Abstract
Partitioning of per- and polyfluoroalkyl substances (PFAS) to indoor materials, including clothing, may prolong the residence time of PFAS indoors and contribute to exposure. During the Indoor PFAS Assessment (IPA) Campaign, we measured concentrations of nine neutral PFAS in air and cotton cloth in 11 homes in North Carolina, for up to 9 months. Fluorotelomer alcohols (i.e., 6:2 FTOH, 8:2 FTOH, and 10:2 FTOH) are the dominant target species in indoor air, with concentrations ranging from 1.8 to 49 ng m−3, 1.2 to 53 ng m−3, and 0.21 to 5.7 ng m−3, respectively. In cloth, perfluorooctane sulfonamidoethanols (i.e., MeFOSE and EtFOSE) accumulated most significantly over time, reaching concentrations of up to 0.26 ng cm−2 and 0.24 ng cm−2, respectively. From paired measurements of neutral PFAS in air and suspended cloth, we derived cloth–air partition coefficients (Kca) for 6:2, 8:2, and 10:2 FTOH; ethylperfluorooctane sulfonamide (EtFOSA); MeFOSE; and EtFOSE. Mean log(Kca) values range from 4.7 to 6.6 and are positively correlated with the octanol–air partition coefficient. We investigated the effect of the cloth storage method on PFAS accumulation and the influence of home characteristics on air concentrations. Temperature had the overall greatest effect. This study provides valuable insights into PFAS distribution, fate, and exposure indoors.
Keywords: Indoor air quality, indoor exposure, clothing, sinks, residential, cotton, sorption, textile
Graphical Abstract
INTRODUCTION
The indoor environment can be an important contributor to population exposures to per- and polyfluoroalkyl substances (PFAS).1,2 Exposures may occur via inhalation of airborne particles and gases; dermal exposure by direct contact with PFAS-containing materials or from the air; ingestion of food, water, and dust; and mouthing of materials.3 PFAS are manufactured chemicals, many of which are persistent in the environment and bioaccumulative.4–6 Currently, it is estimated that thousands of different PFAS exist, either for use in industry and product manufacture or as precursors or breakdown products.1,6–9 PFAS are commonly used in consumer products and articles as water- and stain-resistant or nonstick coatings and as surfactants.1,10–12 Examples of products treated with PFAS include furnishings, carpets, functional apparel, outdoor gear, cookware, food-contact materials, paints, and cleaning products. Exposure to several PFAS has been linked to a number of adverse health outcomes in humans and wildlife;1,13 however, the toxic potential of the majority of PFAS has not been studied to date.14 While diet and contaminated drinking water are often considered main pathways of human exposure to PFAS,1 the colocation of people and PFAS-containing products and materials in homes, where most people spend the majority of their time,15 makes the indoor environment a likely place for substantial inhalation and perhaps dermal PFAS exposure.
Existing studies measuring PFAS in residential indoor air (i.e., studies from Asia, Europe, and Canada and one study from the United States) have concluded that neutral PFAS dominate indoor PFAS concentrations; specifically, the fluorotelomer alcohols (FTOHs) 6:2 FTOH and 8:2 FTOH are present in indoor air with concentrations in the range of 1–100 ng m−3 in residential settings.16–25 Other neutral PFAS, such as fluoroalkyl sulfonamides (FASAs), fluoroalkyl sulfonamidoethanols (FASEs), and fluorotelomer acrylates (FTACs), have also been measured in residential indoor air.16–28 Biotransformation of neutral PFAS can result in intermediates and products that accumulate in the human body, as has been shown, for example, for 6:2 FTOH.29,30 Previous studies31,32 found positive associations between FTOH concentrations in indoor air and concentrations of perfluoroalkyl acids such as perfluorooctanoic acid (PFOA) and perfluorononanoic acid (PFNA) in human serum. PFOA and PFNA have been linked to a range of adverse health effects.33 However, our knowledge of the concentrations, composition, occurrence, and fate of neutral PFAS in residential indoor air is still very limited, especially in the United States (U.S.).
Clothing could be a mediator of exposure to PFAS, as has been shown for a variety of semivolatile organic compounds (SVOCs; e.g., phthalates,34,35 nicotine,36 and other compounds35,37,38). People are in continuous and intimate contact with different types of clothing materials. Clean clothing can act as a barrier to exposures by serving as a sink for chemicals present in the indoor air, as has been shown for phthalates and nicotine, for example.36,39 However, over time, these clothing items can accumulate SVOCs from the indoor air, and clothing can then become a secondary source with the potential to prolong exposure to the accumulated compounds, contributing to dermal exposure and to exposure via other pathways (e.g., ingestion via mouthing of fabrics and inhalation).36,39,40 In addition, PFAS have been found in clothing that has been treated to repel water or stains.41–47 These clothing items are likely additional sources of PFAS in indoor environments.40,47,48
Thus, it is likely that clothing plays an important role in human PFAS exposure; however, the partitioning of PFAS between air and initially PFAS-free, untreated clothing has not been studied to date. Cloth–air partition coefficients (Kca) are key to advancing our understanding of the impact of clothing on exposure.49,50 Kca values have been derived or directly measured for phthalates, flame retardants, methamphetamine, and volatile organic compounds (VOCs) for partitioning to cotton, polyester, rayon, linen, and some blends, either in chamber experiments or by deploying clothing in realistic indoor settings.34,35,38,51–54 The benefit of measuring Kca in realistic environments is that the influence of changing environmental conditions and competition with other indoor species is reflected in the resulting partition coefficients, thus making them more directly applicable in exposure assessments. However, no Kca values have been measured for PFAS to date.
To address these knowledge gaps, we conducted a field campaign with the first concurrent residential measurements of neutral PFAS in air and untreated cloth. Below, we describe the variations in concentrations and compositions of nine neutral PFAS in air and cloth sampled over up to 9 months in 11 U.S. (North Carolina) homes. We quantify Kca for six neutral PFAS based on paired measurements in indoor air and cloth. This analysis was designed to further inform PFAS exposure models that can provide population-based exposure predictions and contribute to more comprehensive policies for PFAS exposure mitigation.
MATERIALS AND METHODS
The Indoor PFAS Assessment (IPA) Campaign.
The overall goal of the IPA Campaign was to improve our understanding of the mechanisms that govern PFAS behavior, fate, and transport indoors. Thus, we designed a study that included a comprehensive suite of measurements across multiple indoor environmental compartments and seasons. A list of all samples collected during the IPA Campaign is provided in the Supporting Information (SI), Table S1. To the best of our knowledge, this campaign is the most comprehensive residential indoor PFAS measurement campaign to date, in terms of the number of indoor compartments sampled concurrently and over an extended period of time. Herein, we focus on nine neutral PFAS measured in total-air and gas-phase samples, as well as in cloth and clothing samples. These nine neutral target species have been found in indoor air in previous studies.18,20,21,24,26 Subsequent publications will address other aspects of the IPA Campaign.
Eleven nonsmoking, detached, single-family homes in the Chapel Hill/Durham area of North Carolina were sampled between July 4, 2021 and May 20, 2022 (UNC Chapel Hill IRB 20–2771), with staggered start dates. Initially, a convenience sample of 11 homes was recruited; one home (Home 82) left the field campaign after one month, so that 10 homes completed the study. Each home was visited multiple times over a 6–9-month period, as illustrated in Figures S1 and S2. Before sampling, informed consent was given and a home survey was administered to collect information about building characteristics and typical occupant activities (Figure S3). Temperature and relative humidity (RH) were measured continuously throughout each home’s 6–9-month study participation. At the end of each home’s participation, a Home Exit Survey (Figure S4) was conducted to address any changes that may have occurred in the home during the study.
Active 6-day sampling periods took place three times in each home: at the beginning of each home’s study participation (t = 0), after 3 months, and after 6 months (Figure S1). During active sampling, CO2 was measured continuously, and participants completed daily activity checklists (Figure S5), detailing their cooking, cleaning, laundering, and heating/cooling/ventilation behaviors as well as occupancy. At the end of each 6 day sampling period, an activity survey (Figure S6) was conducted to capture additional information about activities and behaviors potentially related to PFAS emissions, concentrations, or removal during sampling.
Key characteristics of the 11 study homes (taken from surveys and measured environmental conditions) are provided in Table S2. Home sizes were approximately 70–240 m2 (740–2620 ft2). All homes had central heating and air conditioning (HAC) systems, 1–4 occupants, and 0–3 pets. Estimated air change rates (ACH) for each home averaged 0.2–0.6 h−1, with variations (within home) of 0.02 h−1 (10%) to 0.3 h−1 (66%). Window opening behavior (Table S3) was quite variable, with most window opening (for more than 15 min) occurring in the fall and winter months. Several occupants did not open their windows at all during the sampling periods, and one reported opening windows on 14 occasions in late summer during the first 6-day sampling period. Carpet or rugs were found in all homes, and hardwood flooring, including bamboo, was the most common flooring type (Table S2). Additional survey data will be reported in subsequent papers.
Chemicals.
Names, CAS RNs, and vendors of the nine neutral PFAS analytes and six mass-labeled neutral PFAS standards are listed in Table S4. Table S5 summarizes relevant physicochemical properties. We included estimates of vapor pressures and octanol–air partition coefficients derived with the OPEn structure–activity/property Relationship App (OPERA), as implemented in the U.S. Environmental Protection Agency’s CompTox Chemicals Dashboard, because of its excellent performance in cross-model evaluation.55 HPLC-grade methanol (99.9% purity) and GC-grade hexane (99.9% purity) were purchased from Fisher Scientific (Waltham, MA).
Air Sampling.
PFAS in indoor air were sampled 1 m above floor level in the main living area of each home on three occasions by pulling air (0.3 m3 h−1) for ~72 h (21.2 m3 average sample volume) through a polyurethane foam (PUF)-XAD2-PUF sandwich cartridge (ORBO 1500 Precleaned Small PUF/Amberlite XAD-2/PUF Cartridge, Catalog No. 21233-U, Supelco, Bellefonte, PA). There were two exceptions: Home 82 was sampled only on one occasion, and Home 01 was sampled on two occasions. In some cases, a quartz-fiber filter (QFF, 32 mm diameter, Catalog No. 21038, Supelco, Bellefonte, PA; prebaked at 550 °C for 12 h) was attached upstream of the PUF-XAD2-PUF cartridge to remove particles, so that the PUF-XAD2-PUF cartridge collected only the gas phase. Further details regarding air sampling and sample handling and storage can be found in section S1. In total, 21 total-air samples (PUF-XAD2-PUF alone) and 25 gas-phase samples (prefiltered) as well as field blanks, replicates, and breakthrough samples were collected (Table S6).
Cloth Sampling.
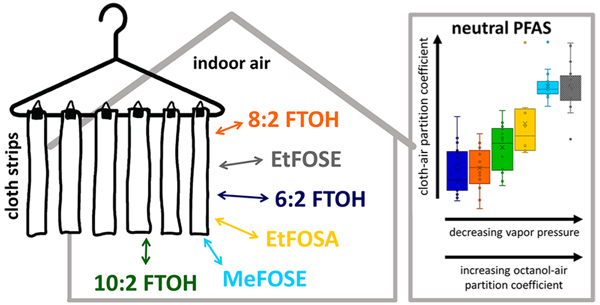
Two types of passive cotton cloth samplers (samplers A and B) were deployed during the first sampling visit (t = 0) at each home, allowed to accumulate PFAS, and retrieved at several time points across each home’s 6–9-month study participation. A precleaned, 100% cotton cloth (TS Designs, Burlington, NC; areal density 0.016 g cm−2, thickness 0.058 cm, bulk density 0.27 g cm−3) was used for this purpose. Details regarding the cloth’s preparation, deployment, sampling, and storage are listed in section S2. Briefly, sampler A consists of six clean cotton cloth strips (~28 cm × 3.8 cm) hanging freely from a stainless-steel clothes hanger below an aluminum roof to minimize the deposition of large particles via gravitational settling (Figure S7A). At each home, two hangers (12 cloth strips total) were placed in the closet of the master bedroom. During deployment of the hangers, two cloth strips per home were brought to each home and immediately retrieved as “t = 0” samples. Then, two (duplicate) cloth strip samples were retrieved after 24 h (t = 1), 6 days (t = 2), 1 month (t = 3), 3 months (t = 4), 6 months (t = 5), and at the end of each home’s participation in the field campaign (t = 6). A total of 143 cloth strip samples (incl. 71 duplicates) and 27 cloth strip field blanks were collected and subsequently analyzed.
Sampler B consists of 14 clean cotton pieces (~28 cm × 15 cm) that were folded once and distributed between stacked clothing items, most commonly in a dresser drawer in the master bedroom (Figure S7B). During deployment, two additional cloth pieces were brought to each home and immediately retrieved as “t = 0” samples without placement between clothing. Then, two cloth pieces were retrieved after 24 h (t = 1), 6 days (t = 2), 1 month (t = 3), 3 months (t = 4), 6 months (t = 5), and at the end of each home’s participation in the field campaign (t = 6). Additional clean cotton pieces were brought to the homes on one to three occasions per home to serve as field blanks. In total, 146 cloth piece samples and 13 field blanks were collected; 91 cloth piece samples and 13 field blanks were analyzed.
All study participants also donated one piece of 100% cotton clothing for which the label did not indicate that it was treated with PFAS and that had been laundered and stored in their home for at least three months without being worn. In total, 11 pieces of household clothing items were collected (see section S2 for details).
Indoor and Outdoor Environmental Conditions.
Carbon dioxide (CO2; 6-day sampling periods only; Extech SD800, Extech Instruments, Nashua, NH), temperature, and RH (entire campaign; 5 min intervals; HOBO UX100–011A, Onset, Bourne, MA) were measured in the main living area of each home. ACH estimates were derived from the CO2 data using the approach described by Bekö et al.56 Outdoor temperature and RH for July 2021 to May 2022 were retrieved from the weather station at Raleigh-Durham International Airport (KRDU) using the University of Utah’s MesoWest Web site (mesowest.utah.edu).
Sample Processing and Analysis.
Extraction and analysis of PUF-XAD2-PUF cartridges, cloth strips, sections of the cloth pieces, and sections of clothing items as well as blanks, duplicate samples, PUF-XAD2-PUF breakthrough samples, and positive storage controls of cloth samples are described in detail in section S3. Briefly, all samples and blanks were spiked with 20 μL of a mass-labeled recovery standard mixture (5 ng μL−1) and extracted twice by sonication (20 min) by using a 3:1 (v/v) hexane/methanol solvent mixture. A 50 mL polypropylene (PP) syringe (BD, Franklin Lakes, NJ) was used to squeeze remaining solvent from each substrate (i.e., cloth, clothing, PUF-XAD2-PUF) before extract cleanup with ENVI-Carb (Supelclean ENVI-Carb SPE Bulk Packing, Supelco, Bellefonte, PA). The extracts of each sample were combined and immediately concentrated under a gentle stream of high-purity nitrogen. PUF-XAD2-PUF extracts were reduced to ~1000 μL, and a 200 μL aliquot was used for analysis. Cloth and clothing extracts were concentrated to ~300 μL and analyzed. Details on quality control measures, including extraction efficiencies, breakthrough, detection limits, and precision, are reported in section S4 and Tables S7–S13. Method detection limits (MDLs, Table S9) range from 0.03 ng m−3 to 0.2 ng m−3 for PUF-XAD2-PUF air samples and 0.001 ng cm−2 to 0.1 ng cm−2 for cloth and clothing samples. MDL is given by the larger of the instrument detection limit (IDL) and the field-blank-based detection limit (FDL). The IDL is the mean concentration plus three times the standard deviation of repeat injections of the lowest calibration standard used (0.001 ng μL−1). The FDL is the mean plus three standard deviations of the field blank values for that type of sample, if available.
We analyzed the samples for nine neutral PFAS analytes and six neutral mass-labeled PFAS standards in selected-ion monitoring (SIM) mode using an Agilent 8890 gas chromatograph (GC) with an Agilent DB-WAX column (30 m length, 0.25 mm ID, 0.25 μm film thickness) and an Agilent 5977B mass-spectrometry detector (MSD) in electron ionization (EI) mode (Table S14). Samples were injected using pulsed splitless injection (injection volume 2 μL). A subset (18% and 15% of air and cloth sample extracts, respectively) was analyzed in replicate. Each analyte and mass-labeled standard was quantified using authentic standards and a seven-point calibration curve ranging from 0.001 ng μL−1 to 1.0 ng μL−1. Peak integration was performed using Agilent Enhanced ChemStation (Version F.01.03.2357) software. Reported concentrations were corrected for recoveries of the mass-labeled standards (section S4); they were not corrected for field blanks or breakthrough.
Calculation of Accumulation Rates and Partition Coefficients.
Accumulation rates (ng h−1 m−2) of each neutral PFAS in the suspended cloth strips were calculated for each home and each sampling time step as
| (1) |
where and (ng cm−2) are the area-based concentrations of species in the cloth at sampling times and , respectively, and (h) indicates the number of hours in between the two sampling times. Positive rates show an accumulation in the cloth, while negative rates indicate a loss.
The cloth–air partition coefficient, , is defined as the concentration of a chemical in the cloth divided by the gas-phase concentration of the chemical at equilibrium. For cloth, the partition coefficient can be normalized by area, mass, or volume of the cloth material.38 The mass-normalized partition coefficient, (m3 g−1), is used here as the basis to derive (unitless) for each PFAS as follows:38
| (2) |
where (ng cm−2) is the area-based concentration of species in the cloth at time , is the areal density of the cotton cloth (g cm−2), and is the gas-phase concentration (ng m−3) at time . If a species was detected but concentrations were below the MDL, the measured value, rather than zero or MDL/2, was used. If a species was not detected, then the concentration was set to zero. is then calculated as
| (3) |
where is the bulk density of the cotton cloth (g m−3).
Across-Home and within-Home Variability.
Across-home variability (AHV) and within-home variability (WHV) of measured PFAS concentrations in indoor air were calculated as pooled coefficients of variation (%) for either paired (n = 2) or n > 2 samples. Details can be found in the SI, section S5.
RESULTS AND DISCUSSION
Neutral PFAS in Indoor Air and Suspended Cloth.
FTOHs are the dominant measured PFAS in all indoor air samples, accounting for 94% (on average) of the mass of the nine measured neutral PFAS in the total-air (PUF-XAD2-PUF, no filter) samples (Figures 1 and S8; Table S15); in contrast, FOSEs are the dominant species in the suspended cloth strips, accounting for 62% (on average) of the mass of measured neutral PFAS (Figures 2 and S9; Table S16).
Figure 1.
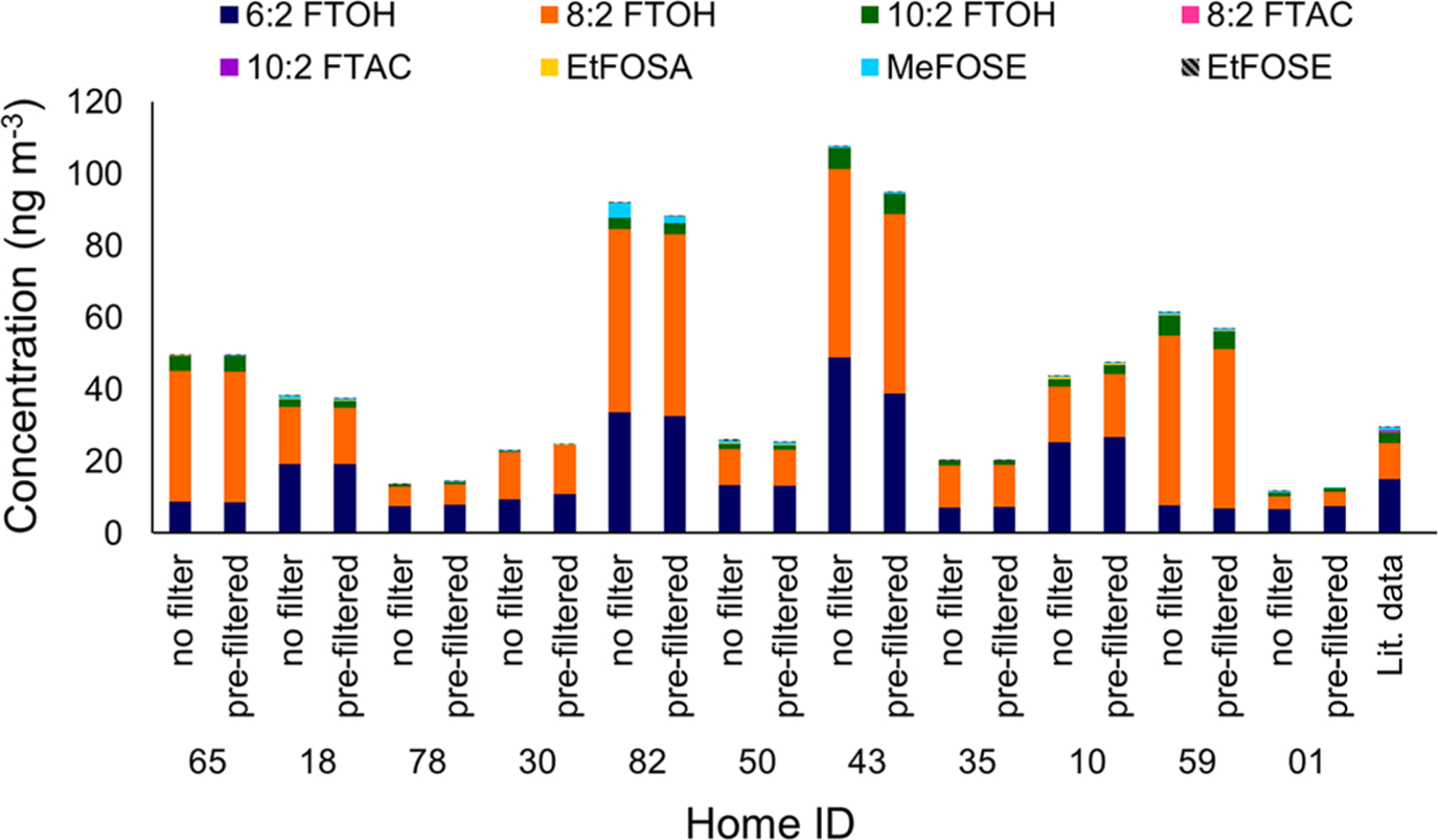
Concentration profiles of neutral PFAS measured in air samples in 11 homes during the first sampling visit. The label “no filter” refers to total-air samples, and “pre-filtered” refers to gas-phase samples. The bar showing the results for “Lit. data” is based on multiple studies.16–18,20–25 MeFOSA was not detected in any samples and, therefore, not shown. Results are shown in the order in which samples were collected at the homes.
Figure 2.
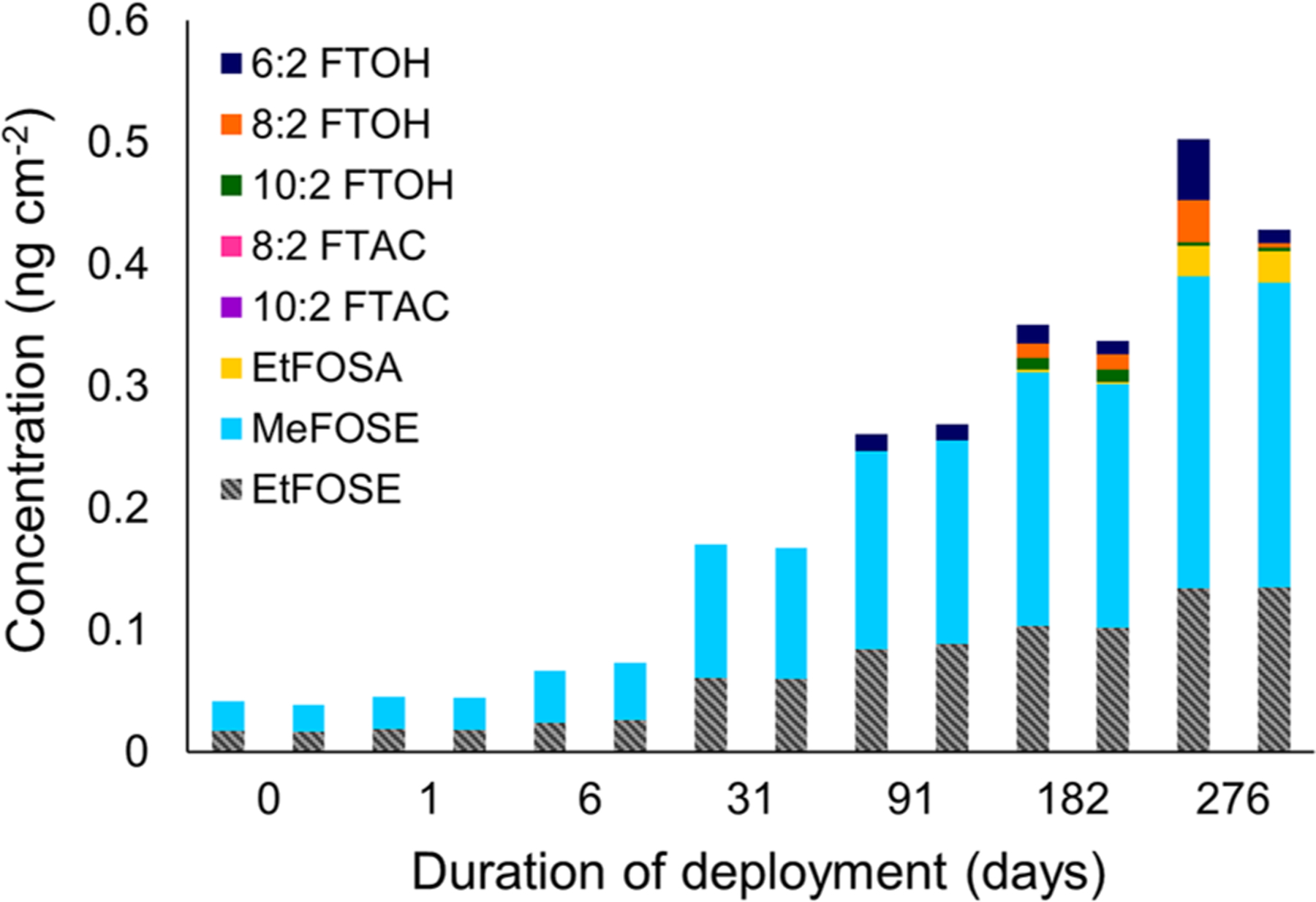
Concentration profiles of neutral PFAS measured in suspended duplicate cloth strips (sampler A), sampled from Home 18 over the duration of the field campaign. MeFOSA was not detected in any samples and is therefore not shown.
The FTOHs (6:2 FTOH, 8:2 FTOH, and 10:2 FTOH), MeFOSE, and EtFOSE were present above the MDL in 100% of the total-air samples (Table S15). Concentrations of 6:2 FTOH and 8:2 FTOH in total-air samples range from 1.2 to 53 ng m−3 (means: 13 ng m−3 and 15 ng m−3, respectively), whereas concentrations of 10:2 FTOH are an order of magnitude lower (mean: 2.2 ng m−3). Mean MeFOSE and EtFOSE concentrations in the total air are 0.60 ng m−3 and 0.19 ng m−3, respectively. In contrast, 8:2 FTAC, 10:2 FTAC, and EtFOSA are above MDL in one-third or less of total-air samples, with mean concentrations in the range of 0.06 ng m−3 (10:2 FTAC) to 0.18 ng m−3 (8:2 FTAC). MeFOSA was not detected in any air sample. Overall, the concentrations are on the same order of magnitude and follow similar trends as reported in previous studies on PFAS in indoor air, with high detection frequencies of FTOHs and FOSEs, and 6:2 FTOH and 8:2 FTOH as the dominant neutral species.16–18,20–25
There is no statistically significant difference between total-air and gas-phase samples (Figures 1 and S8) for six of the eight detected PFAS, according to paired two-sided t-tests or Wilcoxon signed-rank tests, depending on the sample distribution (95% confidence intervals; section S6; Table S17). QFFs collect particles with >99.99% efficiency and are known to adsorb some organic gases.57–60 Therefore, based on the difference between total-air and gas-phase concentrations, at a minimum, 88% of neutral PFAS found in total-air samples are associated with the gas phase. Statistically significant differences are observed only for MeFOSE and EtFOSE, which are less volatile than the other detected PFAS (Table S4), indicating some removal of FOSEs by the upstream QFF. On average, MeFOSE and EtFOSE account for 3.7% of the sum of measured neutral PFAS in total air (∑(neutral PFAS)air) and 3.6% in the gas phase (∑(neutral PFAS)gas). Removal of FOSEs by filters has also been reported in the literature, while FTOHs have not been found in the airborne particle phase indoors.61
Neutral PFAS accumulated in the suspended cloth strips (sampler A) as they were exposed in homes during the campaign (Figures 2 and S9). The highest measured sum of neutral PFAS concentration (∑(neutral PFAS)susp), 0.52 ng cm−2, was measured in Home 50 after 6 months of deployment. The increase in PFAS concentrations on the cloth strips is mostly due to the accumulation of MeFOSE and EtFOSE. MeFOSE (76% > MDL) and EtFOSE (63% > MDL) dominate ∑(neutral PFAS)susp concentrations in most homes, with ∑(neutral PFAS)susp concentrations that increase from 0 to 0.13 ng cm−2 after 1 day of exposure to 0.16–0.47 ng cm−2 after (on average) 244 days of exposure. In contrast to the air samples, 6:2 FTOH concentrations (1% > MDL) in suspended cloth strips remained below the MDL in most homes even after several months of deployment. We found that 8:2 FTOH, 10:2 FTOH, and EtFOSA were detected above the MDLs of 20%, 34%, and 5%, respectively, in the cloth strips. FTACs and MeFOSA were not detected in any of the strips. The characteristic time for 6:2 FTOH indicates that it takes only a few hours to reach equilibrium between air and cloth (Table S18), but its high vapor pressure (Table S5) suggests that partitioning favors the gas phase.
While FOSEs dominate ∑(neutral PFAS)susp concentrations on average, this is not true for Home 78. In this unusual case, 8:2 FTOH and 10:2 FTOH concentrations are on the same order of magnitude as MeFOSE and EtFOSE concentrations and the ∑(neutral PFAS)susp peak after 3 months (0.25 ng cm−2 on average). The highest ∑(neutral PFAS)air and ∑(neutral PFAS)gas concentrations in Home 78 were also measured at the 3-month time point. MeFOSE and EtFOSE air concentrations are generally lower in Home 78 than in other homes. It was also observed that due to the small size of the closet in Home 78, strips frequently touched other items kept in the closet, some of which may have been FTOH sources.
Equilibration Time of Suspended Cloth Strips.
PFAS accumulated in the suspended cloth strips (sampler A) over a period of months, accumulating quickly at first and more slowly at longer deployment times. Figure 3 shows the mean accumulation rates of six neutral PFAS over the 8 month (on average) study period. The average accumulation rate of the FTOHs becomes negative between 1 and 6 days, indicating that equilibrium was reached during that time period. Fluctuations in the accumulation rates afterward (e.g., 6:2 FTOH) indicate that FTOHs react within hours or days to changes in air concentrations and environmental conditions, consistent with the expectation that the characteristic times to equilibrium for 6:2 FTOH, 8:2 FTOH, and 10:2 FTOH are on the order of hours to days (Table S18), based on their estimated Koa values (Table S5). In contrast, mean accumulation rates of the FOSEs approach zero but remain positive until between 6 and 8 months of accumulation. This indicates that the FOSEs in the cloth need, on average, at least 6 months to come to equilibrium with the indoor air, which is in agreement with their lower volatility and higher Koa, resulting in characteristic times (τ) of up to 3 months and 6 months for MeFOSE and EtFOSE, respectively (Table S18).62 To calculate τ, Kca was approximated by Koa, assuming that the cotton acts as a uniform organic phase.62 It will be shown in the following section that this approximation is reasonable and that Kca is very close to Koa for the PFAS and cotton investigated in this study.
Figure 3.
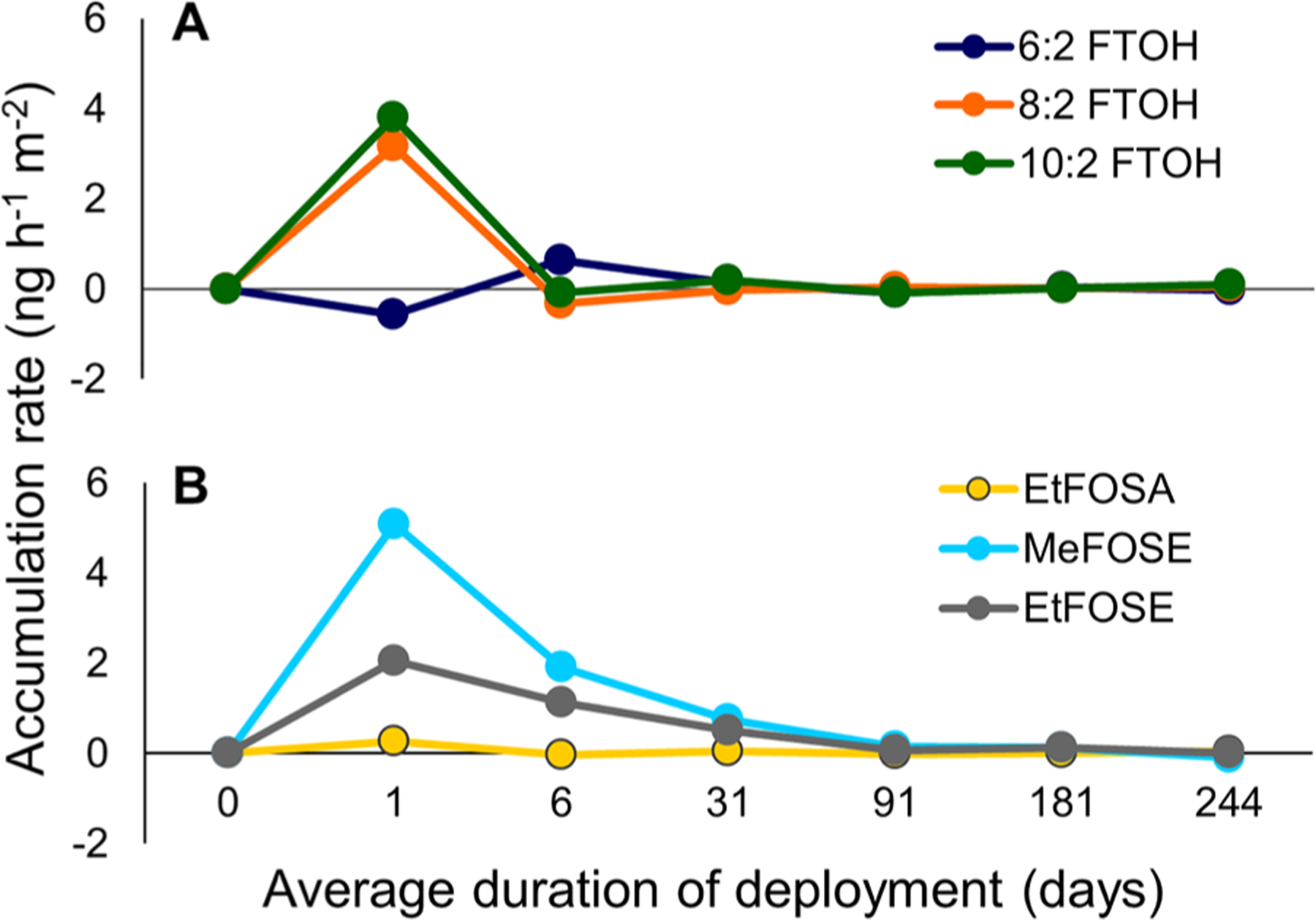
Mean accumulation rates of neutral PFAS in suspended cloth strips (sampler A) based on average concentrations in the cloth strips from the 11 IPA Campaign homes, for (A) 6:2 FTOH, 8:2 FTOH, and 10:2 FTOH and (B) EtFOSA, MeFOSE, and EtFOSE.
Cloth–Air Partition Coefficients.
Cloth–air partition coefficients (as mean log(Kca)) range from 4.7 (6:2 FTOH) to 6.7 (MeFOSE) and the partitioning of PFAS from air to cloth increases with increasing log(Koa) and decreasing vapor pressure (Figure 4; Tables 1 and S19). Concentrations of 6:2 FTOH, 8:2 FTOH, 10:2 FTOH, EtFOSA, MeFOSE, and EtFOSE in paired suspended cloth strips (sampler A) and air samples, collected at t = 3 months and t = 6 months, were used to calculate Kca (see eqs 2 and 3). For t = 3 months, total-air data were used in the calculation of log(Kca), while for t = 6 months, gas-phase data were used because of data availability. As described earlier, the difference between total-air and gas-phase concentrations is overall relatively small. Note that for 6:2 FTOH and EtFOSA, the majority of concentrations measured in the cloth and used for calculating log(Kca) are below the MDL.
Figure 4.
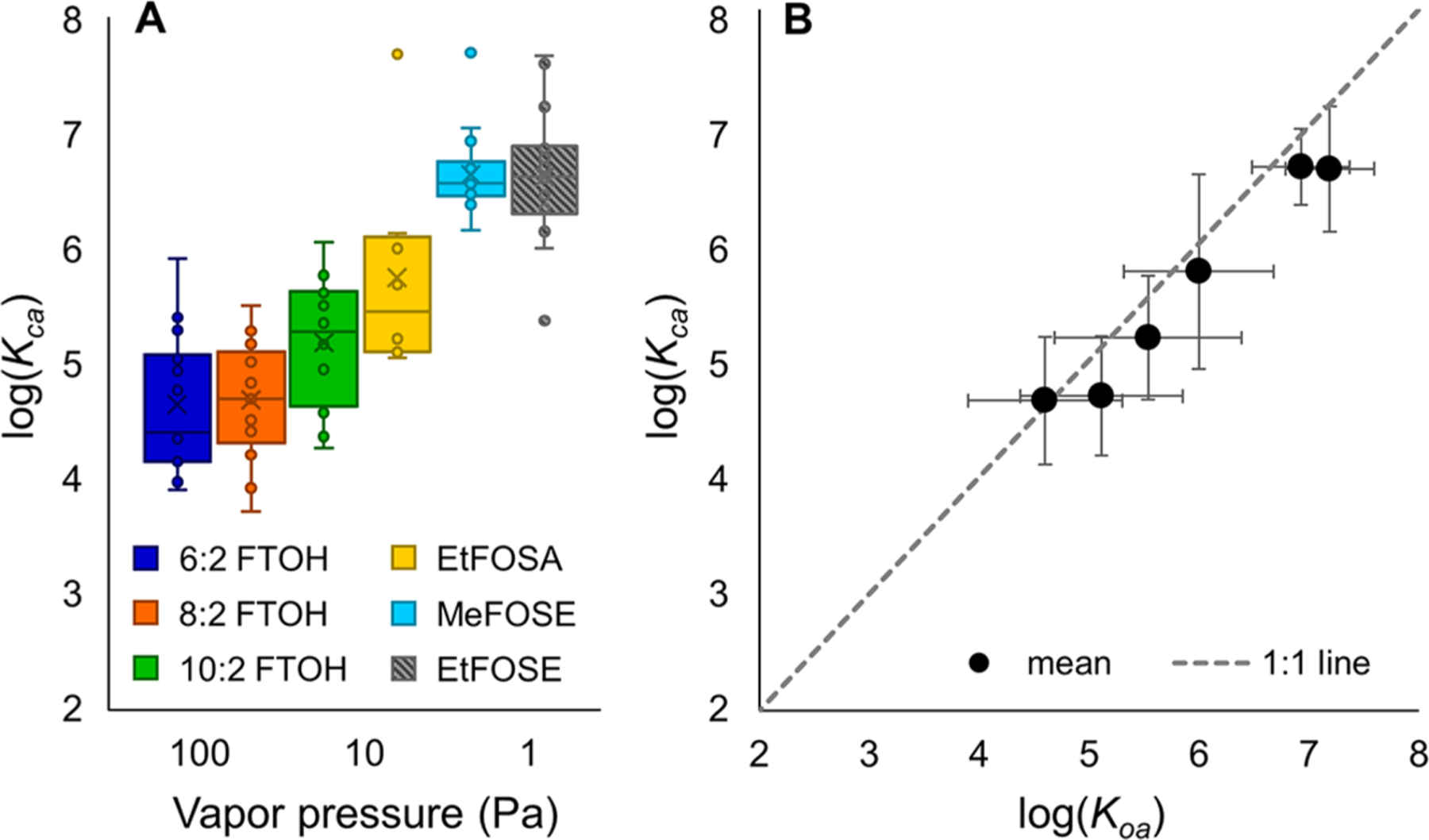
(A) Boxplot showing the log(Kca) of 6:2 FTOH, 8:2 FTOH, 10:2 FTOH, EtFOSA, MeFOSE, and EtFOSE with decreasing vapor pressure. The box indicates the 25th and 75th percentiles. The line inside the box is the median. The × represents the mean, and the whiskers indicate the minimum and maximum. Points outside the range of the whiskers are considered outliers. (B) Log(Kca) based on 3-month and 6-month measurements over log(Koa) derived from measured and predicted literature values (geometric mean and standard deviation, R2 = 0.98).28,63–69
Table 1.
Geometric Means and Standard Deviations of log(Kca) for Neutral PFAS, Calculated from Measurements of PFAS in Indoor Air and Cloth
| PFAS |
||||||
|---|---|---|---|---|---|---|
| log(Kca) | 6:2 FTOH | 8:2 FTOH | 10:2 FTOH | EtFOSA | MeFOSE | EtFOSE |
| mean ± std. dev. | 4.7 ± 0.6 | 4.7 ± 0.5 | 5.2 ± 0.5 | 5.8 ± 0.8 | 6.7 ± 0.3 | 6.6 ± 0.5 |
| median | 4.4 | 4.7 | 5.3 | 5.5 | 6.6 | 6.6 |
To the best of our knowledge, these are the first measurements of Kca for PFAS. However, we can use related PFAS partition coefficients to place these results in context. Shoeib et al.61 measured PUF–air and sorbent-impregnated PUF (SIP)–air partition coefficients for FTOHs and FOSEs. These partition coefficients follow the same trend as the Kca values reported in this study—smaller partition coefficients for the FTOHs and higher, very similar partition coefficients for MeFOSE and EtFOSE.61 In another study, partitioning of neutral PFAS to polyethylene (PE) sheets was studied, and the PE–air partition coefficients also followed the same trend.16 These comparisons, in combination with the relatively small standard deviations, increase the confidence in the Kca values presented here (Table 1).
Figure 4B shows a positive correlation (R2 = 0.98) between log(Kca) and mean log(Koa) values from the literature (Figure 4B; Table S5).28,63–69 This is not unreasonable, given that cotton fibers consist predominantly of organic material. Previous studies have shown positive correlations between log(Kca) and log(Koa) for polybrominated diphenyl ethers (PBDEs),35,37 phthalates, some PCBs,35,38 and homologous series of VOCs.54 However, in these cases, log(Kca) was either below the 1:1 line (for PBDEs, phthalates, and PCBs) or above the 1:1 line (for VOCs; Figure S10). Five of the six neutral PFAS log(Kca) values increase with log(Koa); the log(Kca) values of MeFOSE and EtFOSE are nearly identical despite an evident, yet small, difference in log(Koa). The two smallest FTOHs (6:2 FTOH and 8:2 FTOH) also have very similar log(Kca) values; however, the log(Kca) of 6:2 FTOH has larger uncertainties because a larger portion of the concentrations in cloth are below MDL.
Correlations of log(Kca) with the vapor pressure (Figure S11) and temperature (Section S7; Figures S12 and S13) were also examined. For vapor pressure, one major issue is the lack of consistent data for the vapor pressure of neutral PFAS, as shown by the large error bars in Figure S11, especially for the FOSEs. Despite this, the general trend that can be observed is a decrease in log(Kca) with increasing vapor pressure, leading to reduced partitioning of more volatile species to the cotton cloth. A similar inverse relationship between log(Kca) and vapor pressure has also been observed for PDBEs and VOCs.37,54 Further, we observed an inverse relationship between log(Kca) for all neutral PFAS except EtFOSA and indoor temperature, indicating that these species favor the gas phase over the cloth at elevated temperatures (Section S7; Figures S12 and S13). Although the correlations are modest, they are significant (95% confidence interval) for 6:2 FTOH and MeFOSE, with R2 values of 0.24 and 0.55 and p values of 0.0152 and 0.0002, respectively. A similar relationship was observed for phthalates by Eftekhari and Morrison.53 An inverse relationship of log(Kca) with air concentration can also be observed for all species. However, as shown for 6:2 FTOH and MeFOSE in Figure S14, this relationship is likely caused by the temperature-dependence of both log(Kca) and the air concentration, with higher temperatures resulting in lower partition coefficients and higher air concentrations.
Log(Kca) values have been reported for several groups of compounds, including SVOCs such as phthalates, PCBs, and methamphetamine,34,35,38,51–53 and most recently for VOCs, including carbonyls, carboxylic acids, aromatics, and hydrocarbons.54 For SVOCs, log(Kca) values for cotton clothing are in the range of 5 to 7.5 and are located below the 1:1 line of log(Kca) and log(Koa); i.e., log(Kca) is usually about 1 order of magnitude lower than log(Koa) (Figure S10). VOCs, on the other hand, appear to have cotton log(Kca) values that are roughly 1 order of magnitude higher than their respective log(Koa) values.54 The log(Kca) data measured for neutral PFAS included in this study lay close to the 1:1 line between VOCs and SVOCs, with the less volatile species, MeFOSE and EtFOSE, more closely aligned with the SVOCs and the FTOHs following the VOC trends more closely. These findings reinforce the perception of neutral PFAS as a set of chemicals that exists at the intersection of VOCs and SVOCs; the behavior of neutral PFAS does not fall neatly into either group. This work validates, in part, the use of Koa as a predictor for Kca for neutral PFAS and for use in describing the partitioning of neutral PFAS in air to cotton cloth.
Effect of Cloth Storage Method.
The method of storage influences the distribution and concentrations of PFAS in the cloth as well as the accumulation rate. However, regardless of whether cloth is stored in a drawer or on hangers in a closet, measurable accumulation of PFAS can occur in as little as 24 h. Neutral PFAS were detected with similar frequency in the folded cloth pieces (sampler B) compared to the suspended cloth strips (sampler A), and total measured concentrations were typically on the same order of magnitude, with concentrations in the suspended strips being slightly higher, on average (Table S20; Figure S15). However, the FTOH-to-FOSE ratios were typically higher in the folded cloth pieces than in the strips. The FTOH-to-FOSE ratio is particularly high for Homes 01, 35, and 78. This indicates the potential presence of FTOH sources in the drawer near the folded cloth pieces. PFAS concentrations in collocated, concurrently sampled folded cloth pieces were also more variable than the concentrations in duplicate suspended cloth strips. This can be seen by comparing the Home 18 time series for duplicate suspended cloth strips (Figure 2) with that for duplicate folded pieces, as well as for samples from Home 10 (Figure S15). The greater concentration variability for folded cloth may point to the potential presence of PFAS sources in the drawers as well as the potential influence of kinetic factors on transfer to the folded cloth. It may also reflect the reduced influence of airborne PFAS or the variable degree of disturbance as clothing is placed into or removed from the drawer.
Household Clothing Items.
Neutral PFAS were found in all household-donated clothing items, showing directly that presumably untreated clothing can serve as a sink and potential mediator of exposure for PFAS (Figure S16, Tables S21 and S22, and section S8). Homes with a higher sum of neutral PFAS in the folded cloth (∑(neutral PFAS)folded) typically also have a higher sum of PFAS in the household-donated clothing (∑(neutral PFAS)clothing; e.g., Homes 01, 18, and 82 compared to Home 78). Interestingly, FTOHs were more frequently detected and at higher concentrations (mass-normalized) in the household-donated clothing items (∑(FTOH)clothing) than in the 3- or 6-months folded cloth pieces. In many samples, ∑(FTOH)clothing concentrations contribute more than 50% to ∑(neutral PFAS)clothing concentrations. On average, ∑(neutral PFAS)clothing is higher than the 6-month ∑(neutral PFAS)folded concentrations (in ng g−1 of cloth), although they are on the same order of magnitude. ∑(neutral PFAS)clothing range from 2.3 ng g−1 (Home 78) to 34 ng g−1 (Home 65). Concentrations of the three FTOHs, MeFOSE, and EtFOSE are greater than the MDL in 70% or more of the samples. EtFOSA is above MDL in about one-fifth of the samples (18% > MDL). The FTACs and MeFOSA were not detected. Overall, these findings suggest that the folded cloth pieces serve as a reasonable proxy for clothing stored in homes; however, they may have not been the most suitable fabric to assess the full range of neutral PFAS that accumulate in household clothing. More volatile species (i.e., 6:2 and 8:2 FTOH) might have been less likely to accumulate in the cloth used in the field campaign because the fabric was thinner with a smaller available surface area, and it was selected to be free of surface treatments that may enhance partitioning of volatiles to the cloth.
Factors Influencing PFAS Concentrations in Air.
We expect PFAS concentrations across homes to vary because of differences in furnishings, building materials, home age (and other factors affecting mean home air change rates), and activities that occur very regularly (i.e., daily). We additionally expect within-home variability in PFAS concentrations as a result of changes in occupant activities related to sources (e.g., cooking), indoor conditions (e.g., temperature), and within-home variations in air change rates, such as those that result from window opening, indoor–outdoor temperature differences, or variations in wind speed. Thus, we calculated the within-home variability (WHV) and across-home variability (AHV) in PFAS air concentrations and investigated correlations between air concentrations and building characteristics, environmental factors, air change rates, and window opening behavior in order to better understand the drivers of PFAS concentrations in indoor air.
For all detected compounds except MeFOSE, the WHVs (74–334%) were greater than the respective AHVs (67–311%) for PFAS air concentrations, as shown in Table S23. For example, the WHV (150%) was nearly twice the AHV (84%) for 8:2 FTOH. This suggests a significant role for the influence of occupant activities or environmental conditions in the emission, partitioning, or dilution of these PFAS compounds. In contrast, MeFOSE was more variable across homes (138%) than within homes (37%). This suggests that MeFOSE is likely associated with a different, more permanent type of source, such as building materials or furnishings.
To investigate these relationships further, we present correlations between PFAS air concentrations and building characteristics, window opening behavior, occupancy, and environmental conditions in Tables S24 and S25. ∑(neutral PFAS)air and ∑(neutral PFAS)gas are moderately positively linearly correlated with average indoor temperature (R2 = 0.3 and 0.4, respectively, Figure S17) and also with the outdoor temperature and inversely correlated (moderate) with indoor–outdoor temperature difference (Tables S24 and S25). The correlation of PFAS with indoor temperature may be due to a shift of PFAS partitioning toward the gas phase (Figures S12, S13, and S14) as well as reflect the increased emission (or partitioning) of neutral PFAS from indoor sources or reservoirs to the air at higher temperatures, as demonstrated by Liu et al.42 for FTOHs in chamber experiments. Similar behavior has been shown in several studies for SVOCs70,71 and VOCs.72 Home age is inversely correlated (moderately) to the air concentration of MeFOSE. Together with the stronger AHV for MeFOSE, this finding suggests that newer home construction or newer furnishings are associated with sources of MeFOSE. In contrast, 8:2 FTAC in air is positively correlated with home age, associating 8:2 FTAC more strongly with older homes. The type of flooring is correlated (weak-to-moderate) with 6:2 FTOH. Specifically, houses with laminate in the main living area were more likely to have higher 6:2 FTOH air concentrations. Note that rugs were present in all homes except Home 18, which had a carpeted section. For this reason, rugs were excluded from the correlations. EtFOSA in air is moderately correlated with the air change rate, but we did not observe any correlation with the window opening behavior. Overall, these findings indicate that for most neutral PFAS, occupant activities and indoor conditions, especially changes in temperature (Figure S17), are more important factors influencing variations in total neutral PFAS concentrations in air than building materials and furnishings.
Implications for Human Exposure.
Concentrations of several neutral PFAS measured in indoor air in this study are in the range of typical SVOCs like di(2-ethylhexyl)adipate (DEHA), di(2-ethylhexyl) phthalate (DEHP), butylbenzyl phthalate (BBzP), and tris(chloropropyl)phosphate (TCPP).62,73,74 Because of the ubiquitous presence and relatively high concentrations of neutral PFAS in indoor air, consideration of inhalation exposure as an important pathway of exposure of the general U.S. population is warranted, especially for FTOHs. Biotransformation of neutral PFAS compounds (e.g., FTOHs and FOSEs) to potentially more toxic, persistent, or bioaccumulative intermediates (e.g., 5:3 fluorotelomer carboxylic acid) or products (e.g., PFOA and PFOS) in the human body increases the toxicological relevance of the neutral PFAS inhalation pathway, especially in locations where drinking water sources are not highly contaminated.1,20,29,30,32,75,76
This study shows that neutral PFAS accumulate in initially PFAS-free clothing. Thus, clothing as a secondary PFAS source could potentially facilitate dermal PFAS exposures and prolong indoor inhalation exposures. Generally, dermal absorption of PFAS has been considered a minor route of exposure compared to other pathways, i.e., dietary intake and drinking water, because dermal permeability has been thought to be low for PFAS.1,24,48,77,78 However, a recent study found that direct transdermal absorption of PFAS from air may be of greater relevance than inhalation exposure for multiple neutral PFAS.79 Based on the estimates of the dermal uptake-to-inhalation (D/I) ratio reported by Kissel et al.79 and the criteria established by Weschler and Nazaroff,80 dermal absorption is not expected to exceed inhalation exposure but is expected to contribute non-negligibly to MeFOSE and EtFOSE uptake. Such dermal absorption from air can be mediated by PFAS sorbed to clothing. These findings emphasize the importance of including dermal PFAS exposure in exposure assessments. The cloth–air partition coefficients reported here and their relationship to physicochemical properties and temperature can be used in such exposure models to incorporate the influence of clothing.
Limitations.
The IPA Campaign measured PFAS in multiple indoor compartments in 11 homes over 9 months. To the best of our knowledge, this is the first study to report concurrent measurements of neutral PFAS in air and cloth across multiple homes in the U.S. It is also the first study to report cloth–air partition coefficients for PFAS. However, the study has several limitations. The 11 single-family homes constituted a convenience sample, and the homes studied were located in a relatively small area in North Carolina. Additional measurements in more types and ages of homes and different climactic regions, with occupants spanning across a broader socioeconomic range, would provide a more complete understanding of the factors that influence residential PFAS exposures. Paired measurements of indoor air and cloth were not conducted in the same room, thus, introducing larger uncertainty in the cloth–air partition coefficients. We further assumed that the air concentrations of the target species are lower outdoors than indoors. We are confident this is true because we “cleaned” all cloth strips and pieces by hanging them outside for 3–4 months, and we found that concentrations of target species in the field blanks and t = 0 samples (essentially outdoor samples) were low compared to cloth exposed to indoor air, particularly for MeFOSE and EtFOSE. The literature also supports this assumption. For example, Shoeib et al.81 reported 2 orders of magnitude lower concentrations of MeFOSE and EtFOSE in outdoor air compared to indoor air, and Ahrens et al.82 reported FTOH and FOSE concentrations in the low pg m−3 range in the outdoor air near Toronto, Canada. Also, this study measured nine neutral PFAS. However, thousands of PFAS exist,7 and the indoor concentrations of most are unknown. Although detection limits still pose challenges, total organic fluorine measurements83 and analytical advancements such as real-time chemical ionization mass spectrometry (CIMS)84,85 in combination with integrated measurements of ionic PFAS may prove useful in approaching mass closure. Overall, there is great potential for future research to close knowledge gaps regarding PFAS emissions, partitioning, and exposure indoors.
Supplementary Material
ACKNOWLEDGMENTS
The Indoor PFAS Assessment (IPA) Campaign was funded in part by the Alfred P. Sloan Foundation (G-2017-9794 and G-2020-13937), the National Institute for Occupational Safety and Health (T42-OH008673), and the North Carolina Policy Collaboratory through an appropriation from the North Carolina General Assembly. CMAE was funded by the National Science Foundation Graduate Research Fellowships Program (NSF GRFP, Fellow # 2020305405). Authors gratefully acknowledge the contribution of the residents of the 11 study homes. The views expressed in this article are those of the authors and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.3c04770.
Overview of collected samples; sampling schedules; surveys and checklist; home characteristics; window opening; PFAS analytes and mass-labeled standards; additional sampling, sample processing, and sample analysis details; quality assurance and quality control measures and results; GC-MS parameters; across-home and within-home variabilities; concentration profiles of neutral PFAS in air and cloth; statistical analysis details and results; characteristic times and measured equilibration times; cloth–air partitioning coefficients; comparison and temperature-dependency of log(Kca); details regarding household clothing items; temperature dependency of air concentrations; and Spearman rank correlation coefficients (PDF)
Contributor Information
Clara M. A. Eichler, University of North Carolina at Chapel Hill, Gillings School of Global Public Health, Department of Environmental Sciences and Engineering, Chapel Hill, North Carolina 27599-7400, United States
Naomi Y. Chang, University of North Carolina at Chapel Hill, Gillings School of Global Public Health, Department of Environmental Sciences and Engineering, Chapel Hill, North Carolina 27599-7400, United States
Elaine A. Cohen Hubal, U.S. EPA, Center for Public Health and Environmental Assessment, Research Triangle Park, North Carolina 27711, United States
Daniel E. Amparo, University of North Carolina at Chapel Hill, Gillings School of Global Public Health, Department of Environmental Sciences and Engineering, Chapel Hill, North Carolina 27599-7400, United States
Jiaqi Zhou, University of North Carolina at Chapel Hill, Gillings School of Global Public Health, Department of Environmental Sciences and Engineering, Chapel Hill, North Carolina 27599-7400, United States.
Jason D. Surratt, University of North Carolina at Chapel Hill, Gillings School of Global Public Health, Department of Environmental Sciences and Engineering, Chapel Hill, North Carolina 27599-7400, United States; University of North Carolina at Chapel Hill, College of Arts and Sciences, Department of Chemistry, Chapel Hill, North Carolina 27599-3290, United States
Glenn C. Morrison, University of North Carolina at Chapel Hill, Gillings School of Global Public Health, Department of Environmental Sciences and Engineering, Chapel Hill, North Carolina 27599-7400, United States
Barbara J. Turpin, University of North Carolina at Chapel Hill, Gillings School of Global Public Health, Department of Environmental Sciences and Engineering, Chapel Hill, North Carolina 27599-7400, United States
REFERENCES
- 1.Sunderland EM; Hu XC; Dassuncao C; Tokranov AK; Wagner CC; Allen JG A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol 2019, 29, 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeLuca NM; Minucci JM; Mullikin A; Slover R; Cohen Hubal EA Human exposure pathways to poly- and perfluoroalkyl substances (PFAS) from indoor media: A systematic review. Environ. Int 2022, 162, No. 107149. [DOI] [PubMed] [Google Scholar]
- 3.Holder C; DeLuca N; Luh J; Alexander P; Minucci JM; Vallero DA; Thomas K; Cohen Hubal EA Systematic Evidence Mapping of Potential EXposure Pathways for Per- and Polyfluoroalkyl Substances Based on Measured Occurrence in Multiple Media. Environ. Sci. Technol 2023, 57 (13), 5107–5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conder JM; Hoke RA; de Wolf W; Russell MH; Buck RC Are PFCAs Bioaccumulative? A Critical Review and Comparison with Regulatory Criteria and Persistent Lipophilic Compounds. Environ. Sci. Technol 2008, 42 (4), 995–1003. [DOI] [PubMed] [Google Scholar]
- 5.Evich MG; Davis MJB; McCord JP; Acrey B; Awkerman JA; Knappe DRU; Lindstrom AB; Speth TF; Tebes-Stevens C; Strynar MJ; Wang Z; Weber EJ; Henderson WM; Washington JW Per- and polyfluoroalkyl substances in the environment. Science 2022, 375 (6580), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bălan SA; Mathrani VC; Guo DF; Algazi AM Regulating PFAS as a Chemical Class under the California Safer Consumer Products Program. Environ. Health Perspect. 2021, 129 (2), No. 025001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cordner A; Goldenman G; Birnbaum LS; Brown P; Miller MF; Mueller R; Patton S; Salvatore DH; Trasande L The True Cost of PFAS and the Benefits of Acting Now. Environ. Sci. Technol 2021, 55 (14), 9630–9633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.USEPA PFAS Strategic Roadmap: EPA’s Commitments to Action 2021–2024. https://www.epa.gov/pfas/pfas-strategic-roadmap-epas-commitments-action-2021-2024 (accessed May 30, 2023). [Google Scholar]
- 9.USEPA PFAS structures in DSSTox (update August 2022). https://comptox.epa.gov/dashboard/chemical-lists/PFASSTRUCTV5 (accessed May 30, 2023). [Google Scholar]
- 10.Glüge J; Scheringer M; Cousins IT; DeWitt JC; Goldenman G; Herzke D; Lohmann R; Ng C. a.; Trier X; Wang Z An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Process Impacts 2020, 22 (12), 2345–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cahuas L; Muensterman DJ; Kim-Fu ML; Reardon PN; Titaley IA; Field JA Paints: A Source of Volatile PFAS in Air—Potential Implications for Inhalation Exposure. Environ. Sci. Technol 2022, 56 (23), 17070–17079. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz-Narbonne H; Xia C; Shalin A; Whitehead HD; Yang D; Peaslee GF; Wang Z; Wu Y; Peng H; Blum A; Venier M; Diamond ML Per- and Polyfluoroalkyl Substances in Canadian Fast Food Packaging. Environ. Sci. Technol. Lett 2023, 10 (4), 343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pelch KE; Reade A; Kwiatkowski CF; Merced-Nieves FM; Cavalier H; Schultz K; Wolffe T; Varshavsky J The PFAS-Tox Database: A systematic evidence map of health studies on 29 per- and polyfluoroalkyl substances. Environ. Int 2022, 167, No. 107408. [DOI] [PubMed] [Google Scholar]
- 14.Rericha Y; Simonich MT; Truong L; Tanguay RL Review of the zebrafish as a model to investigate per- and polyfluoroalkyl substance toxicity. Toxicol. Sci 2023, 194 (2), 138–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diffey BL An overview analysis of the time people spend outdoors. Br. J. Dermatol 2011, 164 (4), 848–854. [DOI] [PubMed] [Google Scholar]
- 16.Morales-McDevitt ME; Bečanová J; Blum A; Bruton TA; Vojta S; Woodward M; Lohmann R The Air That We Breathe: Neutral and Volatile PFAS in Indoor Air. Environ. Sci. Technol. Lett 2021, 8 (10), 897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao Y; Zhao Y; Sun H; Chang S; Zhu L; Alder AC; Kannan K Per- and Polyfluoroalkyl Substances (PFASs) in Indoor Air and Dust from Homes and Various Microenvironments in China: Implications for Human Exposure. Environ. Sci. Technol 2018, 52 (5), 3156–3166. [DOI] [PubMed] [Google Scholar]
- 18.Winkens K; Koponen J; Schuster J; Shoeib M; Vestergren R; Berger U; Karvonen AM; Pekkanen J; Kiviranta H; Cousins IT Perfluoroalkyl acids and their precursors in indoor air sampled in children’s bedrooms. Environ. Pollut 2017, 222, 423–432. [DOI] [PubMed] [Google Scholar]
- 19.Padilla-Sánchez JA; Papadopoulou E; Poothong S; Haug LS Investigation of the Best Approach for Assessing Human Exposure to Poly- and Perfluoroalkyl Substances through Indoor Air. Environ. Sci. Technol 2017, 51 (21), 12836–12843. [DOI] [PubMed] [Google Scholar]
- 20.Fromme H; Dreyer A; Dietrich S; Fembacher L; Lahrz T; Völkel W Neutral polyfluorinated compounds in indoor air in Germany – The LUPE 4 study. Chemosphere 2015, 139, 572–578. [DOI] [PubMed] [Google Scholar]
- 21.Liu W; Takahashi S; Sakuramachi Y; Harada KH; Koizumi A Polyfluorinated telomers in indoor air of Japanese houses. Chemosphere 2013, 90, 1672–1677. [DOI] [PubMed] [Google Scholar]
- 22.Goosey E; Harrad S Perfluoroalkyl substances in UK indoor and outdoor air: Spatial and seasonal variation, and implications for human exposure. Environ. Int 2012, 45, 86–90. [DOI] [PubMed] [Google Scholar]
- 23.Kim S-K; Shoeib M; Kim K-S; Park J-E Indoor and outdoor poly- and perfluoroalkyl substances (PFASs) in Korea determined by passive air sampler. Environ. Pollut 2012, 162, 144–150. [DOI] [PubMed] [Google Scholar]
- 24.Shoeib M; Harner T; Webster GM; Lee SC Indoor Sources of Poly- and Perfluorinated Compounds (PFCS) in Vancouver, Canada: Implications for Human Exposure. Environ. Sci. Technol 2011, 45 (19), 7999–8005. [DOI] [PubMed] [Google Scholar]
- 25.Haug LS; Huber S; Schlabach M; Becher G; Thomsen C Investigation on Per- and Polyfluorinated Compounds in Paired Samples of House Dust and Indoor Air from Norwegian Homes. Environ. Sci. Technol 2011, 45, 7991–7998. [DOI] [PubMed] [Google Scholar]
- 26.Harrad S; Wemken N; Drage DS; Abdallah MA-E; Coggins A-M Perfluoroalkyl Substances in Drinking Water, Indoor Air and Dust from Ireland: Implications for Human Exposure Environ. Sci. Technol 2019, 53, 13449–13457. [DOI] [PubMed] [Google Scholar]
- 27.Langer V; Dreyer A; Ebinghaus R Polyfluorinated Compounds in Residential and Nonresidential Indoor Air. Environ. Sci. Technol 2010, 44 (21), 8075–8081. [DOI] [PubMed] [Google Scholar]
- 28.Shoeib M; Harner T; Ikonomou M; Kannan K Indoor and Outdoor Air Concentrations and Phase Partitioning of Perfluoroalkyl Sulfonamides and Polybrominated Diphenyl Ethers. Environ. Sci. Technol 2004, 38, 1313–1320. [DOI] [PubMed] [Google Scholar]
- 29.Dinglasan MJA; Ye Y; Edwards EA; Mabury SA Fluorotelomer Alcohol Biodegradation Yields Poly- and Perfluorinated Acids. Environ. Sci. Technol 2004, 38 (10), 2857–2864. [DOI] [PubMed] [Google Scholar]
- 30.Rice PA; Aungst J; Cooper J; Bandele O; Kabadi SV Comparative analysis of the toxicological databases for 6:2 fluorotelomer alcohol (6:2 FTOH) and perfluorohexanoic acid (PFHXA). Food Chem. Toxicol 2020, 138, No. 111210. [DOI] [PubMed] [Google Scholar]
- 31.Fraser AJ; Webster TF; Watkins DJ; Nelson JW; Stapleton HM; Calafat AM; Kato K; Shoeib M; Vieira VM; McClean MD Polyfluorinated Compounds in Serum Linked to Indoor Air in Office Environments. Environ. Sci. Technol 2012, 46 (2), 1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makey CM; Webster TF; Martin JW; Shoeib M; Harner T; DiX-Cooper L; Webster GM Airborne Precursors Predict Maternal Serum Perfluoroalkyl Acid Concentrations. Environ. Sci. Technol 2017, 51 (13), 7667–7675. [DOI] [PubMed] [Google Scholar]
- 33.Fenton SE; Ducatman A; Boobis A; DeWitt JC; Lau C; Ng C; Smith JS; Roberts SM Per- and Polyfluoroalkyl Substance ToXicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem 2021, 40 (3), 606–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrison GC; Li H; Mishra S; Buechlein M Airborne phthalate partitioning to cotton clothing. Atmos. Environ 2015, 115, 149–152. [Google Scholar]
- 35.Saini A; Okeme JO; Mark Parnis J; McQueen RH; Diamond ML From air to clothing: characterizing the accumulation of semi-volatile organic compounds to fabrics in indoor environments. Indoor Air 2017, 27, 631–641. [DOI] [PubMed] [Google Scholar]
- 36.Bekö G; Morrison G; Weschler CJ; Koch HM; Pälmke C; Salthammer T; Schripp T; Eftekhari A; Toftum J; Clausen G Dermal uptake of nicotine from air and clothing: Experimental verification. Indoor Air 2018, 28, 247–257. [DOI] [PubMed] [Google Scholar]
- 37.Saini A; Rauert C; Simpson MJ; Harrad S; Diamond ML Characterizing the sorption of polybrominated diphenyl ethers (PBDEs) to cotton and polyester fabrics under controlled conditions. Sci. Total Environ 2016, 563–564, 99–107. [DOI] [PubMed] [Google Scholar]
- 38.Morrison GC; Andersen HV; Gunnarsen L; Varol D; Uhde E; Kolarik B Partitioning of PCBs from air to clothing materials in a Danish apartment. Indoor Air 2018, 28 (1), 188–197. [DOI] [PubMed] [Google Scholar]
- 39.Morrison GC; Weschler CJ; Bekö G; Koch HM; Salthammer T; Schripp T; Toftum J; Clausen G Role of clothing in both accelerating and impeding dermal absorption of airborne SVOCs. J. Expo. Sci. Environ. Epidemiol 2016, 26, 113–118. [DOI] [PubMed] [Google Scholar]
- 40.Licina D; Morrison GC; Bekö G; Weschler CJ; Nazaroff WW Clothing-Mediated EXposures to Chemicals and Particles. Environ. Sci. Technol 2019, 53 (10), 5559–5575. [DOI] [PubMed] [Google Scholar]
- 41.Schlummer M; Gruber L; Fiedler D; Kizlauskas M; Müller J Detection of fluorotelomer alcohols in indoor environments and their relevance for human exposure. Environ. Int 2013, 57–58, 42–49. [DOI] [PubMed] [Google Scholar]
- 42.Liu X; Guo Z; Folk EE; Roache NF Determination of fluorotelomer alcohols in selected consumer products and preliminary investigation of their fate in the indoor environment. Chemosphere 2015, 129, 81–86. [DOI] [PubMed] [Google Scholar]
- 43.Gremmel C; Frömel T; Knepper TP Systematic determination of perfluoroalkyl and polyfluoroalkyl substances (PFASs) in outdoor jackets. Chemosphere 2016, 160, 173–180. [DOI] [PubMed] [Google Scholar]
- 44.van der Veen I; Hanning A-C; Stare A; Leonards PEG; de Boer J; Weiss JM The effect of weathering on per- and polyfluoroalkyl substances (PFASs) from durable water repellent (DWR) clothing. Chemosphere 2020, 249, 126100. [DOI] [PubMed] [Google Scholar]
- 45.Schellenberger S; Liagkouridis I; Awad R; Khan S; Plassmann M; Peters G; Benskin JP; Cousins IT An Outdoor Aging Study to Investigate the Release of Per- And Polyfluoroalkyl Substances (PFAS) from Functional Textiles. Environ. Sci. Technol 2022, 56 (6), 3471–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xia C; Diamond ML; Peaslee GF; Peng H; Blum A; Wang Z; Shalin A; Whitehead HD; Green M; Schwartz-Narbonne H; Yang D; Venier M Per- and Polyfluoroalkyl Substances in North American School Uniforms. Environ. Sci. Technol 2022, 56 (19), 13845–13857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Veen I; Schellenberger S; Hanning A-C; Stare A; de Boer J; Weiss JM; Leonards PEG Fate of Per- and Polyfluoroalkyl Substances from Durable Water-Repellent Clothing during Use. Environ. Sci. Technol 2022, 56 (9), 5886–5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poothong S; Papadopoulou E; Padilla-Sánchez JA; Thomsen C; Haug LS Multiple pathways of human exposure to poly- and perfluoroalkyl substances (PFASs): From external exposure to human blood. Environ. Int 2020, 134, 105244. [DOI] [PubMed] [Google Scholar]
- 49.Morrison GC; Weschler CJ; Bekö G Dermal uptake of phthalates from clothing: Comparison of model to human participant results. Indoor Air 2017, 27 (3), 642–649. [DOI] [PubMed] [Google Scholar]
- 50.Cao J; Zhang X; Zhang Y Predicting Dermal Exposure to Gas-Phase Semivolatile Organic Compounds (SVOCs): A Further Study of SVOC Mass Transfer between Clothing and Skin Surface Lipids. Environ. Sci. Technol 2018, 52, 4676–4683. [DOI] [PubMed] [Google Scholar]
- 51.Cao J; Weschler CJ; Luo J; Zhang Y Cm-History Method, a Novel Approach to Simultaneously Measure Source and Sink Parameters Important for Estimating Indoor Exposures to Phthalates. Environ. Sci. Technol 2016, 50 (2), 825–834. [DOI] [PubMed] [Google Scholar]
- 52.Morrison G; Shakila NV; Parker K Accumulation of gas-phase methamphetamine on clothing, toy fabrics, and skin oil. Indoor Air 2015, 25 (4), 405–414. [DOI] [PubMed] [Google Scholar]
- 53.Eftekhari A; Morrison G A high throughput method for measuring cloth-air equilibrium distribution ratios for SVOCs present in indoor environments. Talanta 2018, 183, 250–257. [DOI] [PubMed] [Google Scholar]
- 54.Yu J; Wania F; Abbatt JPD A New Approach to Characterizing the Partitioning of Volatile Organic Compounds to Cotton Fabric. Environ. Sci. Technol 2022, 56 (6), 3365–3374. [DOI] [PubMed] [Google Scholar]
- 55.Lampic A; Parnis JM Property Estimation of Per- and Polyfluoroalkyl Substances: A Comparative Assessment of Estimation Methods. Environ. Toxicol. Chem 2020, 39 (4), 775–786. [DOI] [PubMed] [Google Scholar]
- 56.Bekö G; Lund T; Nors F; Toftum J; Clausen G Ventilation rates in the bedrooms of 500 Danish children. Build. Environ 2010, 45 (10), 2289–2295. [Google Scholar]
- 57.Turpin BJ; Saxena P; Andrews E Measuring and simulating particulate organics in the atmosphere: problems and prospects. Atmos. Environ 2000, 34 (18), 2983–3013. [Google Scholar]
- 58.Ahrens L; Harner T; Shoeib M; Lane DA; Murphy JG Improved Characterization of Gas-Particle Partitioning for Per- and Polyfluoroalkyl Substances in the Atmosphere Using Annular Diffusion Denuder Samplers. Environ. Sci. Technol 2012, 46, 7199–7206. [DOI] [PubMed] [Google Scholar]
- 59.Arp HPH; Goss K-U Irreversible sorption of trace concentrations of perfluorocarboxylic acids to fiber filters used for air sampling. Atmos. Environ 2008, 42 (28), 6869–6872. [Google Scholar]
- 60.Johansson JH; Berger U; Cousins IT Can the use of deactivated glass fibre filters eliminate sorption artefacts associated with active air sampling of perfluorooctanoic acid? Environ. Pollut 2017, 224, 779–786. [DOI] [PubMed] [Google Scholar]
- 61.Shoeib M; Harner T; Lee SC; Lane D; Zhu J Sorbent-Impregnated Polyurethane Foam Disk for Passive Air Sampling of Volatile Fluorinated Chemicals. Anal. Chem 2008, 80, 675–682. [DOI] [PubMed] [Google Scholar]
- 62.Weschler CJ; Nazaroff WW Semivolatile organic compounds in indoor environments. Atmos. Environ 2008, 42 (40), 9018–9040. [Google Scholar]
- 63.Lei YD; Wania F; Mathers D; Mabury SA Determination of Vapor Pressures, Octanol–Air, and Water–Air Partition Coefficients for Polyfluorinated Sulfonamide, Sulfonamidoethanols, and Telomer Alcohols. J. Chem. Eng. Data 2004, 49 (4), 1013–1022. [Google Scholar]
- 64.Dreyer A; Langer V; Ebinghaus R Determination of Octanol-Air Partition Coefficients (Koa) of Fluorotelomer Acrylates, Perfluoroalkyl Sulfonamids, and Perfluoroalkylsulfonamido Ethanols. J. Chem. Eng. Data 2009, 54, 3022–3025. [Google Scholar]
- 65.Goss K-U; Bronner G; Harner T; Hertel M; Schmidt TC The Partition Behavior of Fluorotelomer Alcohols and Olefins. Environ. Sci. Technol 2006, 40, 3572–3577. [DOI] [PubMed] [Google Scholar]
- 66.Thuens S; Dreyer A; Sturm R; Temme C; Ebinghaus R Determination of the Octanol-Air Partition Coefficients (Koa) of Fluorotelomer Alcohols. J. Chem. Eng. Data 2008, 53 (1), 223–227. [Google Scholar]
- 67.Kim M; Li LY; Grace JR; Yue C Selecting reliable physicochemical properties of perfluoroalkyl and polyfluoroalkyl substances (PFASs) based on molecular descriptors. Environ. Pollut 2015, 196, 462–472. [DOI] [PubMed] [Google Scholar]
- 68.Salthammer T; Grimme S; Stahn M; Hohm U; Palm W-U Quantum Chemical Calculation and Evaluation of Partition Coefficients for Classical and Emerging Environmentally Relevant Organic Compounds. Environ. Sci. Technol 2022, 56 (1), 379–391. [DOI] [PubMed] [Google Scholar]
- 69.USEPA CompTox Chemicals Dashboard. https://comptox.epa.gov/dashboard/ (accessed Apr 3, 2022). [Google Scholar]
- 70.Cao J; Zhang X; Little JC; Zhang Y A SPME-based method for rapidly and accurately measuring the characteristic parameter for DEHP emitted from PVC floorings. Indoor Air 2017, 27, 417–426. [DOI] [PubMed] [Google Scholar]
- 71.Liang Y; Liu X; Allen MR The influence of temperature on the emissions of organophosphate ester flame retardants from polyisocyanurate foam: Measurement and modeling. Chemosphere 2019, 233, 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu Z; Ye W; Little JC Predicting Emissions of Volatile and Semivolatile Organic Compounds from Building Materials: A Review. Build. Environ 2013, 64, 7–25. [Google Scholar]
- 73.Salthammer T; Zhang Y; Mo J; Koch HM; Weschler CJ Assessing human exposure to organic pollutants in the indoor environment. Angew. Chem 2018, 57 (38), 12228–12263. [DOI] [PubMed] [Google Scholar]
- 74.Lucattini L; Poma G; Covaci A; de Boer J; Lamoree MH; Leonards PEG A review of semi-volatile organic compounds (SVOCs) in the indoor environment: occurrence in consumer products, indoor air and dust. Chemosphere 2018, 201, 466–482. [DOI] [PubMed] [Google Scholar]
- 75.Butt CM; Muir DCG; Mabury SA Biotransformation pathways of fluorotelomer-based polyfluoroalkyl substances: A review. Environ. Toxicol. Chem 2014, 33 (2), 243–267. [DOI] [PubMed] [Google Scholar]
- 76.Ellis DA; Martin JW; De Silva AO; Mabury SA; Hurley MD; Sulbaek Andersen MP; Wallington TJ Degradation of Fluorotelomer Alcohols: A Likely Atmospheric Source of Perfluorinated Carboxylic Acids. Environ. Sci. Technol 2004, 38 (12), 3316–3321. [DOI] [PubMed] [Google Scholar]
- 77.. Haug LS; Huber S; Becher G; Thomsen C Characterisation of human exposure pathways to perfluorinated compounds—comparing exposure estimates with biomarkers of exposure. Environ. Int 2011, 37, 687–693. [DOI] [PubMed] [Google Scholar]
- 78.Trudel D; Horowitz L; Wormuth M; Scheringer M; Cousins IT; Hungerbühler K Estimating Consumer Exposure to PFOS and PFOA. Risk Anal 2008, 28 (2), 251–269. [DOI] [PubMed] [Google Scholar]
- 79.Kissel JC; Titaley IA; Muensterman DJ; Field JA Evaluating Neutral PFAS for Potential Dermal Absorption from the Gas Phase. Environ. Sci. Technol 2023, 57 (12), 4951–4958. [DOI] [PubMed] [Google Scholar]
- 80.Weschler CJ; Nazaroff WW Dermal Uptake of Organic Vapors Commonly Found in Indoor Air. Environ. Sci. Technol 2014, 48, 1230–1237. [DOI] [PubMed] [Google Scholar]
- 81.Shoeib M; Harner T; Wilford BH; Jones KC; Zhu J Perfluorinated Sulfonamides in Indoor and Outdoor Air and Indoor Dust: Occurrence, Partitioning, and Human Exposure. Environ. Sci. Technol 2005, 39 (17), 6599–6606. [DOI] [PubMed] [Google Scholar]
- 82.Ahrens L; Harner T; Shoeib M; Koblizkova M; Reiner EJ Characterization of Two Passive Air Samplers for Per- and Polyfluoroalkyl Substances. Environ. Sci. Technol 2013, 47 (24), 14024–14033. [DOI] [PubMed] [Google Scholar]
- 83.Koch A; Aro R; Wang T; Yeung LWY Towards a comprehensive analytical workflow for the chemical characterisation of organofluorine in consumer products and environmental samples. Trends Anal. Chem 2020, 123, 115423. [Google Scholar]
- 84.Riedel TP; Lang JR; Strynar MJ; Lindstrom AB; Offenberg JH Gas-Phase Detection of Fluorotelomer Alcohols and Other Oxygenated Per- and Polyfluoroalkyl Substances by Chemical Ionization Mass Spectrometry. Environ. Sci. Technol. Lett 2019, 6, 289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bowers BB; Thornton JA; Sullivan RC Evaluation of iodide chemical ionization mass spectrometry for gas and aerosol-phase per- and polyfluoroalkyl substances (PFAS) analysis. Environ. Sci. Process Impacts 2023, 25, 277–287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


