ABSTRACT
T lymphocytes expressing CD57 and lacking costimulatory receptors CD27/CD28 have been reported to accumulate with aging, chronic infection, and cancer. These cells are described as senescent, with inability to proliferate but enhanced cytolytic and cytokine-producing capacity. However, robust functional studies on these cells taken directly from cancer patients are lacking. We isolated these T cells and their CD27/28+ counterparts from blood and tumor samples of 50 patients with previously untreated head and neck cancer. Functional studies confirmed that these cells have enhanced ability to degranulate and produce IFN-γ. They also retain the ability to proliferate, thus are not senescent. These data suggest that CD27/28-CD57+ CD8+ T cells are a subset of highly differentiated, CD45RA+ effector memory (TEMRA) cells with retained proliferative capacity. Patients with > 34% of these cells among CD8+ T cells in the blood had a higher rate of locoregional disease relapse, suggesting these cells may have prognostic significance.
KEYWORDS: CD28, CD57, cellular senescence, costimulation, head and neck cancer, KLRG1, TEMRA cells
Introduction
As the median age of the world population continues to rise, there is an expanding population of geriatric cancer patients. It is estimated that by 2030, the proportion of cancer patients who are over age 65 will reach 70%.1 Treatment of this patient population can be challenging, due to increased medical complexity and a higher risk of morbidity and mortality associated with surgery and other forms of cancer treatment. Risk factors for head and neck squamous cell carcinoma (HNSCC) include exposure to tobacco, alcohol, and high-risk subtypes of human papillomavirus (HPV). However, advanced age is often the only risk factor among patients over 70 years of age. As such, there is a growing interest in understanding how anti-tumor immune responses change with aging, leading to increased susceptibility to HNSCC and other cancers. Of additional importance, immune checkpoint blockade is a relatively new treatment modality in HNSCC, with equivalent benefit seen in older versus younger patients.2 However, as in other cancers treated with immune checkpoint blockade, biomarkers of response are needed for HNSCC.
A population of T lymphocytes expressing the differentiation marker CD57 and lacking expression of costimulatory receptors CD27/CD28 have been noted to accumulate with aging and chronic infection.3–5 A high proportion of these cells have been documented in some patients with cancer, both within the tumor microenvironment (TME) and the peripheral blood6–8. Whether these cells contribute to the formation of cancer, versus accumulating as a result of chronic stimulation by tumor antigens, is unclear. Emerging studies in different cancer types have suggested that a high proportion of T cells in the peripheral blood expressing CD57 or lacking CD28 expression is associated with poor responses to immune checkpoint blockade and other forms of therapy;8–10 however, other reports have shown the exact opposite, 6,7,11–13 with data suggesting that these cells may be directly beneficial or indirect evidence of a strong anti-tumor immune response. In order to make sense of these discrepancies, a better understanding of the functional phenotypes of these CD27/28-CD57+ T cells is critical.
Early reports suggested that these are senescent T cells, lacking the ability to proliferate and expressing cellular markers of senescence.14 As a result, CD28-negative T cells are commonly called senescent T cells (even in studies where senescence is not assessed). Accumulation of these cells, along with other features of immune aging, have been collectively called “immunosenescence”.8,14,15 However, conflicting studies show that these cells may in fact maintain proliferative capacity.16–18 These CD27/28-CD57+ T cells have also been described to contain high levels of perforin and granzymes,17,19 but it is unclear whether this increase is a result of increased production of enzymes or impaired release of cytolytic granules. It has also been suggested that CD27/28-CD57+ are terminally differentiated effector memory cells that produce large amounts of effector cytokines upon stimulation.17
Conflicting reports regarding the functional phenotypes of these cells may be a result of how they were defined (i.e., looking at CD28 or CD57 in isolation), where they were obtained (human versus murine systems), how they were cultured, and how they were assayed. To better understand and characterize their function, we isolated CD27/28-CD57+ T cells and their CD27/28+ counterparts from peripheral blood and tumor tissue of 50 patients with previously untreated, HPV-negative HNSCC of the oral cavity, larynx, and hypopharynx. We then investigated associations with other shared cellular markers and clinical features, including age and disease stage. Next, we subjected the sorted cell populations to well-validated functional assays to definitively show whether these cells are senescent and/or capable of secreting large amounts of cytolytic enzymes and effector cytokines. Lastly, we also assessed the relationship between the proportion of these cells in the peripheral blood and disease relapse.
Methods
Human subjects
Blood and tumor tissue from patients with previously untreated head and neck cancer were obtained under a protocol approved by the Institutional Review Board at Emory University (Study #00002286) with written informed consent from each patient prior to participation and prior to surgery. Blood and tumor tissue were collected on the day of surgery. The electronic medical record was reviewed and sociodemographic, histopathologic, and tumor staging details were recorded for each subject. Age was recorded as the patient’s age at the time of surgery, and locoregional control was defined as the length of time between surgery and local or regional disease recurrence (where applicable). Pathology reports were used to record histopathologic details including overall disease stage and the presence or absence of high-risk features including perineural/lymphovascular invasion and extracapsular extension.
Isolation of PBMCs and tumor infiltrating lymphocytes
Blood samples were diluted 1:1 in sterile PBS, then centrifuged with Lymphoprep in EasySep tubes (Stem Cell Technologies) to create a density gradient, followed by collection of PBMCs. Tumor samples were mechanically and enzymatically digested using the human tumor digestion kit from Miltenyi per manufacturer instructions, then filtered. Cell pellets from blood or tumor were then resuspended in freezing media consisting of 40% fetal bovine serum, 10% DMSO, and 50% RPMI, then frozen at −80°C until further use.
Cell sorting
PBMCs were labeled for CD3, CD8, CD27, CD28, and CD57, rinsed, then sorted into live CD27/28-CD57+ and CD27/28+ populations of CD3+CD8+ T cells with a BD FACS Aria II SORP Cell Sorter. Cells were then rested in T cell media, consisting of ImmunoCult-XF (Stem Cell Technologies) and 0.33 units/mL DNase (Invitrogen), overnight prior to use in functional assays.
Head and neck cancer cell lines
JHU029 cells were obtained from Dr. David Sidransky at Johns Hopkins University. UM-SCC-74A cells were obtained from Dr. Thomas Carey at the University of Michigan. UPCI SCC-90 cells were purchased from ATCC. Cell lines were validated by short tandem repeat testing and/or HLA typing. For long-term storage, cells were kept in liquid nitrogen. Cells were regularly tested for Mycoplasma contamination and passaged for no more than 3 months or 20 passages before discarding.
Flow cytometry
PBMCs and single-cell suspensions from tumors were rinsed in FACS buffer, then stained with surface antibodies for 30 minutes, rinsed again, fixed, then permeabilized with the eBioscience kit where needed prior to intracellular staining. Samples were then analyzed on a BD Symphony A3 cytometer, then further analyzed using FlowJo software. Clustering and creation of tSNE plots was performed using FlowJo with default settings. Live cells were gated based on negative staining for FVS575 viability dye (BD Biosciences). “Fluorescence minus one” controls were tested for each multicolor flow panel. Antibodies used for flow cytometry and FACS sorting were from Cell Signaling Technology (anti-p16INK4A, AF647 clone D7C1M), Biorbyt (anti-β-galactosidase, FITC, polyclonal rabbit IgG), Sigma Aldrich (anti-Phosphatidylserine, AF488, clone 1H6), Miltenyi (anti-CD45RO, APC/Vio770, clone UCHL1), BD Biosciences (anti-CD3, BUV563, clone HIT3α; anti-CD4, BUV496, clone SK3; anti-CD8, BUV395, clone RPA-T8; anti-CD28, BB700, clone L293; anti-CD45RA, BUV805, clone 5H9; anti-interferon-γ, BUV737, clone 4S.B3), or Biolegend (anti-TIGIT, BV421, clone A15153G; anti-KLRG1, BV711, clone 2F1/KLRG1; anti-CD57, BV785, clone QA17A04; anti-granzyme B, PE/Dazzle 594, clone QA16A02; anti-CD27, Pe/Cy7, clone O323; anti-perforin, APC/Fire750, clone B-D48; anti-CCR7 (CD197), BV650, clone G043H7; anti-CD62L, AF647, clone DREG-56; anti-CD107a, FITC, clone H4A3; anti-Ki67, PE, clone Ki-67).
T cell proliferation assay
PBMCs or sorted CD8+ T cells from the peripheral blood were labeled with CellTrace Violet (ThermoFisher) per manufacturer instructions, then incubated in DNase (0.33 units/mL) and stimulated with CD2/CD3/CD28 beads (Miltenyi) per manufacturer instructions and human IL-2 (50 IU/ml) or IL-15 (20 ng/ml), both from Biolegend and repleted every 2 days, for 4–5 days. Cells were then labeled for CD3, CD8, CD27, CD28, and CD57, then analyzed by flow cytometry.
T cell degranulation and cytokine assays
PBMCs or sorted CD8+ T cells from the peripheral blood were cultured for 4 hours with eBioscience Cell Stimulation Cocktail (PMA/ionomycin, 1:500) with or without GolgiPlug (brefeldin A, BD Biosciences, 1:1000). Cells were then stained for intracellular granzyme B, perforin, and IFN-γ, in addition to surface CD107a. Samples were then analyzed by flow cytometry.
Statistical analyses
Experimental data were analyzed by Student’s t test, one- or two-way ANOVA with post-hoc Tukey analyses where appropriate. The relationship between T cell populations and disease recurrence were analyzed with a receiver operating curve, followed by Chi squared test. GraphPad Prism software was used for statistical testing, with p < .05 considered statistically significant.
Results
CD27/28-CD57+ T cells are enriched in the peripheral blood and TME of HNSCC patients
Peripheral blood mononuclear cells (PBMCs) and tumor cell suspensions were analyzed by flow cytometry and gated into CD4+ and CD8+ subsets of CD27/28-CD57+ T cells versus their CD27/28+ counterparts for each sample (Figure 1a). Prior reports suggest that T cells lacking CD28 and/or expressing CD57 also express high levels of KLRG1 and TIGIT.4,20 We found that KLRG1 expression was indeed high on both CD4+ and CD8+ T cells (versus CD27/28+ cells) within the peripheral blood, but the difference on CD8+ tumor infiltrating lymphocytes (TIL) was not statistically significant (Figure 1b). TIGIT expression was higher among CD8+ versus CD4+ T cells in the blood, but lower among CD4+ TIL; there was no difference in TIGIT expression among CD8+ TIL (Figure 1c). Overall, the proportion of CD27/28-CD57+ T cells was higher in the peripheral blood versus TIL, but there was a strong correlation between the proportions in the blood versus TIL (Figure 1d).
Figure 1.
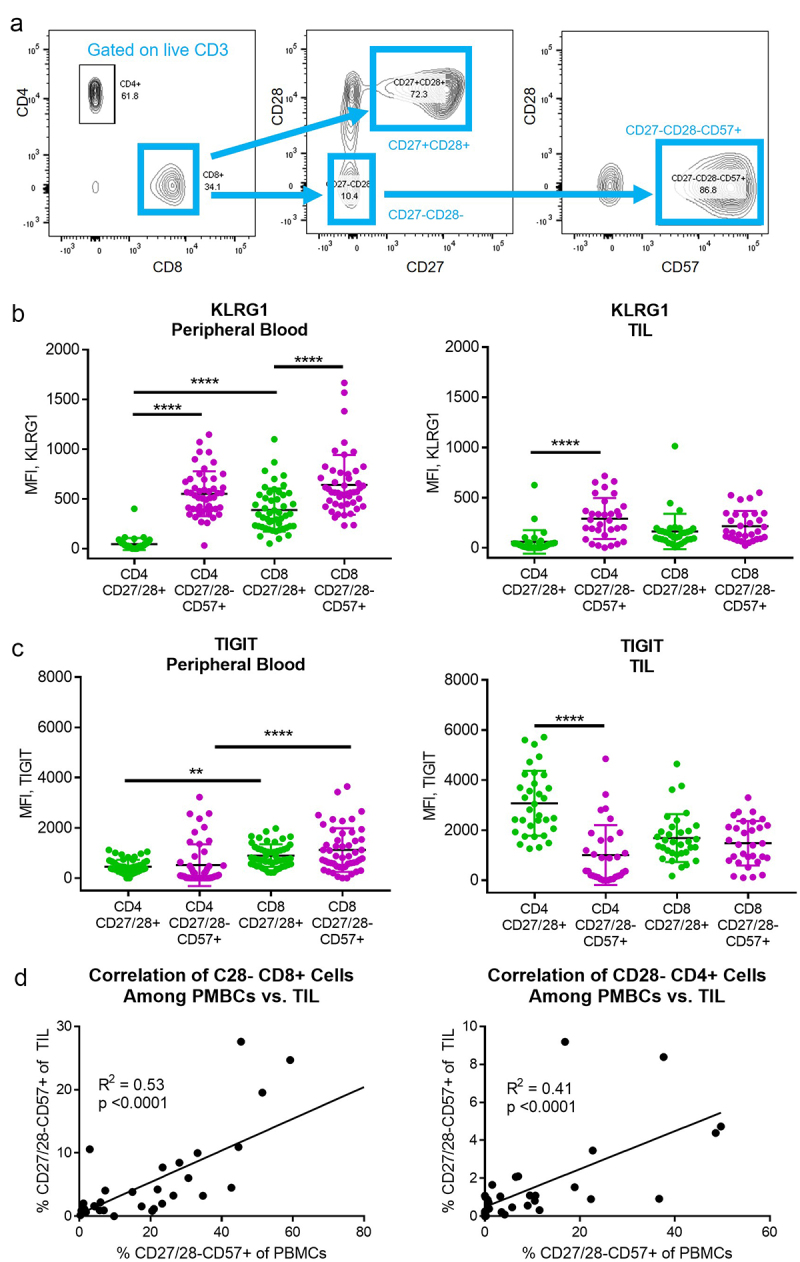
CD27/28-CD57+ T cells and their CD27/28+ counterparts were isolated from the peripheral blood and tumor tissue of HNSCC patients. (a) Flow cytometry gating strategy used for CD8+ and CD4+ subsets. (b) KLRG1 expression among T cell subsets in the peripheral blood (left) and tumor infiltrating lymphocytes (TIL, right). MFI, mean fluorescence intensity. (c) TIGIT expression among T cell subsets in the peripheral blood (left) and TIL (right). (d) Correlation (Pearson correlation coefficient, squared) between percent of CD27/28-CD57+ T cells in the peripheral blood versus TIL for CD8+ subsets (left) and CD4+ subsets (right). Data in B and C represent mean ± one SD. **p < .01, ***p < .001, ****p < .0001 by two-way ANOVA with post hoc Tukey analyses.
CD27/28-CD57+ T cells are enriched in HNSCC patients over age 75
Within the peripheral blood and TIL, there was no significant correlation between age and the proportion of CD27/28-CD57+ T cells (Figure 2). However, the mean proportion of CD27/28-CD57+ cells was significantly higher in the CD8+ subset among patients over age 70 (not shown) and in both CD4+ and CD8+ subsets among patients over age 75 (Figure 2c,f). These data suggest that the prevalence of these cells may be more pronounced in head and neck cancer patients over age 75. However, several outliers were noted to have high levels of these cells during the sixth decade of life, suggesting that some patients experience these changes earlier for reasons that are unclear. Multiple studies have shown high numbers of these cells in patients who are seropositive for CMV, EBV, HIV, and other viruses.4,19,21–23 Thus, the degree to which a particular person has been exposed to infection and other sources of chronic antigen stimulation during their lifetime may impact this aspect of immune aging. High numbers of CD27/28-CD57+ T cells could also be a result of the cancer itself, which is also a source of chronic antigen stimulation. In keeping with this idea, we did see a possible association between high numbers of CD27/28-CD57+ CD8+ T cells and advanced (stage IV) disease (Figure 2g), although this did not reach statistical significance, and there was no association between disease stage and composition of CD4+ cells (Figure 2h). Looking at other clinical factors, we saw a significant difference in the percent of CD27/28-CD57+ T cells in oral cavity tumors versus larynx tumors, but only in the CD8+ subset (Figure 2i,j). Interestingly, the distribution among oral cavity tumors appears to be bimodal (Figure 2i). Since few hypopharynx tumors were included in the cohort, we did not include those tumors in the comparison. When comparing based on patient sex, there was no difference in T cell populations in male versus female patients (Figure 2k,l). Interestingly, we noted a higher percentage of CD27/28-CD57+ CD8+ T cells in cases with perineural invasion, but not with other high-risk pathologic features (lymphovascular invasion and extracapsular nodal spread; Figure 2m,n). Given the role of CD8+ cytotoxic T lymphocytes in tumor control and the lack of any difference in CD4+ subsets according to disease stage, we focused most of our functional analyses on CD8+ T cells.
Figure 2.
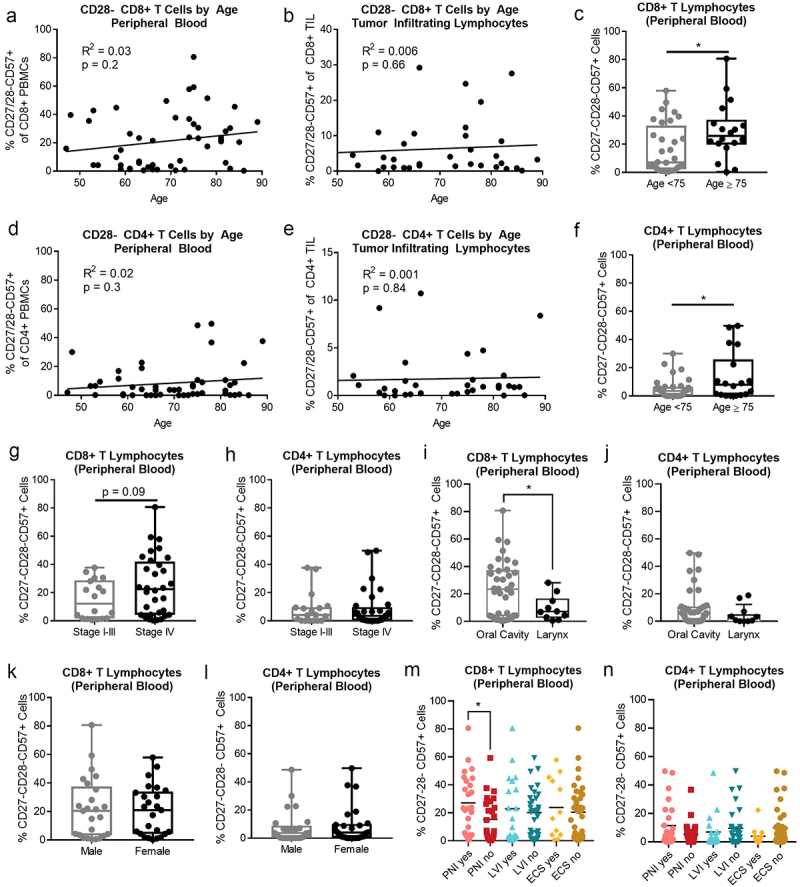
CD27/28-CD57+ T cells are more abundant in the peripheral blood of HNSCC patients over age 75. (a) Correlation between percent of CD27/28-CD57+ CD8+ T cells in the peripheral blood versus age. (b) Correlation between percent of CD27/28-CD57+ CD8+ T cells in the tumor versus age. (c) Percent of CD27/28-CD57+ CD8+ T cells in the peripheral blood of patients <age 75 versus ≥age 75. (d) Correlation between percent of CD27/28-CD57+ CD4+ T cells in the peripheral blood versus age. (e) Correlation between percent of CD27/28-CD57+ CD4+ T cells in the tumor versus age. (f) Percent of CD27/28-CD57+ CD4+ T cells in the peripheral blood of patients <age 75 versus ≥age 75. (g, h) Percent of CD27/28-CD57+ CD8+ T cells (G) and CD4+ T cells (h) in the peripheral blood of patients according to disease stage. (i, j) Percent of CD27/28-CD57+ CD8+ T cells (i) and CD4+ T cells (j) in the peripheral blood of patients according to anatomic site. (k, l) Percent of CD27/28-CD57+ CD8+ T cells (k) and CD4+ T cells (l) in the peripheral blood of patients according to sex. (m, n) Percent of CD27/28-CD57+ CD8+ T cells (m) and CD4+ T cells (n) in the peripheral blood of patients according to histopathologic features including perineural invasion (PNI), lymphovascular invasion (LVI) and extracapsular spread (ECS). Box plot whiskers depict min and max. *p < .05 by student’s t test.
CD27/28-CD57+ T cells exhibit mildly impaired proliferation but are not senescent
Because CD27/28-CD57+ T cells have been described as senescent, we examined intracellular markers associated with senescence. An isoform of β-galactosidase (β-gal) known as “senescence-associated” β-gal is thought to function best at low pH, and its origin is linked to the GLB1 gene that produces the β-D-galactosidase within lysosomes.24 High β-gal is often associated with, though not required for, cellular senescence.24 p16INK4A has also been associated with cellular senescence.25 To look for β-gal and p16 enrichment, we performed intracellular staining for these markers and measured their expression with flow cytometry. We first verified that our p16 antibody was specific for the intended target by demonstrating robust staining in HNSCC cells positive for human papillomavirus, which are known to express high levels of p16 (Supplementary Figure S1). To validate p16 and β-gal as markers of senescence in our HNSCC cells, we then subjected cell lines to a low dose of doxorubicin, a well-established method for inducing senescence,26–28 and noted that β-gal and p16 increased along with morphologic changes associated with senescence (Supplementary Figures S2,3). These experiments suggested that senescent cells do stain strongly for p16 and β-gal by flow cytometry.
When we stained for p16 and β-gal in T cells from patient tumor and blood samples, we noted that these markers were not enriched in CD27/28-CD57+ T cells versus their CD27/28+ counterparts. This led us to question whether these cells are truly senescent. Given that we had seen a shift in the composition of CD8+ T cells in the peripheral blood of patients over age 75, we performed clustering analysis on the TIL from all patients over age 75 (Figure 3a,b). Although there was an obvious cluster of cells lacking CD27/28, enriched for CD57, and mildly enriched for KLRG1, these cells were not enriched for p16 or β-gal. This was confirmed when we compared the levels of these senescence markers across T cell subsets in the peripheral blood and TIL for all patients (Figure 3c–f).
Figure 3.
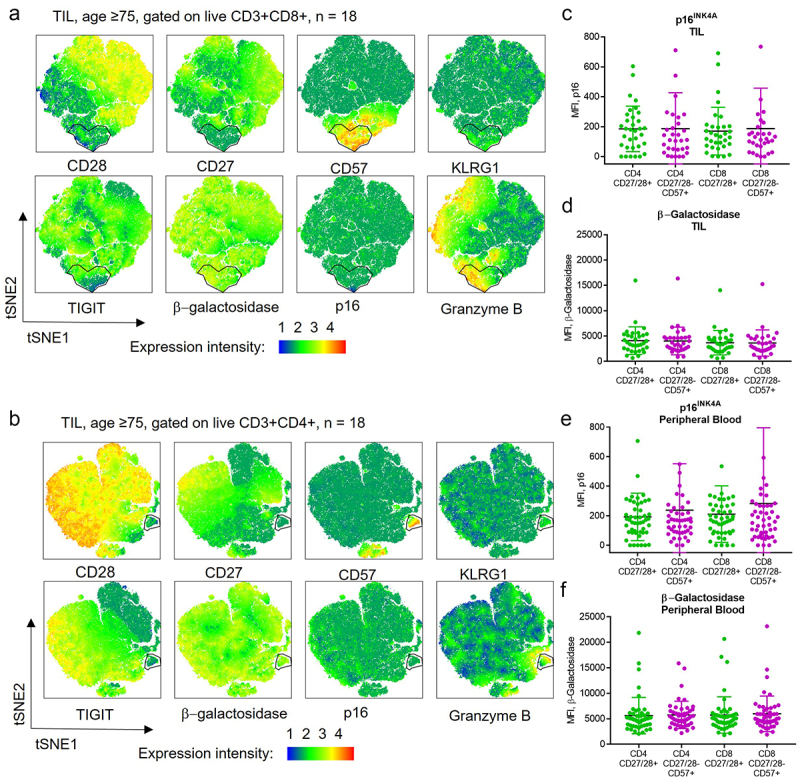
CD27/28-CD57+ T cells are not enriched for markers of senescence. (a, b) Flow cytometry data gated on live CD3+CD8+ (a) and CD3+CD4+ (b) cells from tumor samples of 18 patients ≥ age 75 were concatenated and tSNE plots created. A cluster of CD27/28-CD57+ T cells was identified that was enriched for KLRG1 and granzyme B but not for senescence markers β-galactosidase or p16. (c,d) p16INK4A (c) and β-galactosidase (d) expression among T cell subsets in the TIL. (e, f) p16INK4A (e) and β-galactosidase (f) expression among T cell subsets in the peripheral blood. MFI, mean fluorescence intensity.
We then looked at additional markers of cell death/stress and proliferation. To rule out the possibility that T cells may lose CD27/28 expression and/or gain CD57 while in culture, we sorted CD8+ T cells from peripheral blood samples into CD27/28-CD57+ or CD27/28+ populations. We did note that the CD27/28-CD57+ cells expressed higher levels of phosphatidylserine (Figure 4a), which may occur during apoptosis or cellular stress.29 However, the two populations expressed similar levels of the proliferative marker Ki67 (Figure 4b). We then labeled the two populations of T cells with CellTrace Violet and cultured them with CD2/3/28 beads and IL-2 for up to 5 days. When we performed flow cytometry on the treated cells five days after the sort, we noted that the sorted CD27/28+ T cells did not lose CD27 or CD28 during the culture period. We also noted that the proliferative capacity of CD27/28-CD57+ CD8+ T cells was intact but slightly reduced versus the CD27/28+ cells (Figure 4c), demonstrating that these cells are not senescent. Since these cells do not appear to change their CD2728/57 expression in culture, and since they do typically coexist together, we next stained all PBMCs with CellTrace Violet and cultured them together with different stimuli. We specifically compared proliferation with IL-2 versus IL-15, which has been reported to induce robust expansion of CD57+ T cells.23 We noted that proliferation of CD27/28-CD57+ CD8+ T cells was slightly better with IL-15 (Figure 4d). Interestingly, when cultured together, all of the cells appeared to proliferate less readily, with about a third of even the CD27/28+ cells failing to divide at all over the same period of time. This finding raises the possibility that the CD27/28-CD57+ cells may exert some type of inhibitory effect on the effector functions of CD27/28+ T cells, although further studies are needed to explore this idea.
Figure 4.
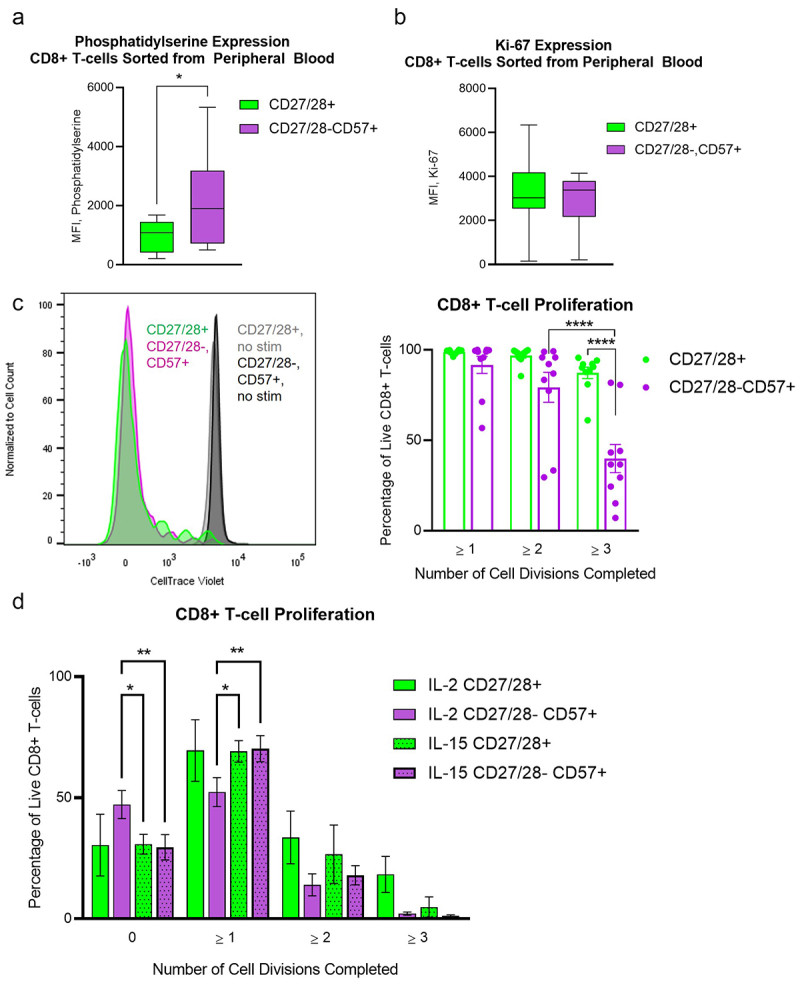
CD27/28-CD57+ T cells have mildly increased expression of phosphatidylserine but are not senescent. (a) Box plots for surface phosphatidylserine expression by flow cytometry on CD8+ T cells sorted from the peripheral blood into CD27/28-CD57+ and CD27/28+ populations. (b) Box plots for Ki-67 expression by flow cytometry on CD8+ T cells sorted from the peripheral blood into CD27/28-CD57+ and CD27/28+ populations. (c) CellTraceTrace Violet dilution to depict cell division among CD27/28-CD57+ and CD27/28+ populations of CD8+ T cells from the peripheral blood stimulated with CD2/3/28 beads and cultured with IL-2, as shown by representative histograms (left) and quantification (right). (d) Comparison of proliferation achieved with IL-2 versus IL-15 when all lymphocytes were stimulated with CD2/3/28 beads and cultured together for 4 days. Data are combined from four independent experiments using blood from nine individual patients, except for D (two experiments using blood from three patients). Data represent mean ± one SD, box plot whiskers in a and B represent min and max. *p < .05, **p < .01, ****p < .0001 by student’s t test (a, b) or two-way ANOVA with post-hoc Tukey analyses (c, d).
CD27/28-CD57+ T cells may have enhanced cytolytic potential and secrete more IFN-γ upon stimulation than their CD27/28+ counterparts
Numerous studies have shown high levels of granzyme and perforin within T cells lacking CD28 and/or expressing CD57 17,19,21,23. We confirmed this finding in both CD4+ and CD8+ subsets within peripheral blood and TIL (Figure 5a–c).
Figure 5.
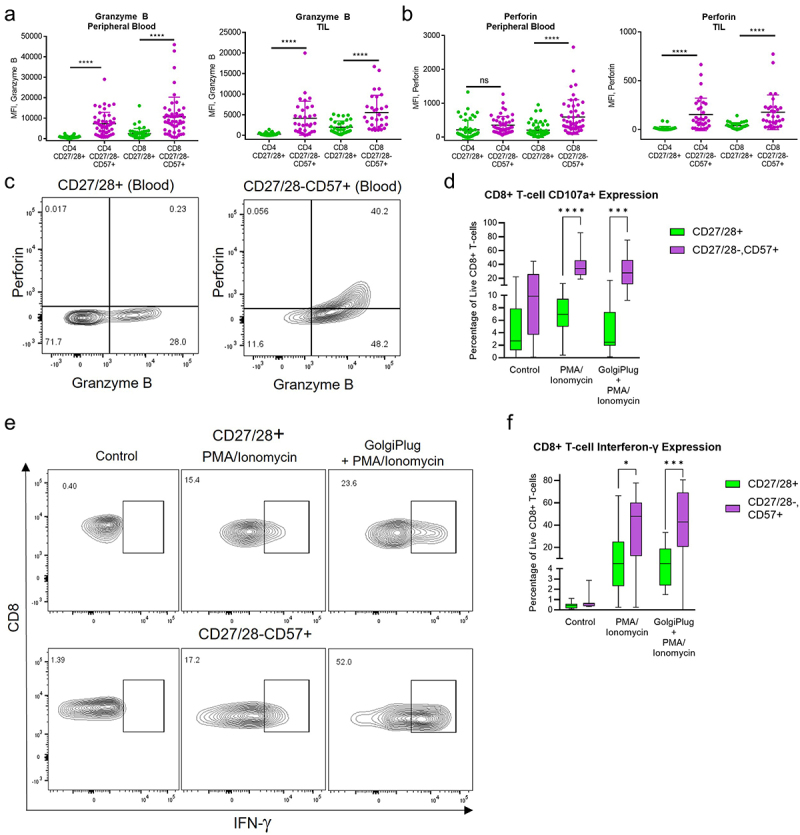
CD27/28-CD57+ T cells secrete high amounts of cytolytic enzymes and cytokines upon stimulation. (a) Granzyme B expression among T cell subsets in the peripheral blood (left) and TIL (right). MFI, mean fluorescence intensity. (b) Perforin expression among T cell subsets in the peripheral blood (left) and TIL (right). (c) Representative flow plots showing baseline expression of granzyme B and perforin among CD8+ T cell subsets in the peripheral blood. (d) Box plots for surface CD107a expression among CD8+ T cell subsets in the peripheral blood after no treatment (control) versus treatment with PMA/ionomycin with or without GolgiPlug. (e) Representative flow plots of IFN-γ expression in CD8+ T cell subsets from the peripheral blood treated with PMA/ionomycin with or without GolgiPlug vs. control. (f) Box plots for quantification of IFN-γ expression among CD8+ T cell subsets from the peripheral blood. *p < .05, ***p < .001, ****p < .0001 by two-way ANOVA with post hoc Tukey analyses.
CD107a (also known as LAMP1) is expressed along the inner membrane leaflet of intracellular lytic vesicles, becoming exposed on the cell surface of immune effector cells upon fusion of these vesicles to the cell surface and release of cytolytic enzymes. Expression of CD107a on the surface of a T cell is considered a robust surrogate marker of degranulation.30 To our knowledge, expression of CD107a has not been investigated along with granzyme/perforin in CD27/28-CD57+ T cells. When we compared CD107a expression in CD27/28-CD57+ versus CD27/28+ CD8+ T cells, we found that CD107a was significantly higher in the CD27/28-CD57+ T cells upon stimulation (Figure 5d). Taken together, these data suggest that the CD27/28-CD57+ T cells produce large quantities of cytolytic enzymes and are capable of releasing them upon stimulation.
Several studies have reported robust production and secretion of effector cytokines, particularly IFN-γ, by CD27/28-CD57+ T cells.17,23 To investigate whether this is a feature of CD27/28-CD57+ T cells from HNSCC patients, we stimulated CD8+ T cells from the peripheral blood in the presence or absence of GolgiPlug and assessed intracellular IFN-γ by flow cytometry (Figure 5e,f). The level of intracellular IFN-γ was substantially higher in the CD27/28-CD57+ T cells versus their CD27/28+ counterparts. Thus, it appears that this distinct subset of T cells that have been portrayed in the literature as fragile and past their prime31 do excel at some very specific effector functions, including the production of effector enzymes and cytokines.
CD27/28-CD57+ T cells are likely to be a highly differentiated subpopulation of TEMRA cells
Costimulation is an early, critical component of T cell activation upon stimulation of the T cell receptor by a peptide-MHC complex.9,32 Loss of CD27 and CD28 constitutes a late event, and terminally differentiated T cells often lack these costimulatory receptors. It has been suggested that these CD27/28-CD57+ T cells are oligoclonal, terminally differentiated, CD45RA-positive effector memory (TEMRA) cells that accumulate as a result of chronic antigen stimulation.21,23,31,33,34 Consistent with other reports,35 flow cytometric analysis of our blood samples from HNSCC patients showed that these cells are universally positive for CD45RA and negative for CCR7 (Figure 6), which are classic features of TEMRA cells along with lack of CD27 expression.36 Thus, our data indicate that these CD27/28-CD57+ T cells are likely to be a subset of TEMRA cells.
Figure 6.
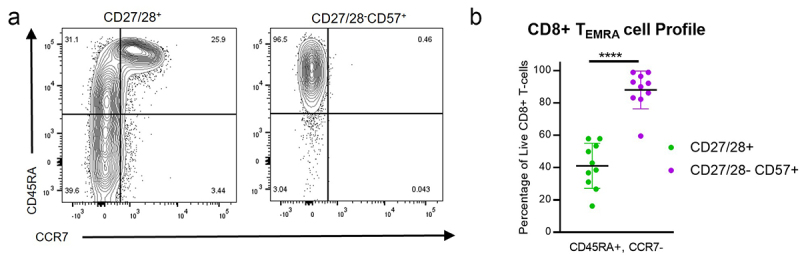
CD27/28-CD57+ T cells are a subset of TEMRA cells. (a) Representative flow plots for CD45RA and CCR7 expression among CD8+ T cell subsets from the peripheral blood. (b) Quantification of TEMRA cells (CD45RA+CCR7-) among CD8+ T cell subsets from the peripheral blood. Data represent mean ± one SD. Data represent 4 independent experiments with PBMCs from a total of 10 individual patients. ****p < .0001 by student’s t test.
CD27/28-CD57+ T cells are associated with early locoregional recurrence after surgery
Studies on the prognostic significance of T cells lacking CD27/28 and expressing CD57 in patients treated with immune checkpoint blockade have shown mixed results.6–8 We next analyzed our dataset of 50 patients (Table 1) to determine whether there was any association between the proportion of these cells in the blood and locoregional (LR) disease relapse. We focused our analysis on LR relapse within 6 months rather than the development of distant metastatic disease, since distant sites were not specifically treated in this surgical cohort. We first performed a receiver operating curve to assess for any relationship between the percent of CD8+ T cells in the blood that were CD27/28-CD57+ (Figure 7a). We then chose a cutoff point of 34%, which was found to have 80% sensitivity and specificity. The rate of LR relapse in patients with < 34% CD27/28-CD57+ CD8+ T cells in the blood was 2.7%, compared with over 30% of the patients with these cells comprising > 34% of the CD8+ T cells (Figure 7b). The one-year locoregional control rate was also significantly reduced in patients with > 34% of these cells in the blood (Figure 7c). These results suggest that patients wherein a third or more of the circulating CD8+ T cells lack CD27/28 and express CD57 may be at risk for early disease relapse and lower locoregional disease control.
Table 1.
Demographic and clinicopathologic details of the included patients.
| Characteristic | n = 50 |
|---|---|
| Patient Demographics | |
| Age, mean (SD) | 69 (10.7) |
| Gender | |
| Male, n (%) | 25 (50) |
| Female, n (%) | 25 (50) |
| Race | |
| White, n (%) | 39 (78) |
| Black, n (%) | 8 (16) |
| Asian, n (%) | 2 (4) |
| Ethnicity | |
| Hispanic, n (%) | 1 (2) |
| Non-Hispanic, n (%) | 49 (98) |
| Anatomic sites, n (%) | |
| Oral Cavity | 38 (76) |
| Hypopharynx | 2 (4) |
| Larynx | 10 (20) |
| Tumor Grade, n (%) | |
| G1 | 10 (20) |
| G2 | 35 (70) |
| G3 | 5 (10) |
| pT Stage (AJCC 8thedition), n (%) | |
| T1 | 3 (6) |
| T2 | 7 (14) |
| T3 | 11 (22) |
| T4a | 24 (48) |
| T4b | 5 (10) |
| pN Stage (AJCC 8thedition), n (%) | |
| N0 | 27 (54) |
| N1 | 3 (6) |
| N2a | 2 (4) |
| N2b | 7 (14) |
| N2c | 1 (2) |
| N3b | 10 (20) |
| Overall Stage | |
| I | 3 (6) |
| II | 4 (8) |
| III | 11 (22) |
| IVA | 20 (40) |
| IVB | 12 (24) |
| High-risk Pathologic Features | |
| Positive margins, n (%) | 2 (4) |
| Perineural invasion, n (%) | 25 (50) |
| Lymphovascular invasion, n (%) | 19 (38) |
| Extracapsular extension, n (%) | 12 (24) |
Figure 7.
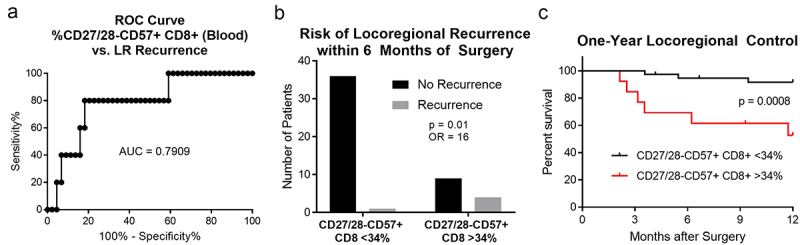
CD27/28-CD57+ T cells are associated with early locoregional disease relapse in surgically-treated HNSCC. We performed a receiver operating curve (ROC) analysis (a) comparing the proportion of CD8+ T cells in the blood that are CD27/28-CD57+ versus the incidence of locoregional (LR) recurrence within 6 months after surgery. After selecting 34% as the cutoff with 80% sensitivity and specificity for LR recurrence, we then found that patients with > 34% of these cells within the peripheral blood were more likely to have LR disease recurrence within 6 months (b). On Kaplan-Meier analysis, patients with > 34% of these cells within the peripheral blood (red) had significantly lower locoregional disease control (c). AUC, area under the curve; OR, odds ratio.
Discussion
Our data show that a distinct subset of TEMRA cells lacking costimulatory receptors and expressing CD57 accumulate in the peripheral blood of some patients with head and neck cancer. These cells have reduced ability to proliferate but are not senescent, and they retain a strong ability to perform some important effector functions, including production of cytolytic enzymes and IFN-γ. Our results suggest that CD27/28-CD57+ T cells in both CD4+ and CD8+ subsets are more prevalent in the peripheral blood versus tumor, and in most cases the proportion of these cells in the blood can be used to predict the relative proportion of these cells among TIL. We found that these cells in the blood had increased expression of KLRG1, which has recently been associated with poor responses to neoadjuvant immunotherapy in head and neck cancer.37 Contrary to other reports,4 we did not find increased expression of TIGIT in these cells.
The proportion of these cells within the peripheral blood, which is logistically more practical to assess versus tumor specimens, has been previously used as a biomarker of prognosis and treatment response in cancer patients.6-8 At this point, based on our studies and others,38,39 it is unclear whether advanced disease leads to accumulation of these CD27/28-CD57+ T cells, or vice versa. To our knowledge, our study is the first to explore the prognostic significance of these cells in surgically-treated cancer patients. Our results suggest that patients with more than a third of their CD8+ T cells in the blood comprised of these cells may be at risk for early disease relapse after oncologic head and neck surgery. This information could have implications for patient counseling and shared decision making, particularly in older patients with a higher risk of postoperative morbidity and mortality. However, the relationship between age and the proportion of these cells in the peripheral blood was not as striking as we had expected, with our results showing a statistically significant but overall modest increase in patients over age 75.
He and Sharpless describe cellular senescence as “a stress-induced, durable cell-cycle arrest of previously replicative cells”.25 Numerous studies and review articles refer to CD27/28-CD57+ T cells as senescent, though primary data in support of this notion are limited. The idea that these cells are senescent is largely based on a series of experiments culturing T cells (mostly murine) ex vivo with tumor cells or regulatory T cells, which forces the cells to lose CD28 and gain senescence markers including p16 and β-gal.15,40–44 The loss of CD28 and gain of CD57 upon chronic antigen stimulation is a phenomenon that occurs in T cells from humans and other primates, but not in mice17; thus, murine T cells may not be the best system for studying the phenotype of CD27/28-CD57+ T cells. To our knowledge, there is a lack of any data showing that CD27/28-CD57+ T cells taken directly from a human cancer patient (from blood or the TME, where tumor cells and regulatory T cells are present) are enriched for these markers. Experiments showing inability of CFSE-labeled, anti-CD3-treated CD27/28-CD57+ T cells to proliferate have been described.17,45 However, Chong et al. demonstrated that CFSE dye is toxic to these cells, which are clearly able to proliferate when cultured with IL-2.16 More recently, a study of CD28-CD57+ CD8+ T cells from blood samples of patients seropositive for HIV and CMV showed that these cells also proliferate well upon exposure to IL-15.23 We also found that these cells are able to proliferate reasonably well when cultured with IL-2 or IL-15. Taken together, data from our study and others16,18,23 clearly show that CD27/28-CD57+ T cells are able to proliferate (i.e., are not senescent) when cultured under the right conditions. In light of these findings and other definitive experiments in the literature,17 we posit that these cells should not be called “senescent” T cells. Similarly, the term “immunosenescence” should not be used to refer to all aspects of immune aging.
Our study has some limitations. There are numerous assays to assess cellular senescence, and we could not perform all of them in this study. However, by the strictest definition, i.e., inability to divide/proliferate, our experiments suggest that these T cells are not senescent. Although our work confirms increased expression of perforin and granzymes in addition to increased CD107a expression suggesting that CD27/28-CD57+ T cells have enhanced cytolytic potential, we have not been able to directly demonstrate cytolysis of target cells. True comparison of cytolytic capacity among CD27/28-CD57+ cells and their CD27/28+ counterparts is challenging without neoantigen-specific, HLA-matched tumor cells to culture along with the above T cell subsets for a given patient. Although we have been trying to create cell lines from tumor tissue so that they can be cultured along with sorted T cells, this has been technically challenging. A prior study using CD28-negative T cells from HNSCC patients with partially HLA-matched tumor cell lines showed that these T cells are capable of killing tumor cells.46 Our prognostic data thus far are limited by short follow-up. Long-term follow-up and multivariate analyses are needed to determine whether the proportion of CD27/28-CD57+ CD8+ T cells in the blood can be used as an independent predictor of treatment response and survival outcomes in HNSCC. Finally, the potential mechanism by which these cells may have a detrimental effect on prognosis is unclear. Our proliferation data suggest that the CD27/28-CD57+ cells may have inhibitory effects on some of the effector functions of CD27/28+ T cells; however, these data are not sufficient to draw definitive conclusions. It is also possible that these cells are simply bystander cells specific for chronic viral antigens, causing detrimental effects simply by outnumbering the effector T cells available for anti-tumor immunity. Our ongoing studies will attempt to answer these important questions.
These cells also appear to be associated with advanced disease stage. Whether these CD28-negative TEMRA cells contribute to the pathogenesis of cancer, or conversely, whether they accumulate as a result of aggressive cancer with chronic antigen stimulation, is unclear. Further, conflicting results seen in studies on the effects of these cells on prognosis and treatment response6-8 suggest that the role of these cells may be cancer- and context-specific. Additional human studies are needed in a variety of cancer types, and such studies should attempt to adjust for important clinical variables, including age, sex, race/ethnicity, and disease characteristics including stage, treatment, and the presence or absence of high-risk features.
Supplementary Material
Acknowledgments
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank Aaron Rae and Lisa Bixby of the Flow Cytometry for their technical assistance. We also thank Rachel Payton, Sydney Morgan, Margaret Johns, Xiu Wang, Patricia Wood and physicians, nurse anesthetists, and physician assistants in the Emory Departments of Anesthesiology and Pathology for their assistance with blood and tumor specimen procurement.
Funding Statement
This work was funded by Winship Cancer Institute, the Department of Otolaryngology – Head and Neck Surgery at Emory University School of Medicine, and the Emory Woodruff Health Sciences Center for Health in Aging. Research reported in this publication was supported in part by the Winship Cancer Tissue and Pathology Resource, Emory Pediatrics/ Winship Flow Cytometry Core, Emory Integrated Genomics Core, and NIH/NCI under award number [P30CA138292].
Disclosure statement
NCS discloses consulting fees from Sensorion, Regeneron, and GeoVax in addition to research funding from Astex Pharmaceuticals. The authors have no conflicting financial interests related to this work.
Data availability statement
Data will be provided upon reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/2162402X.2024.2367777
References
- 1.Kadambi S, Loh KP, Dunne R, Magnuson A, Maggiore R, Zittel J, Flannery M, Inglis J, Gilmore N, Mohamed M. et al. Older adults with cancer and their caregivers — current landscape and future directions for clinical care. Nat Rev Clin Oncol. 2020;17(12):742–12. doi: 10.1038/s41571-020-0421-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saba NF, Blumenschein G Jr., Guigay J, Licitra L, Fayette J, Harrington KJ, Kiyota N, Gillison ML, Ferris RL, Jayaprakash V. et al. Nivolumab versus investigator’s choice in patients with recurrent or metastatic squamous cell carcinoma of the head and neck: efficacy and safety in CheckMate 141 by age. Oral Oncol. 2019;96:7–14. doi: 10.1016/j.oraloncology.2019.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kared H, Martelli S, Ng TP, Pender SL, Larbi A. CD57 in human natural killer cells and T-lymphocytes. Cancer Immunol Immunother. 2016;65(4):441–452. doi: 10.1007/s00262-016-1803-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huff WX, Kwon JH, Henriquez M, Fetcko K, Dey M. The evolving role of CD8+CD28−immunosenescent T cells in cancer immunology. Int J Mol Sci. 2019;20(11):2810. doi: 10.3390/ijms20112810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mondal AM, Horikawa I, Pine SR, Fujita K, Morgan KM, Vera E, Mazur SJ, Appella E, Vojtesek B, Blasco MA. et al. p53 isoforms regulate aging- and tumor-associated replicative senescence in T lymphocytes. J Clin Invest. 2013;123(12):5247–5257. doi: 10.1172/JCI70355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fehlings M, Jhunjhunwala S, Kowanetz M, O’Gorman WE, Hegde PS, Sumatoh H, Lee BH, Nardin A, Becht E, Flynn S. et al. Late-differentiated effector neoantigen-specific CD8+ T cells are enriched in peripheral blood of non-small cell lung carcinoma patients responding to atezolizumab treatment. J Immunother Cancer. 2019;7(1):249. doi: 10.1186/s40425-019-0695-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fehlings M, Kim L, Guan X, Yuen K, Tafazzol A, Sanjabi S, Zill OA, Rishipathak D, Wallace A, Nardin A. et al. Single-cell analysis reveals clonally expanded tumor-associated CD57 + CD8 T cells are enriched in the periphery of patients with metastatic urothelial cancer responding to PD-L1 blockade. J Immunother Cancer. 2022;10(8):e004759. doi: 10.1136/jitc-2022-004759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrara R, Naigeon M, Auclin E, Duchemann B, Cassard L, Jouniaux JM, Boselli L, Grivel J, Desnoyer A, Mezquita L. et al. Circulating T-cell immunosenescence in patients with advanced non–small cell lung cancer treated with single-agent PD-1/PD-L1 inhibitors or platinum-based chemotherapy. Clin Cancer Res. 2021;27(2):492–503. doi: 10.1158/1078-0432.CCR-20-1420. [DOI] [PubMed] [Google Scholar]
- 9.Kamphorst AO, Wieland A, Nasti T, Yang S, Zhang R, Barber DL, Konieczny BT, Daugherty CZ, Koenig L, Yu K. et al. Rescue of exhausted CD8 T cells by PD-1–targeted therapies is CD28-dependent. Science. 2017;355(6332):1423–1427. doi: 10.1126/science.aaf0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Characiejus D, Pasukoniene V, Jonusauskaite R, Azlauskaite N, Aleknavicius E, Mauricas M, Otter WD. Peripheral blood CD8highCD57+ lymphocyte levels may predict outcome in melanoma patients treated with adjuvant interferon-alpha. Anticancer Res. 2008;28(2B):1139–1142. [PubMed] [Google Scholar]
- 11.Characiejus D, Pasukoniene V, Kazlauskaite N, Valuckas KP, Petraitis T, Mauricas M, Den Otter W. Predictive value of CD8highCD57+ lymphocyte subset in interferon therapy of patients with renal cell carcinoma. Anticancer Res. 2002;22(6B):3679–3683. [PubMed] [Google Scholar]
- 12.Kunert A, Basak EA, Hurkmans DP, Balcioglu HE, Klaver Y, van Brakel M, Oostvogels AAM, Lamers CHJ, Bins S, Koolen SLW. et al. CD45RA+CCR7− CD8 T cells lacking co-stimulatory receptors demonstrate enhanced frequency in peripheral blood of NSCLC patients responding to nivolumab. J Immunother Cancer. 2019;7(1):149. doi: 10.1186/s40425-019-0608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Characiejus D, Pasukoniene V, Jacobs JJ, Eidukevicius R, Jankevicius F, Dobrovolskiene N, Mauricas M, Van Moorselaar RJ, Den Otter W. Prognostic significance of peripheral blood CD8highCD57+ lymphocytes in bladder carcinoma patients after intravesical IL-2. Anticancer Res. 2011;31(2):699–703. [PubMed] [Google Scholar]
- 14.Liu X, Hoft DF, Peng G. Senescent T cells within suppressive tumor microenvironments: emerging target for tumor immunotherapy. J Clin Invest. 2020;130:1073–1083. doi: 10.1172/JCI133679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye J, Ma C, Hsueh EC, Eickhoff CS, Zhang Y, Varvares MA, Hoft DF, Peng G. Tumor-derived γδ regulatory T cells suppress innate and adaptive immunity through the induction of immunosenescence. J Immunol. 2013;190(5):2403–2414. doi: 10.4049/jimmunol.1202369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chong LK, Aicheler RJ, Llewellyn-Lacey S, Tomasec P, Brennan P, Wang EC. Proliferation and interleukin 5 production by CD8hi CD57+ T cells. Eur J Immunol. 2008;38(4):995–1000. doi: 10.1002/eji.200737687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strioga M, Pasukoniene V, Characiejus D. CD8+ CD28− and CD8+ CD57+ T cells and their role in health and disease. Immunology. 2011;134(1):17–32. doi: 10.1111/j.1365-2567.2011.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed R, Miners KL, Lahoz-Beneytez J, Jones RE, Roger L, Baboonian C, Zhang Y, Wang ECY, Hellerstein MK, McCune JM. et al. CD57+ memory T cells proliferate in vivo. Cell Rep. 2020;33(11):108501. doi: 10.1016/j.celrep.2020.108501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weng NP, Akbar AN, Goronzy J. CD28− T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30(7):306–312. doi: 10.1016/j.it.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pieren DKJ, Smits NAM, Postel RJ, Kandiah V, de Wit J, van Beek J, van Baarle D, Guichelaar T. Co-expression of TIGIT and Helios marks immunosenescent CD8(+) T cells during aging. Front Immunol. 2022;13:833531. doi: 10.3389/fimmu.2022.833531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang W, Morris AB, Peek EV, Karadkhele G, Robertson JM, Kissick HT, Larsen CP. CMV status drives distinct trajectories of CD4+ T cell differentiation. Front Immunol. 2021;12:620386. doi: 10.3389/fimmu.2021.620386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Focosi D, Bestagno M, Burrone O, Petrini M. CD57+ T lymphocytes and functional immune deficiency. J Leukoc Biol. 2010;87(1):107–116. doi: 10.1189/jlb.0809566. [DOI] [PubMed] [Google Scholar]
- 23.Morris SR, Chen B, Mudd JC, Panigrahi S, Shive CL, Sieg SF, Cameron CM, Zidar DA, Funderburg NT, Younes SA. et al. Inflammescent CX3CR1+CD57+CD8+ T cells are generated and expanded by IL-15. JCI Insight. 2020;5. doi: 10.1172/jci.insight.132963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee BY, Han JA, Im JS, Morrone A, Johung K, Goodwin EC, Kleijer WJ, DiMaio D, Hwang ES. Senescence-associated β-galactosidase is lysosomal β-galactosidase. Aging Cell. 2006;5(2):187–195. doi: 10.1111/j.1474-9726.2006.00199.x. [DOI] [PubMed] [Google Scholar]
- 25.He S, Sharpless NE. Senescence in health and disease. Cell. 2017;169(6):1000–1011. doi: 10.1016/j.cell.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fallah M, Mohammadi H, Shaki F, Hosseini-Khah Z, Moloudizargari M, Dashti A, Ziar A, Mohammadpour A, Mirshafa A, Modanloo M. et al. Doxorubicin and liposomal doxorubicin induce senescence by enhancing nuclear factor kappa B and mitochondrial membrane potential. Life Sci. 2019;232:116677. doi: 10.1016/j.lfs.2019.116677. [DOI] [PubMed] [Google Scholar]
- 27.Rebbaa A, Zheng X, Chou PM, Mirkin BL. Caspase inhibition switches doxorubicin-induced apoptosis to senescence. Oncogene. 2003;22(18):2805–2811. doi: 10.1038/sj.onc.1206366. [DOI] [PubMed] [Google Scholar]
- 28.Sliwinska MA, Mosieniak G, Wolanin K, Babik A, Piwocka K, Magalska A, Szczepanowska J, Fronk J, Sikora E. Induction of senescence with doxorubicin leads to increased genomic instability of HCT116 cells. Mech Ageing Dev. 2009;130:24–32. doi: 10.1016/j.mad.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 29.Shin HW, Takatsu H. Phosphatidylserine exposure in living cells. Crit Rev Biochem Mol Biol. 2020;55:166–178. doi: 10.1080/10409238.2020.1758624. [DOI] [PubMed] [Google Scholar]
- 30.Lorenzo-Herrero S, Sordo-Bahamonde C, Gonzalez S, Lopez-Soto A. CD107a degranulation assay to evaluate immune cell antitumor activity. Methods Mol Biol. 2019;1884:119–130. doi: 10.1007/978-1-4939-8885-3_7. [DOI] [PubMed] [Google Scholar]
- 31.Vallejo AN, Weyand CM, Goronzy JJ. T-cell senescence: a culprit of immune abnormalities in chronic inflammation and persistent infection. Trends Mol Med. 2004;10:119–124. doi: 10.1016/j.molmed.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Esensten JH, Helou YA, Chopra G, Weiss A, Bluestone JA. CD28 costimulation: from mechanism to therapy. Immunity. 2016;44(5):973–988. doi: 10.1016/j.immuni.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salumets A, Tserel L, Rumm AP, Turk L, Kingo K, Saks K, Oras A, Uibo R, Tamm R, Peterson H. et al. Epigenetic quantification of immunosenescent CD8 + TEMRA cells in human blood. Aging Cell. 2022;21(5):e13607. doi: 10.1111/acel.13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pangrazzi L, Reidla J, Carmona Arana JA, Naismith E, Miggitsch C, Meryk A, Keller M, Krause AAN, Melzer FL, Trieb K. et al. CD28 and CD57 define four populations with distinct phenotypic properties within human CD8 + T cells. Eur J Immunol. 2020;50(3):363–379. doi: 10.1002/eji.201948362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tarazona R, DelaRosa O, Alonso C, Ostos B, Espejo J, Pena J, Solana R. Increased expression of NK cell markers on T lymphocytes in aging and chronic activation of the immune system reflects the accumulation of effector/senescent T cells. Mech Ageing Dev. 2000;121(1–3):77–88. doi: 10.1016/s0047-6374(00)00199-8. [DOI] [PubMed] [Google Scholar]
- 36.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 37.Luoma AM, Suo S, Wang Y, Gunasti L, Porter CBM, Nabilsi N, Tadros J, Ferretti AP, Liao S, Gurer C. et al. Tissue-resident memory and circulating T cells are early responders to pre-surgical cancer immunotherapy. Cell. 2022;185(16):2918–2935 e2929. doi: 10.1016/j.cell.2022.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zelle-Rieser C, Thangavadivel S, Biedermann R, Brunner A, Stoitzner P, Willenbacher E, Greil R, Johrer K. T cells in multiple myeloma display features of exhaustion and senescence at the tumor site. J Hematol Oncol. 2016;9(1):116. doi: 10.1186/s13045-016-0345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suen H, Brown R, Yang S, Weatherburn C, Ho PJ, Woodland N, Nassif N, Barbaro P, Bryant C, Hart D. et al. Multiple myeloma causes clonal T-cell immunosenescence: identification of potential novel targets for promoting tumour immunity and implications for checkpoint blockade. Leukemia. 2016;30(8):1716–1724. doi: 10.1038/leu.2016.84. [DOI] [PubMed] [Google Scholar]
- 40.Li L, Liu X, Sanders KL, Edwards JL, Ye J, Si F, Gao A, Huang L, Hsueh EC, Ford DA. et al. TLR8-mediated metabolic control of human treg function: a mechanistic target for cancer immunotherapy. Cell Metab. 2019;29(1):103–123 e105. doi: 10.1016/j.cmet.2018.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X, Mo W, Ye J, Li L, Zhang Y, Hsueh EC, Hoft DF, Peng G. Regulatory T cells trigger effector T cell DNA damage and senescence caused by metabolic competition. Nat Commun. 2018;9(1):249. doi: 10.1038/s41467-017-02689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Montes CL, Chapoval AI, Nelson J, Orhue V, Zhang X, Schulze DH, Strome SE, Gastman BR. Tumor-induced senescent T cells with suppressor function: a potential form of tumor immune evasion. Cancer Res. 2008;68:870–879. doi: 10.1158/0008-5472.CAN-07-2282. [DOI] [PubMed] [Google Scholar]
- 43.Ye J, Huang X, Hsueh EC, Zhang Q, Ma C, Zhang Y, Varvares MA, Hoft DF, Peng G. Human regulatory T cells induce T-lymphocyte senescence. Blood. 2012;120(10):2021–2031. doi: 10.1182/blood-2012-03-416040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ye J, Ma C, Hsueh EC, Dou J, Mo W, Liu S, Han B, Huang Y, Zhang Y, Varvares MA. et al. TLR8 signaling enhances tumor immunity by preventing tumor-induced T-cell senescence. EMBO Mol Med. 2014;6(10):1294–1311. doi: 10.15252/emmm.201403918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, Casazza JP, Kuruppu J, Migueles SA, Connors M. et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101(7):2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 46.Tsukishiro T, Donnenberg AD, Whiteside TL. Rapid turnover of the CD8(+)CD28(-) T-cell subset of effector cells in the circulation of patients with head and neck cancer. Cancer Immunol Immunother. 2003;52(10):599–607. doi: 10.1007/s00262-003-0395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be provided upon reasonable request.


