Abstract
Purpose
Both hypertension and diabetes are known to increase the wall-to-lumen ratio (WLR) of retinal arterioles, but the differential effects are unknown. Here, we study the timing and relative impact of hypertension versus diabetes on the WLR in diabetic retinopathy (DR) to address this unresolved question.
Methods
This prospective cross-sectional study compared the retinal arteriolar WLR in 17 healthy eyes, 15 with diabetes but no apparent DR (DM no DR), and 8 with diabetic macular edema (DME) and either nonproliferative or proliferative DR. We imaged each arteriole using adaptive optics scanning laser ophthalmoscopy and measured the WLR using ImageJ. Multiple linear regression (MLR) was performed to estimate the effects of hypertension, diabetes, and age on the WLR.
Results
Both subjects with DM no DR and subjects with DME had significantly higher WLR than healthy subjects (0.36 ± 0.08 and 0.42 ± 0.08 vs. 0.29 ± 0.07, 1-way ANOVA P = 0.0009). MLR in healthy subjects and subjects with DM no DR showed hypertension had the strongest effect (regression coefficient = 0.08, P = 0.009), whereas age and diabetes were not significantly correlated with WLR. MLR in all three groups together (healthy, DM no DR, and DME) showed diabetes had the strongest effect (regression coefficient = 0.05, P = 0.02), whereas age and hypertension were not significantly correlated with WLR.
Conclusions
Hypertension may be an early driver of retinal arteriolar wall thickening in preclinical DR, independent of age or diabetes, whereas changes specific to DR may drive wall thickening in DME and later DR stages.
Translational Relevance
We offer a framework for understanding the relative contributions of hypertension and diabetes on the vascular wall, and emphasize the importance of hypertension control early in diabetes even before DR onset.
Keywords: diabetic retinopathy (DR), hypertension, retinal vessels, adaptive optics
Introduction
Hypertension and diabetes often co-exist; individuals with diabetes are twice as likely to have hypertension compared to those without diabetes, and patients with hypertension are more likely to develop diabetes compared to normotensive individuals.1 Moreover, hypertension and diabetes lead to macrovascular complications, such as coronary artery disease and stroke, as well as microvascular complications, including hypertensive and diabetic retinopathy (DR).2–4 Hypertensive retinopathy is characterized by arteriolar narrowing, arterio-venous nicking, increased arteriolar opacity, retinal hemorrhages, and microaneurysms.5 Clinically detectable DR is defined by retinal microaneurysms, hemorrhages, and exudates,5 and hypertension has been shown to play a role in the development of DR.6 Notably, there are microvascular changes that precede clinically detectable retinopathy.7–10 Arteriolar remodeling may be the earliest sign of preclinical eye disease in hypertension,8,11,12 and subclinical DR may be characterized by wall thickening9 along with other microvascular changes, such as our recently reported retinal arteriolar vascular smooth muscle cell loss.10 In particular, hypertension and diabetes both induce vascular wall remodeling in the retinal microvasculature.9,13
A range of vascular biomarkers have been proposed to characterize retinal vascular pathology, including parietal thickness, wall cross sectional area, and wall to lumen ratio (WLR).14 The WLR represents the ratio comparing a particular vessel's wall thickness to its lumen diameter. In this study, we use WLR to quantify vascular wall remodeling because it is independent of vessel size.12 Changes in WLR can be visualized and quantified in humans in vivo using adaptive optics (AO) imaging, a technique that allows direct visualization of the retinal vasculature in high resolution.14,15 Previous studies using AO established the correlation between hypertension and increased WLR,16–19 as well as the relationship between DR and increased WLR.9,20,21 However, the timing and relative impact of diabetes versus hypertension on WLR remains unresolved in the literature, specifically whether there is a difference in WLR between healthy subjects and those with diabetes mellitus (DM) before the onset of clinical retinopathy (DM no DR), and if so, whether the increase in WLR is mainly driven by diabetes or hypertension. The majority of previous studies used flood-illuminated AO and reported that the WLR is significantly higher in subjects with DM no DR compared to healthy subjects.20,22,23 In contrast, a relatively large study using scanning laser doppler flowmetry (SLDF) did not find a difference in WLR between control subjects and those with early type 2 diabetes without irreversible end-organ damage, after adjusting for age and gender.24 Furthermore, previous studies often did not focus on DME specifically9,21 or excluded individuals who had received prior therapy for DME.20
As a result of these controversial reports, the timing and relative contribution of hypertension versus diabetes on vascular remodeling in the retina remains unclear. Here, we use AO scanning laser ophthalmoscopy (AOSLO) to quantify the WLR in a cross-sectional cohort in three groups: healthy subjects, subjects with DM no DR, and subjects with diabetic macular edema (DME) and either nonproliferative or proliferative DR. Then, we estimate the relative influence of diabetes and hypertension on the WLR in these three study groups. Given the association of age with increased WLR,16–18 we also included age in our model. With this work, we aimed to understand the pathophysiologic course of increased WLR in DR and the relative contribution of hypertension, diabetes, and age to this process, which could potentially guide the development of future treatments to delay DR progression.
Methods
Participants
This is a prospective cross-sectional study conducted at the Department of Ophthalmology at Northwestern University in Chicago, Illinois, between June 2021 and March 2023. All participants gave written informed consent before image acquisition. The study was approved by the Institutional Review Board of Northwestern University and conducted in accordance with the Health Insurance Portability and Accountability Act regulations and the Declaration of Helsinki tenets.
The study sample is the same as that used in our recent study elucidating the timeline of retinal vascular mural cell loss in DR.10 Briefly, three cohorts were recruited: healthy subjects with no retinal pathology (healthy), subjects with DM no DR, and patients with DME with either nonproliferative or proliferative DR. Inclusion criteria included diagnosis of either type 1 or type 2 diabetes mellitus for the DM no DR and DME groups, and aged between 18 and 80 years old for all subjects. Individuals were excluded if they had prediabetes (HbA1c between 5.7% and 6.4%), glaucoma, or refractive error greater than 3 diopters. To confirm that subjects in the DM no DR cohort did not have clinically apparent retinopathy, we used the International Clinical Diabetic Retinopathy Disease Severity Scale25 to evaluate all available imaging studies (including infrared photography and/or pseudo-color ultrawide-field fundus photographs) in order to exclude subjects with any signs of retinopathy, including microaneurysms. Individuals in the DME group were clinically assessed by board-certified ophthalmologists and found to have either extrafoveal or center-involved DME, based on an optical coherence tomography (OCT) central subfield thickness of more than 260 µm. Demographic and clinical data were obtained from the electronic medical records.
Image Acquisition and Analysis
Complete details of the image acquisition protocol can be found in our previous work.10 Briefly, after dilating the pupil, we measured the axial length using an IOLMaster 700 (ZEISS, Jena, Germany) in each eye to account for its magnification and scale our metrics accurately. Next, we obtained 6 × 6 mm2 optical coherence tomography angiography (OCTA) images centered on the fovea to identify the individual vessels of interest. The OCTA images were acquired using the RTVue-XR Avanti system (Optovue Inc., Fremont, CA, USA) with split-spectrum amplitude-decorrelation angiography software (SSADA; version 2017.1.0.151).26 Machine specifications included a light source centered on 840 nm with a bandwidth of 45 nm and an A-scan rate of 70,000 scans per second. Finally, we imaged each vessel of interest using the Apaeros Retinal Imaging AOSLO System (Boston Micromachines Corporation, Cambridge, MA, USA). To enhance vascular wall visibility, an offset confocal aperture was used.27,28 We obtained 100 video frames for each vessel, which were then aligned and averaged to generate the final images.15
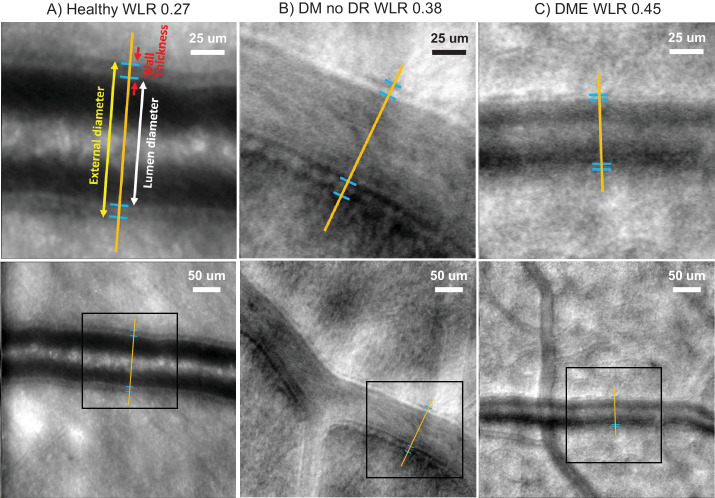
For each vessel, the external diameter and lumen diameter were measured using ImageJ (developed by Wayne Rasband, National Institutes of Health [NIH], Bethesda, MD, USA; available in the public domain at http://rsb.info.nih.gov/ij/index.html). The wall thickness was calculated as the difference between the external diameter and lumen diameter. The WLR was calculated as the wall thickness divided by the lumen diameter. Figure 1 shows example arterioles with annotated wall thicknesses and external and lumen diameters.
Figure 1.
Example adaptive optics scanning laser ophthalmoscopy images of retinal arterioles. Example arterioles with annotated wall thicknesses and external and lumen diameters. (A) Depicts an example arteriole from a healthy eye with a low wall to lumen ratio (WLR) of 0.27. (B) Shows an example vessel from a patient with diabetes mellitus without retinopathy (DM no DR) with a higher WLR of 0.38. (C) Depicts an example arteriole from a diabetic macular edema (DME) eye with a WLR of 0.45. The top row displays a magnified view of the inset regions from the bottom row. Contrast was adjusted for clarity.
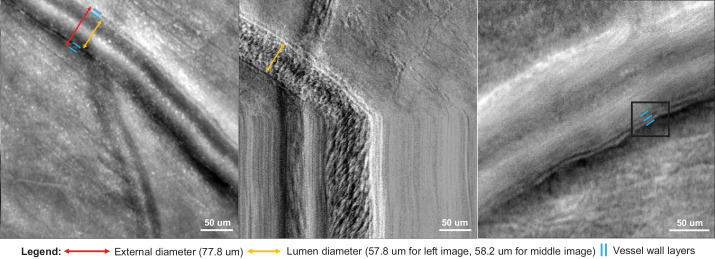
Some of the images had three clear interfaces in the vessel wall (presumably the luminal surface of the endothelial cell layer, basement membrane, and outer vessel wall), whereas others only had two visible interfaces. For vessels with two interfaces, we sought to identify whether the innermost surface was the endothelial cell layer or basement membrane. To do so, we compared an offset aperture image with the corresponding XT (blood flow zymography) image,29,30 which shows the streak of red cells coursing within the lumen of the vessel and the internal flow diameter (Fig. 2). Using these images, we determined that the visible innermost surface on the offset aperture images was the innermost surface of the lumen, consistent with the luminal surface of the endothelial cell layer (rather than the basement membrane).
Figure 2.
The inner vessel wall layer as the endothelial cell layer. For AOSLO images with two visible vessel wall layers (left image, indicated by the blue lines), we found that the inner layer is the endothelial cell layer rather than the basement membrane. We used the corresponding XT image (middle image), where the vertical scan is momentarily stopped and thus diagonal streaks represent moving cells, to find the lumen diameter. Here, the lumen diameter is 58.2 um. The inner diameter of the offset confocal aperture image on the left is 57.8 um, highlighting that the inner layer is indeed the endothelial cell layer. The image on the right illustrates a vessel with three clear interfaces in the vessel wall indicated by the three blue lines. These layers are presumably the luminal surface of the endothelial cell layer, basement membrane, and outer vessel wall. Contrast was adjusted for clarity.
Statistical Analysis
We used Python software version 3.8.8 (Python Software Foundation, Wilmington, DE, USA) to perform statistical analysis. To determine whether the demographic data are normally distributed, Shapiro-Wilk tests were used. To compare continuous variables across all three study groups, 1-way ANOVA was performed, whereas Student's t-test was used to compare continuous variables between two study groups. To compare categorical variables across all three study groups, the Chi-square test was performed, whereas the Fisher exact test was used to compare categorical variables between two study groups. Pearson correlations were used to evaluate potential associations between confounding variables and the WLR. Student's t-test was also used to compare pairwise differences in WLR among healthy, DM no DR, and DME groups. Multiple linear regression (MLR) using the method of Ordinary Least Squares was used to estimate the effect of diabetes, age, and hypertension on the WLR. The P values were considered statistically significant if they were less than 0.05.
Results
Demographic and Clinical Characteristics
The study included 40 subjects (17 healthy subjects, 15 subjects with DM no DR, and 8 subjects with DME) and a total of 47 eyes (20 healthy subjects, 19 subjects with DM no DR, and 8 subjects with DME). Table 1 summarizes the demographic and clinical characteristics of the study participants. Complete details regarding participant characteristics can also be found in our previous work.10 Briefly, all demographic data were normally distributed (Shapiro-Wilk tests, P > 0.05) except for time since DM diagnosis (P = 0.006). Among the three study groups, age, hypertension diagnosis, external vessel diameter, and lumen vessel diameter were significantly different (1-way ANOVA P = 0.002, 0.02, 0.02, and 0.01 respectively), whereas sex, race/ethnicity, and axial length were comparable across the three groups. For the DME group, DR severity varied widely: one eye had mild nonproliferative DR (NPDR), two had moderate NPDR, two had severe NPDR, and three had proliferative DR.
Table 1.
Clinical and Demographic Characteristics of Participant Groups
| Subject Characteristics | Healthy | DM No DR | DME | P Value |
|---|---|---|---|---|
| Patients | 17 | 15 | 8 | – |
| Eyes | 20 | 19 | 8 | – |
| Age, y, mean ± SD [range] | 38.4 ± 10.8 [18–59] | 48.4 ± 12.1 [25–67] | 56.8 ± 10.2 [36-72] | 0.002a,b |
| Sex, male/female (% male) | 5/12 (29%) | 8/7 (53%) | 4/4 (50%) | 0.35c |
| Hypertension yes/no (% yes) | 3/14 (18%) | 7/8 (47%) | 6/2 (75%) | 0.02a,c |
| Race/ethnicity | 0.08c | |||
| Non-Hispanic White | 14 | 9 | 3 | |
| Non-Hispanic Black | 0 | 3 | 4 | |
| Hispanic | 2 | 1 | 1 | |
| Non-Hispanic Asian | 1 | 2 | 0 | |
| DM type, type I/type II (% type I) | – | 4/11 (27%) | 1/7 (13%) | 0.62d |
| Time since DM diagnosis, y, mean ± SD | – | 8.3 ± 8.5 | 16.4 ± 7.9 | 0.046a,e |
| DR severity | – | – | 1 mild NPDR, 2 moderate NPDR, 2 severe NPDR, 3 PDR | – |
| Last HbA1c, mean ± SD | – | 6.3 ± 0.9 | 7.9 ± 1.5 | < 0.001a,e |
| External vessel diameter, µm, mean ± SD [range] | 95.5 ± 25.7 [57.5–157.6] | 91.6 ± 13.7 [34.2–135.4] | 68.9 ± 24.9 [40.9–127.0] | 0.02a,b |
| Lumen vessel diameter, µm, mean ± SD | 74.7 ± 22.0 | 67.9 ± 10.8 | 49.1 ± 18.6 | 0.01a,b |
| Wall thickness, µm, mean ± SD | 20.8 ± 4.7 | 23.7 ± 4.6 | 19.9 ± 6.8 | 0.18b |
| Axial length, mm, mean ± SD | 23.9 ± 1.0 | 24.2 ± 0.9 | 23.7 ± 1.3 | 0.54b |
DM, diabetes mellitus; DM no DR, diabetes mellitus without apparent diabetic retinopathy; DME, diabetic macular edema; HbA1c, hemoglobin A1c; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; SD, standard deviation.
Statistically significant.
One-way ANOVA (healthy versus DM no DR versus DME).
Chi-square test (healthy versus DM no DR versus DME [for the Chi-square test for race/ethnicity, Non-Hispanic Black, Hispanic, and Non-Hispanic Asian were grouped together as all are racial/ethnic minority groups]).
Fisher exact test (DM no DR versus DME).
Student's t-test (DM no DR versus DME).
Impact of Diabetes, Age, and Hypertension on WLR: Univariate Analysis
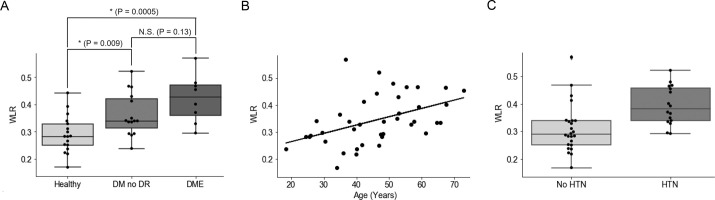
Healthy subjects, subjects with DM no DR, and subjects with DME had an average WLR of 0.29 ± 0.07, 0.36 ± 0.08, and 0.42 ± 0.08, respectively (1-way ANOVA P = 0.0009; Fig. 3A). Pairwise comparisons showed that subjects with DM no DR and subjects with DME had significantly higher WLR compared to healthy subjects (P = 0.009 and 0.0005 respectively), whereas DM no DR and DME eyes did not differ (P = 0.13). Using univariate analysis, we found that age was significantly correlated with WLR (Pearson correlation R2 = 0.209, P value = 0.003; Fig. 3B). The healthy controls ranged from 18 to 59 years old, the subjects with DM no DR were 25 to 67 years old, and the subjects with DME were 36 to 72 years old. Moreover, there was a statistically significant relationship between hypertension and WLR (Fig. 3C). Those without hypertension had an average WLR of 0.31 ± 0.09, whereas those with hypertension had an average WLR of 0.39 ± 0.07 (t-test P value = 0.003).
Figure 3.
Impact of diabetes, age, and hypertension on the wall to lumen ratio. (A) Boxplot comparing wall to lumen ratio (WLR) in three groups: healthy subjects, those with diabetes without diabetic retinopathy (DM no DR), and subjects with diabetic macular edema (DME). Although DM no DR subjects and DME subjects have a higher WLR than healthy subjects (P = 0.009 and 0.0005 respectively), there is no significant difference in WLR for subjects with DME compared with subjects with DM no DR (P = 0.13). The asterisk symbol (*) indicates statistical significance from Student's t-test (P < 0.05). N.S. stands for not significant. (B) Linear regression shows that WLR is significantly correlated with age (Pearson correlation R2 = 0.209, P = 0.003). (C) T-test shows that hypertension is significantly associated with WLR (P = 0.003).
Multiple Linear Regression Analysis
Hypertension (yes or no), DR status (healthy versus DM no DR versus DME), and age were the three parameters included in the MLR model. When we performed MLR on the healthy subjects and subjects with DM no DR, we found that hypertension had the strongest effect on WLR with a coefficient term of 0.075 and P value of 0.009, whereas age and diabetes were not significantly correlated with the WLR (P value of 0.15 for both; Table 2). When we added DME eyes, we found that diabetes had the strongest effect on WLR with a coefficient term of 0.047 and P value of 0.016, whereas age and hypertension were not significantly correlated with the WLR (P value of 0.46 and 0.21, respectively; Table 3). The regression coefficient plot of this MLR analysis is shown in Figure 4.
Table 2.
Multiple Linear Regression – Effect of Diabetes, Age, and Hypertension on Wall to Lumen Ratio (Healthy and DM No DR Eyes Only)
| Variables | Coefficient Term | Standard Error | P Value | Confidence Interval [0.025 to 0.975] |
|---|---|---|---|---|
| Diabetes | 0.036 | 0.024 | 0.15 | −0.013 to 0.085 |
| Age, y | 0.002 | 0.001 | 0.15 | −0.001 to 0.004 |
| Hypertension | 0.075 | 0.027 | 0.009* | 0.021 to 0.129 |
P values obtained from multiple linear regression using the method of ordinary least squares.
Statistically significant.
Table 3.
Multiple Linear Regression – Effect of Diabetes, Age, and Hypertension on Wall to Lumen Ratio (Healthy, DM no DR, and DME Eyes)
| Variables | Coefficient Term | Standard Error | P Value | Confidence Interval [0.025 to 0.975] |
|---|---|---|---|---|
| Diabetes | 0.047 | 0.019 | 0.02* | 0.009 to 0.085 |
| Age, y | 0.0009 | 0.001 | 0.46 | −0.001 to 0.003 |
| Hypertension | 0.038 | 0.030 | 0.21 | −0.022 to 0.097 |
P values obtained from multiple linear regression using the method of ordinary least squares.
Statistically significant.
Figure 4.
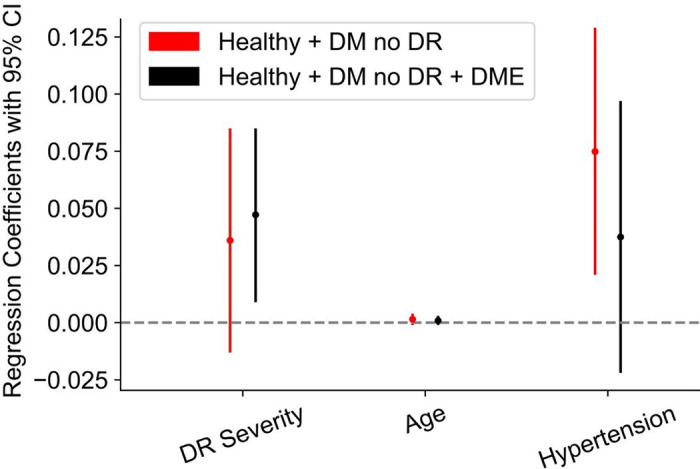
Regression coefficient plot of the contributions of diabetes, age, and hypertension on the wall to lumen ratio. Regression coefficient plot from multiple linear regression (MLR). MLR on healthy subjects versus subjects with DM no DR only (red) show that hypertension has the strongest effect on WLR with a coefficient term of 0.075 (95% confidence interval [CI] = 0.021-0.129) and P value of 0.009, whereas age and diabetes were not significantly correlated with the WLR (P value of 0.15 for both). MLR on all three groups together (black) shows that DR status has the strongest effect on WLR with a coefficient term of 0.047 (95% CI = 0.009-0.085) and P value of 0.02, whereas age and hypertension were not significantly correlated with the WLR (P value of 0.46 and 0.21, respectively). Three parameters were included in the MLR model: hypertension (yes or no), DR status (healthy versus DM no DR versus DME), and age.
Discussion
In this study, we used AOSLO in vivo to quantify the WLR of retinal arterioles in the eyes of healthy, DM no DR, and DME subjects. First, from univariate analysis, we found that DM no DR and DME subjects had significantly higher WLR compared to healthy subjects, whereas DM no DR and DME subjects did not differ. Furthermore, age and hypertension were significantly correlated with WLR. Then, we performed MLR to study the relative contributions of age, hypertension, and diabetes to the WLR. When analyzing only healthy versus DM no DR subjects, hypertension had the strongest effect on WLR, whereas DR status had the strongest effect when analyzing all three groups together.
Notably, our finding that DM no DR subjects have significantly higher WLR compared to healthy subjects sheds light on the potential source for disagreements in the current literature, where some studies found no difference in WLR, whereas others did. For instance, using SLDF, Kannenkeril et al. did not find a difference in WLR between subjects with early type 2 diabetes (111 patients) and control subjects (54 healthy).24 They did not discuss the specific stage of DR in their patients, but noted that they presented without “irreversible end-organ damage,”24 suggesting they likely did not have DR. In contrast, Ueno et al. reported that the mean WLR was significantly higher in the DM no DR group (47 patients) compared to the control group (24 subjects).20 However, it is unclear whether either of these groups accounted for hypertension in their analysis. Arichika et al. found a significantly greater retinal arteriolar wall thickness in patients with type 2 diabetes without clinically apparent DR than in the control group, similar to our finding.31 Then, using multivariate regression analysis, they found that the HbA1c level was independently associated with wall thickness.31 Our results are consistent with Ueno et al.,20 Arichika et al.,31 and others.22,23 One possible reason for the discrepancy between our results and those of Kannenkeril et al. could be related to the use of SLDF, which has lower reproducibility compared to AOSLO.32 Another limitation of SLDF is its low resolution and inability to directly visualize the vessel walls; therefore, when using SLDF, the WLR is calculated indirectly by comparing reflection and perfusion images.33
Our finding of an association between age/hypertension and WLR is expected and consistent with previous literature. Arichika et al. studied retinal arterioles in 51 healthy subjects and 22 patients with hypertension and found that WLR showed a strong correlation with age as well as with blood pressure.16 Similarly, Koch et al. imaged the supero-temporal retinal arteriole in 49 individuals and found that WLR was positively correlated with blood pressure and age (accounting for 43% of the variability of WLR).17 Finally, a meta-analysis comparing patients with hypertension to healthy controls found a significantly higher WLR in patients with hypertension (I2 = 94%, P < 0.001).14 Our results showing subjects with DME (with either nonproliferative or proliferative DR) have significantly higher WLR compared to healthy subjects is also well-supported by the literature. Burns et al. found extensive capillary remodeling in subjects with mild or moderate DR (increased WLR in 7 subjects with diabetes versus 7 healthy subjects).21 Ueno et al. studied 24 healthy eyes, 47 eyes with DM no DR, 36 eyes with mild or moderate NPDR, 22 eyes with severe NPDR, and 32 eyes with PDR and found that the WLR is higher in PDR than in all of the other groups, whereas the WLR is lower in the control group compared to all of the other groups.20
Because age, hypertension, and diabetes are all shown to impact the WLR, we next performed MLR to parse out the timing and relative contribution of each of these variables on the WLR. In particular, the timing of the impact of diabetes on WLR is unresolved in the literature. For example, using SLDF, Stefanski et al. found that new-onset diabetes is associated with an increase in retinal capillary flow, whereas structural changes (as shown via increased WLR) are seen later (>10 years duration of diabetes).34 In addition, using SLDF, Jumar et al. found that early stages of type 2 diabetes did not exhibit hypertrophic remodeling (no increased wall thickness) in the retinal arterioles, while those with diabetes for more than 5 years did have these changes.35 In our study, we add to the scientific literature by showing that in healthy subjects and subjects with DM no DR, hypertension was a strong predictor of WLR, independent of age or diabetes. This finding suggests that hypertension may be an early driver of wall thickening independent of age or diabetes, whereas changes specific to diabetes may drive wall thickening in later stages of DR and DME. One strength of our study was the diverse group of patients, where a proportion of the subjects in each of the three study groups (control, DM no DR, and DME) had hypertension (see Table 1). Previous studies often excluded those with hypertension from the control group9 or from the study as a whole,22 making it difficult to draw conclusions regarding the impact of DM and hypertension versus hypertension alone on retinal arteriolar remodeling. Our dataset, which includes healthy subjects with hypertension and subjects with DM no DR and subjects with DME without hypertension, allowed us to parse out the individual contributions of hypertension versus diabetes on the WLR in subclinical versus clinically apparent DR, which is a novel result from this study. Furthermore, our use of AOSLO with an offset confocal aperture configuration (which was also used by Sapoznik et al.9 and Hillard et al.19) is unique as it allows for greater resolution of the retinal arteriolar walls compared to previous AO imaging studies that used flood-illuminated imaging devices.20,22 Whereas standard flood illuminated AO approaches (eg, the rtx1 imaging device) can only visualize the lumen and external diameters,20,22 the offset confocal aperture can visualize the vascular wall fine structure,15 including all three layers of the vessel wall when the images are of sufficient quality (see Fig. 2). Improved technology in the future may reveal the basement membrane in even higher resolution, allowing an improved understanding of the contribution of the different vessel wall structures (eg, endothelial cells, basement membrane versus smooth muscle cells) to the overall wall thickening in DR.
A variety of mechanisms have been proposed to contribute to the pathologically increased WLR in hypertension and diabetes. In hypertension, increased hemodynamic load is thought to lead to increased vascular resistance to blood flow, an adaptive response where the arteriolar lumen narrows and the wall thickens.12,36 This process optimizes wall stress to protect arterioles from elevated blood pressure.13 Another mechanism for the increase in WLR in hypertension is eutrophic remodeling, which occurs in response to increased wall stress and chronic vasoconstriction, leading to rearrangement of the smooth muscle cells in the vascular wall to align more closely, leading to a smaller lumen.11,13 In more severe hypertension, an additional process of hypertrophic remodeling occurs due to smooth muscle cell growth.13,37,38 In diabetes, hyperglycemia causes basement membrane thickening,39 which can lead to an increased WLR. This basement membrane thickening is thought to arise via several mechanisms, including protein kinase C activation, increased growth factor activity, and accumulation of advanced glycation end-products, which may increase the expression of basement membrane components and decrease the rate of basement membrane degradation.39 Hypertrophic remodeling, characterized by vascular smooth muscle cell hypertrophy or hyperplasia, is also present in DR.40 In terms of the impact of age on WLR, it has been hypothesized that structural remodeling occurs during normal aging, including microvascular wall thickening and basement membrane collagen deposition, perhaps due to age-related endothelial dysfunction.41 Interestingly, in our MLR analysis, we found that age was not a statistically significant contributor to the increase in WLR in DM no DR and DME eyes.
Overall, hypertension and diabetes work synergistically in remodeling the retinal arterioles,12 which can be quantified by WLR, an important biomarker of these changes. In our previous work, we showed that arteriolar vascular mural cell loss occurs in preclinical DR and proposed a model for the progression of vascular mural cell compromise in human DR.10 Here, we add to this picture of preclinical and clinical DR the increase in WLR seen in preclinical DR, likely driven by hypertension, whereas diabetes likely drives vascular remodeling in clinical DR and DME (as well as capillary pericyte loss). This further highlights the importance of optimizing blood pressure control in individuals with DM even prior to the onset of DR.42 Our study sheds light on, and begins to address, an important gap in our knowledge regarding the relative contribution of hypertension (early) and diabetes (later) in the course of DR. Understanding the pathogenesis of preclinical DR is important for the development of treatments to prevent DR onset and progression.
Our study faced several limitations. One limitation was the small sample size due to our strict criteria for image quality. With the small sample size, we could not adjust for additional variables other than diabetes, age, and hypertension, such as last HbA1c or length of time since DM diagnosis. The small sample size also precluded our ability to stratify subjects by DM type (type 1 vs. 2), which is another important area of future study, as it is unknown whether the vasculature may be affected in different ways in type 1 versus type 2 diabetes.43 Furthermore, our DME group included subjects with varying DR severity, including mild, moderate, and severe NPDR, as well as PDR. Future work with larger sample sizes could focus on dividing subjects with DME into subgroups based on DR severity. Finally, we did not stratify patients based on whether they had received treatment (eg, for hypertension, diabetes, DR, or DME) which could have confounding effects on the WLR. Future studies could focus on the impact and timing of antihypertensive drugs or anti-VEGF therapy on retinal vascular remodeling.
Conclusions
We found that hypertension had the strongest effect on WLR in subjects with DM no DR, whereas DR status had the strongest effect when analyzing healthy patients, subjects with DM no DR, and subjects with DME together. This shows that hypertension may be an early driver of wall thickening independent of age or diabetes, whereas changes specific to DR may drive wall thickening later in the course of DR and DME.
Acknowledgments
A. A. Fawzi is supported in part by NIH Grant R01EY31815. B. B. Huang is supported in part by NIH Grant 1T35DK126628-01, a grant from the Illinois Society for the Prevention of Blindness (SP0076776), and an Alpha Omega Alpha Carolyn L. Kuckein Student Research Fellowship.
Disclosure: B.B. Huang, None; A.A. Fawzi, Roche/Genentech (C, N), Boehringer Ingelheim (C, N), RegenXbio (C, N), 3Helix (C, N), Optos Inc. (C, N), Boehringer Ingelheim (F)
References
- 1. Petrie JR, Guzik TJ, Touyz RM.. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018; 34(5): 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008; 26(2): 77–82. [Google Scholar]
- 3. Wong TY, Mitchell P. Hypertensive retinopathy. N Engl J Med. 2004; 351(22): 2310–2317. [DOI] [PubMed] [Google Scholar]
- 4. Rask-Madsen C, King GL.. Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metab. 2013; 17(1): 20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grosso A, Cheung N, Veglio F, Wong TY.. Similarities and differences in early retinal phenotypes in hypertension and diabetes. J Hypertens. 2011; 29(9): 1667. [DOI] [PubMed] [Google Scholar]
- 6. Fraser-Bell S, Symes R, Vaze A.. Hypertensive eye disease: a review. Clin Experiment Ophthalmol. 2017; 45(1): 45–53. [DOI] [PubMed] [Google Scholar]
- 7. Ikram MK, Cheung CY, Lorenzi M, et al.. Retinal vascular caliber as a biomarker for diabetes microvascular complications. Diabetes Care. 2013; 36(3): 750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Park JB, Schiffrin EL.. Small artery remodeling is the most prevalent (earliest?) form of target organ damage in mild essential hypertension. J Hypertens. 2001; 19(5): 921–930. [DOI] [PubMed] [Google Scholar]
- 9. Sapoznik KA, Gast TJ, Carmichael-Martins A, Walker BR, Warner RL, Burns SA.. Retinal arteriolar wall remodeling in diabetes captured with AOSLO. Transl Vis Sci Technol. 2023; 12(11): 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang BB, Fukuyama H, Burns SA, Fawzi AA.. Imaging the retinal vascular mural cells in vivo: elucidating the timeline of their loss in diabetic retinopathy. Arterioscler Thromb Vasc Biol. 2024; 44(2): 465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Intengan HD, Schiffrin EL.. Structure and mechanical properties of resistance arteries in hypertension: role of adhesion molecules and extracellular matrix determinants. Hypertens Dallas Tex 1979. 2000; 36(3): 312–318. [DOI] [PubMed] [Google Scholar]
- 12. Rizzoni D, Agabiti Rosei E. Small artery remodeling in hypertension and diabetes. Curr Hypertens Rep. 2006; 8(1): 90–95. [DOI] [PubMed] [Google Scholar]
- 13. Ritt M, Harazny JM, Ott C, et al.. Analysis of retinal arteriolar structure in never-treated patients with essential hypertension. J Hypertens. 2008; 26(7): 1427. [DOI] [PubMed] [Google Scholar]
- 14. Bakker E, Dikland FA, van Bakel R, et al.. Adaptive optics ophthalmoscopy: a systematic review of vascular biomarkers. Surv Ophthalmol. 2022; 67(2): 369–387. [DOI] [PubMed] [Google Scholar]
- 15. Chui TYP, Gast TJ, Burns SA.. Imaging of vascular wall fine structure in the human retina using adaptive optics scanning laser ophthalmoscopy. Invest Ophthalmol Vis Sci. 2013; 54(10): 7115–7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arichika S, Uji A, Ooto S, Muraoka Y, Yoshimura N.. Effects of age and blood pressure on the retinal arterial wall, analyzed using adaptive optics scanning laser ophthalmoscopy. Sci Rep. 2015; 5: 12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koch E, Rosenbaum D, Brolly A, et al.. Morphometric analysis of small arteries in the human retina using adaptive optics imaging: relationship with blood pressure and focal vascular changes. J Hypertens. 2014; 32(4): 890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rosenbaum D, Mattina A, Koch E, et al.. Effects of age, blood pressure and antihypertensive treatments on retinal arterioles remodeling assessed by adaptive optics. J Hypertens. 2016; 34(6): 1115–1122. [DOI] [PubMed] [Google Scholar]
- 19. Hillard JG, Gast TJ, Chui TYP, Sapir D, Burns SA.. Retinal arterioles in hypo-, normo-, and hypertensive subjects measured using adaptive optics. Transl Vis Sci Technol. 2016; 5(4): 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ueno Y, Iwase T, Goto K, et al.. Association of changes of retinal vessels diameter with ocular blood flow in eyes with diabetic retinopathy. Sci Rep. 2021; 11(1): 4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burns SA, Elsner AE, Chui TY, et al.. In vivo adaptive optics microvascular imaging in diabetic patients without clinically severe diabetic retinopathy. Biomed Opt Express. 2014; 5(3): 961–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cristescu IE, Zagrean L, Balta F, Branisteanu DC.. Retinal microcirculation investigation in type I and II diabetic patients without retinopathy using an adaptive optics retinal camera. Acta Endocrinol Buchar. 2019; 15(4): 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matuszewski W, Gontarz-Nowak K, Harazny JM, Bandurska-Stankiewicz E.. Evaluation of morphological changes in retinal vessels in type 1 diabetes mellitus patients with the use of adaptive optics. Biomedicines. 2022; 10(8): 1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kannenkeril D, Bosch A, Harazny J, et al.. Early vascular parameters in the micro- and macrocirculation in type 2 diabetes. Cardiovasc Diabetol. 2018; 17(1): 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilkinson CP, Ferris FL, Klein RE, et al.. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003; 110(9): 1677–1682. [DOI] [PubMed] [Google Scholar]
- 26. Jia Y, Tan O, Tokayer J, et al.. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012; 20(4): 4710–4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chui TYP, VanNasdale DA, Burns SA.. The use of forward scatter to improve retinal vascular imaging with an adaptive optics scanning laser ophthalmoscope. Biomed Opt Express. 2012; 3(10): 2537–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dubra A, Sulai Y.. Reflective afocal broadband adaptive optics scanning ophthalmoscope. Biomed Opt Express. 2011; 2(6): 1757–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhong Z, Petrig BL, Qi X, Burns SA.. In vivo measurement of erythrocyte velocity and retinal blood flow using adaptive optics scanning laser ophthalmoscopy. Opt Express. 2008; 16(17): 12746–12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhong Z, Song H, Chui TYP, Petrig BL, Burns SA.. Noninvasive measurements and analysis of blood velocity profiles in human retinal vessels. Invest Ophthalmol Vis Sci. 2011; 52(7): 4151–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arichika S, Uji A, Murakami T, Suzuma K, Gotoh N, Yoshimura N.. Correlation of retinal arterial wall thickness with atherosclerosis predictors in type 2 diabetes without clinical retinopathy. Br J Ophthalmol. 2017; 101(1): 69–74. [DOI] [PubMed] [Google Scholar]
- 32. De Ciuceis C, Agabiti Rosei C, Caletti S, et al.. Comparison between invasive and noninvasive techniques of evaluation of microvascular structural alterations. J Hypertens. 2018; 36(5): 1154. [DOI] [PubMed] [Google Scholar]
- 33. Harazny JM, Ritt M, Baleanu D, et al.. Increased wall:lumen ratio of retinal arterioles in male patients with a history of a cerebrovascular event. Hypertension. 2007; 50(4): 623–629. [DOI] [PubMed] [Google Scholar]
- 34. Stefański A, Wolf J, Harazny JM, et al.. Impact of type 1 diabetes and its duration on wall-to-lumen ratio and blood flow in retinal arterioles. Microvasc Res. 2023; 147: 104499. [DOI] [PubMed] [Google Scholar]
- 35. Jumar A, Ott C, Kistner I, et al.. Early signs of end-organ damage in retinal arterioles in patients with type 2 diabetes compared to hypertensive patients. Microcirculation. 2016; 23(6): 447–455. [DOI] [PubMed] [Google Scholar]
- 36. Schiffrin EL. Remodeling of resistance arteries in essential hypertension and effects of antihypertensive treatment. Am J Hypertens. 2004; 17(12 Pt 1): 1192–1200. [DOI] [PubMed] [Google Scholar]
- 37. Intengan HD, Schiffrin EL.. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertens Dallas Tex 1979. 2001; 38(3 Pt 2): 581–587. [DOI] [PubMed] [Google Scholar]
- 38. Lehmann MV, Schmieder RE.. Remodeling of retinal small arteries in hypertension. Am J Hypertens. 2011; 24(12): 1267–1273. [DOI] [PubMed] [Google Scholar]
- 39. Roy S, Ha J, Trudeau K, Beglova E.. Vascular basement membrane thickening in diabetic retinopathy. Curr Eye Res. 2010; 35(12): 1045–1056. [DOI] [PubMed] [Google Scholar]
- 40. Rizzoni D, Rosei EA.. Small artery remodeling in diabetes mellitus. Nutr Metab Cardiovasc Dis. 2009; 19(8): 587–592. [DOI] [PubMed] [Google Scholar]
- 41. Scioli MG, Bielli A, Arcuri G, Ferlosio A, Orlandi A. Ageing and microvasculature. Vasc Cell. 2014; 6: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Do DV, Han G, Abariga SA, Sleilati G, Vedula SS, Hawkins BS.. Blood pressure control for diabetic retinopathy. Cochrane Database Syst Rev. 2023; 3(3): CD006127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zaccardi F, Webb DR, Yates T, Davies MJ.. Pathophysiology of type 1 and type 2 diabetes mellitus: a 90-year perspective. Postgrad Med J. 2016; 92(1084): 63–69. [DOI] [PubMed] [Google Scholar]