1.
The incidence of Richter transformation in relapsed/refractory chronic lymphocytic leukemia (CLL) is up to 15% and Richter transformation is a frequent cause of treatment failure on ibrutinib (IBR) [1]. IBR has been promising in CLL cell growth suppression by inhibiting Bruton tyrosine kinase (BTK) [2]. However, resistance can occur due to specific mutations in BTK or PLCG2 genes [3]. Another agent, venetoclax (VEN) has shown excellent efficacy in relapsed refractory CLL [4]. In Richter transformation, the effectiveness of VEN alone or in combination with conventional chemotherapy has been demonstrated, although research in this area is limited and based on only a few cases [5, 6, 7]. BTK/PLCG2 mutations play a role in IBR‐resistant CLL progression. However, there is limited knowledge about BTK or PLCG2 gene mutation statuses in Richter transformation.
A 72‐year‐old male was diagnosed with CLL characterized by a deletion in chromosome 17p [del(17p)] and a complex karyotype. After 12 months of diagnosis, he started IBR treatment. He initially showed a partial response. Two years after starting IBR, CLL cells in the peripheral blood increased, and he developed bilateral cervical lymphadenopathy, along with elevated levels of lactate dehydrogenase (LDH) and soluble interleukin‐2 receptor (sIL‐2R). Fluorodeoxyglucose‐positron emission tomography (FDG‐PET) revealed systemic lymphadenopathy. Bone marrow examination showed increased atypical lymphocytes, displaying a CD5+CD10‐19+sIgκ+ phenotype, with del(17p) present in 27% of all nucleated cells, as confirmed by fluorescence in situ hybridization. The pathological examination of the cervical lymph nodes showed a diffuse proliferation of large lymphocytes that tested positive for CD5, CD19, CD20, CD22, CD23, CD45, and BCL‐2 and negative for MUM‐1 and BCL‐6, with a Ki‐67 index of ∼50%. A biopsy confirmed the transformation into diffuse large B‐cell lymphoma (Figure 1). Genetic testing was conducted on lymph nodes and bone marrow samples collected simultaneously; TP53 mutation (R283P) was found in lymph nodes [variant allele frequency (VAF): 0.85] and bone marrow specimens (VAF: 0.77). However, PLCG2 mutation (D1144G) was only detected in the lymph node specimens (VAF: 0.07). In this case, a targeted gene panel was used in Japan. The sensitivity of allele mutation detection was 0.05. A single polymerase chain reaction was not performed. Additionally, we could not measure the IGHV mutation.
FIGURE 1.
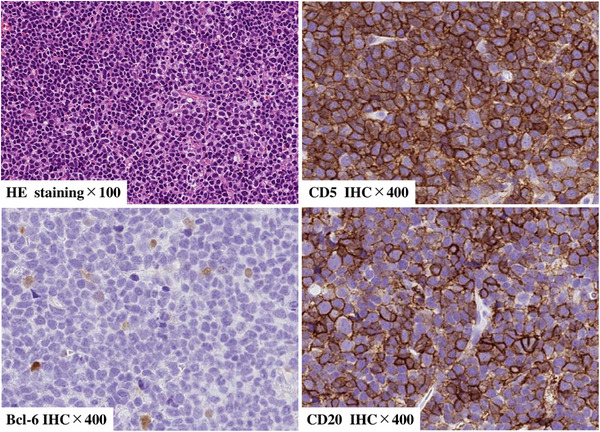
Pathological findings of lymph node (at Richter transformation)The histological examination revealed extensive lymphocyte infiltrations with positive staining for CD20 and CD5 and negative staining for BCL‐6.
The patient's treatment involved VEN usage for gradually increasing doses up to 200 mg (with fluconazole concomitant use) as per guidelines, while the use of IBR gradually reduced (Figure 2). The patient's clinical symptoms (fever and others) and lymphadenopathy showed no signs of improvement with VEN initiation and IBR dose reduction. The laboratory values indicated a worsening trend, with elevated LDH and sIL‐2R, reaching 511 U/L (normal; 124–222 IU/L) and 4,247 U/mL (normal; 122–496 U/mL), respectively. Initially, the plan was to taper off IBR, but due to worsening CLL lesions (especially the presence of leukemic cells in the peripheral blood and bone marrow), IBR was continued at 420 mg/day in combination with 200 mg/day VEN. Subsequently, there was a marked improvement in the peripheral blood and lymph node lesions. This suggests that VEN is highly effective against Richter transformation, while IBR remains effective in treating existing CLL lesions in the bone marrow since no PLCG2 mutations were found. Following VEN initiation, RIT was administered every 4 weeks for six doses, initiated 6 months after VEN treatment began. At the end of RIT, FDG‐PET and bone marrow examination confirmed that the patient achieved complete remission, which was maintained at 24 months post‐treatment follow‐up. During the treatment course, neutropenia of CTCAE Grade1 was observed, and events such as febrile neutropenia and infection were not observed. Blood count has recovered to normal range at CR. At that time, peripheral blood flow cytometry‐minimal residual disease (MRD) was negative.
FIGURE 2.
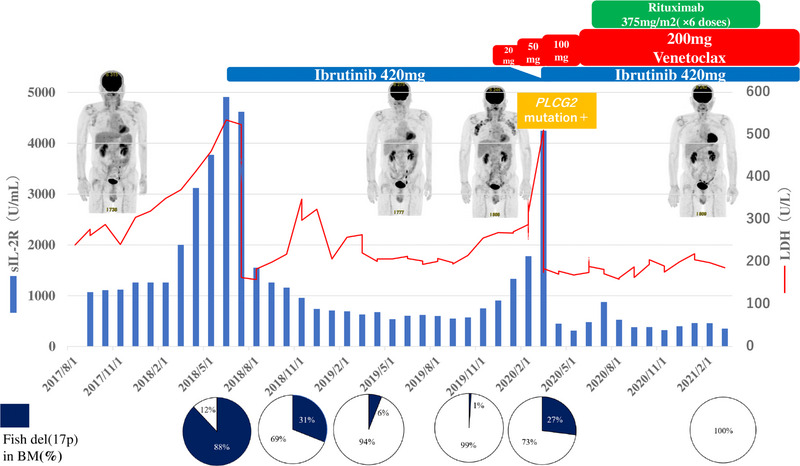
Clinical course from the initial diagnosis to Richter transformation 1 year after the disease onset, chronic lymphocytic leukemia (CLL) required treatment and ibrutinib (IBR) was initiated. After IBR initiation, the markers and del17p showed signs of improvement; however, during the treatment, CLL transformed into Richter transformation. Consequently, the markers and del17p worsened again. At this time, a biopsy of the lymph node tissue revealed the acquisition of a PLCG2 mutation. Upon reducing the IBR dose and commencing venetoclax (VEN), the lymphadenopathy exhibited improvement, but the tumor markers worsened. Subsequently, the IBR dose was reinstated, and VEN treatment continued, resulting in improvement. An additional dose of RIT was administered, leading the patient to achieve complete remission.
This case represents a rare report of a PLCG2 mutation specifically appearing only in the lymph nodes and not in the bone marrow during IBR treatment for CLL, leading to Richter transformation. The patient had complex karyotype and TP53 abnormalities during the initial treatment, indicating a potential for a shorter duration of response compared to typical cases [8]. Despite approximately 2 years of disease control with IBR, the patient eventually progressed to Richter transformation, with an IBR‐resistant PLCG2 mutation detected in the lymph node lesions. CLL is known to exhibit genomic instability and clonal evolution, which can lead to the development of Richter transformation [9]. Richter transformation from CLL is associated with TP53, NOTCH1 mutation, CDKN2A deletion, and MYC translocation [10]. Conventional cytotoxic anticancer treatments for Richter transformation have demonstrated unsatisfactory results, highlighting the need for new and innovative therapies [11]. In our patient, PLCG2 mutations were detected only in the lymph node lesions, where more aggressive Richter transformation findings were observed, while no mutation was found in the bone marrow specimens; this aligns with the clinical course, which showed a transient increase in CLL cells in the peripheral blood after discontinuing IBR treatment. The three‐drug combination therapy of IBR + VEN + RIT has effectively maintained a long‐term complete remission in this patient. This case represents the first report of a patient with Richter transformation with differing PLCG2 mutation statuses between the bone marrow and lymph nodes.
The combination of VEN and IBR has been documented to effectively reduce the resting and dividing subpopulations in most cases, making it a significant treatment approach for CLL [12]. In a particular study, high MRD‐negative rates were achieved with the two‐drug combination of IBR and VEN [13]. There are also reports of triple therapy with obinutuzumab, IBR, and VEN, which achieved negative MRD with high safety in high‐risk CLL patients, suggesting earlier initiation of multidrug combination therapy is also promising [14]. In conclusion, this report highlights the effectiveness of VEN and RIT even in cases of Richter transformation with PLCG2 mutations that have developed resistance to IBR. Furthermore, the results indicate that IBR remains significantly effective in treating existing residual CLL lesions in Richter transformation.
AUTHOR CONTRIBUTIONS
Takafumi Tsushima collected the data and wrote the manuscript. Junichiro Yuda and Takafumi Tsushima provided patient care. All the authors have reviewed and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
All procedures were performed according to the ethical standards of the institutional and national research committee and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the institutional review board.
PATIENT CONSENT STATEMENT
The author has confirmed patient consent statement is not needed for this submission.
CLINICAL TRIAL REGISTRATION
The authors have confirmed clinical trial registration is not needed for this submission.
ACKNOWLEDGMENTS
The authors would like to thank the patient and the medical staff of National Cancer Center Hospital East. We thank Enago (www.enago.jp/) for English language editing.
DATA AVAILABILITY STATEMENT
Data generated during and/or analyzed during the current study are available upon reasonable request from authors.
REFERENCES
- 1. Condoluci A, Rossi D. Biology and treatment of richter transformation. Front Oncol. 2022;12:829983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davids MS, Brown JR. Ibrutinib: a first in class covalent inhibitor of Bruton's tyrosine kinase. Future Oncol. 2014;10:957–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Quinquenel A, Fornecker LM, Letestu R, Ysebaert L, Fleury C, Lazarian G, et al. Prevalence of BTK and PLCG2 mutations in a real‐life CLL cohort still on ibrutinib after 3 years: a FILO group study. Blood. 2019;134:641–644. [DOI] [PubMed] [Google Scholar]
- 4. Jones JA, Mato AR, Wierda WG, Davids MS, Choi M, Cheson BD, et al. Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: an interim analysis of a multicentre, open‐label, phase 2 trial. Lancet Oncol. 2018;19:65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davids MS, Rogers KA, Tyekucheva S, Wang Z, Pazienza S, Renner SK, et al. Venetoclax plus dose‐adjusted R‐EPOCH for Richter syndrome. Blood. 2022;139:686–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rutherford SC, Abramson JS, Bartlett NL, Barta SK, Khan N, Joyce R, et al. Venetoclax with dose‐adjusted EPOCH‐R as initial therapy for patients with aggressive B‐cell lymphoma: a single‐arm, multicentre, phase 1 study. Lancet Haematol. 2021;8:e818–e827. [DOI] [PubMed] [Google Scholar]
- 7. Morschhauser F, Feugier P, Flinn IW, Gasiorowski R, Greil R, Illés Á, et al. A phase 2 study of venetoclax plus R‐CHOP as first‐line treatment for patients with diffuse large B‐cell lymphoma. Blood. 2021;137:600–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kittai AS, Miller C, Goldstein D, Huang Y, Abruzzo LV, Beckwith K, et al. The impact of increasing karyotypic complexity and evolution on survival in patients with CLL treated with ibrutinib. Blood. 2021;138:2372–2382. [DOI] [PubMed] [Google Scholar]
- 9. Condoluci A, Rossi D. Genomic instability and clonal evolution in chronic lymphocytic leukemia: clinical relevance. J Natl Compr Canc Netw. 2020;19:227–233. [DOI] [PubMed] [Google Scholar]
- 10. Fabbri G, Khiabanian H, Holmes AB, Wang J, Messina M, Mullighan CG, et al. Genetic lesions associated with chronic lymphocytic leukemia transformation to Richter syndrome. J Exp Med. 2013;210:2273–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tadmor T, Levy I. Richter transformation in chronic lymphocytic leukemia: update in the era of novel agents. Cancers. 2021;13:5141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lu P, Wang S, Franzen CA, Venkataraman G, McClure R, Li L, et al. Ibrutinib and venetoclax target distinct subpopulations of CLL cells: implication for residual disease eradication. Blood Cancer J. 2021;11:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wierda WG, Allan JN, Siddiqi T, Kipps TJ, Opat S, Tedeschi A, et al. Ibrutinib plus venetoclax for first‐line treatment of chronic lymphocytic leukemia: primary analysis results from the minimal residual disease cohort of the randomized phase II CAPTIVATE study. J Clin Oncol. 2021;39:3853–3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huber H, Edenhofer S, von Tresckow J, Robrecht S, Zhang C, Tausch E, et al. Obinutuzumab (GA‐101), ibrutinib, and venetoclax (GIVe) frontline treatment for high‐risk chronic lymphocytic leukemia. Blood. 2022;139:1318–1329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data generated during and/or analyzed during the current study are available upon reasonable request from authors.


