Abstract
The sequence-specific RNA-binding protein Pumilio (Pum) controls Drosophila development; however, the network of mRNAs that it regulates remains incompletely characterized. In this study, we use knockdown and knockout approaches coupled with RNA-seq to measure the impact of Pum on the transcriptome of Drosophila cells in culture. We also use an improved RNA coimmunoprecipitation method to identify Pum-bound mRNAs in Drosophila embryos. Integration of these data sets with the locations of Pum-binding motifs across the transcriptome reveals novel direct Pum target genes involved in neural, muscle, wing, and germ cell development and in cellular proliferation. These genes include components of Wnt, TGF-β, MAPK/ERK, and Notch signaling pathways, DNA replication, and lipid metabolism. We identify the mRNAs regulated by the CCR4–NOT deadenylase complex, a key factor in Pum-mediated repression, and observe concordant regulation of Pum:CCR4–NOT target mRNAs. Computational modeling reveals that Pum binding, binding site number, clustering, and sequence context are important determinants of regulation. In contrast, we show that the responses of direct mRNA targets to Pum-mediated repression are not influenced by the content of optimal synonymous codons. Moreover, contrary to a prevailing model, we do not detect a role for CCR4–NOT in the degradation of mRNAs with low codon optimality. Together, the results of this work provide new insights into the Pum regulatory network and mechanisms and the parameters that influence the efficacy of Pum-mediated regulation.
Keywords: CCR4–NOT, Pumilio, RNA-binding proteins, deadenylase, repression
INTRODUCTION
Eukaryotic transcriptomes are regulated by RNA-binding proteins (RBPs) that control processing, localization, stability, and—for mRNAs—translation. Pumilio (Pum) proteins are one such RBP class that regulates specific mRNAs in the cytoplasm. In metazoans, Pum orthologs are essential for the development and control of cellular proliferation and stem cell differentiation (Arvola et al. 2017; Goldstrohm et al. 2018). Their dysfunction contributes to diseases such as the neurodegenerative disease spinocerebellar ataxia (SCA47), infertility, and cancer (Gennarino et al. 2015, 2018; Goldstrohm et al. 2018). To better understand the regulatory roles played by Pum proteins in an accessible and well-studied model system, here we focus on Drosophila melanogaster Pum, which controls germline stem cell proliferation, embryonic development, and neurological functions (Arvola et al. 2017). Pum serves as an archetype for understanding posttranscriptional regulatory mechanisms and their impact on the transcriptome.
Pum proteins are defined by a conserved RNA-binding domain with eight repeated triple α-helical units. Their RNA recognition mechanism is well-understood, with each repeat presenting three amino acids that specifically contact a ribonucleotide base (Wang et al. 2002; Weidmann et al. 2016). The specificity, affinity, and 3D structure of Drosophila Pum-bound to RNA have been extensively characterized, thereby defining the Pumilio response element (PRE) with consensus 5′-UGUANAUA (where N = A,G,C, or U) (Zamore et al. 1997, 1999; Gerber et al. 2006; Laver et al. 2015; Weidmann et al. 2016).
Pum is a repressor that reduces the translation and stability of select target mRNAs (Arvola et al. 2017). The PRE is necessary and sufficient to confer Pum-mediated regulation when placed into the 3′ UTR of a reporter gene (Weidmann and Goldstrohm 2012). In this context, Pum represses protein expression by accelerating mRNA degradation and by antagonizing the translation-promoting activities of the poly(A) tail and poly(A)-binding protein (Weidmann and Goldstrohm 2012; Weidmann et al. 2014; Burow et al. 2015; Arvola et al. 2020).
Although Pum-regulated mRNAs have been identified by genetic and biochemical analyses (for review, see Arvola et al. 2017), the potential impact of Drosophila Pum on the transcriptome remains to be fully characterized. Robust functional evidence is limited to a handful of key Pum target genes linked to phenotypes in embryos (e.g., hunchback) (Murata and Wharton 1995; Wreden et al. 1997; Forbes and Lehmann 1998; Wharton et al. 1998), the germline (e.g., Cyclin B, mei-P26) (Kadyrova et al. 2007; Joly et al. 2013), neuromuscular junction (e.g., eIEF4E, GluRIIA) (Menon et al. 2004, 2009), and brain (e.g., paralytic, hid) (Mee et al. 2004; Bhogal et al. 2016). Two lines of evidence suggest a broader regulatory role of Pum. First, PRE motifs are prevalent in the transcriptome, with more than 3724 genes having one or more PREs, which are distributed in 5′ UTR, 3′ UTR, and protein coding sequence (CDS) (Supplemental Table S1; Arvola et al. 2017). Second, hundreds of PRE-containing mRNAs that coimmunoprecipitate with Pum from embryos or adult ovaries were identified by microarray analyses (Gerber et al. 2006; Laver et al. 2015). Nevertheless, regulation of these PRE-containing mRNAs by Pum remains largely unexplored.
Analysis of PRE-containing reporter genes established that Pum accelerates mRNA degradation by binding and recruiting the CCR4–NOT deadenylase complex (Weidmann et al. 2014; Arvola et al. 2020). CCR4–NOT catalyzes the removal of the 3′ polyadenosine tail of mRNAs. The poly(A) tail can stabilize mRNAs and promote their translation (Passmore and Coller 2022). Removal of the poly(A) by deadenylases initiates mRNA decay pathways (Meyer et al. 2004; Temme et al. 2004, 2010, 2014; Goldstrohm and Wickens 2008). In vivo, several Pum target mRNAs were shown to be degraded by CCR4–NOT (Wreden et al. 1997; Joly et al. 2013; Arvola et al. 2017, 2020). However, the impact of the Pum:CCR4–NOT regulatory mechanism on the fly transcriptome has not yet been investigated. Moreover, the broader effects of CCR4–NOT on transcript levels in Drosophila, and the extent to which its activity occurs through interactions with Pum versus other factors, is not well-understood. Across eukarya, CCR4–NOT-mediated deadenylation has emerged as a crucial node for mRNA regulation by multiple RBPs and microRNAs (Goldstrohm and Wickens 2008; Jonas and Izaurralde 2015; Raisch and Valkov 2022). In addition, CCR4–NOT is implicated in codon optimality-mediated mRNA decay, based on evidence that yeast CCR4–NOT interacts with ribosomes whose elongation is slowed by suboptimal codon content (Buschauer et al. 2020; Bae and Coller 2022; Wu and Bazzini 2023). The potential relationship between CCR4–NOT activity and codon optimality in metazoans remains to be determined. Additionally, the potential relevance of codon optimality to Pum-mediated mRNA decay is unknown.
To better understand the Pum regulatory network, both in terms of its targets and mechanisms of action, we measured the impact of Pum on the transcriptome using RNA-seq combined with two loss-of-function strategies: transient RNAi-mediated depletion and CRISPR–Cas9-based genetic knockout. Integrative analysis of the resulting differential expression data revealed a collection of PRE-enriched, Pum-bound, directly regulated target mRNAs with key functions in neural and germ cell development, transposon suppression, metabolism, cell proliferation, and signaling. Our analysis provides insights into the effectiveness of Pum-mediated repression in relation to the location, sequence context, number, and clustering of PREs. We also measured the effect of CCR4–NOT depletion on the transcriptome. The intersection of Pum and CCR4–NOT-regulated mRNAs supports the role of the deadenylase complex in the regulation of natural Pum target mRNAs. Analysis of the mRNAs regulated by Pum and CCR4–NOT identified both shared and unique functional classes of target genes related to developmental pathways and potential cis-acting regulatory features. Remarkably, optimal synonymous codons did not exhibit a direct functional relationship to regulation by either Pum or CCR4–NOT. Collectively, our results provide new insights into the Pum regulatory network and mechanisms.
RESULTS
RNA-seq identifies new Pumilio-regulated mRNAs
To identify mRNAs that are regulated by Pum, we knocked down its expression in the embryo-derived Drosophila Line 1 (DL1) cells using RNAi (Pum knockdown [KD]) and measured the impact on RNA levels using RNA-seq. We reasoned that although not a complete loss of function, this transient depletion of Pum is likely to be less prone to adaptive changes in gene expression due to the speed with which it can be accomplished. We performed RNA-seq on libraries generated from poly(A)-selected RNAs using the Click-seq method (Routh et al. 2015). Differential expression in the Pum KD condition was determined relative to the nontargeting control (NTC) RNAi using dsRNA corresponding to the Escherichia coli lacZ gene (Weidmann and Goldstrohm 2012; Arvola et al. 2020; Haugen et al. 2022). Three biological replicates were analyzed for each condition. For this experiment, DL1 cells were modified using CRISPR–Cas9 genome editing to introduce a myc epitope tag into the pumilio gene (Supplemental Fig. S1), which enabled confirmation of depletion of Pum protein by western blotting (Fig. 1A). Knockdown of pumilio mRNA was also confirmed by RT-qPCR and RNA-seq (Fig. 1B). The observed level of Pum depletion is consistent with previous studies that demonstrated stabilization of PRE-containing reporter mRNAs and alleviation of Pum-mediated repression in Drosophila (Weidmann et al. 2014; Arvola et al. 2020; Haugen et al. 2022).
FIGURE 1.
Identification of Pum-regulated transcripts in response to Pum knockdown in Drosophila cells. (A) Western blot of RNAi knockdown of endogenous myc-tagged Pum protein from three biological replicate samples of DL1 Pum-myc cells. RNAi with NTC dsRNA, corresponding to E. coli lacZ, served as a negative control. Western blot of tubulin served as a loading control. (B) RNAi knockdown of Pum mRNA was confirmed by RT-qPCR and RNA-seq. Mean log2 fold change (LFC) values ± standard error of the mean (SEM) are plotted relative to the NTC RNAi condition. n = 3. (C) Volcano plot of statistical significance (q-value) versus mean LFC of RNA levels in Pum RNAi relative to NTC RNAi, measured by RNA-seq. Vertical dashed lines indicate a LFC value of ±log2(1.3). A statistical significance threshold (q-value ≤ 0.05) is shown with a horizontal dashed line. Red or blue markers (×) indicate genes passing both statistical significance and fold change thresholds in positive (Up) or negative (Down) directions, respectively. (D) Plot of mean normalized RNA-seq read counts per kilobase of Pum RNAi versus NTC. Significantly up-regulated and down-regulated genes are highlighted. (E) Identification of RNA sequence motifs significantly correlated with changes in transcript abundance, analyzed using the finding informative regulatory element (FIRE) algorithm. Target RNAs were sorted into nine equally populated bins, in order of significant-weighted log fold change values (i.e., the Wald statistic). The enrichment or depletion of the identified motif in each bin is shown with the blue–yellow color scale. Significant enrichment is observed for a motif that is highly identical to the documented Pum-binding site, the PRE, in transcripts that are strongly up-regulated by knockdown of Pum (Up in Pum KD). The Z-score output by FIRE indicates the information content of the optimized motif. The E-value output by TOMTOM indicates how confidently the motif matched with a known RBP.
The levels of more than 8600 genes were measured in this experiment. The results are summarized in Table 1, with full data reported in Supplemental Table S2. Differentially expressed mRNAs were identified using a ≥1.3-fold change threshold and, as a cutoff for statistical significance, an FDR-adjusted P-value of ≤0.05 (Fig. 1C,D). We note that this threshold was established in previous functional studies showing that a single PRE reproducibly confers 1.3-fold repression by Pum (Van Etten et al. 2012; Weidmann and Goldstrohm 2012; Bohn et al. 2018; Enwerem et al. 2021). By these criteria, 44 genes were significantly up-regulated in the Pum KD condition. For example, the aquaporin big brain (bib), which is involved in neurogenesis and modulates Notch signaling, was among the most highly up-regulated genes (>3.2-fold, P-adj = 0.005). In contrast, only 17 genes were significantly decreased in transcript abundance by Pum KD. Given the documented role of Pum in the repression of gene expression (Arvola et al. 2017), we primarily focus our analysis on the up-regulated genes. Considerations and limitations of this RNAi approach are addressed in the Discussion.
TABLE 1.
Significantly affected genes by Pum, Not1, and Pop2 knockdown and Pum knockout
| Up | Down | |
|---|---|---|
| RNAi knockdowns | ||
| Pum KD | 44 | 17 |
| Not1 KD | 2301 | 2175 |
| Pop2 KD | 1673 | 1789 |
| Overlap of Not1 + Pop2 KD | 1522 | 1523 |
| Overlap of Not1 + Pop2 + Pum KD | 19 | 10 |
| CRISPR–Cas9 knockouts | ||
| Pum KO1 | 1776 | 2173 |
| Pum KO2 | 1573 | 1878 |
The number of genes that were significantly up- or down-regulated in each knockdown and knockout experiment are reported. Significance was determined based on whether the gene had a q-value ≤ 0.05 and a ±fold change ≥1.3. Complete data sets are listed in Supplemental Table S2 for Pum KD and Supplemental Table S3 for Pum KO.
Enrichment of PRE motifs in Pumilio-regulated mRNAs
If the mRNAs that are up-regulated by Pum KD are direct targets, then they should contain one or more binding sites for Pum. Using the well-characterized RNA-binding specificity of Pum (5′-UGUANAUA, which constitutes a PRE) (Arvola et al. 2017), we cataloged the PRE-containing genes in the Drosophila genome, documenting the number and location of PREs within the annotated transcripts. Table 2 provides an overview, and the complete list is reported in Supplemental Table S1.
TABLE 2.
Summary of PRE-containing genes in Drosophila melanogaster
| Number with PREs | Range | Mean | |
|---|---|---|---|
| Genes w/PRE | 3724 (of 17,225) | 1–15 | 1.5 |
| 5′ UTR w/PRE | 694 | 1–5 | 1.1 |
| CDS w/PRE | 532 | 1–11 | 1.1 |
| 3′ UTR w/PRE | 2600 | 1–12 | 1.4 |
| lncRNA w/PRE | 316 | 1–11 | 1.4 |
| microRNA w/PRE | 6 | 1 | 1 |
The number of genes containing Pumilio response element (PRE) sequences is listed, along with the range of PREs and the overall mean. Unique genes with one or more mRNA isoforms that contain a PRE are included in the total number. The complete data set is reported in Supplemental Table S1.
We found that 31 of the 44 genes up-regulated ≥1.3-fold by Pum KD contain one or more PREs (Table 3). These genes encode proteins involved in neurodevelopment, germ cell development, transposon suppression, metabolism, cell proliferation, and signaling (i.e., Notch, Wnt, TGF-β, and MAPK/ERK pathways). The location and number of PREs in each Pum-repressed mRNA are shown in Table 3. It is notable that there is a statistically significant enrichment in the intersection between genes that are Pum-regulated and those containing a PRE in the 3′ UTR (Table 4). Indeed, most of the genes that were up-regulated in response to Pum KD have PREs located in the 3′ UTR (29 of 31). The remaining two genes up-regulated by Pum KD (CBP, Jheh2) have a single PRE in their 5′ UTR—and none elsewhere in the transcript—suggesting the potential for Pum repression via 5′ UTR-binding sites, although an indirect effect on these two transcripts remains possible. We also noted that of the 17 genes that were significantly down-regulated in response to Pum KD, only two contain PREs: the focal adhesion-associated factor Paxillin (Pax) and muscle-specific protein 300 kDa (Msp300).
TABLE 3.
Drosophila genes that are significantly up-regulated in response to Pum RNAi and contain one or more PREs
| Pum-repressed, PRE-containing gene | PRE location | |||||
|---|---|---|---|---|---|---|
| Flybase ID | Symbol | Summary | 5′ UTR | CDS | 3′ UTR | Bound |
| FBgn0000077 | amx | Neurodevelopment, Notch pathway | 0 | 0 | 2 | + |
| FBgn0001128 | gpdh1 | Glycerol-3-phosphate dehydrogenase 1, metabolism | 0 | 0 | 2 | + |
| FBgn0002973 | numb | Neurodevelopment, Notch pathway | 0 | 0 | 1 | + |
| FBgn0011704 | RnrS | Deoxynucleotide biosynthesis, ribonucleoside diphosphate reductase, small subunit | 0 | 0 | 1 | + |
| FBgn0024889 | Kap-α1 | Spermatogenesis, nuclear import, karyopherin | 0 | 0 | 1 | + |
| FBgn0024994 | Ugalt | UDP-galactose transporter | 0 | 0 | 3 | + |
| FBgn0028360 | cdc7 | Promotes DNA replication, protein kinase | 0 | 0 | 2 | + |
| FBgn0031491 | α4GT1 | α1,4-Galactosyltransferase 1, glycosphingolipid biosynthesis | 0 | 0 | 1 | + |
| FBgn0036502 | CG7841 | Function and phenotype unknown | 0 | 0 | 2 | + |
| FBgn0037110 | ORMDL | Negative regulator of sphingolipid synthesis | 0 | 0 | 2 | + |
| FBgn0039135 | CG13603 | Function and phenotype unknown | 0 | 0 | 1 | + |
| FBgn0000180 | bib | Neurodevelopment, Notch pathway | 0 | 1 | 1 | |
| FBgn0003079 | Raf | Oncogene, serine–threonine kinase | 0 | 0 | 2 | |
| FBgn0003716 | tkv | Germ cell development, TGF β receptor for dpp signaling | 0 | 0 | 3 | |
| FBgn0010473 | tutl | Neurodevelopment, axon guidance, motor control, IgG superfamily transmembrane protein | 0 | 0 | 1 | |
| FBgn0011288 | snap25 | SNARE complex, neurotransmitter release | 0 | 1 | 2 | |
| FBgn0015766 | Msr-110 | Integral membrane component, molecular function unknown, expressed in the digestive system, embryo germ band, prothoracic gland | 0 | 0 | 1 | |
| FBgn0024245 | dnt | Muscle development, cell morphogenesis, differentiation, Ryk family of Wnt receptor tyrosine kinase | 0 | 0 | 1 | |
| FBgn0025626 | CG4281 | Function unknown, expressed in the gonad and digestive system | 0 | 0 | 2 | |
| FBgn0026144 | CBP | Sarcoplasmic calcium-binding protein | 1 | 0 | 0 | |
| FBgn0031401 | papi | piRNA pathway, repression of transposable elements during meiosis | 0 | 0 | 1 | |
| FBgn0034405 | Jheh2 | Juvenile hormone epoxide hydrolase 2 | 1 | 0 | 0 | |
| FBgn0034611 | MFS16 | Integral membrane component, molecular function unknown | 0 | 0 | 2 | |
| FBgn0035696 | Best2 | Predicted chloride transport channel | 0 | 1 | 2 | |
| FBgn0038460 | CG18622 | Function and phenotype unknown | 0 | 0 | 4 | |
| FBgn0039045 | Ctns | Amino acid transmembrane transport, Cystine/H+ symporter | 0 | 0 | 1 | |
| FBgn0040334 | Tsp3A | Notch signaling, tetraspanin superfamily transmembrane protein | 0 | 0 | 1 | |
| FBgn0040348 | CG3703 | GTPase activator, expressed in embryonic brain and larval ventral nerve cord | 0 | 0 | 1 | |
| FBgn0041629 | Hexo2 | β-N-acetylglucosaminidase involved in carbohydrate metabolism | 0 | 0 | 1 | |
| FBgn0264090 | CG43759 | Function unknown | 0 | 0 | 3 | |
| FBgn0283509 | Phm | Neuropeptide biosynthesis, peptidylglycine-α-hydroxylating monooxygenase | 0 | 0 | 2 | |
The FlyBase gene name and symbol for each gene are listed, along with a summary of its function and the location of PREs within the transcript. For genes with multiple mRNA isoforms, PRE parameters are based on the longest isoform. Genes that were also up-regulated in both Pum KO cell lines are indicated in bold text. Genes with transcripts bound by Pum in embryos, identified by RIP-seq, are indicated with a “+.”
TABLE 4.
Functional and statistical relationship of Pum-regulated, bound, and PRE-containing genes
| PRE location: | 5′ UTR | CDS | 3′ UTR | |
|---|---|---|---|---|
| Pum-regulated and PRE-containing | P-value | 0.778 | 0.171 | <0.001 |
| log2(FE) | −0.403 | 1.105 | 1.953 | |
| Pum-regulated and Pum-bound | P-value | <0.001 | <0.001 | <0.001 |
| log2(FE) | 1.943 | 1.977 | 1.956 | |
| PRE-containing and Pum-bound | P-value | <0.001 | 0.013 | <0.001 |
| log2(FE) | 1.220 | 0.605 | 1.249 | |
| Pum-regulated, PRE-containing, and Pum-bound | P-value | <0.001 | ||
| log2(FE) | 0.575 | |||
(UTR) Untranslated region, (CDS) coding sequence, (FE) fold enrichment. P-values from permutation test, n = 1000.
We sought to identify cis-RNA elements associated with the observed changes in gene expression caused by Pum KD in the RNA-seq data set. To do so, we applied the motif discovery tool FIRE, which evaluates the significance (assessed via mutual information) of the presence or absence of a motif while making as few prior assumptions as possible (Elemento et al. 2007). We used FIRE to search for motifs in the 3′ and 5′ UTR regions that were informative of the effects of Pum KD on transcript levels. To determine if these motifs correspond to known binding sites of RBPs, we used the tool TOMTOM (Gupta et al. 2007) to cross-reference the motifs discovered by FIRE to the CISBP-RNA database, which catalogs known RBP motifs, including for Drosophila RBPs (Ray et al. 2013). Motifs were considered to be both significantly informative for the gene expression changes and a confident match to known RBP motifs if they had a Z-score ≥ 50 from FIRE and an E-value ≤ 0.05 from TOMTOM, respectively. The results revealed that an overrepresented motif in the 3′ UTR of genes up-regulated by Pum KD significantly matches the previously characterized RNA-binding specificity of Pum (Fig. 1E; Gerber et al. 2006; Weidmann et al. 2016; Arvola et al. 2017; Wolfe et al. 2020) and is the strongest motif identifiable in the Pum KD data. We noted that several additional motifs were identified by this analysis (Supplemental Fig. S2), although their recognition by RBPs and functional relationship to Pum are unknown. Taken together, our results identify multiple new Pum-regulated target genes and emphasize the importance of 3′ UTR PREs in Pum-mediated repression.
Differential gene expression analysis in Pumilio knockout cells
As an additional approach to identify Pum-regulated target mRNAs, we used CRISPR–Cas9 to inactivate the pumilio gene (Haugen et al. 2022). Two Pum knockout (KO) DL1 cell lines were clonally isolated and genotyped. Pum KO1 contains a 20-bp deletion and an 81-bp insertion after glutamine 725 that appends eight additional amino acids and a premature stop codon (Supplemental Fig. S3A–D). Pum KO2 has a 10-bp deletion that creates a frameshift and premature stop codon after methionine 726, as we recently reported (Haugen et al. 2022). Both Pum KO1 and KO2 proteins lack an RNA-binding domain, thereby rendering them nonfunctional. Both Pum knockouts also produce significantly less pumilio mRNA, as measured by RT-qPCR (Supplemental Fig. S3E; Haugen et al. 2022) and RNA-seq (Supplemental Fig. S4A,B), consistent with their propensity to be degraded by the nonsense-mediated decay. We then performed RNA-seq on three replicates of each Pum KO line and compared differential gene expression relative to parental wild-type (WT) DL1 cells. RNA levels from 9924 genes were quantifiable. The complete loss of function of Pum was anticipated to result in the up-regulation of a greater number of transcripts and, indeed, many more genes were significantly affected compared to the Pum KD experiments (Supplemental Fig. S4, summarized in Table 1, with complete data set reported in Supplemental Table S3). Overall, 1247 genes were significantly up-regulated ≥1.3-fold in both the Pum KO1 and KO2 lines (Supplemental Table S3).
We anticipated that the up-regulated genes would be enriched for direct Pum targets in a manner consistent with Pum-mediated repression. Of the genes with significantly increased expression in both Pum KOs, 399 contain PREs (Supplemental Table S3), including documented Pum targets hunchback (hb) and brat (Wharton and Struhl 1991; Murata and Wharton 1995; Harris et al. 2011; Arvola et al. 2017). Although its expression in WT DL1 cells is low, hunchback was among the most highly up-regulated transcripts in the Pum KO experiments, increasing by eightfold to 14-fold (P-adj < 0.05). When we examined the overlap of significantly up-regulated genes captured by both KO and KD approaches, we found 16 PRE-containing genes, including Raf, bib, numb, and tutl (Table 3). These mRNAs encode factors involved in germ cell, muscle, and neurodevelopment and key signaling pathways. These observations provide corroborating evidence for Pum-mediated repression of a novel collection of direct target mRNAs.
Although the preceding results from the Pum KOs are informative, we also found the reason for the cautious interpretation of the knockout cell line data. First, the significantly affected genes in the KOs were skewed toward down-regulation (45% up vs. 55% down), contrary to the expected effect of loss of Pum activity. This contrasts with the results of transient Pum KD, wherein the majority of affected genes were up-regulated (72% up vs. 28% down). Second, the majority of differentially expressed genes in the Pum KOs (68%) do not appear to be direct Pum targets in that they lack PREs, again unlike the case of the transient knockdown. Third, contrary to expectation, the PRE motif is not significantly overrepresented in the genes that are up-regulated by knockout of Pum. Instead, our de novo motif enrichment analysis using FIRE and TOMTOM identified several overrepresented motifs, including those corresponding to Pabp2 and B52/SRp55 RBPs, both of which participate in mRNA processing and regulation (Supplemental Fig. S2; Roth et al. 1991; Mayeda et al. 1992; Ring and Lis 1994; Benoit et al. 1999). However, the patterns of their motif enrichment did not correspond to up-regulation by Pum KO and, therefore, likely result from indirect or secondary effects.
Our observations led us to suspect that constitutive loss of Pum and/or a technical issue may have contributed to the changes in gene expression. Several caveats are worth consideration. Pum is essential for viability in animals, and although we succeeded in knocking out Pum in the cultured cells, adaptive changes in gene expression may have occurred to compensate for the loss of Pum function. Further, Cas9 may have persisted in the Pum KO cells with the potential to cause off-target effects. Indeed, examination of the RNA-seq data for the presence of Cas9 mRNA indicated that the Pum KO1 and KO2 lines had average TPMs of 22.5 and 13.4, respectively, placing it in the ∼75th percentile of expressed genes. Deeper inspection of transcriptomes revealed higher expression of DNA damage response genes (e.g., CG7457/FBgn0035812, a human TONSL ortholog) in the Pum KO lines, potentially reflecting DNA damage by Cas9. We note that the CRISPR–Cas9 KOs used here were generated using standard procedures in the field (see Materials and Methods); we suggest that evaluation of residual Cas9 expression may be prudent in other contexts. As a result of these considerations, we chose to primarily focus our analysis on the RNAi data set, which did not show signs of competing or adaptive effects.
Expression of the Raf oncogene is regulated by Pumilio
Based on the observed up-regulation of Raf mRNA in both the Pum KD and KO RNA-seq data sets, we performed further validation for this newly identified target. Raf encodes a serine–threonine kinase, which activates the MAPK/ERK pathway to regulate proliferation and differentiation (Hayashi and Ogura 2020). The Raf gene produces two mRNA isoforms, Raf-RA and Raf-RE, which are transcribed by separate promoters and differ solely in their 5′ UTRs. Both Raf mRNAs share the same protein CDS and 460 nt 3′ UTR, which contains two PREs. To validate Pum regulation, we measured changes in Raf mRNA and protein levels in response to Pum KD. As a Drosophila Raf antibody is not available, we engineered a V5 epitope tag on the N terminus of the Raf CDS using CRISPR–Cas9 in the DL1 cells wherein the pumilio gene was myc-tagged. We clonally isolated a homozygous V5-Raf cell line, which was confirmed by genotyping, sequencing, and western blotting (Fig. 2; Supplemental Fig. S5). The effect of Pum knockdown on Raf mRNA and protein was then measured relative to a NTC RNAi. To assess reproducibility, these measurements were made in three independent experiments, each with three biological replicates. Depletion of Pum-myc protein was confirmed by western blot (Fig. 2A). To measure changes in Raf mRNA, we performed RT-qPCR on total cellular RNA using optimized primers that detect both mRNA isoforms. Consistent with the RNA-seq results, Pum KD significantly increased Raf mRNA abundance (Fig. 2B). The magnitude of the increase of Raf mRNA determined by RT-qPCR (fold change = 1.7, P < 0.001) closely matched the RNA-seq data (fold change = 1.6, P-adj = 0.029). In parallel, we performed quantitative western blotting on titrated amounts of cell extracts to measure changes in V5-tagged Raf protein between Pum KD and NTC conditions (see Materials and Methods for details). Pum KD significantly increased Raf protein to the same degree as Raf mRNA level (Fig. 2B,C). Together with the RNA-seq Pum KD and KO data, these results support Pum-mediated repression of endogenous Raf expression. Further support is provided by a recent study wherein we showed that Pum represses a luciferase-based reporter mRNA with the Raf 3′ UTR, dependent on the two PREs (Haugen et al. 2022).
FIGURE 2.

Repression of Raf mRNA and protein levels by Pum. (A) A western blot confirmed depletion of endogenous myc-tagged Pum by RNAi relative to NTC negative control in three biological replicate samples of DL1 V5-Raf, Pum-myc cells. (B) Increased expression of Raf mRNA and protein levels in Pum RNAi samples relative to NTC was measured by RT-qPCR and quantitative western blotting, respectively. Mean LFC values ±SEM are plotted relative to the NTC RNAi condition. RT-qPCR measurements were made from nine replicate samples. Quantitative western blot measurements were made in three independent experiments, each of which had three biological replicate samples with four technical replicate measurements per condition. Significance calling is as follows: (*) P < 0.05, (***) P < 0.001, using unpaired, two-tailed Student's t-test. Details of quantitation are described in the Materials and Methods section. (C) Representative western blots of V5-tagged endogenous Raf protein in three biological replicate samples from an RNAi experiment wherein the effect of Pum RNAi was compared to NTC control in DL1 V5-Raf, Pum-myc cells. The indicated amount of total cellular protein per lane, as measured by DC Lowry assay, for each replicate was analyzed in the western blots.
Integrative data analysis identifies direct Pumilio-regulated target mRNAs
As an additional parameter to characterize the direct regulation of target genes, we incorporated new experimental evidence for Pum binding to mRNAs in the context of early embryos (0–3 h), a context where Pum has well-documented regulatory roles (Lehmann and Nusslein-Volhard 1987; Arvola et al. 2017). To do so, Pum was immunoprecipitated using a synthetic monoclonal Fab antibody (Laver et al. 2015), and Pum-associated mRNAs were identified by RNA-seq (RIP-seq). Transcript enrichment in the Pum RIP-seq was determined relative to a negative control immunoprecipitate (see Materials and Methods), with an enrichment cutoff of >1.5-fold and FDR-corrected P-value < 0.05. Of the 7034 expressed genes, 695 transcripts from 646 unique genes were significantly enriched in Pum immunoprecipitates (Supplemental Table S4). It is noteworthy that the expressed genes detected in embryos substantially overlapped with those that we detected in DL1 cells using RNA-seq: 6498 genes were expressed in both contexts (Supplemental Table S4).
We then performed de novo motif enrichment analysis using FIRE. In this application, genes were stratified into equivalent-sized bins based on their log2 fold enrichment. FIRE identified a motif that is significantly overrepresented in Pum-bound transcripts and matches the PRE consensus (Fig. 3A). Indeed, a total of 329 of the Pum-bound genes contain PREs (Supplemental Table S4). The Pum transcript itself was among the most highly enriched, consistent with a previous report of autoregulation (Laver et al. 2015). FIRE analysis detected a significant overrepresentation of the PRE motif in the 3′ UTR of Pum-bound transcripts (Fig. 3A) but not in the 5′ UTR or CDS. Further analysis of all PRE-containing and Pum-bound transcripts indicates that Pum is significantly more likely to bind mRNAs that have a PRE, regardless of where that PRE is located, although there is an enrichment bias toward 3′-UTR PREs, followed by 5′-UTR PREs, over CDS PREs (Table 4).
FIGURE 3.
Analysis of Pum-bound mRNAs identifies PRE-containing, Pum-repressed direct targets. (A) Identification of a significantly overrepresented RNA sequence motif in Pum-enriched transcripts from Drosophila embryos identified by RIP-seq, analyzed using the FIRE algorithm. Target RNAs were sorted into nine equally populated bins, in order of log2 fold enrichment values in Pum RIP-seq data, as indicated at the top (the red sector in each bin indicates the range of log fold changes contained in that bin), and the over- or underrepresentation of the identified motif in each bin is shown with the blue–yellow color scale. Significant overrepresentation is observed for a motif that is highly identical to the documented Pum-binding site, the PRE, in the 3′ UTR of transcripts that are strongly enriched in the Pum RIP-seq. The Z-score output by FIRE indicates the information content of the optimized motif. The E-value output by TOMTOM indicates how confidently the motif matched with a known RBP. (B) Overlapping density distributions of the RNA abundance changes in response to Pum knockdown, plotted as significance-weighted log fold change (Wald statistic), for genes that were classified as bound (blue) or unbound (red) by Pum RIP-seq, based on a LFC ≥ 1.5 and a q-value ≤ 0.05. (C) Venn diagram comparing the overlap of significantly up-regulated genes by RNAi of Pum, measured by RNA-seq, with expressed PRE-containing genes and Pum-bound genes, identified by RIP-seq. Gene sets are reported in Supplemental Table S4. P-values for the significance of two-set or three-set gene overlaps were calculated via one-sided permutation tests (n = 1000), as described in the Materials and Methods section. For significance calling: (*) P < 0.05, (**) P < 0.01, (***) P < 0.001.
We investigated whether evidence of Pum binding to transcripts was informative in relation to increased transcript abundance in the Pum KD RNA-seq data. Based on the RIP-seq data, RNA binding by Pum had a statistically significant association with increased RNA abundance caused by Pum KD (Fig. 3B; Table 4). Thus, Pum binding to a transcript is informative of Pum-mediated repression of its abundance, consistent with direct regulation.
We then sought to identify the highest confidence direct Pum target RNAs by integrating three categories of evidence: (1) up-regulation in response to Pum KD, (2) Pum binding from RIP-seq, and (3) the presence of one or more PREs. Of the 44 genes that were significantly up-regulated by Pum KD, 31 have PREs and 12 genes were detected in Pum-bound transcripts (Fig. 3C; Supplemental Table S4). A total of 11 transcripts matched all three criteria, including amx, numb, and cdc7 (Table 3). The statistical significance of the observed overlaps in Figure 3C of the up-regulated, bound, and PRE-containing categories was assessed by performing a nonparametric permutation test, wherein the observed overlap was compared to a null distribution generated by shuffling the membership of genes in each of the three categories being considered (N = 1000 permutations), showing a significant enrichment in the overlaps of those groups.
Concordant regulatory effects of Pumilio and CCR4–NOT
We previously showed that the CCR4–NOT complex is necessary for Pum repression of PRE-containing reporter genes; specifically, RNAi of Pop2 or Not1 subunits reduced Pum:PRE mediated repression and mRNA degradation (Arvola et al. 2020). Pop2 is one of two catalytic subunits in the CCR4–NOT complex that plays an important role in mRNA deadenylation in the cytoplasm (Temme et al. 2010, 2014; Pekovic et al. 2023). Not1 is the structural backbone upon which the CCR4–NOT complex assembles. Further, multiple repression domains of Pum directly bind to the Not1, 2, and 3 subunits of CCR4–NOT (Arvola et al. 2020; Haugen et al. 2022). Importantly, the effect of the Pum:CCR4–NOT mechanism on natural target mRNAs remained unknown. If Pum uses CCR4–NOT to repress most mRNAs, then depletion of Pop2 or Not1 should increase the levels of Pum target mRNAs. In addition to its role in mRNA decay by Pum, CCR4–NOT participates in other regulatory processes, and, therefore, we anticipated that depletion of Not1 or Pop2 would stabilize a broader spectrum of transcripts (Chicoine et al. 2007; Eulalio et al. 2009; Temme et al. 2014; Raisch and Valkov 2022).
To measure the impact of CCR4–NOT on the transcriptome, Pop2 and Not1 were knocked down by RNAi using previously established conditions (Arvola et al. 2020). RNA-seq was performed on three replicates for each RNAi condition and compared to the control (NTC). Notably, this experiment was conducted in parallel with the Pum KD described in Figure 1. Knockdown of Not1 and Pop2 led to significantly increased transcript abundances of 2301 and 1673 genes (Fig. 4A–D; Table 1; Supplemental Table S2), respectively, in a manner consistent with the role of CCR4–NOT in RNA decay. Knockdown of Not1 and Pop2 themselves was confirmed by RT-qPCR and RNA-seq (Fig. 4E).
FIGURE 4.
Identification of CCR4–NOT-regulated transcripts in response to knockdown of Not1 or Pop2 in Drosophila cells. Volcano plots of RNA level changes measured by RNA-seq in Not1 (A) and Pop2 (C) RNAi conditions relative to NTC RNAi. The LFC (RNAi/NTC) is shown on the x-axis. Vertical dashed lines indicate a LFC value of ±log2(1.3). A statistical significance threshold (q-value ≤ 0.05) is shown with a horizontal dashed line. Red or blue markers (×) indicate genes passing both statistical significance and fold change thresholds in positive (Up) or negative (Down) directions, respectively. Scatterplots of RNA levels measured in the Not1 (B) and Pop2 (D) RNAi conditions (y-axis) versus the NTC control (x-axis). (E) RNAi knockdown of Not1 and Pop2 mRNA was confirmed by RT-qPCR and RNA-seq. Mean LFC values ± standard error of the mean (SEM) are plotted relative to the NTC RNAi condition. n = 3. (F) Venn overlaps of genes from all three KD data sets that were significantly up. Significance was based on the same thresholds as in A–D. All two-set and three-set overlaps were significantly enriched ((**) P ≤ 0.01, (***) P < 0.001, permutation test n = 1000), compared to chance (null hypothesis = no correlation between the KDs).
We then assessed the overlap of mRNAs stabilized by Pum, Not1, and Pop2 depletion. A total of 8596 genes were measured across all three data sets. We found that Not1 KD and Pop2 KD had a statistically significant overlap of 1522 up-regulated genes, consistent with their mutual function in the CCR4–NOT complex (Fig. 4F; Table 1; Supplemental Table S5); it is particularly striking that the identified transcripts affected by Pop2 knockdown are mostly a subset of Not1 targets. Comparison of Pum, Not1, and Pop2 KD data sets revealed a statistically significant overlap of 19 significantly up-regulated genes (Supplemental Table S5), including the high-confidence Pum targets listed in Table 3 (bib, numb, tkv, amx, Tsp3A, Raf, cdc7, RnrS, CG13603, and CBP). Together, these data sets reveal new PRE-containing natural transcripts that are negatively regulated by Pum and CCR4–NOT.
Gene ontology enrichment analysis of Pumilio and CCR4–NOT data sets
To gain insight into the network of genes affected by Pum, we performed gene ontology (GO) term enrichment analysis on our RNA-seq data using iPAGE (Goodarzi et al. 2009). iPAGE allows the identification of annotations (GO terms) that have significant mutual information with expression profiles or other quantitative data. Key advantages of this mutual information approach include (1) that any correlation structure can be detected (even if it is not monotonic (e.g., enrichments specifically among unchanged genes or among both up- and down-regulated genes) and (2) that redundant terms can be removed in a principled way by requiring each new GO term to add significant new information (assessed based on the conditional mutual information), thus avoiding overcalling of related GO terms. Transcripts were separated into five equally populated bins based on their significance-weighted log fold change (i.e., Wald statistic) in response to Pum KD. GO terms that were significantly over- or underrepresented in those categories were identified, as indicated by the heatmap (Fig. 5A).
FIGURE 5.
Gene ontology enrichment analysis of differentially expressed transcripts in response to Pum and CCR4–NOT knockdown relative to negative control RNAi (NTC). (A) GO terms showing significant mutual information with the observed LFCs upon Pum knockdown, measured in our RNA-seq data set. The genes were sorted into five equally populated bins, in order of their Wald statistic (scaled log fold change), ranging from strongly down-regulated on the left (Down in KD) to strongly up-regulated on the right (Up in KD). GO terms showing significant mutual information with the profiles of transcript abundance changes, calculated using iPAGE, are listed on the right. The color of each cell shows the magnitude of the P-value for the significance of the enrichment (red) or depletion (blue) at that cell. Scale is shown on the right-hand side of the figure; black bordered cells individually show P-values ≤ 0.05 after Bonferroni correction across their row. The plotting and highlighting conventions described here apply to all panels of the figure except for D, which is a discrete analysis and lacks the indication of Bonferroni corrected P-values ≤ 0.05. (B) As in A, for Not1 knockdown data. The dashed line indicates the omission of GO terms which are not strongly enriched in the “Up in KD” bin; see Supplemental Figure S6 for the complete output. (C) As in A, for Pop2 knockdown data; untruncated output is shown in Supplemental Figure S7. (D) GO term enrichment analysis using iPAGE of four categories of genes based on their down-regulation (Down in all 3) or up-regulation (Up in all 3) upon knockdown of Pum, Not1, and Pop2. The “neutral” category encompasses genes that were not differentially expressed in any knockdown. The “Different directions” category includes the genes that responded in opposition between any two knockdown conditions.
The analyses revealed significantly affected molecular and biological processes and pathways modulated by Pum. Genes up-regulated by Pum KD (and, thus, normally repressed by Pum) were significantly overrepresented with GO terms including sodium ion transport, wing disc morphogenesis, and cell adhesion (Fig. 5A). In addition, genes that mediate proteolysis to regulate the cell cycle were overrepresented, including the anaphase-promoting complex and Skp1-cullin 1-F-box (SCF) ubiquitin ligase complex. In contrast, RNA-related processes, such as cytoplasmic translation and RNA helicase activity, were overrepresented in the down-regulated genes.
We also performed separate pathway analyses using the RNA-seq data sets from Not1 KD (Fig. 5B) and Pop2 KD (Fig. 5C). A diverse group of GO terms was overrepresented in up-regulated gene sets, many of which are identical or related between Pop2 KD and Not1 KD, consistent with their coordinated function in the CCR4–NOT complex. Among those in common are signaling pathways, posttranslational modifications, cell adhesion and migration, and intracellular trafficking. GO terms overrepresented in the down-regulated genes included glycolytic process, cytoplasmic translation, and rRNA processing (see Supplemental Figs. S6 and S7 for expanded lists).
In addition, we performed a comparative GO term enrichment analysis of the Pum KD, Not1 KD, and Pop2 KD gene sets. Genes were binned into four categories based on their responses in the three conditions (Fig. 5D): (1) up-regulated genes in Pum and Not1 and Pop2 knockdowns (Up in all 3), (2) genes that did not change in any of the knockdowns (Neutral in all 3), (3) genes that decreased in all knockdowns (Down in all 3), and (4) genes that responded in different directions among the three knockdowns (Different directions). The “Up in all 3” category had enriched categories involved in morphogenesis of spermathecum and compound eye, sodium ion transmembrane transport, ganglion mother cell fate determination, negative regulation of growth, and cellular response to starvation (Fig. 5D), suggesting that the Pum:CCR4–NOT mechanism normally represses these processes. In contrast, categories related to translation were overrepresented in the “Down in all 3” gene list, including cytoplasmic translation, poly(A) binding, and translation initiation, suggesting a concerted down-regulation of the protein synthesis machinery in response to depletion of Pum, Not1, and Pop2 (Fig. 5D). The observed down-regulation of those genes is inconsistent with them being direct targets of the three RNA decay factors that we knocked down. Instead, their decreases may be secondary responses to the genes with increased expression (Up in all 3). Expression of the translation machinery is known to be coordinately regulated at all levels (i.e., transcription, RNA processing, translation, and RNA decay) in response to nutrients, growth, stress, and starvation (Petibon et al. 2021). Based on the results of this GO term enrichment analysis, we speculate that the observed up-regulation of the “negative regulation of growth” and/or “cellular response to starvation” categories by Pum, Not1, and Pop2 KDs may contribute to the subsequent down-regulation of the translation machinery. Taken together, these results provide new insights into the overlapping and unique regulatory networks of Pum and CCR4–NOT. Future research in vivo will be important to pursue these new relationships and their potential phenotypic consequences.
Functional association of PRE features with Pumilio-mediated repression
To further our understanding of Pum repression, we investigated contextual features of Pum-responsive mRNAs. We restricted our analysis to the Click-seq RNAi data to ensure that we were building the models on the most robust experimental data. We initially focused on the number of PRE sites, adenine and uracil (AU) content, and the relative location of PRE sites within the 3′ UTR, which we previously found were strong predictors of binding and transcript destabilization by Pum orthologs, PUM1 and PUM2, in human cells (Wolfe et al. 2020). We began by investigating the relationship of these features in the 3′ UTRs to the Pum RNAi KD differential expression results in Drosophila cells. Each feature was assessed in relation to the Wald statistic for change in transcript abundance, which provides similar information to the log fold changes but incorporates statistical robustness of the result in a single-number summary for each gene.
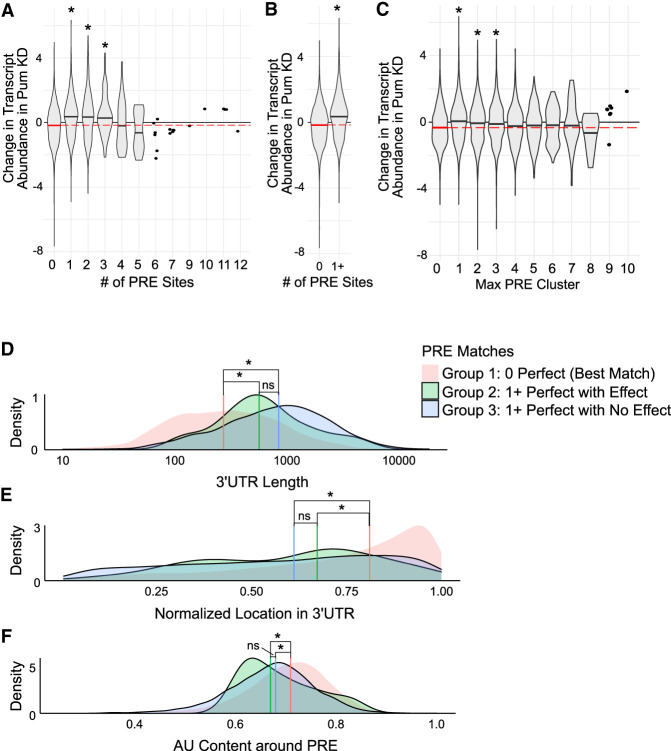
First, we examined the relationship between the number of PREs in the 3′ UTR of a transcript and regulation by Pum. The abundance of transcripts with up to three PREs significantly increased in response to Pum KD (Fig. 6A), consistent with direct repression by Pum. Transcripts with four to nine PREs were associated with a decrease in median RNA abundance, but these differences were not statistically significant and the number of transcripts with four or more PREs comprised <0.7% of all transcripts considered, compared to 21.1% of transcripts with one or more PRE sites (detailed in Supplemental Table S4). Because low numbers reduce the power of the Wilcoxon rank-sum test, we repeated the test to compare transcripts with zero perfect PRE sites to all transcripts that had one or more PRE sites present in the 3′ UTR. In this case, we found that the overall presence of one or more PRE sites led to a significant increase in RNA abundance upon Pum depletion (Fig. 6B).
FIGURE 6.
Functional associations between PRE features and differential gene expression in response to knockdown of Pum. (A–C) Density distributions of log fold changes (Wald statistic) in transcript abundance in Pum KD for transcripts (A) with different numbers of 3′-UTR PREs sites, (B) with or without a PRE site in the 3′ UTR, and (C) with different maximum numbers of 3′-UTR PRE sites clustered within a 100-nt window. Density medians are indicated with black lines within each violin. The null median is shown as a dashed red line across the width of each panel. Statistical significance (P-value ≤ 0.05, Wilcoxon rank-sum test) is indicated by an asterisk above each violin. The number of transcripts in each violin plot is reported in Supplemental Table S4. (D–F) Overlapping density distributions of (D) 3′-UTR length, (E) the normalized location of a 3′-UTR PRE, and (F) the %AU content within a 100-nt window of 3′-UTR PREs, for genes that had no perfect PREs (red), genes that had at least one PRE and passed the significance thresholds (LFC ± log2(1.3) and q-value ≤ 0.05) (green), and genes that had at least one PRE but did not pass the significance thresholds (blue). Density medians are indicated by their corresponding color-coded vertical line. Statistical significance (P-value ≤ 0.05, Wilcoxon rank-sum test) is indicated by an asterisk (ns = “not statistically significant”, i.e., P-value > 0.05).
We next examined the potential functional relationship of clustering of PREs in 3′ UTRs. PRE clustering was assessed within a sliding window of 100 nt. The maximum number of clustered PREs showed a statistically significant increase in the median RNA abundance in response to Pum KD up to three clustered PREs, but not when there were four or more clustered PREs (Fig. 6C), possibly due to the same issues with small sample size noted above (Supplemental Table S4).
We examined three additional sequence-context features of interest that might modulate the effects of PREs on transcript stability: 3′-UTR length, the length-normalized location of PRE sites, and the percent AU sequence content surrounding PRE sites. For this analysis, we divided transcripts into three groups defined in Table 5, based on the presence versus absence of PREs and of a significant impact of Pum knockdown on transcript stability. We then tested whether there were differences in the median values of the three groups for each feature. The PRE-containing mRNAs (groups 2 and 3) had significantly different median values for 3′-UTR length (Fig. 6D), PRE location (Fig. 6E), and AU content (Fig. 6F), relative to the median value of these features for mRNAs without a PRE (group 1). The results show that PREs tend to occur in longer 3′ UTRs (Fig. 6D), toward the 3′ end (Fig. 6E), and in a context with slightly less than average AU content (Fig. 6F). However, between the groups that contained PREs and either were significantly impacted by Pum depletion (group 2) or were not (group 3), there was no significant difference in median values for any of the three sequence-context features enumerated above. Therefore, this analysis did not detect a statistically significant role of 3′-UTR length, PRE location, or AU content as determinants of Pum-mediated repression in Drosophila cells.
TABLE 5.
Transcript group criteria for feature evaluation
| Group | Characteristics | Definition |
|---|---|---|
| 1 | 0 Perfect | Transcripts that had no perfect PRE sites present in the 3′ UTR |
| 2 | 1 + Perfect with effect | Transcripts that had at least one perfect PRE site present and the differential expression showed a significant effect (LFC ≥ log2(1.3), q-value ≤ 0.05) |
| 3 | 1 + Perfect with no effect | Transcripts that had at least one perfect PRE site present but the differential expression showed no significant effect (LFC ≥ log2(1.3), q-value ≤ 0.05) |
Defining criteria for the transcript groups 1, 2, and 3: Groups were defined based on whether or not the 3′ UTR of the transcript contained a PRE site that perfectly matched the consensus sequence 5′-UGUANAUA and whether or not the transcript appeared significantly repressed by Pum.
Direct repression of mRNAs by Pumilio does not significantly correlate with codon optimality
Codon optimality relates synonymous codon usage to the efficiency of translation and degradation of mRNAs in eukaryotic organisms (Bae and Coller 2022; Wu and Bazzini 2023). We considered the possibility that the stability of a transcript specified by its codon content may modulate its response to Pum-mediated mRNA decay. We investigated the potential relationship using previously established Drosophila codon stabilization coefficients (Burow et al. 2018) to bin transcripts based on their overall percentage of optimal codons (Supplemental Table S7). Consistent with the destabilizing effect of low codon optimality, our control RNA-seq data showed that transcripts with low optimality generally have lower abundances than those with high optimality (Supplemental Fig. S8).
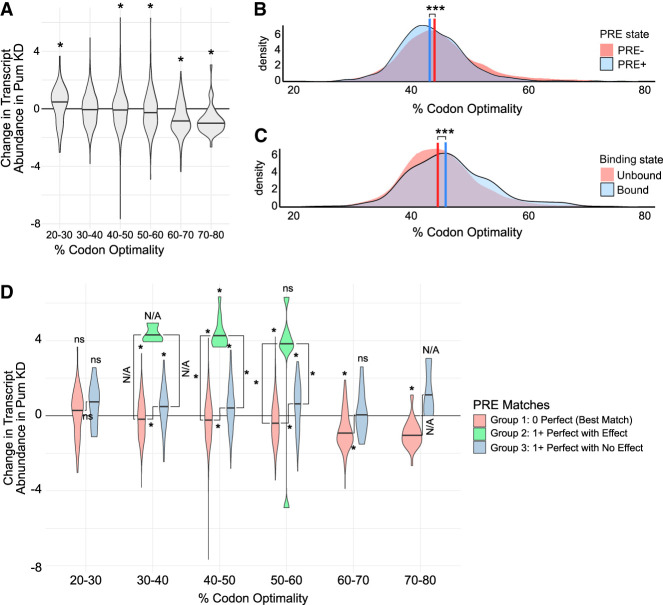
We analyzed the relationship of optimal codon content of transcripts to their response to Pum depletion, measured by the Wald statistic. Overall, codon optimality showed a linear relationship with changes in RNA abundance caused by Pum KD (Fig. 7A): mRNAs with a low percentage of optimal codons increased slightly, whereas those with a higher percentage of optimal codons decreased. In particular, mRNAs with 20%–30% codon optimalities had significantly increased relative abundance in the Pum KD, suggesting a potential relationship to Pum-mediated regulation. Unexpectedly, mRNAs with the highest codon optimality range (60%–80%) decreased upon Pum KD, which is inconsistent with them being direct Pum targets; we return to this observation below. We note that, in the absence of spike-in normalization, RNA-seq only measures relative, not absolute, changes in transcript abundance, as discussed in a recent review (Wolfe et al. 2019).
FIGURE 7.
Codon optimality does not significantly correlate with direct mRNA repression by Pum. (A) Density distributions as violin plots of normalized log fold changes in mRNA abundance (Wald statistic) in Pum KD for transcripts binned by codon optimality. Density medians are indicated with black lines within each violin. Statistical significance (P-value ≤ 0.05, Wilcoxon rank-sum test) is indicated by an asterisk above each relevant violin. (B) Overlapping density distributions of the codon optimality for transcripts with (PRE+, in blue) or without (PRE−, in red) perfect PREs in the 3′ UTR. Distribution medians are indicated by vertical lines: 44.9% for PRE− and 44.1% for PRE+. A statistically significant difference between the distribution median and zero is denoted by an asterisk. (***) P < 0.001, Wilcoxon signed-rank test. (C) As in B, but for mRNAs classified as unbound (red) or bound (blue) by Pum RIP-seq, based on a log2 fold enrichment ≥1.5 and a q-value ≤ 0.05. Distribution medians are indicated by vertical lines: 44.5% for unbound and 45.8% for Pum-bound. Statistical significance is denoted by an asterisk. (***) P < 0.001, Wilcoxon rank-sum test. (D) Density distributions as violin plots of normalized log fold changes in transcript abundance (Wald statistic) in Pum KD for transcripts binned by codon optimality and divided into three groups, as defined in Table 5. Density medians are indicated with black lines within each violin. A statistically significant difference between the distribution median and zero is denoted by an asterisk above the respective violin (P-value < 0.05, Wilcoxon signed-rank test). Within each % codon optimality bin, statistically significant differences between distribution medians are denoted with asterisks alongside the black lines indicating which medians were compared (P-value < 0.05, Wilcoxon rank-sum test). Here, we use “ns,” to indicate median-to-zero or median-to-median comparisons that were not statistically significant. Not assessed “N/A” indicates median-to-zero or median-to-median comparisons that lacked a large enough sample size to test robustly (n ≤ 3). The placement of “ns” and “N/A” labels follows the same convention as for asterisks.
These initial observations led us to further scrutinize the potential relationship between direct Pum-regulated target mRNAs and codon optimality. First, we considered the possibility that transcripts with low codon optimality may be enriched for Pum-binding sites. The results showed a statistically significant difference between the distributions for PRE-containing mRNAs (median codon optimality = 44.1%, n = 1163) in relation to those that lack a PRE (median codon optimality = 44.9%, n = 5929) mRNAs (P-value = 6.5 × 10−7, Wilcoxon rank-sum test) (Fig. 7B). Thus, PRE-containing mRNAs have lower codon optimalities than those that lack a PRE. Second, we explored the association of Pum-binding to codon optimality. To do so, the codon optimalities for mRNAs were plotted in relation to Pum:mRNA-binding state classified in the Pum RIP-seq data. The results showed a statistically significant difference between the distributions for Pum-bound mRNAs (median codon optimality = 45.8%, n = 398) relative to unbound mRNAs (median codon optimality = 44.5%, n = 4013) (P-value = 0.00037, Wilcoxon rank-sum test) (Fig. 7C). Thus, Pum-bound mRNAs have more optimal codons than coexpressed, unbound transcripts. Although the observed differences are statistically significant, the magnitudes are small (Δ = −0.8% for PRE+ vs. PRE− and Δ = 1.3% for Pum-bound vs. unbound) and in opposition. Importantly, the distributions in Figure 7B,C show that the bulk of transcripts fall within the 30%–60% codon optimality range, as detailed in Supplemental Table S7. Very few PRE-containing, Pum-bound mRNAs are present in genes with 20%–30% and 60%–80% codon optimality ranges. Together, the results suggest that the observed changes in mRNA abundance in the low and high codon optimality bins seen in Figure 7A are unlikely to be the result of preferential direct targeting by Pum.
To further distinguish the direct effects of Pum KD on transcripts from secondary effects, we analyzed the relationship of codon optimality for mRNAs that have PREs and either responded to Pum KD (group 2) or did not (group 3) to those that lack PREs (group 1) (as defined in Table 5). The results are shown in Figure 7D, wherein the response of each group of transcripts to Pum KD is plotted within the respective codon optimality ranges. To assess the significance of the observed differences, several statistical comparisons were made. First, the significance of changes in mRNA abundance caused by Pum KD was assessed relative to the negative control RNAi (labeled at the top of each violin plot in Fig. 7D). Second, the significance of changes in mRNA abundance was assessed between each group (indicated by brackets in Fig. 7D). Note that several statistical comparisons were not possible due to the very small number of data points and are, therefore, labeled Not Assessed (N/A) in Figure 7D.
The results showed that PRE-containing, Pum-regulated mRNAs increased in abundance to the same magnitude in the 30%–40%, 40%–50%, and 50%–60% codon optimality bins (Fig. 7D, green violins), thus the direct effects of Pum on transcript stability do not change over the 30%–60% codon optimality range. For the mRNAs in the 20%–30% codon optimality range, the changes in mRNA abundance associated with Pum KD were not statistically significant relative to the negative control RNAi condition, nor were there significant differences between those mRNAs with or without PREs (Fig. 7D). Likewise, the changes in abundances of the small number of PRE-containing mRNAs in the 60%–80% codon optimality range did not significantly change in response to Pum KD. In contrast, the mRNAs in the 60%–80% range that lack PREs (group 1) significantly decreased in relative mRNA abundance in response to Pum KD, indicating that these decreases are secondary, indirect effects (Fig. 7D, red violins). Overall, the results in Figure 7 indicate that direct Pum-mediated repression of PRE-containing mRNAs is not affected by codon optimality in Drosophila cells.
Low codon optimality does not specify the responsiveness of mRNAs to CCR4–NOT
The molecular basis of codon optimality-mediated mRNA decay remains unknown. Based on research in budding yeast, the CCR4–NOT complex has been proposed to sense low codon optimality to initiate mRNA decay (Buschauer et al. 2020; Bae and Coller 2022). However, the role of CCR4–NOT in codon-mediated mRNA decay in higher eukaryotes remains to be determined. We, therefore, interrogated the relationship of percent codon optimality to the change in transcript abundance caused by the knockdown of Not1 (Fig. 8A) and Pop2 (Fig. 8B). Neither Not1 nor Pop2 depletion significantly affected the median abundance of mRNAs with very low codon optimality (20%–30%) (Fig. 8A,B). This is contrary to the expected result: If CCR4–NOT were essential for codon-mediated decay, these mRNAs would have been significantly stabilized. We did observe significant, albeit small in magnitude, increases in abundances of mRNAs with 40%–50% optimal codons in the Not1 KD and 30%–50% for Pop2 KD. Overall, our results do not support the hypothesis that CCR4–NOT is essential for the degradation of mRNAs with low codon optimality.
FIGURE 8.
Low codon optimality does not confer the responsiveness of mRNAs to CCR4–NOT. (A) Density distributions of normalized log fold changes in mRNA abundance (Wald statistic) caused by Not1 KD for transcripts binned by their content of optimal codons. Density medians are indicated with black lines within each violin. Statistical significance of the changes, measured relative to negative control NTC RNAi condition (P-value ≤ 0.05, Wilcoxon rank-sum test) is indicated by an asterisk above each relevant violin. (B) As in A, for Pop2 knockdown data. (C) GO terms showing significant mutual information with genes sorted by the content of optimal codons, calculated using iPAGE, are listed on the right. The color of each cell shows the magnitude of the P-value for the significance of the enrichment (red) or depletion (blue) at that cell, scale is shown on the left-hand side of the figure; black bordered cells individually show P-value ≤ 0.05 after Bonferroni correction across their row.
Unexpectedly, transcripts with high codon optimality (50%–80%) were significantly decreased in relative abundance by knockdown of Not1 or Pop2, especially in the 60%–70% and 70%–80% bins (Fig. 8A,B). This effect is inconsistent with the role of CCR4–NOT in mRNA decay. Curiously, this effect mirrors that observed with Pum KD (Fig. 7A,C). Because no other evidence or mechanism supports a direct role of Pum and CCR4–NOT in stabilizing mRNAs with optimal codons, we searched for an alternative explanation by analyzing the characteristics of those affected genes. First, relatively few genes are contained in this range: 195 genes in the 60%–70% optimal codons range and 44 genes in the 70%–80% bin (Supplemental Table S7). Second, these genes are highly enriched for GO terms related to cytoplasmic translation and metabolism (Fig. 8C). The cytoplasmic translation GO term was identical to that detected in the genes that were coordinately down-regulated by Pum + Not1 + Pop2 knockdowns (Fig. 5D, “Down in all 3 KD”). When we compared the genes in the “Down in all 3 KD” category to those with 60%–80% optimal codons, we found 36 in common (Supplemental Table S7). Those genes encode 34 protein subunits of the large and small ribosomal complexes and two translation factors, poly(A)-binding protein and Tma7. Collectively, these results and the GO term enrichment analysis in Figure 5 lead us to speculate that the up-regulation of negative growth regulators and starvation response factors seen in the Pum, Not1, and Pop2 KDs (Fig. 5D) may in turn down-regulate the translation machinery, which is encoded by mRNAs with high codon optimality. In this model, the decreased abundance of translation machinery mRNAs is a secondary effect coincident with their high codon optimality.
Computational modeling of Pumilio repression
We applied linear regression modeling to assess which features combined to contribute to Pum-mediated regulation of mRNA levels, fitting the Wald statistics from the Pum KD data set as a function of potentially informative transcript features. We used a leave-one-out analysis to determine the relative importance of the following eight features: (1) Pum binding, (2) PRE count, (3) 3′-UTR length, (4) max clustered PREs, (5) AU content, (6) percent codon optimality, (7) PRE location, and (8) normalized PRE location. The Bayesian information criterion (BIC) was used to compare the performance of the models. The first six features were found to be informative, and the BIC for the six-feature model was better than the model using all eight features, with an approximate 11-point drop in BIC. The informative features for the Pum KD data, in descending order of impact upon removal from the model, were Pum-binding (RIP-seq log2 fold enrichment) values (ΔBIC = 112.57), AU content around the PRE site (ΔBIC = 80.77), codon optimality (ΔBIC = 53.09), number of perfect PRE sites (ΔBIC = 43.61), the number of PRE sites clustered together (ΔBIC = 27.22), and 3′-UTR length (ΔBIC = 18.19) (Fig. 9). We also tested a more complex generalized additive model (GAM) with the same six features but found that it produced a less informative model. In both models, the percent variance in the expression that was data explained by the input features was between 11% and 12% (derived from the adjusted R-squared value), indicating that one or more unknown features contributing to Pum-mediated regulation are missing from the model. The small number of significantly impacted genes captured in the Pum KD data set may also contribute to this limitation; we address this point further in the Discussion. It is important to note that although the analysis in Figure 7 indicates that codon optimality does not modulate the direct repressive activity of Pum, the nature of the model fitted here predicts the effects of Pum KD on both direct and indirect Pum regulation, and codon optimality is indeed informative for indirect effects of Pum KD (Fig. 7D).
FIGURE 9.

Computational modeling of Pum-mediated regulation of mRNAs identifies informative features. Leave-one-out analysis of a linear regression model applied to six influential features of Pum repression, labeled on the x-axis. The information contributed to the model by each feature was quantified with the change in the Bayesian Information Criterion (ΔBIC), where the difference was calculated as BIC1 left out − BICfull model, resulting in a positive ΔBIC value for informative features in the model.
DISCUSSION
Drosophila Pum has long served as an archetype of an RNA-binding regulator with crucial biological functions (Arvola et al. 2017). This study identifies new transcripts that are up-regulated in response to Pum depletion and deletion. By integrating multiple criteria, we winnowed that list to define high-confidence, functionally repressed, direct Pum target mRNAs. The functions of proteins encoded by these Pum-repressed target genes provide important insights into the regulatory roles of Pum (Table 3). We found that Pum represses genes involved in neural, muscle, and germ cell development. Pum-repressed targets also function in wing morphogenesis, cellular proliferation, and differentiation. Components of the DNA replication (e.g., cdc7, RnrS), lipid (e.g., Gpdh1), and sphingolipid metabolism (e.g., α4GT1, ORMDL), and transposable element suppression pathways (e.g., papi) are directly repressed by Pum. Key components of signaling pathways are repressed by Pum including Wnt (e.g., dnt), TGF-β (e.g., tkv), and MAPK/ERK (e.g., Raf). Strikingly, four Notch signaling components are direct Pum targets (amx, bib, numb, Tsp3A). A previous study provides additional evidence for Pum-mediated repression of four targets (bib, RnrS, Kap-alpha1, and CG4281) in embryonic neural progenitors (Burow et al. 2015). Future work should explore the functional role of Pum regulation of the high-confidence targets in vivo, in the context of development and physiology. Intriguingly, some of the same Pum-repressed signaling pathways identified in our study (Wnt, TGF-β, MAPK) are targeted by Pum orthologs in other species (Bohn et al. 2018; Goldstrohm et al. 2018). For example, human and Caenorhabditis elegans Pum orthologs regulate MAPK/ERK signaling in stem cells (Lee et al. 2007).
Pum repression of Raf is relevant to the shared role of these regulators in wing morphogenesis. Pum was previously shown to negatively affect MAPK/ERK signaling by epidermal growth factor receptor to control wing development (Kim et al. 2012). Our results establish that Raf is a high-confidence direct target of Pum repression, providing a means by which Pum can modulate MAPK-dependent wing development. Regulation of Raf by Pum proteins may be conserved in mammals, which have three Raf homologs (ARAF, BRAF, RAF1). The ARAF mRNA has two PREs in its 3′ UTR and was reported to be bound by Pum proteins in both human and mouse, whereas RAF1 and BRAF do not have PREs, although the association of BRAF mRNA with human PUMs has been reported (Galgano et al. 2008; Zhang et al. 2017; Bohn et al. 2018).
Although our approach successfully identified new directly regulated Pum targets, the list of significantly repressed RNAs is modest compared to the breadth of transcripts that contain PRE(s) or are bound by Pum in embryos. We consider several relevant factors that likely effect this outcome. First, Pum may repress some mRNAs by additional mechanisms, such as inhibition of translation in lieu of mRNA decay (Weidmann et al. 2014; Arvola et al. 2017), which would not be apparent in our analysis. Second, the endogenous level of Pum in WT cultured cells may be at a limiting threshold, in which case mRNAs could escape repression unless the level of active Pum became elevated. In support of this possibility, we previously showed that overexpression of Pum strengthened repression of target mRNAs in a dose-dependent manner (Weidmann and Goldstrohm 2012; Arvola et al. 2020; Haugen et al. 2022). Third, Pum activity may be modulated by a factor(s) that is absent from our experimental system. For example, the Nanos RBP can promote Pum-mediated repression but is expressed at an exceedingly low level in DL1 cells (Supplemental Table S2, nos; Weidmann et al. 2016; Arvola et al. 2017). Fourth, we considered that residual Pum in the knockdown condition might be sufficient to maintain repression of some RNAs. To address this issue, we created Pum knockout cell lines and measured differential gene expression relative to WT cells. As anticipated, substantially more genes were significantly differentially expressed in the Pum knockout cells, and although many of those contain PREs (including bona fide direct Pum targets like hunchback and Raf), the PRE was not overrepresented in the up-regulated transcripts. We suspect that adaptive changes in gene expression may have occurred during clonal isolation of the Pum knockout cells that buffer the loss of Pum activity. We also found evidence of residual Cas9 expression in these cells, which could contribute to off-target effects (Guo et al. 2023). For future analysis of regulated mRNA decay, a rapid, efficient, and transient means of depletion of a regulatory protein would be ideal, such as conditional degrons.
It is important to acknowledge that the magnitude of effects from any single RNA-binding factor on transcript abundances is often modest. Such is the case for repression by microRNAs (Eichhorn et al. 2014). For bona fide Pum targets, previous studies have repeatedly demonstrated increases in transcript abundance on the order of 30% (Van Etten et al. 2012; Weidmann and Goldstrohm 2012; Bohn et al. 2018; Enwerem et al. 2021), and in the present work we found that the newly identified high-confidence Pum target Raf has a ∼1.6-fold increase in transcript level upon Pum KD (Fig. 2). It is likely that many transcripts affected by Pum-dependent regulation are missed because of small effect sizes. Additional evidence for this notion comes from the fact that bulk analysis across changes in transcript abundance without imposing statistical significance and fold change cutoffs (as, e.g., in the FIRE-based motif analysis of Fig. 1E and gene set enrichment analyses in Fig. 5) reveals strong, systematic, and meaningful Pum effects across a much broader range of transcripts than the set which can be confidently identified as direct Pum-repressed targets (Table 3).
Our data provide insight into mRNA features that contribute to repression by Pum. Foremost, the location, number, and clustering of PREs in a transcript are functionally important. In particular, PREs located in the 3′ UTR are significantly correlated with repression by Pum, as is the number and clustering of PREs up to three sites. Notably, we also observed functional relationships between the location, number, and clustering of PREs to repression by human Pum proteins (Bohn et al. 2018; Wolfe et al. 2020). Our computational modeling of Pum repression emphasizes six important functional determinants. Three relate to the Pum:RNA interaction, including evidence of Pum binding, the number of perfect PRE sites, the number of PRE sites clustered together, and PRE location. The fourth, length of 3′ UTR, may relate to the propensity of longer UTRs to contain more regulatory sequences and structures than shorter ones. The fifth, surrounding AU content of PREs, may reflect the reduced stability of AU base pairs relative to GC-rich sequences. Such AU content may facilitate the accessibility of functional PREs. The sixth determinant, percent codon optimality, is discussed below and likely encompasses indirect effects of Pum. The modeling also indicates that additional determinants remain to be identified to accurately predict the regulation of a gene by Pum without functional data. This remains a universal challenge for the gene regulation field. We speculate that collaborative activities of other modes of regulation (e.g., translational control), other RNA-binding factors, or RNA structural features, which remain to be discovered, may help specify targets of Pum-mediated repression.
Pum repression is mediated by direct recruitment of the CCR4–NOT deadenylase complex (Arvola et al. 2020; Haugen et al. 2022). Before this work, the Pum:CCR4–NOT repression mechanism was established using reporter mRNAs and supported by a single example of a natural target mRNA in ovaries, mei-P26 (Joly et al. 2013; Weidmann et al. 2014; Arvola et al. 2020). Our results extend the Pum:CCR4–NOT mechanism to a group of natural target mRNAs in Drosophila cells. Interestingly, roughly half of the Pum-repressed mRNAs we identified were not significantly affected by depletion of the Pop2 and Not1 CCR4–NOT components. Residual CCR4–NOT in the RNAi conditions may have been sufficient to support Pum activity. Alternatively, we speculate that Pum may repress these transcripts by another mechanism, such as decapping-mediated mRNA decay and/or antagonism of poly(A)-binding protein (Blewett and Goldstrohm 2012; Weidmann et al. 2014; Arvola et al. 2020).
Our results also emphasize the broader effect of CCR4–NOT on the transcriptome, consistent with the growing list of pathways and RNA-binding regulatory proteins that use CCR4–NOT (D'Orazio and Green 2021; Raisch and Valkov 2022). Many transcripts increased in abundance when Not1 or Pop2 were depleted, corresponding with the role of CCR4–NOT in initiating the mRNA decay. Many genes also decreased in abundance in response to CCR4–NOT depletion, likely representing secondary consequences downstream from direct CCR4–NOT targets. We observed enrichment of specific classes of CCR4–NOT-affected genes, indicating that one or more regulatory mechanisms coordinate the CCR4–NOT-mediated decay of groups of functionally related transcripts. Future analysis of CCR4–NOT-mediated mRNA metabolism is worth pursuing using new technologies for measuring dynamic changes in poly(A) tail length and RNA metabolism.
We considered the possibility that the inherent stability of a transcript, as controlled by its codon optimality, might modulate the responsiveness to Pum-mediated decay. Our results do not support a significant functional relationship between Pum-repressed direct target mRNAs and their codon content. This was surprising because we previously observed that mRNAs with low codon optimality are more likely to be susceptible to degradation by Pum orthologs, PUM1 and PUM2, in human cells (Wolfe et al. 2020). Several considerations in the current study may have limited our ability to detect significant codon-level effects. Foremost, relatively few mRNAs were regulated by Pum KD in the Drosophila cells in comparison to the hundreds of mRNAs regulated by human PUM1 and PUM2. Second, our analysis measured changes in steady-state mRNA levels, whereas Wolfe et al. measured mRNA decay kinetics. That said, an in vivo study supports our conclusion: Although Pum target mRNAs had significantly faster decay rates than nontargets in Drosophila neurons, no significant relationship to codon optimality was detected (Burow et al. 2018).
Presently, the molecular mechanism of codon optimality-mediated decay remains to be elucidated. Evidence from yeast indicates that the CCR4–NOT complex is a central player (Buschauer et al. 2020; Bae and Coller 2022; Wu and Bazzini 2023), but that model is untested in higher eukaryotes. Our results do not support a crucial role for CCR4–NOT in codon-mediated decay. Although depletion of Not1 or Pop2 stabilized many transcripts, there were either no, or minor, effects on mRNAs with low to average codon optimality. Future research will be necessary to elucidate the precise mechanism of codon-mediated mRNA degradation in metazoans, and the Drosophila will be a powerful model system in which to do so.
MATERIALS AND METHODS
Cell culture
This study used DL1 cells (Drosophila Genomics Resource Center) (Schneider 1972), which were grown at 25°C in Schneider's Drosophila medium (SDM, Gibco) supplemented with glutamine (1× GlutaMAX, Gibco), 1× antibiotic–antimycotic containing 100 units/mL of penicillin, 100 µg/mL of streptomycin, and 0.25 µg/mL of Amphotericin B (Thermo Fisher), and 10% heat-inactivated fetal bovine serum (FBS, GenClone).
Generation of DL1 Pum-myc and V5-Raf cell lines
A myc epitope tag was engineered onto the C terminus of the pumilio (FBgn0003165) coding region using CRISPR–Cas9 and homologous recombination in the DL1 cell line. A guide RNA site targeting exon 13 was identified using Benchling CRISPR RNA guide software. The single guide RNA plasmid pAc sgRNA Cas9 Not1 was created by inserting annealed primers RJH284 Pum exon 13 sg1 Fwd 5′-ttcgAGGAAATAACAAATTAAGCC and RJH285 Pum exon 13 sg1 Rev 5′-aacGGCTTAATTTGTTATTTCCTc into the BspQ1 site (Bassett et al. 2013; Haugen et al. 2022). Note that the 5′ “ttc” or “aac” (in lowercase) are BsqQI cohesive overhangs and the g:c base pair was included as part of the U6 promoter. To integrate the tag, a single-stranded homology-directed repair donor template (IDT) containing Pumilio homology arms (in capital letters), a cleavage site for the human rhinovirus (HRV) 3C protease (in lowercase italics), a myc epitope tag (lowercase underlined), and in-frame stop codon (bold, capital letters) was created with the following sequence: Pum-myc ssODN: 5′-ACAGCAGCTTGGGTCCCATTGGACCCCCGACCAACGGCAACGTTGTGctggaggtgctgttccagggccccgaacaaaaactcatctcagaagaggatctgTAAAGGAAATAACAAATTAAGCCAAGCAGTCAAAGGAAACTTCTTTCTCGAATCGCAGTATAGTTTTTAGAAGCTGTAGAGCTTAACATAAACAACAAG.
DL1 cells (2 × 106 per well) were plated in 2 mL of SDM in one well of a 6-well plate. After 24 h, cells were transfected with 4 µL FuGene HD (Promega), 1 µg sgRNA-Cas9 plasmid DNA, 40 pmol ssODN template, and 100 µL serum-free SDM. After incubation for 48 h at 25°C, the medium was replaced with fresh SDM containing 5 µg/mL puromycin (Gibco). Seventy-two hours later, when cells approached confluence, they were expanded into a 10-cm dish and allowed to continue growing to 80% density. Clonal lines were then isolated by limiting dilution. The Pum-myc cell line was identified by western blot detection with anti-myc (Fig. 1) and confirmed by PCR amplification and sequencing of Pum exon 13 from genomic DNA (Supplemental Fig. S1).
To measure the effect of Pum on Raf protein expression, the Pum-Myc DL1 cell line was used to create the V5-tagged Raf cell line. The Raf gene (FBgn0003079) encodes two transcript variants, both of which code for identical proteins. A single-guide RNA plasmid pAc sgRNA Cas9 Raf sg1 was created by inserting annealed primers RJH 327 Raf sg1 Fwd 5′-ttcgTAGATCGCTGTCGCCTTCGG and RJH 328 Raf sg1 Rev 5′-aacCCGAAGGCGACAGCGATCTAc into the BspQ1 site. Note that the 5′ “ttc” or “aac” (in lowercase) are BsqQI cohesive overhangs and the g:c base pair was included as part of the U6 promoter. The V5 tag was integrated onto the N terminus of the Raf coding region, just after the translation initiation codon by cotransfecting the V5-Raf ssODN oligo: 5′-TTCGGGGTCATGGTCACAGCGCATAGTATATAGGATAAAGCAACACCATGggtaagcctatccctaaccctctcctcggtctcgattctacgTCCAGCGAGTCCAGCACCGAAGGCGACAGCGATCTATACGATCCTTTGGCCGAGGAGCTGCACAACGTCCAGCTCGTCAAACATGTGACCCGCGAGAATATTGATGCC. The V5 tag is indicated by underlined, lowercase text and the translation initiation site for Raf in bold text. A homozygous V5-Raf clone was isolated and verified by genotyping, sequencing, and western blot analysis (Fig. 2; Supplemental Fig. S5). The following PCR primers that span the exon 3–intron 3 junction, and encompassing the site of V5 tag integration, were used for genotyping and sequencing: RJH323 Raf ex3 Fwd 5′-GCTTGCAAGTGTGTGGG and RJH324 Raf in3 Rev 5′-GGTAGTGTTCAGCTCGGC.
Pumilio knockout cell lines
Two homozygous pumilio knockout DL1 lines were used in this study. The strategy and tools for generating the CRISPR–Cas9-induced indels and the details of the first clonal line were described in Haugen et al. (2022). That clonal line (designated Pum KO2 herein) contained a homozygous 10 bp deletion in pumilio exon 9, which causes a frameshift after methionine 726 that creates a truncated, nonfunctional 765-amino acid protein that lacks the RNA-binding domain. Here, we report an additional homozygous knockout clonal line. This pumilio knockout was verified by sequencing a PCR product spanning exon 9 that was amplified from genomic DNA using primers RJH 191 Pum exon 9 fwd primer 5′-AACTGTTTCGCTCGCAGAATCCG and RJH 192 Pum exon 9 rev primer 5′-TGATACGGCTGATTCTCGGCACC (Supplemental Fig. S3A). This new pumilio knockout line (designated Pum KO1) has a 20 bp deletion of the pumilio CDS and insertion of 81 additional base pairs, resulting in a net gain of 61 bp (Supplemental Fig. S3B,C). Therefore, the resulting frameshift after glutamine 725 in this mutant pumilio gene adds eight amino acids followed by a stop codon (Supplemental Fig. S3D). This 733-amino acid protein would be nonfunctional due to the absence of the C-terminal RNA-binding domain. Additionally, the mRNA produced would be subject to nonsense-mediated mRNA decay, consistent with our observation of reduced pumilio mRNA in the knockout cells relative to WT (Supplemental Fig. S3E). The RT-qPCR assay and primers for exons 9 and 11 were previously reported (Haugen et al. 2022).
RNA interference
RNAi was performed in Pum-myc-tagged DL1 cells. Double-stranded RNA (dsRNA) corresponding to Pum, Not1, and Pop2 were designed using the SnapDragon web-based tool provided by the Harvard Drosophila RNAi Screening Center (https://www.flyrnai.org/snapdragon) to minimize potential off-target regions. The effectiveness of these dsRNAs was previously established (Van Etten et al. 2012; Weidmann and Goldstrohm 2012; Arvola et al. 2020). Templates for in vitro transcription were PCR-amplified with primers that add opposing T7 promoters to each DNA strand. The NTC dsRNA, corresponding to the E. coli lacZ gene, was described previously (Weidmann and Goldstrohm 2012). Primers for generating the Pum, Not1, Pop2, and LacZ dsRNAs were previously described (Weidmann and Goldstrohm 2012; Arvola et al. 2020) including:
T7 LacZ F: 5′-GGATCCTAATACGACTCACTATAGGGTGACGTCTCGTTGCTGCATAAAC,
T7 LacZ R: 5′-GGATCCTAATACGACTCACTATAGGGGGCGTTAAAGTTGTTCTGCTTCATC,
T7 Pum F: 5′-GGATCCTAATACGACTCACTATAGGGGTCAAGGATCAGAATGGCAATCATGT,
T7 Pum R: 5′-GGATCCTAATACGACTCACTATAGGGCTTCTCCAACTTGGCATTGATGTGC,
T7 Not1 F: 5′ GGATCCTAATACGACTCACTATAGGGCAAGGACTTCGCCCTGGATG,
T7 Not1 R: 5′-GGATCCTAATACGACTCACTATAGGGCATTTGGCTGAGACAAATCCGTCG,
T7 Pop2 F: 5′-GGATCCTAATACGACTCACTATAGGGGACACCGAGTTTCCAGGCG,
T7 Pop2 R: 5′-GGATCCTAATACGACTCACTATAGGGAAGAAGGCCATGCCCGTCAGC.
The dsRNAs were transcribed from these templates using a HiScribe T7 high-yield RNA synthesis kit (New England Biolabs). The dsRNAs were then treated with DNase and purified using RNA Clean & Concentrator-25 (Zymo Research).
For RNAi experiments, cells (3 × 106 in 1 mL of serum-free SDM per well of a 6-well dish) were bathed with 20 µg of dsRNA corresponding to either Pum, Not1, Pop2, or LacZ NTC for 60 min. Then 2 mL of SDM containing 5% heat-inactivated FBS was added to each well. After 48 h, the cells were harvested by centrifugation and suspended in 2 mL phosphate buffered saline (PBS). RNA was purified from 1.5 mL of the cells, and the remaining 0.5 mL was reserved for western blot analysis.
Western blotting
Cells (∼7.5 × 105) were harvested by centrifugation at 900g for 4 min and lysed in radioimmunoprecipitation assay (RIPA) buffer (25 mM Tris-HCl pH 7.6, 1 mM EDTA, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) containing 2× complete protease inhibitor cocktail (Roche). Lysates were then cleared of cellular debris by centrifugation at 21,000g for 10 min. The protein concentration of the supernatant was measured using the detergent-compatible (DC Lowry) protein assay kit according to the manufacturer's directions (Bio-Rad). For each sample, 20 µg of total protein extract was combined with an equal volume of 2× SDS loading buffer and then heated at 85°C for 10 min. Samples were then separated by SDS-PAGE and blotted to Immobilon P membranes (Millipore). Membranes were blocked for 1 h and then the primary antibody (as indicated in Figs. 1A and 2A,C) was applied for 1 h at room temperature or overnight at 4°C on a rocking platform. Antibodies, their dilution factor, and buffer condition are listed below. Membranes were washed three times for 10 min, and then horseradish peroxidase (HRP)-linked secondary antibody was applied for 1 h at room temperature. After three washes of 10 min each, the chemiluminescent substrate was added to the membrane, which was then imaged with a ChemiDoc Touch instrument (Bio-Rad).
Antibodies
The following antibodies were used for western blot analysis at the indicated dilutions in either blotto (1× PBS containing 10 mM Na2HPO4 and 1.8 mM KH2PO4 at pH 7.4, 137 mM NaCl, 2.7 mM KCl, 0.1% tween-20, and 5%, w/v, nonfat powdered milk) or TBST (1× Tris-HCl buffered saline containing 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% tween-20, and 5%, w/v, bovine serum albumin), as specified by the antibody's manufacturer.
Mouse anti-Tubulin (Cell Signaling Technologies 3873) at 1:1000 in blotto.
Rabbit anti-Myc (Cell Signaling Technologies 2278S) at 1:5000 in TBST.
Mouse anti-V5 primary antibody (Invitrogen R960-25) at 1:5000 dilution in blotto.
Goat anti-rabbit-HRP secondary antibody (Cell Signaling Technologies 7074P2 or Sigma AP187P) at 1:5000 in blotto.
Goat anti-mouse-HRP (Thermo Fisher 31430) at 1:5000 in blotto.
Quantitative western blotting
To measure the effect of Pum knockdown on endogenous Raf protein levels (Fig. 2), RNAi of Pum was performed using Pum-myc, V5-Raf DL1 cells. The addition of a V5 tag to Raf was necessary because an antibody to Raf was not available. The cells were plated in 6-well plates at a density of 1 × 106 cells/mL. To initiate RNAi, cells were bathed with 10 µg of Pum dsRNA, or NTC LacZ dsRNA, for 1 h in 1 mL of serum-free medium. Thereafter, the medium was exchanged for 2 mL complete serum-containing medium, and cells were incubated at 25°C for 90 h. Cells were then harvested by removing the medium and washing the cells with 1× PBS. The cells were lysed in 150 µL RIPA buffer with 2× complete protease inhibitor cocktail (Roche) for 10 min on ice, followed by mechanical disruption using a sterile pellet pestle for 20 sec. Cellular debris was then removed by centrifugation for 10 min at 21,000g at 4°C. The supernatant was then collected, and the total protein concentration was determined by DC Lowry assay.
For quantitation of V5-Raf protein levels, total protein was analyzed by SDS-PAGE for the NTC samples (5, 10, 15, 20, and 25 µg) and Pum RNAi samples (5, 10, 15, and 20 µg), as indicated in Figure 2. The linear detection range of titration of total protein for the cell extracts was established in optimization experiments (not shown). For each biological replicate, the titrations of total protein were separated on 12% SDS-PAGE gel followed by blotting to Immobilon PVDF membrane (Millipore). Membranes were dried and total protein was stained with Sypro Ruby (Thermo Fisher) and imaged. Blots were then washed three times in blotto for 1 h to block and remove the stain. The blots were then probed with anti-V5 antibody overnight at 4°C. The blots were then washed three times with blotto, for 5 min per wash, and then were probed with HRP-labeled goat anti-mouse antibody for 1 h at room temperature. Blots were then washed twice with blotto and then 1× TBS. After incubation with Immobilon ECL substrate (Millipore), the blots were imaged using a ChemiDoc Touch.
Western blot images were analyzed using Image Lab software (Bio-Rad). We performed three independent experiments, each with three biological replicates, and measurements were obtained for four amounts of cell extract (5, 10, 15, and 20 µg of total protein), for a total of 36 measurements. For each sample, the measured amount of V5-Raf per band was normalized to the total protein in that lane, which was measured before sample loading by DC Lowry assay and on the membrane by staining with Sypro Ruby. We then determined the fold change in normalized V5-Raf protein level in the Pum RNAi condition relative to NTC (Pum KD/NTC) for the same amount of total protein on the same western blot. For example, the V5-Raf band volume for the 5 µg Pum KD sample was compared to the equivalent 5 µg NTC sample on the same blot from the same biological replicate.
Click-seq
Click-seq libraries were generated following the established method (Routh et al. 2015, 2017). RNA was purified using the Maxwell RSC simply RNA tissue extraction kit (Promega) with on-bead DNase I digestion. The RNA concentration was determined using a NanoDrop spectrophotometer. RNA samples were submitted for Agilent TapeStation analysis, and RNA integrity number (RIN) values were 8.7–10. Total RNA was poly(A) selected using the NEBNext Poly(A) mRNA Magnetic Isolation Module (New England Biolabs).
Reverse transcription (RT) was performed in a 20 µL reaction following the manufacturer's protocol with Superscript III Reverse Transcriptase (Invitrogen) with the following components: 2 µg RNA, 1 µL of 5 mM AzVTP:dNTPs (at a ratio of 1:5 azido-nucleotides AzATP, AzCTP, AzGTP [AzVTP] to dNTPs), 1 µL 50 µM Illumina 6N p7 adapter: 5′-GTGACTGGAGTTCAGACGTGTGCTCTTCCGATCTNNNNNN, 5× Superscript First Strand buffer, DTT, and RNase OUT (Invitrogen). The RNA template was removed after cDNA synthesis by incubating for 20 min at 37°C with 10 units RNase H (New England Biolabs). Azido-terminated cDNA was purified using a Clean and Concentrator kit (Zymo Research) and eluted in 10 µL of 50 mM HEPES buffer, pH 7.2.
To form the click-adapter-linked cDNA, 10 µL of cDNA was incubated for 30 min at room temperature with 20 µL DMSO, 3 µL of 5 µM click-adapter (5′ Hexynyl-12(N)AGATCGGaaGAGCGTCGTGTAGGGaaAGAGTGTAGATCTCGGTGGTCGCCGTATCATT), 0.4 µL premixed 50 mM vitamin C and 2 µL of 10 mM Cu-TBTA (Lumiprobe). The alkyne-azide cycloaddition of the adapter was catalyzed twice, then the click-linked cDNA was purified with a DNA Clean and Concentrator kit (Zymo Research).
To anneal the remaining Illumina adapters (indexing primer CaaGCAGaaGA CGGCATACGAGATnnnnnnGTGACTGGAGTTCAGACGTGT, where nnnnnn is the index sequence, and universal primer aaTGATACGGCGACCACCGAG), PCR reactions were set up using 2.5 µL each of 5 µM primers, 5 µL click-ligated cDNA, 25 µL 2× OneTaq Standard Buffer Master Mix (New England Biolabs) in 50 µL total reactions. Optimized cycling parameters were 4 min at 94°; 30 sec at 53°; 10 min at 68°; 30 sec at 94°; 30 sec at 53°; 2 min at 68° × 20–22 cycles; 5 min at 68°. Amplicons were size selected at 200–300 bp on a 2% agarose gel, and then gel purified with the Gel DNA recovery kit (Zymo Research). Final yields were determined with a QuBit fluorometer (Thermo Fisher). Sequencing was performed on pooled samples using single-end 75-bp reads on the NextSeq 500 (Illumina) with a high-density v2.5 flow cell at the University of Texas Medical Branch Next Generation Sequencing Core Facility.
Click-seq data processing
For the Click-seq samples (Routh et al. 2017; Elrod et al. 2019), sequencing adapters were trimmed and unique molecular identifiers (UMIs) annotated using fastp (version 0.14.1) (Chen et al. 2018), and then reads were aligned to the UCSC dm6 genome using hisat2 (version 2.1.0) (Kim et al. 2019). The alignments were then deduplicated using UMI-tools (version 1.0.1) (Smith et al. 2017) and differential expression was analyzed using DESeq2 (version 1.23.110) (Love et al. 2014) and featureCounts (Rsubreads 1.30.9) (Liao et al. 2014). Significance calling was based on adjusted P-values (P-adj) and a biological significance cutoff of 1.3-fold change. RNA-seq data were deposited to the Gene Expression Omnibus as entry GSE241940.
Identification of PRE-containing genes
FlyBase release 6.38 (February 2021) was used for gene annotations of CDSs, 3′ UTRs, 5′ UTRs, ncRNAs, miRNAs, and miscRNAs (Supplemental Table S1). PRE processing was performed in R using the seqinR package. Data were then exported to Excel, and gene matching based on FBgn, gene name, or gene locus was performed to assign PRE numbers to each gene from our RNA-seq data sets. Transcripts in RNA-seq data sets that could not be matched to any of the above-mentioned FlyBase sequences were discarded; these included but were not limited to genes with withdrawn gene status, pseudogenes, some tRNAs, and mitochondrially encoded genes.
RIP-seq
The Pum RIP experiment used 0- to 3-h-old embryos with three biological replicates. Immunoprecipitation of Pum was carried out as previously described (Laver et al. 2015). The synthetic antibodies, Fab Pum-4 and negative control Fab C1 (Na et al. 2016), were used for Pum RIP and control-RIP, respectively. Fab C1 is against human EGFR and has been used in our previously published RIP experiments (Laver et al. 2013, 2015, 2020). Immunoprecipitated RNA and total RNA were extracted using TRIzol (Invitrogen) following the manufacturer's protocol. The concentration and quality of the RNA samples were checked with Qubit RNA HS Assay Kits (Thermo Fisher Scientific) and an Agilent Bioanalyzer.
Ribosomal RNA was depleted using the following approach. All D. melanogaster 5S, 5.8S, 18S, 28S rRNA, and 7SL RNA sequences were downloaded from FlyBase (http://ftp.flybase.net/releases/FB2018_04/dmel_r6.23/fasta/). Each isoform sequence was split into multiple ∼60-nt DNA oligos covering the entire length of its reverse complement strand without overlap. The oligo probes were chemically synthesized by Integrated DNA Technologies Inc. We pooled together equimolar amounts of each of these oligonucleotides to generate oligo probe mixes used for rRNA depletion (Supplemental Table S6).
The protocol for depletion of rRNA and the other RNAs was as published (Adiconis et al. 2013) with minor changes: To deplete target RNAs, we added 1000 ng of pooled oligos to 1000 ng of total RNA, incubated the mixture in 1× hybridization buffer (100 mM NaCl, 50 mM Tris-HCl, pH 7.4) in a final volume of 5 μL at 95°C for 2 min, and then slowly lowered the temperature at −0.1°C/sec to 50°C. We then added 5 μL preheated RNase H reaction buffer (100 mM NaCl, 50 mM Tris-HCl, pH 7.4, 20 mM MgCl2) with 5 units Hybridase Thermostable RNase H (MACLAB, H39500) to the mix, incubated for 30 min at 50°C, and then placed it on ice. We added 10 µL DNase mixture (2× TURBO DNase I Reaction Buffer, 2 units TURBO DNase) to the samples and incubated them for 30 min at 37°C. The samples were cleaned up with 2.2× volume of Ampure XP beads (Beckman Coulter) or Sera-Mag beads for the next step library preparation.
RNA-seq libraries were prepared using the Next Ultra II Directional RNA Library Prep Kit (New England Biolabs) for Illumina with 96 Unique Dual Index Primer Pairs according to the manufacturer's protocol. The libraries were sequenced on an Illumina NovaSeq 6000 SP (PE100) at the Next Generation Sequencing Facility, the Centre for Applied Genomics, The Hospital for Sick Children, Toronto, Ontario, Canada. RIP-seq data were deposited to the Gene Expression Omnibus as entry GSE240494.
Reverse transcription and quantitative polymerase chain reaction
RNA was purified from cells using the Maxwell RSC simplyRNA tissue extraction kit (Promega) and the concentration was determined using a NanoDrop spectrophotometer (Thermo Fisher). To confirm Pum knockdown, RT-qPCR analysis of pumilio exon 9 and exon 11 in WT and Pum KO DL1 cells was performed according to the method previously described (Haugen et al. 2022).
A total of 10 µg RNA was taken from each sample for RT using GoScript (Promega) reverse transcriptase (5 µg for RT, 5 µg for “no RT” negative control samples). RT reactions were primed with random hexamers and carried out according to the manufacturer's protocol. cDNA was then diluted with 100 µL water to a final concentration of ∼41 ng/µL. Quantitative PCR (qPCR) was performed using Go-Taq qPCR Master Mix (Promega) with 2 µL of cDNA or “no RT” sample in a 20 µL reaction volume using 100 or 200 nM final concentration qPCR primers, as indicated for primer sets listed below. Reactions were performed on a Bio-Rad CFX96 instrument using the following cycling parameters: 3 min at 98°C, (10 sec at 95°C, 30 sec at 62/63/64°C, 40 sec at 72°C + image) × 39, 60°C–90°C melt curve + image. The fold change induced by RNAi of each mRNA was calculated relative to NTC RNAi condition using the measured Ct values according to the method established by Pfaffl (2001). Ct values for each target mRNA were normalized to the internal control mRNA encoding ribosomal protein RpL32. Measurements for each target mRNA were repeated in three experiments with three biological replicates each. Significance calling is as follows: *P < 0.05, **P < 0.01, ***P < 0.001 using unpaired, two-tailed Student's t-test.
The following primer sets were used to measure Pop2, Not1, Raf, and RpL32 mRNAs.
Pop2 qPCR primer set produced a 133-bp amplicon 133 bp with 100% amplification efficiency (measured according to the method of Pfaffl [2001]) at 64°C and 200 nM:
RJH510 Pop2 Fwd 5′-AAGTTTAACCTGAGCGAGGACATG
RJH511 Pop2 Rev 5′-CAGAGCTCATCAGCAGTTCGG
Not1 qPCR primer set produced a 157 bp amplicon with 97% efficiency at 64°C and 200 nM:
RJH512 Not1 Fwd 5′-GGACGTGTGCATGGAACTTGATC
RJH513 Not1 Rev 5′-CAGCTGACCTTCCGTGTTTGC
Raf qPCR primer set produced a 195 bp amplicon with 100% efficiency at 62°C and 100 nM:
RJH472 Raf Fwd 5′-AGACCTCCTTTGCCGCATCC
RJH473 Raf Rev 5′-GATGCGCGGCCCAATTAAAA
RpL32 qPCR primer set produced an 85 bp amplicon with 100% efficiency at 62°C and 100 nM:
RC133 RpL32 Fwd 5′-GCCCAAGGGTATCGACAACA
RC134 RpL32 Rev 5′-GCGCTTGTTCGATCCGTAAC
Determination of data set overlap significance
P-values for the significance of two-set or three-set gene overlap in the Venn diagrams (Figs. 3C and 4F) were calculated via one-sided permutation tests (n = 1000). These tests compared the observed overlap to the overlaps resulting from shuffled, randomized gene sets by taking the data and permuting the gene labels before subsetting based on significance criteria 1000 times for 1000 total comparisons. Fold enrichment was calculated for the genes shared between two sets and three sets with
where the expected value was calculated for two-set overlaps with
and calculated for three-set overlaps with the inclusion of a third term:
In the case of the overlaps between different experimental types of data, in which the total number of genes captured was different for the Click-seq and RIP-seq experiments, the total genes captured variable was replaced by the shared intersection of the total genes captured by each experiment.
Hybrid transcriptome alignment
To check for the presence of Cas9 mRNA reads in the Pum KO1 and KO2 samples, we built a hybrid transcriptome index for kallisto (v0.44.0) (Bray et al. 2016), containing both the FlyBase (Gramates et al. 2022) D. melanogaster (r6.38) transcripts along with the sequences for the guide RNAs and mRNAs used in the KO experiments. Each KO sample was run individually, with the arguments ‐‐single ‐‐rf-stranded -l 200.0 -s 50.0. TPM values for reads mapping to each Cas9 mRNA were subsequently pulled from the *abundance.tsv files from kallisto and averaged to calculate an average TPM over the Cas9 mRNA for each KO sample.
GO term analysis
GO term enrichment analysis was performed using iPAGE (Goodarzi et al. 2009) with database files built off of the FlyBase dmel r6.38 genome. For analyses within a single experimental data set (Fig. 5A–C), genes were discretized into five bins based on RNA stability in Pum KD (i.e., ‐‐ebins=5), and otherwise iPAGE was run with default parameters. For analysis across experimental data sets (Fig. 5D), genes were discretized into four categories based on whether they shared the same directionality in all three RNAi knockdown data sets. The “Down in all 3” and “Up in all 3” categories were based on whether the genes met our significance criteria (LFC ≥ ±log2(1.3), q-value ≤ 0.05) in either direction. Genes belonging to the “Neutral in all 3” category, consistently did not meet our significance criteria in any of the three KD data sets. Genes that had any other combination of directionalities across data sets were added to the “Different directions” category. iPAGE was run with the argument ‐‐exptype=discrete and otherwise with default parameters.
Naive motif discovery and analysis
We identified motifs that were informative of the observed gene regulatory changes in our Click-seq expression data, from each of the Pum, Not1, and Pop2 knockdowns, using a development version of FIRE (Elemento et al. 2007; Goodarzi et al. 2009). We used FIRE to evaluate both the 5′ UTR and 3′ UTR regions for all three data sets. The FIRE-discovered motifs were then cross-referenced against the CISBP-RNA database of known RBP motifs in D. melanogaster using the TOMTOM motif comparison tool to quantify the similarity of our discovered motifs to the motifs already known (Gupta et al. 2007). Motifs were considered to be both informative and a good match to known RBPs if they had a Z-score ≥ 50 from FIRE and an E-value ≤ 0.05 from TOMTOM; only motifs passing these criteria were included in our subsequent analysis.
Modeling gene regulation
The following features were generated for each transcript as previously described (Wolfe et al. 2020). (1) The number of perfect PRE matches present. (2) The maximum number of PREs clustered together. (3) The AU content surrounding the PRE site(s). (4) The absolute distance of the PRE into the 3′ UTR. (5) The relative location of the PRE in the 3′ UTR. (6) Percent codon optimality (data were obtained from previously reported values in Drosophila [Burow et al. 2018]). (7) Pum-binding data from RIP-seq data in the form of log2 fold enrichment values. (8) The length of the 3′ UTR. In cases in which there was no perfect PRE match in a transcript, the features pertaining to PRE location (i.e., 3–5 above) were calculated based on the sequence context around the motif that most closely matched a PRE, if one existed with a maximum of five mismatches. The resulting statistical analysis was also duplicated while allowing for up to one mismatch (insertion, deletion, or substitution) from a perfect PRE motif. The eight features described above were used as the independent variables for attempting to model our dependent variable, which was the Wald statistic observed from the Pum KD Click-seq experiment, in regression analysis.
Linear regression models were fitted to the Pum KD expression data using the lm( ) function from the stats (v4.2.0) package in R (v4.2.3; R Core Team 2023) (https://www.R-project.org/). GAMs were fitted using the gam( ) function from the mgcv (v1.8-40) package with the method argument set to “REML” (Wood 2011). The importance of individual features was evaluated with the BIC applied to leave-one-out variations of the linear or GAM. The BIC was calculated using the BIC( ) function also from the R stats (v4.2.0) package (Wood 2011).
SUPPLEMENTAL MATERIAL
Supplemental material is available for this article.
ACKNOWLEDGMENTS
We thank Katherine McKenney, Isioma Enwerem, Robert Connacher, and Elise Dunshee for their insightful comments, suggestions, and technical assistance. This research was funded by the National Institute of General Medical Sciences, the National Institutes of Health (grant nos. R01 GM105707 to A.C.G. and R35 GM128637 to P.L.F.), and the Canadian Institutes for Health Research (Project grants PJT-159702 and PJT-190124 to H.D.L.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. Additional support was provided by the University of Minnesota Medical School (A.C.G.) and the University of Texas Medical Branch (E.J.W.).
Author contributions: R.J.H. and A.C.G.: conceptualization, investigation, resources, formal analysis, writing, review, editing, and visualization; A.C.G.: project administration and supervision; C.B. and P.L.F.: data analysis, formal analysis, writing, review, editing, visualization, and software; P.L.F.: supervision; N.D.E., M.K.J., P.J., and E.J.W.: experiments, data analysis, and writing, review; E.J.W.: supervision; H.L., H.D.L., and C.A.S.: investigation, resources, formal analysis, writing, review, data analysis.
Footnotes
Article is online at http://www.rnajournal.org/cgi/doi/10.1261/rna.079813.123.
REFERENCES
- Adiconis X, Borges-Rivera D, Satija R, DeLuca DS, Busby MA, Berlin AM, Sivachenko A, Thompson DA, Wysoker A, Fennell T, et al. 2013. Comparative analysis of RNA sequencing methods for degraded or low-input samples. Nat Methods 10: 623–629. 10.1038/nmeth.2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvola RM, Weidmann CA, Tanaka Hall TM, Goldstrohm AC. 2017. Combinatorial control of messenger RNAs by Pumilio, Nanos and brain tumor proteins. RNA Biol 14: 1445–1456. 10.1080/15476286.2017.1306168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvola RM, Chang CT, Buytendorp JP, Levdansky Y, Valkov E, Freddolino PL, Goldstrohm AC. 2020. Unique repression domains of Pumilio utilize deadenylation and decapping factors to accelerate destruction of target mRNAs. Nucleic Acids Res 48: 1843–1871. 10.1093/nar/gkz1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae H, Coller J. 2022. Codon optimality-mediated mRNA degradation: linking translational elongation to mRNA stability. Mol Cell 82: 1467–1476. 10.1016/j.molcel.2022.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AR, Tibbit C, Ponting CP, Liu JL. 2013. Highly efficient targeted mutagenesis of Drosophila with the CRISPR/Cas9 system. Cell Rep 4: 220–228. 10.1016/j.celrep.2013.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit B, Nemeth A, Aulner N, Kuhn U, Simonelig M, Wahle E, Bourbon HM. 1999. The Drosophila poly(A)-binding protein II is ubiquitous throughout Drosophila development and has the same function in mRNA polyadenylation as its bovine homolog in vitro. Nucleic Acids Res 27: 3771–3778. 10.1093/nar/27.19.3771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhogal B, Plaza-Jennings A, Gavis ER. 2016. Nanos-mediated repression of hid protects larval sensory neurons after a global switch in sensitivity to apoptotic signals. Development 143: 2147–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blewett NH, Goldstrohm AC. 2012. An eIF4E-binding protein promotes mRNA decapping and is required for PUF repression. Mol Cell Biol 32: 4181–4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn JA, Van Etten JL, Schagat TL, Bowman BM, McEachin RC, Freddolino PL, Goldstrohm AC. 2018. Identification of diverse target RNAs that are functionally regulated by human Pumilio proteins. Nucleic Acids Res 46: 362–386. 10.1093/nar/gkx1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray NL, Pimentel H, Melsted P, Pachter L. 2016. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol 34: 525–527. 10.1038/nbt.3519 [DOI] [PubMed] [Google Scholar]
- Burow DA, Umeh-Garcia MC, True MB, Bakhaj CD, Ardell DH, Cleary MD. 2015. Dynamic regulation of mRNA decay during neural development. Neural Dev 10: 11. 10.1186/s13064-015-0038-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burow DA, Martin S, Quail JF, Alhusaini N, Coller J, Cleary MD. 2018. Attenuated codon optimality contributes to neural-specific mRNA decay in Drosophila. Cell Rep 24: 1704–1712. 10.1016/j.celrep.2018.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschauer R, Matsuo Y, Sugiyama T, Chen YH, Alhusaini N, Sweet T, Ikeuchi K, Cheng J, Matsuki Y, Nobuta R, et al. 2020. The Ccr4-Not complex monitors the translating ribosome for codon optimality. Science 368: eaay6912. 10.1126/science.aay6912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Zhou Y, Chen Y, Gu J. 2018. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34: i884–i890. 10.1093/bioinformatics/bty560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicoine J, Benoit P, Gamberi C, Paliouras M, Simonelig M, Lasko P. 2007. Bicaudal-C recruits CCR4-NOT deadenylase to target mRNAs and regulates oogenesis, cytoskeletal organization, and its own expression. Dev Cell 13: 691–704. 10.1016/j.devcel.2007.10.002 [DOI] [PubMed] [Google Scholar]
- D'Orazio KN, Green R. 2021. Ribosome states signal RNA quality control. Mol Cell 81: 1372–1383. 10.1016/j.molcel.2021.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichhorn SW, Guo H, McGeary SE, Rodriguez-Mias RA, Shin C, Baek D, Hsu SH, Ghoshal K, Villén J, Bartel DP. 2014. mRNA destabilization is the dominant effect of mammalian microRNAs by the time substantial repression ensues. Mol Cell 56: 104–115. 10.1016/j.molcel.2014.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elemento O, Slonim N, Tavazoie S. 2007. A universal framework for regulatory element discovery across all genomes and data types. Mol Cell 28: 337–350. 10.1016/j.molcel.2007.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrod ND, Jaworski EA, Ji P, Wagner EJ, Routh A. 2019. Development of Poly(A)-ClickSeq as a tool enabling simultaneous genome-wide poly(A)-site identification and differential expression analysis. Methods 155: 20–29. 10.1016/j.ymeth.2019.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enwerem III, Elrod ND, Chang CT, Lin A, Ji P, Bohn JA, Levdansky Y, Wagner EJ, Valkov E, Goldstrohm A. 2021. Human Pumilio proteins directly bind the CCR4-NOT deadenylase complex to regulate the transcriptome. RNA 27: 445–464. 10.1261/rna.078436.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulalio A, Huntzinger E, Nishihara T, Rehwinkel J, Fauser M, Izaurralde E. 2009. Deadenylation is a widespread effect of miRNA regulation. RNA 15: 21–32. 10.1261/rna.1399509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes A, Lehmann R. 1998. Nanos and Pumilio have critical roles in the development and function of Drosophila germline stem cells. Development 125: 679–690. 10.1242/dev.125.4.679 [DOI] [PubMed] [Google Scholar]
- Galgano A, Forrer M, Jaskiewicz L, Kanitz A, Zavolan M, Gerber AP. 2008. Comparative analysis of mRNA targets for human PUF-family proteins suggests extensive interaction with the miRNA regulatory system. PLoS ONE 3: e3164. 10.1371/journal.pone.0003164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennarino VA, Singh RK, White JJ, De Maio A, Han K, Kim JY, Jafar-Nejad P, di Ronza A, Kang H, Sayegh LS, et al. 2015. Pumilio1 haploinsufficiency leads to SCA1-like neurodegeneration by increasing wild-type Ataxin1 levels. Cell 160: 1087–1098. 10.1016/j.cell.2015.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennarino VA, Palmer EE, McDonell LM, Wang L, Adamski CJ, Koire A, See L, Chen CA, Schaaf CP, Rosenfeld JA, et al. 2018. A mild PUM1 mutation is associated with adult-onset ataxia, whereas haploinsufficiency causes developmental delay and seizures. Cell 172: 924–936.e11. 10.1016/j.cell.2018.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber AP, Luschnig S, Krasnow MA, Brown PO, Herschlag D. 2006. Genome-wide identification of mRNAs associated with the translational regulator PUMILIO in Drosophila melanogaster. Proc Natl Acad Sci 103: 4487–4492. 10.1073/pnas.0509260103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstrohm AC, Wickens M. 2008. Multifunctional deadenylase complexes diversify mRNA control. Nat Rev Mol Cell Biol 9: 337–344. 10.1038/nrm2370 [DOI] [PubMed] [Google Scholar]
- Goldstrohm AC, Hall TMT, McKenney KM. 2018. Post-transcriptional regulatory functions of mammalian Pumilio proteins. Trends Genet 34: 972–990. 10.1016/j.tig.2018.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodarzi H, Elemento O, Tavazoie S. 2009. Revealing global regulatory perturbations across human cancers. Mol Cell 36: 900–911. 10.1016/j.molcel.2009.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramates LS, Agapite J, Attrill H, Calvi BR, Crosby MA, Dos Santos G, Goodman JL, Goutte-Gattat D, Jenkins VK, Kaufman T, et al. 2022. FlyBase: a guided tour of highlighted features. Genetics 220: iyac035. 10.1093/genetics/iyac035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Ma X, Gao F, Guo Y. 2023. Off-target effects in CRISPR/Cas9 gene editing. Front Bioeng Biotechnol 11: 1143157. 10.3389/fbioe.2023.1143157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Stamatoyannopoulos JA, Bailey TL, Noble WS. 2007. Quantifying similarity between motifs. Genome Biol 8: R24. 10.1186/gb-2007-8-2-r24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RE, Pargett M, Sutcliffe C, Umulis D, Ashe HL. 2011. Brat promotes stem cell differentiation via control of a bistable switch that restricts BMP signaling. Dev Cell 20: 72–83. 10.1016/j.devcel.2010.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen RJ, Arvola RM, Connacher RP, Roden RT, Goldstrohm AC. 2022. A conserved domain of Drosophila RNA-binding protein Pumilio interacts with multiple CCR4-NOT deadenylase complex subunits to repress target mRNAs. J Biol Chem 298: 102270. 10.1016/j.jbc.2022.102270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S, Ogura Y. 2020. ERK signaling dynamics in the morphogenesis and homeostasis of Drosophila. Curr Opin Genet Dev 63: 9–15. 10.1016/j.gde.2020.01.004 [DOI] [PubMed] [Google Scholar]
- Joly W, Chartier A, Rojas-Rios P, Busseau I, Simonelig M. 2013. The CCR4 deadenylase acts with Nanos and Pumilio in the fine-tuning of Mei-P26 expression to promote germline stem cell self-renewal. Stem Cell Rep 1: 411–424. 10.1016/j.stemcr.2013.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas S, Izaurralde E. 2015. Towards a molecular understanding of microRNA-mediated gene silencing. Nat Rev Genet 16: 421–433. 10.1038/nrg3965 [DOI] [PubMed] [Google Scholar]
- Kadyrova LY, Habara Y, Lee TH, Wharton RP. 2007. Translational control of maternal Cyclin B mRNA by Nanos in the Drosophila germline. Development 134: 1519–1527. 10.1242/dev.002212 [DOI] [PubMed] [Google Scholar]
- Kim SY, Kim JY, Malik S, Son W, Kwon KS, Kim C. 2012. Negative regulation of EGFR/MAPK pathway by Pumilio in Drosophila melanogaster. PLoS ONE 7: e34016. 10.1371/journal.pone.0034016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Paggi JM, Park C, Bennett C, Salzberg SL. 2019. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol 37: 907–915. 10.1038/s41587-019-0201-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver JD, Li X, Ancevicius K, Westwood JT, Smibert CA, Morris QD, Lipshitz HD. 2013. Genome-wide analysis of Staufen-associated mRNAs identifies secondary structures that confer target specificity. Nucleic Acids Res 41: 9438–9460. 10.1093/nar/gkt702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver JD, Li X, Ray D, Cook KB, Hahn NA, Nabeel-Shah S, Kekis M, Luo H, Marsolais AJ, Fung KY, et al. 2015. Brain tumor is a sequence-specific RNA-binding protein that directs maternal mRNA clearance during the Drosophila maternal-to-zygotic transition. Genome Biol 16: 94. 10.1186/s13059-015-0659-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver JD, Ly J, Winn AK, Karaiskakis A, Lin S, Nie K, Benic G, Jaberi-Lashkari N, Cao WX, Khademi A, et al. 2020. The RNA-binding protein Rasputin/G3BP enhances the stability and translation of its target mRNAs. Cell Rep 30: 3353–3367.e7. 10.1016/j.celrep.2020.02.066 [DOI] [PubMed] [Google Scholar]
- Lee MH, Hook B, Pan G, Kershner AM, Merritt C, Seydoux G, Thomson JA, Wickens M, Kimble J. 2007. Conserved regulation of MAP kinase expression by PUF RNA-binding proteins. PLoS Genet 3: e233. 10.1371/journal.pgen.0030233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann R, Nüsslein-Volhard C. 1987. Involvement of the pumilio gene in the transport of an abdominal signal in the Drosophila embyro. Nature 329: 167–170. 10.1038/329167a0 [DOI] [Google Scholar]
- Liao Y, Smyth GK, Shi W. 2014. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30: 923–930. 10.1093/bioinformatics/btt656 [DOI] [PubMed] [Google Scholar]
- Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15: 550. 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeda A, Zahler AM, Krainer AR, Roth MB. 1992. Two members of a conserved family of nuclear phosphoproteins are involved in pre-mRNA splicing. Proc Natl Acad Sci 89: 1301–1304. 10.1073/pnas.89.4.1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mee CJ, Pym EC, Moffat KG, Baines RA. 2004. Regulation of neuronal excitability through pumilio-dependent control of a sodium channel gene. J Neurosci 24: 8695–8703. 10.1523/JNEUROSCI.2282-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon KP, Sanyal S, Habara Y, Sanchez R, Wharton RP, Ramaswami M, Zinn K. 2004. The translational repressor Pumilio regulates presynaptic morphology and controls postsynaptic accumulation of translation factor eIF-4E. Neuron 44: 663–676. 10.1016/j.neuron.2004.10.028 [DOI] [PubMed] [Google Scholar]
- Menon KP, Andrews S, Murthy M, Gavis ER, Zinn K. 2009. The translational repressors Nanos and Pumilio have divergent effects on presynaptic terminal growth and postsynaptic glutamate receptor subunit composition. J Neurosci 29: 5558–5572. 10.1523/JNEUROSCI.0520-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S, Temme C, Wahle E. 2004. Messenger RNA turnover in eukaryotes: pathways and enzymes. Crit Rev Biochem Mol Biol 39: 197–216. 10.1080/10409230490513991 [DOI] [PubMed] [Google Scholar]
- Murata Y, Wharton RP. 1995. Binding of pumilio to maternal hunchback mRNA is required for posterior patterning in Drosophila embryos. Cell 80: 747–756. 10.1016/0092-8674(95)90353-4 [DOI] [PubMed] [Google Scholar]
- Na H, Laver JD, Jeon J, Singh F, Ancevicius K, Fan Y, Cao WX, Nie K, Yang Z, Luo H, et al. 2016. A high-throughput pipeline for the production of synthetic antibodies for analysis of ribonucleoprotein complexes. RNA 22: 636–655. 10.1261/rna.055186.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passmore LA, Coller J. 2022. Roles of mRNA poly(A) tails in regulation of eukaryotic gene expression. Nat Rev Mol Cell Biol 23: 93–106. 10.1038/s41580-021-00417-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekovic F, Rammelt C, Kubíková J, Metz J, Jeske M, Wahle E. 2023. RNA binding proteins Smaug and Cup induce CCR4-NOT-dependent deadenylation of the nanos mRNA in a reconstituted system. Nucleic Acids Res 51: 3950–3970. 10.1093/nar/gkad159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petibon C, Malik Ghulam M, Catala M, Abou Elela S. 2021. Regulation of ribosomal protein genes: an ordered anarchy. Wiley Interdiscip Rev RNA 12: e1632. 10.1002/wrna.1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45. 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisch T, Valkov E. 2022. Regulation of the multisubunit CCR4-NOT deadenylase in the initiation of mRNA degradation. Curr Opin Struct Biol 77: 102460. 10.1016/j.sbi.2022.102460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray D, Kazan H, Cook KB, Weirauch MT, Najafabadi HS, Li X, Gueroussov S, Albu M, Zheng H, Yang A, et al. 2013. A compendium of RNA-binding motifs for decoding gene regulation. Nature 499: 172–177. 10.1038/nature12311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring HZ, Lis JT. 1994. The SR protein B52/SRp55 is essential for Drosophila development. Mol Cell Biol 14: 7499–7506. 10.1128/mcb.14.11.7499-7506.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth MB, Zahler AM, Stolk JA. 1991. A conserved family of nuclear phosphoproteins localized to sites of polymerase II transcription. J Cell Biol 115: 587–596. 10.1083/jcb.115.3.587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routh A, Head SR, Ordoukhanian P, Johnson JE. 2015. ClickSeq: fragmentation-free next-generation sequencing via click ligation of adaptors to stochastically terminated 3′-azido cDNAs. J Mol Biol 427: 2610–2616. 10.1016/j.jmb.2015.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routh A, Ji P, Jaworski E, Xia Z, Li W, Wagner EJ. 2017. Poly(A)-ClickSeq: click-chemistry for next-generation 3-end sequencing without RNA enrichment or fragmentation. Nucleic Acids Res 45: e112. 10.1093/nar/gkx286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider I. 1972. Cell lines derived from late embryonic stages of Drosophila melanogaster. J Embryol Exp Morphol 27: 353–365. 10.1242/dev.27.2.353 [DOI] [PubMed] [Google Scholar]
- Smith T, Heger A, Sudbery I. 2017. UMI-tools: modeling sequencing errors in unique molecular identifiers to improve quantification accuracy. Genome Res 27: 491–499. 10.1101/gr.209601.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temme C, Zaessinger S, Meyer S, Simonelig M, Wahle E. 2004. A complex containing the CCR4 and CAF1 proteins is involved in mRNA deadenylation in Drosophila. EMBO J 23: 2862–2871. 10.1038/sj.emboj.7600273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temme C, Zhang L, Kremmer E, Ihling C, Chartier A, Sinz A, Simonelig M, Wahle E. 2010. Subunits of the Drosophila CCR4-NOT complex and their roles in mRNA deadenylation. RNA 16: 1356–1370. 10.1261/rna.2145110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temme C, Simonelig M, Wahle E. 2014. Deadenylation of mRNA by the CCR4-NOT complex in Drosophila: molecular and developmental aspects. Front Genet 5: 143. 10.3389/fgene.2014.00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten J, Schagat TL, Hrit J, Weidmann CA, Brumbaugh J, Coon JJ, Goldstrohm AC. 2012. Human Pumilio proteins recruit multiple deadenylases to efficiently repress messenger RNAs. J Biol Chem 287: 36370–36383. 10.1074/jbc.M112.373522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, McLachlan J, Zamore PD, Hall TM. 2002. Modular recognition of RNA by a human pumilio-homology domain. Cell 110: 501–512. 10.1016/S0092-8674(02)00873-5 [DOI] [PubMed] [Google Scholar]
- Weidmann CA, Goldstrohm AC. 2012. Drosophila Pumilio protein contains multiple autonomous repression domains that regulate mRNAs independently of Nanos and brain tumor. Mol Cell Biol 32: 527–540. 10.1128/MCB.06052-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidmann CA, Raynard NA, Blewett NH, Van Etten J, Goldstrohm AC. 2014. The RNA binding domain of Pumilio antagonizes poly-adenosine binding protein and accelerates deadenylation. RNA 20: 1298–1319. 10.1261/rna.046029.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidmann CA, Qiu C, Arvola RM, Lou TF, Killingsworth J, Campbell ZT, Tanaka Hall TM, Goldstrohm AC. 2016. Drosophila Nanos acts as a molecular clamp that modulates the RNA-binding and repression activities of Pumilio. Elife 5: e17096. 10.7554/eLife.17096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton RP, Struhl G. 1991. RNA regulatory elements mediate control of Drosophila body pattern by the posterior morphogen nanos. Cell 67: 955–967. 10.1016/0092-8674(91)90368-9 [DOI] [PubMed] [Google Scholar]
- Wharton RP, Sonoda J, Lee T, Patterson M, Murata Y. 1998. The Pumilio RNA-binding domain is also a translational regulator. Mol Cell 1: 863–872. 10.1016/S1097-2765(00)80085-4 [DOI] [PubMed] [Google Scholar]
- Wolfe MB, Goldstrohm AC, Freddolino PL. 2019. Global analysis of RNA metabolism using bio-orthogonal labeling coupled with next-generation RNA sequencing. Methods 155: 88–103. 10.1016/j.ymeth.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe MB, Schagat TL, Paulsen MT, Magnuson B, Ljungman M, Park D, Zhang C, Campbell ZT, Goldstrohm AC, Freddolino PL. 2020. Principles of mRNA control by human PUM proteins elucidated from multimodal experiments and integrative data analysis. RNA 26: 1680–1703. 10.1261/rna.077362.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SN. 2011. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc Series B 73: 3–36. 10.1111/j.1467-9868.2010.00749.x [DOI] [Google Scholar]
- Wreden C, Verrotti AC, Schisa JA, Lieberfarb ME, Strickland S. 1997. Nanos and pumilio establish embryonic polarity in Drosophila by promoting posterior deadenylation of hunchback mRNA. Development 124: 3015–3023. 10.1242/dev.124.15.3015 [DOI] [PubMed] [Google Scholar]
- Wu Q, Bazzini AA. 2023. Translation and mRNA stability control. Annu Rev Biochem 92: 227–245. 10.1146/annurev-biochem-052621-091808 [DOI] [PubMed] [Google Scholar]
- Zamore PD, Williamson JR, Lehmann R. 1997. The Pumilio protein binds RNA through a conserved domain that defines a new class of RNA-binding proteins. RNA 3: 1421–1433. [PMC free article] [PubMed] [Google Scholar]
- Zamore PD, Bartel DP, Lehmann R, Williamson JR. 1999. The PUMILIO–RNA interaction: a single RNA-binding domain monomer recognizes a bipartite target sequence. Biochemistry 38: 596–604. 10.1021/bi982264s [DOI] [PubMed] [Google Scholar]
- Zhang M, Chen D, Xia J, Han W, Cui X, Neuenkirchen N, Hermes G, Sestan N, Lin H. 2017. Post-transcriptional regulation of mouse neurogenesis by Pumilio proteins. Genes Dev 31: 1354–1369. 10.1101/gad.298752.117 [DOI] [PMC free article] [PubMed] [Google Scholar]