ABSTRACT
Recently, an outbreak of highly pathogenic avian influenza A (H5N1), which carries the clade 2.3.4.4b hemagglutinin (HA) gene and has been prevalent among North American bird populations since the winter of 2021, was reported in dairy cows in the United States. As of 24 May 2024, the virus has affected 63 dairy herds across nine states and has resulted in two human infections. The virus causes unusual symptoms in dairy cows, including an unexpected drop in milk production, and thick colostrum-like milk. Notably, The US Food and Drug Administration reported that around 20% of tested retail milk samples contained H5N1 viruses, with a higher percentage of positive results from regions with infected cattle herds. Data are scant regarding how effectively pasteurization inactivates the H5N1 virus in milk. Therefore, in this study, we evaluated the thermal stability of the H5 clade 2.3.4.4b viruses, along with one human H3N2 virus and other influenza subtype viruses, including H1, H3, H7, H9, and H10 subtype viruses. We also assessed the effectiveness of pasteurization in inactivating these viruses. We found that the avian H3 virus exhibits the highest thermal stability, whereas the H5N1 viruses that belong to clade 2.3.4.4b display moderate thermal stability. Importantly, our data provide direct evidence that the standard pasteurization methods used by dairy companies are effective in inactivating all tested subtypes of influenza viruses in raw milk. Our findings indicate that thermally pasteurized milk products do not pose a safety risk to consumers.
KEYWORDS: Pasteurization, Influenza A virus, H5N1, clade 2.3.4.4b, milk
Most recently, in the United States, the list of species susceptible to H5N1 viruses has expanded to include goats and cattle, particularly dairy cattle, which were previously considered resistant to influenza A virus. The H5N1 outbreak in dairy cattle has now spread to 63 herds in nine states, resulting in two human infections [1,2]. This expansion of susceptible mammalian species highlights the pandemic potential of the clade 2.3.4.4b virus. Notably, researchers from the U.S. Department of Agriculture (USDA) have detected very high virus concentrations in milk [3]. Moreover, the US Food and Drug Administration (FDA) reported that about 20% of retail milk samples tested were positive for H5N1, with a higher rate of positive results from areas with infected cattle herds, further suggesting the possibility of transmission from cow to cow or possibly from cows to humans via milk. While the USDA and FDA have both stated that pasteurization effectively inactivates viruses and that the commercial milk supply is safe, limited data are available regarding the efficacy of pasteurization against H5N1 virus in raw milk. Therefore, here, we examined the effectiveness of pasteurization against influenza A viruses.
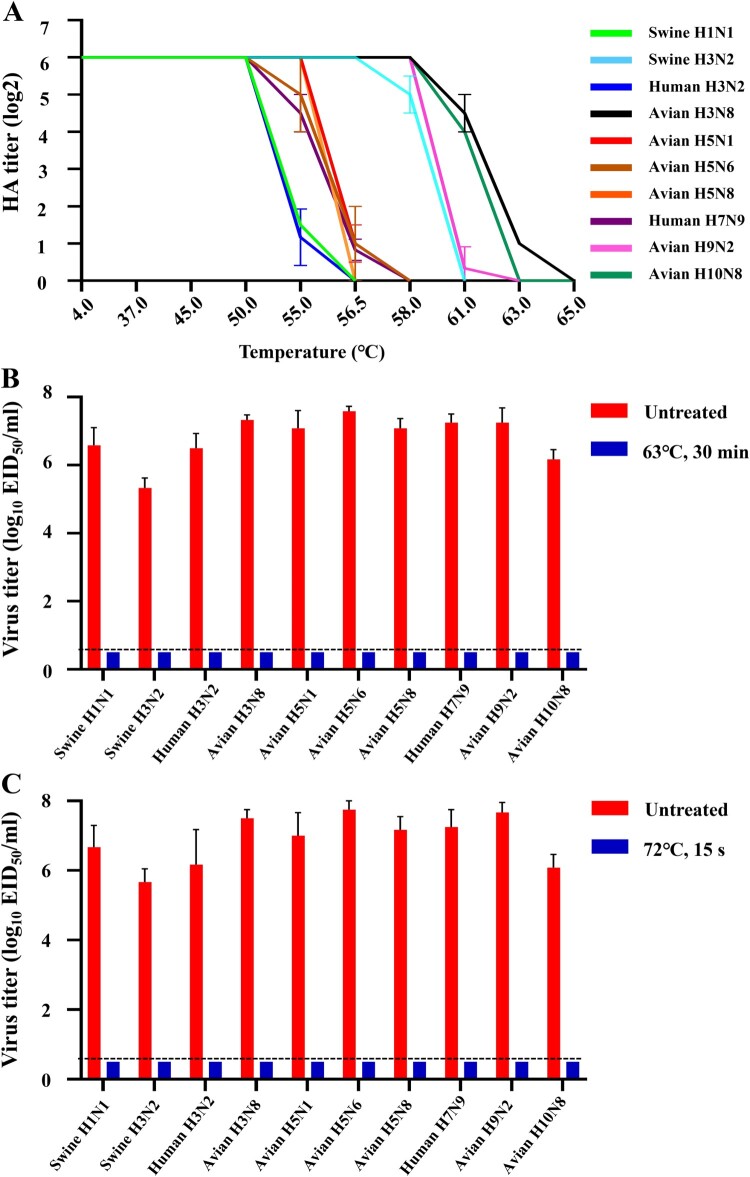
In our study, we initially tested the thermal stability of three H5 clade 2.3.4.4b viruses bearing the N1, N6, or N8 gene, along with one human H3N2 virus and other influenza subtype viruses with human-infecting potential, including H1N1, H3N8, H7N9, H9N2, and H10N8 viruses [4–6]. Based on the data from three independent experiments (Figure 1A), the influenza viruses were categorized into three groups: Group 1 contained four viruses with the highest thermal stability (one avian H3N8, one avian H10N8, one avian H9N2, and one swine H3N2 virus); Group 2 comprised four viruses with moderate thermal stability (three H5 clade 2.3.4.4b viruses bearing the N1, N6, or N8 gene, and one human H7N9 virus); and Group 3 contained two viruses with the lowest thermal stability (one swine H1N1 and one human seasonal H3N2 virus). Our data thus demonstrate that influenza viruses exhibit different thermal stability, with H5 clade 2.3.4.4b viruses showing moderate thermal stability.
Figure 1.
Effectiveness of heat treatment on different influenza A viruses. (A) Thermal stability of various subtypes of influenza A virus. For the thermal stability analysis, 50 μl of test virus was diluted with phosphate buffered saline to contain 6 log2 HA units of virus, which was measured using 0.5% chicken red blood cells (cRBC). Virus aliquots in PCR tubes were then treated in a PCR machine preheated to 63 °C, and the samples were incubated at the indicated temperature for 10 min. The HA titers of the samples were measured using 0.5% (cRBC) after heat treatment. Data are presented as means and are representative of three independent experiments. Pasteurization analysis of different subtypes of influenza A virus at 63 °C for 30 min (B) or 72°C for 15 s (C). Briefly, 2.5 μl of fresh virus allantoic fluid was added to 47.5 μl of raw milk collected from health diary cows and then aliquoted into five PCR tubes. After heat treatments in a PCR machine preheated to either 63°C or 72°C, the five aliquots were combined into one sample and the inactivation efficacy was analyzed by inoculating the sample into 9-day-old embryonated eggs to detect live viruses. Control samples were prepared in the same manner as treated samples but underwent no heat treatment. Data are shown as means ± SD (n = 3) and are representative of three independent experiments. The dashed lines in these panels indicate the lower limit of detection. A/swine/Hebei/165/2017 (H1N1), Swine H1N1; A/swine/Gansu/234/2011 (H3N2), Swine H3N2, A/Kansas/14/2017 (H3N2), Human H3N2; A/chicken/Hunan/S2076/2022 (H3N8), Avian H3N8; A/goose/Xinjiang/S4159/2023 (H5N1), Avian H5N1; A/duck/Guizhou/S4702/2021 (H5N6), Avian H5N6; A/duck/Hebei/S1070/2021 (H5N8), Avian H5N8; A/Anhui/01/2013 (H7N9), Human H7N9; A/chicken/Guangxi/S11583/2019 (H9N2), Avian H9N2; and A/duck/Zhejiang/S1159/2017 (H10N8), Avian H10N8.
In the dairy industry, the original method of pasteurization is vat pasteurization, which heats milk in a large tank to 63 °C for at least 30 min. Today, the most common method is high-temperature short-time (HTST) pasteurization, which subjects milk to at least 72 °C for no less than 15 s, followed by rapid cooling [7]. We tested the effectiveness of both vat pasteurization and HTST in inactivating influenza A viruses mixed in raw milk collected from health diary cattles. All H1, H3, H5, H7, H9, and H10 subtype influenza viruses tested were completely inactivated at 63 °C for 30 min or at 72 °C for only 15 s (Figure 1B,C). Our data show that heat treatment can effectively inactivate as much as 107.75 EID50/ml of H5 virus in raw milk. These data provide direct evidence that pasteurization effectively inactivates all influenza viruses in raw milk, confirming the safety of commercial milk for consumption.
Discussion
USDA scientists have reported that their analysis suggests the viruses in different cows likely originated from a single source, suggesting inter-cow transmission [3]. Potential transmission routes include milking practices and herd transport. A spokesperson for the Idaho State Department of Agriculture said that the virus likely emerged after importing cows from Texas [8]. The detection of the virus in raw milk also implies possible transmission to other cows or even humans via milk droplets. While USDA and FDA officials have emphasized that contamination of commercial milk by cattle H5N1 virus is not a concern, the question of whether pasteurization can inactivate bird flu has caused apprehension among consumers. On reviewing the scientific literature, we confirmed that both highly pathogenic and low pathogenic influenza viruses can be inactivated by pasteurization in various substrates, such as fat-free egg products, allantoic fluid, or blood plasma [9]. The efficacy of pasteurization in inactivating various viruses, including Adenovirus, Murine norovirus, MERS-CoV, Herpes simplex virus, Foot and mouth disease virus, Hepatitis A virus, Poliovirus, and Bovine immunodeficiency virus, has been documented [9]. However, limited studies have reported on the inactivation efficacy of influenza viruses in milk by pasteurization. In this study, we provide direct evidence that pasteurization is sufficient to inactivate all tested influenza A viruses in raw milk collected from healthy dairy cattles, addressing a common concern among milk consumers and those with food safety concerns. However, whether the pasteurization processes used in industry can completely inactivate influenza A viruses in raw milk collected from sick cattle, which is colostrum-like and too thick, remains to be investigated.
When an outbreak of HPAI occurs in poultry, the usual response is to cull the entire flock, which has led to over 90 million birds being destroyed since February 8, 2022, due to the H5N1 clade 2.3.4.4b virus [10]. Culling drastically reduces the poultry supply and, along with consumer concerns about egg product safety, severely impacts the poultry industry. Similarly, the dairy industry faces challenges with the H5N1 virus infecting about 10% of cows in each affected herd, causing symptoms such as decreased milk production and thicker milk [11], which are more severe in older or lactating cows. The Texas Animal Health Commission reports that up to 40% of milk production losses occur in infected herds. Since the H5N1 virus is causing illness in cows, and with herds affected in multiple states, new federal restrictions on the movement of dairy cows between states are putting economic pressure on farmers. While the situation is concerning for the dairy industry, no effective control strategies, such as vaccination, could be implemented. Furthermore, there is ongoing concern that this problem could escalate and potentially impact milk production. Currently, the milk supply is stable, but it is strongly recommended that only pasteurized milk and dairy products be consumed.
Funding Statement
The study was supported by the National Key Research and Development Program of China [grant numbers 2021YFD1800200, 2021YFC2301700], the National Natural Science Foundation of China [grant number 32192451], the Innovation Program of the Chinese Academy of Agricultural Sciences [grant number CAAS-CSLPDCP-202301], and the earmarked fund [grant number CARS-41].
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.CDC . CDC reports second human case of H5 bird flu tied to dairy cow outbreak. [accessed 2024 May 28]. Available from: https://www.cdc.gov/media/releases/2024/s0522-human-case-h5.html
- 2.CDC . H5N1 bird flu: current situation summary. [accessed 2024 May 28]. Available from: https://www.cdc.gov/flu/avianflu/avian-flu-summary.htm
- 3.Science . [accessed 2024 Apr 21]. Available from: https://www.science.org/content/article/bird-flu-may-be-spreading-cows-milking-and-herd-transport
- 4.Cui P, Shi J, Yan C, et al. Analysis of avian influenza A (H3N8) viruses in poultry and their zoonotic potential, China, September 2021 to May 2022. Euro Surveill. 2023;28(41). doi: 10.2807/1560-7917.ES.2023.28.41.2200871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi J, Zeng X, Cui P, et al. Alarming situation of emerging H5 and H7 avian influenza and effective control strategies. Emerg Microbes Infect. 2023;12(1):2155072. doi: 10.1080/22221751.2022.2155072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Q, Shi J, Deng G, et al. H7N9 influenza viruses are transmissible in ferrets by respiratory droplet. Science. 2013;341(6144):410-414. doi: 10.1126/science.1240532 [DOI] [PubMed] [Google Scholar]
- 7.IDFA . Pasteurization. [accessed 2018 Apr 21]. Available from: https://www.idfa.org/pasteurization
- 8.UNMC . Global Center for Health Security. [accessed 2018 Apr 21]. Available from: https://www.unmc.edu/healthsecurity/transmission/2024/04/10/idaho-dairy-officials-report-avian-flu-affected-cows-are-recovering/
- 9.Pitino MA, O'Connor DL, McGeer AJ, et al. The impact of thermal pasteurization on viral load and detectable live viruses in human milk and other matrices: a rapid review. Appl Physiol Nutr Metab. 2021;46(1):10–26. doi: 10.1139/apnm-2020-0388 [DOI] [PubMed] [Google Scholar]
- 10.USDA . Animal and Plant Health Inspection Service. [accessed 2018 Apr 21]. Available from: https://www.aphis.usda.gov/livestock-poultry-disease/avian/avian-influenza/hpai-detections/commercial-backyard-flocks [DOI] [PubMed]
- 11.Science . [accessed 2024 Apr 21]. Available from: https://www.science.org/content/article/us-dairy-farm-worker-infected-as-bird-flu-spreads-to-cows-in-five-states