Abstract
Fungal diseases are a major and growing public health concern, but despite that, there are only four major classes of drugs to treat primary fungal pathogens. The pipeline of new antifungals in clinical development is relatively thin compared to other disease classes. One approach to rapidly identify and provide novel treatment options is to repurpose existing drugs as antifungals. However, such proposed drug repurposing candidates often suffer from suboptimal efficacy and pharmacokinetics for fungal diseases. Herein, we briefly review the current antifungal drug pipeline and recent approaches to optimize existing drugs into novel molecules with unique modes of action relative to existing antifungal drug classes.
Keywords: Anti-fungal drug development, Drug repurposing, Lead optimization, SOSA
Teaser
The use of selective optimization of side activities of repurposed drug candidates has great potential for developing novel anti-fungal therapies.
Introduction
The global incidence of fungal diseases is estimated to kill 1.5 million and affect the health of over 1 billion people annually.1 Despite the large number of people affected, there are only four major classes of drugs to treat the main fungal pathogens Aspergillus, Candida, Cryptococcus species, Pneumocystis jirovecii and the endemic fungi Histoplasma capsulatum and Mucoromycetes. This limited arsenal of anti-fungals is made more dire by the increasing number of immunocompromised patients susceptible to invasive fungal infections due to advanced medical treatments.2,3 There is also an increase in fungal infections following natural disasters such as mucormycosis following tornadoes and associated with other infectious diseases such as COVID-19.4,5 Finally, the development of drug-resistant strains as well as identification of novel drug-resistance fungal species observed, such as Candida auris, first identified in Japan and since expanded to five continents, further demonstrates the need for new anti-fungal therapies.6-8
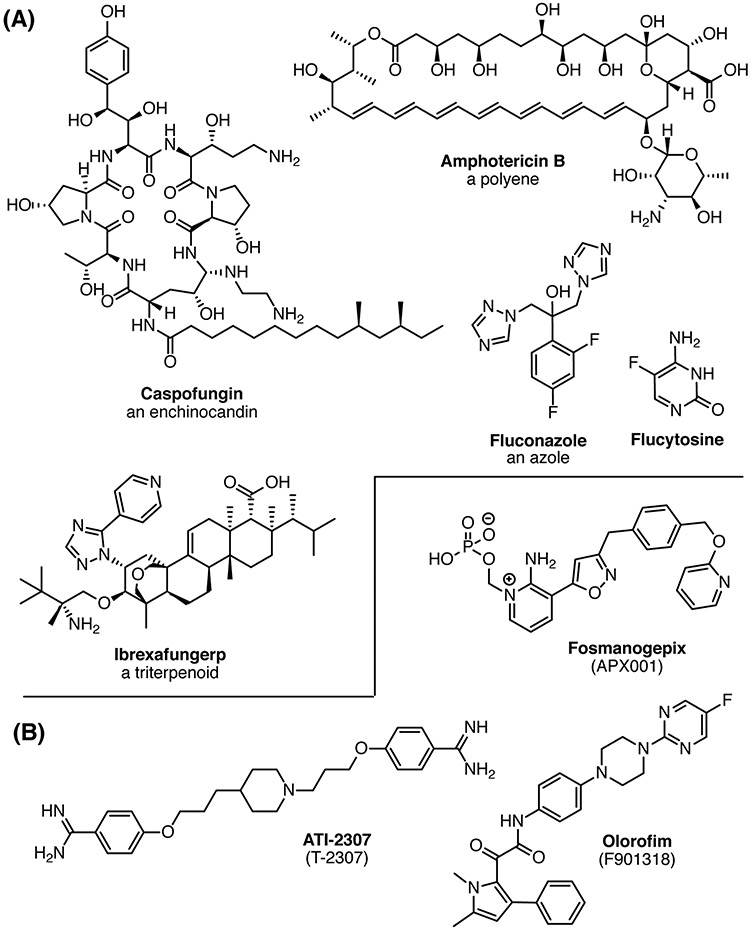
The current therapies for treating systemic fungal infections include the azoles, echinocandins, polyenes and flucytosine (Fig. 1A). All four of these drug classes have limitations in efficacy, toxicity, and development of resistance so there is an urgent need for new anti-fungal therapies to meet the growing threat to human health. The current pipeline for anti-fungals is sparse due to lack of funding and perceived commercial potential, but has shown improvement in the past few years with the recent approval of ibrexafungerp and three candidates with novel activities or site of action that are currently or will soon be evaluated in clinical trials (Fig. 1A,B).
Figure 1. (A) Examples of approved drugs for systemic fungal infections. (B) Current new antifungal drugs in clinical trials.
Fosmanogepix (formerly known as APX001) is a pro-drug whose active form, manogepix, inhibits Gwt1, a protein required for inositol acetylation in the glycosylphosphatidylinositol (GPI) biosynthesis pathway.9 Fosmanogepix is currently in a Phase II trial to evaluate its efficacy for treatment of invasive fungal infections caused by Aspergillus spp. or rare molds (ClinicalTrials.gov Identifier: NCT04240886).
Olorofim (F901318) was developed by F2G, Inc. It was originally identified in a high throughput screen of >300,000 small molecules for inhibition of A. fumigatis. One chemical series identified in this screen showed potency against A. fumigatis but almost none against Candida spp.10 The initial hits in this series were developed by a traditional medicinal chemistry program using structure–activity relationships based on in vitro activity to develop F901318. The target of F901318, dihydroorotate dehydrogenase, was identified by a genetic screen.10 Olorofim is currently being evaluated in a Phase IIb trial for treatment of invasive fungal infections due to Lomentospora prolificans, Scedosporium Spp., Aspergillus Spp. and other resistant fungi in patients lacking alternative treatment options (NCT03583164). It is also in a Phase III trial to evaluate its efficacy compared to amphotericin B treatment in patients with invasive fungal disease caused by Aspergillus spp. (NCT05101187).
T-2307 is an acrylamidine that was identified from a small molecule library screen and optimized in a medicinal chemistry campaign to improve the in vitro and in vivo antifungal activities.11 Although its mechanism of action was not known at the time of optimization, it was recently demonstrated that T-2307 primarily inhibits the respiratory complexes III and IV in yeast mitochondria.12 The rights to T-2307 were purchased by Appili Therapeutics and renamed ATI-2307. ATI-2307 has fungicidal activity against C. neoformans infections and was tested in a Phase I trial. The company is seeking a Neglected Tropical Disease Priority Review Voucher from the FDA and planning to begin a Phase II trial in 2022.13
An important caveat is that all therapies in clinical development have efficacy against only a sub-set of fungal pathogens, so the pipeline needs to be enlarged to ensure there are enough different drug treatment options to cover the range of fungal infections. The time to develop a completely novel chemical scaffold into an FDA approved therapeutic can be well over a decade and development costs are close to one billion dollars.14 Thus, repurposing approved drugs or close derivatives of approved drugs to new targets is an attractive option for possibly bringing a drug to market quicker, with potentially increased chances of success in a clinical trial. Direct drug repurposing has been reviewed recently by Zhang et. al. and is beyond the scope of this review.15 Instead, this review focuses on recent efforts to optimize antifungal activity of drug repurposing hits.
Review of repurposing screens 2019-2021
An efficient method for identification of repurposing candidates is to screen chemical libraries of approved drugs for antifungal activity. There have been two recent reviews on direct drug repurposing, so we will highlight only a few studies published since 2019 that have pursued this avenue to identifying new antifungal drug candidates.16,17
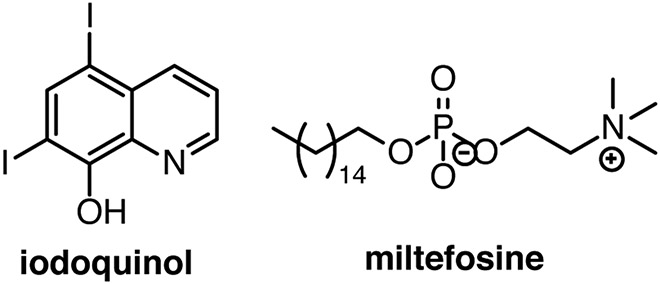
Candida auris emerged in 2009 as a serious nosocomial invasive fungal infection threat due to its inherent multidrug resistance. The Medicines for Malaria Venture (MMV) pathogen box, a library of 400 drug-like molecules known to be active against neglected infectious diseases, was screened for inhibition of C. auris.18 This screen identified 12 molecules that inhibited at least 60% biofilm formation or planktonic growth, leading to iodoquinol and miltefosine being selected as the two main leading repurposing candidates (Fig. 2). Miltefosine was the more promising lead with IC50 values between 1 and 6 μg/mL for inhibition of planktonic, biofilm, and pre-formed biofilm reduction.
Figure 2. Repurposing candidates from the Medicines for Malaria Venture Pathogen Box.
Another approach is to apply virtual screening methodology to identify new antifungal leads. Joshi and coworkers conducted an in silico screen of the Selleck FDA-approved drug library (1930 compounds) against the essential enzyme dihydrofolate reductase of C. albicans (CaDHFR) using deep-learning, molecular docking and similarity searching followed by molecular dynamics simulation leading to the identification of paritaprevir, lumacaftor and rifampin.19 While such an approach may yield useful leads, no confirmation of antifungal activity has been reported to date.
An alternative approach to identifying compounds with intrinsic antifungal activity is to screen approved drugs for synergy with existing antifungal drugs. This approach has revealed drugs such as the antibiotic paromomycin, the antimalarial primaquine the anti-inflammatory ibuprofen and the statin pitavastatin—such compounds do not have intrinsic antifungal activity themselves and are classified as chemosensitizers.20,21
Limitations of repurposing without optimization
Clinical trials of two repurposed FDA-approved drugs, tamoxifen and sertraline, as adjuvant treatments for cryptococcal meningitis were recently completed. Tamoxifen showed no benefit in clearing Cryptococcus from the cerebral spinal fluid, and the trial with sertraline was terminated early due to serious adverse events.22,23 The key advantage to repurposing existing drugs is reduced development costs because of the pharmacokinetic and toxicologic data collected from preclinical and from human clinical trials. However, such repurposed drugs were originally optimized for other disease indications leading to suboptimal antifungal potencies. As such, their efficacy, and perhaps pharmacokinetic properties, have not been optimized for antifungal targets which puts demonstration of clinical efficacy at risk. Furthermore, many repurposing compounds will have known efficacy against a human target that may lead to undesired side effects.
Alternative approaches to direct drug repurposing
An alternative approach to direct repurposing is to optimize a compound for its secondary effect termed “selective optimization of side activities” (SOSA).24 The rationale for SOSA is that almost all drugs currently in use in human therapy have one or more pharmacological side effects, often against unrelated targets. While historically, these “side activities” were identified during clinical use, a derivative of this approach for infectious diseases is executed in two steps in the lab. The first is to screen a limited chemical library of diverse approved drug molecules for antifungal activity. Rather than direct repurposing, hits are then optimized by a medicinal chemistry campaign to increase the affinity for a new target and decrease affinity for the original human target(s). It should be noted, however, that a derivative of an approved drug will need to undergo full clinical evaluation as any novel drug candidate.
A related approach is termed analog-based drug design (ABDD). This approach takes existing drugs for a particular disease area and modifies them to address liabilities and shortcomings of existing drugs such as to combat drug resistance. This could also be considered akin to next-generation and “me-too” approaches and has been recently reviewed for antifungal diseases.25 While ABDD is an appropriate approach, herein, we focus on SOSA approaches for antifungals as these approaches will provide, by definition, novel antifungal chemotypes and mechanisms of action.
Recent antifungal SOSA examples
Analogs of the Antimalarial Drug Mefloquine Are Broad-Spectrum Antifungals.
Mefloquine is an orally active antimalarial drug approved by the US FDA in 1989 (Fig. 3). The antibacterial and antifungal activity of a limited set of analogs of mefloquine was reported by Kunin and Ellis in 2000.26 Over 400 mefloquine analogs from The Walter Reed Army Institute of Research (WRAIR) collection were tested in this study. While mefloquine itself was not particularly active against C. albicans some analogs were identified with strong potency versus C. albicans and C. neoformans, comparable to amphotericin B (e.g., WR166391). A structure-activity relationship (SAR) analysis indicated that the piperidine methanol side chain was essential to antimicrobial activity.
Figure 3. Antimalarial drug mefloquine and analogs with more potent antifungal potency.
Chemical changes are colored blue. MICs reported for mefloquine and 4377 are from 27 and for WR166391 from 26.
No further work on antifungal properties of mefloquine analogs was reported until 2020. In an effort to identify drug candidates for cryptococcal meningitis, the Krysan group was attracted to mefloquine due to its properties as an orally active and CNS-penetrant antifungal lead as well as the availability of analogs from the National Cancer Institute Developmental Therapeutics Program for structure-activity relationships.27 Thirteen analogs were tested for antifungal activity, with four showing improved potency relative to mefloquine. The most potent compound, 4377, had broad spectrum antifungal activity and was not affected by resistance mechanisms affecting current antifungal drugs. Furthermore, mefloquine and analogs were shown to be fungicidal when combined with fluconazole making them attractive for further development.
Optimizing FK506 Analogues for anti-fungal activity.28
The potential anti-fungal activity of the immunosuppressive drug FK506 or tacrolimus was first identified over two decades ago.29,30 The complex natural product chemistry and potent immunosuppressive activity made developing analogs of this drug difficult. However, recently published crystal structures of multiple fungal calcineurins and advances in metabolic engineering has allowed progress in improving the fungal potency of FK506 analogs while simultaeously reducing the immunosuppressive activity.31 One strategy is to use biosynthetic engineering of the genes in the polyketide synthesis cluster found in several Streptomyces species to produce diverse FK506 analogs. Boem and co-workers demonstrated that with modifications to both the FK506-binding protein 12 and calcineurin-binding regions they could generate a FK506 analog with >900-fold decrease in immunosuppressive activity but maintain antifungal activity.28 The anti-fungal activity was generally better against C. neoformans and C. albicans compared to A. fumigatus and also showed synergy with fluconazole.
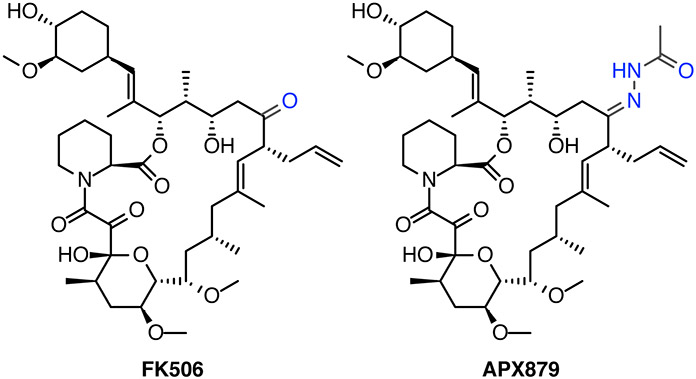
The structural determinations of calcineurin-FKBP12-FK506 complexes from multiple fungal pathogens has revealed conformational differences from the human FKBP12 structure and further identified a single phenylalanine residue in fungal FKPB12 that is not found in the human enzyme and is critical for binding calcineurin.31 Using these structural differences, an analog of FK506, APX879, was synthesized semi-synthetically from FK506 to replace the proximal C22-carbonyl with an acetohydrazine (Fig. 4), which was part of a series of analogs predicted to sterically clash with the His88 in the human enzyme. Treatment with APX879 compared to FK506 was demonstrated to be less immunosuppressive in a cell culture assay of IL-2+ T-cell production and animal models of antigen response. APX879 is much less toxic than FK506 and combinations of APX879 plus fluconazole extended survival by several days and reduced fungal burden in the brain in a cryptococcal mouse infection study. However, a caveat to both of these studies is that neither group has demonstrated that the combination of the FK506 analogs and fluconazole are fungicidal or that they will work at concentrations that will not still be immunosuppressive. It remains to be seen if the therapeutic window between antifungal activity and immunosuppressive activity can be widened to a clinically useful level.
Figure 4. FK506 and semi-synthetic derivative APX879.
Chemical differences are colored blue.
Repurposing antipsychotic drugs as anti-fungals.
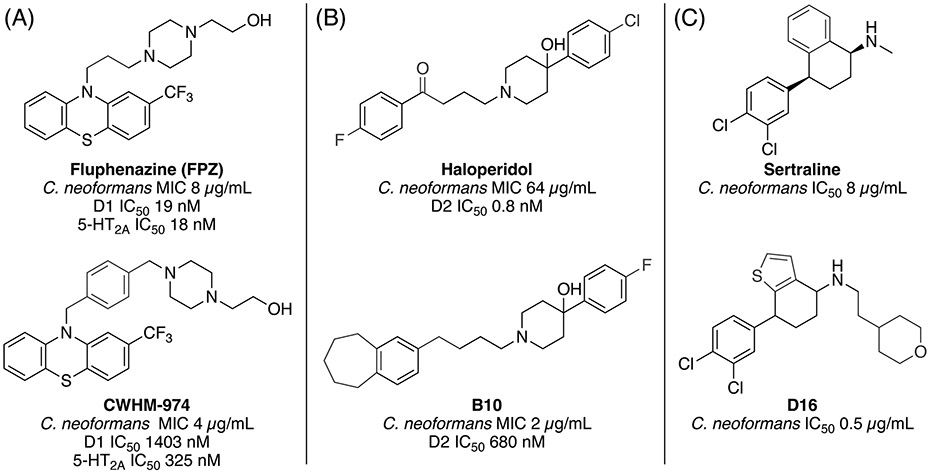
The antifungal side activity of antipsychotic phenothiazines has been known for more than 30 years.32,33 Antipsychotic agents possess an inherent property desirable for treating certain fungal infections such as cryptococcal meningitis since they can readily penetrate the blood-brain barrier. Recently, Krysan and coworkers pursued a repurposing approach by screening a library of FDA-approved drugs and identified thioridazine and trifluoperazine as potential anticryptococcal agents that are orally bioavailable and penetrate the CNS.34 The relatively modest in vitro potency of existing phenothiazine drugs would require concentrations higher than would be considered safe in humans. Thus, improvement of their antifungal potency and reduction in side-effect profile is essential. Montoya and coworkers reported the first structure-activity relationship (SAR) analysis of existing and novel analogs of trifluoperazine.27,35 The SAR of existing phenothiazines showed the related drug fluphenazine (FPZ) to have a potency of 8 μg/mL against C. neoformans, a modest 2-fold improvement over thioridazine and trifluoperazine. A limited medicinal chemistry effort of only 11 analogs led to the discovery of CWHM-974 which further improved potency to 4 μg/mL which simultaneously reduced binding affinity by 10- to 100-fold versus dopamine D1-5 and six serotonin receptors when compared to FPZ (Fig. 5A). Furthermore, CWHM-974 showed improved potency against both fluconazole-sensitive and -resistant strains of C. albicans.
Figure 5. Examples of recent antipsychotic drug SOSA efforts. (A) Fluphenazine and analog CWHM-974 with improved antifungal potency and reduced affinity for dopamine and serotonin receptors. (B) Haloperidol and analog B10 with improved antifungal potency and dopamine selectivity. (C) Sertraline and analog D16 with improved antifungal potency.
Chemical differences are colored blue.
Haloperidol, an antipsychotic, was identified as a potential anti-fungal agent in screen of the Enzo library of off-patent drugs and the oncology library from the Institute for Molecular Medicine Finland for activity against C. albicans.36 Haloperidol was used as the starting point for a medicinal chemistry campaign to increase its anti-fungal activity while also reducing its inhibition of the dopamine D2 receptor (D2).37 Ji and colleagues developed benzocylane derivatives of haloperidol and tested for inhibition of C. albicans and C. neoformans. One derivative, B10, showed an 8-16-fold decrease in the MIC50 and a >850-fold reduction in inhibition of D2 and synergy with FLC in C. albicans (Fig. 5B). However, the haloperidol derivative still inhibited the D2 receptor at 0.6 μM, which is well below the MIC50 concentration. The growth curves of B10, even in combination with FLC, showed a less than 2-log drop in CFU, indicating that the combination is likely only fungistatic. The moderate level of inhibition is reflected in animal studies where there was only a modest improvement in survival or reduction in fungal CFUs in the brain. In a related study, the same group started from their benzocylane scaffold to develop derivatives that synergize with FLC and tested against multiple Candida species.38 The best derivative, B2, was also tested against other Candida species. It was strongly synergistic in C. albicans but only additive in C. glabrata and C. auris. Administration of high concentrations of B2 in combination with 16 μg/mL FLC inhibited biofilm growth. However, in a mouse model of infection, the combination of FLC and B2 only decreased the kidney fungal burden by 2 logs, suggesting that the combination may only be fungistatic.
As mentioned earlier, the antipsychotic sertraline failed in clinical trials as an antifungal agent. Li and coworkers have recently used a scaffold-hopping approach to identify sertraline analogs with improved potency against Cryptococcus that do not rely on the benzo-cyclohexane scaffold known to be important for binding the serotonin transporter (Fig. 5C).39 Structural optimization led to compound D16, which exhibits significantly greater potency than sertraline against C. neoformans both in vitro and in vivo. Furthermore, D16 was shown to block the biosynthesis of ergosterol by inhibition of Δ5,6-desaturase, a novel antifungal mechanism, although the compound was found to be fungistatic. Selectivity vs. the serotonin transporter was not reported but optimization is reportedly still in progress.
Summary and future directions
Direct repurposing of approved drugs for antifungal applications, either as antifungal drugs themselves or as chemosensitizers to improve the efficacy of approved antifungal drugs against resistant strains is an attractive option. However, such approaches are limited by the inherently weak efficacy of the limited numbers of approved drugs with such activity. Alternatively, the SOSA approach applies medicinal chemistry optimization to improve the potency and side effect profile of existing drugs, opening the way for customizing them to treating fungal diseases. To date, there are only a few examples reported for antifungal applications despite the clear advantages of the SOSA approach for developing novel antifungal drug candidates. Some challenges include incomplete understanding of the mechanism of action which in some cases may be due to disruption of multiple pathways, resulting in challenging lead optimization programs. Also, known side effects, or even primary pharmacology, as in the case of the antipsychotics, requires additional effort to remove such properties while simultaneously improving antifungal potency. We have illustrated the potential and promising recent progress that has been accomplished using the SOSA approach. Significantly more optimization effort will be required to identify more potent, selective fungicidal compounds with oral efficacy in vivo that are suitable for advancement to clinical trials.
Acknowledgments
We would like to thank John Tavis for providing comments on the manuscript. Writing this review is supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R21 AI164578 (MJM) and R01 AI123407 (MJD).
References:
- 1.Bongomin F, Gago S, Oladele RO, Denning DW. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J Fungi (Basel). 2017;3(4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casadevall A. Fungal Diseases in the 21st Century: The Near and Far Horizons. Pathog Immun. 2018;3(2):183–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seagle EE, Williams SL, Chiller TM. Recent Trends in the Epidemiology of Fungal Infections. Infect Dis Clin North Am. 2021;35(2):237–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benedict K, Park BJ. Invasive fungal infections after natural disasters. Emerg Infect Dis. 2014;20(3):349–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frias-De-Leon MG, Pinto-Almazan R, Hernandez-Castro R, Garcia-Salazar E, Meza-Meneses P, Rodriguez-Cerdeira C, et al. Epidemiology of Systemic Mycoses in the COVID-19 Pandemic. J Fungi (Basel). 2021;7(7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Satoh K, Makimura K, Hasumi Y, Nishiyama Y, Uchida K, Yamaguchi H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol. 2009;53(1):41–4. [DOI] [PubMed] [Google Scholar]
- 7.Jeffery-Smith A, Taori SK, Schelenz S, Jeffery K, Johnson EM, Borman A, et al. Candida auris: a Review of the Literature. Clin Microbiol Rev. 2018;31(1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farmakiotis D, Kontoyiannis DP. Epidemiology of antifungal resistance in human pathogenic yeasts: current viewpoint and practical recommendations for management. Int J Antimicrob Agents. 2017;50(3):318–24. [DOI] [PubMed] [Google Scholar]
- 9.Hata K, Horii T, Miyazaki M, Watanabe NA, Okubo M, Sonoda J, et al. Efficacy of oral E1210, a new broad-spectrum antifungal with a novel mechanism of action, in murine models of candidiasis, aspergillosis, and fusariosis. Antimicrob Agents Chemother. 2011;55(10):4543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliver JD, Sibley GEM, Beckmann N, Dobb KS, Slater MJ, McEntee L, et al. F901318 represents a novel class of antifungal drug that inhibits dihydroorotate dehydrogenase. Proc Natl Acad Sci U S A. 2016;113(45):12809–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitsuyama J, Nomura N, Hashimoto K, Yamada E, Nishikawa H, Kaeriyama M, et al. In vitro and in vivo antifungal activities of T-2307, a novel arylamidine. Antimicrob Agents Chemother. 2008;52(4):1318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamashita K, Miyazaki T, Fukuda Y, Mitsuyama J, Saijo T, Shimamura S, et al. The Novel Arylamidine T-2307 Selectively Disrupts Yeast Mitochondrial Function by Inhibiting Respiratory Chain Complexes. Antimicrob Agents Chemother. 2019;63(8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Appilitherapeutics.com. Appili Therapeutics. Accessed 11/4/2021, 2021. https://www.appilitherapeutics.com/ati-2307
- 14.Wouters OJ, McKee M, Luyten J. Estimated Research and Development Investment Needed to Bring a New Medicine to Market, 2009-2018. JAMA. 2020;323(9):844–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Q, Liu F, Zeng M, Mao Y, Song Z. Drug repurposing strategies in the development of potential antifungal agents. Appl Microbiol Biotechnol. 2021;105(13):5259–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wall G, Lopez-Ribot JL. Screening Repurposing Libraries for Identification of Drugs with Novel Antifungal Activity. Antimicrob Agents Chemother. 2020;64(9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miro-Canturri A, Ayerbe-Algaba R, Smani Y. Drug Repurposing for the Treatment of Bacterial and Fungal Infections. Front Microbiol. 2019;10:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wall G, Herrera N, Lopez-Ribot JL. Repositionable Compounds with Antifungal Activity against Multidrug Resistant Candida auris Identified in the Medicines for Malaria Venture's Pathogen Box. J Fungi (Basel). 2019;5(4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joshi T, Pundir H, Chandra S. Deep-learning based repurposing of FDA-approved drugs against Candida albicans dihydrofolate reductase and molecular dynamics study. J Biomol Struct Dyn. 2021:1–17. [DOI] [PubMed] [Google Scholar]
- 20.Vallieres C, Singh N, Alexander C, Avery SV. Repurposing Nonantifungal Approved Drugs for Synergistic Targeting of Fungal Pathogens. ACS Infect Dis. 2020;6(11):2950–8. [DOI] [PubMed] [Google Scholar]
- 21.Eldesouky HE, Salama EA, Li X, Hazbun TR, Mayhoub AS, Seleem MN. Repurposing approach identifies pitavastatin as a potent azole chemosensitizing agent effective against azole-resistant Candida species. Sci Rep. 2020;10(1):7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ngan NTT, Thanh Hoang Le N, Vi Vi NN, Van NTT, Mai NTH, Anh DV, et al. An open label randomized controlled trial of tamoxifen combined with amphotericin B and fluconazole for cryptococcal meningitis. Elife. 2021;10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boulware DR, Nalintya E, Rajasingham R, Kirumira P, Naluyima R, Turya F, et al. Adjunctive sertraline for asymptomatic cryptococcal antigenemia: A randomized clinical trial. Med Mycol. 2020;58(8):1037–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wermuth CG. Selective optimization of side activities: the SOSA approach. Drug Discov Today. 2006;11(3-4):160–4. [DOI] [PubMed] [Google Scholar]
- 25.Dangi M, Khichi A, Jakhar R, Chhillar AK. Growing Preferences towards Analog-based Drug Discovery. Curr Pharm Biotechnol. 2021;22(8):1030–45. [DOI] [PubMed] [Google Scholar]
- 26.Kunin CM, Ellis WY. Antimicrobial activities of mefloquine and a series of related compounds. Antimicrob Agents Chemother. 2000;44(4):848–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montoya MC, Beattie S, Alden KM, Krysan DJ. Derivatives of the Antimalarial Drug Mefloquine Are Broad-Spectrum Antifungal Molecules with Activity against Drug-Resistant Clinical Isolates. Antimicrob Agents Chemother. 2020;64(3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beom JY, Jung JA, Lee KT, Hwangbo A, Song MC, Lee Y, et al. Biosynthesis of Nonimmunosuppressive FK506 Analogues with Antifungal Activity. J Nat Prod. 2019;82(8):2078–86. [DOI] [PubMed] [Google Scholar]
- 29.Odom A, Del Poeta M, Perfect J, Heitman J. The immunosuppressant FK506 and its nonimmunosuppressive analog L-685,818 are toxic to Cryptococcus neoformans by inhibition of a common target protein. Antimicrob Agents Chemother. 1997;41(1):156–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Onyewu C, Blankenship JR, Del Poeta M, Heitman J. Ergosterol biosynthesis inhibitors become fungicidal when combined with calcineurin inhibitors against Candida albicans, Candida glabrata, and Candida krusei. Antimicrob Agents Chemother. 2003;47(3):956–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juvvadi PR, Fox D 3rd, Bobay BG, Hoy MJ, Gobeil SMC, Venters RA, et al. Harnessing calcineurin-FK506-FKBP12 crystal structures from invasive fungal pathogens to develop antifungal agents. Nat Commun. 2019;10(1):4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eilam Y, Polacheck I, Ben-Gigi G, Chernichovsky D. Activity of phenothiazines against medically important yeasts. Antimicrob Agents Chemother. 1987;31(5):834–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spitzer M, Griffiths E, Blakely KM, Wildenhain J, Ejim L, Rossi L, et al. Cross-species discovery of syncretic drug combinations that potentiate the antifungal fluconazole. Mol Syst Biol. 2011;7:499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Butts A, DiDone L, Koselny K, Baxter BK, Chabrier-Rosello Y, Wellington M, et al. A repurposing approach identifies off-patent drugs with fungicidal cryptococcal activity, a common structural chemotype, and pharmacological properties relevant to the treatment of cryptococcosis. Eukaryot Cell. 2013;12(2):278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montoya MC, DiDone L, Heier RF, Meyers MJ, Krysan DJ. Antifungal Phenothiazines: Optimization, Characterization of Mechanism, and Modulation of Neuroreceptor Activity. ACS Infect Dis. 2018;4(4):499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stylianou M, Kulesskiy E, Lopes JP, Granlund M, Wennerberg K, Urban CF. Antifungal application of nonantifungal drugs. Antimicrob Agents Chemother. 2014;58(2):1055–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ji C, Liu N, Tu J, Li Z, Han G, Li J, et al. Drug Repurposing of Haloperidol: Discovery of New Benzocyclane Derivatives as Potent Antifungal Agents against Cryptococcosis and Candidiasis. ACS Infect Dis. 2020;6(5):768–86. [DOI] [PubMed] [Google Scholar]
- 38.Yang W, Tu J, Ji C, Li Z, Han G, Liu N, et al. Discovery of Piperidol Derivatives for Combinational Treatment of Azole-Resistant Candidiasis. ACS Infect Dis. 2021;7(3):650–60. [DOI] [PubMed] [Google Scholar]
- 39.Li W, Yun Z, Ji C, Tu J, Yang W, Li J, et al. Discovery of Novel Sertraline Derivatives as Potent Anti-Cryptococcus Agents. J Med Chem. 2022; [DOI] [PubMed] [Google Scholar]