Abstract
We studied the replication and cytopathicity in SCID-hu mice of R5 human immunodeficiency virus type 1 (HIV-1) biological clones from early and late stages of infection of three patients who never developed MT-2 cell syncytium-inducing (SI; R5X4 or X4) viruses. Several of the late-stage non-MT-2 cell syncytium-inducing (NSI; R5) viruses from these patients depleted human CD4+ thymocytes from SCID-hu mice. Earlier clones from the same patients did not deplete CD4+ thymocytes from SCID-hu mice as well as later clones. We studied three R5 HIV-1 clones from patient ACH142 in greater detail. Two of these clones were obtained prior to the onset of AIDS; the third was obtained following the AIDS diagnosis. In GHOST cell infection assays, all three ACH142 R5 HIV-1 clones could infect GHOST cells expressing CCR5 but not GHOST cells expressing any of nine other HIV coreceptors tested. Furthermore, these patient clones efficiently infected stimulated peripheral blood mononuclear cells from a normal donor but not those from a homozygous CCR5Δ32 individual. Statistical analyses of data obtained from infection of SCID-hu mice with patient ACH142 R5 clones revealed that only the AIDS-associated clone significantly depleted CD4+ thymocytes from SCID-hu mice. This clone also replicated to higher levels in SCID-hu mice than the two earlier clones, and a significant correlation between viral replication and CD4+ thymocyte depletion was observed. Our results indicate that an intrinsic property of AIDS-associated R5 patient clones causes their increased replication and cytopathic effects in SCID-hu mice and likely contributes to the development of AIDS in patients who harbor only R5 quasispecies of HIV-1.
Human immunodeficiency virus type 1 (HIV-1) enters cells by binding to the cell surface glycoprotein CD4 and then to one of several seven-transmembrane trimeric GTP-binding protein coupled chemokine receptors or related molecules which are known as HIV-1 coreceptors (reviewed in references 6 and 23). Twelve coreceptors, CCR2b, CCR3, CCR5, CCR8, CXCR4, CX3CR1, BONZO/STRL33/Tymstr, BOB/GPR15, GPR1, APJ, HCMV-US28, and BLTR, have been reported to function in HIV-1 infection or syncytium formation in tissue culture (2, 3, 12, 13, 21, 22, 24–26, 28, 29, 31, 34, 35, 49, 50, 53, 57, 60). Recent work, however, has shown that CCR5 and CXCR4 are the predominant coreceptors used for infection by primary isolates (5, 72, 73). Many studies have shown that R5X4 or X4 isolates of HIV-1, which can use CXCR4 to enter cells, are more pathogenic in tissue culture, hu-PBL-SCID mice, and SCID-hu mice and are associated with more rapid progression to AIDS and death in infected individuals (10, 15, 16, 38, 41, 55, 56, 62, 65–67). Nevertheless, approximately half of people infected with clade B HIV-1 who develop AIDS never acquire detectable X4 HIV-1 quasispecies (4, 11, 45, 65). It is enigmatic that most studies of primary R5 isolates of HIV-1 have detected little if any pathogenesis attributable to these viral isolates in tissue culture, yet many AIDS patients die from infection with exclusively R5 HIV-1 quasispecies. Many R5 isolates of HIV-1 replicate more slowly and to lower titers in stimulated peripheral blood mononuclear cells (PBMC) than R5X4 or X4 isolates (15, 68). Nevertheless, some R5 isolates from later in the course of infection, particularly after an AIDS diagnosis has been made, replicate more rapidly and to higher titers in tissue culture than typical R5 isolates from early in the course of infection (68).
A hallmark of HIV-1 pathogenesis in infected individuals is the loss of CD4+ peripheral blood T cells. This may result from direct or indirect killing of mature CD4+ T cells or from depletion of T-cell precursors or from both. Infection of the thymus may play a significant role in T-cell depletion by lessening the body's ability to generate T cells (17, 40, 46, 59, 63). Kourtis and colleagues showed that infection of the thymus in HIV-1-infected children is frequently seen and is associated with rapid progression (46). We have studied the infection of human thymocytes by HIV-1 using the SCID-hu thymus/liver model system. The SCID-hu (thymus/liver) mouse is created by surgical implantation of human fetal thymus and liver tissue into SCID mice (51). These mice develop a conjoint thymus/liver graft which has the morphology of normal human thymus with small islands of hematopoietic tissue. Thymus/liver grafts maintain normal human thymopoiesis for over a year. Previous studies have shown that infection of human thymus liver grafts in SCID-hu mice leads to a pathogenic phenotype: the depletion of CD4+ thymocytes (1, 9). Furthermore, the SCID-hu mouse model of HIV-1 infection accurately reflects viral phenotypes seen in infected people. For example, the nef gene has a significant effect on replication and pathogenesis in SCID-hu mice, and late-stage X4 patient isolates are more pathogenic than earlier R5 HIV-1 isolates from the same patients (10, 37, 41).
We tested the replication and cytopathic effects of early, intermediate, and late R5 HIV-1 isolates from three Amsterdam cohort patients in SCID-hu mice. In each case the latest isolate followed an AIDS diagnosis; these isolates are referred to hereafter as R5-AIDS isolates. We also tested the replication of three isolates in a panel of GHOST cells bearing diverse HIV coreceptors and in PBMC derived from normal donors and from a donor homozygous for a 32-bp inactivating deletion in the CCR5 gene (CCR5Δ32). Our results demonstrate that R5-AIDS biological clones of HIV-1 replicate to higher levels and are more cytopathic for human CD4+ thymocytes in SCID-hu mice than earlier R5 HIV-1 biological clones from the same patients.
MATERIALS AND METHODS
Virus isolation; production and titration of viral stocks.
HIV-1 patient biological clones were obtained from the Amsterdam (The Netherlands) cohort studies. Patients were selected because of the absence of the X4 phenotype during the entire course of their infection despite progression to AIDS and death. HIV-1 was cloned from patients by limiting dilution of patient PBMC cocultured with stimulated normal donor PBMC. Viral stocks were amplified by infection of two day phytohemagglutinin (PHA) and interleukin-2 (IL-2)-stimulated healthy donor PBMC. One half of the virus-containing supernatants were removed every 2 days and replaced with fresh medium containing IL-2. Fresh stimulated PBMC were added 7 days postinfection if viral titers of the collected supernatants had not peaked. Virus-containing supernatants were aliquoted and frozen at −80°C until needed. In some cases CD8+ cells were depleted from PBMC prior to infection using the mouse monoclonal CD8 antibody OKT-8 (American Type Culture Collection). Healthy PBMC were incubated with OKT-8. Cells with bound antibody were removed using magnetic beads coated with goat anti-mouse immunoglobulin (Dynal A.S., Oslo, Norway). Cells in the supernatant were washed and placed in culture. CD8+ cell-depleted PBMC cultures were infected with patient clones as described above. The tissue culture infectious dose (TCID) of virus containing supernatants was measured by infection of P4-2 cells (14). P4-2 cells were infected with a known volume of virus containing supernatant and incubated at 37°C. After 48 h, the cells were fixed with 0.2% glutaraldehyde and stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). The stained cells were incubated overnight. The TCID was obtained by the number of blue cells counted on the plate divided by the volume used for infection.
Preparation and maintenance of SCID-hu mice.
SCID-hu thymus/liver mice were created by implantation of human fetal thymus and liver fragments under the kidney capsule of C.B-17 SCID mice as originally described by McCune and colleagues (51). SCID and SCID-hu mice were maintained in microisolater cages on racks with HEPA-filtered air blown into each cage (Allentown Caging, Allentown, Pa.). The mice were implanted with 1-mm3 pieces of human fetal thymus and liver when they were 6 to 8 weeks old. Tissue at 16 to 24 weeks of gestational age was obtained from Advanced Bioscience Resources (Alameda, Calif.). One piece of fetal thymus and two of fetal liver were inserted under the left kidney capsule of each mouse, using a 16-gauge cancer implant needle set (Popper and Sons, New Hyde Park, N.Y.). The grafts were left undisturbed for 4 to 6 months prior to infection with HIV-1.
Infection of SCID-hu mice with HIV-1 and biopsy of infected grafts.
Mice were anesthetized with ketamine and xylazine (8 and 0.8 μg, respectively per g of body weight) injected intraperitoneally prior to infection or biopsy. Methoxyfluorane was used if additional anesthesia was necessary, and buprenone was administered to minimize postoperative discomfort for all surgical procedures. Thymus/liver grafts were exteriorized and measured with a caliper. Only grafts larger than or equal to 0.5 cm in diameter were used. Freshly titered HIV-1 stocks were diluted in Iscove's medium with 2% fetal calf serum, and 400 to 2,000 TCID50 was injected directly into the thymus/liver grafts in a volume of 50 to 100 μl. SCID-hu mice were biopsied at 3, 6, 9, and 12 weeks postinfection. For each biopsy, the grafts were again exteriorized and one-quarter to one-half of the tissue, depending on the size of the graft, was removed. A single-cell suspension was made by mincing the tissue with two scalpels in Iscove's medium (Life Technologies, Rockville, Md.) supplemented with 2% fetal bovine serum (Omega Scientific, Tarzana, Calif.) and gentamicin (50 μg/ml; Life Technologies). The cells were filtered through 70-μm nylon mesh and transported on ice from the BL2+ mouse facility to the BL3 laboratory.
Flow cytometry.
Cells were washed twice in phosphate-buffered saline (PBS), counted, and aliquoted (106 cells per well) into 96-well V-bottom plates (Costar, Cambridge, Mass.). Fluorochrome-conjugated monoclonal antibodies (MAbs) were added to each well, and the plates were agitated and incubated 30 to 60 min in the dark at 4°C. MAbs used together were CD7-fluorescein isothiocyanate (FITC), CD4-phycoerythrin (PE) (CalTag, South San Francisco, Calif.), CD8-peridinin chlorophyll protein (PerCP) (BDIS, San Jose, Calif.), CD8-FITC (BDIS), CD4-PE, and CD3-PerCP (BDIS). Following incubation with MAb, the cells were washed twice with 200 μl of PBS, resuspended in 100 μl of PBS–2% formaldehyde, and incubated for 16 h at 4°C in the dark. Samples were diluted with PBS, and 104 cells, discriminated by their 90° and low-angle light scattering properties, were analyzed with a FACScan flow cytometer fitted with a helium-neon laser, appropriate filters for the fluorochromes used, and CellQuest software.
Statistical methods.
Statistical analysis of the percentage of CD4 CD8 double-positive (DP) cells, and the CD4 single-positive (SP)/CD8 SP ratio, in thymus/liver grafts 6 weeks after mock infection or infection with patient ACH142 clones 8G9, 32D2, *E11 (CCR5 +/+ grafts), and *E11 (CCR5 +/Δ32 grafts), or the X4 HIV-1 molecular clone NL4-3, was by one-way analysis of variation (ANOVA). In order to maintain an experimental type I error rate of less than or equal to 0.05, the t tests for between-group comparisons were adjusted by Tukey's honest significant difference (HSD) criterion (48). Data from mock-infected and NL4-3-infected SCID-hu thymus/liver grafts from other experiments were included in these analyses to increase the power of the statistical comparisons. Statistical computations were carried out in SAS version 6.12 with Proc Mixed or Prism 2.0 software (SAS Institute, Inc., Cary, N.C.; GraphPad Software, San Diego, Calif.).
Quantitative PCR.
Genomic DNA was purified using a QIAamp blood kit (Qiagen, Valencia, Calif.) from approximately 107 thymocytes from each biopsy except when cell numbers obtained from biopsied material were too low, in which case as close to 107 thymocytes as possible were used. PCR amplification was performed by an initial denaturation step at 94°C for 2 min followed by 23 cycles of 94°C for 30 s and 65°C for 1 min with primers M667 and AA55, specific for R/U5 region of HIV-1 long terminal repeat (LTR) (70), using a model PT-200 thermocycler (MJ Research, Watertown, Mass.). Primers specific for the human β-globin gene were used to detect cellular DNA. In each case, one of the two primers used was labeled on the free 5′ phosphate by using T4 bacteriophage polynucleotide kinase (New England Biolabs, Beverly, Mass.) and [γ-32P]ATP. A standard curve for the number of HIV-1 copies was generated for each PCR with fivefold dilutions of EcoRI digested nSVNX-JRCSF mixed with genomic DNA from 105 PBMC. A standard curve for the number of β-globin copies was generated for each PCR with fivefold dilutions of genomic DNA from PBMC. In both cases, the standard curve was used only for the range of values over which a linear regression gave an r2 value of greater than or equal to 0.98. Radiolabeled PCR products were resolved by electrophoresis on 6% polyacrylamide–1× Tris-borate-EDTA gels. HIV-1 and β-globin copy numbers were obtained from the standard curve, using a model 425 PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.).
Preparation and infection of PBMC.
Whole blood was collected from both a normal CCR5 homozygous individual and a CCR5Δ32 homozygous individual. PBMC from each donor were isolated from whole blood by density gradient centrifugation. Briefly, whole blood was diluted 1:2 with PBS, layered over Histopaque-1077 (Sigma-Aldrich, St. Louis, Mo.), and centrifuged at 400 × g for 1 h. The opaque interface containing mononuclear cells was removed, and mononuclear cells were washed two times with PBS. Cells were resuspended at a concentration of 2 × 106 cells/ml in RPMI 1640 with 10% fetal calf serum, PHA, and gentamicin. After 2 days, IL-2 (10 U/ml) was added to the cell culture. On day 3, stimulated PBMC were washed two times with PBS and resuspended at a concentration of 106 cells/ml in RPMI 1640 with 10% fetal calf serum. Then 2.5 × 104 cells (25 μl) were added to each well of a 96-well plate. Viral stocks were diluted to equivalent titers, and 25 μl of a diluted virus stock containing Polybrene (8 μg/μl) was added to each of four wells with normal CCR5 homozygous PBMC and four wells with CCR5Δ32 homozygous PBMC. Infected cell cultures were incubated at 37° for 2 h, and then 200 μl of RPMI 1640 with 10% fetal calf serum, IL-2 (10 U/ml), and gentamicin was added. Supernatants were collected, and an equal volume of medium was replaced every day for 2 weeks. The concentration of p24 in the collected supernatants was measured by p24 enzyme-linked immunosorbent assay (ELISA) (NEN Life Science Products, Boston, Mass.).
GHOST cell assay.
GHOST cells were obtained from the NIH AIDS Research and Reference Reagents Program; they were donated by V. KewalRamani and D. Littman. GHOST-CCR1, -CCR2b, -CCR3, -CCR4, -CCR5, -CCR8, -CXCR4, -V28/CX3CR1, -BONZO/STRL33, and -BOB/GPR15 cell lines, 5 × 104 cells per well in 12-well plates, were infected with 0.5 ml of one of three R5 patient ACH142 clones, HIV-1/NL4-3, or HIV-1/JR-CSF in the presence of Polybrene (4 μg/μl) according to the protocol provided by the donors. Cells were harvested 48 h postinfection, and green fluorescent protein (GFP) fluorescence was measured by flow cytometry. For assay of viruses cultured in SCID-hu mice, 2 × 107 thymocytes were cocultured with 2 × 107 PHA-activated PBMC for 3 days. Supernatants were collected and used to infect 5 × 104 GHOST-CCR5 or GHOST-CXCR4 cells in 12-well plates. GHOST cells were incubated for 48 h and then removed for flow cytometric assay of GFP expression.
RESULTS
R5 HIV-1 biological clones isolated from patients at different stages of infection were used to infect SCID-hu mice.
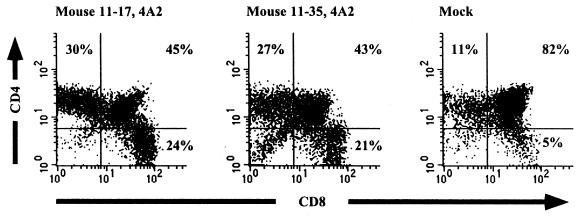
HIV-1 biological clones were isolated at early, middle, and late stages of disease from three patients who never developed X4 phenotype virus despite progressing to AIDS and death (Table 1). Several biological clones were isolated from each time point and tested for syncytium induction phenotype by the MT-2 cell syncytium formation assay. At each time point no X4 virus was detected by this assay. Initially we chose to study one HIV-1 biological clone from an early stage of infection and a second clone from a later stage of infection from two patients. In each case, the later HIV-1 clone was isolated after the patient experienced a significant drop in the number of peripheral CD4+ T cells and in one case following an AIDS diagnosis. SCID-hu thymus/liver grafts were infected with 400 TCID50 of the following R5 patient clones: ACH142-8A4, ACH142-32A7, AMS198-1C10, AMS198-1A11, and AMS198-4A2. The R5 AIDS clone AMS198-4A2 depleted CD4+ thymocytes from two of three human thymus liver grafts in SCID-hu mice by 13 weeks postinfection, while ACH142-32A7 caused mild CD4+ thymocyte depletion in one of three grafts at this time point (Fig. 1). These results suggested that late-stage AIDS-associated R5 HIV-1 clones were pathogenic for human thymocytes. To further test this hypothesis, a second set of SCID-hu infections was performed with 1,000 to 2,000 TCID50 of three R5-AIDS clones from two patients: ACH424-23A1, ACH424-23G2, and ACH142-*E11. In this experiment, mild depletion of CD4 DP and SP thymocytes was observed 4 weeks after infection with ACH424-23A1 in one of three grafts (mouse 24-17 [Table 2]). By 8 weeks postinfection, marked depletion of human CD4 SP and DP thymocytes was observed in this same mouse, and CD4 DP cells were depleted in one of two grafts infected with ACH142-*E11 (Mice 24-7 and 24-17 [Table 2]). Moderate depletion of mature CD4 SP cells only was also seen 8 weeks postinfection in one of two ACH424-23G2-infected grafts (mouse 24-9 [Table 2]). At 12 weeks postinfection, CD4+ thymocyte depletion was seen in all surviving infected mice, two infected with ACH424-23A1 and two infected with ACH424-23G2 (Table 2). The mock-infected mouse, 24-11, retained 84% CD4 CD8 DP cells and a CD4 SP/CD8 SP ratio of 2.0.
TABLE 1.
Isolation of HIV-1 biological clones from three patients who did not develop X4 phenotype virusa
| Patient | Date | No. of CD4+ cells/μl | CD4+/ CD8+ | Biological clone | TCID50/ml |
|---|---|---|---|---|---|
| ACH424b | 9/1/88 | 660 | 0.45 | 9F4 | 3.31 × 103 |
| 9H1 | 5.01 × 103 | ||||
| 5/21/92 | 110 | 0.17 | 23A1 | 1.66 × 104 | |
| 23G2 | 2.51 × 104 | ||||
| ACH142c | 8/18/86 | 720 | 1.06 | 8A4 | 7.41 × 103 |
| 8G9 | 5.01 × 103 | ||||
| 8/25/92 | 310 | 0.09 | 32A7 | 3.72 × 104 | |
| 32D2 | 2.51 × 104 | ||||
| 4/28/94 | 300 | 0.1 | *E11 | 3.72 × 104 | |
| AMS198d | 9/4/89 | 430 | 3.3 | 1C10 | 8.32 × 104 |
| 1A11 | 5.01 × 103 | ||||
| 7/1/91 | 250 | 1.2 | 3F4 | 1.66 × 104 | |
| 3C7 | 2.51 × 104 | ||||
| 9/16/93 | 30 | 0.27 | 4A2 | 1.12 × 104 |
R5 HIV-1 biological clones were isolated from three patients in the Amsterdam cohort at different stages of disease. At each time point, blood was drawn and the numbers of CD4+ and CD8+ T cells per microliter were determined. Biological clones were isolated by limiting dilution of PBMC at each time point, stocks were made, and the titer was measured on PBMC.
Seroconversion, 8/22/88; AIDS, 10/18/91 Candida esophagitis); deceased, 2/09/94.
Seroconversion, before 1986; AIDS, 1991; deceased, 9/16/93.
Seroconversion, before 11/22/84; AIDS, 12/15/93 (Kaposi's sarcoma); deceased, 4/28/94.
FIG. 1.
CD4 and CD8 expression of thymocytes isolated from two SCID-hu thymus/liver grafts infected with the R5-AIDS HIV-1 biological clone AMS198-4A2 and from one mock-infected thymus/liver graft. Cells were isolated 13 weeks postinfection and stained with fluorochrome-conjugated CD4 and CD8 MAbs. Expression of the corresponding cell surface antigens was measured by flow cytometry. Percentages of CD4 SP CD8 SP and CD4 CD8 DP cells are shown.
TABLE 2.
Summary of flow cytometric data from 4, 8, and 12 weeks after infection of SCID-hu mice with R5-AIDS HIV-1 clone ACH142-*E11, ACH424-23G2, or ACH424-23A1a
| Mouse | Virus | Cell distribution
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 wk
|
8 wk
|
12 wk
|
|||||||||||
| % CD4+ | % CD8+ | % CD4+ CD8+ | CD4+/CD8+ | % CD4+ | % CD8+ | % CD4+ CD8+ | CD4+/CD8+ | %CD4+ | % CD8+ | % CD4+ CD8+ | CD4+/CD8+ | ||
| 24-3 | *E11 | 12 | 7 | 80 | 1.7 | 14 | 10 | 76 | 1.4 | ||||
| 24-5 | 23G2 | 10 | 5 | 85 | 2.0 | 11 | 8 | 80 | 1.4 | ||||
| 24-6 | 23A1 | 16 | 6 | 77 | 2.7 | 12 | 7 | 81 | 1.7 | ||||
| 24-7 | *E11 | 13 | 7 | 79 | 1.9 | 19 | 14 | 31 | 1.4 | ||||
| 24-9 | 23G2 | 19 | 9 | 70 | 2.1 | 10 | 10 | 79 | 1.0 | 25 | 27 | 42 | 0.9 |
| 24-10 | 23A1 | 10 | 6 | 84 | 1.7 | 15 | 9 | 74 | 1.7 | 13 | 16 | 21 | 0.8 |
| 24-11 | Mock | 13 | 4 | 82 | 3.3 | 9 | 5 | 86 | 1.8 | 10 | 5 | 84 | 2.0 |
| 24-15 | *E11 | 11 | 4 | 84 | 2.8 | ||||||||
| 24-17 | 23A1 | 22 | 21 | 50 | 1.0 | 19 | 50 | 29 | 0.4 | 43 | 36 | 26 | 1.2 |
Cells were stained with fluorochrome-conjugated CD4 and CD8 MAbs, and antigen expression was measured by flow cytometry. Data are not shown for one mouse at 8 weeks and five mice at 12 weeks due to their death.
We chose to study three R5 HIV-1 biological clones from patient ACH142 in greater detail.
ACH142-*E11 depleted CD4+ thymocytes from human thymus/liver grafts in SCID-hu mice, while the earlier clones ACH142-8A4 and ACH142-32A7 did not. Furthermore, ACH142-*E11 achieved the highest viral load of any R5-AIDS clone in the experiment described above and in Table 2 (data not shown). The early-stage virus 8G9 was isolated in 1986, at least 21 months after seroconversion, when the patient's peripheral CD4+ T-cell count was 720/μl and his peripheral CD4+ T-cell/peripheral CD8+ T-cell ratio was 1.06 (Table 1). The intermediate-stage virus 32D2 was isolated in 1992 when the CD4+ T-cell count had fallen to 310 cells/μl and the ratio of peripheral CD4+ to CD8+ T cells was 0.09. The patient was diagnosed with AIDS in December 1993 and died in April 1994. *E11 was isolated at autopsy when the CD4+ and CD8+ T-cell numbers were at levels similar to those seen in 1992 (Table 1). The three patient ACH142 HIV-1 biological clones that we studied, and all other HIV-1 isolates and clones derived from this patient, were negative in an MT-2 cell syncytium formation assay.
Patient ACH142-derived HIV-1 biological clones have different growth characteristics in PBMC from normal donors.
We characterized the in vitro growth characteristics of the three HIV-1 biological clones isolated from patient ACH142 at three different time points during the course of disease in normal donor PBMC (Fig. 2A). Each biological clone was used to infect 2-day PHA- and IL-2-stimulated normal donor PBMC at a multiplicity of infection (MOI) of 0.01. Viral growth was measured by the presence of the viral capsid protein p24 in the supernatants of infected cell cultures. For all three biological clones, p24 production peaked on day 6 postinfection. The early virus 8G9 did not grow as well as the middle or late HIV-1 clones. Both 32D2 and *E11, however, could infect normal PBMC with efficiency and kinetic characteristics similar to the control virus, NL4-3.
FIG. 2.
HIV-1 capsid (p24) production by PBMC infected with HIV-1 ACH142-8G9, ACH142-32D2, ACH142-*E11, or NL4-3, at an MOI of 0.01. PBMC were isolated from a CCR5+ homozygous donor (A) and a CCR5Δ32 homozygous donor (B). p24 concentration was measured on supernatants collected on days 3, 6, 9, and 12 postinfection using a commercial ELISA kit (NEN Life Science Products).
ACH142 biological clones do not infect PBMC from an individual homozygous for the CCR5Δ32 allele.
We hypothesized that late-stage R5 patient clones may gain increased pathogenesis or increased replication capacity by using other coreceptors, in addition to CCR5, for entry into CD4+ T cells. We tested this hypothesis by attempting to infect primary cells which do not express CCR5. We isolated PBMC from a donor who is homozygous for the CCR5Δ32 mutation, which inactivates CCR5, and stimulated the PBMC with PHA and IL-2 for 2 days. We infected the PBMC at an MOI of 0.01 with the three primary HIV-1 biological clones, the R5 molecular clone JR-CSF, and the X4 molecular clone NL4-3. Only infection with the X4 molecular clone NL4-3 resulted in production of viral p24 antigen, indicative of viral replication (Fig. 2B). The peak of p24 production by NL4-3 was 9 days postinfection. These results suggest that all three patient ACH142 biological clones require the CCR5 coreceptor for infection of PBMC.
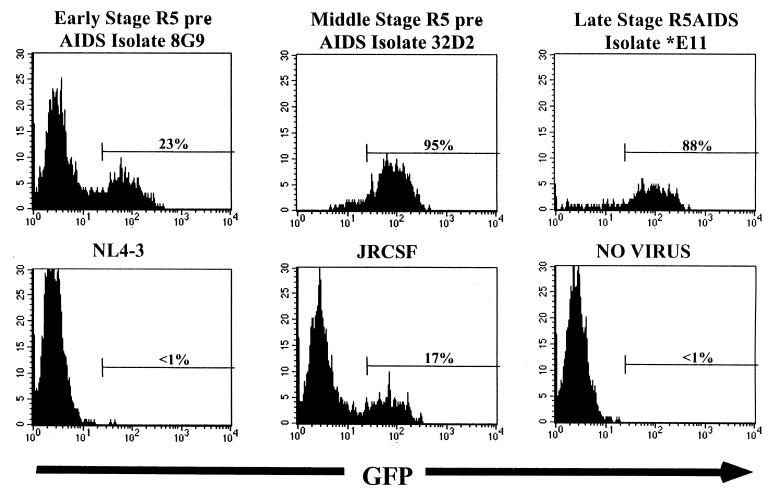
Primary HIV-1 biological clones from patient ACH142 infect a CCR5-expressing GHOST cell line but not GHOST cell lines expressing CCR1, CCR2b, CCR3, CCR4, CCR5, CCR8, CXCR-4, BOB, BONZO, or V28.
PBMC from a CCR5Δ32 donor may not express a full complement of HIV-1 coreceptors. To further test the ability of ACH142 clones to enter cells via coreceptors other than CCR5, we attempted to infect GHOST cell lines expressing different known HIV coreceptors with each clone. GHOST cells expressing most of the known HIV coreceptors were obtained from the NIH AIDS Research and Reference Reagents Program. Infection was measured by the production of GFP detected by flow cytometry. For the three patient ACH142 biological clones 8G9, 32D2, and *E11 and the R5 molecular clone JR-CSF, GFP was detected 48 h postinfection only in a GHOST cell line expressing the CCR5 coreceptor (Fig. 3). The X4 molecular clone NL4-3 did not infect GHOST-CCR5 cells or any other GHOST cell line except GHOST-CXCR4. No GFP was detected in GHOST cell lines expressing CCR1, CCR2b, CCR3, CCR4, CCR8, BOB, BONZO or V28. We conclude that CCR5 is the only known coreceptor used by the three biological clones derived from different stages of infection of patient ACH142.
FIG. 3.
GFP expression of CCR5-expressing GHOST cells infected with HIV-1 clone ACH142-8G9, ACH142-32D2, ACH142-*E11, NL4-3, or JR-CSF. GHOST cells were infected with 0.5 ml of viral stocks in the presence of Polybrene (4 μg/ml). Forty-eight hours after infection, cells were harvested and fixed, and GFP expression was measured by flow cytometry with a FACScan.
R5 patient ACH142 biological clones from early, middle, and late stages of infection differ in CD4+ cell depletion in the SCID-hu mouse.
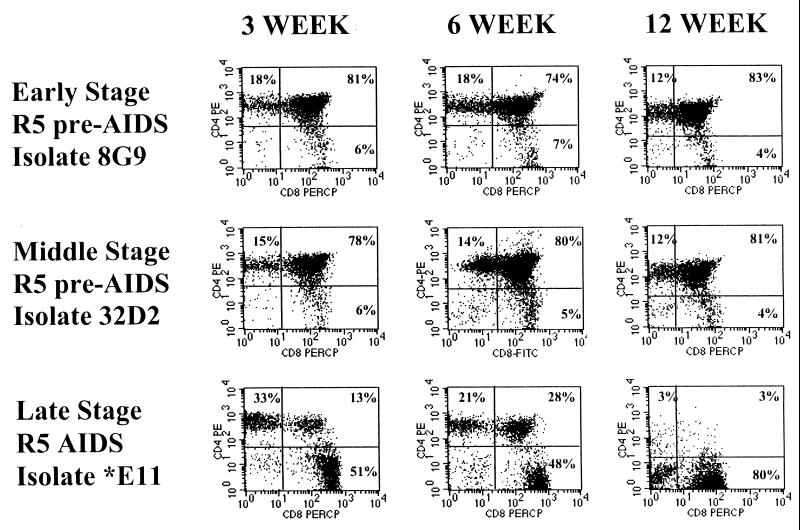
Replication and pathogenesis of the three biological clones was measured in the SCID-hu mouse model. We infected human thymus/liver grafts implanted in SCID-hu mice with the early- or middle-stage R5 pre-AIDS or the late-stage R5-AIDS biological clones by injection of 1,000 TCID50 of virus directly into the thymus/liver grafts. Mice were biopsied and thymocytes were isolated at 3, 6, and 12 weeks postinfection. CD4 and CD8 cell surface antigens were detected by flow cytometry with directly conjugated fluorescently labeled MAbs. CD4+ thymocyte depletion was observed in R5-AIDS clone *E11-infected grafts once at 3 weeks, often at 6 weeks, and always at 12 weeks postinfection (Fig. 4). In Fig. 4, the *E11-infected graft which showed CD4+ thymocyte depletion at 3 weeks is shown. This graft was almost completely depleted of CD4+ thymocytes at 12 weeks postinfection, leaving only CD8 SP and double-negative cells. For the R5 pre-AIDS clones 8G9 and 32D2, only limited cytopathic effects were seen on one occasion for each clone at 12 weeks postinfection. CD4+ thymocyte depletion in the grafts was assayed by measuring the ratio of CD4 SP to CD8 SP thymocytes and the percent CD4 CD8 DP cells (Table 3). Statistical analyses described below show that as a group, the *E11-infected grafts were significantly depleted of CD4+ cells at 6 weeks postinfection. We established cutoff values for each of these measures to define significant CD4 depletion in individual infected grafts. These values allow us to define pathogenesis as changes in these measures which are unlikely to occur by chance in the mock-infected grafts. Thus, a CD4 SP/CD8 SP ratio of less than 1.25 or a CD4 CD8 DP cell percentage below 55 was considered indicative of CD4+ thymocyte depletion. The probability of a mock-infected graft having a CD4 SP/CD8 SP ratio of less than 1.25 is 0.04, while the probability of a mock-infected graft having a CD4 CD8 DP cell percentage below 55 is 0.0007. None of the mice infected with the R5 pre-AIDS clone 8G9 or 32D2 displayed evidence of CD4+ cell depletion by these criteria at 3 or 6 weeks postinfection (Table 3; Fig. 5). In contrast, at 3 weeks postinfection we observed CD4 SP thymocyte depletion, judged by the CD4 SP/CD8 SP ratio, in one of seven mice infected with the late-stage R5-AIDS virus *E11. Six weeks postinfection, five of seven mice infected with the R5-AIDS virus *E11 showed depletion of CD4 SP cells and two showed depletion of CD4 CD8 DP cells as well by these criteria. An *E11 dose of 1,000 TCID50 was not sufficient, however, to cause depletion of CD4+ thymocytes from four SCID-hu mice bearing human thymus/liver grafts which were heterozygous for the CCR5Δ32 mutation (Table 3; Fig. 5). Following infection of SCID-hu mice with patient ACH142 clones, thymocytes recovered 6 weeks postinfection were cocultured with PHA-activated PBMC. Supernatants of these cocultures were used to infect GHOST-CCR5 and GHOST-CXCR4 cells to determine if the viral tropism had been changed by culture in SCID-hu thymus/liver grafts. In no case was evidence of a switch in tropism seen (data not shown).
FIG. 4.
Representative CD4 and CD8 expression on thymocytes from thymus/liver grafts 3, 6, and 12 weeks after infection with HIV-1 ACH142-8G9, ACH142-32D2, or ACH142-*E11. Thymus/liver grafts were biopsied, a single-cell suspension was made, and the cells were stained with fluorochrome-conjugated CD4 and CD8 MAbs. Expression of the corresponding cell surface antigens was measured by flow cytometry. The data are representative of those summarized in Table 3.
TABLE 3.
Summary of CD4 and CD8 expression on human thymocytes derived from SCID-hu thymus/liver grafts 3 and 6 weeks after infection with HIV-1 ACH142-8G9, ACH142-32D2, and ACH142-*E11a
| Mouse | Virus | Cell distribution
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 3 wk
|
6 wk
|
||||||||
| % CD4+ | % CD8+ | % CD4+ CD8+ | CD4+/CD8+ | % CD4+ | % CD8+ | % CD4+ CD8+ | CD4+/CD8+ | ||
| 38-5 | Mock | 8 | 4 | 87 | 2.0 | 15 | 6 | 78 | 2.5 |
| 38-26 | Mock | 10 | 5 | 85 | 2.0 | 10 | 6 | 81 | 1.7 |
| 47-14 | Mock | 16 | 6 | 78 | 2.7 | 13 | 4 | 83 | 3.3 |
| 38-6 | 8G9 | 12 | 6 | 81 | 2.0 | 18 | 8 | 74 | 2.3 |
| 47-16 | 8G9 | 9 | 4 | 86 | 2.3 | 9 | 6 | 85 | 1.5 |
| 47-18 | 8G9 | 19 | 8 | 73 | 2.4 | 18 | 7 | 74 | 2.6 |
| 49-5 | 8G9 | 13 | 4 | 83 | 3.3 | 12 | 5 | 83 | 2.4 |
| 49-30 | 8G9 | 16 | 5 | 78 | 3.2 | 14 | 7 | 78 | 2.0 |
| 38-8 | 32D2 | 17 | 7 | 73 | 2.4 | 19 | 12 | 60 | 1.6 |
| 47-11 | 32D2 | 27 | 11 | 60 | 2.5 | 6 | 3 | 89 | 2.0 |
| 47-12 | 32D2 | 13 | 4 | 83 | 3.3 | 17 | 7 | 75 | 2.4 |
| 47-19 | 32D2 | 19 | 10 | 69 | 1.9 | 12 | 6 | 82 | 2.0 |
| 49-11 | 32D2 | 13 | 3 | 83 | 4.3 | 14 | 5 | 80 | 2.8 |
| 38-12 | *E11 | 20 | 9 | 71 | 2.2 | 23 | 15 | 59 | 1.5 |
| 38-14 | *E11 | 12 | 5 | 83 | 2.4 | 13 | 12 | 73 | 1.1 |
| 38-18 | *E11 | 23 | 10 | 68 | 2.3 | 18 | 15 | 63 | 1.2 |
| 38-20 | *E11 | 19 | 9 | 71 | 2.1 | 33 | 34 | 23 | 1.0 |
| 30-42 | *E11 | 33 | 51 | 13 | 0.6 | 21 | 48 | 28 | 0.4 |
| 49-13 | *E11 | 13 | 5 | 81 | 2.6 | 16 | 6 | 77 | 2.7 |
| 49-17 | *E11 | 14 | 5 | 79 | 2.8 | 14 | 16 | 67 | 0.9 |
| 46-13 +/Δ32 | *E11 | 9 | 5 | 85 | 1.8 | ||||
| 46-15 +/Δ32 | *E11 | 8 | 5 | 86 | 1.6 | ||||
| 46-27 +/Δ32 | *E11 | 12 | 6 | 81 | 2.0 | ||||
| 46-29 +/Δ32 | *E11 | 8 | 4 | 88 | 2.0 | ||||
| 38-25 | NL4-3 | 18 | 14 | 66 | 1.3 | 22 | 44 | 9 | 0.5 |
| 30-24 | NL4-3 | 11 | 5 | 83 | 2.2 | 27 | 54 | 3 | 0.5 |
| 49-28 | NL4-3 | 13 | 7 | 79 | 1.9 | 31 | 34 | 20 | 0.9 |
Thymus/liver implants from several donors were infected with 1,000 TCID50 of HIV-1 ACH142-8G9, ACH142-32D2, ACH142-*E11, or NL4-3. Implants were from CCR5 +/+ homozygous donors except for implant 46, which was derived from a CCR5 +/Δ32 heterozygous donor. The CCR5 +/Δ32 grafts were biopsied only at 6 weeks postinfection.
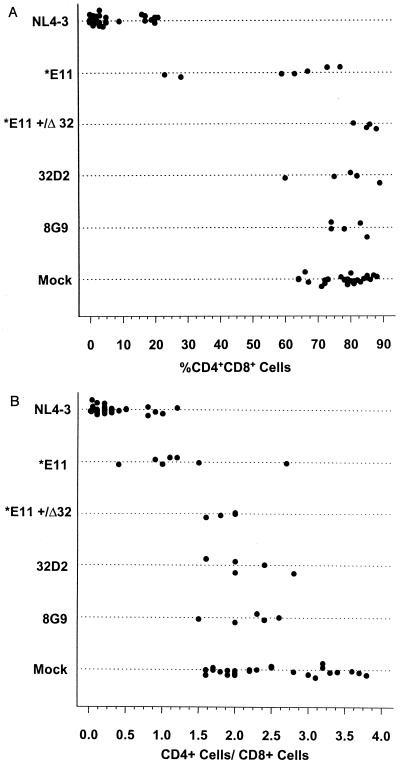
FIG. 5.
Plot comparing percent CD4 CD8 DP thymocytes (A) or CD4 SP/CD8 SP thymocyte ratio (B) at 6 weeks after infection of thymus/liver grafts infected with HIV-1 ACH142-8G9, ACH142-32D2, ACH142-*E11, or NL4-3 or mock infected.
Statistical analyses by one-way ANOVA and Tukey's HSD criterion-adjusted t tests were carried out to compare each group of infected grafts with all other groups.
Statistical comparisons (Tables 4 and 5) revealed that the percent CD4 CD8 DP cells found 6 weeks after infection of thymus/liver grafts with *E11 was significantly different from the value found for 8G9-, 32D2-, or mock-infected grafts (P < 0.001, P < 0.002, or P < 0.001). This was not true, however, for 8G9- or 32D2-infected grafts or for CCR5+/Δ32 grafts infected with *E11, which did not result in a percentage of CD4 CD8 DP cells 6 weeks later that was significantly different from the value for mock-infected grafts (Table 4; Fig. 5A). The percentage of cells remaining 6 weeks after infection of CCR5 +/+ grafts with *E11 which were CD4 CD8 DP was also significantly less than the percentage of DP cells among the cells which remained in *E11-infected CCR5 +/Δ32 grafts (P < 0.001). Similarly, the CD4 SP/CD8 SP ratio 6 weeks after infection with the R5-AIDS clone *E11 was significantly lower than for mock-infected grafts (P < 0.001), but the CD4 SP/CD8 SP ratio difference between *E11- and 8G9- or 32D2-infected grafts was only marginally significant (P < 0.055 [Table 4; Fig. 5B]). Infection with the R5 pre-AIDS clone 8G9 or 32D2, however, did not lead to a significant difference in the CD4 SP/CD8 SP ratio compared to mock-infected grafts. For both of these measures, NL4-3-infected grafts were significantly different from all other groups.
TABLE 4.
Statistical analysis of percent CD4 CD8 DP thymocytes and CD4 SP/CD8 SP thymocyte ratio 6 weeks after infection of thymus/liver grafts with HIV-1 ACH142-8G9, ACH142-32D2, ACH142-*E11, or NL4-3, or mock infectiona
| Between-group comparison | Δ Mean | Δ SE | Lower 95% CL | Upper 95% CL | Probability Δ = 0 |
|---|---|---|---|---|---|
| % CD4 CD8 DP thymocytes | |||||
| Mock-8G9 | −1.24 | 4.51 | −14.46 | 11.97 | 1.000 |
| Mock-32D2 | 0.36 | 4.51 | −12.86 | 13.57 | 1.000 |
| Mock-E11 (CCR5 +/+ grafts) | 21.84 | 3.93 | 10.33 | 33.36 | <0.001 |
| Mock-E11 (CCR5 +/Δ32) | −7.44 | 4.97 | −21.99 | 7.10 | 0.666 |
| Mock–NL4-3 | 71.42 | 2.46 | 64.22 | 78.62 | <0.001 |
| 8G9-32D2 | 1.60 | 5.86 | −15.57 | 18.77 | 1.000 |
| 8G9-E11 (CCR5 +/+ grafts) | 23.09 | 5.43 | 7.19 | 38.98 | <0.001 |
| 8G9-E11 (CCR5 +/Δ32) | −6.20 | 6.22 | −24.41 | 12.01 | 0.918 |
| 8G9–NL4-3 | 72.67 | 4.48 | 59.55 | 85.78 | <0.001 |
| 32D2-E11 (CCR5 +/+ grafts) | 21.49 | 5.43 | 5.59 | 37.38 | <0.002 |
| 32D2-E11 (CCR5 +/Δ32) | −7.80 | 6.22 | −26.01 | 10.41 | 0.809 |
| 32D2–NL4-3 | 71.07 | 4.48 | 57.95 | 84.18 | <0.001 |
| E11 (CCR5 +/+ graft)–E11 (CCR5 +/Δ32) | −29.29 | 5.81 | −46.30 | −12.27 | <0.001 |
| E11 (CCR5 +/+ graft)–NL4-3 | 49.58 | 3.89 | 38.18 | 60.98 | <0.001 |
| E11 (CCR5 +/Δ32)–NL4-3 | 78.87 | 4.94 | 64.41 | 93.32 | <0.001 |
| CD4 SP/CD8 SP thymocyte ratio | |||||
| Mock-8G9 | 0.34 | 0.26 | −0.42 | 1.10 | 0.780 |
| Mock-32D2 | 0.34 | 0.26 | −0.42 | 1.10 | 0.780 |
| Mock-E11 (CCR5 +/+ grafts) | 1.24 | 0.23 | 0.58 | 1.91 | <0.001 |
| Mock-E11 (CCR5 +/Δ32) | 0.65 | 0.29 | −0.19 | 1.49 | 0.219 |
| Mock-NL4-3 | 2.19 | 0.20 | 1.77 | 2.60 | <0.001 |
| 8G9-32D2 | 0.00 | 0.34 | −0.99 | 0.99 | 1.000 |
| 8G9–E11 (CCR5 +/+ grafts) | 0.90 | 0.31 | −0.01 | 1.82 | 0.055 |
| 8G9–E11 (CCR5 +/Δ32) | 0.31 | 0.36 | −0.74 | 1.36 | 0.953 |
| 8G9–NL4-3 | 1.85 | 0.26 | 1.09 | 2.60 | <0.001 |
| 32D2–E11 (CCR5 +/+ grafts) | 0.91 | 0.34 | −0.01 | 1.82 | 0.055 |
| 32D2-E11 (CCR5 +/Δ32) | 0.31 | 0.36 | −0.74 | 1.36 | 0.953 |
| 32D2-NL4-3 | 1.85 | 0.26 | 1.09 | 2.60 | <0.001 |
| E11 (CCR5 +/+ graft)–E11 (CCR5 +/Δ32) | −0.59 | 0.33 | −1.57 | 0.39 | 0.490 |
| E11 (CCR5 +/+ graft)–NL4-3 | 0.95 | 0.22 | 0.29 | 1.60 | 0.001 |
| E11 (CCR5 +/Δ32)–NL4-3 | 1.54 | 0.28 | 0.71 | 2.37 | <0.001 |
Corresponding summary measures are given in Table 5.
TABLE 5.
Summary measures to quantitatively describe the distribution of values analyzed in Table 4
| Virus | Status | n | % CD4 CD8 DP thymocytes
|
CD4 SP/CD8 SP thymocyte ratio
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Median | Minimum | Maximum | Q(0.25) | Q(0.75) | Mean | SEM | Median | Minimum | Maximum | Q(0.25) | Q(0.75) | |||
| Mock | Nonpathogenic | 27 | 77.6 | 1.3 | 79.0 | 64.0 | 88.0 | 72.5 | 82.5 | 2.5 | 0.1 | 2.3 | 1.6 | 3.8 | 2.0 | 3.2 |
| 8G9 | Unknown | 5 | 78.8 | 2.3 | 78.0 | 74.0 | 85.0 | 74.0 | 83.0 | 2.2 | 0.2 | 2.3 | 1.5 | 2.6 | 2.0 | 2.4 |
| 32D2 | Unknown | 5 | 77.2 | 4.9 | 80.0 | 60.0 | 89.0 | 75.0 | 82.0 | 2.2 | 0.2 | 2.0 | 1.6 | 2.8 | 2.0 | 2.4 |
| E11 (CCR5 +/+ grafts) | Unknown | 7 | 55.7 | 8.1 | 63.0 | 23.0 | 77.0 | 43.5 | 70.0 | 1.3 | 0.3 | 1.1 | 0.4 | 2.7 | 1.0 | 1.4 |
| E11 (CCR5 +/Δ32) | Unknown | 4 | 85.0 | 1.5 | 85.5 | 81.0 | 88.0 | 84.0 | 86.5 | 1.9 | 0.1 | 1.9 | 1.6 | 2.0 | 1.8 | 2.0 |
| NL4-3 | Pathogenic | 30 | 6.1 | 1.3 | 3.0 | 0.0 | 21.0 | 1.0 | 8.0 | 0.3 | 0.1 | 0.2 | 0.0 | 1.2 | 0.1 | 0.4 |
R5 patient clones from early, middle, and late stages of infection differ in replication in the SCID-hu mouse.
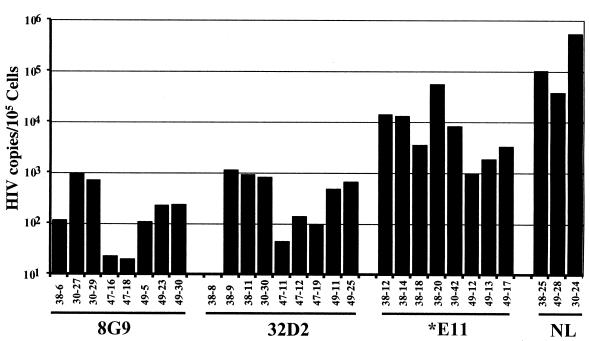
From each biopsy, genomic DNA was isolated from thymocytes and used to determine the amount of viral DNA present. Quantitative PCR was performed with primers which amplify the HIV-1 LTR region and the human globin gene. With known amounts of HIV-1 and globin DNA, a standard curve was generated from which the amounts of globin and HIV-1 DNA in the biopsy samples were extrapolated. Viral DNA recovered from the human thymus/liver grafts reached its highest level, of any time measured, at 6 weeks postinfection (Fig. 6). The level of HIV-1 DNA detected in 8G9- and 32D2-infected grafts, however, was on average 10-fold lower than that found in *E11-infected grafts and more than 100-fold lower than the level of viral DNA obtained from NL4-3-infected thymus/liver grafts.
FIG. 6.
HIV-1 DNA copies per 105 thymus/liver cells in infected thymus liver grafts derived from SCID-hu mice, determined by quantitative PCR. Genomic DNA was isolated and subjected to 22 cycles of quantitative PCR using a primer pair specific for HIV-1 DNA and a primer pair specific for the human β-globin gene. In each case, one primer was end labeled with 32P. PCR products were resolved on a 6% polyacrylamide gel. Quantitation was performed by comparison to standard curves of the PCR products of known amounts of both HIV-1 plasmid DNA and human genomic DNA, using a PhosphorImager.
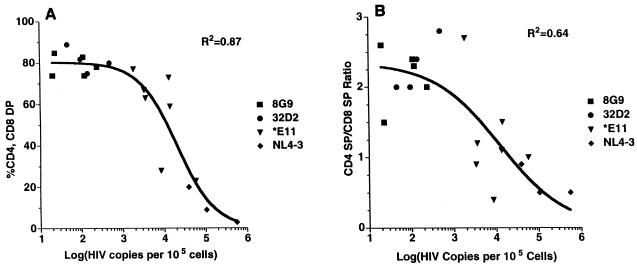
The level of HIV-1 viral DNA detected in thymus/liver grafts exhibited a sigmoid relationship with the percentage of CD4 CD8 DP cells found.
This nonlinear relationship was highly significant (R2 = 0.87) [Fig. 7A]) and gave a better fit to the data than could be achieved with a straight line. Similarly, the level of HIV-1 viral DNA detected 6 weeks postinfection was highly correlated with the CD4 SP/CD8 SP ratio and exhibited a sigmoid relationship that was more significant than could be achieved with a linear regression (r2 = 0.64 [Fig. 7B]). These nonlinear relationships indicate that a threshold effect pertains to the replication of HIV-1 needed for cytopathic effects in SCID-hu mice similar to, but not as extreme as, the “all or nothing” binding of oxygen by hemoglobin. The inflection point of each sigmoid curve indicates the threshold value of viral replication—the point at which the slope of the curve is steepest—where a small increase in viral replication yields a large increase in cytopathic effect. For Fig. 7A, the relationship of percent CD4 CD8 DP cells to HIV-1 DNA copies, the threshold, or 50% effective copy number, is 20,550 copies of HIV-1 DNA per 105 cells with 95% confidence limits of 11,100 to 38,070. Similarly, the sigmoid plot of viral load versus the CD4 SP/CD8 SP ratio gave a 50% effective copy number of 12,220 per 105 cells with 95% confidence limits of 1,826 and 81,750.
FIG. 7.
Scatter plot of percent CD4 CD8 DP thymocytes (A) or CD4 SP/CD8 SP thymocyte ratio (B) versus HIV-1 DNA copies per 105 thymus/liver cells recovered 6 weeks after infection with the indicated HIV-1 clones. A nonlinear regression was determined and plotted using a four-parameter logistic equation and Prism software (GraphPad Software).
DISCUSSION
Our results show that infection with R5-AIDS HIV-1 clones has more severe cytopathic effects on human thymocytes in SCID-hu mice than infection with earlier pre-AIDS R5 clones from the same patients. The development of increased pathogenicity during the course of natural infection is a paradigm which has emerged from the study of HIV and simian immunodeficiency virus (SIV). It has been long known that later patient isolates of HIV-1 often replicate more efficiently and are more cytopathic in tissue culture than earlier isolates (15, 16, 65–67). This is particularly true of X4 HIV-1 isolates but has also been documented to a more limited extent for R5 HIV-1 isolates (68). Overbaugh and colleagues have shown that a similar pattern of increased cytopathicity developing during the course of infection occurs with SIV during the infection of rhesus macaques (42, 61). These findings suggest that the biological constraints which select the fittest HIV or SIV species change during the protracted course of infection in each individual. Therefore, the fittest quasispecies of HIV-1 immediately after infection are not the same as those which are fittest several years later. This must be so since two amino acid substitutions can convert an R5 HIV-1 strain to R5X4, yet this does not usually occur for many years postinfection despite the higher replicative capacity of X4 HIV-1 isolates and the rapid evolution of HIV-1 (20, 30). The changes which convert an early-stage R5 pre-AIDS HIV-1 isolate to a more pathogenic R5-AIDS isolate, such as those we have studied, are not yet known. The development of such isolates, however, must similarly be initially limited by the host immune system and selected later in the course of infection when antiviral immunity may be less effective and other factors such as opportunistic infections have changed the host milieu.
In a recent study by Berkowitz et al., no cytopathic effects were seen in SCID-hu mice 2.5 and 5 weeks after infection with three R5-AIDS patient isolates, including a different clone from patient ACH424 whose clones we also studied (8). This is likely explained by the shorter time course of SCID-hu infections used in this study compared to the experiments reported here. We rarely saw depletion of human thymocytes 3 weeks after infection with R5-AIDS HIV-1 clones and saw consistent cytopathic effects only at 6 weeks postinfection or later.
Despite the cytopathic effects of ACH142 R5-AIDS clone *E11 infection on normal human thymocytes in SCID-hu mice, infection by this virus did not deplete CD4+ thymocytes from CCR5 +/Δ32 heterozygous grafts. This result indicates that the lower level of CCR5 present in these grafts limits viral replication or cytopathic effects (7, 18, 44, 54, 71). Similarly, HIV-1-infected CCR5 +/Δ32 individuals have lower viral load and progress more slowly to AIDS (19, 27, 36, 52, 58). The fact that X4 viruses often replicate to higher levels in many systems including SCID-hu mice suggests that interaction with CCR5 may be rate limiting for HIV-1 replication even with the higher level of CCR5 found in a CCR5 +/+ host (10, 15, 38, 67). Our results further indicate that *E11 could not readily evolve to efficiently use another coreceptor for the infection of human thymocytes during 6 weeks of culture in CCR5 +/Δ32 thymus/liver grafts despite the presence of other coreceptors on human thymocytes (CCR3, CCR8, CCR9, CXCR4, and APJ) (7, 18, 26, 32, 33, 43, 54, 69, 71). This result also confirms our findings from tissue culture assays with GHOST cells and CCR5Δ32/Δ32 PBMC that *E11 can enter cells only via CCR5.
In this study, we found that viral replication was highly correlated with CD4+ thymocyte depletion in SCID-hu mice and that these correlations were highly significant. Furthermore, these data support our conclusion in the accompanying report that a threshold of viral replication must be surpassed for cytopathic effects to be evident in the SCID-hu system (10). We observed a bimodal distribution of the points when viral DNA copies were plotted against the two measures of cytopathicity we have used (Fig. 7). For both the percent CD4 CD8 DP cells and the CD4 SP/CD8 SP ratio plotted as a function of HIV-1 DNA copy number, our data suggest that the relationship is sigmoidal, implying that little cytopathic effect of HIV-1 infection is seen until a threshold level of viral replication (one copy of HIV-1 DNA for every 5 to 8 cells) is achieved. At this threshold, increases in viral replication yield large effects on thymocyte depletion, while at higher levels of viral replication increases in the two measures of pathogenesis occur more slowly. Our data are not perfect in this regard; in Fig. 7A we see CD4 CD8 DP thymocyte depletion in one graft with fewer copies of HIV-1 DNA than two others which are not depleted. This may be because we biopsied the mice at 3-week intervals and missed the peak of viral replication or the maximum thymocyte depletion. Nevertheless, only the *E11- and NL4-3-infected grafts achieved a level of viral replication near or exceeding one copy of HIV-1 DNA for every five cells, which is close to what we noted in the accompanying report to be a threshold level of viral replication required for depletion of CD4 CD8 DP cells in the SCID-hu system (10). The CD4 SP/CD8 SP ratio is a more sensitive measure of cytopathic effects mediated by R5 HIV-1 infection, and perturbations of this value occur at a lower viral load of approximately one copy of HIV-1 DNA for every 8 cells (Fig. 7B).
The relationship between viral replication and CD4+ thymocyte depletion that we saw is consistent with models of both indirect and direct killing of CD4+ thymocytes in SCID-hu mice which have been proposed (39, 47, 64). Further study with both R5 and X4 strains of HIV-1 will be required to elucidate the mechanism or mechanisms of thymocyte depletion seen in the SCID-hu model and by inference in the infected thymuses of HIV-1-infected individuals.
ACKNOWLEDGMENTS
We thank Robert Berkowitz and Mike McCune for sharing data prior to publication and for comments on the manuscript. We also thank Bill Ross, University of Virginia FACS Core Laboratory, for flow cytometry, and we thank Graciela Gamez-Torre, Jayanand Vasudevan, and Brigitta Zoltay for help with SCID-hu mice.
This work was supported by NIH grants AI39943, AI40981, and AI38186. R.M.S. was supported by a University of Virginia M.D./Ph.D. Program and Infectious Diseases training grant.
REFERENCES
- 1.Aldrovandi G M, Feuer G, Gao L, Jamieson B, Kristeva M, Chen I S, Zack J A. The SCID-hu mouse as a model for HIV-1 infection. Nature. 1993;363:732–736. doi: 10.1038/363732a0. [DOI] [PubMed] [Google Scholar]
- 2.Alkhatib G, Berger E A, Murphy P M, Pease J E. Determinants of HIV-1 coreceptor function on CC chemokine receptor 3. Importance of both extracellular and transmembrane/cytoplasmic regions. J Biol Chem. 1997;272:20420–20426. doi: 10.1074/jbc.272.33.20420. [DOI] [PubMed] [Google Scholar]
- 3.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 4.Asjo B, Morfeldt-Manson L, Albert J, Biberfeld G, Karlsson A, Lidman K, Fenyo E M. Replicative capacity of human immunodeficiency virus from patients with varying severity of HIV infection. Lancet. 1986;ii:660–662. [PubMed] [Google Scholar]
- 5.Bazan H A, Alkhatib G, Broder C C, Berger E A. Patterns of CCR5, CXCR4, and CCR3 usage by envelope glycoproteins from human immunodeficiency virus type 1 primary isolates. J Virol. 1998;72:4485–4491. doi: 10.1128/jvi.72.5.4485-4491.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger E A. HIV entry and tropism: the chemokine receptor connection. AIDS. 1997;11(Suppl. A):S3–S16. [PubMed] [Google Scholar]
- 7.Berkowitz R D, Beckerman K P, Schall T J, McCune J M. CXCR4 and CCR5 expression delineates targets for HIV-1 disruption of T cell differentiation. J Immunol. 1998;161:3702–3710. [PubMed] [Google Scholar]
- 8.Berkowitz R D, van't Wout A B, Kootstra N A, Moreno M E, Linquist-Stepps V D, Bare C, Stoddart C A, Schuitemaker H, McCune J M. R5 strains of human immunodeficiency virus type 1 from rapid progressors lacking X4 strains do not possess X4-type pathogenicity in human thymus. J Virol. 1999;73:7817–7822. doi: 10.1128/jvi.73.9.7817-7822.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonyhadi M L, Rabin L, Salimi S, Brown D A, Kosek J, McCune J M, Kaneshima H. HIV induces thymus depletion in vivo. Nature. 1993;363:728–732. doi: 10.1038/363728a0. [DOI] [PubMed] [Google Scholar]
- 10.Camerini D, Su H-P, Gamez-Torre G, Johnson M L, Zack J A, Chen I S Y. Human immunodeficiency virus type 1 pathogenesis in SCID-hu mice correlates with syncytium-inducing phenotype and viral replication. J Virol. 2000;74:3196–3204. doi: 10.1128/jvi.74.7.3196-3204.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng-Mayer C, Seto D, Tateno M, Levy J A. Biologic features of HIV-1 that correlate with virulence in the host. Science. 1988;240:80–82. doi: 10.1126/science.2832945. [DOI] [PubMed] [Google Scholar]
- 12.Choe H, Farzan M, Konkel M, Martin K, Sun Y, Marcon L, Cayabyab M, Berman M, Dorf M E, Gerard N, Gerard C, Sodroski J. The orphan seven-transmembrane receptor apj supports the entry of primary T-cell-line-tropic and dualtropic human immunodeficiency virus type 1. J Virol. 1998;72:6113–6118. doi: 10.1128/jvi.72.7.6113-6118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 14.Clavel F, Charneau P. Fusion from without directed by human immunodeficiency virus particles. J Virol. 1994;68:1179–1185. doi: 10.1128/jvi.68.2.1179-1185.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connor R I, Mohri H, Cao Y, Ho D. Increased viral burden and cytopathicity correlate temporally with CD4+ T-lymphocyte decline and clinical progression in human immunodeficiency virus type 1-infected individuals. J Virol. 1993;67:1772–1777. doi: 10.1128/jvi.67.4.1772-1777.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Courgnaud V, Laure F, Brossard A, Bignozzi C, Goudeau A, Barin F, Brechot C. Frequent and early in utero HIV-1 infection. AIDS Res Hum Retroviruses. 1991;7:337–341. doi: 10.1089/aid.1991.7.337. [DOI] [PubMed] [Google Scholar]
- 18.Dairaghi D J, Franz-Bacon K, Callas E, Cupp J, Schall T J, Tamraz S A, Boehme S A, Taylor N, Bacon K B. Macrophage inflammatory protein-1beta induces migration and activation of human thymocytes. Blood. 1998;91:2905–2913. [PubMed] [Google Scholar]
- 19.Dean M, Carrington M, Winkler C, Huttley G A, Smith M W, Allikmets R, Goedert J J, Buchbinder S P, Vittinghoff E, Gomperts E, Donfield S, Vlahov D, Kaslow R, Saah A, Rinaldo C, Detels R, O'Brien S J. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 20.De Jong J J, De Ronde A, Keulen W, Tersmette M, Goudsmit J. Minimal requirements for the human immunodeficiency virus type 1 V3 domain to support the syncytium-inducing phenotype: analysis by single amino acid substitution. J Virol. 1992;66:6777–6780. doi: 10.1128/jvi.66.11.6777-6780.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 22.Deng H K, Unutmaz D, KewalRamani V N, Littman D R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 23.Doms R W, Peiper S C. Unwelcomed guests with master keys: how HIV uses chemokine receptors for cellular entry. Virology. 1997;235:179–190. doi: 10.1006/viro.1997.8703. [DOI] [PubMed] [Google Scholar]
- 24.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R C, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 25.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC- CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 26.Edinger A L, Hoffman T L, Sharron M, Lee B, Yi Y, Choe W, Kolson D L, Mitrovic B, Zhou Y, Faulds D, Collman R G, Hesselgesser J, Horuk R, Doms R W. An orphan seven-transmembrane domain receptor expressed widely in the brain functions as a coreceptor for human immunodeficiency virus type 1 and simian immunodeficiency virus. J Virol. 1998;72:7934–7940. doi: 10.1128/jvi.72.10.7934-7940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eugen-Olsen J, Iversen A K, Garred P, Koppelhus U, Pedersen C, Benfield T L, Sorensen A M, Katzenstein T, Dickmeiss E, Gerstoft J, Skinhoj P, Svejgaard A, Nielsen J O, Hofmann B. Heterozygosity for a deletion in the CKR-5 gene leads to prolonged AIDS-free survival and slower CD4 T-cell decline in a cohort of HIV-seropositive individuals. AIDS. 1997;11:305–310. doi: 10.1097/00002030-199703110-00007. [DOI] [PubMed] [Google Scholar]
- 28.Farzan M, Choe H, Martin K, Marcon L, Hofmann W, Karlsson G, Sun Y, Barrett P, Marchand N, Sullivan N, Gerard N, Gerard C, Sodroski J. Two orphan seven-transmembrane segment receptors which are expressed in CD4-positive cells support simian immunodeficiency virus infection. J Exp Med. 1997;186:405–411. doi: 10.1084/jem.186.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 30.Fouchier R A, Groenink M, Kootstra N A, Tersmette M, Huisman H G, Miedema F, Schuitemaker H. Phenotype-associated sequence variation in the third variable domain of the human immunodeficiency virus type 1 gp120 molecule. J Virol. 1992;66:3183–3187. doi: 10.1128/jvi.66.5.3183-3187.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frade J M R, Llorente M, Mellado M, Alcami J, Gutierrez-Ramos J C, Zaballos A, Real G, Martinez A C. The amino-terminal domain of the CCR2 chemokine receptor acts as coreceptor for HIV-1 infection. J Clin Investig. 1997;100:497–502. doi: 10.1172/JCI119558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franz-Bacon K, Dairaghi D J, Boehme S A, Sullivan S K, Schall T J, Conlon P J, Taylor N, Bacon K B. Human thymocytes express CCR-3 and are activated by eotaxin. Blood. 1999;93:3233–3240. [PubMed] [Google Scholar]
- 33.Goya I, Gutierrez J, Varona R, Kremer L, Zaballos A, Marquez G. Identification of CCR8 as the specific receptor for the human beta-chemokine I-309: cloning and molecular characterization of murine CCR8 as the receptor for TCA-3. J Immunol. 1998;160:1975–1981. [PubMed] [Google Scholar]
- 34.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay C R, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 35.Horuk R, Hesselgesser J, Zhou Y, Faulds D, Halks-Miller M, Harvey S, Taub D, Samson M, Parmentier M, Rucker J, Doranz B J, Doms R W. The CC chemokine I-309 inhibits CCR8-dependent infection by diverse HIV-1 strains. J Biol Chem. 1998;273:386–391. doi: 10.1074/jbc.273.1.386. [DOI] [PubMed] [Google Scholar]
- 36.Huang Y, Paxton W A, Wolinsky S M, Neumann A U, Zhang L, He T, Kang S, Ceradini D, Jin Z, Yazdanbakhsh K, Kunstman K, Erickson D, Dragon E, Landau N R, Phair J, Ho D D, Koup R A. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 37.Jamieson B D, Aldrovandi G M, Planelles V, Jowett J B, Gao L, Bloch L M, Chen I S, Zack J A. Requirement of human immunodeficiency virus type 1 nef for in vivo replication and pathogenicity. J Virol. 1994;68:3478–3485. doi: 10.1128/jvi.68.6.3478-3485.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jamieson B D, Pang S, Aldrovandi G M, Zha J, Zack J A. In vivo pathogenic properties of two clonal human immunodeficiency virus type 1 isolates. J Virol. 1995;69:6259–6264. doi: 10.1128/jvi.69.10.6259-6264.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jamieson B D, Uittenbogaart C H, Schmid I, Zack J A. High viral burden and rapid CD4+ cell depletion in human immunodeficiency virus type 1-infected SCID-hu mice suggest direct viral killing of thymocytes in vivo. J Virol. 1997;71:8245–8253. doi: 10.1128/jvi.71.11.8245-8253.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joshi V V, Oleske J M. Pathologic appraisal of the thymus gland in acquired immunodeficiency syndrome in children. A study of four cases and a review of the literature. Arch Pathol Lab Med. 1985;109:142–146. [PubMed] [Google Scholar]
- 41.Kaneshima H, Su L, Bonyhadi M L, Connor R I, Ho D D, McCune J M. Rapid-high, syncytium-inducing isolates of human immunodeficiency virus type 1 induce cytopathicity in the human thymus of the SCID-hu mouse. J Virol. 1994;68:8188–8192. doi: 10.1128/jvi.68.12.8188-8192.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimata J T, Kuller L, Anderson D B, Dailey P, Overbaugh J. Emerging cytopathic and antigenic simian immunodeficiency virus variants influence AIDS progression. Nat Med. 1999;5:535–541. doi: 10.1038/8414. [DOI] [PubMed] [Google Scholar]
- 43.Kitchen S G, Zack J A. CXCR4 expression during lymphopoiesis: implications for human immunodeficiency virus type 1 infection of the thymus. J Virol. 1997;71:6928–6934. doi: 10.1128/jvi.71.9.6928-6934.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kitchen S G, Zack J A. Distribution of the human immunodeficiency virus coreceptors CXCR4 and CCR5 in fetal lymphoid organs: implications for pathogenesis in utero. AIDS Res Hum Retroviruses. 1999;15:143–148. doi: 10.1089/088922299311565. [DOI] [PubMed] [Google Scholar]
- 45.Koot M, Vos A H, Keet R P, de Goede R E, Dercksen M W, Terpstra F G, Coutinho R A, Miedema F, Tersmette M. HIV-1 biological phenotype in long-term infected individuals evaluated with an MT-2 cocultivation assay. AIDS. 1992;6:49–54. doi: 10.1097/00002030-199201000-00006. [DOI] [PubMed] [Google Scholar]
- 46.Kourtis A P, Ibegbu C, Nahmias A J, Lee F K, Clark W S, Sawyer M K, Nesheim S. Early progression of disease in HIV-infected infants with thymus dysfunction. N Engl J Med. 1996;335:1431–1436. doi: 10.1056/NEJM199611073351904. [DOI] [PubMed] [Google Scholar]
- 47.Kovalev G, Duus K, Wang L, Lee R, Bonyhadi M, Ho D, McCune J M, Kaneshima H, Su L. Induction of MHC class I expression on immature thymocytes in HIV-1-infected SCID-hu Thy/Liv mice: evidence of indirect mechanisms. J Immunol. 1999;162:7555–7562. [PMC free article] [PubMed] [Google Scholar]
- 48.Kuel R O. Statistical principles of research design and analysis. Belmont, Calif: Duxbury Press; 1994. [Google Scholar]
- 49.Liao F, Alkhatib G, Peden K W, Sharma G, Berger E A, Farber J M. STRL33, A novel chemokine receptor-like protein, functions as a fusion cofactor for both macrophage-tropic and T cell line-tropic HIV-1. J Exp Med. 1997;185:2015–2023. doi: 10.1084/jem.185.11.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loetscher D F, Amara D F, Oberlin D F, Brass D F, Legler D F. TYMSTR, a putative chemokine receptor selectively expressed in activated T cells, exhibits HIV-1 coreceptor function. Curr Biol. 1997;7:652–660. doi: 10.1016/s0960-9822(06)00292-2. [DOI] [PubMed] [Google Scholar]
- 51.McCune J M, Namikawa R, Kaneshima H, Shultz L D, Lieberman M, Weissman I L. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 52.Michael N L, Chang G, Louie L G, Mascola J R, Dondero D, Birx D L, Sheppard H W. The role of viral phenotype and CCR-5 gene defects in HIV-1 transmission and disease progression. Nat Med. 1997;3:338–340. doi: 10.1038/nm0397-338. [DOI] [PubMed] [Google Scholar]
- 53.Owman C, Garzino-Demo A, Cocchi F, Popovic M, Sabirsh A, Gallo R C. The leukotriene B4 receptor functions as a novel type of coreceptor mediating entry of primary HIV-1 isolates into CD4-positive cells. Proc Natl Acad Sci USA. 1998;95:9530–9534. doi: 10.1073/pnas.95.16.9530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pedroza-Martins L, Gurney K B, Torbett B E, Uittenbogaart C H. Differential tropism and replication kinetics of human immunodeficiency virus type 1 isolates in thymocytes: coreceptor expression allows viral entry, but productive infection of distinct subsets is determined at the postentry level. J Virol. 1998;72:9441–9452. doi: 10.1128/jvi.72.12.9441-9452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Penn M L, Grivel J C, Schramm B, Goldsmith M A, Margolis L. CXCR4 utilization is sufficient to trigger CD4+ T cell depletion in HIV-1-infected human lymphoid tissue. Proc Natl Acad Sci USA. 1999;96:663–668. doi: 10.1073/pnas.96.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Picchio G R, Gulizia R J, Wehrly K, Chesebro B, Mosier D E. The cell tropism of human immunodeficiency virus type 1 determines the kinetics of plasma viremia in SCID mice reconstituted with human peripheral blood leukocytes. J Virol. 1998;72:2002–2009. doi: 10.1128/jvi.72.3.2002-2009.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pleskoff O, Treboute C, Brelot A, Heveker N, Seman M, Alizon M. Identification of a chemokine receptor encoded by human cytomegalovirus as a cofactor for HIV-1 entry. Science. 1997;276:1874–1878. doi: 10.1126/science.276.5320.1874. [DOI] [PubMed] [Google Scholar]
- 58.Rappaport J, Cho Y Y, Hendel H, Schwartz E J, Schachter F, Zagury J F. 32 bp CCR-5 gene deletion and resistance to fast progression in HIV-1 infected heterozygotes. Lancet. 1997;349:922–923. doi: 10.1016/S0140-6736(05)62697-9. [DOI] [PubMed] [Google Scholar]
- 59.Rosenzweig M, Clark D P, Gaulton G N. Selective thymocyte depletion in neonatal HIV-1 thymic infection. AIDS. 1993;7:1601–1605. doi: 10.1097/00002030-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 60.Rucker J, Edinger A L, Sharron M, Samson M, Lee B, Berson J F, Yi Y, Margulies B, Collman R G, Doranz B J, Parmentier M, Doms R W. Utilization of chemokine receptors, orphan receptors, and herpesvirus-encoded receptors by diverse human and simian immunodeficiency viruses. J Virol. 1997;71:8999–9007. doi: 10.1128/jvi.71.12.8999-9007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rudensey L M, Kimata J T, Benveniste R E, Overbaugh J. Progression to AIDS in macaques is associated with changes in the replication, tropism, and cytopathic properties of the simian immunodeficiency virus variant population. Virology. 1995;207:528–542. doi: 10.1006/viro.1995.1113. [DOI] [PubMed] [Google Scholar]
- 62.Schuitemaker H, Koot M, Kootstra N A, Dercksen M W, de Goede R E, van Steenwijk R P, Lange J M, Schattenkerk J K, Miedema F, Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J Virol. 1992;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seemayer T A, Laroche A C, Russo P, Malebranche R, Arnoux E, Guerin J M, Pierre G, Dupuy J M, Gartner J G, Lapp W S, et al. Precocious thymic involution manifest by epithelial injury in the acquired immune deficiency syndrome. Hum Pathol. 1984;15:469–474. doi: 10.1016/s0046-8177(84)80082-9. [DOI] [PubMed] [Google Scholar]
- 64.Su L, Kaneshima H, Bonyhadi M, Salimi S, Kraft D, Rabin L, McCune J M. HIV-1-induced thymocyte depletion is associated with indirect cytopathogenicity and infection of progenitor cells in vivo. Immunity. 1995;2:25–36. doi: 10.1016/1074-7613(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 65.Tersmette M, Goede R E Y D, Al B J M B, Winkel I M, Gruters R A, Cuypers H T, Huisman H G, Miedema F. Differential syncytium-inducing capacity of human immunodeficiency virus isolates: frequent detection of syncytium-inducing isolates in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. J Virol. 1988;62:2026–2032. doi: 10.1128/jvi.62.6.2026-2032.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tersmette M, Gruters R A, de Wolf F, de Goede R E, Lange J M, Schellekens P T, Goudsmit J, Huisman H G, Miedema F. Evidence for a role of virulent human immunodeficiency virus (HIV) variants in the pathogenesis of acquired immunodeficiency syndrome: studies on sequential HIV isolates. J Virol. 1989;63:2118–2125. doi: 10.1128/jvi.63.5.2118-2125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tersmette M, Lange J M, de Goede R E, de Wolf F, Eeftink-Schattenkerk J K, Schellekens P T, Coutinho R A, Huisman J G, Goudsmit J, Miedema F. Association between biological properties of human immunodeficiency virus variants and risk for AIDS and AIDS mortality. Lancet. 1989;i:983–985. doi: 10.1016/s0140-6736(89)92628-7. [DOI] [PubMed] [Google Scholar]
- 68.van't Wout A B, Blaak H, Ran L J, Brouwer M, Kuiken C, Schuitemaker H. Evolution of syncytium-inducing and non-syncytium-inducing biological virus clones in relation to replication kinetics during the course of human immunodeficiency virus type 1 infection. J Virol. 1998;72:5099–5107. doi: 10.1128/jvi.72.6.5099-5107.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zaballos A, Gutierrez J, Varona R, Ardavin C, Marquez G. Cutting edge: identification of the orphan chemokine receptor GPR-9-6 as CCR9, the receptor for the chemokine TECK. J Immunol. 1999;162:5671–5675. [PubMed] [Google Scholar]
- 70.Zack J A, Arrigo S J, Weitsman S R, Go A S, Haislip A, Chen I S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 71.Zaitseva M B, Lee S, Rabin R L, Tiffany H L, Farber J M, Peden K W, Murphy P M, Golding H. CXCR4 and CCR5 on human thymocytes: biological function and role in HIV-1 infection. J Immunol. 1998;161:3103–3113. [PubMed] [Google Scholar]
- 72.Zhang L, He T, Huang Y, Chen Z, Guo Y, Wu S, Kunstman K J, Brown R C, Phair J P, Neumann A U, Ho D D, Wolinsky S M. Chemokine coreceptor usage by diverse primary isolates of human immunodeficiency virus type 1. J Virol. 1998;72:9307–9312. doi: 10.1128/jvi.72.11.9307-9312.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Y J, Dragic T, Cao Y, Kostrikis L, Kwon D S, Littman D R, KewalRamani V N, Moore J P. Use of coreceptors other than CCR5 by non-syncytium-inducing adult and pediatric isolates of human immunodeficiency virus type 1 is rare in vitro. J Virol. 1998;72:9337–9344. doi: 10.1128/jvi.72.11.9337-9344.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]