilot/Feasibility Study of a Peer-Led Diabetes Self-Management and Support Intervention for Black Men With Type 2 Diabetes by Alana M. Ewen, Jaclynn M. Hawkins, Katherine A. Kloss, Robin Nwankwo, Martha M. Funnell, Srijani Sengupta, Nelson Jean Francois and Gretchen Piatt in American Journal of Men's Health
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Michigan Men’s Diabetes Project is supported by the National Institute on Aging (2P30AG024824–16) and Blue Cross Blue Shield of Michigan Foundation (003063.MG)
Trial Registration: Registered at ClinicalTrials.gov with an ID NCT04760444 on February 17, 2021.
ORCID iD: Alana M. Ewen  https://orcid.org/0000-0003-4078-0972
https://orcid.org/0000-0003-4078-0972
Data Availability: Data are available upon reasonable request.
Supplemental Material: Supplemental material for this article is available online.
References
- Amuda A. T., Berkowitz S. A. (2019). Diabetes and the built environment: Evidence and policies. Current Diabetes Reports, 19(7), 35. 10.1007/s11892-019-1162-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balls-Berry J., Watson C., Kadimpati S., Crockett A., Mohamed E. A., Brown I., Soto M. V., Sanford B., Halyard M., Khubchandani J., Dacy L., Davis O. I. (2015). Black men’s perceptions and knowledge of diabetes: A Church-affiliated barbershop focus group study. Journal of Racial and Ethnic Health Disparities, 2(4), 465–472. 10.1007/s40615-015-0094-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck J., Greenwood D. A., Blanton L., Bollinger S. T., Butcher M. K., Condon J. E., Cypress M., Faulkner P., Fischl A. H., Francis T., Kolb L. E., Lavin-Tompkins J. M., MacLeod J., Maryniuk M., Mensing C., Orzeck E. A., Pope D. D., Pulizzi J. L., Reed A. A., Rhinehart A. S. (2017). Standards Revision Task Force (2017). 2017 national standards for diabetes self-management education and support. The Diabetes Educator, 43(5), 449–464. 10.1177/0145721717722968 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2024). United States Diabetes Surveillance System. https://gis.cdc.gov/grasp/diabetes/diabetesatlas.html
- Centers for Disease Control and Prevention. (2023). National Diabetes Statistics Report: Estimates of diabetes and its burden in the United States. https://www.cdc.gov/diabetes/data/statistics-report/index.html
- Courtenay W. H. (2000). Constructions of masculinity and their influence on men’s well-being: A theory of gender and health. Social Science & Medicine, 50(10), 1385–1401. 10.1016/s0277-9536(99)00390-1 [DOI] [PubMed] [Google Scholar]
- Davis J., Fischl A. H., Beck J., Browning L., Carter A., Condon J. E., Dennison M., Francis T., Hughes P. J., Jaime S., Lau K. H. K., McArthur T., McAvoy K., Magee M., Newby O., Ponder S. W., Quraishi U., Rawlings K., Socke J., . . .Villalobos S. (2022). 2022 National standards for diabetes self-management education and support. The Science of Diabetes Self-Management and Care, 48(1), 44–59. 10.1177/26350106211072203 [DOI] [PubMed] [Google Scholar]
- Debussche X., Besançon S., Balcou-Debussche M., Ferdynus C., Delisle H., Huiart L., Sidibe A. T. (2018). Structured peer-led diabetes self-management and support in a low-income country: The ST2EP randomised controlled trial in Mali. PLOS ONE, 13(1), Article e0191262. 10.1371/journal.pone.0191262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming P. J., Lee J. G., Dworkin S. L. (2014). “Real men don’t”: Constructions of masculinity and inadvertent harm in public health interventions. American Journal of Public Health, 104(6), 1029–1035. 10.2105/AJPH.2013.301820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funnell M. M. (2023). Growing up: A 50-year journey from compliance to collaboration. The Science of Diabetes Self-Management and Care, 49(4), 324–325. 10.1177/26350106231169695 [DOI] [PubMed] [Google Scholar]
- Funnell M. M., Nwankwo R., Gillard M. L., Anderson R. M., Tang T. S. (2005). Implementing an empowerment-based diabetes self-management education program. The Diabetes Educator, 31(1), 53–61. 10.1177/0145721704273166 [DOI] [PubMed] [Google Scholar]
- Funnell M. M., Tang T. S., Anderson R. M. (2007). From DSME to DSMS: Developing empowerment-based diabetes self-management support. Diabetes Spectrum, 20(4), 221–226. [Google Scholar]
- Harris P. A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J. G. (2009). Research electronic data capture (REDCap): A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42(2), 377–381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey I. S., Alston R. J. (2011). Understanding preventive behaviors among mid-Western African-American men: A pilot qualitative study of prostate screening. Journal of Mens’ Health, 8(2), 140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins J., Kloss K., Funnell M., Nwankwo R., Schwenzer C., Smith F., Piatt G. (2021). Michigan Men’s diabetes project (MenD): Protocol for a peer leader diabetes self-management education and support intervention. BMC Public Health, 21(1), Article 562. 10.1186/s12889-021-10613-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins J., Watkins D. C., Kieffer E., Spencer M., Espitia N., Anderson M. (2015). Psychosocial factors that influence health care use and self-management for African American and Latino men with type 2 diabetes: An exploratory study. Journal of Men’s Studies, 23, 161–176. https://doi.org/1060826515582495 [Google Scholar]
- Hawkins J., Watkins D. C., Kieffer E., Spencer M., Piatt G., Nicklett E. J., Lebron A., Espitia N., Palmisano G. (2017). An exploratory study of the impact of gender on health behavior among African American and Latino men with type 2 diabetes. American Journal of Men’s Health, 11(2), 344–356. 10.1177/1557988316681125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler M. (2007). Overview of peer support models to improve diabetes self-management and clinical outcomes. Diabetes Spectrum, 20(4), 214–221. [Google Scholar]
- Heisler M. (2010). Different models to mobilize peer support to improve diabetes self management and clinical outcomes: Evidence, logistics, evaluation considerations and needs for future research. Family Practice, 27(Suppl. 1), i23–i32. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hurt T. R., Seawell A. H., O’Connor M. C. (2015). Developing effective diabetes programming for Black men. Global Qualitative Nursing Research, 2, 2333393615610576. https://doi.org/22333393615610576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju C., Shi R., Yao L., Ye X., Jia M., Han J., Yang T., Lu Q., Jin H., Cai X., Yuan S., Xie B., Yu X., Coufal M. M., Fisher E. B., Sun Z. (2018). Effect of peer support on diabetes distress: A cluster randomized controlled trial. Diabetic Medicine: A Journal of the British Diabetic Association, 35(6), 770–775. 10.1111/dme.13625 [DOI] [PubMed] [Google Scholar]
- Kloss K. A., Funnell M. M., Nwankwo R. (2022). Going beyond education: A practical framework for diabetes self-management and decision-making. ADCES in Practice, 10(5), 8–12. 10.1177/2633559X221114871 [DOI] [Google Scholar]
- Kroenke K., Spitzer R. L., Williams J. B. (2003). The Patient Health Questionnaire-2: Validity of a two-item depression screener. Medical Care, 41(11), 1284–1292. 10.1097/01.MLR.0000093487.78664.3C [DOI] [PubMed] [Google Scholar]
- Lee S., Schorr E., Hadidi N. N., Kelley R., Treat-Jacobson D., Lindquist R. (2018). Power of peer support to change health behavior to reduce risks for heart disease and stroke for African American men in a faith-based community. Journal of Racial and Ethnic Health Disparities, 5(5), 1107–1116. 10.1007/s40615-018-0460-7 [DOI] [PubMed] [Google Scholar]
- Liburd L. C., Namageyo-Funa A., Jack L., Jr. (2007). Understanding “masculinity” and the challenges of managing type-2 diabetes among African-American men. Journal of the National Medical Association, 99(5), 550–558 [PMC free article] [PubMed] [Google Scholar]
- Lorig K., Ritter P. L., Villa F. J., Armas J. (2009). Community-based peer-led diabetes self-management: A randomized trial. The Diabetes Educator, 35(4), 641–651. 10.1177/0145721709335006 [DOI] [PubMed] [Google Scholar]
- Maulsby C., Millett G., Lindsey K., Kelley R., Johnson K., Montoya D., Holtgrave D. (2013). A systematic review of HIV interventions for black men who have sex with men (MSM). BMC Public Health, 13, Article 625. 10.1186/1471-2458-13-625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michigan Department of Community Health. (2019). Diabetes in Michigan 2019. https://www.michigan.gov/documents/mdhhs/diabetes-in-Michigan-update-2019_658300_7.pdf
- Michigan Department of Health & Human Services, Division for Vital Records & Health Statistics. (2022). National Center for Health Statistics, U.S. Census Populations With Bridged Race Categories. https://www.mdch.state.mi.us/osr/deaths/DiabetesUS.asp
- Moskowitz D., Thom D. H., Hessler D., Ghorob A., Bodenheimer T. (2013). Peer coaching to improve diabetes self-management: Which patients benefit most? Journal of General Internal Medicine, 28, 938–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muvuka B., Combs R. M., Ayangeakaa S. D., Ali N. M., Wendel M. L., Jackson T. (2020). Health literacy in African-American communities: Barriers and strategies. Health Literacy Research and Practice, 4(3), e138–e143. 10.3928/24748307-20200617-01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoro F. O., Veri S., Davis V. (2018). Culturally appropriate peer-led behavior support program for African Americans with type 2 diabetes. Frontiers in Public Health, 6, Article 340. 10.3389/fpubh.2018.00340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Participant resource pool. (n.d.). Michigan Center for Urban African American Aging Research. https://mcuaaar.org/cores/community-liaison-and-recruitment-core/participant-resource-pool/
- Pearson N., Naylor P. J., Ashe M. C., Fernandez M., Yoong S. L., Wolfenden L. (2020). Guidance for conducting feasibility and pilot studies for implementation trials. Pilot and Feasibility Studies, 6(1), 167. 10.1186/s40814-020-00634-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers for Progress. (2010). Peer support in health and health care: A guide to program development and management. American Academy of Family Physicians Foundation. [Google Scholar]
- Piatt G. A., Anderson R. M., Brooks M. M., Songer T., Siminerio L. M., Korytkowski M. M., Zgibor J. C. (2010). 3-year follow-up of clinical and behavioral improvements following a multifaceted diabetes care intervention: Results of a randomized controlled trial. The Diabetes Educator, 36(2), 301–309. 10.1177/0145721710361388 [DOI] [PubMed] [Google Scholar]
- Piatt G. A., Provenzano A. M., Nwankwo R., Hall D., Kloss K. A., Hawkins J. M., Funnell M. M. (2021). 62-OR: Fostering sustainability through diabetes self-management support in African-American churches: Results of the Praise Diabetes project. Diabetes, 70(Suppl. 1), 62.33115827 [Google Scholar]
- Piatt G. A., Rodgers E. A., Xue L., Zgibor J. C. (2018). Integration and utilization of peer leaders for diabetes self-management support: Results from project SEED (Support, Education, and Evaluation in Diabetes). The Diabetes Educator, 44(4), 373–382. 10.1177/0145721718777855 [DOI] [PubMed] [Google Scholar]
- Piatt G. A., Seidel M. C., Chen H. Y., Powell R. O., Zgibor J. C. (2012). Two-year results of translating the diabetes prevention program into an urban, underserved community. The Diabetes Educator, 38(6), 798–804. 10.1177/0145721712458834 [DOI] [PubMed] [Google Scholar]
- Piatt G. A., Zgibor J. C. (2010). Project SEED: Support, education, and evaluation in diabetes. University of Michigan; University of Pittsburgh. [Google Scholar]
- Polonsky W. H., Fisher L., Earles J., Dudl R. J., Lees J., Mullan J., Jackson R. A. (2005). Assessing psychosocial distress in diabetes: Development of the diabetes distress scale. Diabetes Care, 28(3), 626–631. 10.2337/diacare.28.3.626 [DOI] [PubMed] [Google Scholar]
- Powers M. A., Bardsley J., Cypress M., Duker P., Funnell M. M., Fischl A. H., Maryniuk M. D., Siminerio L., Vivian E. (2016). Diabetes self-management education and support in type 2 diabetes: A joint position statement of the American Diabetes Association, the American Association of Diabetes Educators, and the Academy of Nutrition and Dietetics. Clinical Diabetes: A Publication of the American Diabetes Association, 34(2), 70–80. 10.2337/diaclin.34.2.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presley C., Agne A., Shelton T., Oster R., Cherrington A. (2020). Mobile-enhanced peer support for African Americans with type 2 diabetes: A randomized controlled trial. Journal of General Internal Medicine, 35, 2889–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz K. F., Altman D. G., Moher D., & CONSORT Group. (2010). CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. British Medical Journal, 340, c332. 10.1136/bmj.c332 [DOI] [PubMed] [Google Scholar]
- Sherman L. D., Hawkins J. M., Bonner T. (2017). An analysis of the recruitment and participation of African American men in type 2 diabetes self-management research: A review of the published literature. Social Work in Public Health, 32(1), 38–48. 10.1080/19371918.2016.1188742 [DOI] [PubMed] [Google Scholar]
- Simmons D., Prevost A. T., Bunn C., Holman D., Parker R. A., Cohn S., Donald S., Paddison C. A., Ward C., Robins P., Graffy J. (2015). Impact of community based peer support in type 2 diabetes: A cluster randomised controlled trial of individual and/or group approaches. PLOS ONE, 103(3), Article e0120277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer M. S., Hawkins J., Espitia N. R., Sinco B., Jennings T., Lewis C., Palmisano G., Kieffer E. (2013). Influence of a community health worker intervention on mental health outcomes among low-income Latino and African American adults with type 2 diabetes. Race and Social Problems, 5(2), 137–146. 10.1007/s12552-013-9098-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun A. C., Tsoh J. Y., Saw A., Chan J. L., Cheng J. W. (2012). Effectiveness of a culturally tailored diabetes self-management program for Chinese Americans. The Diabetes Educator, 38(5), 685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T. S., Funnell M. M., Gillard M., Nwankwo R., Heisler M. (2011). The development of a pilot training program for peer leaders in diabetes: Process and content. The Diabetes Educator, 37(1), 67–77. 10.1177/0145721710387308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T. S., Funnell M. M., Noorulla S., Oh M., Brown M. B. (2012). Sustaining short-term improvements over the long-term: Results from a 2-year diabetes self-management support (DSMS) intervention. Diabetes Research and Clinical Practice, 95(1), 85–92. 10.1016/j.diabres.2011.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T. S., Funnell M. M., Oh M. (2012). Lasting effects of a 2-year diabetes self-management support intervention: Outcomes at 1-year follow-up. Preventing Chronic Disease, 9, E109. 10.5888/pcd9.110313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T. S., Funnell M. M., Sinco B., Spencer M. S., Heisler M. (2015). Peer-led, empowerment-based approach to self-management efforts in diabetes (PLEASED): A randomized controlled trial in an African American community. The Annals of Family Medicine, 13(Suppl. 1), S27–S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T. S., Nwankwo R., Whiten Y., Oney C. (2012). Training peers to deliver a church-based diabetes prevention program. The Diabetes Educator, 38(4), 519–525. 10.1177/0145721712447982 [DOI] [PubMed] [Google Scholar]
- Tang T. S., Nwankwo R., Whiten Y., Oney C. (2014). Outcomes of a church-based diabetes prevention program delivered by peers: A feasibility study. The Diabetes Educator, 40(2), 223–230. 10.1177/0145721713520569 [DOI] [PubMed] [Google Scholar]
- Tian Y. (2023). Health risk behaviors within the state of Michigan: 2021 Behavioral Risk Factor Survey (35th Annual Report). Michigan Department of Health and Human Services, Lifecourse Epidemiology and Genomics Division. [Google Scholar]
- Toobert D. J., Hampson S. E., Glasgow R. E. (2000). The summary of diabetes self-care activities measure: Results from 7 studies and a revised scale. Diabetes Care, 23(7), 943–950. 10.2337/diacare.23.7.943 [DOI] [PubMed] [Google Scholar]
- University of Michigan Medical School. (n.d.). Self-serve data tools. https://research.medicine.umich.edu/our-units/data-office-clinical-translational-research/self-serve-data-tools
- Whitley H. P., Yong E. V., Rasinen C. (2015). Selecting an A1C point-of-care instrument. Diabetes Spectrum: A Publication of the American Diabetes Association, 28(3), 201–208. 10.2337/diaspect.28.3.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoom at U-M. (n.d.). Information and Technology Services. https://its.umich.edu/communication/videoconferencing/zoom
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jmh-10.1177_15579883241258318 for The Michigan Men’s Diabetes Project Randomized Clinical Control Trial: A Pilot/Feasibility Study of a Peer-Led Diabetes Self-Management and Support Intervention for Black Men With Type 2 Diabetes by Alana M. Ewen, Jaclynn M. Hawkins, Katherine A. Kloss, Robin Nwankwo, Martha M. Funnell, Srijani Sengupta, Nelson Jean Francois and Gretchen Piatt in American Journal of Men's Health