Iron is critical during host-microbe interactions1–4. Restriction of available iron by the host during infection is an important defense strategy, described as nutritional immunity5. However, this poses a conundrum for externally facing, absorptive tissues like the gut epithelium or the plant root epidermis that generate environments favoring iron bioavailability. For instance, plant roots acquire iron mostly from the soil and when iron deficient, increase iron availability through mechanisms that include rhizosphere acidification and secretion of iron chelators6–9. Yet, the elevated iron bioavailability would also be beneficial for the growth of bacteria which threaten plant health. Here we report that microbial patterns such as flagellin lead to suppression of root iron acquisition via a localized degradation of the systemic iron deficiency signaling peptide Iron Man 1 (IMA1). This response is also elicited when bacteria enter root tissues, but not when they dwell on the outer root surface. IMA1 itself has a role in modulating immunity in root and shoot, affecting the levels of root colonization and the resistance to a bacterial foliar pathogen. Our findings uncover an adaptive molecular mechanism of nutritional immunity that affects iron bioavailability and uptake, as well as immune responses.
Iron is an essential nutrient for organismal growth throughout all branches of life 10,11. Although iron is among the most common elements on our planet, its bioavailability in most environments is low and is a limiting factor for growth. Consequently, strong competition for iron is common between organisms. In mammals, iron levels have a direct impact on the composition of gut microbiota 1,2 and can modulate host inflammatory responses restricting iron availability for pathogenic microbes5. In plants, iron-limited soil environments trigger the activation of coumarin secretion in roots, which contributes to the alteration of the root-associated microbiota 3,4. Conversely, the soil-borne bacterial community can also have a significant impact on root iron acquisition 3,12,13. On the other side of the spectrum, pathogens can compete for and restrict iron for plants 14,15, plant immunity responses affect bacterial iron homeostasis 16, and beneficial microbes can produce siderophores that pathogens cannot use, consequently suppressing pathogen growth 17,18. Overall, iron plays an important but complex role in regulating plant-microbe interactions. However, much about this multifaced interaction that includes plants, beneficial or commensal and pathogenic microbes in the rhizosphere 19 remains to be learned.
Flg22 represses root responses to -Fe
We had previously found a pronounced interplay of responses to low iron and immunity within the first few hours of roots exposed to an iron depletion environment 20. To investigate this interplay at a longer timescale, we grew Arabidopsis seedlings in iron sufficient and low iron media with and without the elicitor flg22, a peptide fragment of bacterial flagellin (Fig. 1a). Compared to seedlings grown under iron sufficient conditions (+Fe), seedlings grown on low iron conditions (-Fe) were slightly more chlorotic and contained less iron. This was drastically exacerbated when co-treated with flg22 (Fig. 1a-c, Extended Data Fig.1a), but not when treated with a non-immunogenic form of flagellin, Flg20 (Extended Data Fig.1b-c). We therefore hypothesized that flg22 abolishes root iron uptake. To test this hypothesis, we measured ferric chelate reductase (FCR) activity and expression of the IRON REGULATED TRANSPORTER1 (IRT1), which are part of the canonical iron deficiency response in Arabidopsis and facilitate iron uptake. While FCR activity in roots was induced under -Fe without flg22 treatment, FCR activity was not induced in -Fe in the presence of flg22 (Fig. 1d, Extended Data Fig. 1d). Likewise, lack of iron triggered the induction of the pIRT1::NLS-2xYpet marker20 in the epidermal cells of the differentiation zone, and treatment with flg22, but not flg20, abolished these responses (Fig. 1e-f, Extended Data Fig. 1e). IRT1 protein levels reflected this response (Fig. 1g, Extended Data Fig. 1f-h). This was dependent on flg22 reception by the FLS2 immune receptor as flg22-induced chlorosis, and flg22-triggered FCR activity and IRT1 repression in -Fe conditions were abolished in fls2 mutants (Extended Data Fig. 1i-m). Overall, these data demonstrated that flg22-elicited immune responses can repress the iron deficiency program.
Figure 1.
flg22 represses iron uptake during iron deficiency. (a-c) 15-day-old Arabidopsis seedling leaves in sufficient iron (50 μM) or low iron (10 μM) with or without low levels of flg22 (10 nM) treatment. (a) Shoots; Scale bar 1cm, (b) total chlorophyll concentration of Col-0 shoots, (c) Iron concentration of Col-0 seedlings; 3 biological replicates. Error bars: s.e.m. Statistically analysis: two-sided t-test.
(d) Ferric chelate reductase activity in Col-0 roots grown for 7 days under +Fe conditions and transferred to +Fe, +Fe with flg22, -Fe and -Fe with flg22 liquid media for 2 days. 5 biological replicates. Error bars: s.e.m. Different letters indicate statistically significant differences between different conditions analyzed by one-way ANOVA and Tukey’s test (p<0.05).
(e) Promoter activity of IRT1 in the root of pIRT1::NLS-2xYpet seedlings in response to +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatment. seedlings were grown on the +Fe medium and after 5 days transferred to the different liquid media for 24 hours treatment. MZ: Meristematic zone; EZ: elongation zone; Green: Nuclear localized Ypet; Red: propidium iodide (PI) cell wall stain. For each treatment, a representative single confocal section (single image, GFP/PI), Maximum Intensity Z-Projection (Z-max, GFP only), a single optical section of the transverse view, and the Z-projection of the transverse section are shown. Scale bar, 50 μm.
(f) Raw signal intensity quantification of pIRT1 reporter. Different letters indicate statistically significant differences between different conditions by one-way ANOVA and Tukey’s test (p<0.05).
(g) Western blots showing IRT1 protein levels in Col-0 roots grown in +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatment. Arrow indicates the IRT protein band. The asterisk indicates a non-specific band. Tubulin protein: internal control.
The intriguing effect of flg22-triggered repression on iron deficiency responses prompted us to test other microbe-associated molecular patterns (MAMPs). The bacteria derived MAMPs, flg22 and elf18 triggered immune responses in the root tip and chitin, a fungal-derived MAMP, triggered immune responses in the differentiation zone as indicated by the upregulation of the reporters for several defense genes such as FRK1, MYB51 and CYP71A12 (Extended Data Fig. 2a-c). Like flg22, elf18 also repressed FCR activity and IRT1 activation upon -Fe in differentiated epidermal cells (Extended Data Fig. 2d-f). However, chitin treatment neither repressed FCR activity nor IRT1 activation in -Fe (Extended Data Fig. 2d-f). Taken together, our results suggest that different MAMPs modulate iron deficiency responses in the root in a specific manner.
IMA1 facilitates flg22 -Fe crosstalk
The induction of FCR and IRT1 upon iron deficiency is controlled by the transcription factor FIT21. However, flg22 was still able to strongly repress IRT1 protein accumulation in plants that constitutively overexpress FIT (Extended Data Fig. 2g), suggesting that this response to flg22 is not dependent on a regulation of FIT. To elucidate the program responsible for the flg22 triggered repression of IRT1, we conducted an RNAseq of roots grown under +/− Fe and/or +/− flg22. During the response to flg22, the expression of a large portion of iron responsive genes were modulated (Fig. 2a). The most significantly overlapping gene sets were constituted by genes that were downregulated in response to -Fe and upregulated in response to -Fe/+flg22 (hypergeometric test; P<2.98*10−139), and vice versa (hypergeometric test; P<2.08*10−92) (Extended Data Fig. 3a). K-means clustering of all differentially expressed genes (DEGs) that responded to either flg22, -Fe, or both (-Fe with flg22 double treatment) led to 5 clusters whose genes showed distinct expression patterns (Extended Data Fig. 3b). Cluster 5 was of most interest to us as it contained genes that were induced by -Fe, but strongly repressed by additional flg22 treatment. A gene ontology (GO) enrichment analysis for the genes contained in cluster 5 revealed that this gene list was highly enriched for the GO term “response to iron starvation”. Among genes in cluster 5 were many of the canonical iron deficiency responsive genes, including those related to iron deficiency signaling (BTS, PYE, bHLH38, bHLH39, bHLH100, bHLH101), iron uptake-related components (OPT3, NAS4, F6’H1, MYB10, IRT1, FRO2, MYB72, AHA7) and mobile iron deficiency signaling (IMA1, IMA2, IMA3) (Fig. 2b). As expected, these responses were not observable in fls2 mutant plants that don’t perceive flg22 (Extended Data Fig. 3c-f). The extensive and distinct interplay of gene expression changes induced by flg22 and -Fe suggested that flg22 represses iron deficiency signaling by regulating key components of the iron deficiency signaling pathway.
Figure 2.
flg22 represses iron deficiency responses through IMA1.
(a) Venn diagram of differentially expressed genes from RNAseq experiment.
(b) Heat map of mean-centered Z-scores for well-known iron responsive genes (3 independent biological repeats): iron deficiency signaling components, iron uptake components and long-distance signaling components.
(c-e) Phenotypes in 15-day-old Col-0 and IMA1ox seedlings in response to sufficient iron (50 μM) or low iron (10 μM) with or without low level flg22 (10 nM) treatment. (c) Shoots; Scale bar, 1cm. (d) Total chlorophyll concentrations of shoots. (e) Iron concentration of seedlings. (d,e) 3 biological replicates; Error bar: s.e.m. P-values from two-tailed Student t-test.
(f) Western blots showing IRT1 protein levels in Col-0 and IMA1ox roots in response to +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatment. Internal control Tubulin.
(g) Quantitative analysis of ferric chelate reductase activities in Col-0 and IMA1ox roots grown for 7 days under +Fe conditions and transferred to +Fe, +Fe with flg22, -Fe and -Fe with flg22 liquid media for 2 days. 5 biological replicates. Error bars: s.e.m. Different letters indicate statistically significant differences between different conditions analyzed by one-way ANOVA and Tukey’s test (p<0.05).
(h) Heat map of mean-centered Z-scores (normalized to Col-0 +Fe) for well-known iron-responsive genes.
Notably, flg22 repressed the upregulation of three IMAs in the root upon -Fe treatment (Fig. 2b). As IMAs act upstream of most known iron deficiency responses including FIT22, we reasoned that IMAs might be downstream of the flg22 signaling relay. We therefore tested the overexpression line IMA1ox in which the iron deficiency pathway is constitutively activated in our conditions. Flg22-triggered leaf chlorosis and reduced iron concentration in the -Fe treated seedlings were largely restored in IMA1ox seedlings (Fig. 2c-e). Moreover, iron deficiency-induced IRT1 accumulation and FCR activation were insensitive to flg22 treatment in -Fe conditions in the IMA1ox line (Fig. 2f-g). An RNAseq experiment using UBQ10::mCitrine-IMA1 and WT showed that unlike in Col-0 where a large proportion of genes that were upregulated under -Fe conditions were downregulated under -Fe/+flg22 (227/457, 49.7%; p-value: 2.08*10−92, hypergeometric test), there was no significant overlap of such genes in UBQ10::mCitrine-IMA1 (Extended Data Fig. 4a, lower panel, 50/457, 10.9%; p-value: 0.99, hypergeometric test). The -Fe induced genes in Col-0 were upregulated in UBQ10::mCitrine-IMA1 under +Fe condition, and shows less sensitivity to flg22 treatment (Extended Data Fig. 4b-d). Consistent with previous findings 23, expression levels of IMA2 and IMA3 were decreased in UBQ10::mCitrine-IMA1, compared to Col-0 throughout all treatment conditions, suggesting that there might be a gene dosage compensation mechanism within the IMA gene family (Fig. 2h). We also examined the expression levels of key flg22-dependent PTI (Pattern triggered immunity) components24,25 and found that some of these were also affected by IMA1 overexpression (Extended Data Fig. 4e). Taken together, our data show that the repression of iron deficiency responses by flg22 is abolished when IMA1 is continuously expressed, that the flg22 modulation of iron deficiency responses involve a downregulation of IMAs, and that IMA1 signaling can perturb expression of PTI response genes.
flg22 causes IMA1 depletion
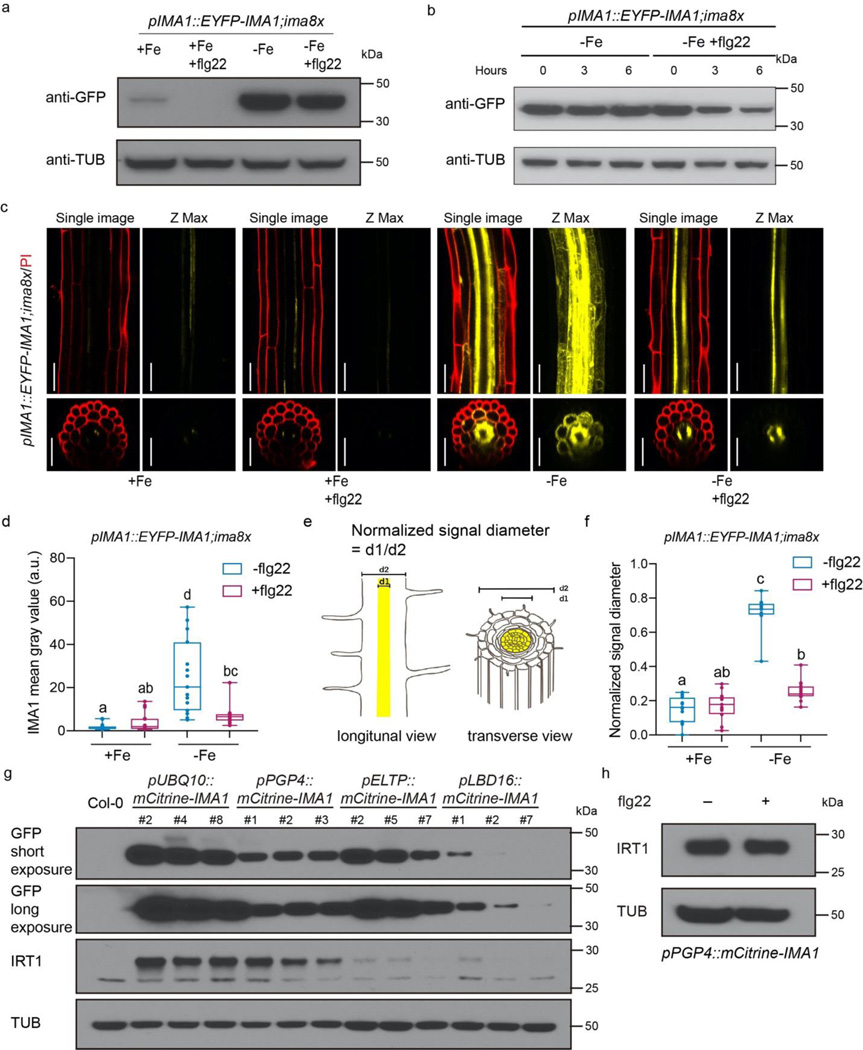
IMA1 is a phloem mobile signal that is triggered upon iron deficiency 22. To investigate whether and how flg22 abolishes IMA function, we obtained the IMA octuple mutant ima8x, and the rescue line, pIMA1::EYFP-IMA; ima8x 22. As expected, we observed that IRT1 was not induced in iron deficiency condition in the ima8x mutant line and that the IMA1 transgene successfully rescued IRT1 induction in ima8x (Extended Data Fig. 5a). As in wildtype, flg22 was able to repress IRT1 accumulation and FCR induction in pIMA1::EYFP-IMA;ima8x (Extended Data Fig. 5a-b). To our surprise, the highly increased EYFP-IMA1 protein levels that we observed upon -Fe treatment in whole root tissue, were only slightly reduced upon flg22 treatment (Fig. 3a). To investigate this partial depletion of IMA1 protein in higher detail, we pretreated pIMA1::EYFP-IMA;ima8x with -Fe to strongly elevate IMA1 protein levels, and only subsequently treated with flg22. Consistent with the previous experiment, flg22 treatment for 6 hours only partially depleted the -Fe treatment-induced IMA1 in the root (Fig. 3b). As iron deficiency responses can be highly localized, we conducted confocal microscopy of EYFP-IMA1 in the early differentiation zone of the root, which is the region of IRT1 induction under iron deficient conditions26,27. As expected, -Fe highly induced IMA1 protein in stele, as well as in the pericycle, endodermis and cortex tissues in the roots of these seedlings (Fig. 3c-d). Concomitant treatment with -Fe and flg22 strongly reduced IMA1 protein accumulation in the endodermis and cortex and partially in the pericycle, but it did not strongly reduce IMA1 level in the vasculature (Fig. 3c-d). To quantify this pattern, we measured the IMA1 signal diameter, and IMA1 signal intensity profile compared to the width of the whole root (Fig. 3e, Extended Data Fig.5h). Under -Fe, the IMA signal spread throughout the whole width of the root. In +Fe or when treated with flg22 in -Fe, it was restricted around the vasculature (Fig. 3f, Extended Data Fig.4h). As expected, these responses were abolished in the fls2 receptor mutant that does not perceive flg22 (Extended Data Fig. 5c-h). We then set out to understand the mechanism for this cell-type specific depletion of IMA1 upon flg22 treatment. To study a potential transcriptional regulation, we obtained pIMA1::mCitrine-NLS-mCitrine plants. The IMA1 transcriptional activity was low under +Fe, and -Fe strongly induced IMA1 transcription in all cell layers (Extended Data Fig.6a-b). Strikingly, flg22 led to a further induction of IMA1 transcriptional activity in the ground tissue under -Fe (Extended Data Fig.6a,c). This suggested that the depletion of IMA1 in the ground tissue was more likely to be regulated at the post-transcriptional level.
Figure 3.
flg22 spatially represses IMA1 in the ground tissue of the root.
(a,b) IMA1 protein levels in pIMA1::EYFP-IMA1;ima8x roots. Internal control: Tubulin. (a) Response to +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatment. (b) Time course: Seedlings were pre-treatment with -Fe for 36 hours, then treated with -Fe or -Fe+flg22 (1μm flg22) for 0, 3 and 6 hours.
(c) IMA1 distribution in response to +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatment in differentiation zone of the root. Five-day-old pIMA1::EYFP-IMA1;ima8x seedlings were grown on +Fe medium and then transferred to liquid treatment medium for 24 hours. Yellow: EYFP-IMA1 signals; Red: Propidium iodide (PI) cell wall stain. For each treatment, a representative single confocal section (single image, EYFP/PI), a maximal Z-projection of the Z-stack (Z-max, EYFP only), a single optical section of the transverse view, and the Z-projection of the transverse section is shown. Scale bar, 50 μm.
(d) Quantification of IMA1 fluorescence signal intensity in the differentiation zone of roots (n=15 biologically independent seedlings). Statistical analysis: Kruskal Wallis/two-tailed test followed by Multiple pairwise comparisons using the Steel-Dwass-Critchlow-Fligner procedure/two-tailed test (p<0.05).
(e) Schematic of quantification method for normalized signal diameter.
(f) Quantification of normalized IMA1 signal diameter in different treatment conditions in differentiation zones of the roots (n=15 biologically independent seedlings). Statistical analysis: Kruskal Wallis/two-tailed test followed by Multiple pairwise comparisons using the Steel-Dwass-Critchlow-Fligner procedure/two-tailed test (p<0.05).
(g,h) Western blots showing IRT1 protein levels in roots of transgenic plant with different tissue-specific promoter driving IMA1 expression. Internal control Tubulin. (g) All seedlings were grown in +Fe to avoid the endogenous IRT1 induction by low iron. Three independent lines of each transgenic plants are shown (refer to extended data fig. 6e). (h) pPGP4::mCtirine-IMA1 roots in response to +Fe and +Fe with flg22 treatment.
IMA1 had been shown to be a mobile signal22, and intercellular molecular movement can be regulated in plants by flg22-dependent callose deposition at plasmodesmata28. To test whether this could explain the flg22-mediated repression of -Fe responses, we applied 2-deoxy-d-glucose (DDG), a well-characterized callose synthase inhibitor29. To assess this, we used IRT1 expression as a read-out as the repression of IMA1 in the ground tissue coincided with the flg22 elicited repression of IRT1 and FCR. We couldn’t observe a rescue of the IRT1 protein level (Extended Data Fig. 6d). This suggested that flg22 mediated callose deposition may not play a role in regulating the iron deficiency responses.
We then ectopically expressed mCitrine-IMA1 in a cell type specific manner (Extended Data Fig. 6e) and measured the IRT1 induction under iron sufficient condition, as this allowed us to exclude the effect of endogenous IMA, which is only induced by iron deficiency. IRT1 was strongly induced in pUBQ10::mCitrine-IMA1 (expressing in all cell layers), and pPGP4::mCitrine-IMA1 lines (preferentially expressing in the epidermis and partially in the cortex) (Fig. 3g). This suggested that the presence of IMA1 in the epidermis/cortex is required and sufficient to induce IRT1 in the root. IRT1 was not strongly induced in the pLBD16::mCitrine-IMA1 (pericycle) or in pELTP::mCitrine-IMA1 lines (endodermis) compared to Col-0 wildtype plants (Fig. 3g). Interestingly and unlike pIMA1::EYFP-IMA1;ima8x, the signal of pELTP::mCitrine-IMA1 did not extend to the outer cell layers under both +Fe and -Fe conditions, suggesting that IMA1 needs to be locally expressed in the cortex and epidermis to induce IRT1 (Extended data Fig. 7a-c).
We then tested whether constitutive presence of IMA1 in the epidermis and cortex was sufficient to abolish the repression of iron deficiency responses by flg22. pPGP4::mCitrine-IMA1 roots driving IMA1 continuously in the epidermis and cortex root tissues were insensitive to the flg22-mediated IRT1 repression under +Fe conditions (Fig. 3h). We noted however, that the IMA1 levels were sightly decreased upon flg22 treatment in -Fe in the pPGP4::mCitrine-IMA1 lines, suggesting IMA1 protein was degraded in this condition (Extended Data Fig. 7d). Taken together, our data strongly suggested that flg22 treatment leads to the repression of IRT1 and FCR activation under iron deficiency through downregulation of IMA1 in the outer tissue layers (ground tissue).
BTSLs deplete IMA1 in the ground-tissue
We next investigated whether the partial degradation of IMA1 that we had observed in -Fe flg22 conditions, acted through the ubiquitin dependent protein degradation pathway. MG132, an inhibitor of 26S proteasome, strongly reduced the protein degradation of IMA1 under -Fe (Extended Data Fig. 8a). As a previous study had shown that BRUTUS (BTS), a regulator of iron homeostasis, ubiquitinates IMA1 to mediate IMA1 degradation to regulate iron homeostasis 30, we hypothesized that BTS is required to mediate flg22-triggered IMA1 degradation. However, even though bts-1 roots showed induced IRT1 expression and FCR activity compared to Col-0 under +Fe and -Fe conditions, IRT1 expression and FCR activity, as well as IMA1 in the ground tissue were still repressed upon flg22 treatment in bts-1 mutant plants (Extended Data Fig. 8b-d). This suggested that BTS is not required for the flg22 mediated IMA1 degradation and iron deficiency response repression. There are two BTS homologs in Arabidopsis (BTSL1 and BTSL2) that are expressed in the root and function redundantly to regulate iron homeostasis 31. To test whether BTSL1 and BTSL2 are involved in regulating flg22-mediated iron responses, we phenotyped the btsl1,2 double mutant in response to -Fe and flg22. Compared to Col-0, the btsl1,2 mutant plants developed less leaf chlorosis in response to flg22 under low iron conditions, suggesting that btsl1,2 plants are less sensitive to the flg22-triggered repression of iron deficiency responses (Fig. 4a-c). Consistent with the chlorosis phenotype, FCR activity and IRT1 protein accumulation were significantly less responsive to flg22 treatment in -Fe conditions in btsl1,2 mutant plants compared Col-0 (Fig. 4d-e). These data suggested that BTSL1 and BTSL2 are required for regulating flg22-mediated repression of iron deficiency responses.
Figure 4.
flg22 represses iron deficiency responses via IMA1 degradation through BTSL1 and BTSL2.
(a-c) 15-day-old seedlings in +Fe (50 μM) or -Fe (10 μM) in without or low level flg22 (10 nM) treatment. (a) Shoots; Scale bar, 1cm. (b) Total chlorophyll concentrations of shoots. (c) Iron concentration of seedlings. (b,c) 3 biological replicates; Error bar: s.e.m. Two-tailed Student t-test.
(d) Ferric chelate reductase activities in Col-0 and btsl1,2 roots grown for 7 days under +Fe and transferred to +Fe, +Fe with flg22, -Fe and -Fe with flg22 liquid media for 2 days. 5 biological replicates. Error bar: s.e.m. One-way ANOVA and Tukey’s test (p<0.05).
(e) Western blots showing IRT1 protein levels in Col-0 and btsl1,2 roots in +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatment. Control: Tubulin.
(f) Transverse confocal microscopy sections of root differentiation zones of pBTSL1-GFP and pBSTL2-GFP in response to +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatments. Green: GFP channel; scale bar, 50 μm.
(g) Confocal microscopy images of IMA1 in +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatment in root differentiation zone. 5-day-old pIMA1::EYFP-IMA1;ima8x and pIMA1::EYFP-IMA1;btsl1,2 seedlings grown on +Fe medium, then transferred to liquid treatment media for 24 hours. Yellow: EYFP-IMA1; Red: Propidium iodide (PI) cell wall stain. Shown per treatment: representative single confocal section (single image, EYFP/PI), maximal Z-projection (Z-max, EYFP only), single optical section of the transverse view, Z-projection of the transverse section. Scale bar, 50 μm.
(h-i) IMA1 fluorescence signal intensity (h) and normalized IMA1 signal diameter (i) in +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatment in root differentiation zone of pIMA1::EYFP-IMA1;ima8x and pIMA1::EYFP-IMA1;btsl1,2. Same dataset as shown in Fig.3 e&f of pIMA1::EYFP-IMA1;ima8x as images were taken at the same time. Statistical analysis: Kruskal Wallis/two-tailed test followed by Steel-Dwass-Critchlow-Fligner procedure/two-tailed test (p<0.05).
Next, we analyzed the expression pattern of BTSL1 and BTSL2 using plant carrying pBTSL1-GFP and pBTSL2-GFP transcriptional reporters 31. We observed that the expression levels of BTSL1 and BTSL2 were induced by -Fe (Fig. 4f). BTSL1 is mainly expressed in the epidermis in response to iron deficiency. flg22 strongly repressed BTSL1 expression under -Fe. This was in contrast to BTSL2, which is expressed in the entire ground tissue in iron deficiency conditions. flg22 treatment didn’t repress BTSL2 expression (Fig. 4f). Since the spatial expression patterns of BTSL1 and BTSL2 coincided with the area in which IMA1 was repressed upon flg22 treatment, we hypothesized that BTSL1 and BTSL2 are involved in the flg22-regulated IMA1 depletion in the ground tissue. IMA1 protein level was reduced upon flg22 treatment in pIMA1::EYFP-IMA1;ima8x, but not in pIMA1::EYFP-IMA1;btsl1,2 (Extended Data Fig. 8e-f). Consistent with our hypothesis, the strong increase of IMA1 in the ground tissue upon iron deficiency was not abolished by flg22 treatment in btsl1,2 (Fig. 4g-i, Extended Data Fig. 8g). Cycloheximide (CHX) treatment decreased IMA1 level under -Fe, particularly in the epidermis and cortex, similarly to the effect of flg22 indicating that IMA1 is degraded in low iron conditions. This reduction of IMA1 by either CHX or flg22 was restored in btsl1,2 (Extended Data Fig. 8h-j) indicating that BTSL1&2 are required for degrading IMA1 protein under these conditions.
Taken together, our data indicate the flg22-induced suppression of the iron deficiency response is mediated predominantly through a BTSL1/2-dependent IMA1 degradation in the ground tissue of the root in the differentiation zone (Extended Data Fig. 8k).
IMA1 has root and shoot immune functions
The iron deficiency response and an effective immune response trigger antagonistic responses in the root. For instance, the iron deficiency response includes the acidification of the rhizosphere for promoting iron solubility9. However, the defense response leads to an alkalization of the root meristem, which further promotes immunity 32. Our data suggested that IMA1 might be a central player at the interface of both pathways. While the role of IMA1 in iron deficiency signaling has been well characterized, a role of IMA1 in the regulation of immunity had not been described yet. We therefore first investigated if IMA1 played a role in regulating the rhizosphere acidification capacity. We found that under +Fe conditions, Col-0 and ima8x exhibited no acidified roots whereas UBQ10::mCitrine-IMA1 displayed constitutive root acidification (Fig. 5a-b). Iron deficiency triggered root acidification around the root tip in Col-0, but not in ima8x. flg22 treatment repressed root acidification in Col-0, but not in UBQ10::mCitrine-IMA1 (Fig. 5a-b). This suggested that IMA depletion is required for abolishing the root acidification that is triggered by flg22.
Figure 5.
IMA1 dependent rhizosphere acidification regulates root immune-responses and bacterial colonization.
(a,b) Rhizosphere acidification responses of Col-0, ima8x and UBQ10::mCitrine-IMA1 in +Fe and -Fe treatment. Bromocresol purple was used as pH indicator.
(b) Standard media acidification by Col-0, ima8x and UBQ10::mCitrine-IMA1 in response to +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatments. 6 biological replicates. Error bar: s.e.m. Statistical analysis: one-way ANOVA and Tukey’s test (p<0.05). Two-tailed Student t-test shows statistically significant difference of media acidification of Col-0 and UBQ10::mCitrine-IMA1 under +Fe.
(c,d) Quantification of root length of Col-0, ima8x and UBQ10::mCitrine-IMA1 in +Fe and +Fe+flg22 conditions (c) without MES-KOH and (d) with 1mM MES-KOH. P-values: two-tailed Student t-test.
(e) Quantification of flg22-mediated root growth responses (+flg22/-flg22, root length of +flg22 divided by mean of -flg22) of Col-0, ima8x and UBQ10::mCitrine-IMA1 with/without MES-KOH. Horizontal line: mean; Error bars s.e.m. P-values: two-tailed Student t-test.
(f) CYP71A12 transcript level in +Fe and +Fe with flg22 by quantitative RT-PCR. Roots were treated with 1μM flg22 for 1 hour. Normalized to ACT2. 3 biological replicates. Error bars: s.e.m. Statistical analysis: one-way ANOVA and Tukey’s test (p<0.05).
(g) Western blots showing MAPK phosphorylation by flg22 in Col-0, ima8x and UBQ10::mCitrine-IMA1 roots in response to flg22. The roots were treated with 1μM flg22 for 0, 5 and 10 minutes. Internal control Tubulin.
(h) Colonization of 7-day-old Arabidopsis roots at 1 d post-inoculation by CHA0 under +Fe, -Fe and non-available iron (nAvFe) conditions. 3 biological replicates. Error bars: s.e.m. Statistical analysis: one-way ANOVA and Tukey’s test (p<0.05).
Although UBQ10::mCitrine-IMA1 plants showed slightly shorter roots when they were grown on iron sufficient medium compared to Col-0 and ima8x, they exhibited less sensitivity to flg22-mediated root growth inhibition, whereas ima8x exhibited enhanced flg22-mediated root growth inhibition (Fig. 5c,e). Consistent with the data that the activity of the flg22 receptor FLS2 is dependent on environmental conditions, such as root apoplastic pH 33, the less sensitive response of UBQ10::mCitrine-IMA1 to flg22 was restored by adding the pH buffering reagent MES (Fig. 5d-e) We then checked expression of CYP71A12, which is a flg22 response gene whose promoter activation had been shown to be dampened by low pH 33. CYP71A12 induction by flg22 was impaired in UBQ10::mCitrine-IMA1 plants, but was stronger in ima8x compared to Col-0 (Fig. 5f). In line with this, roots of UBQ10::mCitrine-IMA1 plants showed less MAPK phosphorylation upon flg22 treatment compared to Col-0 under iron sufficient conditions (Fig. 5g). Overall, our data suggest that IMA1 and its effect on rhizosphere acidification is important for root responses to flg22. Iron availability has been shown to mediate root colonization by rhizobacterium Bacillus velezensis SQR9 34. As our data suggested that IMA1 might be a central player in mediating iron and immune responses, we tested if IMA1 plays a role in mediating host-microbe interaction. The rhizobacterium Pseudomonas protegens CHA0 (a model commensal Pseudomonas protegens strain which produces flagellin) showed less colonization on the root of ima8x, whereas it colonized roots of the UBQ10::mCitrine-IMA1 line to a higher extent (Fig. 5h). Taken together, this suggests that IMA1 functions at the nexus of iron deficiency and root-microbe interactions and that higher levels of IMA can facilitate elevated bacterial growth on roots.
Since IMA1 is considered a mobile signal that relays information from the shoot to the root 22, we checked if IMA1 also plays a role in coordinating iron and immune responses in the shoot. IMA1 protein level was low in the shoot under +Fe conditions. -Fe led to accumulation of IMA1 protein in the shoot in epidermal cells, mesophyll cells, and abundantly in in the vascular tissue (Extended Data Fig. 9a). Treatment with flg22 under -Fe conditions decreased IMA1 protein in the epidermal cells and mesophyll cells but not in the vascular tissue (Extended Data Fig. 9a), suggesting that like in the root, there are also cell-type specific regulatory mechanisms for IMA1 in the shoot. By analyzing IMA1ox dependent transcriptome changes in the shoot from a published dataset 22, we found that besides iron-responsive genes, immune response and systemic acquired resistance genes were enriched among the upregulated genes (Extended Data Fig. 9b). This suggests that IMA1 may also play a role in regulating immune programs in the shoot. However, we didn’t observe any strong differences in flg22-elicited MAPK phosphorylation in the shoot when comparing UBQ10::mCitrine-IMA1 and Col-0 wildtype (Extended Data Fig. 9c), suggesting that the perception of flg22 or PTI signaling activation is not affected in UBQ10::mCitrine-IMA1 in the shoot. To further explore this, we measured several PTI markers in shoot tissue using qPCR after plants had been exposed to flg22 for a short time (1 hour). Immunity marker genes were slightly upregulated without flg22, and the activation was more robust with flg22 in UBQ10::mCitrine-IMA1 compared to Col-0. Moreover, ethylene and jasmonic acid biosynthesis and signaling pathways were not hyperactive in UBQ10::mCitrine-IMA1. While the observed effects in the shoot were moderate and more thorough investigations are needed, one explanation for this might be that overexpression of IMA1 in the shoot induces a sub-set of systemic defense responses, which is different from the root. Based on this idea, we then speculated that if the systemic defense responses were indeed induced in the shoot of the IMA1 overexpressor, these plants might then be more resistant to foliar pathogen attacks. Consistent with this idea, UBQ10::mCitrine-IMA1 plants were more resistant to foliar bacterial pathogen Pseudomonas syringae pv. tomato DC3000 (Extended Data Fig. 9e). Taken together, IMA1 plays an important role to coordinate iron deficiency and immunity, but the mechanism of action in roots and shoots may be different and might depend on the type of microorganism.
Iron, root surface and invading bacteria
Bacterial populations can positively or negatively impact plant fitness through interactions that relate to iron. For example, commensal bacterial strains can aid roots with iron acquisition 3,35, whereas pathogens are thought to be in competition for available iron 17. Recently, a spatially defined gating mechanism for cell damage dependent immune receptor activation that contributed to the distinction of commensal/beneficial and pathogenic bacteria was discovered 36. Our data had shown that iron uptake and defense programs are connected by an IMA related mechanism in a spatially restricted manner. Thus, we wanted to test whether the repression of the iron signaling by a bacterial MAMP is dependent on the location of the bacteria. For this, we inoculated roots with CHA0-mcherry/gfp2 under -Fe. Unlike with the flg22 treatment, the pIRT1 reporter as well as the IRT1 protein induction were not fully repressed by CHA0 colonization (Fig. 6a-b, Extended Data Fig.10a), showing that root colonization by this strain of commensal bacteria does not strongly repress iron deficiency responses. Moreover, CHA0 colonization did not repress, but it even enhanced IMA1 protein induction by -Fe, whereas flg22 partially repressed IMA1 protein induction (Fig. 6c, Extended Data Fig.10b). When CHA0 was colonizing solely the surface of the root, IMA1 remained present in the outer cell layer, whereas flg22 treatment confined IMA1 into the stele (Fig. 6e-f). This indicates that this commensal/beneficial bacterial strain colonization on the root surface does not necessarily repress iron deficiency responses and that the plant is still able to take up iron.
Figure 6.
IMA1 accumulation is distinctly modulated by surface dwelling or invading bacteria.
(a) pIRT1::NLS-2xYpet roots in +Fe, +Fe+CHA0, -Fe, -Fe+CHA0 and -Fe+flg22. Red: Cell surface-localized CHA0-mcherry bacteria; Green: nuclear localized Ypet. Representative single confocal section (single image, GFP/mCherry), a maximal Z-projection of the Z-stack (Z-max, GFP only) and a single optical section of the transverse view are shown. Scale bar, 50 μm.
(b) Normalized signal quantification in pIRT1::NLS-2xYpet roots in +Fe, +Fe+CHA0, -Fe, -Fe+CHA0 and -Fe+flg22. One-way ANOVA and Fisher’s LSD test (two-sided, p<0.05).
(c) IMA1 distribution in +Fe, +Fe+CHA0, -Fe, -Fe+CHA0 and -Fe+flg22 in root differentiation zone of pIMA1::EYFP-IMA1;ima8x. Red: root surface-localized CHA0-mcherry bacteria; Yellow: EYFP-IMA1 (yellow channel). Representative single confocal section (single image, EYFP/mCherry), maximal Z-projection of the Z-stack (Z-max, EYFP only), single optical section of the transverse view are shown. Scale bar, 50 μm.
(d) Normalized IMA1 signal intensity in +Fe, +Fe+CHA0, -Fe, -Fe+CHA0 and -Fe+flg22. One-way ANOVA and Fisher’s LSD test (two-sided, p<0.05).
(e) Normalized IMA1 signal intensity in epidermis-cortex cell layers in +Fe, +Fe+CHA0, -Fe, -Fe+CHA0 and -Fe+flg22. One-way ANOVA and Fisher’s LSD test (two-sided, p<0.05).
(f) Normalized IMA1 signal diameter quantification in +Fe, +Fe+CHA0, -Fe, -Fe+CHA0 and -Fe+flg22. One-way ANOVA and Fisher’s LSD test (two-sided, p<0.05).
(g) Representative images of EYFP-IMA1 in +Fe, +Fe+CHA0, -Fe, -Fe+CHA0 at emerging lateral root primordia. Red: Interior-localized CHA0-mcherry bacteria; Yellow: EYFP-IMA1 signals. Asterisks: CHA0-mcherry entered the roots through lateral root primordia site. Arrow: Weak IMA1 signal in cortex. Scale bar, 50 μm. Single confocal section (single image, EYFP/mCherry), maximal Z-projection of the Z-stack (Z-max, EYFP only), single optical section of the transverse view are shown. Scale bar, 50 μm.
(h) Normalized IMA1 signal intensity quantification in +Fe, +Fe+CHA0, -Fe, -Fe+CHA0 and -Fe+flg22 in lateral root primordia region. One-way ANOVA and Fisher’s LSD test (two-sided, p<0.05).
During our inoculations, we noticed that in some cases, the CHA0 (which is considered a non-pathogenic bacterial strain) entered the roots at lateral root primordia, a region where cracks can naturally occur36. Consistent with previous findings36, higher immune responses were detected when CHA0 colonized at lateral root primordia compared with growth on the surface of differentiation zone (Extended Data Fig. 10c-d). When looking at such cases systematically, we found that when CHA0 entered the primary root through the LRP, IMA1 accumulation in the primary root was strongly reduced (Fig. 6g-h). Altogether, our data suggest that locally gated MAMP responsiveness can lead to spatially confined IMA1 repression and thereby might contribute to allow roots to locally shut off iron deficiency responses when internally colonized or to continue with iron acquisition in the presence of non-invasive, surface-dwelling bacteria (Extended Data Fig. 10e).
Discussion
Our results reveal that the extended presence of the flg22 MAMP can abolish major components of the iron deficiency response in Arabidopsis thaliana. This seems puzzling, as transporting bioavailable iron into the root and therefore sequestering it, could deplete a potential pathogen of iron. However, these responses are only mounted when bioavailable iron is scarce in the environment (otherwise the plant wouldn’t be iron deficient), therefore continuing to make iron bioavailable by exuding iron binding compounds and protons might benefit pathogens too. Taken together, we propose that the antagonistic function between the IMA1-mediated iron deficiency response and the flg22-elicited defense response might be critical to avoid making iron bioavailable for potential pathogens and to avoid impairing plant defense responses. Our findings also indicate that the modulation of iron deficiency responses is not a constitutive response that is triggered merely by the presence of bacteria but one that is triggered according to the presence of cues indicative of threats (e.g. sustained presence of high levels of flg22 or tissue damage). This finely tuned modulation would appear to be important to maintain a healthy rhizosphere during iron limiting conditions, as acidic (reducing) conditions and coumarins generally promote iron solubility in the rhizosphere. Shutting iron acquisition down might constitute a way to avoid enabling harmful bacteria the easy access to iron, but at the same time limits available iron for the plant itself as well as beneficial bacteria. However, limiting iron availability in the rhizosphere generally might contain the risk for promoting the virulence of bacteria found in the rhizosphere, if it resembles the situation in the mammalian gut, where metabolic cooperativity and iron levels have been shown to suppress virulence 37.
A close linkage of nutrient stress response and the plant immune system has been observed for plant responses to phosphate38–40 and points towards a general and complex intertwinement of nutrient acquisition and plant immune responses. For the conduit between iron and the immune system, IMAs appear to be a key component mediating a set of complex and multifaceted functions. On one hand IMA local degradation allows for shutting down root acidification, thereby enabling root responses to flg22, such as growth arrest and full phosphorylation of MAPKs. Consistent with these data, lack of IMA led to less colonization of the root with a surface-dwelling commensal bacteria strain. On the other hand, IMA1 overexpressing plants were more resistance to foliar Pseudomonas syringae pv. tomato DC3000. It will be interesting to explore in the future to which extent modulation of iron acquisition and storage affects microbiome composition and microbial virulence in roots and shoots of plants.
Materials and Method
Plant material and plant growth conditions
For all experiments, Arabidopsis thaliana ecotype Columbia (Col-0) was used as wild-type control. Plant seeds were sterilized in 70% ethanol for 8 minutes and washed with sterilized MilliQ water. Seeds were sowed on agar medium and stratified at 4 °C for 3 days in the dark. Seedlings were grown in Percival growth chamber (GENEVA SCIENTIFIC) at 22 °C and 16 hours light, 8 hours dark cycle. Iron sufficient (+Fe) medium was prepared according to the recipe of standard media as previously described41. The Gruber et al. medium contains: 750 μM of MgSO4−7H2O, 625 μM of KH2PO4, 1000 μM of NH4NO3, 9400 μM of KNO3, 1500 μM of CaCl2−2H2O, 0.055 μM of CoCl2−6H2O, 0.053 μM of CuCl2−2H2O, 50 μM of H3BO3, 2.5 μM of KI, 50 μM of MnCl2−4H2O, 0.52 μM of Na2MoO4−2H2O, 15 μM of ZnCl2, 75 μM of Na-Fe-EDTA (+Fe) or 10 μM of Na-Fe-EDTA (phenotype analysis), 1000 μM of MES adjusted to pH 5.5 with KOH, 0.5% Sucrose and 1% Difco Agar (BD, Cat# 214530, only for seedling growth on solid medium).
Generation of transgenic lines
To generate the different promoter driving IMA1 transgenic plants, the upstream promoter region based on the previous study42 of UBQ10 (AT4G05320, 1986 bp), PGP4 (AT2G47000, 2174 bp), ELTP (At2g48140, 464 bp) and LBD16 (AT2G42430, 2564 bp) was cloned into p5’ (pDONR P4-P1r), the mCitrine CDS without stop codon was cloned into p221 (pDONR 221) and the IMA1 CDS with stop codon was cloned into p3’ (pDONR P2r-P3) through BP reactions. The destination construct was combined using pB7m34GW, p221-mCitrine, p3’-IMA1 and one of the p5’ vector using the multiple gateway LR reaction. The destination constructs were transformed into Col-0 and selected via Basta resistance to obtain homozygous T3 transgenic lines. To generate pIMA1::mCitrine-NLS-mCitrine, the same promoter region of pIMA1::EYFP-IMA1;ima8x used in previous study43 was cloned into p5’ (pDONR P4-P1r) through BP reactions. The destination construct was combined using pB7m34GW, p221-mCitrine, p3’-NLS-mCitrine and p5’-IMA1pro using the multiple gateway LR reaction. The destination constructs were transformed into Col-0 and selected via Basta resistance to obtain homozygous T3 transgenic lines. To generate pIMA1::EYFP-IMA1 in bts-1, fls2 and btsl1,2 mutant background, pIMA1::EYFP-IMA1;ima8x was crossed to bts-1, fls2 and btsl1,2 mutants, respectively, and the F3 homozygous transgenic plants were obtained for experimental analysis.
Elicitor preparation and treatment
Flg22 oligopeptide (QRLSTGSRINSAKDDAAGLQIA) and flg20 oligopeptide (QRLSTGSRINSAKDDAAGLQ) were synthesized by the Salk Peptide Synthesis Core. The elf18 oligopeptide (Ac-SKEKFERTKPHVNVGTIG) was obtained from EZbiolab. The peptides were dissolved in deionized water. Chitin (Frontier Scientific, Cat. JK399372) was dissolved in deionized water in 4°C with overnight rotating.
For elicitor treatment, flg22 and flg20 was added to the liquid medium to obtain the respective final concentration (depending on the time scale of the assay 2μM,1μM, 100nM or 10nM as described in the figure legends). Chitin was diluted to 1mg/mL as the final concentration. Seedlings were treated in +Fe (75μM FeEDTA) or no Fe (0μM FeEDTA, 50μM FerroZine (ACROS Organics, Cat. 410570050)) liquid medium containing the elicitors for 24 hours, unless otherwise specified.
Protein extraction and western blot.
7-day-old seedlings grown on +Fe plates were transferred to liquid medium with +Fe (75μM Fe), +Fe+flg22 (75μM Fe + 100nM flg22), -Fe (50μM FerroZine), -Fe+flg22 (50μM FerroZine + 100nM flg22) and treated for 24 hours. For the protein extraction and western blot procedure, previously published method was used with minor modifications 44. For IRT1 and EYFP-IMA1 detection, 15 roots cut from the pretreated seedlings were harvested immediately in liquid nitrogen. The samples were ground with liquid nitrogen and lysed directly in 80μL total protein extraction buffer (50mM Tris-Cl pH 7.4, 150mM NaCl, 5mM EDTA, 1% SDS and 1% TritonX-100 supplemented with 1x NuPAGE™ LDS Sample Buffer (Invitrogen™, Cat. NP0008) and 1x NuPAGE™ Sample Reducing Agent (Invitrogen™, Cat. NP0009) for 15 minutes on ice. The protein samples were denatured by heating for 10 minutes at 90°C and centrifuge at 13000rpm for 10 minutes. The supernatant protein samples were separated by NUPAGE 10% Bis-Tris Plus Gel (Invitrogen™, Cat.NW00105BOX) and transferred onto Nitrocellulose membrane by iBlot 2 Dry Blotting system (Invitrogen™, Cat. IB23001). IRT1 was detected by western blot with corresponding antibody (primary antibody, anti-IRT1 (Agrisera, Cat. No. AS111780) 1:2000 diluted in 5% non-fat milk; secondary antibody: Goat Anti-Rabbit IgG (H + L)-HRP Conjugate (Bio-Rad, Cat. No. 170–6515) 1:5000 in 5% non-fat milk). EYFP-IMA1, mCitrine-IMA1 and free GFP that expressed in CHA0 bacteria were detected by anti-GFP HRP conjugate antibody (Miltenyi Biotec, Cat. No. 130–091-833, 1:2000 diluted in 5% non-fat milk). FIT-3xHA was detected by anti-HA HRP conjugate antibody (Roche, Cat. No. 12013819001, 1:2000 diluted in 5% non-fat milk). The same membrane was stripped by the following steps (i) wash the membrane with 1M NaOH for 5 minutes, (ii) wash the membrane with 1x TBST, 5 minutes for 3 times. Then the membrane was re-blotted with Tubulin antibody as the internal control (Invitrogen™, Cat. 32–2500, 1:5000 in 5% non-fat milk; Goat Anti-Mouse IgG (H + L)-HRP Conjugate (Bio-Rad, Cat. No. 170–6516), 1:5000 in 5% non-fat milk).
Western blot for MAPK activity analysis
20 seedlings of 7-day-old light-grown Col-0 and UBQ10::mCtritine-IMA1 on the +Fe plates without MES were treated in Fe sufficient liquid medium without MES with 1 μM flg22 peptide for 5 and 10 minutes. The root and shoot parts of the seedlings were harvested separately then the samples were ground with liquid nitrogen and lysed directly in 80μL (for root samples) or 150μL (for shoot samples) total protein extraction buffer (Same as described above). The phosphorylation status of MPK3,6 was detected by western blot with corresponding antibody (phospho-P44/42 MAPK antibodies (Cell Signaling, Cat. No. #4370) 1:2000 diluted in 1% BSA, Merck/Calbiochim, Cat. No.12657; Goat Anti-Rabbit IgG (H + L)-HRP Conjugate (Bio-Rad, Cat. No. 170–6515) 1:5000 in 5% non-fat milk). The same membrane was re-blotted with Tubulin antibody (root samples) (Invitrogen™, Cat. 32–2500, 1:5000 in 5% non-fat milk; Goat Anti-Mouse IgG (H + L)-HRP Conjugate (Bio-Rad, Cat. No. 170–6516), 1:5000 in 5% non-fat milk as an internal control.
Ferric chelate reductase activity measurement
7-day-old seedlings grown on +Fe plates were transferred to liquid medium with +Fe (75μM Fe), +Fe+flg22 (75μM Fe + 100nM flg22), -Fe (50μM FerroZine), or -Fe+flg22 (50μM FerroZine + 100nM flg22) and treated for 2 days. The seedlings were washed with deionized water for 5 times to remove any residual chemicals and 8–10 seedlings were pooled as one sample. The seedlings were incubated in the assay solution containing 0.1mM Fe (III)-EDTA and 0.3mM FerroZine in the dark for 1 hour. The ferric chelate reductase activity was measured by spectrophotometry (Beckman Coulter, DU 730) with the absorbance (562 nm) of the Fe (II)-Ferrozine complex. The results were calculated on a root fresh weight basis by the formula: FCR Activity (nmol/(g.hour))=OD(562)/29800*V(ml)/Fw(g)/T(hour)*106
Total chlorophyll concentration measurement
The total chlorophyll content measurement is based on spectrophotometric analysis. Briefly, four shoots of the plants were harvested and pooled as one sample. Any liquid was carefully removed from the sample before the fresh weight was measured. Chlorophyll was extracted with 80% acetone in TissueLyser until the pellet became white. The leaf extracts were measured by spectrophotometry (Beckman Coulter, DU 730, 1 cm width cuvettes) with the absorbance (663 nm and 647nm), respectively. The results were calculated on a root fresh weight basis by the formula:
Total chlorophyll (a+b) (μg/mL)=(7.15 *A663)+(18.71*A647)
Total chlorophyll concentration= (Total chlorophyll * extract volume (mL))/ Fw (g)
Iron concentration measurement
The total iron content was measured by a spectrophotometric method that was described previously45. The seedlings were harvested and rinsed with de-ionized water for 5 times and dried in an oven at 65°C for two days. After measuring the dry weight, the tissues were digested with 65% (v/v) HNO3 at 95°C for 6 hours, followed by adding 30% (v/v) H2O2 at 56°C for 2 hours. The iron content was analyzed in the assay solution (1 mM bathophenanthroline disulfonate (BPDS), 0.6 M sodium acetate, and 0.48 M hydroxylamine hydrochloride). The FeCl3 solution was used to prepare the standard curve. The resulting Fe2+-BPDS3 complex was measured with absorbance (535nm) using a microplate reader. The iron content in each sample was calculated by the absorbance values against the standard curve and normalized by the dry weight (DW).
Confocal microscopy for pIRT1::NLS-2xYPet and pIMA1::EYFP-IMA1 with PI staining
Seedlings were precultured on +Fe (75μM Fe) solid media for 4 days. For the treatment, around 8–10 seedlings were transferred to 5mL of +Fe (75μM Fe), +Fe+flg22 (75μM Fe + 100nM flg22), -Fe (50μM FerroZine), and -Fe+flg22 (50μM FerroZine + 100nM flg22) liquid medium in a 6-well plate and treated for 24 hours. For PI staining, pre-treated seedlings were stained with PI solution (2μg/mL, dissolved in MilliQ water) for 5 minutes and rinsed in water. Imaging experiment was performed on a Zeiss LSM710 confocal microscope with C-Apochromat 40x/1.20 W Korr M27 water objective lens. Ypet was excited with a 488 nm laser and fluorescence emission was filtered by a 505/550 nm filter. The PI signal is excited with either 488 or 514nm laser and fluorescence emission was filtered by a 600/650 nm filter. For the quantification of the raw intensity of the Ypet signal, the process has been automatized using the Fiji® software Macro, MACRO_Min_pIRT1_LSM710_PI-staining
For EYFP-IMA1 imaging in the leaf, 4-day-old pIMA1::EYFP-IMA1;ima8x seedlings were incubated in 5mL of +Fe (75μM Fe), +Fe+flg22 (75μM Fe + 100nM flg22), -Fe (50μM FerroZine), and -Fe+flg22 (50μM FerroZine + 100nM flg22) liquid medium in a 6-well plate for 24 hours. Before imaging, cotyledons were excised and mounted in water, abaxial side of epidermis, mesophyll and vasculature regions were imaged using C-Apochromat 40x/1.20 W Korr M27 water objective lens. The bright field was imaged at the same time as a control. YFP was excited with a 514 nm laser and fluorescence emission was filtered by a 520/570 nm filter.
Microscopy setup for EYFP-IMA1 quantification
Imaging experiments except when indicated below, were performed with the following Zeiss LSM710 confocal microscope set up: inverted Zeiss microscope using a Plan-Apochromat 20x/0.8 M27 objective lens. YFP was excited with a 514 nm laser (60mW) and fluorescence emission was filtered by a 519/580 nm filter.
Calculation of the EYFP-IMA1 mean gray values
Eight Z-stack images were acquired in bright field and YFP signal representing a root section between 35 and 45μm. The brightfield images were processed with a Z-projection using the standard deviation method. From these images, the root area was detected using the plugin “Wavelet a trou” (http://www.ens-lyon.fr/RDP/SiCE/METHODS.html)46. The YFP images were processed with a Z-projection using the sum method. On those images the root area previous determined was reported and the mean gray value measured in this area to obtain the Mean gray value of the YFP channel. The process has been automatized using the Fiji® software Macro, MACRO_Intensity_IMA1.
Calculation of the EYFP-IMA1 diameter
Eight Z-stack images were acquired in bright field and YFP signal representing a section between 35 and 45μm. The brightfield images were processed with a Z-projection using standard deviation method. The YFP images were processed with a Z-projection using the sum method. On Fiji® software, the root width was calculated using the straight line and on the same zone the width of the YFP signal was determined. The ratio of the YFP width was divided by the root width and multiplied by 100 to obtain the percentage of YFP-IMA1 tissue lateral diffusion.
Rhizosphere acidification assay and pH quantification
Col-0, ima8x and UBQ10::mCitrine-IMA1 seedlings were precultured on +Fe (75μM Fe) solid medium for 7 days. The seedlings were transferred to +Fe solid medium or no Fe (0μM Fe, 50 μM FerroZine) solid medium for 3 days before the pH assay was carried out. The seedlings were then placed on a 1% agar plate containing 0.05% (w/v) bromocresol purple (pH 6.5 adjusted with NaOH) for 24 hours before being photographed.
Quantification of rhizosphere pH was conducted with a spectrophotometric assay that was published with some modification47,48. 7-day-old seedlings grown on +Fe plates without MES were transferred to liquid medium with +Fe (75μM Fe), +Fe+flg22 (75μM Fe + 100nM flg22), -Fe (50μM FerroZine), or -Fe+flg22 (50μM FerroZine + 100nM flg22) without MES and treated for 2 days. Then the seedlings were transferred to the same liquid media supplemented with pH indicator bromocresol purple (0.005%) for 1 day in 48-well plates. Proton extrusion capacity was analyzed by reading the absorption at 590 nm (A590) with an automated microplate reader.
CHA0 Bacteria strain growth condition and inoculation
The GFP or mCherry-labeled Pseudomonas protegens strain, CHA0-gfp2 (CHA0::attTn7-gfp2; Gmr) and CHA0-mCherry (CHA0::attTn7-mCherry; Gmr) were used for the bacteria inoculation assay49. The CHA0-gfp2 or CHA0-mCherry strain was cultured in liquid LB medium (Miller’s LB Broth, Research products international, Cat. L24040) supplemented with 25 μg/ml gentamycin at 28°C overnight. Bacteria cells were harvested by centrifugation (1 minute, 5000 rpm) and resuspended in sterile MilliQ water for 5 times to prevent potential element contamination from the LB medium.
For the bacteria inoculation experiment, 4-day-old seedlings (imaging for IMA1 in DZ) or 7 -day-old seedlings (imaging for IMA1 in LRP) were precultured on +Fe (75μM Fe) solid medium were treated with liquid medium. Around 8–10 seedlings were transferred to 5mL of (Gruber medium with 0.25% sucrose) +Fe (75μM Fe), +Fe+CHA0 (75μM Fe + CHA0-gfp2 or CHA0-mCherry), -Fe (100μM FerroZine), -Fe+CHA0 (100μM FerroZine + CHA0-gfp2 or CHA0-mCherry), and -Fe+flg22 (100μM FerroZine + 100nM flg22) liquid medium in a 6-well plate and treated for 24 hours. For the liquid treatment with CHA0, the bacterial suspension was added in the well to a final OD600 of 0.05.
The effect of iron on CHA0 colonization of Arabidopsis roots was performed according to the previous studies with some modification49–51. Briefly, 7 days old seedlings of Col-0, ima8x and UBQ10::mCitrine-IMA1 were transferred to 5mL of (Gruber medium without MES and sucrose) +Fe (50μM FeEDTA pH 5.5), -Fe (100μM FerroZine), non-available iron (nAvFe 50μM FeCl3 with pH 7.0) liquid medium in a 6-well plate and treated for 24 hours with prewashed CHA0-mcherry at the final OD 0.02. After the treatment, the roots were gently washed with sterilized de-ionized water to remove the non-attached bacteria and the root length was measured. The roots were harvested in 1mL extraction buffer (10 mM MgCl2, 0.01% Silwet L-77) and homogenized using TissueLyser with stainless steel beads. The samples were diluted serially from 101 to 106, and then spread on LB agar plates supplemented with 30μg/mL gentamycin. The CFUs were counted after 30 hours incubation at 28°C. The calculated CFUs were normalized by the root length.
Pseudomonas syringae pv. tomato DC3000 infection assay
Arabidopsis thaliana Col-0 and UBQ10::mCitrine-IMA1 were grown in a chamber at 22°C with a 12-h light period and 60–70% relative humidity for 30–31 days in the soil. Pseudomonas syringae pv. tomato DC3000 was cultured in the King’s B (KB) liquid medium with antibiotics (Rifampicin and Tetracyclin) at 28°C. Bacteria were harvested by centrifugation and resuspended in sterile water to an OD600 of 0.001 (approximately 5 × 106 colony forming unit (CFU) ml−1). A. thaliana leaves (2–3 fully-expanded leaves per plant) were infiltrated with bacterial suspensions using a needleless syringe. Two days after bacterial infiltration, two leaf discs (0.13 cm2) per leaf were homogenized in 200 μl of MgSO4, and a dilution series was streaked on KB plates. The plates were incubated at 28ºC for approximately two days before CFUs were counted.
Quantitative RT-PCR
7-day-old seedlings grown on +Fe solid medium were transferred to 5mL +Fe, +Fe +flg22, -Fe, and -Fe +flg22 liquid medium in a 6-well plate and treated for 24 hours unless otherwise specified. The root total RNA was isolated from around 15 roots (as a pool for one biological replicate) by using Spectrum™ Plant Total RNA Kit (Sigma-Aldrich, Cat. STRN250). 500ng of total RNA was reverse transcribed to cDNA using Maxima™ H Minus cDNA Synthesis Master Mix with dsDNase (Thermo Fisher, Cat. M1682). qRT-PCR was performed with Bio-Rad CFX384 Real-Time System and Luna qPCR mix (New England Biolabs, M3003L) according to the manufacturer’s instructions. All the primers used for qRT-PCR analysis are listed in Supplementary Table 1.
RNAseq and data analysis
For transcriptomic analysis, 7-day-old seedlings grown on +Fe medium plates were transferred to 5mL +Fe, +Fe +flg22, -Fe, and -Fe +flg22 liquid medium in a 6-well plate and treated for 24 hours. 20 roots were pooled harvested as one biological replicate, with a total of three independent replicates per condition in each genotype (total sample numbers=36). The root samples were ground in liquid nitrogen and the RNA was extracted with the Spectrum™ Plant Total RNA Kit (Sigma-Aldrich, Cat. STRN250). The RNA quality and quantity were determined using a 2100 Bioanalyzer tape station (Agilent Technologies) and Qubit Fluorometer (Invitrogen). The sequencing libraries were generated by the Salk Next Generation Sequencing Core according to Illumina manufacturer’s instructions. Sequencing was performed using the Illumina Nextseq2000 platform.
Read alignment and generation of counts
RNA-seq short reads were mapped to the TAIR 10 reference genome which were obtained from the Arabidopsis Information resource web site (http://www.arabidopsis.org) 52 using the Splice Transcripts Alignments to Reference (STAR) version 2.7.0a 53. A STAR index was built using the following parameters before mapping:
$ STAR --runThreadN 4 \ --runMode genomeGenerate \ --genomeDir ara_star_index \ --genomeFastaFiles <TAIR10.fa>\ --sjdbGTFfile <TAIR10.gtf> \ --sjdbOverhang 99
Then, RNA-seq reads in the FASTQ files were aligned to TAIR 10 and raw count files were generated using the following STAR command:
$ STAR --genomeDir ara_star_index \ --runThreadN 8 \ --sjdbOverhang 99 \ --sjdbGTFfile <TAIR10.gtf> \ --outSAMtype BAM SortedByCoordinate \ --outFileNamePrefix /output/small_ \ --outReadsUnmapped Fastx \ --quantMode GeneCounts \ --readFilesIn <fastq>
A custom R script (min_R_codes_for_manuscript) was used to combine counts per gene from count data produced from STAR cross all samples.
Differential expression analysis
Normalization of the read counts and differential gene expression analysis were performed using the R package, edgeR (version 3.36.0) 54. The CPM (counts per million) function from edgeR was used to normalize the counts and differentially expressed genes (DEGs) by comparing the +Fe and -Fe treatment without or with flg22 were identified using glmLRT function from edgeR. A false discovery rate (FDR < 0.05) and logFC (> 0 or < 0) were used as the criteria values for identification of up-regulated and down-regulated DEGs.
The DEG analysis was conducted by comparing different combinations of iron and flg22 treatments in wild type using ANOVA with Benjamini-Hochberg -corrected (FDR < 0.05) plus maximal absolute value of log2-converted fold change between pairs of treatment conditions larger than 1. Differentially expressed genes were visualized and k-means clustering method was used to classify DEGs via the ComplexHeatmap 55 package in R. The cluster number (k=5) was determined by total within-cluster sum of squared error and Bayesian information criterion (BIC). The boxplots were used to display scaled expression of genes from each cluster.
Gene ontology enrichment analysis was conducted using online tools of GENEONTOLOGY website: http://geneontology.org/
Statistical analysis
Statistical significance of overlap between DEGs from was assessed by hypergeometric distribution test. Hypergeometric test function in R was used to calculate statistical significance:
$ phyper(q-1, m, n-m, k, lower.tail = FALSE, log.p = FALSE)
q = the number of genes in common between two sets
m = the number of genes in Set 1;
n = the total number of genes in RNA-Seq counts table (33,602)
k = the number of genes in Set 2
Statistical analysis was performed by Graphpad Prism 9 and XLSTAT 2020.4.1.1032
Box & whiskers plots
For all the Box & whiskers plots (unless specified in the figure legend), the upper and lower boundary show min to max. The horizontal line in the box represents the median value. The upper quartile represents 75th percentile and the lower quartile represents 25th percentile.
Statistics and Reproducibility
Each experiment was repeated independently for at least 3 times with consistent results except for the western blot of Figure 3g were repeated for two times and showing consistent results.
Extended Data
Extended Data figure 1.
Flg22 represses iron uptake through FLS2.
(a) Phenotype of 15-day-old Arabidopsis seedling leaves response to different iron concentration (shown in figures) with or without low levels of flg22 (10 nM) treatment. Scale bar 1cm.
(b-c) Phenotype of 15-day-old Arabidopsis seedling leaves response to sufficient iron (50 μM) or low iron (10 μM) with or without low levels of flg20 or flg22 (10 nM) treatment. (b) Shoots; Scale bar 1cm, (c) Total chlorophyll concentration of Col-0 shoots. Bar chart centers show means of 3 biological replicates. Error bar: s.e.m. The numbers correspond to P-values were analyzed by two-tailed Student t-test.
(d) Quantitative analysis of ferric chelate reductase activities in Col-0 roots grown for 7 days under +Fe conditions and transferred to -Fe, -Fe with flg20 and -Fe with flg22 liquid medium for 2 days. The bar chart centers show means of 5 biological replicates. Error bar: s.e.m. Different letters indicate statistically significant differences between different conditions analyzed by one-way ANOVA and Tukey’s test (p<0.05).
(e) Promoter activity of IRT1 in the root of pIRT1::NLS-2xYpet seedlings in response to -Fe, -Fe with flg20 and -Fe with flg22 treatment. Seedlings were grown on the +Fe medium and after 5 days transferred to the different liquid media for 24 hours treatment. Green: Nuclear localized Ypet; Red: propidium iodide (PI) cell wall stain. For each treatment, a representative single confocal section (single image, GFP/PI), Maximum Intensity Z-Projection (Z-max, GFP only), a single optical section of the transverse view, and the Z-projection of the transverse section are shown. Scale bar, 50 μm.
(f-h) Western blots showing IRT1 protein levels in Col-0 and irt1–1 roots grown in +Fe and -Fe (f) or Col-0 under +Fe, -Fe and -Fe with different concentrations of flg22 treatment (g) or Col-0 under +Fe, -Fe and -Fe with flg20 or flg22 treatment (h). Arrow indicates the IRT1 protein band. The asterisk indicates non-specific band. Tubulin protein was blotted as an internal control.
(i-k) Phenotype of 15-day-old Arabidopsis Col-0 and fls2 seedling leaves in response to sufficient iron (50 μM) or low iron (10 μM) with or without low levels of flg22 (10 nM) treatment. The numbers correspond to p-values that were analyzed by two-tailed Student t-test. (i) Shoots; Scale bar 1cm, (j) total chlorophyll concentration of Col-0 shoots, (k) Iron concentration of Col-0 seedlings; bar chart centers show means of 3 biological replicates. Error bar: s.e.m.
(l) Quantitative analysis of ferric chelate reductase activities in Col-0 and fls2 roots grown for 7 days under +Fe conditions and transferred to +Fe, +Fe with flg22, -Fe and -Fe with flg22 liquid medium for 2 days. The bar chart centers show means of 5 biological replicates. Error bar: s.e.m. Different letters indicate statistically significant differences between different conditions analyzed by one-way ANOVA and Tukey’s test (p<0.05).
(m) Western blots showing IRT1 protein levels in Col-0 and fls2 roots in +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatment. The tubulin protein was blotted as an internal control.
Extended Data figure 2.
MAMPs regulate iron uptake through distinct mechanisms.
(a-b) Promoter activity of FRK1 in roots of pFRK1::NLS-3xmVenus seedlings in response to -Fe and -Fe with flg22 treatment (a) or promoter activity of MYB51 in roots of pMYB51::NLS-3xmVenus seedlings in response to -Fe and -Fe with elf18 treatment (b). 7-day-old seedlings are treated with -Fe and -Fe with 100nM flg22 (a) or -Fe and -Fe with 100nM elf18 (b) for 24 hours in liquid media. Green: Nuclear localized mVenus signals; Red: propidium iodide (PI) cell wall stain. In each treatment, a representative single confocal section (single image, GFP/PI) and Maximum Intensity Z-Projection (Z-max, GFP only) is shown. Scale bar, 50 μm.
(c) Promoter activity of CYP71A12 in roots of pCYP71A12::GUS seedlings in response to -Fe and -Fe with chitin treatment. 7-day-old seedlings are treated with -Fe and -Fe with 1mg/mL chitin for 24 hours in liquid media. Scale bar, 0.5cm.
(d) Quantitative analysis of ferric chelate reductase activities in Col-0 roots grown for 7 days under +Fe conditions and transferred to -Fe, -Fe only or -Fe with flg22, elf18 and chitin liquid medium for 2 days. The bar chart centers show means of 5 biological replicates. Error bars, s.e.m. Different letters indicate statistically significant differences between different conditions analyzed by one-way ANOVA and Tukey’s test (p<0.05).
(e) Western blots showing IRT1 protein levels in Col-0 roots grown +Fe, -Fe only or -Fe with flg22, elf18 and chitin treatment. Tubulin protein was blotted as an internal control.
(f) Promoter activity of IRT1 in the root of pIRT1::NLS-2xYpet seedlings in response to -Fe only or -Fe with flg22, elf18 and chitin treatment. Green: Nuclear localized Ypet signals; Red: propidium iodide (PI) cell wall stain. In each treatment, the Z-stack scan is processed to single confocal section (single image, GFP/PI), maximal Z-projection (Z-max, GFP only). Scale bar, 50 μm.
(g) Western blots showing IRT1 protein levels in 35S::3xHA-FIT roots in response to +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatment. HA-tagged FIT protein was blotted using anti-HA antibody. Tubulin protein was blotted as an internal control.
Extended Data figure 3.
Flg22 antagonistically regulates the iron deficiency transcriptional landscape through FLS2.
(a) Venn diagram of up/down-regulated genes of -Fe vs +Fe, +Fe+flg22 vs +Fe, and -Fe+flg22 vs -Fe, respectively. The statistical analysis of p-value was calculated by hypergeometric test (one-sided).
(b) Heat map of mean-centered Z-scores for 1290 differentially expressed genes identified across different treatments (+Fe, +Fe+flg22, -Fe and -Fe+flg22), arranged by k-means clustering. Box plot indicates the relative expression level based on median centered Z-score in different clusters. The GO terms analysis was performed using PANTHER17.0 (Fisher’s Exact test, p-value<0.05) and indicated on the right side of the heatmap.
(c&d) Heat map of mean-centered Z-scores for differentially expressed genes (cluster 5 (c) and cluster 1 (d) in WT and fls2) identified across different treatments (+Fe, +Fe+flg22, -Fe and -Fe+flg22), arranged by k-means clustering.
(e) Heat map of mean-centered Z-scores (normalized by Col-0 +Fe) for well-known iron responsive genes in response to +Fe, +Fe+flg22, -Fe and -Fe+flg22 in Col-0 and fls2 roots.
(f) Gene expression analysis in response to +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatment in Col-0 and fls2 roots by quantitative RT-PCR. The gene expression level is normalized to ACT2 internal control. The bar chart centers show means of 3 biological replicates. Error bars, s.e.m. Different letters indicate statistically significant differences between different conditions analyzed by one-way ANOVA and Tukey’s test (p<0.05).
Extended Data figure 4.
Flg22 antagonistically regulates the iron deficiency transcriptional landscape through IMA1.
(a&b) Venn diagram of the iron deficiency up-regulated genes in Col-0 vs down-regulated genes of -Fe+flg22 vs -Fe in Col-0 vs down-regulated genes of -Fe+flg22 vs -Fe in UBQ10::mCitrine-IMA1 (a). Venn diagram of the iron deficiency up-regulated genes in Col-0 vs upregulated genes in UBQ10::mCitrine-IMA1 in +Fe vs Col-0 +Fe (b). The statistical analysis of p-value was calculated by hypergeometric test.
(c&d) Heat map of mean-centered Z-scores for differentially expressed genes (cluster 5 and cluster 1 refer to extended data fig.3b in WT and UBQ10::mCitine-IMA1) identified across different treatments (+Fe, +Fe+flg22, -Fe and -Fe+flg22), arranged by k-means clustering.
(e) Heat map of mean-centered Z-scores (normalized by Col-0 +Fe) for well-known PTI components in response to +Fe, +Fe+flg22, -Fe and -Fe+flg22, respectively.
Extended Data figure 5.
Flg22 spatially regulates IMA1 protein level in the ground tissue of the root through FLS2.
(a) Western blots showing IRT1 protein levels in Col-0, ima8x and pIMA1::EYFP-IMA1;ima8x in different treatment conditions. Seedlings were treated with +Fe and -Fe (Col-0 and ima8x) and +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatment (pIMA1::EYFP-IMA1;ima8x). Tubulin protein was blotted as an internal control.
(b) Quantitative analysis of ferric chelate reductase activities in pIMA1::EYFP-IMA1;ima8x roots grown for 7 days under +Fe conditions and transferred to +Fe, +Fe with flg22, -Fe and -Fe with flg22 liquid medium for 2 days. The bar chart centers show mean of 5 biological replicates. Error bars, s.e.m. Different letters indicate statistically significant differences between different conditions analyzed by one-way ANOVA and Tukey’s test (p<0.05).
(c) IMA1 distribution in response to +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatment in differentiation zone of the root. 5-day-old pIMA1::EYFP-IMA1;ima8x and pIMA1::EYFP-IMA1;fls2 seedlings were grown on the +Fe medium and then transferred to different liquid media for 24 hours treatment. The cytosolic and nuclear localized EYFP-IMA1 signals (yellow channel) are visualized with propidium iodide (PI, cell wall staining, red channel). For each treatment, a representative single confocal section (single image, EYFP/PI), a maximal Z-projection of the Z-stack (Z-max, EYFP only), a single optical section of the transverse view, and the Z-projection of the transverse section is shown. Scale bar, 50 μm.
(d-e) Quantification of IMA1 fluorescence signal intensity (d) and normalized IMA1 signal diameter (e) in response to +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatment in differentiation zone of the root in pIMA1::EYFP-IMA1;ima8x and pIMA1::EYFP-IMA1;fls2 (n=15 biologically independent seedlings from 3 biological repeats). The same dataset from Figure 3d&f of pIMA1::EYFP-IMA1;ima8x were used here as the images for quantification were taken at the same time. Different letters indicate statistically significant differences between different conditions analyzed by Kruskal Wallis/two tailed test followed by Multiple pairwise comparisons using the Steel-Dwass-Critchlow-Fligner procedure/two-tailed test (p<0.05).
(f) Western blots showing IMA1 protein levels in the roots of pIMA1::EYFP-IMA1;ima8x and pIMA1::EYFP-IMA1;fls2 in +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatments. Tubulin protein was blotted as an internal control.
(g) Western blots showing IMA1 protein levels in pIMA1::EYFP-IMA1;ima8x and pIMA1::EYFP-IMA1;fls2 roots. The seedlings were pre-treated with -Fe for 36 hours, then treated with -Fe+flg22 (1μm flg22) for 0, 3 and 6 hours. Tubulin protein was blotted as an internal control.
(h) Representative image of EYFP-IMA1 signal intensity profile in response to +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatment in differentiation zone of root in pIMA1::EYFP-IMA1;ima8x (left panel) and pIMA1::EYFP-IMA1;fls2 (right panel). The white line in YFP channel indicates the line for signal quantification. The red dashed lines indicate the boundary between the ground tissue and the stele. Scale bar, 50 μm.
Extended Data figure 6.
Flg22 does not fully repress IMA1 transcription in the ground tissue and flg22 dependent callose deposition is not required for IRT1 repression.
(a) Promoter activity of IMA1 in the root of pIMA1::mCitrine-NLS-mCitrine seedlings in response to +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatment. 5-day-old seedlings were grown on the +Fe medium and then transferred to the different liquid media for 24 hours treatment. The nuclear localized mCitrine signals (Yellow channel) are visualized with propidium iodide (PI, red channel). For each treatment, a representative single confocal section (single image, GFP/PI), Maximum Intensity Z-Projection (Z-max, GFP only), a single optical section of the transverse view, and the Z-projection of the transverse section is shown. Scale bar, 50 μm.
(b-c) Normalized IMA1 promoter activity quantification in all cell layers (b) or in epidermis-cortex cell layers (c) in response to +Fe, +Fe+flg22, -Fe and -Fe+flg22 treatments (n=9, 3 repeats). Different letters indicate statistically significant differences between different conditions analyzed by one-way ANOVA and Fisher’s LSD test(two-sided, p<0.05).
(d) Western blots showing IRT1 protein levels in Col-0 roots is in response to +Fe, +Fe with flg22, -Fe, -Fe with flg22 treatment and -Fe with flg22 and DDG treatment. Tubulin protein was blotted as an internal control.
(e) Representative images of cell-layer specific IMA1 expression transgenic plants. 3 individual lines are shown under +Fe condition. Scale bar, 100 μm.
Extended Data figure 7.
IMA1 is not regulated through cell-to-cell movement but regulated through protein level in the epidermis-cortex under -Fe by flg22.
(a) Representative images of roots of plants with different tissue-specific promoters driving IMA1 expression. 5-day-old transgenic seedlings were grown on +Fe solid medium and then transferred to +Fe or +Fe+flg22 liquid medium for 24 hours treatment. For each treatment, the cytosolic and nuclear-localized mCitrine-IMA1 signals (yellow channel) are visualized with longitudinal section and transverse section. Scale bar, 50 μm.
(b) Representative images of roots of plants with pIMA1, pELTP or pPGP4 promoters driving IMA1 expression in response to +Fe, +Fe+flg22, -Fe and -Fe+flg22. For each treatment, mCitrine-IMA1 signals (yellow channel) and bright field are visualized with longitudinal section. Scale bar, 50 μm.
(c) Quantification of normalized IMA1 signal diameter in different treatment conditions in differentiation zones of the roots. Different letters indicate statistically significant differences between different conditions analyzed by one-way ANOVA and Fisher’s LSD test (two-sided, p<0.05).
(d) Quantification of normalized IMA1 signal intensity of pPGP4::mCitrine-IMA1 in different treatment conditions. Different letters indicate statistically significant differences between different conditions analyzed by one-way ANOVA and Fisher’s LSD test (two-sided, p<0.05).
Extended Data figure 8.
Flg22 dependent IMA1 degradation in the ground tissue is regulated by BTSL1 and BTSL2 but not by BTS.
(a) Western blots showing IMA1 protein is degraded through ubiquitin-dependent proteasome mechanism under -Fe condition. The pIMA1::EYFP-IMA1;ima8x was pre-treated with -Fe with DMSO only or -Fe with 10 μM MG132 for 36 hours, subsequently with 100 μM Cycloheximide (CHX) for the indicated time period. Tubulin protein was blotted as an internal control.
(b) Western blots showing IRT1 protein levels in Col-0 and bts-1 roots in +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatment. Tubulin protein was blotted as an internal control.
(c) Quantitative analysis of Ferric Chelate Reductase activities in Col-0 and bts-1 roots grown for 7 days under +Fe conditions and transferred to +Fe, +Fe with flg22, -Fe and -Fe with flg22 liquid medium for 2 days. The bar chart centers show mean of 5 biological replicates. Error bars, s.e.m. Different letters indicate statistically significant differences between different conditions analyzed by one-way ANOVA and Tukey’s test (p<0.05).
(d) IMA1 distribution in bts-1 in response to +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatment in differentiation zone of the root. 5-day-old pIMA1::EYFP-IMA1;bts-1 seedlings were grown on the +Fe medium and then transferred to different liquid medium for 24 hours treatment. The cytosolic and nuclear localized EYFP-IMA1 signals (yellow channel) are visualized with propidium iodide (PI, red channel). For each treatment, a representative single confocal section (single image, EYFP/PI), a maximal Z-projection of the Z-stack (Z-max, EYFP only), a single optical section of the transverse view, and the Z-projection of the transverse section is shown. Scale bar, 50 μm.
(e) Western blots showing IMA1 protein levels in the roots of pIMA1::EYFP-IMA1;ima8x and pIMA1::EYFP-IMA1;btsl1,2 in +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatments. Tubulin protein was blotted as an internal control.
(f) Western blots showing IMA1 protein levels in pIMA1::EYFP-IMA1;ima8x and pIMA1::EYFP-IMA1;btsl1,2 roots. The seedlings were pre-treated with -Fe for 36 hours, then treated with -Fe+flg22 (1μm flg22) for 0, 3 and 6 hours. Tubulin protein was blotted as an internal control.
(g) Representative image of EYFP-IMA1 signal intensity profile in response to +Fe, +Fe with flg22, -Fe and -Fe with flg22 treatment in differentiation zone of roots in pIMA1::EYFP-IMA1;ima8x and pIMA1::EYFP-IMA1;btsl1,2. The white line in YFP channel indicates the line for signal quantification. The red dashed lines indicate the boundary between the ground tissue and the stele. Scale bar, 50 μm.
(h-j) Quantification of normalized total IMA1 signal intensity (h), normalized IMA1 signal intensity in epidermis and cortex (i) and IMA1 signal diameter (j) in differentiation zone of roots in pIMA1::EYFP-IMA1;ima8x and pIMA1::EYFP-IMA1;btsl1,2. The seedlings were pre-treated with +Fe/-Fe 36 hours with/without flg22 and treated with 100 μM CHX for 2 hours before imaging. Different letters indicate statistically significant differences between different conditions analyzed by one-way ANOVA and Fisher’s LSD test (two-sided, p<0.05).
(k) Schematic of flg22-mediated IMA1 depletion in the outer cell layers (epidermis, cortex, and endodermis) and IRT1 repression in epidermis in response to +Fe, -Fe and -Fe with flg22 treatment respectively. By contrast, IMA1 is not fully degraded in btsl1,2 in the outer cell layers upon flg22 treatment, resulting in IRT1 level maintenance in epidermis.
Extended Data figure 9.
IMA1 mediates defense responses to different pathogens in the shoot.
(a) Confocal images of pIMA1::EYFP-IMA1;ima8x responses to +Fe, +Fe with flg22, -Fe and -Fe with flg22 in the shoot. Three different zones were imaged: epidermal cells, mesophyll cells and vasculature. The cytosolic and nuclear localized EYFP-IMA1 signals (yellow channel) are visualized with bright field (bright field channel). For each treatment, a representative single confocal section is shown. Scale bar, 50 μm
(b) Gene ontology analysis of upregulated genes in IMA1ox compared with Col-0 in the shoot. GO term analysis is performed by using PANTHER17.0 (p-value<0.05). X axis, fold enrichment.
(c) Western blots showing MAPK phosphorylation by flg22 in Col-0, ima8x and UBQ10::mCitrine-IMA1 shoot in response to flg22. The shoot parts were treated with 1μM flg22 for 0, 5 and 10 minutes. Tubulin protein was blotted as an internal control.
(d) Gene expression analysis in response to +Fe and +Fe with flg22 by quantitative RT-PCR. The gene expression level is normalized to ACT2 internal control. The shoot parts were treated with 1μM flg22 for 1 hour. The bar chart centers show means of 3 biological replicates and error bars show s.e.m. Different letters indicate statistically significant differences between different conditions analyzed by one-way ANOVA and Tukey’s test (p<0.05).
(e) Growth of Pseudomonas syringae pv. tomato DC3000 in the leaves of Col-0 and UBQ10::mCitrine-IMA1. Bacteria were syringe-infiltrated at OD600=0.001, and bacterial colony forming units (CFUs) were counted 0 and 48 hours after inoculation (hpi). n=22–24 biological replicates from three independent experiments. Different letters indicate statistically significant differences (adjusted P < 0.01; two-tailed Student’s t-test followed by Benjamini–Hochberg method). Results are shown as box plots with boxes displaying the 25th–75th percentiles, the center line indicating the median and whiskers extending to the minimum and maximum values no further than 1.5× interquartile range.
Extended Data figure 10.
IRT1 and IMA1 accumulation is distinctly regulated by CHA0 or flg22 peptide.
(a) Western blots showing IRT1 protein levels in Col-0 roots in response to +Fe, +Fe+CHA0, -Fe, -Fe+CHA0 and -Fe+flg22 treatments. The inoculation of CHA0-gfp2 was detected by anti-GFP western blot. Tubulin protein was blotted as an internal control.
(b) Western blots showing IMA1 protein levels in pIMA1::EYFP-IMA1;ima8x roots in response to +Fe, +Fe+CHA0, -Fe, -Fe+CHA0 and -Fe+flg22 treatment. The EYFP-IMA1 and the inoculation of CHA0-gfp2 were detected by anti-GFP western blot. Tubulin protein was blotted as an internal control.
(c) The promoter activity of FRK1 in pFRK1::NLS-3xmVenus roots in responses to +Fe, +Fe+CHA0, -Fe, -Fe+CHA0 in differentiation zone or at lateral root primordia. Scale bar, 50 μm.
(d) Quantification of the total signal intensity of the promoter activity of FRK1 in response to +Fe, +Fe+CHA0, -Fe, -Fe+CHA0 in differentiation zone or at lateral root primordia. Different letters indicate statistically significant differences between different conditions analyzed by one-way ANOVA and Fisher’s LSD test(p<0.05).
(e) Graphical summary of main findings. Red color gradient indicates IMA1 abundance. Left and middle columns: IMA1 and IRT expression in the root under +Fe (upper left), -Fe (lower left), +Fe + flg22 (upper middle), -Fe + flg22 (lower middle). In +Fe IMA1 and IRT1 are expressed at very low levels in the early differentiation zone of the root. In -Fe, IMA1 highly accumulates in the ground tissue of the root under; roots actively lower the rhizosphere pH and increase iron availability, but this decreases PTI responsiveness. In -Fe and flg22 treatment, IMA1 is degraded in a BTSL dependent manner. The rhizosphere pH is not decreased. Right column: IMA1 accumulation in relation to relation to root and shoot host-microbe interactions. Upper right: In +Fe conditions IMA1 levels are low and don’t respond to commensal bacteria CHA0. Lower right: High levels IMA1 enhances a subset of PTI in the shoot. In -Fe IMA1 is accumulated in the root. In the early differentiation zone, surface dwelling CHA0 bacteria don’t affect IMA1 levels; high IMA1 levels increase bacterial colonization on the surface. When CHA0 colonizes inner root tissues at lateral root primordia cracks, IMA1 decreases in cells adjacent to this colonization. IMA1 accumulation is distinctly regulated by CHA0 in early differentiation zone compared to emerging LR region. The differences in IMA1 signal intensity between the depictions in the panels relating to CHA0 are due to different experiment setting (half sucrose concentration and different laser power for real bacteria experiment; see methods section).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Janneke Balk (John Innes Centre) for providing genetic materials btsl1,2 (btsl1-1 btsl2-2), pBTSL1::GFP and pBTSL2::GFP. We thank Dr. Louis Grillet and Dr. Wolfgang Schmidt (Institute of Plant and Microbial Biology, Academia Sinica) for providing genetic materials ima8x, IMA1ox and pIMA1::EYFP-IMA1;ima8x. We thank Dr. Christoph Keel (University of Lausanne) for providing bacteria materials CHA0-gfp2 and CHA0-mcherry. We thank the Salk Peptide Synthesis Core for synthesizing flg22 and flg20 peptides and the Salk Next Generation Sequencing Core Facility for NGS library preparation & sequencing. We also thank Sanghwa Lee, Charlotte Miller, Nicole Gibbs for critical comments on the manuscript. This study was funded by the National Institute of General Medical Sciences of the National Institutes of Health (grant number R01GM127759 to W. Busch), start-up funds from the Salk Institute for Biological Studies (W. Busch), funds from the Hess Chair in Plant Science (W. Busch), a long-term postdoctoral fellowship (LT000340/2019 L) by the Human Frontier Science Program Organization to M.P. Platre, funds from the Taiwan’s Ministry of Science and Technology (grant number 111-2917-I-564-021 to H. Tsai), funds from the Human Frontiers Science Program (HFSP) Long-term Fellowship (grant number LT000661/2020-L to T.Nobori), and it was supported by the NGS Core Facility of the Salk Institute with funding from NIH-NCI CCSG: P30 014195, the Chapman Foundation and the Helmsley Charitable Trust. J.R.Ecker is an Investigator of the Howard Hughes Medical Institute. L.Armengot is supported by a Maria Zambrano postdoctoral fellowship by de Ministerio de Universidades and the European Union - NextGenerationEU. Research at CRAG was supported by grant MCIN/AEI/PID2019-108595RB-I00 funded by MCIN/AEI/10.13039/501100011033, grant TED2021-131457B-I00 funded by MCIN/AEI/ 10.13039/501100011033 and by the “European Union NextGenerationEU/PRTR”, through the “Severo Ochoa Programme for Centres of Excellence in R&D” (CEX2019-000917 funded by MCIN/AEI/ 10.13039/501100011033), and by the CERCA Pro-gram/Generalitat de Catalunya (N.S.Coll).
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
Code availability
Scripts for imaging quantification in Fiji software and code for RNAseq analysis in R program are available at https://github.com/cm010713/immunity-iron-project. All bioinformatic tools used in this study are cited in the materials and methods section.
Data availability
Raw sequencing data of RNAseq have been uploaded to NCBI GEO database: GSE213557. Uncropped gel and blot source data are provided in Supplementary Figure 1. Source data (with statistical analysis) are provided with this paper in the supplementary files. Gene sequence for RNAseq reads mapping were obtained from TAIR10 reference genome.
References
- 1.Yilmaz B. & Li H. Gut Microbiota and Iron: The Crucial Actors in Health and Disease. Pharmaceuticals (Basel) 11, doi: 10.3390/ph11040098 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seyoum Y, Baye K. & Humblot C. Iron homeostasis in host and gut bacteria - a complex interrelationship. Gut Microbes 13, 1–19, doi: 10.1080/19490976.2021.1874855 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harbort CJ et al. Root-Secreted Coumarins and the Microbiota Interact to Improve Iron Nutrition in Arabidopsis. Cell Host Microbe 28, 825–837 e826, doi: 10.1016/j.chom.2020.09.006 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stringlis IA et al. MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health. Proceedings of the National Academy of Sciences of the United States of America 115, E5213–e5222, doi: 10.1073/pnas.1722335115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganz T. & Nemeth E. Iron homeostasis in host defence and inflammation. Nature Reviews Immunology 15, 500–510, doi: 10.1038/nri3863 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson NJ, Procter CM, Connolly EL & Guerinot ML A ferric-chelate reductase for iron uptake from soils. Nature 397, 694–697, doi: 10.1038/17800 (1999). [DOI] [PubMed] [Google Scholar]
- 7.Eide D, Broderius M, Fett J. & Guerinot ML A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proceedings of the National Academy of Sciences 93, 5624–5628, doi:doi: 10.1073/pnas.93.11.5624 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vert G. g. et al. IRT1, an Arabidopsis Transporter Essential for Iron Uptake from the Soil and for Plant Growth. The Plant Cell 14, 1223–1233, doi: 10.1105/tpc.001388 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt W, Michalke W. & Schikora A. Proton pumping by tomato roots. Effect of Fe deficiency and hormones on the activity and distribution of plasma membrane H+-ATPase in rhizodermal cells. Plant, Cell & Environment 26, 361–370, doi: 10.1046/j.1365-3040.2003.00967.x (2003). [DOI] [Google Scholar]
- 10.Hentze MW, Muckenthaler MU, Galy B. & Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell 142, 24–38, doi: 10.1016/j.cell.2010.06.028 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Brown JC Iron chlorosis in plants. Advances in Agronomy 13, 329–369 (1961). [Google Scholar]
- 12.Romera FJ et al. Induced Systemic Resistance (ISR) and Fe Deficiency Responses in Dicot Plants. Front Plant Sci 10, 287, doi: 10.3389/fpls.2019.00287 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zamioudis C. et al. Rhizobacterial volatiles and photosynthesis-related signals coordinate MYB72 expression in Arabidopsis roots during onset of induced systemic resistance and iron-deficiency responses. Plant J 84, 309–322, doi: 10.1111/tpj.12995 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aznar A, Patrit O, Berger A. & Dellagi A. Alterations of iron distribution in Arabidopsis tissues infected by Dickeya dadantii. Molecular Plant Pathology 16, 521–528, doi: 10.1111/mpp.12208 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xing Y. et al. Bacterial effector targeting of a plant iron sensor facilitates iron acquisition and pathogen colonization. Plant Cell 33, 2015–2031, doi: 10.1093/plcell/koab075 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nobori T. et al. Transcriptome landscape of a bacterial pathogen under plant immunity. Proceedings of the National Academy of Sciences of the United States of America 115, E3055–e3064, doi: 10.1073/pnas.1800529115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu S. et al. Competition for iron drives phytopathogen control by natural rhizosphere microbiomes. Nat Microbiol 5, 1002–1010, doi: 10.1038/s41564-020-0719-8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meziane HI, LC VDS, N. L. VA, Höfte M. & Bakker PA Determinants of Pseudomonas putida WCS358 involved in inducing systemic resistance in plants. Mol Plant Pathol 6, 177–185, doi: 10.1111/j.1364-3703.2005.00276.x (2005). [DOI] [PubMed] [Google Scholar]
- 19.Verbon EH et al. Iron and Immunity. Annual Review of Phytopathology 55, 355–375, doi: 10.1146/annurev-phyto-080516-035537 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Platre MP et al. The receptor kinase SRF3 coordinates iron-level and flagellin dependent defense and growth responses in plants. Nature Communications 13, 4445, doi: 10.1038/s41467-022-32167-6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colangelo EP & Guerinot ML The essential basic helix-loop-helix protein FIT1 is required for the iron deficiency response. Plant Cell 16, 3400–3412, doi: 10.1105/tpc.104.024315 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grillet L, Lan P, Li W, Mokkapati G. & Schmidt W. IRON MAN is a ubiquitous family of peptides that control iron transport in plants. Nature plants 4, 953–963, doi: 10.1038/s41477-018-0266-y (2018). [DOI] [PubMed] [Google Scholar]
- 23.Kroh GE & Pilon M. Connecting the negatives and positives of plant iron homeostasis. New Phytologist 223, 1052–1055, doi: 10.1111/nph.15933 (2019). [DOI] [PubMed] [Google Scholar]
- 24.Ngou BPM, Ahn H-K, Ding P. & Jones JDG Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature 592, 110–115, doi: 10.1038/s41586-021-03315-7 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Yuan M. et al. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 592, 105–109, doi: 10.1038/s41586-021-03316-6 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubeaux G, Neveu J, Zelazny E. & Vert G. Metal Sensing by the IRT1 Transporter-Receptor Orchestrates Its Own Degradation and Plant Metal Nutrition. Mol Cell 69, 953–964.e955, doi: 10.1016/j.molcel.2018.02.009 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Martín-Barranco A, Spielmann J, Dubeaux G, Vert G. & Zelazny E. Dynamic Control of the High-Affinity Iron Uptake Complex in Root Epidermal Cells. Plant Physiol 184, 1236–1250, doi: 10.1104/pp.20.00234 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faulkner C. et al. LYM2-dependent chitin perception limits molecular flux via plasmodesmata. Proceedings of the National Academy of Sciences of the United States of America 110, 9166–9170, doi: 10.1073/pnas.1203458110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vatén A. et al. Callose biosynthesis regulates symplastic trafficking during root development. Dev Cell 21, 1144–1155, doi: 10.1016/j.devcel.2011.10.006 (2011). [DOI] [PubMed] [Google Scholar]
- 30.Li Y. et al. IRON MAN interacts with BRUTUS to maintain iron homeostasis in Arabidopsis. Proceedings of the National Academy of Sciences 118, e2109063118, doi:doi: 10.1073/pnas.2109063118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodríguez-Celma J. et al. Arabidopsis BRUTUS-LIKE E3 ligases negatively regulate iron uptake by targeting transcription factor FIT for recycling. Proceedings of the National Academy of Sciences 116, 17584–17591, doi:doi: 10.1073/pnas.1907971116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu L. et al. Extracellular pH sensing by plant cell-surface peptide-receptor complexes. Cell 185, 3341–3355.e3313, doi: 10.1016/j.cell.2022.07.012 (2022). [DOI] [PubMed] [Google Scholar]
- 33.Yu K. et al. Rhizosphere-Associated Pseudomonas Suppress Local Root Immune Responses by Gluconic Acid-Mediated Lowering of Environmental pH. Current Biology 29, 3913–3920.e3914, doi: 10.1016/j.cub.2019.09.015 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Liu Y. et al. Plant commensal type VII secretion system causes iron leakage from roots to promote colonization. Nature Microbiology, doi: 10.1038/s41564-023-01402-1 (2023). [DOI] [PubMed] [Google Scholar]
- 35.Verbon EH et al. Rhizobacteria-Mediated Activation of the Fe Deficiency Response in Arabidopsis Roots: Impact on Fe Status and Signaling. Frontiers in Plant Science 10, doi: 10.3389/fpls.2019.00909 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou F. et al. Co-incidence of Damage and Microbial Patterns Controls Localized Immune Responses in Roots. Cell 180, 440–453.e418, doi: 10.1016/j.cell.2020.01.013 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanchez KK et al. Cooperative Metabolic Adaptations in the Host Can Favor Asymptomatic Infection and Select for Attenuated Virulence in an Enteric Pathogen. Cell 175, 146–158.e115, doi: 10.1016/j.cell.2018.07.016 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hiruma K. et al. Root Endophyte Colletotrichum tofieldiae Confers Plant Fitness Benefits that Are Phosphate Status Dependent. Cell 165, 464–474, doi: 10.1016/j.cell.2016.02.028 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castrillo G. et al. Root microbiota drive direct integration of phosphate stress and immunity. Nature 543, 513–518, doi: 10.1038/nature21417 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dindas J. et al. Direct inhibition of phosphate transport by immune signaling in Arabidopsis. Current biology : CB 32, 488–495.e485, doi: 10.1016/j.cub.2021.11.063 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gruber BD, Giehl RFH, Friedel S. & von Wirén N. Plasticity of the Arabidopsis Root System under Nutrient Deficiencies Plant Physiology 163, 161–179, doi: 10.1104/pp.113.218453 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wyrsch I, Domínguez-Ferreras A, Geldner N. & Boller T. Tissue-specific FLAGELLIN-SENSING 2 (FLS2) expression in roots restores immune responses in Arabidopsis fls2 mutants. New Phytologist 206, 774–784, doi: 10.1111/nph.13280 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Grillet L, Lan P, Li W, Mokkapati G. & Schmidt W. IRON MAN is a ubiquitous family of peptides that control iron transport in plants. Nature plants 4, 953–963, doi: 10.1038/s41477-018-0266-y (2018). [DOI] [PubMed] [Google Scholar]
- 44.Cao M. et al. TMK1-mediated auxin signalling regulates differential growth of the apical hook. Nature 568, 240–243, doi: 10.1038/s41586-019-1069-7 (2019). [DOI] [PubMed] [Google Scholar]
- 45.Gautam CK, Tsai H-H & Schmidt W. A Quick Method to Quantify Iron in Arabidopsis Seedlings. Bio-protocol 12, e4342, doi: 10.21769/BioProtoc.4342 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bayle V, Platre MP & Jaillais Y. Automatic Quantification of the Number of Intracellular Compartments in Arabidopsis thaliana Root Cells. Bio-protocol 7, doi: 10.21769/BioProtoc.2145 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santi S. & Schmidt W. Dissecting iron deficiency-induced proton extrusion in Arabidopsis roots. New Phytol 183, 1072–1084, doi: 10.1111/j.1469-8137.2009.02908.x (2009). [DOI] [PubMed] [Google Scholar]
- 48.Gujas B, Alonso-Blanco C. & Hardtke CS Natural Arabidopsis brx loss-of-function alleles confer root adaptation to acidic soil. Current biology : CB 22, 1962–1968, doi: 10.1016/j.cub.2012.08.026 (2012). [DOI] [PubMed] [Google Scholar]
- 49.Zhou F. et al. Co-incidence of Damage and Microbial Patterns Controls Localized Immune Responses in Roots. Cell 180, 440–453.e418, doi: 10.1016/j.cell.2020.01.013 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y. et al. Plant commensal type VII secretion system causes iron leakage from roots to promote colonization. Nature Microbiology, doi: 10.1038/s41564-023-01402-1 (2023). [DOI] [PubMed] [Google Scholar]
- 51.Harbort CJ et al. Root-Secreted Coumarins and the Microbiota Interact to Improve Iron Nutrition in Arabidopsis. Cell Host Microbe 28, 825–837 e826, doi: 10.1016/j.chom.2020.09.006 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berardini TZ et al. The Arabidopsis information resource: Making and mining the “gold standard” annotated reference plant genome. Genesis 53, 474–485, doi: 10.1002/dvg.22877 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dobin A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics (Oxford, England) 29, 15–21, doi: 10.1093/bioinformatics/bts635 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robinson MD, McCarthy DJ & Smyth GK edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics (Oxford, England) 26, 139–140, doi: 10.1093/bioinformatics/btp616 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gu Z, Eils R. & Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics (Oxford, England) 32, 2847–2849, doi: 10.1093/bioinformatics/btw313 (2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw sequencing data of RNAseq have been uploaded to NCBI GEO database: GSE213557. Uncropped gel and blot source data are provided in Supplementary Figure 1. Source data (with statistical analysis) are provided with this paper in the supplementary files. Gene sequence for RNAseq reads mapping were obtained from TAIR10 reference genome.