Abstract
Stimulant laxatives are well established as first- or second-line treatments for constipation and although they have a reliable therapeutic effect, alleged safety concerns still exist, particularly with long-term use. The potential harmful effects on the gastrointestinal system (including carcinogenicity) of the long-term use of diphenylmethane [bisacodyl, sodium picosulfate (SPS)] and senna stimulant laxatives were assessed in a comprehensive review of the publications identified in literature searches performed in PubMed and Embase up to and including June 2023. We identified and reviewed 43 publications of interest. While stimulant laxatives at supratherapeutic doses have been shown to cause structural alterations to surface absorptive cells in animals and humans, these effects are reversible and not considered clinically relevant. No formal long-term studies have demonstrated morphological changes in enteric neural elements or intestinal smooth muscle with bisacodyl or SPS in humans. Furthermore, there is no convincing evidence that stimulant laxatives are associated with the development of colon cancer, and in fact, chronic constipation itself has been reported to potentially increase the risk of colon cancer, therefore, the use of stimulant laxatives might reduce this risk. Many studies suggesting a possible harmful effect from laxatives were limited by their failure to consider confounding factors such as concomitant neurological disease, metabolic disorders, and age. These findings highlight the lack of evidence for the harmful effects of laxatives on the colon, and thus, the benefits of treatment with stimulant laxatives, even in the long-term, should be reconsidered for the management of patients with constipation.
Keywords: anthranoid laxatives, bisacodyl, constipation, diphenylmethane laxatives, safety, senna and sennosides, stimulant laxatives
Plain language summary
Do stimulant laxatives damage the gut?
Stimulant laxatives are widely used treatments for constipation that work by causing the muscles in the gut to contract and so move stool more effectively. Examples of these treatments include senna, bisacodyl and sodium picosulfate. Treatments such as these are typically available without a doctor’s prescription and have a long history of helping people relieve their constipation. However, some concerns have been expressed about the safety of these treatments, particularly when they are used for a long time. We did a critical review of published studies of the safety of stimulant laxatives to try to find out whether there is any strong evidence for harm being caused by these treatments. We found 43 papers with information on the gut safety of stimulant laxatives. These studies looked at whether the treatments are associated with changes to gut structure or function and at whether there might be a link between these treatments and bowel cancer. Unfortunately, many of the studies were of poor quality. For instance, they did not look for things, in addition to the laxatives, that could have affected the results, such as the age of the patients, other medications they were taking or whether they had other health conditions that might have affected the bowel. Also, the populations in which the studies were done differed a lot, so they were hard to compare with one another. However, we did not find any strong evidence suggesting that stimulant laxatives damage the gut or cause cancer. We therefore concluded that the harms associated with stimulant laxatives are likely to have been overstated, and that patients should not be denied the benefits of stimulant laxatives for constipation, especially as they have been on the market for a very long time with no serious problems emerging.
Introduction
Stimulant laxatives, including bisacodyl, sodium picosulfate (SPS), and senna, are effective in treating constipation.1–8 These agents act by increasing intestinal motility and secretion through a local effect on the nerve plexus and smooth muscle of the intestine and are well established as first- or second-choice constipation treatments,9–16 either alone or in combination with bulking or osmotic laxatives such as polyethylene glycol (PEG) or milk of magnesia.9,11,14,17,18
At the recommended doses, the potential risk of major side effects with these agents (e.g. dehydration or electrolyte disturbance) is negligible. 11 The most frequently reported electrolyte disturbance is hypokalemia; however, this is only associated with misuse or abuse of laxatives or use of laxatives for colon cleansing. 19 While bulk-forming agents, softeners, and osmotic laxatives are well accepted for long-term use, safety concerns have been raised about the long-term use of stimulant laxatives. 20 Some studies have indicated that bisacodyl, SPS, and senna may lead to structural damage to surface epithelial cells, 21 the myenteric plexus, and intestinal smooth muscle,20–23 leading to impaired colonic function.24,25 There has also been speculation that these laxatives might cause colon and other forms of cancer.6,26–28
Phenolphthalein was used as a laxative until the 1990s, when animal studies suggested that it might have carcinogenic potential, leading to its withdrawal. Phenolphthalein has some structural similarities to bisacodyl, which led to bisacodyl, senna, and other stimulant laxatives being reclassified by the Food and Drug Administration (FDA) from category I (monograph) to category III (more data needed) agents. Bisacodyl and senna were later restored to category I status after further studies demonstrated the lack of genotoxicity and carcinogenicity of these agents.
Although stimulant laxatives have a reliable therapeutic effect and are among the most widely used medications worldwide, alleged safety concerns still exist, 20 which may deprive some patients of an effective long-term treatment modality for their constipation.21,29 While bisacodyl, SPS, and senna have a long post-marketing use as over-the-counter laxatives,1,5,24 current literature has not been systematically scrutinized in depth, especially regarding the quality of the studies claiming potential harm to the lower intestine. Therefore, the present review aimed to address this knowledge gap and critically analyze available data regarding whether these laxatives might potentially damage the colon or cause colon cancer.
Methods
Literature searches for articles in English were performed in PubMed and Embase up to and including June 2023 without chronological restriction and using the search terms ‘stimulant laxatives (cathartics)’, ‘bisacodyl’, ‘SPS’, and ‘senna’ in combination with ‘chronic disease’ [Medical Subject Headings (MeSH) terms], ‘constipation/drug therapy’ (MeSH terms), and ‘chronic constipation’. These terms were searched in various combinations, that is, with constipation including drug therapy (including dosage and administration), metabolism, morphological changes, chronic disease, intestinal mucosa ultrastructure, gastrointestinal motility (including drug effects and colon diagnostic imaging/case-control studies), and in combination with the terms ‘colorectal’ and ‘renal neoplasms’ and related topics and terms ‘adverse effects of stimulant laxatives’, ‘myenteric plexus/drug effects’. The topic ‘bowel preparation or bowel cleansing’ was expressly excluded.
The searches yielded 68 results in PubMed and 126 in Embase. Publications were considered relevant for inclusion if they covered a case report or a study containing treatment safety-related information. Studies were selected if they reported on preclinical or clinical aspects of epithelial, morphological, or anatomical alterations of the intestinal wall and enteric nervous system (ENS), potentially attributable to long-term or chronic intermittent treatment with stimulant laxatives, including senna and sennosides, diphenylmethanes including bisacodyl, SPS, and also phenolphthalein. Reports mentioning these alterations were considered as they might subsequently result in functional impairment, genotoxicity, and carcinogenicity. Duplicates were removed, and those publications that did not report any clinically relevant morphological changes of enteric neural elements or intestinal smooth muscle in response to long-term or chronic intermittent treatment with stimulant laxatives were excluded.
In addition, a series of studies associated with the development of bisacodyl were included in our analysis, even if they had not all been published. These assessed the non-clinical toxicology 30 and the genotoxic, mutagenic, and carcinogenic potential of bisacodyl (Data on File), and some included a comparison with phenolphthalein. 31
Results
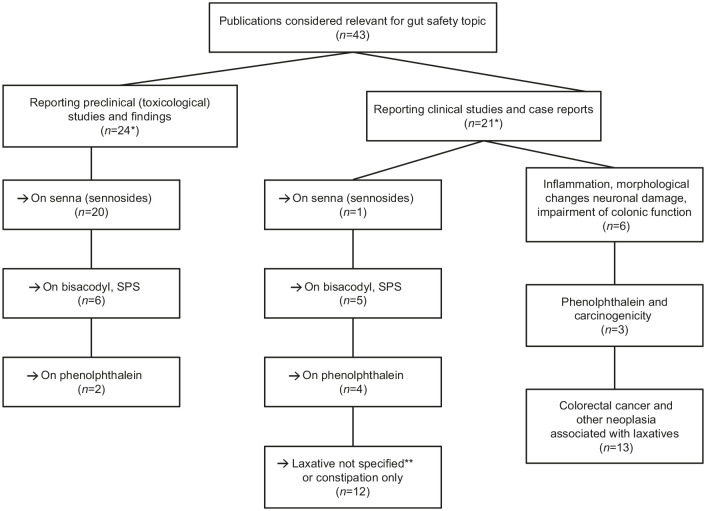
Overall, 43 papers were reviewed in depth (24 reported preclinical/in vitro studies and 21 reported clinical studies) from 1968 to 2022 (Figure 1). Two papers32,33 reported preclinical and clinical studies. These publications were evaluated for information on the effects of long-term treatment with bisacodyl or SPS, senna, or phenolphthalein on the following: morphological alterations of the intestinal wall; damage to the ENS; intestinal functional impairment; genotoxicity; or carcinogenicity.
Figure 1.
Publications included in the analysis.
*Two papers each reported preclinical and clinical studies; some studies reported on more than one compound of interest.
**Individual laxatives were not defined in some publications.
SPS, sodium picosulfate.
A summary of the findings from preclinical and clinical studies is provided in Tables 1 and 2, with further details of the evaluated studies provided in Supplemental Tables 1 and 2.
Table 1.
Summary of key preclinical studies of stimulant laxative toxicity (bisacodyl, senna).
| Citation | Study design | Duration | Key endpoints | Key findings | Conclusion |
|---|---|---|---|---|---|
| Genotoxicity/mutagenicity studies | |||||
| Heidemann et al., 1993 34 | Genotoxicity tests in vitro and in vivo on crude senna, senna extract, sennosides, rhein, and aloe-emodin | Not applicable |
In vitro tests: mouse spot test; CA in vivo; Ames/Salmonella sp.; Ames/E. coli; HGPRT; CA/CHO; MLA In vivo tests: CA/rat; MNT/rat; MNT/mouse; mouse spot test Ex vivo tests: UDS/rat |
Fructus sennae: negative (mouse spot; CA rat; MNT rat) Senna extract: positive (SAL; CHO); negative/equivocal (HGPRT) Sennosides: negative (SAL and E. coli/CHO/ML); negative (MNT mice) Rhein: negative (SAL; CHO); negative/equivocal (ML); negative in mouse MNT Aloe-emodin: positive (SAL; CHO), one positive and two negative tests (HGPRT); negative (mouse spot; CA/rat; MNT/mouse; rat UDS) |
Senna extracts and aloe-emodin were genotoxic in some in vitro tests; no genotoxic effect in in vivo tests Crude senna, sennosides, and rhein* were not genotoxic in in vitro or in vivo conditions |
| Mengs, 1988 35 | Mutagenicity testing of sennosides | Not applicable | Ames test (Salmonella typhimurium; E. coli); MLA; CHO; MNT | No effects (max concentration or dose) in the Ames test (5000 µg/plate), MLA (5000 µg/ml nonactivation; 1000 µg/ml with activation); CHO (5000 µg/ml nonactivation; 4000 µg/ml with activation); MNT (2500 mg/kg) | The sennosides tested were not mutagenic in the test systems employed |
| Brusick and Mengs, 1997 36 | Review article of genotoxic risk of senna [senna extract; sennosides (A, B, C, D)]; rhein; crude senna; aloe-emodin | Not applicable |
In vitro tests: Ames/Salmonella sp.; Ames/E. coli; MLA; HGPRT assay; CA/CHO; rat hepatocyte UDS; cell transformation (C3H/M2 cells) In vivo tests: MNT/rat; CA/rat bone marrow; somatic cell mutation/mouse; UDS/rat hepatocyte; MNT/mouse; CA/rat bone marrow metaphase |
Sennosides: negative (five tests) Rhein: negative (four tests); one test was equivocal (MLA) Senna extract: positive (Ames; CA); negative (HGPRT) Crude senna: negative (three in vivo tests) Aloe-emodin: positive in all in vitro tests (except in one HGPRT experiment); negative (four in vivo tests) |
Therapeutic doses of senna laxative produced in healthy human volunteers: aloe-emodin not detected in plasma (lower limit of quantification 0.5 mg/ml), using same assumptions for effects in rodents results in human safety margins for somatic genotoxicity of ~20,000 |
| NTP, 2001 37 | Genotoxicity studies on emodin | Not applicable |
In vitro tests: Ames/Salmonella sp; CA/CHO In vivo tests: MNT/rat bone marrow erythrocytes; MNT/mouse bone marrow erythrocytes; MNT/mouse peripheral blood erythrocytes |
Emodin was mutagenic in S. typhimurium strain TA100 with S9 activation (not in strain TA98 ± S9) CA was seen in CHO cells ± S9 Negative tests: rat bone marrow MNT; mouse bone marrow, and peripheral blood erythrocytes |
Mutagenicity potential did not result in carcinogenesis (see NTP, 2001 below) |
| Stoll et al., 2006 31 | Mutagenicity testing of bisacodyl | Not applicable | Micronucleus test: SHE test | Micronucleus test: no effect on percent PCEs SHE: No change in transformation frequency at any dose level |
Bisacodyl has no mutagenic potential |
| Inflammation, morphology, nerve damage | |||||
| Saunders et al., 1977 38 | Impact of bisacodyl on upper jejunum sections from humans, and jejunal, ileal, colonic areas in rats | Not applicable | Net water transport LM and EM of colon samples |
Bisacodyl induced net water secretion (p < 0.01) in human intestinal segments In rats: concentration-related inhibition of water absorption in all regions by bisacodyl; LM – focal alterations in surface absorptive cells; EM: mucosal damage |
Laxative effect of bisacodyl may be related to water absorption inhibition Morphological changes may be related to how bisacodyl decreases water transport |
| Carcinogenicity studies | |||||
| Lydén-Sokolowski et al., 1993 39 | Rat carcinogenicity study with a purified senna extract (sennosides) in drinking water | 2 years Daily doses consumed: 0, 5, 15, and 25 mg/kg |
Tumors – specifically GI tract, liver, kidneys, and adrenals | No treatment-related effects in macroscopic findings No differences in microscopic findings (high-dose group versus controls) No treatment-related increase in neoplasms |
There was no relationship between long-term administration of purified senna extract and tumor development of the GI tract, liver, kidneys, and adrenals of rats |
| Siegers et al, 1993 40 | To evaluate the tumorigenic potential of sennosides and aloin alone and their tumor-promoting potential in an established murine model of colorectal tumorigenesis (DMH) | 20 weeks DMH 20 mg/kg SC for 10 weeks. Sennosides 0.03% in diet (equivalent to 100 mg/kg/day – mild laxative effect) Aloin 0.03% in diet |
Colorectal tumors Hepatic and nephrotoxicity |
No. of tumor-bearing animals: senna only 0/20; senna + DMH 7/19; DMH alone 10/19; aloin alone 1/20; aloin + DMH 7/20 Incidence and growth of colorectal tumors were not affected by senna or aloin DMH-induced hepatotoxic and nephrotoxicity effects, which were enhanced by aloin but not senna |
DMH-induced colorectal tumors were not impacted by aloin or sennoside-enriched diets Aloin- and sennoside-fed mice had no significant changes in hepato- or nephrotoxicity |
| NTP, 2001 37 | Rat and mouse carcinogenicity studies with emodin via the die | 2 years Rat study: 0, 280, 830, or 2500 ppm emodin Mouse study: 0, 160, 312, or 625 ppm emodin in males and 0, 312, 625, or 1250 ppm emodin in females |
Tumors | Rat study: Zymbal gland carcinoma (three females, 2500 ppm), which exceeded current historical controls (equivocal finding); negative trends in mononuclear cell leukemia Mouse study: low incidences of renal tubule adenoma and carcinoma in male mice (one of each in the 312 and 625 ppm groups; rare tumor in male mice, possible association with emodin) |
No evidence of carcinogenic activity of emodin in male rats and female mice; equivocal evidence of carcinogenic activity in female rats and male mice |
| Borrelli et al., 2005 41 | Carcinogenicity study of senna pod extract in rats | 2 years Positive control AOM Senna pod extract (by gavage): 30 or 60 mg/kg on 6 days/week |
Tumors | Colon ACF and colon tumors: not present in controls or either dose of senna; present in all groups dosed with AOM; significantly decreased in AOM + senna pod extract versus AOM alone | Senna is devoid of carcinogenic potential in rats; senna may act as an anti-tumoral agent against colon carcinogenesis |
| Mitchell et al., 2006 42 | Rat carcinogenicity study with senna | 2 years Senna at 0, 25, 100, and 300 mg/kg/day (oral gavage) |
Tumors | Increased tubular basophilia and epithelial hypertrophy in the kidneys No alterations in the colonic nervous plexus or increase of cell proliferation in the large intestine No treatment-related neoplastic changes were observed in any organ examined |
Senna is not considered to be carcinogenic after daily administration for 2 years at doses up to 300 mg/kg/day in rats |
| Surh et al., 2013 43 | To assess carcinogenicity of senna in a haplo-insufficient mouse model | 40 weeks Senna fed in the diet at 0, 100, 300, 3000, 10,000 ppm |
Tumors | Significant increases in epithelial hyperplasia (males, females): cecum 10,000 ppm 22/25 and 19/25; colon 3000 ppm 3/24 (not significant) and 7/25, 10,000 ppm 25/25 and 25/25; rectum 10,000 ppm 1/25 and 1/25 (not significant) | Large intestine is the major target of senna-induced toxicity in wild-type and p53+/− mouse models No neoplastic changes seen |
| Borrelli et al, 2001 44 | Subchronic carcinogenic study in rats – to assess ability of bisacodyl and cascara in inducing ACF and tumors in the colon. | 13 weeks Positive control AOM Bisacodyl: 4.3 or 43 mg/kg/day on 6 days/week ± AOM Cascara: 140 or 420 mg/kg/day on 6 days/week ± AOM |
ACF and tumors in the colon | Both doses bisacodyl + AOM did not modify ACF appearance but increased number of crypts/focus versus AOM alone Cascara (both doses) + AOM – no increase in ACF or crypt/focus versus AOM alone AOM alone induced colon tumors as expected Neither bisacodyl nor cascara at either dose (absence of AOM) induced tumors Compared with AOM alone, low-dose bisacodyl and both cascara doses + AOM had no effect on tumors Compared with AOM alone, high-dose bisacodyl + AOM significantly increased number of tumors ~10-fold (of the nine rats in this group, 78% had tumors) |
When bisacodyl, at either dose, was given without AOM initiating treatment, there was no evidence of tumor development Bisacodyl has a possible promoting effect on colon rat carcinogenesis, particularly at diarrheal dose Cascara had no promoting or initiating activity at a laxative and diarrheagenic dose |
Sennosides are active components of senna; rhein is the active metabolite of senna; (aloe)-emodin is an anthraquinone (stimulant laxative). A more complete summary of these studies is available in Supplemental Table 1.
ACF, aberrant crypt foci; AOM, azoxymethane; CA, chromosome aberration test; CHO, Chinese hamster ovary cells; DMH, 1,2-dimethylhydrazine; EM, electron microscopy; GI, gastrointestinal; HGPRT, hypoxanthine-guanine phosphoribosyl transferase; LM, light microscopy; MLA, mouse lymphoma assay; MNT, micronucleus test; NTP, National Toxicology Program; PCE, polychromatic erythrocyte; ppm, parts per million; SAL, Salmonella test; SHE, Syrian hamster embryo test; S9, supernatant of liver homogenate; UDS, unscheduled DNA synthesis.
Table 2.
Evidence from key clinical studies of stimulant laxative toxicity.
| Citation | Study design and aims | Study population | Findings | Conclusions |
|---|---|---|---|---|
| Inflammation, morphology, nerve damage | ||||
| Smith, 1968 32 | Case report | A 57-year-old female with history of plant-based stimulant laxative abuse for 40 years | Terminal 5 cm ileum with colon from caecum to rectum examined Ileocaecal valve was stenosed and incompetent Colon had loss of interhaustral folds Submucosa contained adipose tissues Atrophy of muscle layers Plexus had abnormal neurological structures |
Patient with cathartic syndrome had myenteric plexus damage |
| Joo et al., 1998 23 | To investigate colonic anatomy changes with chronic use of stimulant laxatives (diphenylmethanes, anthraquinones) | Part 1: mean 7.9 years (1–30) of laxative use Part 2: mean 5.2 years (2–19) of laxative use |
Colon changes (haustral fold loss) | Part 1: chronic versus nonusers (% patients): colonic redundancy 35% versus 19%; colonic dilatation 45% versus 23%; loss of haustral marking 28% versus 0% p < 0.005 Part 2: 39% had loss of haustral folds Over both parts, 32% of chronic users had haustral fold loss; no difference in duration/type of laxative use between patients with haustral fold loss versus those without |
| Riemann et al., 1980 22 | To evaluate ultrastructure of submucosal nerve tissue in laxative abuse Anthraquinones (cascara sagrada, aloe, folia sennae, cortex frangulae) or bisacodyl. Average daily dose ~180 mg (70–260 mg) |
Mean abuse = 13.5 years; 8–21 years | Ultrastructure of submucosal nerve tissue | Dose- and time-related severe damage of submucosal nerve fibers Main pathological features: axon ballooning, nerve-specific cell organelle reduction, lysosomal activity, and increased melanin-loaded macrophages Significant decrease in neurosecretory granules in nerve endings versus normal tissue |
| Saunders et al., 1990 45 | Impact of bisacodyl enemas on rectal mucosa in humans | Single biopsies collected at 3, 6, 24, 30, and 48 h post-enema | Histology (LM) | Injury of superficial epithelium and upper third of the crypt within 30 min after bisacodyl Mild inflammation seen histologically for up to 30 h after bisacodyl enema |
| Carcinogenicity | ||||
| Sonnenberg and Müller, 1993 26 | Meta-analysis of 14 case-control studies to evaluate if constipation or laxative use was associated with an increased CRC risk Various laxatives used for different dose frequencies |
Laxative-users (n): patients with CRC (8–165) and non-CRC controls (6–2225) No-laxative-use (n): CRC patients (64–626) and non-CRC controls (94–9135) |
In all but one study, CRC occurred more frequently in constipated patients than non-constipated patients; summary OR (95% CI): 1.48 (1.32–1.66) Use of laxatives also associated with CRC: OR (95% CI): 1.46 (1.33–1.61) The CRC risk was small but significant for both constipation and laxative use |
Increased CRC risk may reflect confounding influence of diet rather than a primary influence of colonic inertia |
| Siegers et al, 1993 46 | A retrospective and a prospective epidemiological study To assess copresence of PMC and colorectal diseases |
Patients undergoing endoscopy N: retrospective, 3049; prospective, 1095 31/33 patients with adenoma or CRC in prospective study abused anthranoid laxatives for 10–30 years |
Retrospective study: no abnormality 3.13% of patients had PMC; adenoma 8.64% (p < 0.01); CRC 3.94% Prospective study: no abnormality 6.9% of patients had PMC; adenoma 9.8%; CRC 18.6% (p < 0.0008) Prospective study: adenoma and CRC were linear functions of age, sex, and PMC; adenomas age (factor 2.47), sex (factor 0.7), PMC (factor 4.57); CRC age (factor 6.14), neither sex nor PMC were significant factors Prospective study: RR: 3.04 (95% CI: 1.18–4.90) for CRC as a result of anthranoid-laxative abuse |
Current data are contradictory as CC per se, together with dietary factors (e.g. low fiber and high fat intake), increase CRC risk in men |
| Kune, 1993 47 | Case-control arm of the Melbourne CRC study To evaluate the possible mutagenicity of anthraquinones |
Histologically confirmed new patients with colorectal adenocarcinoma from April 1980 to April 1981 (685 cases) Community controls were age/sex-matched – randomly selected (723 controls) |
RR of CRC for any anthraquinone use: 1.01 (not significant) RR of CRC for any phenolphthalein use: 1.35 (not significant) |
Use of anthraquinone-stimulant laxatives or phenolphthalein-containing laxatives was not associated with any increased risk of CRC |
| Jacobs and White, 1998 48 | Case-control study To examine associations of colon cancer with constipation and use of commercial laxatives |
Patients with colon cancer (all with invasive adenocarcinoma of the colon = 424). Control patients from same area in Seattle (random-digit dialing) (n = 414) Various durations of constipation and laxative use prior to interview |
Constipation was associated with substantially increased risk of colon cancer: adjusted RR: 2.0 (95% CI: 1.2–3.6) for constipation 12–51×/year, and 4.4 (95% CI: 2.1–8.9) for constipation ⩾52×/year Frequent use of commercial laxatives increased colon cancer risk: RR: 2.5 (95% CI: 1.3–4.7) for ⩾350 lifetime uses Adjusting for constipation and commercial-laxative use, cancer risk associated with laxative use disappeared, and strong association with constipation seen |
Results suggest that frequent constipation may be an important risk factor for colon cancer in middle-aged adults |
| Dukas et al., 2000 49 | A clinical prospective (case-control) study (Nurses’ health study living in the USA) To assess association between bowel-movement frequency, laxative use, and the risk of CRC |
Registered nurses (all female; n = 84,577), 30–55 years at enrollment; follow-up for 12 years | 611 cases of CRC RRs (95% CIs): bowel movements every third day versus daily movements 0.94 (0.69–1.28) for CRC, 0.88 (0.62–1.26) for colon cancer, 1.18 (0.63–2.20) for rectal cancer Compared with women never using laxatives, multivariate RRs (95% CI) associated with weekly to daily laxative use: 1.00 (0.72–1.40) for CRC, 1.09 (0.76–1.57) for colon cancer, 0.68 (0.29–1.57) for rectal cancer |
Findings did not support an association between infrequent bowel movement, laxative use, and CRC |
| Nascimbeni et al., 2002 50 | Prospective case-control study To assess the risk of colon cancer by constipation, anthranoid-laxative use, and PMC |
Patients undergoing resection for SC (n = 55) or DD (n = 41) and matched controls (n = 96) | OR (95% CI) versus controls: 1.9 (0.9–4.1, SC) and 2.8 (1.2–6.3, DD) for constipation; 5.3 (2.1–13, SC) and 4.0 (1.5–11, DD) for anthranoid-laxative use All patients: OR: 3.4 (1.3–8.5) –significant association between PMC and anthranoid-laxative use No association between ACF characteristics and history of constipation, or history of anthranoid, laxative use, or PMC |
Study demonstrates association between ACF frequency and colon cancer; it does not support a cause–effect relationship between CRC with constipation, use of anthranoid laxatives, or PMC |
| Guerin et al., 2014 51 | Retrospective, matched cohort design (CC matched 1:3 with non-CC controls) To evaluate the association of CC and CC severity with CRC |
Participants in US claims database with CC (n = 28,854) and matched controls (n = 86,562) | 1-year CRC prevalence 2.7% (CC patients) and 1.7% (non-CC patients) p < 0.001 Risk of developing CRC (IRR, 95% CI) versus CC free: mild CC: 0.83 (0.62–1.10), severe CC: 1.40 (1.18–1.66), p < 0.05; very severe CC: 2.26 (1.94–2.64), p < 0.05Most indicators of constipation severity were associated with a significant incremental risk of CRC (except for patients with laxative prescriptions) |
CC is associated with significantly higher prevalence and incidence of CRC and benign colorectal neoplasm versus matched CC-free patients. Risks increase with the severity of CC |
| Roberts, 2003 52 | Population-based, case-control study To determine if bowel-movement frequency, laxative use, and type were associated with colon cancer risk in white and black men and women |
Patients with pathologically confirmed invasive adenocarcinoma of the colon 643 cases (349 white, 294 black) and 1048 controls (611 white, 437 black) |
Constipation was associated with risk of colon cancer (OR, CI): 2.36; 1.41–3.93 adjusted for age, race, sex, and relevant confounders The association between constipation and cancer was greater for women: 2.69; 1.46–4.94) than for men: 1.73; 0.61–4.88; and stronger in blacks: 2.90; 1.54–5.46 than whites: 2.02; 1.04–3.91 No association with laxative use and colon cancer: OR: 0.88 (0.69–1.11) Fiber commercial laxatives appeared to exert a protective effect: OR: 0.58 (0.32–1.05) |
Evidence supports a positive association between constipation and increased risk of colon cancer. No apparent association of any laxative use with colon cancer risk. Commercial-fiber laxatives may lower the potential risk of colon cancer |
| Watanabe et al., 2004 53 | Population-based prospective case-control study in Japan To investigate the association between laxative use and CRC risk |
Healthy subjects (n = 41,670) 7 years follow-up |
RR (95% CI) of CRC for constipated subjects versus those daily bowel movements: 1.35 (0.99–1.84), p = 0.006 RR for CRC for laxative-users versus nonusers: 1.31 (0.88–1.95), p = 0.72; and for frequent users (⩾2×/week) 2.75 (1.48–5.09), p = 0.02 RR (95% CI) of colon cancer (constipated versus non-constipated): 1.47 (1.00–2.17), p < 0.05 RR for colon cancer for laxative-users versus nonusers: 1.48 (0.91–2.40), p = 0.11 RR (95% CI) of rectal cancer (constipated versus non-constipated): 1.16 (0.69–1.95), p = 0.59 RR for rectal cancer for laxative-users versus nonusers: 1.04 (0.52–2.10), p = 0.91 |
Supports hypothesis that constipation or laxative use increases the risk of colon cancer |
| Citronberg et al, 2014 27 | Prospective, questionnaire-based study To assess CRC risk with non-fiber and fiber laxatives |
Participants in VITAL study (n = 75,214) followed for 10 years | Non-fiber laxatives: HRs associated with low and high use were 1.49 (95% CI: 1.04–2.14) and 1.43 (95% CI: 0.82–2.28), respectively, versus those who used them <once/year; ptrend = 0.05 Use of non-fiber laxatives was higher than fiber laxatives: OR: 6.04 (95% CI: 5.52–6.60) versus 1.24 (95% CI: 1.10–1.41) in patients with low bowel-movement frequency HRs for CRC were significantly decreased and lowest in individuals who reported using fiber laxatives often (4+ days/week for 4+ years) versus those who reported no use (HR: 0.44, 95% CI: 0.21–0.95); ptrend = 0.19 No statistically significant associations between bowel-movement frequency or constipation and CRC risk |
Risk of CRC increases with non-fiber laxative use and decreases with fiber laxative use. Further studies needed to clarify relationships |
| Citronberg et al., 2018 28 | Retrospective study To examine the association between non-fiber-based laxative use, fiber-based laxative use, and CRC risk |
Participants in CCFC registry (n = 4930 with primary invasive CRC) and controls from the general population (n = 4025) | Non-fiber-based laxatives significantly increased CRC risk versus no use; OR: 2.17, 95% CI: 1.47–3.19 No risk of CRC with fiber-based laxative use: OR: 0.99, 95% CI: 0.80–1.22 |
Risk of CRC increased with non-fiber-based laxative use, while CRC risk was not significantly associated with fiber-based laxative use, compared with nonusers |
A more complete summary of these studies is available in Supplemental Table 2.
ACF, aberrant crypt foci; CC, chronic constipation; CCFC, Colon Cancer Family Registry; CI, confidence interval; CRC, colorectal cancer; DD, diverticular disease; GI, gastrointestinal; HR, hazard ratio; IRR, incidence rate ratio; LM, light microscopy; OR, odds ratios; PMC, pseudomelanosis coli; RR, relative risk; SC, sigmoid colon cancer; VITAL, vitamins and lifestyle.
Preclinical toxicology studies
Clinical observations of melanosis coli or pseudomelanosis coli (PMC) following long-term anthraquinone use have raised the possibility that such conditions might translate into mucosal damage and subsequent tumorigenesis. Bisacodyl and SPS have also come under suspicion because, like senna and sennosides, diphenylmethanes exert a stimulant and secretory effect and may cause intestinal epithelial changes.31,44,54–58 In our analyses, most preclinical studies were acute/short duration and investigated the potential of study treatments to damage the intestinal mucosal wall or to cause cancer. In two studies investigating long-term mucosal alterations of mouse jejunum and colon with sennosides versus a compound known to induce liver and colon tumors in mice, 59 and tumors in the large intestine of rats, 60 myenteric plexus abnormalities were seen only in animals treated with the compound known to induce tumors, 61 suggesting that sennosides do not cause gastrointestinal injury. 62
In a rat study, long-term treatment with sennosides or SPS did not induce chronic changes in colonic motility. 54 In several toxicity studies, sennosides were well tolerated in rats, mice, and dogs, with no specific toxicity to the intestine reported. 35 However, in another rat study, sennosides (but not SPS) at high doses and after long-term dosing, reduced neuropeptide levels in the muscularis externa of the descending colon (considered to be a surrogate marker for impact on intestinal structures, including the ENS). 57
An unpublished expert report by the original marketing authorization holder for bisacodyl summarizing toxicity study findings concluded that teratology studies showed bisacodyl to be unlikely to cause deformities. Bisacodyl has low toxicity in animal studies, with the intestine being the target organ (bisacodyl enhances peristalsis in the colon). Bisacodyl has not been shown to affect the liver, kidneys, heart, circulation, respiration, or sodium, potassium, or hematocrit levels, even at high doses, which may be attributable to its low bioavailability (Data on File).
Genotoxicity studies
Genotoxicity refers to the ability of harmful substances to damage genetic information in cells. 63 In contrast, mutagenicity refers to the ability of a compound to induce a permanent change in DNA structure, that is, a genetic mutation. 63 Regarding genotoxicity, in an analysis of data from rodent and human metabolism and mutation studies with senna, most studies showed no effects, although results from some studies59,60,64 suggested that components of senna products, particularly emodin and aloe-emodin, may have some genotoxic activity. However, response extrapolation estimates supported the conclusion that there are no safety concerns for senna products in humans. 36 Heidemann et al. 34 summarized the results of a number of in vitro and in vivo investigations which included genotoxicity/mutagenicity tests with fructus sennae, senna extract, sennosides, rhein (active metabolite of sennosides), and aloe-emodin. An extract of senna and aloe-emodin was genotoxic in some in vitro test systems, but no genotoxic effects were found in the in vivo tests. Fructus sennae, the sennosides, and rhein did not show any genotoxic effects either in vitro or in vivo. 34 Mengs also reported that sennosides had no mutagenic activity in several test systems. 35 Stoll et al. 31 reported on the genotoxic and carcinogenic responses to phenolphthalein and bisacodyl based on an extensive body of unpublished mutagenicity data reflecting results of a number of preclinical studies. These studies, including a repeated dose micronucleus assay 65 and Chinese hamster ovary cell mutation tests 66 did not detect any mutagenic potential for bisacodyl. Bisacodyl and phenolphthalein were subsequently investigated in the Syrian hamster embryo assay and in the heterozygous p53+/− transgenic mouse. Collectively, these tests indicated that, while phenolphthalein has some carcinogenic potential, there was no evidence of carcinogenic potential for bisacodyl, with no treatment-related neoplasia observed. Moreover, bisacodyl was non-clastogenic. 31
Inflammation, morphological changes, and neuronal damage, including impairment of colonic function
Histological changes in the myenteric plexus in mice exposed to bisacodyl at high doses have been reported. Infusion of bisacodyl was found to alter the morphological appearance of mucosal cells of the rat intestine, and it was suggested that the laxative effect of bisacodyl is related to a reduction of intestinal water absorption secondary to changes in surface absorptive cells of the colon. 38
Carcinogenicity studies
A carcinogen refers to a compound capable of causing cancer. 67 With regards to carcinogenicity, a study by Toyoda et al. 56 in rats found that cell proliferation in almost the entire intestinal epithelia was induced by danthron, sennoside A, and bisacodyl in a dose-dependent manner. Conversely, another study in Sprague-Dawley rats did not find any association between long-term (2-year) administration of senna and damage to the myenteric plexus or the development of gastrointestinal, liver, renal, or adrenal tumors. 39 Similarly, a study in rats found that sennoside, bisacodyl, SPS, or lactulose had no significant effect on ileal or colonic epithelial cell proliferation after up to 12 weeks of continuous treatment. 58 Furthermore, the National Toxicology Program reported that emodin was not carcinogenic in rats and mice. 37
In order to unmask any tumor-promoting effect of stimulant laxatives, some studies co-administered tumor-enhancer substances. In one study measuring the induction of aberrant crypt foci (ACF) in rat colon mucosa as a surrogate marker for a carcinogenic effect, senna, and cascara in the presence of the potent tumor-enhancer 1,2-dimethyl-hydrazine were found to be weak promoters of carcinogenesis at the highest doses applied. 68 In a model of dimethyl-hydrazine-induced colorectal tumors, aloin- or sennoside-enriched diets (0.03%) did not promote the incidence and growth of adenomas and carcinomas after 20 weeks, and no significant changes in serum electrolytes or markers of hepato- or nephrotoxicity were observed in aloin- or sennoside-fed mice. 40 In a study with azoxymethane in healthy rats, senna pod extract for 110 weeks reduced the development of ACF and tumors. 41 The authors suggested that chronic use of senna extract may have protected the gut wall from carcinogenesis. 41 Corroborating these results, no alterations in the colonic nervous plexus, increase in cell proliferation, or treatment-related neoplastic changes in the large intestine were observed when senna was administered for up to 2 years in Sprague-Dawley rats. 42
The US National Toxicology Program tested senna in a genetically modified mouse strain that develops tumor responses more rapidly than standard mice. In this 40-week study, there was no evidence of carcinogenic activity in mice exposed to senna, although it did appear to induce epithelial hyperplasia of the large intestine (colon and cecum).43,69
The carcinogenic potential of supratherapeutic doses of bisacodyl and of cascara was investigated in rats. 44 While bisacodyl had no effect when administered alone at either dose, there was an increase in the number of crypts per focus (but not in the number of tumors) when it was co-administered with azoxymethane. Of the nine rats that received a highly supratherapeutic dose of bisacodyl together with azoxymethane, 78% developed tumors. 44
Dunnick and Hailey have reported multiple carcinogenic effects in rats and mice with phenolphthalein, which was used as a stimulant laxative in the past. In toxicology studies, it increased the incidence of ovarian, adrenal, renal, and hematopoietic neoplasms. Ovarian lesions (stromal cell hyperplasia and stromal cell tumor) were seen at the lowest dose administered to female mice over 2 years. 70 The authors stated that phenolphthalein has estrogenic and clastogenic properties and is a multisite/multispecies carcinogen, with the ovary being a site of particular concern for the development of neoplasms.
Clinical studies and case reports
Inflammation, morphological changes, and neuronal damage, including impairment of colonic function (cathartic colon)
The first case of neuronal impairment was reported in 1968 in a woman who had misused senna for 40 years.32,33 Damage to the myenteric plexus was initially described as caused only by senna; however, these changes were subsequently ascribed to other stimulant laxatives, including bisacodyl.22,23 Bisacodyl and other stimulant laxatives were also implicated in damage to the ENS, 22 altering the anatomical integrity of the gut, resulting in a loss of haustral folds (cathartic colon), 23 inducing inflammation of the human rectal mucosa45,71 and increasing the risk of colorectal cancer (CRC).27,28 Bisacodyl enemas (10 mg) have been reported to result in transient injury, inflammation, irritation, or altered appearance of the rectal mucosa.45,71,72 Riemann et al. 22 described ENS damage among 35 patients who had consumed extremely high doses of (predominantly) stimulant laxatives [18 times the recommended dose 1 for a mean of 13.5 (8–21) years]. The presence of any concomitant disease was not reported for this group. Electron-microscopic pathological changes included a reduction of nerve elements, which was considered to be a morphological correlate to functional colonic impairment. Both anthraquinone derivatives and bisacodyl were considered to lead to reversible degeneration of the submucosal nervous tissue of the ENS, including cathartic colon. 22
Joo et al. 23 also investigated anatomic changes of the colon in 29 long-term users of bisacodyl, phenolphthalein, senna, or casanthranol observed over a mean duration of 7.9 (range 1–30) years. Again, the presence of any concomitant disease was not reported. Approximately half (45%) of the patients showed radiographically documented anatomical changes of colonic redundancy and dilation of colon, with loss of haustral markings, resulting in a variety of enteric symptoms including diarrhea and weakness, metabolic acidosis or alkalosis, and PMC (cathartic colon). This paper did not specify how many patients were taking bisacodyl, SPS, or senna, and no data were provided on the severity of constipation in each group. Subsequently, 39% of 18 chronic users of stimulant laxatives who were studied prospectively were found to have loss of haustral folds. The authors suggested that neuronal injury or damage to colonic longitudinal musculature could be caused by these agents if abused. 23
Müller-Lissner described 41 alleged cases of cathartic colon obtained from 18 papers; all patients were female and began taking laxatives between 1910 and 1960. Since 1960, no cases of cathartic colon have been published, leading to cathartic colon now being regarded as a historical entity that is unlikely to occur with modern laxatives. It is thought that the now-withdrawn agent podophyllin might have been the cause. 73
Carcinogenicity associated with phenolphthalein
Evidence suggests that phenolphthalein may be associated with malignancy in rodents, although at much higher doses than those used therapeutically in humans. 70 Phenolphthalein has also been associated with genotoxicity, oxidative damage, and interaction with estrogen receptors.70,74 Multiple biological properties of phenolphthalein, including its ability to form free radicals and its clastogenic and estrogenic activity, may have contributed to the carcinogenic effects observed. Phenolphthalein was, therefore, withdrawn from use as a laxative.
However, several studies have indicated that phenolphthalein is not carcinogenic in humans,74–76 although a slight non-significant risk of ovarian cancer was observed in one study. 75 For example, a prospective study that analyzed self-reported phenolphthalein use in patients who were admitted to hospital in the year following a diagnosis of cancer (n = 18,163) found no association between increased risk of cancer and phenolphthalein use. 74 Similarly, two population-based case-control studies conducted in eastern and western parts of the USA, respectively, found that compared to women who had never used a laxative (n = 731 and n = 424, respectively), the use of a phenolphthalein-based laxative was not associated with an increased risk of epithelial ovarian cancer (n = 410 and n = 536, respectively).75,76 These observations highlight the limitations of rodent carcinogenicity studies as being indicative of the same effect in humans.
Carcinogenicity associated with sennosides, bisacodyl, and SPS or with constipation
A 1993 meta-analysis of 14 case-control studies found a small but significant risk for CRC associated both with constipation [pooled odds ratio (OR): 1.48, 95% confidence interval (CI): 1.32–1.66] and the use of laxatives (OR: 1.46, 95% CI: 1.33–1.61). 26 The authors suggested that these findings reflected the confounding influence of dietary habits rather than the primary influence of colonic inertia or laxative use. However, this analysis did not look at different types of laxatives, and the incidence and mortality rates varied considerably between regions. 26
Two epidemiologic studies (one retrospective n = 3049, one prospective n = 1095) investigated the presence of PMC as a marker of chronic anthranoid-containing laxative abuse, including senna, aloe, cascara, frangula, and rheum, in patients with adenomas and CRC, with contradictory results. 46 PMC rate was not increased among CRC patients (retrospective study), and PMC incidence was increased among patients with adenomas but not in those with carcinomas. However, in the prospective study, PMC incidence was 6.9% for patients with no abnormality seen on endoscopy, 9.8% (p = 0.068) for patients with adenomas and 18.6% for patients with CRC. Data from the prospective study showed a relative risk (RR) for CRC of 3.04 (95% CI: 1.18–4.90) as a result of anthranoid-laxative abuse. The authors concluded that anthranoid-containing laxatives (aloe, cascara, frangula, and rheum) play a role in CRC and they attributed the observed differences between the retrospective and prospective study findings to confounding factors such as constipation per se and dietary factors (low fiber, high fat intake) that increase the risk for CRC in humans. 46 In a recent meta-analysis of five studies, a significantly higher prevalence of hyperplastic/inflammatory polyps and adenomas was reported in the PMC group than in the control group, while the incidence of adenocarcinoma was not significantly different between the two groups. 77 The association of adenomas with PMC can be explained by the ease of detection of even tiny polyps as white spots within a dark-colored colonic mucosa. This observation is now routinely applied in the enhanced detection of polyps by endogenous chromoendoscopy.78–80
Other studies have identified a strong relationship between constipation and CRC. In 1993, The Melbourne CRC Study was a large population-based epidemiological study of CRC incidence, etiology, and survival. 47 In this study, self-reported constipation was significantly more common in those with CRC than among controls (p = 0.05). Commercial-laxative use was similar among 685 patients with CRC and 723 age/sex-matched community-based controls. When laxatives were segregated into groups by type (anthranoids, phenolphthalein, mineral salts, and others), previous laxative intake was also found to be similar between patients with CRC and controls. As the controls were not examined by total colonoscopy, some asymptomatic colorectal adenomas or carcinomas among the control group may have been missed. The analysis showed that family history, previous colorectal polypectomy, and multiple past stressful life events, but not laxative use, predicted CRC risk.47,81 A retrospective case-control study of 424 incident cases of colon cancer and 414 controls reported a four-fold increased risk of colon cancer among individuals with frequent constipation. 48 Cumulative lifetime use of commercial laxatives was also associated with an increased risk of colon cancer, but this association disappeared when the results were adjusted for constipation and commercial-laxative use. The authors concluded that diet may explain much of the international variation in colon cancer incidence and that individual differences in colonic function might also have a role in determining susceptibility. 48
A large prospective study of 84,577 women in the USA also examined the association among bowel-movement frequency, laxative use, and CRC risk. 49 The data showed that frequency of bowel movements and laxative use were not associated with increased incidence of CRC (either for colon or rectal cancer). After controlling for various factors, including age, fiber intake, physical activity, and laxative use, the RRs (95% CI) associated with having bowel movements every third day or less, compared with those with bowel movements once daily, were 0.94 (0.69–1.28) for CRC, 0.88 (0.62–1.26) for colon cancer, and 1.18 (0.63–2.20) for rectal cancer. Compared with women who never used laxatives, the multivariate RRs (95% CI) associated with weekly to daily laxative use were 1.00 (0.72–1.40; CRC), 1.09 (0.76–1.57; colon cancer), and 0.68 (0.29–1.57; rectal cancer). 49
A prospective case-control study in patients with sigmoid cancer, diverticular disease, and matched controls confirmed the association of ACF frequency with colon cancer but did not support the hypothesis of a cause–effect relationship of CRC with constipation, anthranoid-laxative use, or PMC. 50 However, a strong association between constipation and CRC and benign colonic neoplasia (BCN) was found in a large, retrospective study of over 100,000 subjects. 51 Patients with constipation displayed a significantly higher risk of developing CRC than controls, and this risk increased with the severity of constipation. 51 Regular review by a gastroenterologist and treatment with laxatives appeared to reduce the risk of CRC and BCN compared with patients with less severe constipation. 82 These findings were corroborated by a population-based, case-control study including 643 cases and 1048 controls. Constipation (⩽3 bowel movements/week) was associated with an increased adjusted risk of colon cancer [OR (95% CI) 2.36 (1.41–3.93)]. 52 The association was greater for women than for men [OR: 2.69 (1.46–4.94) versus 1.73 (0.61–4.88)] and in black people than in white people. Notably, there was no association with laxative use, and commercial-fiber laxatives appeared to exert a protective effect in a small subgroup. 52
Two Japanese studies investigated the association between constipation or laxative use and CRC risk.53,83 In one study of 41,670 subjects, the RR (95% CI) of CRC for constipated subjects (<1 stool/day) compared with those with daily bowel movements was 1.35 (0.99–1.84); for laxative-users, the RR was 1.31 (0.88–1.95), and for frequent users (⩾2-times/week) the RR was 2.75 (1.48–5.09). When CRC was divided into colon cancers or rectal cancers, a significant association of colon cancer alone was found with bowel-movement frequency. 53 In the other study of 212 cases of CRC Dukes’ stage A and 833 community controls, the adjusted OR (95% CI) of CRC was 1.51 (1.02–2.25) for self-reported constipation, 1.60 (1.05–2.44) for functional constipation, and 1.24 (0.81–1.90) for infrequent bowel movements (<1 stool/day). 83
The association among CRC incidence, constipation, and laxative use was also investigated in another two studies.27,28 In the prospective study, of 75,214 subjects, 558 developed CRC, 413 were laxative nonusers. The hazard ratios (HRs) for CRC associated with low (1–4 times/year, n = 68) and high (⩾5 times, n = 26) long-term use (average 10-year non-fiber laxative use) were 1.49 (95% CI: 1.04–2.14) and 1.43 (95% CI: 0.82–2.28), respectively. The duration of administration was not differentiated. HRs for CRC were significantly decreased and were lowest in individuals who reported using fiber laxatives often (4+ days/week for 4+ years) versus those who reported no use (HR: 0.44 95% CI: 0.21–0.95). 27 In the retrospective study involving almost 9000 subjects, fiber-based laxative use was slightly more common among controls [4.7% (n = 190) versus 4.1% (n = 198)], while non-fiber-based laxatives were more commonly used among those with CRC [1.9% (n = 94) versus 1.0% (n = 39)]. 28
Discussion
Structural changes, nerve damage, and functional impairment of the intestine
Among the possible effects of excessive use of stimulant laxatives are morphological changes, neuronal damage, and functional impairment of the intestine. However, reports of histopathological changes and damage to the ENS caused by stimulant laxatives are scarce and often difficult to interpret. Although enteric nerve damage and smooth muscle atrophy have been reported, it is unclear whether these findings were attributable to a pre-existing primary motility disorder, concomitant disease, or the chronic use of laxatives.29,32 Stimulant laxatives have been shown to cause structural alterations to surface absorptive cells in both animals and humans at supratherapeutic doses of the medication. However, unlike constipation itself, as discussed later, these changes are not associated with any safety concerns as reported in the literature 21 and no formal long-term studies have been conducted on this concern. PMC, which can sometimes develop with chronic use of senna laxatives, is a harmless, reversible pigmentation of the colonic mucosa without functional relevance. 2
In the studies reporting damage to the myenteric plexus with laxatives,22,23 it was not discussed whether these findings could have resulted from autonomic neuropathy, which in most cases is caused by long-term diabetes or other underlying diseases. As these conditions typically result in constipation, these patients often take stimulant laxatives.84–86 The irritating effect of bisacodyl on the intestinal structure reported by Saunders et al.38,45 and Meisel et al. 72 is transient and attributable to high local concentrations that are only achievable when bisacodyl is instilled locally. Additionally, the studies by Saunders et al.38,45 are limited as the doses used were unspecified; also, Saunders et al. demonstrated damage to the small intestine with laxatives, which are enteric-coated and therefore are only released in the colon. While the retrospective study findings on chronic use of anthraquinone derivatives and bisacodyl by Riemann et al. 22 should be acknowledged, no prospective, controlled studies have demonstrated morphological changes of enteric neural elements or intestinal smooth muscle by bisacodyl or SPS in humans. Furthermore, studies reporting on the absorption and enterohepatic circulation are based on bisacodyl solution, not tablets, and include doses that are not consistent with those currently used in clinical practice.38,87
In a review of more than 70 publications describing 240 cases of stimulant laxative abuse, no cases of cathartic colon were reported, nor could any published case of cathartic colon be identified in which laxative intake started after 1960. Certainly, no randomized, prospective, controlled studies have demonstrated morphological changes of enteric neural elements or intestinal smooth muscle by senna, bisacodyl, or SPS. Nor did the chronic use of senna cause any structural and/or functional alteration of the enteric nerves or the smooth intestinal muscle.73,88,89 Thus, the available evidence indicates that chronic use of stimulant laxatives is unlikely to damage the colon.2,90
An important consideration when interpreting the findings of studies that report potential safety concerns with stimulant laxatives is that many of these22,23,32,38,45 did not control for potentially important confounding factors such as age and concomitant diseases. Age is obviously an important factor when considering a possible relationship between laxative use and cancer incidence since both increase with age. Similarly, comorbidities (notably diabetes) that are known to cause constipation affect the ENS and are associated with the development of damage to the myenteric plexus.91–93 Diabetes is associated with the development of autonomic neuropathy affecting the intrinsic neurons, interstitial cells of Cajal and smooth muscle, and can result in neuron loss/dysfunction and subsequent gastrointestinal complications (constipation has been reported in up to 60% of diabetics with autonomic polyneuropathy). 86
Colorectal cancer
With regard to any potential association between CRC and laxative use, it should be recognized that available evidence indicates a link between constipation itself and CRC risk rather than laxative use. A meta-analysis by Power et al. 94 showed a significantly increased prevalence of constipation in subjects with CRC compared with controls without CRC in 17/20 case-control studies, while another meta-analysis of case-control studies by Sonnenberg and Müller found an association between constipation and colon cancer with a pooled OR (95% CI) of 1.48 (1.32–1.66). 26 An association between constipation and CRC was also found in a large, controlled study 51 and in two case-control studies.48,53 In the Melbourne CRC study, self-reported constipation was significantly more common among subjects with CRC than in controls (p = 0.05). 47 It can be postulated that this association between constipation and CRC is perhaps attributable to more prolonged contact between carcinogens in the lumen and the intestinal mucosa in subjects with constipation, 82 or it may be the result of factors associated with constipation, particularly a diet poor in fiber and vegetables, which has been found to increase the risk of CRC. 50 In line with our current study, a recent systematic review on the use of anthraquinone laxatives reported that it is not possible to conclude on the association of anthraquinone laxatives with CRC due to the lack of robust studies; thus, there is a need for high-quality studies to formally test the association of increased risk of CRC with use of stimulant laxatives. 95
Regarding any link with laxative use, the Melbourne study found no association between anthraquinone or phenolphthalein use and CRC risk, 47 and one case-control study found no association with commercial-laxative use after adjustment for constipation. 48 Two studies, one prospective and one retrospective, suggested that CRC risk increases with non-fiber laxative use and decreases with fiber laxative use.27,28 However, taking into account the small numbers of cases with exposure to non-fiber laxatives, the lack of differentiation among non-fiber laxatives, and the fact that – compared to fiber laxatives – the use of non-fiber laxatives was much higher in patients with low bowel-movement frequency, the scientific relevance of the conclusions in favor of fiber laxatives appears questionable. It is notable that in the prospective study (this was not described for the retrospective study), the use of non-fiber laxatives was much higher than that of fiber laxatives [OR: 6.04 (95% CI: 5.52–6.60) versus 1.24 (1.10–1.41)] among patients with low bowel-movement frequency. 27 Thus, those subjects who had exposure to non-fiber laxatives seem to have had a different severity of constipation, which may suggest differences in the underlying treatment condition(s) and medical history, raising doubts about the comparability of the patient groups with regard to their CRC risk.
This hypothesis is corroborated by another study by Lacy et al., 96 who found that compared with stimulant laxatives (bisacodyl, SPS, senna), other non-stimulant laxatives (PEG, stool softeners, bulk laxatives, and wheat dextrin) provided a lower degree of satisfaction with the medication’s effect on subjects’ constipation and abdominal symptoms. Thus, the severity and chronicity of constipation may have been a relevant confounding factor distorting results of these investigations. Patients with more chronic or severe symptoms of constipation may be more likely to take faster-acting and more potent laxatives and, at the same time, may have a higher susceptibility to develop CRC.
Association of phenolphthalein with CRC
In 1997, the US FDA proposed phenolphthalein to be reclassified as ‘not generally recognized as safe and effective’ following positive rodent carcinogen studies.70,97 Consequently, phenolphthalein was banned by the FDA, and almost all phenolphthalein-containing preparations were voluntarily withdrawn from the market in the USA and Europe. However, retrospective case-control studies have not shown any significant link between phenolphthalein and CRC risk. Moreover, the reported human data did not address any potential effect of their very heavy use or abuse.
Association of anthranoid laxatives with CRC
Although stimulant laxatives induced cell proliferation in a dose-dependent manner in preclinical studies, and some data suggest that senna and cascara glycoside might be weak promoters of rat colon carcinogenesis, 68 it is important to note that a large proportion of the carcinogenicity demonstrated in rodents may be an artifact of chronic cell proliferation induced at the high doses (and comparatively long durations of exposure, given the relative lifespans of rodents and humans) used in routine bioassays. 98 This consideration may explain why more than half of the compounds for a range of medical conditions examined by the US National Toxicology Program at the maximum tolerated dose were deemed to be carcinogenic. 56 Response extrapolation estimates lend support to the conclusion that experimental data from rodent and human metabolism and mutation studies do not indicate that oral consumption of senna laxatives poses systemic risks to somatic cells of humans. 36 This position is supported by the 2-year carcinogenicity study with a senna extract in rats, which found no evidence of tumorigenicity in the intestinal tract36,39 and other studies.41,58,99,100
With regard to clinical studies, while data on the association between chronic anthranoid-laxative use and adenoma and CRC promotion were inconsistent in one study, 46 the balance of the study data, including other studies,47,48–51 does not support the hypothesis of a causal relationship between anthranoid-laxative use or PMC 81 and CRC. The study by Borrelli et al. 41 reported a protective effect of senna from carcinogenesis.
Conclusion
Many years ago, a single case report 32 speculating that stimulant laxatives might cause harm has resulted in continuing concerns about their safety and reluctance to prescribe them, especially over the long term. Some evidence exists of gut wall mucosal damage and changes of colonic submucosal nerves from small and older, mostly retrospective studies where other causes may not have been excluded. However, no well-controlled prospective clinical studies have demonstrated any such changes associated with the administration of diphenyl-methane laxatives, bisacodyl, or SPS in humans. It remains unclear from uncontrolled studies whether the reported changes were attributable to an underlying disease, the primary motility disorder itself, or if the cause was chronic use of laxatives. Thus, there is no good evidence that stimulant laxatives cause structural impairment of enteric nerves or intestinal smooth muscle when used at recommended therapeutic doses.
Similarly, although there is some evidence that constipation itself may be associated with CRC, there are no data showing that stimulant laxatives are an independent risk factor for CRC. While bisacodyl, SPS, and senna have not been found to cause CRC or other cancers, even if used long-term and at high doses, carcinogenic potential was demonstrated for phenolphthalein when administered at chronic high doses in animal studies, resulting in its withdrawal after >100 years of use. However, carcinogenic effects were never demonstrated in humans. Given the potential link between constipation and CRC (which could result, at least in part, from prolonged contact between carcinogens in the lumen and the intestinal mucosa), it has been speculated that the use of laxatives may actually exert some protective effect. 82 A large, retrospective study reported that the risk of CRC (and benign neoplasm) increased with severity of constipation. 51 Interestingly, this study also showed that each indicator of constipation severity was associated with a significant incremental risk of CRC, except for patients under the care of gastroenterologists or those taking prescription laxatives; that is, the cancer risk for these patients was similar to the risk in those with less severe constipation. 51 This observation suggests a potential preventive role for laxatives, as better clinical management and treatment of constipation may decrease the risk of CRC. 82
In summary, there is a lack of compelling evidence on genotoxicity, carcinogenicity, or harmful effects on the colon associated with the recommended use of stimulant laxatives in humans. Many of the studies discussed in this review were of poor design, using only small sample sizes and/or lacked control for confounding factors, and thus, were not highly representative of the real-world use of stimulant laxatives. This indicates a need for well-designed epidemiological and/or clinical studies to formally address this research question.
A recent Rome Foundation working group document reiterated the recommendation of stimulant laxatives as a first-line intervention for functional constipation and highlighted that concerns regarding their long-term safety are unsubstantiated. 101 Bisacodyl and senna have been available as branded medications for more than 70 years, and SPS for more than 50 years. Based on the available data, we conclude that patients should not be denied the benefits of these stimulant laxatives, which have been shown to improve quality of life and are associated with high patient satisfaction when used for constipation. 102 Our critical review demonstrates that there are no significant gut safety concerns regarding the use of stimulant laxatives based on clinical observations over the long timeframe during which they have been available to patients with constipation. Stimulant laxatives have stood the test of time in the treatment of constipation and represent an important component of our therapeutic armamentarium.
Supplemental Material
Supplemental material, sj-docx-1-tag-10.1177_17562848241249664 for Review article: do stimulant laxatives damage the gut? A critical analysis of current knowledge by Peter Whorwell, Robert Lange and Carmelo Scarpignato in Therapeutic Advances in Gastroenterology
Acknowledgments
Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Ian C. Grieve, PhD, and Jackie Phillipson, PhD, of Ashfield MedComms, an Inizio company.
Footnotes
ORCID iDs: Peter Whorwell  https://orcid.org/0000-0002-5220-8474
https://orcid.org/0000-0002-5220-8474
Carmelo Scarpignato  https://orcid.org/0000-0001-5645-857X
https://orcid.org/0000-0001-5645-857X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Peter Whorwell, Neurogastroenterology Unit, Wythenshawe Hospital, Southmoor Road, Wythenshawe, Manchester M23 9LT, UK.
Robert Lange, Sanofi, Frankfurt am Main, Germany.
Carmelo Scarpignato, Department of Health Sciences, United Campus of Malta, Msida, Malta; Faculty of Medicine, Chinese University of Hong Kong, Sha Tin, Hong Kong.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Peter Whorwell: Conceptualization; Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Robert Lange: Conceptualization; Formal analysis; Investigation; Methodology; Writing – review & editing.
Carmelo Scarpignato: Conceptualization; Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Sanofi provided financial support for the publication costs of the article and medical writing support.
Competing interests: P.W. has acted as a consultant or received research funding from Danone, Allergan, Ironwood, Enteromed, and Sanofi, all outside of the submitted work. R.L. is an employee of Sanofi and may hold stock/share interests in Sanofi. C.S. has served as a speaker, consultant, and/or advisory board member for Alfasigma, Pfizer, Takeda, Reckitt Benckiser, and Shionogi, and has in the past received funding from Giuliani Pharmaceuticals and Pfizer.
Availability of data and materials: Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- 1. Mueller-Lissner S, Kamm MA, Wald A, et al. Multicenter, 4-week, double-blind, randomized, placebo-controlled trial of sodium picosulfate in patients with chronic constipation. Am J Gastroenterol 2010; 105: 897–903. [DOI] [PubMed] [Google Scholar]
- 2. Portalatin M, Winstead N. Medical management of constipation. Clin Colon Rectal Surg 2012; 25: 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rao SSC, Brenner DM. Efficacy and safety of over-the-counter therapies for chronic constipation: an updated systematic review. Am J Gastroenterol 2021; 116: 1156–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lindberg G, Hamid SS, Malfertheiner P, et al. World Gastroenterology Organisation global guideline: constipation – a global perspective. J Clin Gastroenterol 2011; 45: 483–487. [DOI] [PubMed] [Google Scholar]
- 5. Kamm MA, Mueller-Lissner S, Wald A, et al. Oral bisacodyl is effective and well-tolerated in patients with chronic constipation. Clin Gastroenterol Hepatol 2011; 9: 577–583. [DOI] [PubMed] [Google Scholar]
- 6. Le J, Ji H, Zhou X, et al. Pharmacology, toxicology, and metabolism of sennoside A, a medicinal plant-derived natural compound. Front Pharmacol 2021; 12: 714586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhao Q, Chen Y-Y, Xu D-Q, et al. Action mode of gut motility, fluid and electrolyte transport in chronic constipation. Front Pharmacol 2021; 12; 630249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Corsetti M, Landes S, Lange R. Bisacodyl: a review of pharmacology and clinical evidence to guide use in clinical practice in patients with constipation. Neurogastroenterol Motil 2021; 33: e14123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. American Gastroenterological Association; Bharucha AE, Dorn SD, Lembo A, et al. American Gastroenterological Association medical position statement on constipation. Gastroenterology 2013; 144: 211–217. [DOI] [PubMed] [Google Scholar]
- 10. Ford AC, Suares NC. Effect of laxatives and pharmacological therapies in chronic idiopathic constipation: systematic review and meta-analysis. Gut 2011; 60: 209–218. [DOI] [PubMed] [Google Scholar]
- 11. Andresen V, Layer P. Medical therapy of constipation: current standards and beyond. Visc Med 2018; 34: 123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vitton V, Damon H, Benezech A, et al. Clinical practice guidelines from the French National Society of Coloproctology in treating chronic constipation. Eur J Gastroenterol Hepatol 2018; 30: 357–363. [DOI] [PubMed] [Google Scholar]
- 13. Black CJ, Ford AC. Chronic idiopathic constipation in adults: epidemiology, pathophysiology, diagnosis and clinical management. Med J Aust 2018; 209: 86–91. [DOI] [PubMed] [Google Scholar]
- 14. Wald A. Constipation: advances in diagnosis and treatment. JAMA 2016; 315: 185–191. [DOI] [PubMed] [Google Scholar]
- 15. Serra J, Pohl D, Azpiroz F, et al. European Society of Neurogastroenterology and Motility guidelines on functional constipation in adults. Neurogastroenterol Motil 2020; 32: e13762. [DOI] [PubMed] [Google Scholar]
- 16. Chang L, Chey WD, Imdad A, et al. American Gastroenterological Association–American College of Gastroenterology Clinical Practice guideline: pharmacological management of chronic idiopathic constipation. Gastroenterology 2023; 164: 1086–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mounsey A, Raleigh M, Wilson A. Management of constipation in older adults. Am Fam Physician 2015; 92: 500–504. [PubMed] [Google Scholar]
- 18. Tack J, Boardman H, Layer P, et al. An expert consensus definition of failure of a treatment to provide adequate relief (F-PAR) for chronic constipation – an international Delphi survey. Aliment Pharmacol Ther 2017; 45: 434–442. [DOI] [PubMed] [Google Scholar]
- 19. Khan S, Khan SU. Adverse drug event of hypokalaemia-induced cardiotoxicity secondary to the use of laxatives: a systematic review of case reports. J Clin Pharm Ther 2020; 45: 927–936. [DOI] [PubMed] [Google Scholar]
- 20. Noergaard M, Traerup Andersen J, Jimenez-Solem E, et al. Long term treatment with stimulant laxatives – clinical evidence for effectiveness and safety? Scand J Gastroenterol 2019; 54: 27–34. [DOI] [PubMed] [Google Scholar]
- 21. Wald A. Is chronic use of stimulant laxatives harmful to the colon? J Clin Gastroenterol 2003; 36: 386–389. [DOI] [PubMed] [Google Scholar]
- 22. Riemann JF, Schmidt H, Zimmermann W. The fine structure of colonic submucosal nerves in patients with chronic laxative abuse. Scand J Gastroenterol 1980; 15: 761–768. [DOI] [PubMed] [Google Scholar]
- 23. Joo JS, Ehrenpreis ED, Gonzalez L, et al. Alterations in colonic anatomy induced by chronic stimulant laxatives: the cathartic colon revisited. J Clin Gastroenterol 1998; 26: 283–286. [DOI] [PubMed] [Google Scholar]
- 24. Bengtsson M, Ohlsson B. Retrospective study of long-term treatment with sodium picosulfate. Eur J Gastroenterol Hepatol 2004; 16: 433–434. [DOI] [PubMed] [Google Scholar]
- 25. Bharucha AE, Wald A. Chronic constipation. Mayo Clin Proc 2019; 94: 2340–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sonnenberg A, Müller AD. Constipation and cathartics as risk factors of colorectal cancer: a meta-analysis. Pharmacology 1993; 47(Suppl. 1): 224–233. [DOI] [PubMed] [Google Scholar]
- 27. Citronberg J, Kantor ED, Potter JD, et al. A prospective study of the effect of bowel movement frequency, constipation, and laxative use on colorectal cancer risk. Am J Gastroenterol 2014; 109: 1640–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Citronberg JS, Hardikar S, Phipps A, et al. Laxative type in relation to colorectal cancer risk. Ann Epidemiol 2018; 28: 739–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Müller-Lissner SA, Kamm MA, Scarpignato C, et al. Myths and misconceptions about chronic constipation. Am J Gastroenterol 2005; 100: 232–242. [DOI] [PubMed] [Google Scholar]
- 30. Ueberberg H, Ballhause H. Dulcolax (Bisacodyl; La 96a): expert report on the pharmacological, toxicological and biochemical documentation. Unpublished internal doc. no. U89-0721. Dr. Karl Thomae GmbH, 1988.
- 31. Stoll RE, Blanchard KT, Stoltz JH, et al. Phenolphthalein and bisacodyl: assessment of genotoxic and carcinogenic responses in heterozygous p53 (+/−) mice and syrian hamster embryo (SHE) assay. Toxicol Sci 2006; 90: 440–450. [DOI] [PubMed] [Google Scholar]
- 32. Smith B. Effect of irritant purgatives on the myenteric plexus in man and the mouse. Gut 1968; 9: 139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith B. Disorders of the myenteric plexus. Gut 1970; 11: 271–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heidemann A, Miltenburger HG, Mengs U. The genotoxicity status of senna. Pharmacology 1993; 47(Suppl. 1): 178–186. [DOI] [PubMed] [Google Scholar]
- 35. Mengs U. Toxic effects of sennosides in laboratory animals and in vitro. Pharmacology 1988; 36(Suppl. 1): 180–187. [DOI] [PubMed] [Google Scholar]
- 36. Brusick D, Mengs U. Assessment of the genotoxic risk from laxative senna products. Environ Mol Mutagen 1997; 29: 1–9. [DOI] [PubMed] [Google Scholar]
- 37. NTP. NTP toxicology and carcinogenesis studies of EMODIN (CAS NO. 518-82-1) feed studies in F344/N rats and B6C3F1 mice. Natl Toxicol Program Tech Rep Ser 2001; 493: 1–278. [PubMed] [Google Scholar]
- 38. Saunders DR, Sillery J, Rachmilewitz D, et al. Effect of bisacodyl on the structure and function of rodent and human intestine. Gastroenterology 1977; 72: 849–856. [PubMed] [Google Scholar]
- 39. Lydén-Sokolowski A, Nilsson A, Sjöberg P. Two-year carcinogenicity study with sennosides in the rat: emphasis on gastro-intestinal alterations. Pharmacology 1993; 47(Suppl. 1): 209–215. [DOI] [PubMed] [Google Scholar]
- 40. Siegers CP, Siemers J, Baretton G. Sennosides and aloin do not promote dimethylhydrazine-induced colorectal tumors in mice. Pharmacology 1993; 47(Suppl. 1): 205–208. [DOI] [PubMed] [Google Scholar]
- 41. Borrelli F, Capasso R, Aviello G, et al. Senna and the formation of aberrant crypt foci and tumors in rats treated with azoxymethane. Phytomedicine 2005; 12: 501–505; discussion 5. [DOI] [PubMed] [Google Scholar]
- 42. Mitchell JM, Mengs U, McPherson S, et al. An oral carcinogenicity and toxicity study of senna (Tinnevelly senna fruits) in the rat. Arch Toxicol 2006; 80: 34–44. [DOI] [PubMed] [Google Scholar]
- 43. Surh I, Brix A, French JE, et al. Toxicology and carcinogenesis study of senna in C3B6.129F1-Trp53 tm1Brd N12 haploinsufficient mice. Toxicol Pathol 2013; 41: 770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Borrelli F, Mereto E, Capasso F, et al. Effect of bisacodyl and cascara on growth of aberrant crypt foci and malignant tumors in the rat colon. Life Sci 2001; 69: 1871–1877. [DOI] [PubMed] [Google Scholar]
- 45. Saunders DR, Haggitt RC, Kimmey MB, et al. Morphological consequences of bisacodyl on normal human rectal mucosa: effect of a prostaglandin E1 analog on mucosal injury. Gastrointest Endosc 1990; 36: 101–104. [DOI] [PubMed] [Google Scholar]
- 46. Siegers CP, von Hertzberg-Lottin E, Otte M, et al. Anthranoid laxative abuse – a risk for colorectal cancer? Gut 1993; 34: 1099–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kune GA. Laxative use not a risk for colorectal cancer: data from the Melbourne Colorectal Cancer Study. Z Gastroenterol 1993; 31: 140–143. [PubMed] [Google Scholar]
- 48. Jacobs EJ, White E. Constipation, laxative use, and colon cancer among middle-aged adults. Epidemiology 1998; 9: 385–391. [PubMed] [Google Scholar]
- 49. Dukas L, Willett WC, Colditz GA, et al. Prospective study of bowel movement, laxative use, and risk of colorectal cancer among women. Am J Epidemiol 2000; 151: 958–964. [DOI] [PubMed] [Google Scholar]
- 50. Nascimbeni R, Donato F, Ghirardi M, et al. Constipation, anthranoid laxatives, melanosis coli, and colon cancer: a risk assessment using aberrant crypt foci. Cancer Epidemiol Biomarkers Prev 2002; 11: 753–757. [PubMed] [Google Scholar]
- 51. Guerin A, Mody R, Fok B, et al. Risk of developing colorectal cancer and benign colorectal neoplasm in patients with chronic constipation. Aliment Pharmacol Ther 2014; 40: 83–92. [DOI] [PubMed] [Google Scholar]
- 52. Roberts M. Constipation, laxative use, and colon cancer in a North Carolina population. Am J Gastroenterol 2003; 98: 857–864. [DOI] [PubMed] [Google Scholar]
- 53. Watanabe T, Nakaya N, Kurashima K, et al. Constipation, laxative use and risk of colorectal cancer: The Miyagi Cohort Study. Eur J Cancer 2004; 40: 2109–2115. [DOI] [PubMed] [Google Scholar]
- 54. Fioramonti J, Dupuy C, Buéno L. In vivo motility of rat colon chronically pretreated with sennosides. Pharmacology 1993; 47(Suppl. 1): 155–161. [DOI] [PubMed] [Google Scholar]
- 55. Mengs U, Rudolph RL. Light and electron-microscopic changes in the colon of the guinea pig after treatment with anthranoid and non-anthranoid laxatives. Pharmacology 1993; 47(Suppl. 1): 172–177. [DOI] [PubMed] [Google Scholar]
- 56. Toyoda K, Nishikawa A, Furukawa F, et al. Cell proliferation induced by laxatives and related compounds in the rat intestine. Cancer Lett 1994; 83: 43–49. [DOI] [PubMed] [Google Scholar]
- 57. Tzavella K, Schenkirsch G, Riepl RL, et al. Effects of long-term treatment with anthranoids and sodium picosulphate on the contents of vasoactive intestinal polypeptide, somatostatin and substance P in the rat colon. Eur J Gastroenterol Hepatol 1995; 7: 13–20. [PubMed] [Google Scholar]
- 58. Geboes K. Laxatives and intestinal epithelial cells: a morphological study of epithelial cell damage and proliferation. Verh K Acad Geneeskd Belg 1995; 57: 51–74; discussion 74–77. [PubMed] [Google Scholar]
- 59. Mori H, Sugie S, Niwa K, et al. Carcinogenicity of chrysazin in large intestine and liver of mice. Jpn J Cancer Res 1986; 77: 871–876. [PubMed] [Google Scholar]
- 60. Mori H, Sugie S, Niwa K, et al. Induction of intestinal tumours in rats by chrysazin. Br J Cancer 1985; 52: 781–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dufour P, Gendre P. Ultrastructure of mouse intestinal mucosa and changes observed after long term anthraquinone administration. Gut 1984; 25: 1358–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dufour P, Gendre P. Long-term mucosal alterations by sennosides and related compounds. Pharmacology 1988; 36(Suppl. 1): 194–202. [DOI] [PubMed] [Google Scholar]
- 63. Ren N, Atyah M, Chen W-Y, et al. The various aspects of genetic and epigenetic toxicology: testing methods and clinical applications. J Transl Med 2017; 15: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Westendorf J, Marquardt H, Poginsky B, et al. Genotoxicity of naturally occurring hydroxyanthraquinones. Mutat Res 1990; 240: 1–12. [DOI] [PubMed] [Google Scholar]
- 65. Hayashi M. The micronucleus test – most widely used in vivo genotoxicity test. Genes Environ 2016; 38: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang S, Hughes JD, Murgolo N, et al. Mutation detection in an antibody-producing Chinese hamster ovary cell line by targeted RNA sequencing. Biomed Res Int 2016; 2016: 8356435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Schrenk D. What is the meaning of ‘a compound is carcinogenic’? Toxicol Rep 2018; 5: 504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mereto E, Ghia M, Brambilla G. Evaluation of the potential carcinogenic activity of Senna and Cascara glycosides for the rat colon. Cancer Lett 1996; 101: 79–83. [DOI] [PubMed] [Google Scholar]
- 69. NTP. Toxicology study of senna (CAS No. 8013-11-4) in C57BL/6NTAC mice and toxicology and carcinogenesis study of senna in genetically modified C3B6.129F1/Tac-Trp53tm1Brd haploinsufficient mice (feed studies). Natl Toxicol Program Genet Modif Model Rep 2012: 1–114. [PMC free article] [PubMed] [Google Scholar]
- 70. Dunnick JK, Hailey JR. Phenolphthalein exposure causes multiple carcinogenic effects in experimental model systems. Cancer Res 1996; 56: 4922–4926. [PubMed] [Google Scholar]
- 71. Hallmann F. Toxicity of commonly used laxatives. Med Sci Monit 2000; 6: 618–628. [PubMed] [Google Scholar]
- 72. Meisel JL, Bergman D, Graney D. Human rectal mucosa: proctoscopic and morphological changes caused by laxatives. Gastroenterology 1977; 72: 1274. [PubMed] [Google Scholar]
- 73. Müller-Lissner S. What has happened to the cathartic colon? Gut 1996; 39: 486–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Coogan PF, Rosenberg L, Palmer JR, et al. Phenolphthalein laxatives and risk of cancer. J Natl Cancer Inst 2000; 92: 1943–1944. [DOI] [PubMed] [Google Scholar]
- 75. Cooper GS, Longnecker MP, Sandler DP, et al. Risk of ovarian cancer in relation to use of phenolphthalein-containing laxatives. Br J Cancer 2000; 83: 404–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cooper GS, Longnecker MP, Peters RK. Ovarian cancer risk and use of phenolphthalein-containing laxatives. Pharmacoepidemiol Drug Saf 2004; 13: 35–39. [DOI] [PubMed] [Google Scholar]
- 77. Katsumata R, Manabe N, Fujita M, et al. Colorectal neoplasms in melanosis coli: a survey in Japan and a worldwide meta-analysis. Int J Colorectal Dis 2021; 36: 2177–2188. [DOI] [PubMed] [Google Scholar]
- 78. Antonelli G, Correale L, Spadaccini M, et al. Dye-based chromoendoscopy for the detection of colorectal neoplasia: meta-analysis of randomized controlled trials. Gastrointest Endosc 2022; 96: 411–422. [DOI] [PubMed] [Google Scholar]
- 79. Nusko G, Schneider B, Ernst H, et al. Melanosis coli – a harmless pigmentation or a precancerous condition? Z Gastroenterol 1997; 35: 313–318. [PubMed] [Google Scholar]
- 80. Repici A, Wallace MB, East JE, et al. Efficacy of per-oral methylene blue formulation for screening colonoscopy. Gastroenterology 2019; 156: 2198–2207.e1. [DOI] [PubMed] [Google Scholar]
- 81. Nusko G, Schneider B, Schneider I, et al. Anthranoid laxative use is not a risk factor for colorectal neoplasia: results of a prospective case control study. Gut 2000; 46: 651–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Scarpignato C, Blandizzi C. Editorial: adequate management may reduce the colorectal cancer risk associated with constipation. Aliment Pharmacol Ther 2014; 40: 562–564. [DOI] [PubMed] [Google Scholar]
- 83. Tashiro N, Budhathoki S, Ohnaka K, et al. Constipation and colorectal cancer risk: the Fukuoka Colorectal Cancer Study. Asian Pac J Cancer Prev 2011; 12: 2025–2030. [PubMed] [Google Scholar]
- 84. Schmidt RE, Nelson JS, Johnson EM., Jr. Experimental diabetic autonomic neuropathy. Am J Pathol 1981; 103: 210–225. [PMC free article] [PubMed] [Google Scholar]
- 85. Jung HK, Kim DY, Moon IH, et al. Colonic transit time in diabetic patients – comparison with healthy subjects and the effect of autonomic neuropathy. Yonsei Med J 2003; 44: 265–272. [DOI] [PubMed] [Google Scholar]
- 86. Rossol S. [Constipation in patients with diabetes mellitus]. MMW Fortschr Med 2007; 149: 39–42. [DOI] [PubMed] [Google Scholar]
- 87. Ewe K. The physiological basis of laxative action. Pharmacology 1980; 20(Suppl. 1): 2–20. [DOI] [PubMed] [Google Scholar]
- 88. Leng-Peschlow E. Senna and its rational use. Pharmacology 1992; 44(Suppl. 1): 1–52. [PubMed] [Google Scholar]
- 89. Morales MA, Hernández D, Bustamante S, et al. Is senna laxative use associated to cathartic colon, genotoxicity, or carcinogenicity? J Toxicol 2009; 2009: 287247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Camilleri M, Ford AC, Mawe GM, et al. Chronic constipation. Nat Rev Dis Primers 2017; 3: 17095. [DOI] [PubMed] [Google Scholar]
- 91. Nezami BG, Srinivasan S. Enteric nervous system in the small intestine: pathophysiology and clinical implications. Curr Gastroenterol Rep 2010; 12: 358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Prasad VG, Abraham P. Management of chronic constipation in patients with diabetes mellitus. Indian J Gastroenterol 2017; 36: 11–22. [DOI] [PubMed] [Google Scholar]
- 93. Lebouvier T, Chaumette T, Paillusson S, et al. The second brain and Parkinson’s disease. Eur J Neurosci 2009; 30: 735–741. [DOI] [PubMed] [Google Scholar]
- 94. Power AM, Talley NJ, Ford AC. Association between constipation and colorectal cancer: systematic review and meta-analysis of observational studies. Am J Gastroenterol 2013; 108: 894–904. [DOI] [PubMed] [Google Scholar]
- 95. Lombardi N, Crescioli G, Maggini V, et al. Anthraquinone laxatives use and colorectal cancer: a systematic review and meta-analysis of observational studies. Phytother Res 2022; 36: 1093–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lacy BE, Shea EP, Manuel M, et al. Lessons learned: chronic idiopathic constipation patient experiences with over-the-counter medications. PLoS One 2021; 16: e0243318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Program NT. NTP toxicology and carcinogenesis studies of phenolphthalein (CAS No. 77-09-8) in F344/N rats and B6C3F1 mice (feed studies). Natl Toxicol Program Tech Rep Ser 1996; 465: 1–354. [PubMed] [Google Scholar]
- 98. Goodman JI. Goodbye to the bioassay. Toxicol Res 2018; 7: 558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Mascolo N, Mereto E, Borrelli F, et al. Does senna extract promote growth of aberrant crypt foci and malignant tumors in rat colon? Dig Dis Sci 1999; 44: 2226–2230. [DOI] [PubMed] [Google Scholar]
- 100. Borrelli F, Aviello G, Capasso R, et al. Senna: a laxative devoid of carcinogenic effects. Arch Toxicol 2006; 80: 873. [DOI] [PubMed] [Google Scholar]
- 101. Brenner DM, Corsetti M, Drossman D, et al. Perceptions, definitions, and therapeutic interventions for occasional constipation: a Rome Working Group Consensus Document. Clin Gastroenterol Hepatol 2024; 22: 397–412. [DOI] [PubMed] [Google Scholar]
- 102. Lacy BE, Delfini R, Fladung B, et al. Prevalence and patterns of laxative use in subjects with self-reported constipation: results from a multinational digestive health survey. Therap Adv Gastroenterol 2024; 17: 17562848241232605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tag-10.1177_17562848241249664 for Review article: do stimulant laxatives damage the gut? A critical analysis of current knowledge by Peter Whorwell, Robert Lange and Carmelo Scarpignato in Therapeutic Advances in Gastroenterology